Abstract
The enrichment of cancer-associated fibroblast (CAFs) in a tumor microenvironment (TME) cultivates a pro-tumorigenic niche via aberrant paracrine signaling and matrix remodeling. A favorable niche is critical to the maintenance of cancer stem cells (CSCs), a population of cells that are characterized by their enhanced ability to self-renew, metastasis, and develop therapy resistance. Mounting evidence illustrates the interplay between CAF and cancer cells expedites malignant progression. Therefore, targeting the key cellular components and factors in the niche may promote a more efficacious treatment. In this study, we discuss how CAF orchestrates a niche that enhances CSC features and the potential therapeutic implication.
Keywords: inflammation, targeted therapy, tumor microenvironment, cancer associated fibroblast, cancer stem cell, cancer stemness
Introduction
Tumors are illustrated as “wounds that do not heal” due to the enrichment of fibroblasts and immune cells at the tumor site, which highly mimics that of an inflammatory response of a non-neoplastic tissue (Dvorak, 1986). Indeed, cancer-associated fibroblast (CAF) constitutes the bulk of the stromal component in solid tumors. Cancer cells hijack the normal physiological function of activated fibroblast functions during wound recovery to fuel malignant development. Given cancer stemness accelerates disease progression and impinges therapy, understanding how CAFs cultivate a niche that bestows cancer stem cell (CSC) features in cancer cells may facilitate novel therapeutic intervention.
Cancer-Associated Fibroblast as a Dynamic Player in Promoting Cancer Stemness
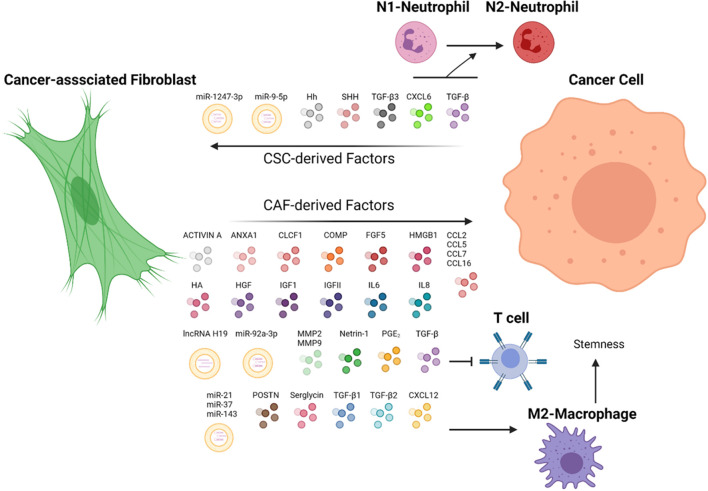
Cancer-associated fibroblast, also known as tumor-associated fibroblast, is generally regarded as fibroblasts that exist within or enveloping the tumor (Öhlund et al., 2014). The presence of CAFs throughout tumor development, from the incipient stage to advanced stages, alludes to CAF participation in tumor initiation and progression (Erez et al., 2010). Compared with non-tumor fibroblast (NF) or peri-tumor fibroblast, CAFs are located more proximal to the neoplastic region and exhibit a greater activation marker, namely actin smooth muscle (αSMA) and fibroblast activation protein (FAP) (Qin et al., 2016, 2019). However, compiling studies indicate CAF displays vast heterogeneity within a tumor with distinct functions, underscoring the importance of identifying the function of how each subtype contributes to malignancy (Öhlund et al., 2017; Costa et al., 2018; Lambrechts et al., 2018). A myriad of elements may affect CAF subtypes, for instance, proximity to the tumor. CAF subsets were distinguished based on their expression of αSMA and IL6 with high-αSMA localized nearer to the tumor and high-IL6 further, indicating juxtacrine and paracrine interaction between cancer cells and fibroblast may stimulate the CAF to differentiate to subtypes with distinct functions (Öhlund et al., 2017). Aside from molecular markers, the secretome of CAF and NF can be discerned as the former secretes a greater abundance of ligands that promote stemness properties of HCC (Jiang et al., 2017; Álvarez-Teijeiro et al., 2018). CAF plays an extensive role in shaping the extracellular matrix (ECM) of the tissues, allowing it to exert its oncogenic influence by modulating the physical properties of the TME. In addition to promoting cancer progression, fibroblasts are shown to be educated by neoplastic cells to their benefits, depicting the dynamic cellular network in the TME (Figure 1).
FIGURE 1.
CAF and cancer cells in a dynamic TME. CAF secretes chemokines that can potentiate stemness and metastasis properties of cancer cells via distinct pathways. In turn, cancer cells have demonstrated the capacity to direct CAF to fuel their malignant properties. Immune cells, such as T cells, macrophages, and neutrophils, are orchestrated by CAF-cancer cell interaction to promote tumor progression. ANXA1, Annexin A1; CLCF1, cardiotrophin-like cytokine factor 1; COMP, cartilage oligomeric matrix protein; CXCL6, C-X-C motif chemokine ligand 6; CXCL12, C-X-C motif chemokine ligand 12; CCL2, CC-chemokine ligand 2; CCL5, CC-chemokine ligand 5; CCL7, CC-chemokine ligand 7; CCL16, CC-chemokine ligand 16; FGF5, fibroblast growth factor 5; HA, hyaluronan; Hh, hedgehog ligand; HMGB1, high-mobility group box protein 1; HGF, hepatocyte growth factor; IGFI, insulin-like growth factor 1; IGFII, insulin-like growth factor 2; IL6, interleukin 6; IL8, interleukin 8; MMP2; matrix metalloproteinase-2; MMP9, matrix metalloproteinase-9; POSTN, periostin; PGE2, prostaglandin E2; SHH, sonic hedgehog ligand; TGF-β, transforming growth factor-β; TGF-β1, transforming growth factor-β 1; TGF-β2, transforming growth factor-β 2; TGF-β3, transforming growth factor-β 3; WNT3a, Wnt Family Member 3A. The figure is created with BioRender.com.
Cancer stem cells are generally deemed as a rare population of cancer cells that share similar features to normal stem cells, including the ability to self-renew and differentiate into lineages that comprise the tumor bulk, thus regarded as tumorigenic, as opposed to non-CSC cancer cells (Reya et al., 2001; Clarke et al., 2006). Common CSC markers include surface markers, such as CD24, CD44, CD90, CD133, EpCAM, LGR5, and aldehyde dehydrogenase (ALDH) ALDH activity (summarized in Walcher et al., 2020). Moreover, expressions of stemness genes, such as OCT4, SOX2, and NANOG, are often deployed to measure cancer stemness. Functional surrogate to assess cancer stemness properties includes in vitro sphere formation and in vivo limiting dilution in which cancer cells are injected at low doses into animal models. Cancer cells with greater stemness are generally considered to have augmented metastasis ability due to their enhanced ability to reconstitute a tumor at the secondary site, and the acquisition of EMT is coupled with elevated stem cell properties, including stem cell markers and tumorigenicity, signifying EMT mimics features of CSC (Mani et al., 2008; Baccelli and Trumpp, 2012; Oskarsson et al., 2014). Emerging evidence illustrates that stemness properties of cancer cells are dependent on their niche (Plaks et al., 2015; Batlle and Clevers, 2017). Factors derived from niche may promote plasticity of non-CSC cancer cells into CSC, whereas depletion of factors may reduce the CSC population, thereby implicating niche factors as potential therapeutic targets (Batlle and Clevers, 2017). Given the CAF fosters the niche by enriching pro-tumor factors and remodeling the matrix of the TME, understanding how a niche facilitates cancer initiation and progression will allow us to contrive therapy targeting the key cellular components and factors sustaining cancer stemness.
