Abstract
Background:
Cancer patients are at higher risk of COVID-19 complications and mortality than the rest of the population. Breast cancer patients seem to have better prognosis when infected by SARS-CoV-2 than other cancer patients.
Methods:
We report a subanalysis of the OnCovid study providing more detailed information in the breast cancer population.
Results:
We included 495 breast cancer patients with a SARS-CoV-2 infection. Mean age was 62.6 years; 31.5% presented more than one comorbidity. The most frequent breast cancer subtype was luminal-like (n = 245, 49.5%) and 177 (35.8%) had metastatic disease. A total of 332 (67.1%) patients were receiving active treatment, with radical intent in 232 (47.6%) of them. Hospitalization rate was 58.2% and all-cause mortality rate was 20.3%. One hundred twenty-nine (26.1%) patients developed one COVID-19 complication, being acute respiratory failure the most common (n = 74, 15.0%). In the multivariable analysis, age older than 70 years, presence of COVID-19 complications, and metastatic disease were factors correlated with worse outcomes, while ongoing anticancer therapy at time of COVID-19 diagnosis appeared to be a protective factor. No particular oncological treatment was related to higher risk of complications. In the context of SARS-CoV-2 infection, 73 (18.3%) patients had some kind of modification on their oncologic treatment. At the first oncological reassessment (median time: 46.9 days ± 36.7), 255 (51.6%) patients reported to be fully recovered from the infection. There were 39 patients (7.9%) with long-term SARS-CoV-2-related complications.
Conclusion:
In the context of COVID-19, our data confirm that breast cancer patients appear to have lower complications and mortality rate than expected in other cancer populations. Most breast cancer patients can be safely treated for their neoplasm during SARS-CoV-2 pandemic. Oncological treatment has no impact on the risk of SARS-CoV-2 complications, and, especially in the curative setting, the treatment should be modified as little as possible.
Keywords: breast cancer, COVID-19, COVID-19 outcomes, OnCovid, SARS-CoV-2
Introduction
In December 2019, a novel SARS-CoV-2 infection was first documented in Wuhan, China, and quickly escalated to a global pandemic by March 2020. As of April 2021, there have been more than 149 million confirmed cases and more than 3 million deaths. In Europe, the number of confirmed cases is more than 51 million, with United Kingdom, Spain, and Italy being three of the most affected countries. 1 In most cases, COVID-19 causes mild flu-like symptoms; however, about 20% of cases experience a more severe form of the disease and 5% become critically ill. 2 Advanced age and presence of comorbidities are associated with severe COVID-19. 3 Patients with cancer infected with SARS-CoV-2 have more severe outcomes compared with patients without cancer. 4 A Belgian population-based analysis with 10,486 COVID-19 patients, 892 of whom with solid tumors, confirmed higher in-hospital mortality from COVID-19 in patients with cancer compared with those without cancer [31.7% versus 20.0%, respectively; adjusted odds ratio (aOR) 1.34; 95% confidence interval (CI) 1.13–1.58]. 5 Similar figures have emerged from a UK cohort study of 20,000 hospitalized COVID-19 patients, where the hazard ratio (HR) for in-hospital death was 1.13 in patients with cancer. 6 A key limitation of these studies as well as many other registries is that mortality rates are derived from patients with a variety of cancer subtypes, different cancer staging, and receiving a wide range of anticancer treatments including chemotherapy, immunotherapy, targeted therapies, and endocrine treatments. The lack of data stratification is a major challenge in providing accurate recommendations for specific oncological scenarios. This is particularly true for breast cancer, where it is key to achieve successful oncological outcomes through a careful multimodal integration of surgery, radiotherapy, chemotherapy, targeted treatments, and hormonal manipulation.
The OnCovid study is an observational European multicenter study which analyzed the characteristics and evolution of 890 patients with cancer and SARS-CoV-2 infection at its first publication (data cut off 1 April 2020). The findings demonstrated a 33.6% mortality rate during the ‘first wave’ of the pandemic. Male sex, older age, and number of comorbidities were identified as negative prognostic factors, while delivery of oncologic treatment did not worsen mortality. Interestingly, patients with breast cancer (n = 258) were characterized by a remarkably lower mortality rate (15.2%), a finding that cannot be solely explained by the protective effect of female sex, given the high rates of mortality reported in gynecological cancers (37.5%). 7
With the goal of providing data focused on patients with breast cancer and SARS-CoV-2 infection, we have designed an ad hoc subanalysis of the OnCovid study providing more detailed information on patients’ characteristics and impact of COVID-19 in this special population. Breast cancer is the most common tumor in women and possesses unique features that differentiate it from other solid tumors. Of note, cure rates are high, and the different subtypes of breast cancer require a wide range of anticancer treatments. The early reports of Chinese patients with SARS-CoV-2 infection and cancer did not have sufficient cases of breast cancer. Preliminary data from a single US hospital suggest that a significant proportion of patients with breast cancer recovers from SARS-CoV-2. 8
To protect patients with cancer from SARS-CoV-2 infection, urgent measures were implemented such as limiting hospital attendance and delaying or disrupting anticancer treatments. 9 For patients receiving active treatment, the Italian Association of Medical Oncology as well as other International Associations suggested to evaluate the risks and benefits of delaying oncological therapy on a case-by-case basis. 10 Individual risks were assessed based on cancer subtype, cancer staging, oncologic treatment, and additional comorbidities that could increase the risk of acute respiratory failure in the context of SARS-CoV-2 infection. It remains unclear whether these recommendations are optimal for breast cancer, given its special characteristics and, in general, the better prognosis, compared with most solid tumors. It is highly relevant to describe actions taken by oncologists in the management of breast cancer patients during the ‘first wave’ of COVID-19 to learn how to improve the management of our patients in this pandemic that is still ongoing. 11
In this report, we describe the features and outcomes of patients with a history of breast cancer and confirmed diagnosis of SARS-CoV-2 infection from 32 European Centers. We compared our findings in this patient subpopulation with previous studies. The aim of this study was to investigate the clinicopathologic features underlying the lower overall mortality observed in patients with breast cancer in previous studies, and to describe the outcome of oncologic treatment decisions upon resolution of the infection. The results from this study can be used to inform future decisions on the oncologic management of this specific patient population.
