Abstract
Background
Early stage liver cancer is often treated with hepatic resection or transplantation for curative intent. Microwave ablation (MWA) is often performed in patients who are poor surgical candidates, patients with limited multifocal disease, disease close to hepatic vasculature, but can also be performed with curative intent in case of small lesions. The purpose of this study is to evaluate safety and efficacy of MWA of liver tumors with final ablation zone ≤5 mm from the heart.
Methods
A retrospective review was conducted on patients with hepatic cancer who underwent MWA between 1/2015 and 6/2019. Patients with a final ablation zone ≤5 mm to the heart were included. For these patients, imaging obtained prior, during and after procedure along with procedure reports were used to identify tumor and ablation characteristics, and electronic medical records were used to identify patient demographics and disease status.
Results
A total of 17 patients had liver tumors with ablation zone ≤5 mm to the heart. Mean lesion size was 18.2 mm (range, 10–33 mm) and mean follow-up period was 10.4 months. Of note 82% of patients had multifocal disease at time of MWA of lesion close to the heart. Two patients had pneumothorax, one of which required chest tube placement. None of the patients had cardiac arrhythmias or other complications. Overall 12/17 of the patients had disease progression within the liver at different sites from ablated lesions. One patient had residual disease and one had local recurrence. In addition, 4/17 patients, had no disease progression or recurrence and one underwent liver transplantation prior to follow-up imaging.
Conclusions
MWA of liver lesions with ablation zone ≤5 mm to the heart is safe and effective, however, it can be technically challenging.
Keywords: Microwave ablation (MWA), hepatic lesions, hepatocellular carcinoma (HCC), liver metastases, heart
Introduction
Incidence of primary liver cancer [comprised mostly of hepatocellular carcinoma (HCC) and cholangiocarcinoma] has been increasing from 2007 to 2016, however, rate of increase has slowed recently (1). It is now fifth most common cause of cancer death in men and seventh most common cause in women (2). HCC accounts for ~75% of primary liver cancers. Overall incidence has tripled from 1980 to 2016 while mortality rate has doubled during the same time period (2). However, liver cancer secondary to metastases is more common than primary liver malignancy. Most common cancers that metastasize to liver include gastrointestinal malignancies particularly colorectal adenocarcinoma followed by adenocarcinomas of unknown origin, breast adenocarcinoma, and lung cancer (3).
Gold standard for treatment of liver cancers (primary or metastatic) is currently surgical resection or transplantation if early stage, localized lesions, and in patients with good liver function (4). However, in poor surgical candidates, non-resectable tumors, or in advanced stage disease, additional treatment modalities are available including ablation, chemoembolization, and radioembolization or a combination of the treatments. Ablation can be curative for patients with early-stage liver cancer (including primary and metastatic disease) and can be first line treatment in patients with small tumor size, location with easy accessibility and distant from biliary tree (5). There are several guidelines in place that recommend using of ablation for curative intent for both primary and metastatic liver cancers (6-8). The Barcelona Clinic Liver Cancer guidelines recommend ablation for single HCC that is <5 cm in diameter or multifocal HCC (up to three lesions) that are <3 cm each in diameter as long as there is no extrahepatic metastatic disease and portal vein invasion (9). The National Comprehensive Cancer Network guidelines for colon cancer recommend ablation for metastatic liver lesions from colon cancer if <3 cm in size, with easy accessibility and distant from biliary tree (6-8). Ablation can also serve as a bridge to transplant and is typically reserved for patients who are poor surgical candidates due to comorbidities such as cirrhosis, portal hypertension, poor liver function, multifocal liver disease, and in prohibitive tumor locations (10). This study will focus on microwave ablation (MWA) of primary HCC and liver metastases.
