Abstract
Objective
We describe a rare case of profound subcutaneous insulin resistance (SIR) presumed due to a paraneoplastic process caused by pancreatic adenocarcinoma that improved with intravenous insulin and tumor resection.
Methods
An 80-year-old man with previously well-controlled type 2 diabetes mellitus had worsening glycemic control (hemoglobin A1C increase of 6.5% to 8.6% over 4 months) following a recent diagnosis of pancreatic adenocarcinoma. His blood glucose was uncontrolled at 600 mg/dL despite rapid up-titration of a subcutaneous basal-bolus insulin regimen totaling 1000 units/d. Extensive evaluation of insulin resistance including insulin antibodies and anti-insulin receptor antibodies was negative. Due to clinical deterioration, the patient underwent pancreaticoduodenectomy before the completion of neoadjuvant chemotherapy. The patient received intravenous insulin before surgery, which resulted in rapid improvement in glycemic control. The patient’s blood glucose normalized, and he was maintained on metformin monotherapy following pancreaticoduodenectomy.
Results
This patient had evidence of SIR in the setting of pancreatic adenocarcinoma. SIR was likely a paraneoplastic process as glycemic control improved after tumor resection. Interestingly, the patient did not have hyperinsulinemia but rather evidence of β-cell dysfunction, which highlights the possibility of exogenous insulin resistance.
Conclusion
Paraneoplastic processes due to pancreatic adenocarcinoma can cause SIR, marked by profound hyperglycemia and deteriorating functional status. It is, therefore important to recognize this rare syndrome and appropriately escalate to a higher level of care and consider proceeding with tumor resection.
Key words: subcutaneous insulin resistance, pancreatic adenocarcinoma, paraneoplastic syndrome
Abbreviations: AG, average blood glucose; CGM, continuous glucose monitor; HOMA-IR, homeostatic model assessment of insulin resistance; IV, intravenous; SD, standard deviation; SIR, subcutaneous insulin resistance; T2DM, type 2 diabetes mellitus
Introduction
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States and has a very poor (9%) 5-year survival rate.1 Type 2 diabetes mellitus (T2DM) is a risk factor for pancreatic cancer, and pancreatic cancer often unmasks impaired glucose tolerance.2, 3, 4 We describe a rare case of profound subcutaneous insulin resistance (SIR) in a patient with poorly differentiated pancreatic adenocarcinoma. The patient’s blood glucose levels responded only to intravenous (IV) insulin and tumor resection, suggesting a paraneoplastic process.
Case Report
An 80-year-old man with T2DM and previous transcatheter aortic valve replacement presented to the diabetes clinic for management of progressive hyperglycemia following a recent diagnosis of pancreatic adenocarcinoma. He had been on metformin monotherapy for several years with hemoglobin A1C levels ranging from 6.0% to 6.5% (42-48 mmol/mol). At the time that the pancreatic mass was discovered (an incidental finding on computed tomography [CT] imaging for transcatheter aortic valve replacement), the patient noted a rapid deterioration in blood glucose control, with self-monitored blood glucose values of >600 mg/dL and hemoglobin A1C levels of 8.6% (70 mmol/mol). His primary care physician prescribed 30 units of insulin glargine and referred the patient to the endocrinology department.
One month prior, CT imaging revealed a 2 × 2.2-cm mass in the pancreatic head/uncinate process. Repeat CT a few weeks later showed biliary obstruction at the distal common duct and mild pancreatic ductal dilatation as a result of the pancreatic mass. He underwent endoscopic retrograde cholangiopancreatography with metal stent placement. Fine-needle biopsy of the mass showed poorly differentiated adenocarcinoma involving the pancreatic parenchyma and duodenal submucosa with metastasis to the lymph node and liver. The carbohydrate antigen 19-9 level was elevated at 779.4 units/mL. The patient underwent neoadjuvant chemotherapy, but the period of chemotherapy was shortened due to severe hyperglycemia.