Methods
The OnCovid registry includes patients older than 18 years with confirmed diagnosis of SARS-CoV-2 infection by reverse-transcriptase polymerase chain reaction (RT-PCR) of a nasopharyngeal swab. 12 For the present subanalysis, we focused exclusively on patients with a history of invasive breast cancer at any time before COVID-19 diagnosis. Patients with insufficient clinical and/or follow-up data were excluded.
At database lock (1 March 2021), the registry included 495 breast cancer patients consecutively diagnosed with COVID-19 in 32 European academic centers between March 2020 and February 2021. A list of participating centers is provided in Supplemental material.
This study is a subanalysis from the main OnCovid registry which included all types of tumors and was granted central approval by the UK Health Research Authority (20/HRA/1608) and by the corresponding research ethics committees at each participating institution outside the United Kingdom. Competent authorities waived prospective informed consent due to the retrospective nature of data collection and the use of anonymized data.
Multisite access and data curation was coordinated by the Medical Statistics Unit in Novara, Italy. Clinical data were collected into a case report form designed using Research Electronic Data Capture software (REDCap, Vanderbilt University). Clinical data were collected from the medical history of the patients and all data were fully anonymized. The clinical definition of the symptoms, clinical syndromes, and complications associated with COVID-19 followed criteria published by the World Health Organization. 13
Patients with active malignancy were defined as those who, at the time of COVID-19 diagnosis, presented with evaluable oncological disease defined by radiologic criteria employed for clinical monitoring breast cancer, including patients receiving neoadjuvant treatment and those with metastatic disease.
Breast cancer subgroups were summarized into luminal-like [hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative (HR+/HER2−)], HER2-positive (HER2+, any HR status), or triple-negative (HR−/HER2−). The definitions for HR and HER2 status followed local diagnostic standards within each institution. Stage of the disease was divided into localized and metastatic depending on the absence or presence of systemic spread (i.e. M1 disease).
We defined menopausal status utilizing as approximation the cut-off of 60 years of age to ensure the menopausal status above 60. 14
Age was analyzed as a continuous variable but we considered too two cut-offs: 60 (as indicator of age at menopause) and 70 (for studying old people).
Patients were defined as receiving active therapy if they were receiving systemic anticancer agents (i.e. chemotherapy, immunotherapy, targeted therapy, endocrine therapy, or combination of previous therapies) with an interval between the last dose of treatment and SARS-CoV-2 diagnosis of less than 4 weeks. Targeted therapies included anti-HER2 therapies, CDK4/6 inhibitors, and PI3K/mTOR inhibitors.
The primary outcomes of this study were mortality and development of complications from SARS-CoV-2 infection during hospitalization. Patients’ overall survival (OS) was computed from the date of SARS-CoV-2 swab positivity to the date of death or last follow-up.
Statistical analysis
Normally distributed data were presented as mean and standard deviation (SD), whereas data following a non-normal distribution were presented as median and interquartile range (IQR). Categorical variables were summarized as counts and percentages.
We examined the association between the study variables and complications using univariable and multivariable logistic regression model. The predictors were included into a multivariable logistic regression model using a stepwise selection process. We used forward selection using a value of p < 0.05 as the entry criterion. The analysis was computed considering all the patients in the study in the analysis for complications and mortality as well.
Univariable and multivariable Cox proportional hazards models stratified by center were used to assess the impact of the factors on risk of death. Results of Cox analysis were presented as HR with a 95% CI and corresponding p values.
A two-sided value of p < 0.05 was considered statistically significant. Analyses of patients’ survival followed Kaplan–Meier methodology.
Analyses were performed using STATA software, version 15 (Stata-Corp. 2017. Statistical Software: Release 15.0. College Station, TX: Stata Corporation).
Results
Demographics and oncologic characteristics
From March 2021 to February 2021, 495 patients with history of breast cancer (either active or in remission) and a diagnosis of SARS-CoV-2 infection were included in 32 centers from United Kingdom (6 sites, n = 132 patients, 26.7%), Italy (15 sites, n = 175 patients, 35.4%), Spain (8 sites, n = 145 patients, 29.3%), Germany (1 site, n = 8 patients, 1.6%), Belgium (1 site, n = 5 patients, 1.0%), and France (1 site, n = 30 patients, 6.0%).
Demographics and breast cancer features are shown in Table 1. Mean age of patients included was 62.6 years (SD 14.7). Approximately half of the patients had never smoked and 31.5% presented more than one comorbidity. The most frequent comorbidities reported were hypertension (33.9%), diabetes (14.6%), and cardiovascular disease (9.9%). Most breast cancers were luminal-like (n = 245, 49.5%), 98 (19.8%) were HER2+, and 63 (12.7%) were triple-negative. Two hundred sixty-six (53.7%) patients had an active neoplasm at the time of SARS-CoV-2 infection, 332 (67.1%) were receiving some oncologic treatment, and in 301 (61%), the interval between last dose and SARS-CoV-2 diagnoses was less than 4 weeks. In 232 (47.6%) patients, therapy was delivered with radical intent and the most frequent systemic treatment was endocrine therapy in 173 (34.9%) patients, followed by targeted therapy in 106 (21.4%). Within the subgroup of targeted treatments, the most common were CDK4/6 inhibitors (24.5%).
Table 1.
Baseline characteristics.