MWA has been shown to be safe and effective in treatment of liver cancer and has similar rates of overall survival and disease-free survival when compared to hepatic resection, with fewer complications, decreased blood loss, decreased time of procedure, and faster recovery (5,11). Size of ablation margin has been shown to be an independent predictor for local tumor progression in both primary and metastatic liver lesions. One study has shown a minimum ablation margin of at least 3 mm to decrease risk of local tumor progression with radiofrequency ablation for primary HCC (12). Most studies have demonstrated a margin of 5-10mm as the optimal ablation margin size (regardless of whether radiofrequency or MWA was used) to decrease risk of local disease progression in case of both primary and metastatic liver lesions (13,14). A recent meta-analysis demonstrated that hepatic resection had lower rates of local tumor recurrence when compared to MWA which was attributed to wider margins obtained with hepatic resection particularly with large tumors, however ablation also resulted in fewer complications compared to surgical resection (5,15). Also notable is that MWA is often performed in patients who are poor surgical candidates due to multiple comorbidities including cirrhosis and portal hypertension, who have multifocal disease, or lesions close to vasculature (5). Ablation in combination with chemotherapy has also shown to offer patients improved overall survival and disease-free survival (similar to results from post-surgical resection) when compared to chemotherapy alone (15). Moreover, MWA has been shown to be safe and effective even when lesions are located close to the heart, diaphragm, and hepatic hilum (16). Only one other study has looked at the safety and efficacy of MWA of liver lesions close to the heart (17). The purpose of this study was to evaluate the safety and efficacy of MWA of hepatic dome liver lesions deemed to pose cardiac risk during MWA, defined as lesions with ablation zones within 5 mm or less from the heart. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tgh-20-314).
Methods
Patient selection and data collection
Following approval by the Institutional Review Board (42415), a retrospective study was performed on all patients who had MWA for hepatic cancer between 1/2015 and 6/2019 at a single tertiary center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of University of Kentucky (42415) and individual consent for this retrospective analysis was waived.
Exclusion criteria included lesions with final ablation zones >5 mm from the heart. For the purpose of this study, lesions with ablation zones ≤5 mm to the heart were considered as lesions with close proximity to heart. To our knowledge, only two studies have focused on lesions close to heart (16,17). In one study, only two lesions were considered in close proximity to heart and both lesions were immediately retreated with TACE post ablation due to residual disease given the proximity to heart and challenging technique (16). The second study compared lesions with ablation zone ≤5 mm to the myocardium and clinically these treated lesions had similar local tumor progression as lesions located in periphery distant from the heart (17). As a result, the current study adapted the inclusion criteria as ablation zone ≤5 mm to the heart. In addition, this criterion also allows for adequate margin size of 5–10 mm that has been shown to be safe and to decrease risk of local tumor progression.
Computed tomography (CT) and magnetic resonance (MR) imaging obtained prior, during and after procedure along with procedure reports were used to identify tumor size, ablation zone size, power of ablation, ablation time, distance from the heart, and presence of residual disease. Electronic medical records were reviewed for patient demographics, complications, clinical success, and disease recurrence for each patient.
A total of 376 unique MWA sessions were identified from January 2015 to June 2019. A total of 17 patients had liver tumors with ablation zone ≤5 mm to the heart; the remaining 359 sessions were excluded due to >5 mm distance from the heart. Of these 17 patients, 12 had primary HCC, 4 patients had liver metastases arising from colorectal cancer and one patient had liver metastasis from bladder cancer. Of these 17 patients, 82% also had multifocal disease (defined as at least two distinct liver lesions) at time of ablation of liver lesion close to the heart. Mean age of patients at time of MWA was 67 years with 82% of the patients being male. Mean lesion size was 18.2 mm (range, 10–33 mm), mean distance of ablation zone to the heart is 3 mm, mean spherical ablation size is 3.7 cm, and median follow-up period was 11 months with a range of 0–23 months (mean of 10.4 months). In this study, residual disease was defined as disease present at the same area of ablation in the immediate 1-month follow-up period indicating a lack of complete response to MWA. Local tumor recurrence was defined as recurrence at the ablation zone found beyond the one-month follow-up (after initial one month imaging demonstrating complete response). Finally, disease progression was defined as disease recurrence at other areas beyond the ablation zone.
