Abstract
Background
The immunogenicity of a two-dose mRNA COVID-19 vaccine regimen is low in kidney transplant (KT) recipients. Here, we provide a thorough assessment of the immunogenicity of a three-dose COVID-19 vaccine regimen in this population.
Methods
We performed a prospective longitudinal study in sixty-one KT recipients given three doses of the BNT162b2 COVID-19 vaccine. We performed semi-structured pharmacovigilance interviews and monitored donor-specific antibodies and kidney function. We compared levels of anti-spike IgG, pseudo-neutralization activity against vaccine homologous and heterologous variants, frequency of spike-specific interferon (IFN)-γ-secreting cells, and antigen-induced cytokine production 28 days after the second and third doses.
Findings
Reactions to vaccine were mild. One patient developed donor-specific anti-HLA antibodies after the second dose which could be explained by non-adherence to immunosuppressive therapy. Spike-specific IgG seroconversion raised from 44·3% (n=27) after the second dose to 62·3% (n=38) after the third dose (p<0·05). The mean level of spike-specific IgG increased from 1620 (SD, 3460) to 8772 (SD, 16733) AU/ml (p<0·0001). Serum neutralizing activity increased after the third dose for all variants of concern tested including the Delta variant (p<0·0001). The frequency of spike-specific IFN-γ-secreting cells increased from 19·9 (SD, 56·0) to 64·0 (SD, 76·8) cells/million PBMCs after the third dose (p<0·0001). A significant increase in IFN-γ responses was also observed in patients who remained seronegative after three doses (p<0·0001).
Interpretation
A third dose of the BNT162b2 vaccine increases both cross-variant neutralizing antibody and cellular responses in KT recipients with an acceptable tolerability profile.
Funding
Nice University Hospital, University Cote d'Azur.
Key words: COVID-19; mRNA vaccine; variants of concern, kidney transplantation; Immunogenicity
Research in context.
Evidence before this study
Three-dose COVID-19 vaccine regimens have been recommended by the French national health authorities in April 2021. Before submitting this article on July 20, 2021, we conducted a search in PubMed for any published article, using key terms “COVID-19” or “SARS-CoV-2” AND “Vaccine”, AND “3 doses” or “Third dose” AND “Kidney Transplant Recipients” or “Transplantation”, without restrictions. In July 2021, two letters reported the prevalence and titres of spike-specific antibodies after a second and third dose of SARS-CoV-2 vaccines in kidney transplant recipients. To date, no study has compared cross-variant neutralizing antibody responses after a second and third vaccine dose in kidney transplant recipients.
Added value of this study
This report demonstrates that a third COVID-19 vaccine dose boosts both cross-variant neutralizing antibodies, including the Delta variant, and T cell immunity in kidney transplant recipients.
Implications of all the available evidence
This study provides evidence that administration of a third dose of COVID-19 mRNA is of benefit for solid organ transplant recipients in terms of vaccine immunogenicity. While these findings support recommending a third dose of vaccine in this population, neutralizing antibody responses after three doses were still relatively low, especially against variants of concern, calling for improved vaccination strategies, including heterologous prime-boost regimens with optimized dosages and time intervals between doses.
Alt-text: Unlabelled box
1. Introduction
Organ transplant recipients with SARS-CoV-2 infection are at increased risk of severe disease and death, and are therefore a priority for vaccination [1]. Messenger RNA (mRNA) COVID-19 vaccines are safe and effective at preventing COVID-19 in the general population [2]. In phase 3 trials, mRNA vaccines efficacy rates were higher than 90% after two doses across most demographic subgroups [3,4]. However, organ transplant recipients were excluded from these trials. Since these early reports, studies have shown that vaccine immunogenicity was severely reduced in this population as a consequence of immunosuppressive drugs. Several fatal cases of COVID-19 have been reported in organ transplant recipients who had received two doses of mRNA vaccines [5,6], the likely result of reduced vaccine immunogenicity. Indeed, less than 20% of transplant recipients developed IgG against the SARS-CoV-2 spike protein after a single vaccine dose [7] and only half of them after two doses [8]. Moreover only 35% of KT recipients generated spike-specific T Helper (Th) 1 cells after the second vaccine dose [9].
In April 2021, the French National Health Authority recommended administering a third dose of mRNA vaccine to be given four weeks after a second dose to improve vaccine responses in organ transplant recipients. Amid the rapid surge of variants of concern, safety assessments and detailed immunogenicity data are still required to weigh up the risk-benefit value of a three-dose COVID-19 regimen in organ transplant recipients. This prospective monocentric longitudinal study conducted in 61 KT recipients given three doses of the BNT162b2 COVID-19 vaccine demonstrates that such a regimen improves vaccine immunogenicity.