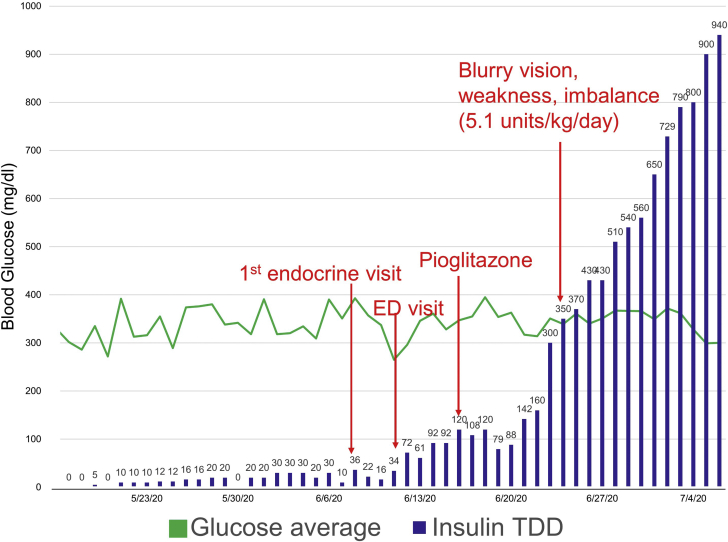
When he was first seen at the endocrinology clinic, the patient’s average blood glucose (AG) and standard deviation (SD), based on fasting and postprandial self-monitored blood glucose, were 445 and 73 mg/dL respectively. He did not achieve adequate glycemic control (AG, 414 mg/dL; SD, 82 mg/dL) despite incremental increases in basal insulin (to 80 units daily), addition of correctional insulin, and maximal dose of the oral insulin-sensitizing agent pioglitazone (Fig. 1). His course was complicated by an emergency department visit for severe hyperglycemia. He was transitioned to U-500 insulin once his daily requirement reached 200 units. He demonstrated proper injection technique and remained hyperglycemic even when the insulin was administered by a health care professional. There was no evidence of scarring or lipohypertrophy at the injection sites.
Fig. 1.
Average daily glucose and insulin requirement from the time of diagnosis of pancreatic adenocarcinoma to 4 days before subtotal pancreatectomy. The daily insulin requirement of the patient markedly increased from 36 units at the time of initial endocrinology evaluation to 940 units in the course of 4 weeks. The patient’s course was complicated by functional decline and visit to the emergency department (ED) for severe hyperglycemia. TDD = total daily dose
The patient underwent extensive evaluation for profound insulin resistance. He did not have acanthosis nigricans, easy bruisability, or migratory necrolytic erythema that would otherwise indicate Cushing syndrome or glucagonoma. There was no physical or laboratory evidence of renal or hepatic dysfunction (including normal bilirubin). The patient’s lipid panel was unremarkable except for moderately low high-density lipoprotein levels (38 mg/dL). The results of his secondary endocrine assessment were as follows: adrenocorticotropic hormone, 13 pg/mL (reference, 6-50 pg/mL); growth hormone, 1.6 ng/mL (reference, <7.1 ng/mL); insulin-like growth factor-1, 70 ng/mL (reference, 34-245 ng/mL); glucagon level, 47 pg/mL (reference, 8-57 pg/mL); and chromogranin A, 209 ng/mL (reference, 24-140 ng/mL). The patient’s calculated homeostatic model assessment of insulin resistance (HOMA-IR) values using the HOMA2 Calculator were as follows: (1) %β, 7.5; %S, 117.8; IR, 0.85 (reference, 0.7-2), for a serum blood glucose value of 277 mg/dL and a serum insulin level of 4.5 μUnit/mL (reference, <19.6 μUnit/mL), and (2) %β, 11.1; %S, 72.0; IR, 1.39 (reference, 0.7-2), for a serum blood glucose value of 277 mg/dL and a fasting c-peptide level of 1.14 ng/mL (reference, 0.80-3.85 ng/mL).5 He had negative insulin antibodies and anti-insulin receptor antibodies (performed by the laboratory of Prof Lutz Schomburg, Charité Universitätsmedizin).
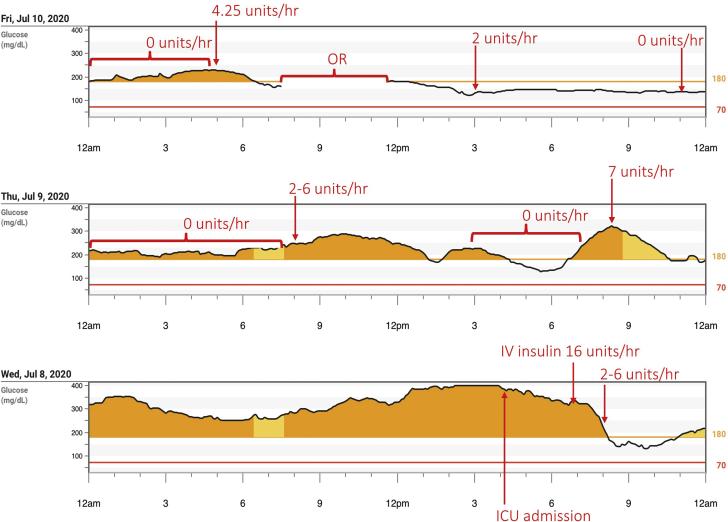
Four weeks after the initial endocrine assessment, the patient was receiving close to 1000 units of insulin per day (Fig. 1) and was experiencing functional decline related to poor glycemic control. He experienced polyuria, polydipsia, blurry vision, impaired balance, and rapid weight loss. This clinical deterioration prompted the decision to pursue pancreaticoduodenectomy (Whipple procedure) before completion of the intended neoadjuvant chemotherapy. The patient was admitted to the surgical intensive care unit for medical optimization 48 hours before the planned tumor resection. He was started on Dexcom G6 continuous glucose monitor (CGM) for real-time glycemic surveillance. The patient had rapid normalization of blood glucose levels within 2 hours of initiating IV insulin (rate of 16 units/h) (Fig. 2). His hourly infusion rate was decreased per protocol (glycemic target, 140-180 mg/dL) and stabilized at 4.25 units/h (Fig. 2). The patient then underwent uncomplicated pancreaticoduodenectomy. His postoperative subcutaneous insulin dosing was glargine 17 units daily and nutritional aspart 3 units. Several weeks later, insulin was discontinued following improvement in blood glucose levels via CGM (average, 218 mg/dL; SD, 58 mg/dL; time in range, 32%). The patient achieved adequate glycemic control (CGM AG, 155 mg/dL; SD, 35 mg/dL; time in range, 77%) on metformin monotherapy (1000 mg twice daily).