Technique
Pre-procedure CT scan was performed to re-identify lesions. In patients whose lesion was difficult to discern, intravenous iodinated contrast media was administered to localize the lesion. Once lesion was identified, sterile technique was performed. A microwave antenna (15/20 cm Medtronic single antenna Emprint™ thermosphere technology, Covidien, Boulder, CO, USA) was inserted into the liver lesion under CT or ultrasound guidance. Once position of the microwave probe was confirmed, MWA was performed at varying powers and times based on lesion location and size. In some patients depending on size of lesion, microwave antenna was repositioned to perform overlapping ablation zones to increase coverage. Ablation area was planned to provide a margin of 5–10 mm for each lesion based on pre-procedural and intraprocedural imaging. In some instances, additional pre and post-procedural contrast-enhanced CT scans were obtained to better assess ablation margins. CT fluoroscopic images were obtained during ablation and post-ablation scan was performed to assess for any acute post-op complications. Technical success is defined as adequate ablation of tumor with acceptable margin and was verified with immediate post procedural CT scan with and/or without contrast administration.
Measuring distance to the heart
Distance from ablation zone to the heart was measured using multiple views to localize center of ablation and its location from the heart. First, the size of ablation zone was identified. Next, using the radiology workstation (McKesson Corporation, Irving, TX, USA), axial images were used to reconstruct the series into coronal and sagittal views to obtain a three-dimensional view of the ablation area. Using intra-procedural axial CT images, the emission point of microwave antenna was used as the center of ablation with crosshairs corresponding to the area in coronal and sagittal views. Distance from the center of the ablation zone to the heart was measured in all three views (axial, coronal, and sagittal). Radius of the final ablation zone and distance from center of ablation to the heart were gathered and only lesions with final ablation zone distance of ≤5 mm to the heart were included. Examples of how the technique was carried out is demonstrated and explained in Figures 1,2.
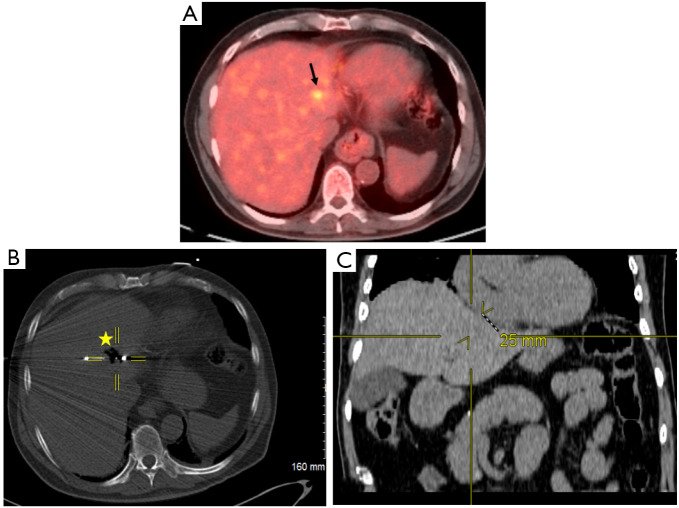
Figure 1.
Localization of primary hepatocellular carcinoma and proximity to the heart. (A) Pre-procedural positron electron tomography/computed tomography (CT) scan demonstrating 11 mm hypermetabolic hepatic lesion (black arrow) in a patient with primary hepatocellular carcinoma. (B) Perioperative CT fluoroscopic imaging demonstrating 4.2 cm spherical zone of ablation (star) with crosshairs centered on the emitter of the microwave antenna. (C) Post-procedural coronal CT image that corresponds to the center of ablation zone. Yellow crosshairs were localized to the same point as center of ablation zone in this coronal view. Distance from center of ablation zone to the heart was measured. It can be noted on the coronal view the center of ablation zone to the heart measures 25 mm in radius corresponding to ≤5 mm distance from the heart.
Figure 2.