2. Methods
2.1. Study design and participants
Sixty-one consecutive KT recipients were included in this prospective monocentric longitudinal study between March 24, 2021 and April 14, 2021 at the Nice University Hospital, Nice, France. Participants contacted the kidney transplantation department on their own to be vaccinated and were not selected by the investigators. All patients received three injections of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) at day 0, day 21, and day 49 as recommended by the French National Authority for Health. Patients with a history of COVID-19 or a positive anti-spike (Receptor Binding Domain, RBD) SARS-CoV-2 serology the day of inclusion were excluded. Blood samples were collected 28 days after the second and the third vaccine dose.
Clinical and laboratory data were collected using the observation booklet completed by the investigating physician in charge of the patient.
2.2. Adverse Events (AE) monitoring
In order to systematically identify eventual AE related to each dose, an advanced practice nurse conducted a telephone follow-up of every participant 72 hours after each dose. Local and systemic adverse reactions solicitation and assessment was based on a semi-structured interview with a questionnaire developed in collaboration with the local pharmacovigilance centre. For each reported AE, time-to-onset, duration, and evolution were recorded.
Classification of a serious AE included a report of one of the following: death or life-threatening illness, occurrence and duration of hospitalization, permanent disability, congenital anomaly or birth defect.
Patients sera were assessed for the presence of anti-HLA donor-specific antibodies 28 days after vaccine injections by Luminex Single Antigen® (One Lambda) as recommended by the French Society of Transplantation. Kidney allograft function was assessed before and after vaccination.
2.3. SARS-CoV-2-specific IgG and IgA
We used a commercial ELISA (ABBOTT) validated for clinical use to assess patient serum IgG titers to SARS-CoV-2 spike. The assay detects IgG directed to the Receptor Binding Domain (RBD) of SARS-CoV-2 spike S1 subunit and was performed according to the manufacturer's instructions. Results were acquired on the Architect I1000 analyser (ABBOTT). Values higher than 50 AU/mL were considered positive as specified by the manufacturer. Seroconversion was defined as conversion from negativity (i.e ≤ 50 AU/mL) to positivity (i.e. > 50 AU/mL).
Serum IgA titers to SARS-CoV-2 Spike protein were determined using the V-PLEX® SARS-CoV-2 Panel 2 (IgA) Kit (MSD), according to the manufacturer's instructions. Data were acquired on the V-PLEX® Sector Imager 2400 plate reader and analysed using the Discovery Workbench 3·0 software (MSD).
2.4. SARS-CoV-2 neutralization assay
The level of SARS-CoV-2 neutralizing antibodies in patients’ serum was estimated by a binding inhibition assay. The multiplex neutralization assay V-PLEX® SARS-CoV-2 Panel 7 (ACE2) Kit (MSD) was used to measure the capacity of patients’ serum to inhibit the binding of the SARS-CoV-2 full-length Spike protein to soluble angiotensin-converting enzyme 2 (ACE2) receptor. Spike protein was derived from the Wuhan sequence or from sequences of the alpha (B1.1.7), beta (B.1.351), gamma (P1) and Delta (B.1.617.2) variants. Assays were performed according to the manufacturer's instructions. Data were acquired on the V-PLEX® Sector Imager 2400 plate reader and analysed using the Discovery Workbench 3·0 software (MSD). Standard curves were generated using standards provided in this kit. Serial 4-fold dilutions of the standards were run to generate a 7-standard concentration set, and the diluent alone was used as a blank. The % inhibition was calculated according to the manufacturer's instructions.
2.5. SARS-CoV-2-specific T cell responses
For measuring the frequency of IFN-γ-secreting SARS-CoV-2-specific T cells, we used the ELISpot Path Human IFN-γ (SARS-CoV-2, S1scan+S2N+SNMO) ALP assay (MABTECH). Briefly, PBMCs (5 × 105 to 1 × 106 cells/well) were incubated for 24 hours at 37°C in a 96-well ELISpot strip plate pre-coated with anti-IFN-γ monoclonal antibody (mAb), a mixture of three pools of SARS-CoV-2 synthetic peptides (SARS-CoV-2 S1, SARS-CoV-2 S2 N and the SARS-CoV-2 SNMO), and anti-CD28 mAbs (0·1 µg/ml).
For measuring cytokine levels produced by SARS-CoV-2-specific T cells, we used the QuantiFERON® SARSCoV-2 Starter Set kit (Qiagen) in which 1 ml of blood was collected in tubes containing a mixture of SARS-CoV-2 peptides. Cells were incubated at 37°C for 24 hours and supernatants were assessed for IFN-γ, IL-2, IL-10 and TGF-beta levels. The V-PLEX® kit (MSD) was used. All assays were performed according to the manufacturer's instructions. Data were acquired on the V-PLEX® Sector Imager 2400 plate reader and analysed using the Discovery Workbench 3·0 software (MSD). Standard curves for each cytokine were generated using standards provided in the kits. As the lower limit of detection (LLOD), we used the median LLOD compiled over multiple plates and corresponding to the concentration calculated based on the signal recorded for the blank plus 2·5 standard deviations. For each cytokine, samples that fell below the LLOD were assigned an arbitrary value equal to half the LLOD.