Fig. 2.
Glucose trends from Dexcom G6 continuous glucose monitor in the perioperative period. Preoperative response to IV insulin and postoperative course with blood glucose (mg/dL) graphed against time. The patient initially received IV insulin at 16 units/h with hourly infusion rate stabilizing at 4.25 units/h before subtotal pancreatectomy. Time in the OR as highlighted in the figure. OR = operating room; ICU = intensive care unit; IV = intravenous.
Discussion
SIR is a rare clinical phenomenon characterized by poor glycemic control in response to subcutaneous insulin but normal sensitivity to IV insulin. It is thought that excessive degradation of subcutaneous insulin, mediated by actions of nonspecific proteases, prevents the hormone from reaching systemic circulation, contributing to insulin resistance.6 Our patient developed marked SIR that appeared to be paraneoplastic as it improved following pancreatic tumor resection.7 In vitro studies of pancreatic adenocarcinoma suggest that tumor-derived factors may influence peripheral insulin resistance.8,9 Two such factors have been shown to release amylin and activate anaerobic glucose metabolism.8,10,11 Furthermore, pancreatic tumor cells demonstrate increased activity of glycogen phosphorylase and downregulation of skeletal muscle glycogen synthase, leading to decreased glycogen storage and hyperglycemia.9 These changes would be expected to cause hyperinsulinemia. In this case, however, our patient did not manifest endogenous hyperinsulinemia. In fact, both his HOMA-IR and HOMA-B, which are measures of insulin resistance and secretion, respectively, showed evidence of β-cell dysfunction due to glucolipotoxicity rather than underlying endogenous insulin resistance. To our knowledge, this is the first report that highlights the possibility of exogenous insulin resistance due to a pancreatic paraneoplastic process.
Insulin resistance encompasses a wide spectrum of disorders characterized by hyperinsulinemia (fasting insulin level > 50-70 μU/mL or peak insulin level > 350 μU/mL after oral glucose tolerance test), acanthosis nigricans, and in women, features of hyperandrogenism.12,13 The most common cause of insulin resistance is obesity/metabolic syndrome, but insulin resistance also occurs due to pathological processes such as acromegaly, Cushing syndrome, and neoplasms.12 Type A and B insulin resistance syndromes are rare phenotypes of extreme insulin resistance due to defects in insulin signaling (type A) or autoantibodies against insulin receptors (type B). Our patient did not have evidence of these conditions.
There are limited data on the treatment of SIR. As implicated in this case, patients with extreme insulin resistance driven by underlying cancer require tumor resection to restore their insulin sensitivity. In an observational study of 16 patients with pancreatic adenocarcinoma, 3 out of 4 patients with diabetes and resectable tumors experienced improved glycemic control following surgery.9 There are limited but encouraging data on aprotinin, a protease inhibitor, in the treatment of SIR. In a study of 5 hospitalized patients with insulin-requiring diabetes complicated by frequent ketoacidosis and insulin requirements up to 2000 units/d, aprotinin administered subcutaneously improved ketonuria and hyperglycemia. Aprotinin is thought to work by preventing the degradation and sequestration of subcutaneous insulin.
One patient in the study developed severe, life-threatening hypoglycemia requiring glucose drip for 10 days.14 More robust data on aprotinin is needed before it can be used for routine management. Alternatively, patients with extreme SIR may benefit from using an inhaled insulin preparation. A case report by Agarwal et al15 showed that inhaled insulin used as nutritional insulin in conjunction with intramuscular glargine improved glycemic control and circumvented the need for frequent intramuscular insulin injections in a patient with type 1 diabetes mellitus and presumed SIR. Inhaled insulin has similar efficacy and decreased risk of hypoglycemia in patients with T2DM.16 Mild to moderate cough is a frequent adverse reaction, so the use of inhaled insulin is limited to patients without underlying lung disease.