Cancer Cells Educate Cancer-Associated Fibroblast to Adopt a Pro-tumor Phenotype
Accumulating research illuminates the cancer cells instigate pro-tumor CAF to foster a pro-favorable niche. Transforming growth factor-β3 (TGF-β3) derived from HNSCC cells can activate CAF to secrete POSTN, leading to greater metastasis ability of the neoplastic cells (Qin et al., 2016). Prostate cancer cells can recruit marrow-derived mesenchymal stem cells (MSCs) and activate them into CAF by TGF-β1 (Barcellos-de-Souza et al., 2016). Of significance, MSC-derived CAF can recruit and induce monocytes into M2 macrophages, illustrating the CAF capacity to govern the TME. Cardiotrophin-like cytokine factor (CLCF1) derived from CAF stimulates the production of C-X-C motif ligand 6 (CXCL6) and TGF-β, which not only escalate stemness properties of cancer cells but also stimulate CLCF1 expression in the CAF, thereby fostering a positive feedback loop that expedites malignancy (Song et al., 2021). Furthermore, CXCL6 and TGF-β can enhance infiltration and development of pro-tumor N2-neutrophils (Song et al., 2021), corroborating CAF capacity to foster an immunosuppressive TME. Sonic hedgehog (SHH) ligand derived from CD24+CD49fhibreast CSCs constructs a pro-tumorigenic TME by activating CAF (Valenti et al., 2017). Cancer cells-derived hedgehog (Hh) instigates the production of pro-tumor paracrine factors and ECM constituents in CAF (Cazet et al., 2018). Autocrine and colorectal cancer cells-derived paracrine signaling of IL34 facilitate the conversion of normal fibroblast into CAF that adopts a pro-tumor secretome that includes stemness-promoting factors, such as Netrin-1 and FGF2 protein (Giulianelli et al., 2019; Sung et al., 2019). The coculture of HNSCC cells and CAF stimulate CAF to generate WNT3A, leading to increased cancer stemness (Le et al., 2019).
Exosomal miRNAs are gaining interest as a mediator between cancer cell-fibroblast crosstalk. Activation of focal adhesion kinase (FAK) signaling by cancer cell-derived miRNA stimulates the production of CAF-derived exosomes that facilitate the spheroid formation and metastasis (Wu et al., 2020). Tumor-derived exosomal miR-1247-3p triggers the activation of fibroblast by fueling NF-κB signaling consequently stimulates the CAF to secrete a greater abundance of IL6 and IL8, instigating the cancer cells to an EMT phenotype with enhanced lung metastases (Fang et al., 2018). Exosomal miR-9-5p elevates IL6 production in CAF, leading to enhanced spheroid-forming ability (Zhang et al., 2020). In summary, targeting the aforementioned factors or exosomes may disrupt the ability of cancer cells to assemble a pro-tumor niche.
Cancer Cell-Educated Cancer-Associated Fibroblast Cultivates a Supportive Secondary Site for Colonization
The TGF-β family is shown to play pivotal roles in allowing cancer cells to colonize secondary sites. TGF-β3 rooted from cancer cells fortifies a favorable niche to colonize a foreign site by stimulating fibroblast-derived POSTN, which is crucial for initiating breast cancer cell colonization at the lung by maintaining CSC properties via the Wnt signaling (Malanchi et al., 2011). In accordance, HNSCC cancer cells secrete TGF-β3 that enhances POSTN secretion from CAF, resulting in the augmented migratory phenotype of cancer cells (Qin et al., 2016). Upon stimulation by TGF-β, CAF confers colorectal cancer cells-enhanced tumor-initiating capacity and reduced tumor latency (Calon et al., 2015). Tumor organoids with elevated TGF-β profoundly enhanced ability to form liver metastases by orchestrating a pro-tumor stroma niche (Calon et al., 2015). Colorectal cancer (CRC) fueled by driver mutations in the Lgr5 + stem cells is accompanied by enrichment of CAF-derived TGF-β that suppresses the tumor-killing T cell populations, thus leading to greater metastases to the liver (Tauriello et al., 2018), highlighting the interplay between CAF, immune cells, and cancer cells. Conversely, the TGF-β-signaling inhibitor significantly dampened the tumor volume and liver metastases of the colon.
Paracrine Signaling of Cancer-Associated Fibroblast Enhances Cancer Stem Cell Features
In a tumor, fibroblast-secreted factors and cytokines, which serve for tissue recovery, are seized by cancer cells. Compiling studies have depicted the critical roles of CAF-derived factors in maintaining the CSC features of cancer cells by promoting stemness pathways (Table 1). HGF is reported to enhance cancer stemness by potentiating the frequency of CD44+, CD47+, and CD90+ HCC CSC and spheroid formation, stemness and EMT gene expressions (Lau et al., 2016; Ding et al., 2018; Yan et al., 2018). Mechanistically, HGF exerts its oncogenic influence via distinct mechanisms in a tissue-dependent manner. HGF augments ERK/FRA1/HEY1, STAT3/TWIST1, and YAP/HIF-1α in HCC, gastric cancer, and pancreatic cancer, respectively (Lau et al., 2016; Ding et al., 2018; Yan et al., 2018). CAF-derived IL6 is demonstrated to fuel stemness as evident in increased spheroid formation, stemness genes markers via STAT3 signaling (Xiong et al., 2018; Zhang et al., 2020). CAF paracrine signaling regulates the AKT pathway via insulin-like growth factor 2 (IGFII), thereby promoting NANOG expression, enhanced tumorigenicity in vivo, and ALDH activity (Chen et al., 2014). The AKT signaling is also mediated by cartilage oligomeric matrix protein (COMP) to fuel EMT and spheroid formation (Li et al., 2018). CC-chemokine ligand 2, 5, 7, and 16 (CCL2, CCL5, CCL7, and CXCL16) potentiate EMT via the Hh and TGF-β signaling (Liu et al., 2016). Serglycin (SRGN), a proteoglycan secreted from cancer cells and CAFs, renders chemoresistance and EMT features via a CD44-dependent NF-κB activation (Guo et al., 2017). The binding of CAF-derived periostin (POSTN) to protein tyrosine kinase 7 (PTK7) drives the β-catenin signaling, leading to increased expression of CSC markers and stemness genes (Yu et al., 2018). Annexin A1 (AnxA1) secreted from CAF potentiates stemness and EMT genes expressions, and spheroid formation (Geary et al., 2014). Prostaglandin E2 (PGE2) produced from fibroblasts drives the expansion of tumor-initiating cells by inducing YAP signaling (Roulis et al., 2020). The cellular crosstalk within the TME was displayed as CAFs recruit tumor-associated macrophages (TAMs) via CXCL12 secretion and induces the M2-phenotype TAMs, subsequently promotes stemness and EMT signature genes (Li et al., 2019). Alteration in specific genes may dictate the impact of CAF on cancer cells. Androgen receptor (AR)-depleted CAF enhances the production of IFN-γ and M-CSF, leading to increased cancer stemness (Liao et al., 2017). Notch1-depleted CAFs augment CD271+ melanoma CSC frequency (Du et al., 2019) and metastasis to the lung (Shao et al., 2016). Together, blocking CAF-derived factors or a downstream signaling cascade may be a therapeutic revenue to diminish cancer stemness.