2.6. Ethics
The study protocol complies with the principles of the Declaration of Helsinki and was approved by the CPP Nord-Ouest institutional review board (IRB), (number A01230-55). Written informed consent was obtained from all participants and all collected data and samples were anonymized and securely stored.
2.7. Statistics
The sample size was calculated as follow: the frequency of KT recipients who were seropositive after the administration of two doses of the BNT162b2 vaccine was estimated to be around 30-40% before the study. We calculated (G*Power 3·1) that 52 patients would be required to demonstrate a difference between the proportion of seropositive patients after two and three vaccine doses with a risk alpha of 5%, a power (1-beta) of 95% and an effect size of 0·5. Assuming a 20% drop-out rate, we calculated that 65 patients would be needed to rule out the null hypothesis.
Data are presented as median with interquartile ranges (IQR) for quantitative variables, or as numbers and percentages for categorical variables. The Shapiro-Wilk and Agostino-Pearson tests were used to verify the distribution of data. Comparison between time points (after the second and third dose) was performed with the Wilcoxon matched-pairs, two-tailed rank test. The Chi-square test was used for comparison of seroconversion rates. Statistical analyses were performed using GraphPad Prism 9·0 (GraphPad Software, Inc., San Diego, CA). Differences were considered significant when p-value was < 0·05. For multivariate analysis, a logistic regression using seroconversion as the outcome and risk factors as independent variables was performed. The variables included in the multivariate analysis were Gender, Age, Retransplantation, Time post-transplantation, Diabetes, Maintenance immunosuppressive therapy, Steroïds, Antiproliferatives, Calcineurins inhibitors, mTOR inhibitors, Creatininemia and Lymphocytes count, × 109/l. Logistic regressions analysis were conducted in R (R Core Team, 2020).
2.8. Role of the funders
Funding sources played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
3. Results
3.1. Characteristics of the study population
Sixty-one KT recipients with no history of COVID-19 and a negative SARS-CoV-2 serology at the time of inclusion were enrolled. Patients’ baseline characteristics are shown in Table 1. Seventeen (27·9%) participants were women and 44 (72·1%) were men. The median age was 58 years (IQR, 47·1-66·1), and the median time since transplantation was 4·5 years (IQR, 1·8-11·3). Thirteen (21·3%) patients had type 2 diabetes. Maintenance immunosuppressive therapy included calcineurin inhibitors (93·4%, 57 of 61 patients), corticosteroids (88·5%, 54 of 61 patients), and antimetabolites (62·3%, 38 of 61 patients, Mycophenolate Mofetil or Mycophenolic Acid, n=33; Azathioprine, n=5). Baseline creatininemia was 145·0 µmol/L (IQR, 106·5-180·5). Thirteen (21·3%) patients had donor-specific antibodies before vaccination. All participants received the BNT162b2 vaccine at day 0, day 21, and day 49.
Table 1.
Baseline characteristics of the study population
| Baseline characteristics | n=61 |
|---|---|
| Males | 44 (72·1) |
| Age, years | 58·0 [47·1-66·1] |
| Retransplantation | 7 (11·5) |
| Time post-transplantation, years | 4·5 [1·8-11·3] |
| Cause of ESKD | |
| Diabetes | 5 (8·2) |
| Vascular | 7 (11·5) |
| Glomerular | 10 (16·4) |
| Polycystic kidney disease | 15 (24·6) |
| Others and unknown | 24 (39·3) |
| Diabetes | 13 (21·3) |
| Obesity (BMI>30kg/m²) | 8 (13·1) |
| Maintenance immunosuppressive therapy | |
| Corticosteroïds | 54 (88·5) |
| Antimetabolites* | 38 (62·3) |
| Calcineurin inhibitors | 57 (93·4) |
| mTOR inhibitors | 6 (9·8) |
| Belatacept | 1 (1·6) |
| Laboratory values | |
| Creatininemia, µmol/L | 145·0 [106·5-180·5] |
| White blood cell count, × 109/l | 6·6 [5·2-8·4] |
| Lymphocytes count, × 109/l | 1·3 [0·8-1·7] |
| Donor-speciifc antibodies | 13 (21·3) |
Data are shown as number and percentage, n (%) or median and interquartile range, m (IQR); ESKD: End-Stage Kidney Disease; BMI: Body Mass Index. * Mycophenolate Mofetil/ Mycophenolic Acid, n=33; Azathioprine, n=5).