| Characteristic | Total (%) |
|---|---|
| Age (years), mean ± SD | 62.6 ± 14.7 |
| Menopausal status (⩾60 versus <60 years) | |
| Premenopausal | 226 (45.7) |
| Postmenopausal | 269 (54.3) |
| Smoking history | |
| Lifetime nonsmoker | 273 (55.2) |
| Former smoker | 85 (17.2) |
| Active smoker | 36 (7.3) |
| Unknown | 101 (20.4) |
| Number comorbidities | |
| 0–1 | 339 (68.5) |
| >1 | 156 (31.5) |
| Comorbidities | |
| Hypertension | 168 (33.9) |
| Diabetes | 72 (14.6) |
| Cardiovascular disease | 49 (9.9) |
| Chronic pulmonary disease | 46 (9.3) |
| Obesity | 21 (4.2) |
| HR (ER and/or PR) | |
| Positive | 338 (68.3) |
| Negative | 90 (18.2) |
| Unknown | 67 (13.5) |
| HER2 | |
| Positive | 98 (19.8) |
| Negative | 312 (63.0) |
| Unknown | 85 (17.2) |
| Breast cancer subtype | |
| Luminal-like disease (HR+/HER2−) | 245 (49.5) |
| HER2+ disease | 98 (19.8) |
| Triple-negative (HR− and HER2−) | 63 (12.7) |
| Tumor stage | |
| Localized | 296 (59.8) |
| Metastatic | 177 (35.8) |
| Unknown | 22 (4.4) |
| Status at COVID-19 diagnosis | |
| Active malignancy | 266 (53.7) |
| Remission | 221 (44.7) |
| Unknown | 8 (1.6) |
| Ongoing anticancer therapy | |
| Yes | 332 (67.1) |
| No | 146 (29.5) |
| Missing | 17 (3.4) |
| Treatment intent and type of therapy | |
| Neoadjuvant chemotherapy | 40 (8.1) |
| Adjuvant chemotherapy | 29 (5.9) |
| Palliative chemotherapy | 71 (14.3) |
| Immunotherapy | 6 (1.2) |
| Radical | 1 (16.7) |
| Palliative | 5 (83.3) |
| Targeted therapy | 106 (21.4) |
| Radical | 30 (28.3) |
| Palliative | 76 (71.7) |
| Endocrine therapy | 173 (34.9) |
| Radical | 84 (48.6) |
| Palliative | 78 (45.0) |
| Unknown | 11 (6.4) |
| Radiotherapy | 14 (2.8) |
| Radical | 9 (64.3) |
| Palliative | 5 (35.7) |
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; PR, progesterone receptor; SD, standard deviation.
Features of COVID-19 disease
Clinical, radiologic, and laboratory features at COVID-19 diagnosis are shown in Table 2. The most common presenting symptoms of SARS-CoV-2 infection were fever (n = 254, 51.3%), cough (n = 216, 43.6%), and dyspnea (n = 151, 30.5%).
Table 2.
Clinical, laboratory, and radiological features.
| Symptoms | n (%) |
| Fever | 254 (51.3) |
| Cough | 216 (43.6) |
| Dyspnea | 151 (30.5) |
| Diarrhea | 57 (11.5) |
| Anosmia/Dysgeusia | 53 (10.7) |
| Fatigue | 105 (21.2) |
| Myalgia | 57 (11.5) |
| Coryzal symptoms | 30 (6.1) |
| Sore throat | 16 (3.2) |
| Headache | 43 (8.7) |
| Nausea or vomiting | 30 (6.1) |
| Laboratory parameters, median (IQR) | |
| Albumin (g/l) | 36.0 (30.0–41.0) |
| Bilirubin (μmol/l) | 8.1 (6.0–12.4) |
| Alanine aminotransferase (IU/l) | 26.0 (18.0–43.0) |
| Sodium (mEq/l) | 138.0 (135.0–140.0) |
| Creatinine (μmol/l) | 65.0 (54.0–80.0) |
| Hemoglobin (g/l) | 116.0 (99.0–129.0) |
| Total leukocyte count (cells 103/mm3) | 5.6 (3.7–9.3) |
| Neutrophil count (cells 103/mm3) | 4.1 (2.5–6.9) |
| Lymphocyte count (cells 103/mm3) | 0.9 (0.6–1.5) |
| Platelet count (cells 103/mm3) | 210.5 (164.0–284.0) |
| Radiologic features (X-ray) | |
| No lesion | 89 (34.2) |
| Ground-glass opacities | 75 (28.9) |
| Focal consolidation | 56 (21.5) |
| Others | 40 (15.4) |
| COVID-19-specific treatment | |
| Chloroquine or hydroxychloroquine | 131 (53.2) |
| Corticosteroids | 71 (28.9) |
| Antibiotics | 201 (81.7) |
| Lopinavir/Ritonavir | 54 (22.0) |
| Remdesivir | 7 (2.9) |
| Tocilizumab | 13 (5.3) |
| COVID-19 complications | |
| Acute respiratory failure | 74 (15.0) |
| ARDS | 33 (6.7) |
| Acute kidney injury | 18 (3.6) |
| Secondary infection | 18 (3.6) |
| Acute cardiac injury | 10 (2.0) |
| Acute liver injury | 5 (1.0) |
ARDS, acute respiratory distress syndrome; IQR, interquartile range.
Radiologic tests were performed in 351 patients (70.9%), X-ray in 260 (74.1%), and/or computed tomography (CT) in 134 (38.2%).
Two hundred eighty-eight (58.2%) patients required hospitalization and in 34 of them (11.8%), intensive or subintensive care unit admission was needed. The median length of hospitalization was 9 days (IQR 6–16) and the median time at an intensive or subintensive care unit was 7 days (IQR 4–11).
Oxygen therapy was administered in 177 (35.8%) patients and 26 patients (5.3%) required mechanical ventilation. Two hundred forty-six (49.7%) patients received empirical therapy for SARS-CoV-2, mostly of them (n = 131, 53.2%) with chloroquine or hydroxychloroquine.
One hundred twenty-nine (26.1%) patients developed one complication derived from COVID-19 and 36 (7.3%) patients developed at least two. Acute respiratory failure was the most common complication (n = 74, 15.0%).
Factors associated with COVID-19 outcomes
We analyzed the association between basal characteristics of the patients, their oncological disease, and the emergence of at least one complication from SARS-CoV-2 infection throughout the observation period. Most nonhospitalized patients had no complications (195/199), but analysis was computed for all the patients in the cohort and not only considering the hospitalized patients (288).
As shown in Table 3, age greater than 70 years (OR 2.43, 95% CI 1.53–3.86, p < 0.0001) and having two or more comorbidities (OR 1.72, 95% CI 1.08–2.77, p = 0.02) were associated with a significantly higher risk of complications. Active anticancer therapy at COVID-19 diagnosis (OR 0.56, 95% CI 0.36–0.88, p = 0.01) was identified as protective factor in front of having COVID-19 complications.
Table 3.