TABLE 1.
Cancer-associated fibroblast (CAF)-derived factors on cancer stemness.
| Factors | Disease | Mechanism | References |
| ACTIVIN A and IGF1 | Breast cancer | CSC secretes SHH to cultivate pro-tumor CAF which then generates ACTIVIN A and IGF1 to promote stemness* | Valenti et al., 2017 |
| ANXA1 | Prostate cancer | Induces EMT and stemness markers | Geary et al., 2014 |
| CCL2, CCL5, CCL7, and CXCL16 | Hepatocellular carcinoma | Promotes Hh and TGF-β signaling in HCC cells | Liu et al., 2016 |
| CLCF1 | Hepatocellular carcinoma | Promotes AKT/ERK/STAT3 signaling. STAT3 target genes, CXCL6, and TGF-β induce CAF’s CLCF1 secretion, thus forming CAF-cancer cell positive feedback* | Song et al., 2021 |
| COMP | Hepatocellular carcinoma | Promotes ERK and AKT signaling in HCC cells | Li et al., 2018; Sun et al., 2019 |
| CXCL12 | Oral squamous cell carcinoma | Induces M2-phenotype of TAMs that promote EMT and stemness in cancer | Li et al., 2019 |
| FGF5 | Triple-negative breast cancer | Cancer cell-derived HH induces FGF5 production from CAF; FGF5 elevates stemness genes expression in cancer cells* | Cazet et al., 2018 |
| HGF | Hepatocellular carcinoma | Promotes c-MET/ERK/FRA1/HEY1 axis in HCC cells* | Lau et al., 2016 |
| Gastric cancer | Promotes c-Met/STAT3/twist1 signaling and EMT signaling | Ding et al., 2018 | |
| Pancreatic Cancer | Promotes c-MET/YAP/HIF-1α signaling | Yan et al., 2018 | |
| Intrahepatic cholangiocarcinoma | Promotes AKT/ERK signaling | Affo et al., 2021 | |
| HMGB1 | Luminal breast cancer | Promotes stemness markers and ALDH1+ population upon binding to TLR4 | Zhao et al., 2017 |
| IGFII | Lung cancer | Promotes IGII/IGF1R/AKT/NANOG signaling | Chen et al., 2014 |
| IL6 | Hepatocellular carcinoma | Promotes STAT3/NOTCH1/NICD/HES1 signaling | Xiong et al., 2018 |
| Intrahepatic cholangiocarcinoma | Promotes STAT3/EZH2 cascade. Neoplastic cell-secreted exosomal miR-9-5p upregulates IL6 production* | Zhang et al., 2020 | |
| IL6 and IL8 | Head and Neck cancer | Promote chemoresistance | New et al., 2017 |
| Hepatocellular carcinoma | HCC cell-derived mi-1247-3p stimulate CAF to produce IL6 and IL8, which promotes stemness and EMT* | Fang et al., 2018 | |
| Breast cancer | CD10+GRP77+ CAF-derived IL6 and IL8 stemness | Su et al., 2018 | |
| Netrin-1 | Non-small cell lung cancer and colon cancer | Promote stemness marker and ALDH1A expression | Sung et al., 2019 |
| Netrin-1 and FGF2 | Colorectal cancer | IL34-acitvated CAF increases Netrin-1 and FGF2 expression | Franze et al., 2020 |
| MMP2 and MMP9 | Prostate carcinoma | Cancer cell-derived IL6 activate CAF to produce MMP2 and MMP9, in turn, promote EMT and stemness* | Giannoni et al., 2010 |
| PGE2 | Intestinal cancer | Promotes pro-oncogenic PTGER4/YAP | Roulis et al., 2020 |
| POSTN | Breast cancer | Activates Wnt signaling | Malanchi et al., 2011 |
| Head and neck cancer | TGF-β3 activated CAF to secrete POSTN | Qin et al., 2016 | |
| Human head and neck squamous cell carcinoma | Promotes PTK7/GSKβ/β-catenin signaling | Yu et al., 2018 | |
| TGF-β | Colorectal cancer | CSC modulates CAF-derived TGF-β to suppress the tumor-killing T cell populations* | Tauriello et al., 2018 |
| TGF-β1 | Pancreatic Cancer | Promotes the upregulation of ATF via the SMAD2/3 pathway | Wei et al., 2021 |
| TGF-β2 | Colorectal cancer | Upregulates GLI2 expression | Tang et al., 2018 |
| SRGN | Non-small cell lung cancer | Promote stemness gene markers in CD44-dependent manner | Guo et al., 2017 |
| WNT3A | Human head and neck squamous cell carcinoma | Neoplastic cells activate WNT3A production in CAF* | Le et al., 2019 |
| Exosomal miR-21, miR-143, and miR-378 | Breast cancer | Enhances stemness and EMT | Donnarumma et al., 2017 |
| Exosomal lncRNA H19 | Colorectal cancer | Sponges miR-141 that target β-catenin | Ren et al., 2018 |
| Exosomal miR-92a-3p | Colorectal cancer | Targets FBXW7 to enhance β-catenin activity and MOAP1 to enhance chemoresistance properties | Hu et al., 2019 |
| Exosomal miR-196a | Head and Neck cancer | Targets CDKN1B and ING5, to promote cisplatin resistance | Qin et al., 2019 |
| Exosomal miR-423-5p | Prostate cancer | Promotes chemoresistance by targeting GREM2, leading to TGF-β pathway activity | Shan et al., 2020 |
*Denotes a cancer cell-to-CAF dialog.
