Abstract
Objective
To compare selective serotonin reuptake inhibitors (SSRIs) and nootropic drugs in the reduction of anxiety and depressive symptoms in post-stroke patients.
Methods
This retrospective cohort study included patients diagnosed with post-stroke depression that were treated with either SSRIs or nootropic drugs (i.e. citicoline or choline alphoscerate). Depression and anxiety were assessed using the Hamilton Rating Scales. Statistical associations between the use of nootropic drugs and mood disorder improvements were determined by measuring assessment scores at 6-months.
Results
A total of 44 post-stroke patients with depression (aged 45–75 years) were enrolled in the study: 20 were treated with SSRIs and 24 received nootropic drugs. From baseline to follow-up, the SSRI group showed a large effect size with regard depression (success rate difference [SRD] 0.57; 95% confidence interval [CI] 0.21, 0.79) and anxiety (SRD 0.49; 95% CI 0.14, 0.74), whereas the nootropic group showed a small effect size for depression (SRD 0.16; 95% CI –0.17, 0.46) and a small effect size for anxiety (SRD 0.36; 95% CI –0.03, 0.62).
Conclusion
The administration of nootropic drugs could be a valid therapeutic strategy to manage post-stroke patients suffering from mild–moderate anxiety or anxious-depressive syndrome, but this requires further research.
Keywords: Anxiety, depression, citicoline, choline, post-stroke
Introduction
Stroke (ischaemic or haemorrhagic) has a severe impact on multiple functional domains and generally causes a greater range of disabilities, affecting the patient’s quality of life and generating negative emotional states. 1 Indeed, if from the motor point of view some patients can return to their pre-stroke functional level, acquiring a greater autonomy in daily living activities, they can present other problems ranging from cognitive deficits to the difficulty in social reintegration. 1
In the past, several studies were focused on motor disabilities and only recently are studies appearing on behavioural, cognitive and emotional impairment. 2 Post-stroke depression (PSD) is a common neuropsychiatric condition in this class of patients, which is a potential mortality risk factor. 3 Indeed, approximately 85% of patients with cerebrovascular disease suffer from PDS. 4 The most common, but not exclusive, symptoms are apathy, fatigue, sleep disturbances and weight alterations, which compromise the quality of life of the subject, hindering their rehabilitation treatment and causing further disabilities and interpersonal problems.5,6 It is crucial to treat depressive symptoms in order to improve the disease recovery. Research has shown an improvement in mood disorders of patients with cerebrovascular diseases through the use of classic antidepressants. 7
The onset of depressive symptoms depends on psychosocial and genetic factors, general vascular damage, the severity of the stroke and disability, cognitive impairment and on the interactions of other conditions. 8 In the vast majority of cases, PSD appears in the first month after the stroke onset and tends to become chronic over time, interfering with the functional recovery. 8 Patients can experience reduced compliance and increased resistance during rehabilitative treatments, because of their low self-esteem and lack of motivation.9,10
The association between PSD and the patient’s cognitive impairment has been shown to be different among the various types of cerebral infarction. 9 Although patients with PSD have Mini Mental Examination State scores lower than non-PSD patients, the scores were significantly lower in patients with partial anterior circulation infarction compared with patients with lacunar circulation infarction or posterior circulation infarction. 11 The association between cognitive impairment and depression is mutual because the relationship is very complex. 12 However, antidepressant treatment stimulates the neurogenesis process and the benefits of treatment might not only cover depressive symptoms but also promote cognitive and motor recovery.13,14
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are frequently chosen for the treatment of depression. Notably, SSRIs are the first-choice drugs since their side-effects are generally well tolerated. 15 Most research supports the efficacy of these antidepressants, 13 but very few studies have evaluated the efficacy of other pharmacological therapies, such as psychostimulants. 16 Indeed, drugs such as citicoline or choline alphoscerate could be used for the treatment of mood disorders in post-ischaemic stroke patients, as supportive drugs to the antidepressant treatment or as an alternative therapy in mild symptomatology. 16
The aim of this study was to investigate whether a therapy based on nootropic drugs could be at least as effective in the reduction of anxiety and depressive symptoms as an antidepressant therapy in patients with PSD.