3.2. Safety and reactogenicity of a three-Dose BNT162b2 vaccine regimen in KT patients
Every participant was contacted after each vaccine dose. The proportions of patients who reported at least one adverse drug reaction after solicitation were 73·8% (45 of 61 patients), 68·9% (42 of 61 patients), and 68·9% (42 of 61 patients) after the first, second, and third vaccine dose respectively (Table 2). Pain at the injection site was the most frequent AE reported in 60·7% (37 of 61 patients), 65·6% (40 of 61 patients), and 67·2% (41 of 61 patients) of patients after the first, second, and third vaccine doses respectively.
Table 2.
Frequency of adverse events after each dose
| Adverse Events | Dose 1 | Dose 2 | Dose 3 |
|---|---|---|---|
| Adverse Drug Reactions | 45 (73·8) | 42 (68·9) | 42 (68·9) |
| Injection-site pain | 37 (60·7) | 40 (65·6) | 41 (67·2) |
| Fatigue | 11 (18·0) | 13 (21·3) | 13 (21·3) |
| Headache | 4 (6·6) | 5 (8·2) | 7 (11·5) |
| Diarrhea | 4 (6·6) | 3 (4·9) | 7 (11·5) |
| Fever | 3 (4·9) | 3 (4·9) | 4 (6·6) |
| Myalgia | 2 (3·3) | 3 (4·9) | 2 (3·3) |
| Rhinorrhea | 1 (1·6) | 3 (4·9) | 2 (3·3) |
| Nausea and vomiting | 1 (1·6) | 2 (3·3) | 1 (1·6) |
| Cough | 0 | 4 (6·6) | 0 |
| Hypertension | 1 (1·6) | 1 (1·6) | 1 (1·6) |
| Anorexia | 1 (1·6) | 0 | 1 (1·6) |
| Vertigo | 1 (1·6) | 0 | 1 (1·6) |
| Local paresthesia | 0 | 2 (3·3) | 0 |
| Abdominal pain | 0 | 2 (3·3) | 0 |
| Rash | 0 | 0 | 1 (1·6) |
| Insomnia | 1 (1·6) | 0 | 0 |
| Serious Adverse Events | 0 | 1 (1·6) | 1 (1·6) |
| De novo donor-specific antibody | 0 | 1 (1·6) | 0 |
| Acute rejection | 0 | 0 | 0 |
| Kidney allograft failure | 0 | 0 | 0 |
Data are shown as number and percentage, n(%).
As for serious adverse events (SAEs), no clinical rejection or kidney graft failure episodes had occurred by the end of the follow-up period (Table 2). None but one of the participants did develop de novo donor-specific antibodies (Table 2). He developed a donor-specific anti-HLA class II (DQB1*06:03) antibody 28 days after the second vaccine dose (Mean Fluorescence Intensity: 5209, which increased to 11318 after the third dose, not shown). This 19-year-old patient retrospectively admitted non-adherence to his immunosuppressive medication during the vaccination period. A kidney graft biopsy did not show any sign of humoral rejection and creatininemia or proteinuria levels had not increased at the time of submission of this article.
3.3. Seroconversion after a third dose of BNT162b2 COVID-19 vaccine
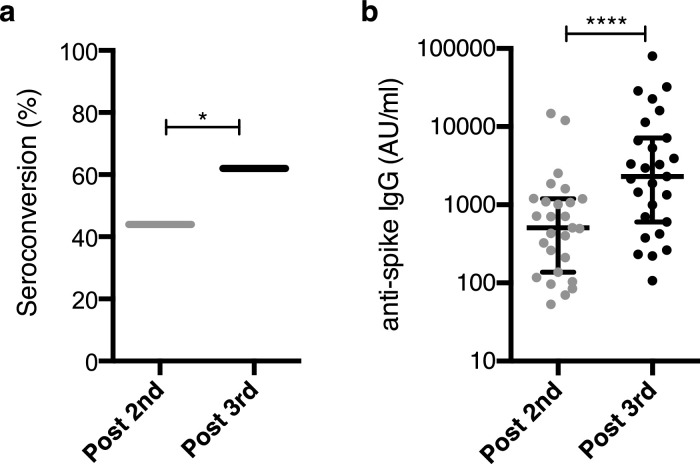
Serum spike-specific IgG and IgA antibody titers were measured 28 days after the second and third doses. Spike-specific IgG seroconversion rate raised from 44·3% (27 of 61 patients) after the second dose to 62·3% (38 of 61 patients) after the third dose (p=0·046, [Chi-square test], Figure 1A). Among the 34 (55·7%) patients who had failed to seroconvert after the second dose, 11 (11/34, 32·3%) seroconverted after the third dose. In patients who had seroconverted after the second dose, levels of IgG to spike (Wuhan) increased from 1620 (SD, 3460) to 8772 (SD, 16733) AU/ml after the third dose (p<0·0001, [Wilcoxon matched-pairs, two-tailed rank test], Figure 1B). Of note, levels of spike-specific IgA and IgG after the third dose were highly correlated (r = 0·87, Supplemental Figure S1).