Cancer-Associated Fibroblast Mediates Cancer Stemness via Extracellular Matrix Remodeling
A fundamental function of activated fibroblast is its participation in ECM homeostasis by synthesizing ECM constituents, including collagen and fibronectin, and producing ECM-degrading proteases to aid communication and trafficking of inflammatory cells (Kalluri, 2016). While the impact of matrix stiffness remains controversial as studies indicate both softer matrix (Tan et al., 2014; Ng et al., 2021) and stiffer matrix (Pang et al., 2016; Tao et al., 2021) can instigate cancer stemness, the role of CAF in mediating the stiffness of the ECM illustrates its ability to affect stemness. Indeed, collagen deposited by CAF functions as a mechano signal to escalate stem cell markers and spheroid formation ability (Cazet et al., 2018). Additionally, hyaluronan (HA), a major constituent of the ECM that boosts CSC self-renewal and EMT (summarized in Chanmee et al., 2015), is shown to be highly produced by CAF (Affo et al., 2021). Given CAFs are a source of ECM-degrading proteases, namely matrix metalloproteinases (MMPs), they can affect CSC properties by remodeling the ECM. Studies demonstrated IL6 derived from prostate cancer cells elicits pro-tumor FAP+CAF (Giannoni et al., 2010). Consequently, these FAP+ CAF secretes MMP2 and MMP9 to potentiate EMT and stemness genes. MMP3 that is predominantly produced by fibroblast (Witty et al., 1995) is demonstrated to promote stem cell population by enhancing canonical Wnt signaling, potentially leading to hyperplastic growth of normal tissues (Kessenbrock et al., 2013), depicting fibroblast can contribute to tumor initiation via ECM remodeling. Future research can focus on ablating the effect of CAF on the ECM, either by targeting ECM constitutes or MMPs, to attenuate the CAF-mediated oncogenic effect.
Cancer-Associated Fibroblast Role in Endowing Drug Resistance
The augmented cancer stemness conferred by CAF may endow cancer resistance to conventional therapy. Patients with breast cancer, who are resistant to chemotherapy, show a greater abundance of CAF marked with a cluster of differentiation 10 (CD10) and G protein-coupled receptor 77 (GRP77) cell surface proteins (Su et al., 2018). CD10+GRP77+ CAFs promote sphere formation, the proportion of ALDH+ and CD44+CD24– breast CSCs, and chemoresistance properties of cancer cells by secreting IL6 and IL8. A similar phenomenon was observed in HNSCC as CAFs undergoing autophagy secrete greater levels of IL6 and IL8 to confer chemoresistance (New et al., 2017). Increased NANOG and SOX2 stemness gene expression in cancer cells treated with CAF-conditioned media may lead to greater cisplatin resistance (Peltanova et al., 2021). CAF-derived TGF-β1 drives self-renewal and gemcitabine resistance by upregulation of activating transcription factor 4 (ATF4) via the SMAD2/3 pathway (Wei et al., 2021). SRGN facilitates cisplatin resistance via inducing NANOG (Guo et al., 2017). In line, CAF-rooted TGF-β2 can elicit stemness and chemoresistance by enhancing GLI Family Zinc Finger 2 (GLI2) (Tang et al., 2018). CAF-derived exosomes enhance the stemness properties of CD133+ colorectal CSCs, leading to greater chemoresistance to fluorouracil and oxaliplatin (Hu et al., 2015). Additionally, exosomal miR-92a-3p derived from colorectal CAF targets anti-tumor F-box/WD repeat-containing protein 7 (FBXW7) and a modulator of apoptosis 1 (MOAP1), thereby endowing chemoresistance to cancer cells (Hu et al., 2019). In line, exosomal miRNA was demonstrated to confer chemoresistance in prostate cancer (Shan et al., 2020) and head and neck cancer (Qin et al., 2019). CAF-derived exosomal long non-coding RNA (lncRNA) H19 was exhibited to enable stemness expression by sponging miR-141 that targets β-catenin (Ren et al., 2018).
Another mechanism that CAF mediates chemoresistance resides in its ability to remodel the ECM. Studies have demonstrated HA upregulates NANOG/STAT signaling, leading to increased expression of multidrug transporter (MDR1) that contributes to chemoresistance (Bourguignon et al., 2008). In accordance, HA-CD44 interaction mediates stemness signaling that governs miRNA regulation of genes involved in chemoresistance in breast cancer cells (Bourguignon et al., 2009) and HNSCC cells (Bourguignon et al., 2012a, b). HA mediates CD44v3highALDH1highHNSCC CSC via an epigenetic alteration to promote cisplatin chemoresistance (Bourguignon et al., 2016). The ability of CAF to mediate matrix stiffness via MMPs may be an area of interest. Given matrix stiffness regulates stemness and chemoresistance (Ng et al., 2021); how a CAF-mediated ECM fosters a niche that endows chemoresistance warrants further investigations.
Single-Cell RNA Sequencing Identified Cancer-Associated Fibroblast Subsets With Distinct Functions on Cancer Stemness
State-of-the-art single-cell transcriptomic profiling of intrahepatic cholangiocarcinoma (ICC) revealed six distinct fibroblast populations (Zhang et al., 2020). Vascular CAFs (vCAF), the most abundant population, were found to secrete IL6 to promote self-renewal of cancer cells. Of note, the characterization of the function of other CAF subtypes identified such as matrix CAFs, which expressed high ECM signature genes, would be an interesting area of research. Lineage tracing coupled with single-cell RNA sequencing (scRNA-seq) in sophisticated animal models categorized CAF into myofibroblast (myCAF) and inflammatory and growth factor-enriched CAF (iCAF) populations that modulate tumor cell proliferation via distinct mechanisms; the former has a high level of hyaluronan synthase 2 (Has2) that can enhance pro-tumor HA; the latter secretes HGF (Affo et al., 2021). scRNA-seq identified prostaglandin-endoperoxide synthase 2 (Ptgs2)-expressing fibroblasts that expand tumor-initiating stem cells via YAP signaling, whereas ablation of Ptgs2 diminished the occurrence of the small intestine and colon cancers (Roulis et al., 2020). Capturing cellular diversity at single-cell resolution ignites exciting research questions as follows: how cellular and non-cellular components of the TME govern CAF identity? How each subtype contributes to disease progression? How CAFs differentiate or undifferentiate into different subtypes?