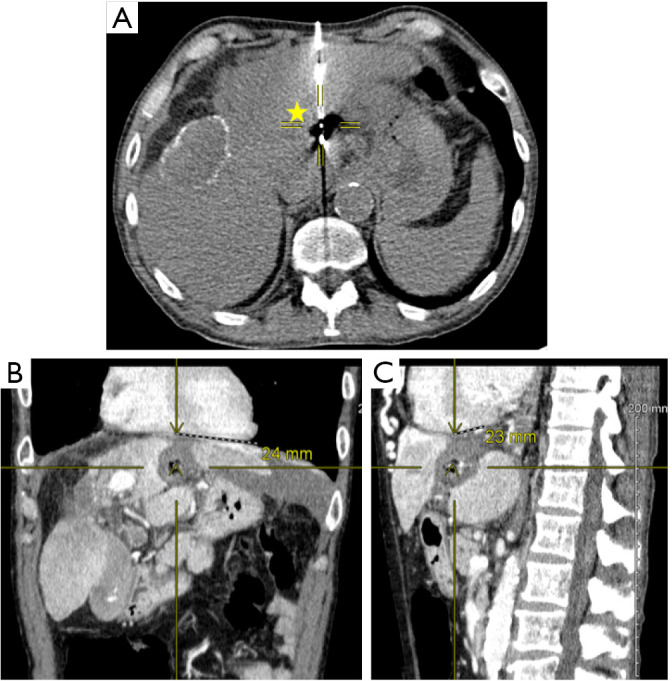
Ablation of hepatocellular carcinoma. The following images are from a patient with 11 mm left hepatic lobe lesion that was primary hepatocellular carcinoma. (A) Peri-operative computed tomography (CT) image demonstrating 4.2 cm spherical ablation zone (yellow star) with crosshairs centered to emission point of microwave antenna. (B,C) Post ablation CT venous phase demonstrating ablation cavity in coronal (B) and sagittal (C) views. Crosshairs and distances are highlighted in coronal (B) and sagittal (C) views demonstrating a distance of 24 and 23 mm respectively between centers of ablation zone to the heart in a patient with 4.2 cm spherical ablation zone. Thus, in both coronal and sagittal views, the distance of the final ablation zone was ≤5 mm to the heart.
Statistical analysis
Statistical analysis was performed using Microsoft Excel version 2016 (Microsoft, Redmond, WA, USA). Overall mean and standard deviations were calculated.
Results
Seventeen patients were included in the current study based on inclusion criteria of ablation zone ≤5 mm to the heart. Only two patients had pneumothorax post-procedure with one patient requiring chest tube placement while the other patient required no further intervention. Based on the Society of Interventional Radiology criteria for complications, none of the patients had major complications and only three patients had minor complications in the form of pneumothorax (18). None of the patients had any cardiac arrhythmias, and no other major or minor complications occurred in these patients as defined by Society of Interventional Radiology criteria. None of the patients were readmitted for MWA complications within the 1-month post-procedure period. There was a 100% technical success rate.
Of note 14/17 (82%) of the patients had multifocal disease at time of MWA with some patients having undergone prior TACE procedures, radioembolization and/or hepatectomy as deemed appropriate following multidisciplinary discussion based on patient’s clinical status and disease progression. As a result, local recurrence and disease progression were made on a per lesion analysis to delineate recurrence related to only lesion of interest close to the heart.
Overall 12/17 (71%) of the patients had disease progression within the liver outside of the ablation zone. While this study’s overall disease progression is high at 71% (12/17), this was confounded by the presence of multifocal lesions in 82% of the patients. Progression occurred anywhere from 1–23 months from time of procedure. One of these 12 patients (8%) had residual disease at the site of ablation zone as well as disease progression at other sites within the liver. This patient had undergone two prior TACE procedures prior to MWA of the lesion close to the heart. Given that patient had residual disease despite MWA, patient underwent a third TACE procedure followed by radioembolization. Another of these 12 patients (8%) had local disease recurrence at 8-month follow-up in the area of ablation zone that was absent at the 2-month or 5-month follow-up imaging. This second patient was treated with TACE followed by radioembolization. One patient (1/17) underwent liver transplantation prior to follow-up imaging. Remaining 4/17 patients (24%) had no residual disease, recurrent disease or disease progression within the liver with follow-up ranging from 4–18 months. Table 1 demonstrates time frame at which residual disease developed as well as mortality and number of patients lost to follow-up at each time period. Deaths were not a result of MWA, but rather due to patients opting for hospice, progression of disease or cardiopulmonary arrest. In most instances, cause of death was not documented, but likely due to overall disease progression.
Table 1. Time frame of disease recurrence/residual disease.
| Follow-up period | Number of patients with no residual disease at site of ablation | Number of deaths | Number of patients—loss to follow-up |
|---|---|---|---|
| 1-month | 15/17 (88%) | 0 | 0 |
| 3-month | 12/17 (71%) | 3 | 0 |
| 6-month | 11/17 (65%) | 0 | 1 |
| 1-year | 7/17 (41%) | 1 | 2 |
Demonstrates time frame at which residual disease developed. Table also provides information regarding number of deaths and number of patients who were lost to follow-up at each time interval.