COVID-19 complications.
| Characteristics | Univariable OR | 95% CI | p value | Multivariable OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Age, <60/⩾60 | 2.93 | 1.89–4.55 | 0.0001 | |||
| Age, <70/⩾70 | 3.17 | 2.10–4.80 | <0.0001 | 2.43 | 1.53–3.86 | <0.0001 |
| Age (continuous variable) | 1.04 | 1.03–1.06 | <0.0001 | 1.04 | 1.03–1.6 | <0.0001 |
| Comorbidities, 0–1/⩾2 | 2.45 | 1.61–3.71 | <0.0001 | 1.72 | 1.08–2.77 | 0.02 |
| Metastasis, no/yes | 1.00 | 0.66–1.52 | 1.00 | |||
| Bone metastasis only/Visceral metastasis | 0.86 | 0.25–2.90 | 0.80 | |||
| HR (positive/negative) | 1.04 | 0.61–1.79 | 0.88 | |||
| HER2 (positive/negative) | 1.38 | 0.80–2.43 | 0.25 | |||
| Ongoing anticancer therapy at COVID diagnosis (no/yes) | 0.50 | 0.33–0.77 | 0.002 | 0.56 | 0.36–0.88 | 0.01 |
| Immunotherapy ongoing (no/yes) | 1.82 | 0.33–10.18 | 0.49 | |||
| Chemotherapy ongoing (no/yes) | 1.05 | 0.62–1.78 | 0.86 | |||
| Targeted therapy ongoing (no/yes) | 0.73 | 0.40–1.32 | 0.30 | |||
| Endocrine therapy ongoing (no/yes) | 1.05 | 0.61–1.81 | 0.87 | |||
| Radiotherapy ongoing (no/yes) | 0.27 | 0.03–2.11 | 0.21 |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; OR, odds ratio.
At the time of the analysis, 387 patients were alive (78.2%). Survival was 78.8% at 3 months and 75.3% at 6 months (Figure 1). Survival was 67.3% at 3 months and 63.1% at 6 months in patients with metastatic disease versus 85.0% and 81.5%, respectively, in patients with early breast cancer.
Figure 1.
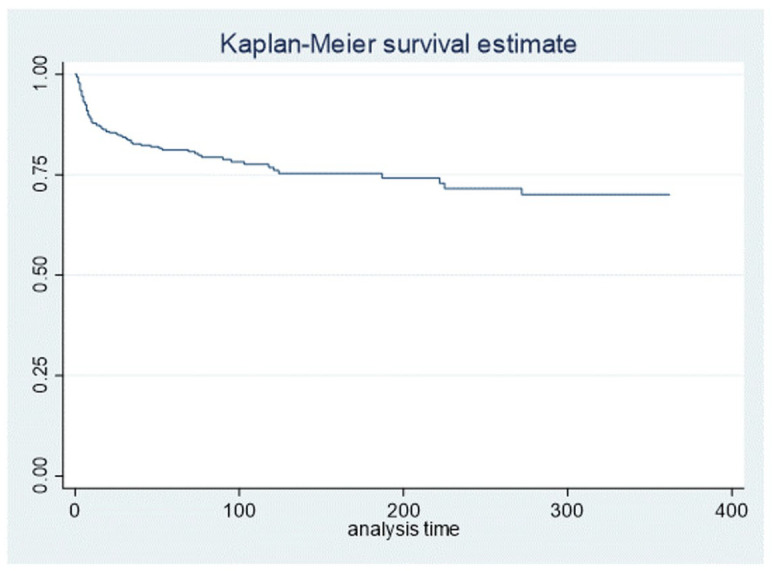
Overall survival analysis
As shown in Table 4, age older than 70 years (HR 2.07, 95% CI 1.26–3.40, p < 0.004), presence of COVID-19 complications (HR 9.18, 95% CI 5.05–16.71, p < 0.0001), and evidence of metastatic disease (HR 2.74, 95% CI 1.62–4.63, p < 0.0001) were associated with higher mortality. Ongoing anticancer therapy at time of COVID-19 diagnosis was included in the analysis, but there is a collinearity between mortality and complications, and for that reason, this factor was not considered in the multivariable analysis.
Table 4.
COVID-19 overall risk mortality.
| Characteristics | Univariable HR | 95% CI | p value | Multivariable HR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Age, <60/⩾60 | 2.43 | 1.43–4.13 | 0.001 | |||
| Age, <70/⩾70 | 3.28 | 1.98–5.42 | <0.0001 | 2.07 | 1.26–3.40 | 0.004 |
| Age (continuous variable) | 1.04 | 1.02–1.06 | <0.001 | 1.05 | 1.03–1.07 | <0.0001 |
| Complications (⩾1 versus 0) | 9.53 | 5.52–16.46 | <0.0001 | 9.18 | 5.05–16.71 | <0.0001 |
| Comorbidities, 0–1/⩾2 | 1.67 | 1.02–2.72 | 0.04 | |||
| Metastasis, no/yes | 2.27 | 1.40–3.67 | 0.001 | 2.74 | 1.62–4.63 | <0.0001 |
| Bone metastasis only/Visceral metastasis | 2.93 | 0.75–11.50 | 0.12 | |||
| HR (positive/negative) | 0.92 | 0.51–1.66 | 0.78 | |||
| HER2 (positive/negative) | 1.62 | 0.80–3.24 | 0.18 | |||
| Ongoing anticancer therapy at COVID diagnosis (no/yes) | 0.50 | 0.30–0.83 | 0.007 | |||
| Immunotherapy ongoing (no/yes) | 1.92 | 0.42–8.80 | 0.40 | |||
| Chemotherapy ongoing (no/yes) | 1.14 | 0.63–2.06 | 0.67 | |||
| Targeted therapy ongoing (no/yes) | 0.71 | 0.34–1.49 | 0.37 | |||
| Endocrine therapy ongoing (no/yes) | 0.91 | 0.49–1.68 | 0.76 | |||
| Radiotherapy ongoing (no/yes) | 0.54 | 0.07–4.11 | 0.55 |
CI, confidence interval; HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Impact of COVID-19 on oncologic treatment, follow-up, and prognosis
In the context of SARS-CoV-2 infection in alive patients at the time of analysis, 11 patients (2.2%) stopped the oncologic treatment permanently and 62 (12.5%) had their treatment regimen modified. Most patients (n = 57, 91.9%) with treatment modifications had their planned dose delayed, while in five patients (8.1%), the dose was reduced and in five patients (8.1%), the therapeutic agents were changed. Of the 11 patients whose treatment was permanently stopped, four (36.4%) were receiving chemotherapy with radical intent, two (18.2%) endocrine therapy, and three (27.3%) palliative chemotherapy. Among the 62 patients whose treatment was modified, 25 (40.3%) were receiving ongoing chemotherapy with radical intent, 17 (27.4%) palliative chemotherapy, and 26 (41.9%) targeted treatment alone or in combination. In 40% of patients, we have almost no information about what definitely happened with cancer treatment.