Potential Therapeutic Strategy and Challenges
Targeting Multiple Cancer-Associated Fibroblast-Derived Factors to Circumvent Stemness
As aforementioned (Table 1), cancer-promoting bioactive molecules derived from stromal cells may be targeted using specific neutralizing antibodies, particularly in combination with chemotherapy. However, the promising preclinical results have yet to yield optimistic results in clinical settings. A Phase 2 trial (NCT00433446) examining siltuximab (CNTO328), an anti-IL6 antibody, showed siltuximab did not have clinical benefit for patients with advanced prostate cancer (Dorff et al., 2010). Similarly, the addition of siltuximab (CNTO328) to mitoxantrone/prednisone regimen did not yield improved clinical (NCT00385827) outcomes compared to mitoxantrone/prednisone alone in treating advanced prostate cancer (Fizazi et al., 2012). The Phase 2, single-arm, clinical trial (NCT00992186) explored the efficacy of carlumab (CNTO 888), antibody targeting (CCL2), in patients with metastatic castration-resistant prostate cancer (CRPC), who had undergone docetaxel treatment (Pienta et al., 2013). However, none of the patients treated with carlumab showed partial or complete remission. One key possibility is that, while the CCL2 level was depleted upon carlumab administration, the level rebounded rapidly within a week, thus suggesting carlumab cannot suppress CCL2 for the clinically meaningful duration (Pienta et al., 2013). Nonetheless, the addition of siltuximab to bortezomib-melphalan-prednisone (VMP) demonstrated marginal clinical benefits in multiple myeloma by statistically improving partial response rate (San-Miguel et al., 2014). Together, these imply more precise stratification of patients and usage of biomarkers may result in better clinical outcomes. Of note, several clinical trials are exploring the clinical utility of neutralizing antibodies that target HGF (NCT04368507), MET (NCT04077099), and IL6R (NCT03999749). Aside from neutralizing antibodies, a combination of inhibitors-targeting receptors, including Osimertinib (an EGFR inhibitor) and Savolitinib (MET inhibitors), are being tested in clinical trials (NCT03778229). Notably, MP0250, a drug candidate targeting HGF and VEGF, which have met the safety requirement and demonstrated clinical efficacy, is being tested in combination with existing drugs to treat multiple myeloma (NCT03136653). Given the TME is often accompanied by an upsurge of cytokines and chemokines, targeting multiple factors may promote treatment efficacy.
Depleting Pro-tumor Cancer-Associated Fibroblast
The ability of CAF to endow stemness of cancer cells makes it an intriguing therapy. CAF is shown to be more positively correlated with gene sets associated with poor prognosis compared with epithelial cancer cells, immune cells, and endothelial cells (Calon et al., 2015), substantiating targeting CAF as a potential therapeutic avenue. Studies have demonstrated that αSMA expression represents a marker for worse prognosis in colorectal cancer, indicating myofibroblast abundance is crucial to disease prediction (Tsujino et al., 2007). In accordance, studies conducted in tongue cancer and oral cancer depicted similar results (Vered et al., 2010; Marsh et al., 2011).
However, the heterogeneity of CAF necessitates precise identification of more specific markers as tumor-restraining CAF exists. Genetic deletion of αSMA in mouse models enhances the progression of pancreatic ductal adenocarcinoma (PDAC) as evidenced by a lower survival rate in αSMA-depleted mice (Ozdemir et al., 2014). Interestingly, the myofibroblast-depleted tumor demonstrated enhanced spheroid-forming ability, indicating a greater proportion of CSC. Indeed, identification of CAF subtypes shows they promote or suppress tumor progression in a tissue-dependent manner (Galbo et al., 2021), corroborating the need for further investigation into more CAF-specific markers. Recent work has illuminated the potential therapeutic benefit of targeting tumor-promoting fibroblasts. Administration of GRP77 neutralizing antibody-targeting CAF depletes CAF-secreted IL6 and IL8, thereby abolishing a stem cell-supporting niche and sensitizing breast cancer cells to doxycycline, leading to significant shrinkage of the tumor volume (Su et al., 2018), highlighting the therapeutic potential of targeting precise tumor-promoting CAF.
Rewire Pro-tumor Cancer-Associated Fibroblast Into Quiescent or Anti-tumor Fibroblast
Reeducating pro-tumor CAF into a quiescent state or even anti-tumor CAF is a tempting strategy. Vitamin D metabolite 1α, 25-dihydroxyvitamin D3 [1,25(OH)2D3] is shown to deplete the oncogenic influence of stromal fibroblast to the cancer cells, while 1,25(OH)2D3-treated stromal fibroblast displayed a gene signature that favors a clinical outcome (Ferrer-Mayorga et al., 2017). VDR activation of the stromal fibroblast using calcipotriol, a vitamin D analog, diminished expression of genes involved in growth factors and cytokines, for instance, the IL6 and POSTN, thereby suppressing a tumor-promoting secretome (Sherman et al., 2014). Additionally, the combination treatment of calcipotriol and gemcitabine markedly prolonged survival in preclinical models. Moreover, high vitamin D receptor (VDR) expression in stromal cells predicts favorable survival (Ferrer-Mayorga et al., 2017). All-trans retinoic acid (ATRA), also known as tretinoin, a vitamin A metabolite, renders pancreatic stellate cells into a quiescent state, which, in turn, secreted a greater level of secreted frizzled-related protein 4 (sFRP4) that negatively modulates Wnt signaling of cancer cells in a paracrine manner (Froeling et al., 2011). Indeed, ATRA has passed the safety in Phase I clinical trial (NCT03307148) and will proceed to Phase II (NCT04241276) as encouraging therapeutic responses were observed (Kocher et al., 2020). Given vitamins are essential for healthy tissue and their toxicity is relatively lower compared to chemotherapy, repurposing vitamin analogs to rewire activated fibroblast into a quiescent state may present a viable therapeutic strategy that can be translated into the clinical settings.
Another strategy to rewire the population of fibroblasts is by reprogramming the fibroblast using growth factors as exemplified in the plasticity of CAF found in PDAC. While IL1 induces fibroblast into having an inflammatory phenotype categorized by an elevated cytokines production, TGF-β antagonizes an IL1-induced phenotype and stimulates the fibroblast to adopt a myofibroblastic phenotype with less tumorigenesis, particularly reduced expression of factors promoting cancer stemness such as Il6 and Cxcl12 (Biffi et al., 2019). Together, this rationalizes the option to rewire tumor-promoting into tumor-restraining fibroblast. To account for the ability of the cancer cell to instigate tumor-promoting CAFs, inhibitors may be deployed to circumvent cancer cell-mediated activation of CAF. Breast cancer cells activate CAF via the hedgehog signaling to cultivate a stem cell niche by ECM remodeling and FGF5 secretion that promote docetaxel chemoresistance (Cazet et al., 2018). As such, targeting the crosstalk via smoothened inhibitors (SMOi), a hedgehog-signaling inhibitor, sensitizes triple-negative breast cancer (TNBC) to docetaxel. Concomitantly, inhibition of hedgehogs abrogates the activated stromal cells, thereby augmenting the efficacy of chemotherapy in treating pancreatic cancer (Olive et al., 2009). However, either genetic depletion or pharmacological inhibition of SHH, a ligand that activates pancreatic CAFs, resulted in less stromal composition but also a more aggressive tumor (Rhim et al., 2014), suggesting treatment should be tailored based on tissue and treatment.