Figure 1.
Spike-specific IgG antibodies. Serum spike-specific IgG antibody titers were measured 28 days after the second and third dose of BNT162b2 vaccine in 61 KT recipients. (a) Spike-specific IgG seroconversion rate after the second and third dose; *, p=0.046, [Chi-square test]. (b) Level of spike-specific IgG after the second and third dose in patients who have seroconverted after the second dose. Data are shown as median and interquartile range (IQR). ****p <0·0001, [Wilcoxon matched-pairs, two-tailed rank test].
The risk factors for failing to seroconvert after a third dose were antiproliferative drugs and lymphopenia in multivariate analysis (OR 12·27 (95% CI, 2·08-111·42), p=0·01 and OR 0·16 (95% CI, 0·04-0·51, p=0·01) respectively, [logistic regression], Table 3).
Table 3.
Multivariate regression of risk factors for anti-spike IgG seronegativity after three doses
| Variables | OR (95% CI) | p-value |
|---|---|---|
| Sexe (male) | 0·48 (0·08 to 2·62) | 0·40 |
| Age (per 1-year increment) | 1·03 (0·97 to 1·09) | 0·34 |
| BMI (kg/m2) | 0·95 (0·77 to 1·16) | 0·61 |
| Retransplantation | 0·59 (0·05 to 6·27) | 0·61 |
| Time post-transplantation (per 1-year increment) | 1·01 (0·91 to 1·06) | 0·79 |
| Diabetes | 0·77 (0·09 to 5·44) | 0·80 |
| Maintenance immunosuppressive therapy | ||
| Steroïds | 1·98 (0·20 to 22·18) | 0·55 |
| Antiproliferatives | 15·64 (2·42 to 173·14) | 0·01 |
| Calcineurins inhibitors | 0·75 (0·02 to 31·95) | 0·86 |
| mTOR inhibitors | 0·91 (0·02 to 19·02) | 0·96 |
| Creatininemia (μmol/l) | 1·01 (0·99 to 1·02) | 0·18 |
| Lymphocytes count, × 109/l | 0·20 (0·04 to 0·65) | 0·02 |
Odds Ratio (OR), 95% confidence intervals (CI) and p-values are shown; mammalian target of rapamycin (mTOR)· Significant associations are highlighted·
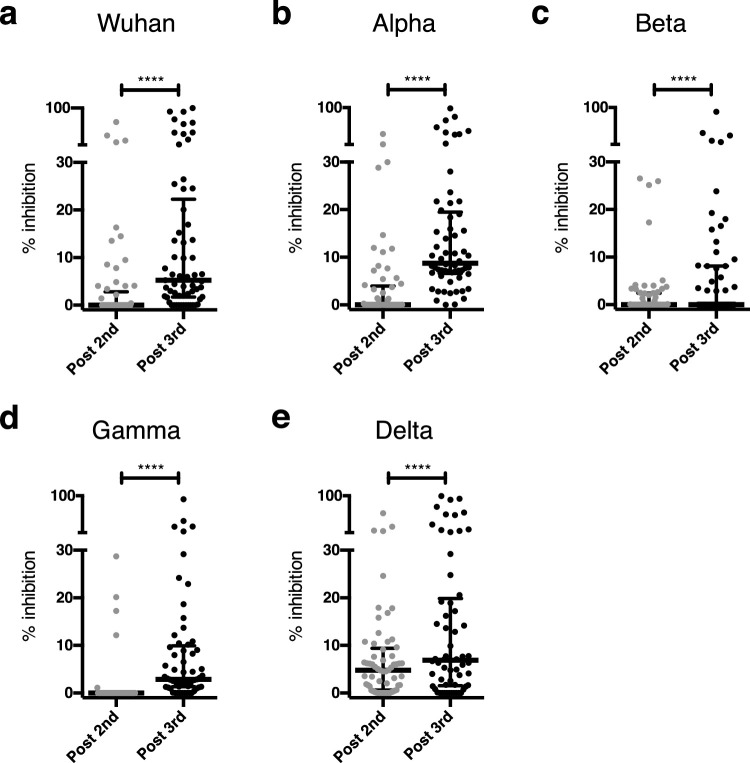
3.4. Neutralizing antibody responses after a third dose of BNT162b2 vaccine
Sera from KT patients were assayed for levels of SARS-CoV-2 neutralizing antibodies by means of a binding-inhibition assay based on inhibition of binding of SARS-CoV-2 spike protein to its ACE2 receptor. Spike protein was derived from the Wuhan sequence or from sequences of the alpha (B1.1.7), beta (B.1.351), gamma (P1) and Delta (B.1.617.2) variants. The serum neutralizing activity increased after the third dose for all variants (p<0·0001, [Wilcoxon matched-pairs, two-tailed rank test], Figure 2A). The mean percentage inhibition increased from 4.7 (SD, 12·9) % to 17.2 (SD, 26·1) % (Wuhan strain), 4.0 (SD, 9·2) % to 17·4 (SD, 21·1) % (alpha variant), 2·4 (SD, 6·0) % to 7·3 (SD, 15·9) % (beta variant), 1·3 (SD, 5·1) % to 9·1 (SD, 15·7) % (gamma variant) and 7·8 (SD, 11·5) % to 17·6 (SD, 25·5) % (delta variant) after the third dose.