Discussion
MWA for liver cancer has been performed at high-risk locations including close to the heart, diaphragm, abdominal wall, bowel, hepatic hilum (4,17). To minimize risk of damage to non-target organs, ancillary maneuvers such as hydrodissection and hydropneumothorax have been used to increase the distance between the ablation zone and organ at risk for damage. Hydrodissection involves injection of fluids into the surrounding third space such as peritoneum to increase distance between ablation zone and non-target organ such as diaphragm, pleura, or even bowel (4,19). Since these fluids collect into a dependent portion, continuous administration is required throughout the length of the procedure to maintain distance created by fluid as well as to provide a cooling effect during the procedure (4). While in some patients the fluid is absorbed over the following few days, some may require paracentesis after the procedure (4,19). Other techniques include artificial pleural effusion and iatrogenic pneumothorax. Former is used for hepatic dome lesions that are in direct contact with diaphragm. In this case, fluid is injected into the pleural space to increase distance between ablation site and diaphragm (19). Similar to the fluid, air can be injected into pleural space to create iatrogenic pneumothorax to minimize damage to lung during ablation (19). Other techniques including traversing through the epicardial fat pad (often >1 cm in diameter in thickness) have been shown to be technically successful in radiofrequency ablation of hepatic dome lesions (17,20).
MWA of lesions in challenging locations has also been demonstrated to be relatively safe and effective in patients without use of ancillary maneuvers (17). In one study, MWA of hepatic dome lesions located close to heart, diaphragm, hepatic hilum were treated using either angulation technique that involves rotation of CT gantry along the craniocaudal direction allowing the passage of the ablation probe in the same angle; second technique used was passage of the microwave probe through the epicardial fat pad if >1 cm in thickness (17). In this study, there were two lesions close to the heart (5.5% of the total cases) with the remaining tumors being predominantly located subcapsularly or in hepatic dome (17). Only minor complications including minor pneumothorax and small hematomas occurred that required no further intervention. This study had a 100% technical success rate with incomplete response in only 8.3% of cases that was attributed to larger size of lesion (17). Another study looked at MWA of lung tissue close to the heart in porcine models (18). This study demonstrated that MWA within 5 mm of the pericardium was associated with an increased risk of cardiac arrhythmias and thermal injury to the cardiac tissue (21). Once cardiac arrhythmias were noted, ablation was immediately terminated which resulted in spontaneous resolution of arrhythmias (21). The distance in this study was measured from the emitter of the antenna to the cardiac tissue in perpendicular direction and from tip of antenna in parallel direction (21).
In addition, the movement of the heart during systole and diastole as well as movement of the lungs and diaphragm with breathing can serve as a protective mechanism in decreasing thermal injury to cardiac tissue, as it has been shown that thermal injury occurs as a compounding effect on gross morphology in porcine models with pulmonary MWA (21). In addition, the same study showed that ablations >5 mm from the pericardium was associated with significantly decreased risk of cardiac complications including arrhythmias and tissue thermal injury (21).
Only one other study looked at MWA of liver cancer close to the heart. In this study, MWA of liver lesions (including HCC, liver metastases, and hemangiomas) with ablation zone extending ≤5 mm to the heart was performed and resulted in no intra-procedural arrhythmias when compared to a control group of >5 mm to the heart (18). In addition, there was no significant difference in local tumor recurrence between both groups (18). The current study also re-demonstrated the safety and efficacy of MWA of hepatic lesions that are located ≤5 mm to the heart. Only two patients had a pneumothorax, one of which required chest tube placement; otherwise no other complications including cardiac arrhythmias occurred in patients with ablation zones ≤5 mm to the heart. While this study’s overall disease progression is high at 71% (12/17), this was confounded by the presence of multifocal lesions in 82% of the patients. Local tumor progression rate of 6% in this study was consistent with published literature (4,5). Therefore, it can be said that MWA of liver lesions close to heart is safe and effective.