Future Perspectives
Advances in single-cell RNA sequencing may not only aid the characterization of cell types based on their molecular profiles and, subsequently, functions but also have the potential to be used to identify new biomarkers for patient stratification and tailor-personalized medicine (Dominguez et al., 2020). Harnessing single-cell RNA-sequencing to profile CAF at the molecular level, future studies can address outstanding questions, including the origin and development of specific CAF subtypes, identification of biomarkers corresponding to each subtype, how each CAF subtype interacts with its niche, and amenable therapeutic opportunities to tackle tumor-promoting CAF. A more holistic approach investigating the CAF molecular profile, for instance, epitranscriptomic and epigenetics (Delaunay and Frye, 2019; Song et al., 2020), may unravel novel insights into how CAF shuttles between cell types. Recent studies have unmasked that the altered epigenetics profile between CAF and NF allows the former to generate greater levels of WNT5A that confers malignancy to the neoplastic cells (Maeda et al., 2020). A critical unmet knowledge gap in our understanding of CAF function is if juxtracrine signaling between CAF and cancer cells affects cancer stemness.
Given the TME is a dynamic region with various cell types actively contributing to tumor progression, merely focusing on targeting a single aspect seems unlikely to yield any long-term therapeutic benefit. For example, targeting niche factors alone may not be sufficient to eradicate the tumor due to the inclination of cancer cells to evolve into a niche-independent malignancy as the disease progresses (Fujii et al., 2016). Therefore, comprehensive characterization of cell types and their respective functions in the TME may pave the way for a multimodal approach to improve cancer treatment.
Author Contributions
JL wrote the review article. SM edited the review article and provided funding support. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This project was supported in part by grants from the Research Grants Council of Hong Kong—Theme-Based Research Scheme (T12-704/16-R), Collaborative Research Fund (C7026-18G), and the Health and Medical Research Fund from Food and Health Bureau of the Hong Kong Government (06172546).
References
- Affo S., Nair A., Brundu F., Ravichandra A., Bhattacharjee S., Matsuda M., et al. (2021). Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 39 866–882.e11. 10.1016/j.ccell.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Teijeiro S., Garcia-Inclan C., Villaronga M. A., Casado P., Hermida-Prado F., Granda-Diaz R., et al. (2018). Factors secreted by cancer-associated fibroblasts that sustain cancer stem properties in head and neck squamous carcinoma cells as potential therapeutic targets. Cancers 10:334. 10.3390/cancers10090334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli I., Trumpp A. (2012). The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 198 281–293. 10.1083/jcb.201202014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-de-Souza P., Comito G., Pons-Segura C., Taddei M. L., Gori V., Becherucci V., et al. (2016). Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-beta1. Stem Cells 34 2536–2547. 10.1002/stem.2412 [DOI] [PubMed] [Google Scholar]
- Batlle E., Clevers H. (2017). Cancer stem cells revisited. Nat. Med. 23 1124–1134. 10.1038/nm.4409 [DOI] [PubMed] [Google Scholar]
- Biffi G., Oni T. E., Spielman B., Hao Y., Elyada E., Park Y., et al. (2019). IL1-induced JAK/STAT signaling Is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9 282–301. 10.1158/2159-8290.CD-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Peyrollier K., Xia W., Gilad E. (2008). Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 283 17635–17651. 10.1074/jbc.M800109200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Spevak C. C., Wong G., Xia W., Gilad E. (2009). Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J. Biol. Chem. 284 26533–26546. 10.1074/jbc.M109.027466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Earle C., Wong G., Spevak C. C., Krueger K. (2012a). Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 31 149–160. 10.1038/onc.2011.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Wong G., Earle C., Chen L. (2012b). Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 287 32800–32824. 10.1074/jbc.M111.308528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Wong G., Shiina M. (2016). Up-regulation of histone methyltransferase, DOT1L, by matrix hyaluronan promotes microRNA-10 expression leading to tumor cell invasion and chemoresistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 291 10571–10585. 10.1074/jbc.M115.700021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A., Lonardo E., Berenguer-Llergo A., Espinet E., Hernando-Momblona X., Iglesias M., et al. (2015). Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 47 320–329. 10.1038/ng.3225 [DOI] [PubMed] [Google Scholar]
- Cazet A. S., Hui M. N., Elsworth B. L., Wu S. Z., Roden D., Chan C. L., et al. (2018). Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 9:2897. 10.1038/s41467-018-05220-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanmee T., Ontong P., Kimata K., Itano N. (2015). Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front. Oncol. 5:180. 10.3389/fonc.2015.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Ho C. C., Chang Y. L., Chen H. Y., Lin C. A., Ling T. Y., et al. (2014). Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 5:3472. 10.1038/ncomms4472 [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Dick J. E., Dirks P. B., Eaves C. J., Jamieson C. H., Jones D. L., et al. (2006). Cancer stem cells - perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 66 9339–9344. 10.1158/0008-5472.CAN-06-3126 [DOI] [PubMed] [Google Scholar]
- Costa A., Kieffer Y., Scholer-Dahirel A., Pelon F., Bourachot B., Cardon M., et al. (2018). Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33 463–479.e10. 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Delaunay S., Frye M. (2019). RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 21 552–559. 10.1038/s41556-019-0319-0 [DOI] [PubMed] [Google Scholar]
- Ding X., Ji J., Jiang J., Cai Q., Wang C., Shi M., et al. (2018). HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 9:867. 10.1038/s41419-018-0922-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C. X., Muller S., Keerthivasan S., Koeppen H., Hung J., Gierke S., et al. (2020). Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 10 232–253. 10.1158/2159-8290.CD-19-0644 [DOI] [PubMed] [Google Scholar]
- Donnarumma E., Fiore D., Nappa M., Roscigno G., Adamo A., Iaboni M., et al. (2017). Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 8 19592–19608. 10.18632/oncotarget.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorff T. B., Goldman B., Pinski J. K., Mack P. C., Lara P. N., Jr., Van Veldhuizen P. J., Jr., et al. (2010). Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin. Cancer Res. 16 3028–3034. 10.1158/1078-0432.CCR-09-3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Shao H., Moller M., Prokupets R., Tse Y. T., Liu Z. J. (2019). Intracellular Notch1 signaling in cancer-associated fibroblasts dictates the plasticity and stemness of melanoma stem/initiating cells. Stem Cells 37 865–875. 10.1002/stem.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315 1650–1659. 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- Erez N., Truitt M., Olson P., Arron S. T., Hanahan D. (2010). Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17 135–147. 10.1016/j.ccr.2009.12.041 [DOI] [PubMed] [Google Scholar]