Figure 2.
Neutralising antibody responses. Serum samples were assayed for SARS-CoV-2 neutralizing antibodies using a pseudo-neutralisation assay based on inhibition of binding of ACE2 receptor to the SARS-CoV-2 spike protein of the Wuhan strain (a) or the alpha (b), beta (c), gamma (d) and delta variants (e). Data are shown as median and interquartile range (IQR). ****p<0•0001 [Wilcoxon matched-pairs, two-tailed rank test].
3.5. T cell responses after a three-dose BNT162b2 vaccine regimen
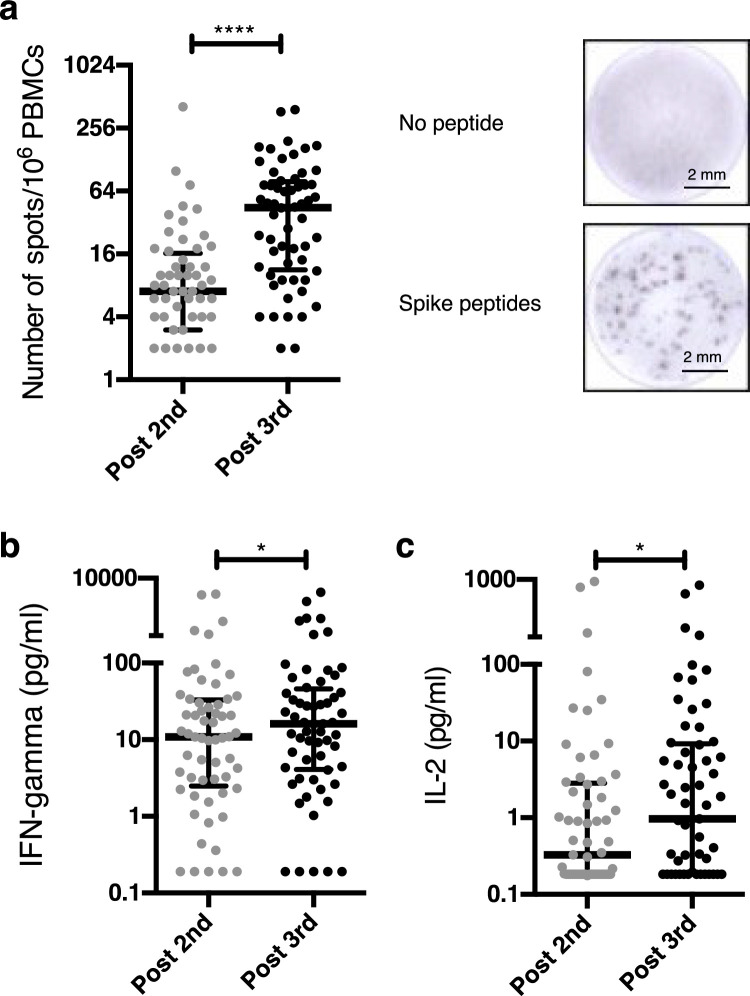
The frequency of blood IFN-γ-secreting cells increased from 7·0 (IQR, 3·0-16·25) cells per million PBMCs 28 days after the second dose to 44·5 (IQR, 11.25-78·5) 28 days after the third dose (p<0·0001, [Wilcoxon matched-pairs, two-tailed rank test], Figure 3A). Similar results were obtained when measuring cell-free IFN-γ levels in supernatants from whole blood cultures of PBMCs stimulated with a SARS-CoV-2 peptide cocktail (Figure 3B). In addition to IFN-γ responses, IL-2 levels also increased between the second and the third vaccine dose. (Figure 3C).
Figure 3.
Spike-specific T cell responses. (a) An ELISpot assay was used to measure the frequency of blood spike-specific IFN-γ-secreting cells 28 days after the second and third dose. ****p<0•0001 [Wilcoxon matched-pairs, two-tailed rank test]. Representative images are shown. Cell-free IFN-γ (b) and IL-2 (c) levels were measured in supernatants from whole blood cultures of PBMCs stimulated with a cocktail of SARS-CoV-2 spike-derived peptides. *p=0•015 and p=0•027 respectively [Wilcoxon matched-pairs, two-tailed rank test]. Data show median and interquartile range.