The mean time between the COVID-19 diagnosis and the first oncological reassessment was 46.9 days (±36.7). At that moment, 246 (49.7%) patients presented Eastern Cooperative Oncology Group (ECOG) 0 or 1 and 255 (51.6%) were reported with full clinical recovery from the infection. Within the 39 patients (7.9%) with long-term SARS-CoV-2-related complications, the most frequent were asthenia (n = 7, 17.9%), dyspnea (n = 8, 20.5%), and arthromyalgia (n = 7, 17.9%).
Discussion
Since December 2019, the emergence and rapid expansion of the SARS-CoV-2 infection has forced oncologic decisions to be primarily based on the prognosis of the malignancy, the risk–benefit assessment of the anticancer therapy, and the predicted outcome of a potential COVID-19 infection. 15 In patients with cancer, the SARS-CoV-2-related mortality rate ranges from 13% to 33.6%.7,16,17 There is some evidence suggesting that patients with cancer are more vulnerable to SARS-CoV-2 infection and at greater risk of complications and death. 18 However studies with larger number of patients including an observational UK study of 800 patients with cancer have shown prognosis to be mainly driven by sex, age, and higher comorbidity burden. This study also found that provision of cancer treatment did not have a significant effect on mortality. 19 In another US study of COVID-19-positive patients which included 117 patients with cancer matched to 468 patients without cancer, there was no difference in mortality (p = 0.894) between the two groups. 20 Collectively, these studies suggest that the poorer prognosis of patients with cancer following SARS-CoV-2 infection might be due to additional risk factors rather than the active malignancy or oncologic therapy. In our study, most patients were low risk and thus account for the good outcomes following SARS-CoV-2 infection.
Recent data suggest that following SARS-CoV-2 infection, patients with breast cancer have better clinical outcomes compared with other malignancies.7,21,22 For this group of patients, we observed a hospitalization rate of 58.2% and a mortality rate of 20.3% compared with the 85.4% and 33.6% observed in patients with all cancers included in the OnCovid project. To our knowledge, the first focused analysis of patients with breast cancer and COVID-19 is a French study which included 41 patients with SARS-CoV-2 receiving anticancer treatment. Of these, 47% were hospitalized and mortality was 9.7%. The authors suggested that mortality rates following SARS-CoV-2 infections were more likely to be driven by separate comorbidities rather than active malignancy or anticancer treatment. 22 In another small report including 27 patients with breast cancer in New York City, the rate of hospitalization was 26% and only one elderly patient with multiple comorbidities died. 8 The difference in mortality and hospitalization rates observed in our study could be explained by the larger number of patients included in our analysis. Comorbidities as well as tumor subtypes were similar in the different studies; however, in the French study, the proportion of metastatic patients was slightly higher. The percentage of patients with a positive PCR test was 100% in our study, 69% in the French, and 81% in the American study. Another difference to note is that the percentage of patients receiving anticancer treatment was 100% in the studies by Vuagnat et al. and Kalinsky et al. and 61% in our study. However, in the French study, active oncologic treatment was defined as receiving active cancer therapy with an interval between last dose and COVID-19 diagnosis within 6 months, while in the American and our study, this interval was 4 months and 4 weeks, respectively.
In line with prior studies, we identified older age (⩾60 or 70 years) and having two or more comorbidities as risk factors for developing COVID-19 complications. Obesity is one of the comorbidities that have been associated with worse prognosis. 23 It has been reported that high body mass index (>40) could predict critical illness. 24 This may be relevant in our patient population, given the positive association between obesity and luminal-like breast cancer. However, given the low obesity incidence found in our study (3%), a significant correlation could not be determined. In a prospective UK cohort study, a significant lower risk of death from COVID-19 was found in patients with breast (OR 0.53) and gynecological cancers (OR 0.36) compared with other tumor types. 25 In our study, all patients were women, younger, and with fewer comorbidities than the cancer population described in most studies. In addition, 59.8% of patients had localized disease, 49.5% had luminal-like breast cancer, and in 44.7% of the cases, the malignancy was in remission at the time of COVID-19 diagnoses. Overall, this is a population of patients with good prognostic features. Within our study population, having COVID-19 complications and the presence of metastatic disease were associated with higher mortality.
No specific anticancer treatment including chemotherapy, endocrine treatment, immunotherapy, targeted therapy, or radiotherapy has been shown to increase the risk of COVID-19 complications. We have observed that ongoing active anticancer treatment at COVID-19 diagnosis is associated with a lower risk of COVID-19 complications and mortality rate. This could be due to the significant benefit of anticancer treatments used in breast cancer, both in the early setting as well as advanced disease. We have a lot of missing information about what happened definitely with oncologic treatments after COVID-19 diagnosis, so we can only draw hypotheses about the possible protective effect of anticancer treatment. This information in combination with the results reported by other authors supports medical decisions into the clinic to continue active systemic treatments in this pandemic era for patients with breast cancer. However, in some instances, treatment modifications may be a good option for patients; particularly in low-risk luminal tumors, surgery could be delayed while patients were receiving endocrine treatment. This type of attitude has been adopted by a vast majority of the physicians treating breast cancer, and although we have not analyzed their outcomes, probably favored the patients in terms of risk/benefit.