One of the limitations in the current study was that ablation zone proximity to heart was estimated using a combination of intraprocedural non-constant imaging and post procedural contrast-enhanced imaging. Also, CT with and without contrast studies were both used depending on availability to obtain post procedural data introducing an element inconsistency. Another limitation of the study is that the inclusion criteria used for the study is distance of the ablation zone of ≤5 mm to the heart. The rationale of choosing this as an exclusion criterion is to not only provide consistency and homogeneity to other studies that have looked at MWA of lesions close to myocardium, but also allow for an adequate ablation margin zone of 5–10 mm to decrease risk of local tumor progression as margin size has been demonstrated previously as an independent predictor of local disease progression. Finally, the small sample size and retrospective single center nature of the data is also a limitation for this study. The safety and efficacy of MWA ablation of liver lesions in close proximity to heart can be further evaluated via a prospective study.
Conclusions
MWA of liver lesions close to heart (with final ablation zone of ≤5 mm to the heart) is safe and effective, however, it can be technically challenging. More studies are needed to evaluate the long term safety and efficacy of thermal ablation close to the heart.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of University of Kentucky (42415) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tgh-20-314
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tgh-20-314
Peer Review File: Available at http://dx.doi.org/10.21037/tgh-20-314
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh-20-314). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Facts & Figures 2020. American Cancer Society. Atlanta, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Ridder J, de Wilt JH, Simmer F, et al. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget 2016;7:55368-76. 10.18632/oncotarget.10552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meloni MF, Chiang J, Laeseke PF, et al. Microwave ablation in primary and secondary liver tumors: technical and clinical approaches. Int J Hyperthermia 2017;33:15-24. 10.1080/02656736.2016.1209694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. World J Surg Oncol 2019;17:98. 10.1186/s12957-019-1632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson AB, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. 10.6004/jnccn.2017.0036 [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw 2019;17:302-10. 10.6004/jnccn.2019.0019 [DOI] [PubMed] [Google Scholar]
- 8.Benson AB, D'angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:563-73. 10.6004/jnccn.2017.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 10.Chamarthy MR, Kalva SP. Liver-Directed therapy for hepatic malignancies. Am J Hematol Oncol 2016;12:14-22. [Google Scholar]
- 11.Zhang M, Ma H, Zhang J, et al. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: a meta analysis. Onco Targets Ther 2017;10:4829-39. 10.2147/OTT.S141968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YS, Lee WJ, Rhim H, et al. The Minimal Ablative Margin of Radiofrequency Ablation of Hepatocellular Carcinoma (> 2 and < 5 cm) Needed to Prevent Local Tumor Progression: 3D Quantitative Assessment Using CT Image Fusion. AJR Am J Roentgenol 2010;195:758-65. 10.2214/AJR.09.2954 [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36:166-75. 10.1007/s00270-012-0377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shady W, Petre EN, Do KG, et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol 2018;29:268-75.e1. 10.1016/j.jvir.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijerink MR, Puijk RS, van Tilborg AAJM, et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol 2018;41:1189-204. 10.1007/s00270-018-1959-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia 2018;34:863-9. 10.1080/02656736.2017.1370728 [DOI] [PubMed] [Google Scholar]
- 17.Carberry GA, Smolock AR, Cristescu M, et al. Safety and Efficacy of Percutaneous Microwave Hepatic Ablation Near the Heart. J Vasc Interv Radiol 2017;28:490-7. 10.1016/j.jvir.2016.12.1216 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M, Solbiati L, Brace CL, et al. Image guided tumor ablation: Standardization of terminology and reporting criteria - A 10-year update. Radiology 2014;273:241-60. 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kambadakone A, Valiyan V, Kordbacheh H, et al. Imaging guided percutaneous interventions in hepatic dome lesions: tips and tricks. World J Hepatol 2017;9:840-9. 10.4254/wjh.v9.i19.840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan DD, Ganguli S, Brecher C, et al. Thinking outside the abdominal box: safe use of the epicardial fat pad window for percutaneous radiofrequency ablation of hepatic dome tumors. J Vasc Interv Radiol 2008;19:133-6. 10.1016/j.jvir.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 21.Carberry GA, Nocerino E, Mason PJ, et al. Pulmonary microwave ablation near the heart: antenna positioning can mitigate cardiac complications in a porcine model. Radiology 2017;282:892-902. 10.1148/radiol.2016160831 [DOI] [PMC free article] [PubMed] [Google Scholar]