Conclusion
To our knowledge, this is the first case report of a possible paraneoplastic process due to pancreatic adenocarcinoma contributing to profound SIR. The association between diabetes and pancreatic cancer has already been established, and this case highlights the possibility of resistance to exogenous insulin rather than underlying endogenous insulin resistance in pancreatic cancer. The mechanism behind the SIR in this case remains unclear, but the improvement following surgery implicates the tumor itself. It is particularly important to recognize SIR in patients with cancer as it may prompt changes in therapeutic plans. Our patient received metformin monotherapy several weeks postoperatively but later developed a modest insulin requirement (12 units of insulin glargine, 5-6 units of aspart insulin) in the setting of new liver metastasis discovered 4 months after pancreaticoduodenectomy. He has been much more responsive to subcutaneous insulin, suggesting that removal of the bulk of his pancreatic adenocarcinoma was responsible for the improved response to subcutaneous insulin.
Acknowledgment
The calculation of HOMA-IR and HOMA-β was at the courtesy of HOMA2 Calculator Software by Hines et al at the University of Oxford. We also would like to acknowledge the laboratory of Professor Lutz Schomburg, Charité Universitätsmedizin, Berlin for processing the anti-insulin receptor antibody laboratory test.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Everhart J., Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 3.Gullo L., Pezzilli R., Morselli-Labate A.M., Italian Pancreatic Cancer Study Group Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331(2):81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 4.Permert J., Ihse I., Jorfeldt L., von Schenck H., Arnquist H.J., Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80(8):1047–1050. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 5.Hines G., Kennedy I., Stevens R., Matthews D., Levy J. A calculator for HOMA. 2004;suppl 1:A222. [Google Scholar]
- 6.Paulsen E.P., Courtney J.W., III, Duckworth W.C. Insulin resistance caused by massive degradation of subcutaneous insulin. Diabetes. 1979;28(7):640–645. doi: 10.2337/diab.28.7.640. [DOI] [PubMed] [Google Scholar]
- 7.Sah R.P., Nagpal S.J., Mukhopadhyay D., Chari S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10(7):423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Knezetic J.A., Strömmer L., Permert J., Larsson J., Adrian T.E. The intracellular mechanism of insulin resistance in pancreatic cancer patients. J Clin Endocrinol Metab. 2000;85(3):1232–1238. doi: 10.1210/jcem.85.3.6400. [DOI] [PubMed] [Google Scholar]
- 9.Permert J., Adrian T.E., Jacobsson P., Jorfelt L., Fruin A.B., Larsson J. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165(1):61–66. doi: 10.1016/s0002-9610(05)80405-2. discussion 66-67. [DOI] [PubMed] [Google Scholar]
- 10.Ding X., Flatt P.R., Permert J., Adrian T.E. Pancreatic cancer cells selectively stimulate islet beta cells to secrete amylin. Gastroenterology. 1998;114(1):130–138. doi: 10.1016/s0016-5085(98)70641-9. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Adrian T.E. A factor from pancreatic and colonic cancer cells stimulates glucose uptake and lactate production in myoblasts. Biochem Biophys Res Commun. 1999;260(3):626–633. doi: 10.1006/bbrc.1999.0955. [DOI] [PubMed] [Google Scholar]
- 12.Tritos N.A., Mantzoros C.S. Clinical review 97: syndromes of severe insulin resistance. J Clin Endocrinol Metab. 1998;83(9):3025–3030. doi: 10.1210/jcem.83.9.5143. [DOI] [PubMed] [Google Scholar]
- 13.Vidal-Puig A., Moller D.E. In: Androgen Excess Disorders in Women. Azziz R., Nestler J.E., Dewailly D., editors. Lippincott Raven; Philadelphia: 1997. insulin resistance: classification, prevalence, clinical manifestations, and diagnosis; pp. 227–236. [Google Scholar]
- 14.Freidenberg G.R., White N., Cataland S., O'Dorisio T.M., Sotos J.F., Santiago J.V. Diabetes responsive to intravenous but not subcutaneous insulin: effectiveness of aprotinin. N Engl J Med. 1981;305(7):363–368. doi: 10.1056/NEJM198108133050702. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal S., Gupta M., Gunn S. Use of inhaled insulin in a patient with subcutaneous insulin resistance syndrome: a rare condition. AACE Clin Case Rep. 2019;5(3):e187–e191. doi: 10.4158/ACCR-2018-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander P.A., Blonde L., Rowe R. Efficacy and safety of inhaled insulin (exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27(10):2356–2362. doi: 10.2337/diacare.27.10.2356. [DOI] [PubMed] [Google Scholar]