During the follow-up period of our study, the mean time of first oncologic reassessment was 45.6 days. Interestingly, 51.6% of patients reported being fully recovered from SARS-CoV-2 infection, while 7.9% described a complication including asthenia (32.3%), dyspnea (17.6%), and arthromyalgia (11.9%). Long-term consequences are also relatively frequent in the general population. In a large cohort study from China at 6 months after symptom onset, 63% patients reported fatigue or muscle weakness, 26% sleep difficulties, and 23% anxiety or depression. 26 In another 3-month follow-up survey, physical decline or fatigue, postactivity polypnea, and alopecia were more common in women. 27 Our data suggest that patients with breast cancer do not develop different or more aggressive COVID-19 complications compared with other COVID-19 patients, at least during the first 1.5 months after the acute infection; however, long-term follow-up studies are needed to confirm this. When interpreting these data, it is also important to consider that most of the persistent symptoms reported in COVID-19 survivors overlap with common toxicities from anticancer treatment.
A limitation of our study is that data collection was made by clinicians in different countries while the pandemic was still ongoing, with sometimes incomplete documentation or missing data due to the unavailability of breast cancer-related information at the site where patient was treated for SARS-CoV-2 infection. In addition, some patients treated by their family physicians or referred to other hospitals without any notification to the responsible oncologist at the time of our analysis might have been excluded from our study. It is important to remark that the vast majority of nonhospitalized patients did not develop serious complications but probably some information was lost in this population.
Our main strength is that this is the largest study specifically analyzing the characteristics and evolution of patients with breast cancer and SARS-CoV-2 infection. Our data suggest that most breast cancer patients can be safely treated for their breast cancer during the SARS-CoV-2 pandemic. Provision of anticancer treatment does not increase the risk of SARS-CoV-2 complications. Collectively the data support continuation of anticancer treatment particularly in the curative setting and in the first-lines of treatment in patients with advanced disease. Regimen modifications and treatment breaks should be minimized prioritizing breast cancer treatment over potential complications of SARS-CoV-2. Follow-up studies are necessary to understand the long-term consequences from COVID-19 in breast cancer patients.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211053416 for COVID-19 in breast cancer patients: a subanalysis of the OnCovid registry by Laia Garrigós, Cristina Saura, Clara Martinez-Vila, Alberto Zambelli, Mark Bower, Barbara Pistilli, Matteo Lambertini, Diego Ottaviani, Nikolaos Diamantis, Ailsa Lumsden, Sonia Pernas, Daniele Generali, Elia Seguí, Gemma Viñas, Eudald Felip, Ana Sanchez, Gianpiero Rizzo, Armando Santoro, Alessio Cortellini, Ylenia Perone, John Chester, Maria Iglesias, Marta Betti, Bruno Vincenzi, Michela Libertini, Francesca Mazzoni, Federica Zoratto, Rossana Berardi, Annalisa Guida, Rachel Wuerstlein, Angela Loizidou, Rachel Sharkey, Juan Aguilar Company, Marta Matas, Chiara Saggia, Lorenzo Chiudinelli, Emeline Colomba-Blameble, Myria Galazi, Uma Mukherjee, Mieke Van Hemelrijck, Mar Marin, Carla Strina, Aleix Prat, Helena Pla, Eva Maria Ciruelos, Alexia Bertuzzi, Lucia del Mastro, Giampiero Porzio, Thomas Newsom-Davis, Isabel Ruiz, Maria Belen Delany, Marco Krengli, Vittoria Fotia, Alessandro Viansone, Neha Chopra, Margarita Romeo, Ramon Salazar, Ignacio Perez, Francesca d’Avanzo, Michela Franchi, Manuela Milani, Fanny Pommeret, Marco Tucci, Paolo Pedrazzoli, Nadia Harbeck, Daniela Ferrante, David J. Pinato and Alessandra Gennari in Therapeutic Advances in Medical Oncology
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.J. Pinato reports personal fees from BMS (travel fees, grant to institution, and lecture fees), Eisai (consultancy, lecture fees, and occasional advisory board), H3B (consultancy and occasional advisory board), Astrazeneca (consultancy and occasional advisory board), MiNa therapeutics (consultancy), Roche (lecture fees), Viiv Healthcare (lecture fees), Bayer Healthcare (lecture fees), and Falk Foundation (lecture fees). A. Zambelli reports personal fees from Lilly (occasional advisory board and travel fees), Novartis (occasional advisory board and travel fees), Pfizer (occasional advisory board), Astrazeneca (occasional advisory board), and Roche (occasional advisory board). M. Bower reports personal fees from Gilead (lecture fees), ViiV (lecture fees), BMS (lecture fees), MSD (lecture fees), and Janssen (lecture fees). M. Lambertini reports personal fees from Roche (consultancy and travel fees), Novartis (consultancy and travel fees), Lilly (consultancy and travel fees), Astrazeneca (consultancy), Pfizer (travel fees), Sandoz (travel fees), and Takeda (travel fees). E. Felip reports personal fees from Pfizer (grant to institution) and Instituto Carlos III (research grant). A. Cortellini reports personal fees from BMS (consultancy), MSD (consultancy), Astrazeneca (consultancy and lecture fees), Roche (consultancy), Astellas (lecture fees), and Novartis (Lecture fees). F. Mazzoni reports personal fees from Roche (lecture fees), Takeda (lecture fees), MSD (lecture fees), BMS (lecture fees), Lilly (lecture fees), and Boehringer (lecture fees). M. Romeo reports personal fees from MSD (consultancy), Pfizer (travel fees), and GSK (occasional advisory board). A. Gennari reports personal fees from Astrazeneca (lecture fees), Lilly (lecture fees), Eisai (lecture fees), Pfizer (lecture fees), Novartis (lecture fees), Teva (lecture fees), and Daiichi Sankyo (lecture fees).
Funding: D.J. Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT), infrastructural and grant support by the Cancer Research UK Imperial Centre and the NIHR Imperial Biomedical Research Centre. A. Gennari is supported by the AIRC IG Grant, No. 14230, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy and acknowledge also support from the UPO Aging Project.
ORCID iDs: Matteo Lambertini  https://orcid.org/0000-0003-1797-5296
https://orcid.org/0000-0003-1797-5296
Sonia Pernas  https://orcid.org/0000-0002-1485-5080
https://orcid.org/0000-0002-1485-5080
Juan Aguilar Company  https://orcid.org/0000-0002-9838-1950
https://orcid.org/0000-0002-9838-1950
Marco Tucci  https://orcid.org/0000-0003-4008-4897
https://orcid.org/0000-0003-4008-4897
Nadia Harbeck  https://orcid.org/0000-0002-9744-7372
https://orcid.org/0000-0002-9744-7372
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Laia Garrigós, Department of Medical Oncology, Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain.