3.6. T cell responses in KT recipients who remained seronegative after the third vaccine dose
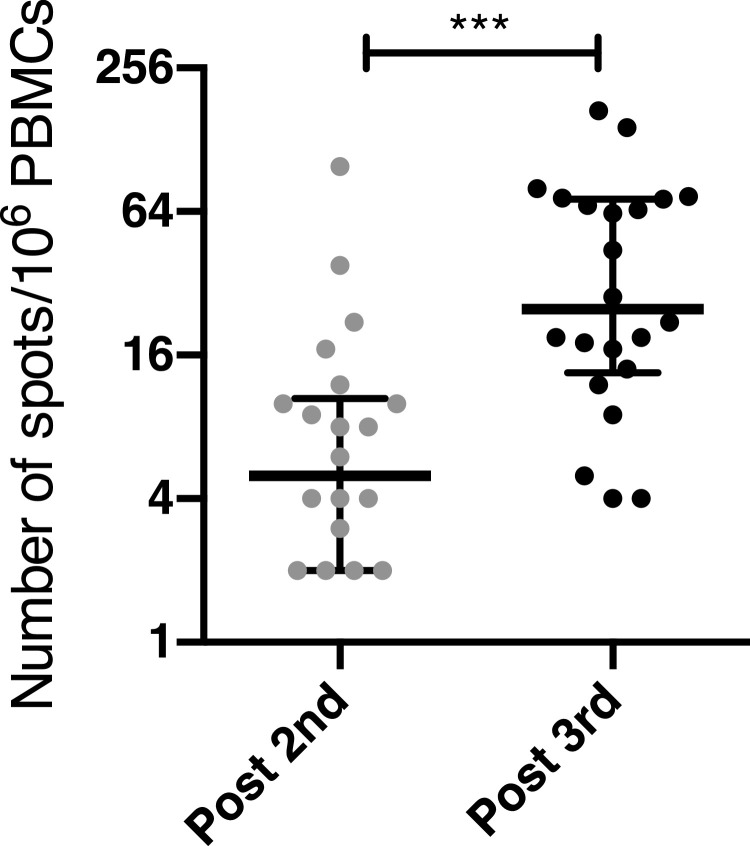
Because repetitive antigen stimulation and complete blockade of the effector immune response may induce antigen-specific tolerance [10,11], we analysed the production of inhibitory cytokines by PBMCs after stimulation with spike peptides in patients who remained seronegative after the third vaccine dose. The levels of IL-10 and TGF-β after spike-specific stimulation did not differ between the second and the third dose in these patients suggesting that the third dose did not induce spike-specific tolerance (Supplemental Figure S2). Inhibitory cytokine levels in these patients were also comparable to those of patients who seroconverted (Supplemental Figure S2). We then compared the frequency of spike-specific IFN-γ-secreting T cells after the second and the third dose in patients who failed to seroconvert after the third dose (Figure 4). This frequency increased by over four-fold between the second and the third dose (p<0·001, [Wilcoxon matched-pairs, two-tailed rank test], Figure 4).
Figure 4.
Spike-specific T cell response in seronegative KT recipients. The frequency of spike-specific IFN-γ-secreting T cells was compared after the second and the third dose of vaccine in KT recipients who have failed to seroconvert after the third dose. Data are shown as median and interquartile range. ***p<0•001, [Wilcoxon matched-pairs, two-tailed rank test].
4. Discussion
In March 2021, the French national health authorities recommended a three-dose regimen of mRNA COVID-19 vaccines in severely immunocompromised individuals amid a reportedly low immunogenicity of these vaccines in solid organ transplant recipients [7,8]. At that time, no data were available on the risks and benefits of an additional vaccine injection in solid-organ transplant recipients. This prospective longitudinal study provides detailed safety and immunological data supporting this recommendation. The results are consistent with those of recent studies, which showed that a 3rd dose of COVID-19 vaccine increased seroconversion rate in organ transplant recipients but did not measure cross-variant neutralizing antibody responses and T cell immunity [12,13].
Overall, the reactogenicity profile of the three-dose vaccine regimen was manageable. The third dose of vaccine did not increase the risk of local or systemic adverse reactions. In one patient who developed a de novo donor-specific antibody during vaccination, the occurrence of a donor-specific humoral response was most likely associated with the patient's non-adherence to immunosuppressive therapy rather than vaccination, in keeping with the WHO-UMC causality assessment system.