- Fang T., Lv H., Lv G., Li T., Wang C., Han Q., et al. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9:191. 10.1038/s41467-017-02583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Mayorga G., Gomez-Lopez G., Barbachano A., Fernandez-Barral A., Pena C., Pisano D. G., et al. (2017). Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut 66 1449–1462. 10.1136/gutjnl-2015-310977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K., De Bono J. S., Flechon A., Heidenreich A., Voog E., Davis N. B., et al. (2012). Randomised phase II study of siltuximab (CNTO 328), an anti-IL6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur. J. Cancer 48 85–93. 10.1016/j.ejca.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Franze E., Di Grazia A., Sica G. S., Biancone L., Laudisi F., Monteleone G. (2020). Interleukin-34 enhances the tumor promoting function of colorectal cancer-associated fibroblasts. Cancers 12:3537. 10.3390/cancers12123537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeling F. E., Feig C., Chelala C., Dobson R., Mein C. E., Tuveson D. A., et al. (2011). Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 141 1486–1497. 10.1053/j.gastro.2011.06.047 [DOI] [PubMed] [Google Scholar]
- Fujii M., Shimokawa M., Date S., Takano A., Matano M., Nanki K., et al. (2016). A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18 827–838. 10.1016/j.stem.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Galbo et al. P. M., Jr., Zang X., Zheng D. (2021). Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin. Cancer Res. 1 2636–2647. 10.1158/1078-0432.CCR-20-4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary L. A., Nash K. A., Adisetiyo H., Liang M., Liao C. P., Jeong J. H., et al. (2014). CAF-secreted annexin A1 induces prostate cancer cells to gain stem cell-like features. Mol. Cancer Res. 12 607–621. 10.1158/1541-7786.MCR-13-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E., Bianchini F., Masieri L., Serni S., Torre E., Calorini L., et al. (2010). Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70 6945–6956. 10.1158/0008-5472.CAN-10-0785 [DOI] [PubMed] [Google Scholar]
- Giulianelli S., Riggio M., Guillardoy T., Perez Pinero C., Gorostiaga M. A., Sequeira G., et al. (2019). FGF2 induces breast cancer growth through ligand-independent activation and recruitment of ERalpha and PRBDelta4 isoform to MYC regulatory sequences. Int. J. Cancer 145 1874–1888. 10.1002/ijc.32252 [DOI] [PubMed] [Google Scholar]
- Guo J. Y., Hsu H. S., Tyan S. W., Li F. Y., Shew J. Y., Lee W. H., et al. (2017). Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene 36 2457–2471. 10.1038/onc.2016.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. L., Wang W., Lan X. L., Zeng Z. C., Liang Y. S., Yan Y. R., et al. (2019). CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 18:91. 10.1186/s12943-019-1019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Yan C., Mu L., Huang K., Li X., Tao D., et al. (2015). Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One 10:e0125625. 10.1371/journal.pone.0125625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Ye F., Yang X., Zong C., Gao L., Yang Y., et al. (2017). Peri-tumor associated fibroblasts promote intrahepatic metastasis of hepatocellular carcinoma by recruiting cancer stem cells. Cancer Lett. 404 19–28. 10.1016/j.canlet.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Kalluri R. (2016). The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16 582–598. 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Dijkgraaf G. J., Lawson D. A., Littlepage L. E., Shahi P., Pieper U., et al. (2013). A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell 13 300–313. 10.1016/j.stem.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher H. M., Basu B., Froeling F. E. M., Sarker D., Slater S., Carlin D., et al. (2020). Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 11:4841. 10.1038/s41467-020-18636-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., et al. (2018). Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24 1277–1289. 10.1038/s41591-018-0096-5 [DOI] [PubMed] [Google Scholar]
- Lau E. Y., Lo J., Cheng B. Y., Ma M. K., Lee J. M., Ng J. K., et al. (2016). Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 15 1175–1189. 10.1016/j.celrep.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Le P. N., Keysar S. B., Miller B., Eagles J. R., Chimed T. S., Reisinger J., et al. (2019). Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol. Carcinog. 58 398–410. 10.1002/mc.22937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang C., Wang Y., Sun L., Liu Z., Wang L., et al. (2018). HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J. Exp. Clin. Cancer Res. 37:231. 10.1186/s13046-018-0908-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Bu W., Meng L., Liu X., Wang S., Jiang L., et al. (2019). CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 378 131–138. 10.1016/j.yexcr.2019.03.013 [DOI] [PubMed] [Google Scholar]
- Liao C. P., Chen L. Y., Luethy A., Kim Y., Kani K., MacLeod A. R., et al. (2017). Androgen receptor in cancer-associated fibroblasts influences stemness in cancer cells. Endocr. Relat. Cancer 24 157–170. 10.1530/ERC-16-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen S., Wang W., Ning B. F., Chen F., Shen W., et al. (2016). Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett. 379 49–59. 10.1016/j.canlet.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Maeda M., Takeshima H., Iida N., Hattori N., Yamashita S., Moro H., et al. (2020). Cancer cell niche factors secreted from cancer-associated fibroblast by loss of H3K27me3. Gut 69 243–251. 10.1136/gutjnl-2018-317645 [DOI] [PubMed] [Google Scholar]
- Malanchi I., Santamaria-Martinez A., Susanto E., Peng H., Lehr H. A., Delaloye J. F., et al. (2011). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481 85–89. 10.1038/nature10694 [DOI] [PubMed] [Google Scholar]
- Mani S. A., Guo W., Liao M. J., Eaton E. N., Ayyanan A., Zhou A. Y., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133 704–715. 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D., Suchak K., Moutasim K. A., Vallath S., Hopper C., Jerjes W., et al. (2011). Stromal features are predictive of disease mortality in oral cancer patients. J. Pathol. 223 470–481. 10.1002/path.2830 [DOI] [PubMed] [Google Scholar]
- New J., Arnold L., Ananth M., Alvi S., Thornton M., Werner L., et al. (2017). Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 77 6679–6691. 10.1158/0008-5472.CAN-17-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K. Y., Shea Q. T., Wong T. L., Luk S. T., Tong M., Lo C. M., et al. (2021). Chemotherapy-enriched THBS2-deficient cancer stem cells drive hepatocarcinogenesis through matrix softness induced histone H3 modifications. Adv. Sci. 8:2002483. 10.1002/advs.202002483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhlund D., Elyada E., Tuveson D. (2014). Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 211 1503–1523. 10.1084/jem.20140692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A. S., Ponz-Sarvise M., et al. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214 579–596. 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive K. P., Jacobetz M. A., Davidson C. J., Gopinathan A., McIntyre D., Honess D., et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324 1457–1461. 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T., Batlle E., Massague J. (2014). Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 14 306–321. 10.1016/j.stem.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir B. C., Pentcheva-Hoang T., Carstens J. L., Zheng X., Wu C. C., Simpson T. R., et al. (2014). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25 719–734. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M. F., Siedlik M. J., Han S., Stallings-Mann M., Radisky D. C., Nelson C. M. (2016). Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 76 5277–5287. 10.1158/0008-5472.CAN-16-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltanova B., Liskova M., Gumulec J., Raudenska M., Polanska H. H., Vaculovic T., et al. (2021). Sensitivity to cisplatin in head and neck cancer cells is significantly affected by patient-derived cancer-associated fibroblasts. Int. J. Mol. Sci. 22:1912. 10.3390/ijms22041912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta K. J., Machiels J. P., Schrijvers D., Alekseev B., Shkolnik M., Crabb S. J., et al. (2013). Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest. New Drugs 31 760–768. 10.1007/s10637-012-9869-8 [DOI] [PubMed] [Google Scholar]