Patients and methods
Study population
This retrospective cohort study evaluated consecutive patients with a diagnosis of depression after a stroke that attended the Centre of Neurovascular Disease of the IRCCS Neurolesi “Bonino-Pulejo”, Messina, Italy from May 2018 to January 2019. The study selected outpatients within 1 year from the stroke onset that had a diagnosis of PSD and were treated with either antidepressants or nootropic drugs (i.e. 1000 mg citicoline once daily orally for 6 months; 600 mg choline alphoscerate once daily orally for 6 months). The exclusion criteria were as follows: (i) previous history of depression; (ii) presence of other neurological or psychiatric illnesses; (iii) presence of cognitive disorders (Montreal Cognitive Assessment [MOCA] < 26); 17 (iv) combined use of antidepressants and nootropic drugs. The electronic health records of all eligible patients were anonymously screened for medications and neuropsychological evaluations from the hospital database. Only patients with complete records were selected for this study.
No study protocol approval was necessary due to the retrospective design of the study. However, all patients provided signed informed consent.
Outpatient procedure
The outpatient procedure involved screening for mood disorders (e.g. depression and anxiety)during the first visit to the centre (baseline) and after 6 months (follow-up). Cognitive functions were screened using the MOCA in order to quickly assess memory, attention, language, praxis abilities and space-time orientation. 17 Anxiety and depression were assessed using the Hamilton Anxiety Rating Scale (HAM-A) and Hamilton Depression Rating Scale (HAM-D). 18 According to the severity of PSD, patients could be treated with antidepressants, such as SSRIs, or with nootropic drugs.
Statistical analyses
All statistical analyses were undertaken using the R statistical package (R version 3.5.0; R Foundation for Statistical Computing, Vienna, Austria) with a 95% of confidence level with a 5% alpha error. Continuous data are presented as mean ± SD and categorical data as frequencies (%). Categorical data were compared using χ2-test with continuity correction. Mann–Whitney U-test was used to compare continuous data. The extent of the difference between two groups of observations was assessed using the effect size success rate difference (SRD) and the corresponding 95% confidence interval (CI). An ‘improvement’ was considered to have occurred if a change in the assessment score allowed the patient to be placed in the next cut-off of the scale (e.g. from moderate at baseline to mild at follow-up). A P-value <0.05 was considered statistically significant.
Results
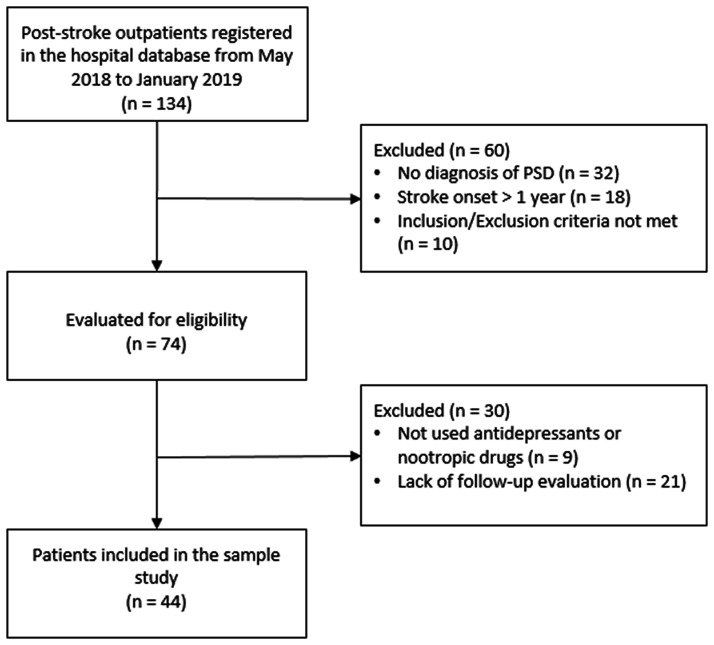
The electronic health records of 134 eligible outpatients with a stroke were anonymously screened (Figure 1). Of these, 60 outpatients were initially screened and excluded due to a lack of a PSD diagnosis or they did not meet the inclusion/exclusion criteria. The remaining 74 patients were further screened and 30 were excluded (nine due to treatment with other medications and 21 due to lack of follow-up evaluations). A total of 44 patients (23 males and 21 females; age range, 45–75 years) were included in this study.
Figure 1.
Patient flow chart showing the screening, exclusion and selection of patients with a diagnosis of post-stroke depression (PSD).