In this series of KT recipients, antibody titers, serum neutralizing activity and T cell responses to SARS-CoV-2 increased after the third vaccine dose. While these results are encouraging and suggest that a three-dose regimen improves vaccine immunogenicity, they should be interpreted with caution. First, serum neutralizing activity against variants of concern and IFN-γ T cell responses remained low after a third vaccine dose. Further assessments of T cell response such as analysis of activation markers, expression of inhibitory coreceptors or regulatory and helper T cell subpopulations would have been interesting. Second, the short duration of this study leaves open questions regarding the long-term safety and duration of immunological responses induced by a third dose of mRNA COVID-19 vaccine. Third, this study is localized and most of the participants were included more than six months after transplantation. This calls for caution in extrapolating these findings to patients who received a transplantation less than six months ago. It is also important to bear in mind that the clinical efficacy of COVID-19 mRNA vaccines in organ transplant recipients is still poorly known at that stage. Furthermore, the cross-protective efficacy of COVID vaccines against infection by certain newly emerging SARS-CoV-2 variants has been shown to decrease although its impact on disease severity has not been fully assessed at that stage. The foregoing considerations underline the limitations of results from phase III efficacy trials which have by and large excluded immunocompromised patients.
Alternative or complementary strategies should be developed to improve the immunogenicity of COVID-19 vaccines in organ transplant recipients. The dose or the route of vaccines could be modified as shown with other vaccines [14,15]. The time interval between doses has a known impact on vaccine immunogenicity [16] and a 4-weeks interval between the second and third doses may be too short. Vaccine strategies based on heterologous prime-boost, e.g. mRNA vaccine followed by adjuvanted protein subunit or vice versa, could improve vaccine immunogenicity as has been shown after sequential administration of heterologous viral vaccines [17], [18], [19], [20], [21]. Finally, short-term lowering of immunosuppressive drugs before vaccination could ameliorate vaccine performance but will have to be balanced against the risk of acute rejection or apparition of donor-specific antibodies. Predictive immunomonitoring tools to differentiate low from high responders before vaccination could be used to tailor immunosuppressive therapy [22].
In conclusion, a third dose of the BNT162b2 mRNA COVID-19 vaccine in KT recipients increases both spike-specific antibody-binding and T cell responses with an acceptable tolerability profile. However, variant-specific neutralizing antibody responses remained low after three doses and underline the importance of barrier measures and of vaccination of patients’ close contacts.
5. Contributors
AS designed the study. FM, MC, AG, HG, LR, MB, MR, EM, JF developed the study methods. FM, NB, GB, CC, GF, PH, VE, BSP, NG, AS contributed to the implementation of the study or data collection. FM, SB, AS conducted the statistical analysis. BSP and AS were investigators. NG and AS ensured data accuracy. FM, MC, AG, CC, NG, AS contributed to the preparation of the report. All authors critically reviewed and approved the final version. All authors had full access to all the data in the study and accepted responsibility to submit for publication.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by grants from the Nice University Hospital (AOI) and the University of Cote d'Azur (IDEX). The authors thank the IBV imaging and cytometry platform for technical assistance, the medical and paramedical staff involved in the care of patients and all study participants.
Data sharing
Anonymized participant data presented in this paper and statistical analysis can be accessed with publication on request to the corresponding author. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement. Data will be made available for a minimum of 5 years.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103679.
Appendix. Supplementary materials
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillard S, Chavarot N, Bertrand D, et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021 doi: 10.1016/j.kint.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. 2021 doi: 10.1111/ajt.16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021 doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL. Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med. 2005;201(7):1101–1112. doi: 10.1084/jem.20041852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishimoto K, Yuan X, Auchincloss H, Jr., Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of action of donor-specific transfusion in inducing tolerance: role of donor MHC molecules, donor co-stimulatory molecules, and indirect antigen presentation. J Am Soc Nephrol. 2004;15(9):2423–2428. doi: 10.1097/01.ASN.0000137883.20961.2D. [DOI] [PubMed] [Google Scholar]
- 12.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021 doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Intern Med. 2021 doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natori Y, Shiotsuka M, Slomovic J, et al. A Double-Blind, Randomized Trial of High-Dose vs Standard-Dose Influenza Vaccine in Adult Solid-Organ Transplant Recipients. Clin Infect Dis. 2018;66(11):1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 15.Liebowitz D, Gottlieb K, Kolhatkar NS, et al. Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect Dis. 2020;20(4):435–444. doi: 10.1016/S1473-3099(19)30584-5. [DOI] [PubMed] [Google Scholar]
- 16.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Normark J, Vikstrom L, Gwon YD, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borobia AM, Carcas AJ, Perez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anywaine Z, Whitworth H, Kaleebu P, et al. Safety and Immunogenicity of a 2-Dose Heterologous Vaccination Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Uganda and Tanzania. J Infect Dis. 2019;220(1):46–56. doi: 10.1093/infdis/jiz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021 doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang JS, Dobano C, VanDamme P, et al. Improving Vaccine-Induced Immunity: Can Baseline Predict Outcome? Trends Immunol. 2020;41(6):457–465. doi: 10.1016/j.it.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.