- Plaks V., Kong N., Werb Z. (2015). The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16 225–238. 10.1016/j.stem.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X., Guo H., Wang X., Zhu X., Yan M., Wang X., et al. (2019). Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 20:12. 10.1186/s13059-018-1604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X., Yan M., Zhang J., Wang X., Shen Z., Lv Z., et al. (2016). TGFbeta3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci. Rep. 6:20587. 10.1038/srep20587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Ding L., Zhang D., Shi G., Xu Q., Shen S., et al. (2018). Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 8 3932–3948. 10.7150/thno.25541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Morrison S. J., Clarke M. F., Weissman I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature 414 105–111. 10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- Rhim A. D., Oberstein P. E., Thomas D. H., Mirek E. T., Palermo C. F., Sastra S. A., et al. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25 735–747. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulis M., Kaklamanos A., Schernthanner M., Bielecki P., Zhao J., Kaffe E., et al. (2020). Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 580 524–529. 10.1038/s41586-020-2166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Miguel J., Blade J., Shpilberg O., Grosicki S., Maloisel F., Min C. K., et al. (2014). Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL6) in multiple myeloma. Blood 123 4136–4142. 10.1182/blood-2013-12-546374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan G., Gu J., Zhou D., Li L., Cheng W., Wang Y., et al. (2020). Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-beta signaling pathway. Exp. Mol. Med. 52 1809–1822. 10.1038/s12276-020-0431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H., Cai L., Moller M., Issac B., Zhang L., Owyong M., et al. (2016). Notch1-WISP-1 axis determines the regulatory role of mesenchymal stem cell-derived stromal fibroblasts in melanoma metastasis. Oncotarget 7 79262–79273. 10.18632/oncotarget.13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. H., Yu R. T., Engle D. D., Ding N., Atkins A. R., Tiriac H., et al. (2014). Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159 80–93. 10.1016/j.cell.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Liu D., Dong S., Zeng L., Wu Z., Zhao P., et al. (2020). Epitranscriptomics and epiproteomics in cancer drug resistance: therapeutic implications. Signal Transduct. Target. Ther. 5:193. 10.1038/s41392-020-00300-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., He J., Pan Q. Z., Yang J., Zhao J., Zhang Y. J., et al. (2021). Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology 73 1717–1735. 10.1002/hep.31792 [DOI] [PubMed] [Google Scholar]
- Su S., Chen J., Yao H., Liu J., Yu S., Lao L., et al. (2018). CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172 841–856.e16. 10.1016/j.cell.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Sun L., Wang Y., Wang L., Yao B., Chen T., Li Q., et al. (2019). Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J. Exp. Clin. Cancer Res. 38 170. 10.1186/s13046-019-1163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P. J., Rama N., Imbach J., Fiore S., Ducarouge B., Neves D., et al. (2019). Cancer-associated fibroblasts produce netrin-1 to control cancer cell plasticity. Cancer Res. 79 3651–3661. 10.1158/0008-5472.CAN-18-2952 [DOI] [PubMed] [Google Scholar]
- Tan Y., Tajik A., Chen J., Jia Q., Chowdhury F., Wang L., et al. (2014). Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat. Commun. 5:4619. 10.1038/ncomms5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y. A., Chen Y. F., Bao Y., Mahara S., Yatim S., Oguz G., et al. (2018). Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 115 E5990–E5999. 10.1073/pnas.1801348115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao B., Song Y., Wu Y., Yang X., Peng T., Peng L., et al. (2021). Matrix stiffness promotes glioma cell stemness by activating BCL9L/Wnt/beta-catenin signaling. Aging 13 5284–5296. 10.18632/aging.202449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello D. V. F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., et al. (2018). TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554 538–543. 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- Tsujino T., Seshimo I., Yamamoto H., Ngan C. Y., Ezumi K., Takemasa I., et al. (2007). Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin. Cancer Res. 13 2082–2090. 10.1158/1078-0432.CCR-06-2191 [DOI] [PubMed] [Google Scholar]
- Valenti G., Quinn H. M., Heynen G., Lan L., Holland J. D., Vogel R., et al. (2017). Cancer stem cells regulate cancer-associated fibroblasts via activation of hedgehog signaling in mammary gland tumors. Cancer Res. 77 2134–2147. 10.1158/0008-5472.CAN-15-3490 [DOI] [PubMed] [Google Scholar]
- Vered M., Dobriyan A., Dayan D., Yahalom R., Talmi Y. P., Bedrin L., et al. (2010). Tumor-host histopathologic variables, stromal myofibroblasts and risk score, are significantly associated with recurrent disease in tongue cancer. Cancer Sci. 101 274–280. 10.1111/j.1349-7006.2009.01357.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcher L., Kistenmacher A. K., Suo H., Kitte R., Dluczek S., Strauss A., et al. (2020). Cancer stem cells- origins and biomarkers: perspectives for targeted personalized therapies. Front. Immunol. 11:1280. 10.3389/fimmu.2020.01280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Lin Q., Lu Y., Li G., Huang L., Fu Z., et al. (2021). Cancer-associated fibroblasts-mediated ATF4 expression promotes malignancy and gemcitabine resistance in pancreatic cancer via the TGF-beta1/SMAD2/3 pathway and ABCC1 transactivation. Cell Death Dis. 12:334. 10.1038/s41419-021-03574-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty J. P., Wright J. H., Matrisian L. M. (1995). Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol. Biol. Cell 6 1287–1303. 10.1091/mbc.6.10.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. J., Hao M., Yeo S. K., Guan J. L. (2020). FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 39 2539–2549. 10.1038/s41388-020-1162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S., Wang R., Chen Q., Luo J., Wang J., Zhao Z., et al. (2018). Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL6/STAT3/Notch signaling. Am. J. Cancer Res. 8 302–316. [PMC free article] [PubMed] [Google Scholar]
- Yan B., Jiang Z., Cheng L., Chen K., Zhou C., Sun L., et al. (2018). Paracrine HGF/c-MET enhances the stem cell-like potential and glycolysis of pancreatic cancer cells via activation of YAP/HIF-1alpha. Exp. Cell Res. 371 63–71. 10.1016/j.yexcr.2018.07.041 [DOI] [PubMed] [Google Scholar]
- Yu B., Wu K., Wang X., Zhang J., Wang L., Jiang Y., et al. (2018). Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 9:1082. 10.1038/s41419-018-1116-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang M., Yang H., Wan L., Wang Z., Wang H., Ge C., et al. (2020). Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 73 1118–1130. 10.1016/j.jhep.2020.05.039 [DOI] [PubMed] [Google Scholar]
- Zhao X. L., Lin Y., Jiang J., Tang Z., Yang S., Lu L., et al. (2017). High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells. J. Pathol. 243 376–389. 10.1002/path.4958 [DOI] [PMC free article] [PubMed] [Google Scholar]