Cristina Saura, Head Breast Cancer Unit, Vall d’Hebron University Hospital and Principal Investigator Breast Group, Vall d’Hebron Institute of Oncology (VHIO), Passeig Vall d’Hebrón 119-129, 08035 Barcelona, Spain.
Clara Martinez-Vila, Department of Oncology, Hospital Althaia Manresa, Barcelona, Spain.
Alberto Zambelli, Oncology Unit, ASST Papa Giovanni XXIII, Bergamo, Italy.
Mark Bower, Department of Oncology and National Centre for HIV Malignancy, Chelsea and Westminster Hospital, London, UK.
Barbara Pistilli, Department of Medical Oncology, Institute Gustave-Roussy, Villejuif, France.
Matteo Lambertini, Department of Medical Oncology, UOC Clinica di Oncologia Medica, IRCCS Policlinico San Martino Hospital, Genoa, Italy; Department of Internal Medicine and Medical Specialties (DiMI), School of Medicine, University of Genova, Genoa, Italy.
Diego Ottaviani, Cancer Division, University College London Hospitals, London, UK.
Nikolaos Diamantis, Medical Oncology, Barts Health NHS Trust, London, UK.
Ailsa Lumsden, Translational Oncology & Urology Research (TOUR), School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK.
Sonia Pernas, Department of Medical Oncology, Catalan Institute of Oncology, L’Hospitalet, Spain; Oncobell Program (IDIBELL), CIBERONC, Hospitalet de Llobregat, Barcelona, Spain.
Daniele Generali, Multidisciplinary Breast Pathology and Translational Research Unit, ASST Cremona, Casalmaggiore, Italy.
Elia Seguí, Department of Medical Oncology, Hospital Clinic, Barcelona, Spain.
Gemma Viñas, Department of Medical Oncology, Catalan Institute of Oncology, University Hospital Josep Trueta, Girona, Spain.
Eudald Felip, Department of Medical Oncology, Catalan Institute of Oncology, Badalona, Spain.
Ana Sanchez, Department Medical Oncology, Hospital XII de Octubre, Madrid, Spain.
Gianpiero Rizzo, Medical Oncology Unit, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy.
Armando Santoro, IRCCS Humanitas Research Hospital, Humanitas Cancer Center, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Milan, Italy.
Alessio Cortellini, Department of Biotechnology and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy; Division of Surgery and Cancer, Hammersmith Hospital, Imperial College London, London, UK.
Ylenia Perone, Medical Oncology, Velindre Cancer Centre, Cardiff, UK.
John Chester, Medical Oncology, Velindre Cancer Centre, Cardiff, UK.
Maria Iglesias, Department of Oncology, Hospital Universitario Son Llatzer, Palma de Mallorca, Spain.
Marta Betti, Research Infrastructure, Research and Innovation Department, Azienda Ospedaliera “SS Antonio e Biagio e Cesare Arrigo”, Alessandria, Italy.
Bruno Vincenzi, Department of Oncology, University “Campus Bio-Medico”, Rome, Italy.
Michela Libertini, Medical Oncology Unit, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy.
Francesca Mazzoni, Department of Oncology, Careggi University Hospital, Florence, Italy.
Federica Zoratto, Medical Oncology, Santa Maria Goretti Hospital, Latina, Italy.
Rossana Berardi, Oncology Clinic, Università Politecnica Delle Marche, Ospedali Riuniti Di Ancona, Ancona, Italy.
Annalisa Guida, Struttura Complessa di Oncologia Medica e Traslazionale, Azienda Ospedaliera Santa Maria di Terni, Italy.
Rachel Wuerstlein, Department of Gynecology and Obstetrics, Breast Center and Gynecological Cancer Center and CCC Munich, University Hospital Munich, Munich, Germany.
Angela Loizidou, Department of Oncology, Institut Jules Bordet, Université Libre de Bruxelles, Belgium.
Rachel Sharkey, Department of Oncology and National Centre for HIV Malignancy, Chelsea and Westminster Hospital, London, UK.
Juan Aguilar Company, Department of Medical Oncology, Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain; Department of Infectious Diseases, Vall d’Hebron University Hospital, Barcelona, Spain.
Marta Matas, Department of Oncology, Hospital Althaia Manresa, Barcelona, Spain.
Chiara Saggia, Department of Translational Medicine, University of Piemonte Orientale, Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara, Italy.
Lorenzo Chiudinelli, Oncology Unit, ASST Papa Giovanni XXIII, Bergamo, Italy.
Emeline Colomba-Blameble, Department of Medical Oncology, Institute Gustave-Roussy, Villejuif, France.
Myria Galazi, Cancer Division, University College London Hospitals, London, UK.
Uma Mukherjee, Medical Oncology, Barts Health NHS Trust, London, UK.
Mieke Van Hemelrijck, Translational Oncology & Urology Research (TOUR), School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK.
Mar Marin, Department of Medical Oncology, Catalan Institute of Oncology, L’Hospitalet (Barcelona), Spain.
Carla Strina, Multidisciplinary Breast Pathology and Translational Research Unit, ASST Cremona, Casalmaggiore, Italy.
Aleix Prat, Department of Medical Oncology, Hospital Clinic, Barcelona, Spain; Translational Genomics and Targeted therapies Group, IDIBAPS, Barcelona, Spain.
Helena Pla, Department of Medical Oncology, Catalan Institute of Oncology, University Hospital Josep Trueta, Girona, Spain.
Eva Maria Ciruelos, Department Medical Oncology, Hospital XII de Octubre, Madrid, Spain.
Alexia Bertuzzi, IRCCS Humanitas Research Hospital, Humanitas Cancer Center, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Milan, Italy.
Lucia del Mastro, Breast Unit, IRCCS Policlinico San Martino Hospital, Genoa, Italy; Department of Internal Medicine and Medical Specialties (DiMI), School of Medicine, University of Genova, Genoa, Italy.
Giampiero Porzio, Medical Oncology Unit, San Salvatore Hospital, L’Aquila, Italy.
Thomas Newsom-Davis, Department of Oncology and National Centre for HIV Malignancy, Chelsea and Westminster Hospital, London, UK.
Isabel Ruiz, Department of Infectious Diseases, Vall d’Hebron University Hospital, Barcelona, Spain.
Maria Belen Delany, Department of Oncology, Hospital Althaia Manresa, Barcelona, Spain.
Marco Krengli, Division of Radiotherapy, Department of Translational Medicine, University of Eastern Piedmont and Hospital “Maggiore della Carità”, Novara, Italy.
Vittoria Fotia, Oncology Unit, ASST Papa Giovanni XXIII, Bergamo, Italy.
Alessandro Viansone, Department of Medical Oncology, Institute Gustave-Roussy, Villejuif, France.
Neha Chopra, Cancer Division, University College London Hospitals, London, UK.
Margarita Romeo, Department of Medical Oncology, Catalan Institute of Oncology, Badalona, Spain.
Ramon Salazar, Department of Medical Oncology, Catalan Institute of Oncology, L’Hospitalet (Barcelona), Spain; Oncobell Program (IDIBELL), CIBERONC. Hospitalet de Llobregat, Spain.
Ignacio Perez, Department of Oncology, Hospital Althaia Manresa, Barcelona, Spain.
Francesca d’Avanzo, Department of Translational Medicine, University of Piemonte Orientale, Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara, Italy.
Michela Franchi, Oncology Unit, ASST Papa Giovanni XXIII, Bergamo, Italy.
Manuela Milani, Multidisciplinary Breast Pathology and Translational Research Unit, ASST Cremona, Casalmaggiore, Italy.
Fanny Pommeret, Department of Medical Oncology, Institute Gustave-Roussy, Villejuif, France.
Marco Tucci, Department of Biomedical Sciences and Human Oncology, University of Bari Aldo Moro, Bari, Italy.
Paolo Pedrazzoli, Medical Oncology Unit, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy.
Nadia Harbeck, Department of Gynecology and Obstetrics, Breast Center and Gynecological Cancer Center and CCC Munich, University Hospital Munich, Munich, Germany.
Daniela Ferrante, Department of Translational Medicine, Unit of Medical Statistics, University of Piemonte Orientale and Cancer Epidemiology, CPO Piemonte, Novara, Italy.
David J. Pinato, Division of Surgery and Cancer, Hammersmith Hospital, Imperial College London, London, UK; Department of Translational Medicine, University of Piemonte Orientale, Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara, Italy.
Alessandra Gennari, Department of Translational Medicine, University of Piemonte Orientale, Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara, Italy; Department of Surgery and Cancer, Imperial College London, London, UK.
References
- 1. Coronavirus disease 2019, https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 17 May 2020).
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer 2020; 139: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Azambuja E, Brandão M, Wildiers H, et al. Impact of solid cancer on in-hospital mortality overall and among different subgroups of patients with COVID-19: a nationwide, population-based analysis. ESMO Open 2020; 5: e000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Discov 2020; 10: 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalinsky K, Accordino MK, Hosi K, et al. Characteristics and outcomes of patients with breast cancer diagnosed with SARS-Cov-2 infection at an academic center in New York City. Breast Cancer Res Treat 2020; 182: 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw 2020; 18: 1–4. [DOI] [PubMed] [Google Scholar]
- 10. Lambertini M, Toss A, Passaro A, et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists’ perspective. ESMO Open 2020; 5: e000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poggio F, Tagliamento M, Di Maio M, et al. Assessing the Impact of the COVID-19 Outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol Pract 2020; 16: e1304–e1314. [DOI] [PubMed] [Google Scholar]
- 12. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 -nCoV by RT-PCR. Euro Surveill 2020; 25: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Who 2020; 2019: 12. [Google Scholar]
- 14. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011; 38: 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanna TP. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol 2020; 17: 268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020; 395: 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020; 31: 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addeo A, Friedlaender A. Cancer and COVID-19: unmasking their ties. Cancer Treat Rev 2020; 88: 102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee LYW, Cazier J, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020; 395: 1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brar G, Pinheiro LC, Shusterman M, et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol 2020; 38: 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei J, Wu M, Liu J, et al. Characteristics and outcomes of COVID-19 infection in 45 patients with breast cancer: a multi-center retrospective study in Hubei, China. Breast 2021; 59: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vuagnat P, Frelaut M, Ramtohul T, et al. COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res 2020; 22: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hussain A, Mahawar K, Xia Z, et al. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract 2020; 14: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020; 21: 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211053416 for COVID-19 in breast cancer patients: a subanalysis of the OnCovid registry by Laia Garrigós, Cristina Saura, Clara Martinez-Vila, Alberto Zambelli, Mark Bower, Barbara Pistilli, Matteo Lambertini, Diego Ottaviani, Nikolaos Diamantis, Ailsa Lumsden, Sonia Pernas, Daniele Generali, Elia Seguí, Gemma Viñas, Eudald Felip, Ana Sanchez, Gianpiero Rizzo, Armando Santoro, Alessio Cortellini, Ylenia Perone, John Chester, Maria Iglesias, Marta Betti, Bruno Vincenzi, Michela Libertini, Francesca Mazzoni, Federica Zoratto, Rossana Berardi, Annalisa Guida, Rachel Wuerstlein, Angela Loizidou, Rachel Sharkey, Juan Aguilar Company, Marta Matas, Chiara Saggia, Lorenzo Chiudinelli, Emeline Colomba-Blameble, Myria Galazi, Uma Mukherjee, Mieke Van Hemelrijck, Mar Marin, Carla Strina, Aleix Prat, Helena Pla, Eva Maria Ciruelos, Alexia Bertuzzi, Lucia del Mastro, Giampiero Porzio, Thomas Newsom-Davis, Isabel Ruiz, Maria Belen Delany, Marco Krengli, Vittoria Fotia, Alessandro Viansone, Neha Chopra, Margarita Romeo, Ramon Salazar, Ignacio Perez, Francesca d’Avanzo, Michela Franchi, Manuela Milani, Fanny Pommeret, Marco Tucci, Paolo Pedrazzoli, Nadia Harbeck, Daniela Ferrante, David J. Pinato and Alessandra Gennari in Therapeutic Advances in Medical Oncology