Of the 44 patients included in this study, 20 were treated with an SSRI (group 1) and 24 received only nootropic drugs (group 2). The demographical and clinical characteristics of the two groups are summarized in Table 1. There were no significant differences between the two groups in terms of age, sex, education, stroke onset and MOCA score at baseline.
Table 1.
Demographic and clinical characteristics of patients (n = 44) with a diagnosis of post-stroke depression that were included in a retrospective study to investigate whether a therapy based on nootropic drugs was at least as effective as an antidepressant therapy.
| Characteristic | Group 1 n = 20 | Group 2n = 24 | SRD | 95% CI |
|---|---|---|---|---|
| Male | 8 (40.0) | 15 (62.5) | –0.225 | –0.56, 0.11 |
| Age, years | 59.9 ± 9.3 | 63.9 ± 9.6 | –0.262 | –0.56, 0.10 |
| Education, years | 9.5 ± 3.9 | 8.6 ± 4.2 | 0.144 | –0.20, 0.45 |
| Time since stroke onset, days | 71.5 ± 12.4 | 65.2 ± 10.1 | 0.079 | –0.28, 0.42 |
| MOCA score | 19.7 ± 6.4 | 20.7 ± 4.3 | –0.010 | –0.36, 0.34 |
| HAM-A score | 17.3 ± 7.5 | 8.3 ± 5.9 | 0.664 | 0.37, 0.84 |
| HAM-D score | 18.3 ± 5.7 | 8.9 ± 5.8 | 0.787 | 0.52, 0.91 |
| Anxiety improvement | 10 (50.0) | 2 (8.3) | 0.417 | 0.12, 0.71 |
| Depression improvement | 16 (80.0) | 11 (45.8) | 0.342 | 0.03, 0.65 |
Data presented as mean ± SD or n of patients (%).
SRD, success rate difference; CI, confidence interval; MOCA, Montreal Cognitive Assessment; HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Depression Rating Scale.
Comparing the two groups at baseline, there were large effect sizes for both anxiety (SRD 0.664; 95%CI 0.37, 0.84) and depression (SRD 0.787; 95%CI 0.52, 0.91) (Table 1). From baseline to follow-up, there was a large effect size for depression in group 1 (SRD 0.57; 95% CI 0.21, 0.79) and a small effect size in group 2 (SRD 0.16; 95% CI –0.17, 0.46); and a large effect size for anxiety in group 1 (SRD 0.49; 95% CI 0.14, 0.74) and a small effect size in group 2 (SRD 0.36; 95% CI = –0.03, 0.62). The proportions of patients with an improvement in anxiety (P < 0.01) and depression (P = 0.04) were significantly higher in group 1 compared with group 2 with medium effect sizes between the two groups.
Discussion
This current study evaluated the overall improvement in mood in patients with PSD after 6 months of treatment following the diagnosis of depression stratified according to the treatment used. The current findings showed an improvement in both anxiety and depression symptoms in the majority of patients that were treated with antidepressants (group 1). These findings were not surprising because citicoline acts on neurotransmitters and results in functional neuroprotective and neurorestorative effects for brain damage. 19 Citicoline is usually used to preserve neurocognitive functions. 20 It is generally well tolerated regardless of the mode of administration or dosage. 21 The current study found that the nootropic group (group 2) were more anxious and depressed at baseline, which might explain the lower levels of improvements observed compared with the group taking antidepressants (group 1). In future randomized controlled trials, the random distribution of baseline scores in study groups should avoid this problem. Choline and citicoline were initially administered to patients with PSD that complained of mild impairment in cognitive performance. 22 Nootropics are substances that increase cognitive ability by improving brain skills and function. 23
The psychostimulant citicoline is biologically active, increases levels of dopamine and acetylcholine and inhibits apoptosis associated with cerebral ischaemia. 23 An experimental stroke model demonstrated the contribution of citicoline to the production of the re-growth of dendritic spines, which was correlated with functional recovery. 24 Other interesting research based on animal models demonstrated that citicoline enhanced learning and memory tasks, reducing cognitive deficits after brain injury. 24
Although research has demonstrated the efficacy of citicoline on the improvement of cognitive impairment and on the enhancement of neuroplasticity, 25 just a few studies have investigated its effect on mood disorder improvement. 23 For example, a randomized clinical trial demonstrated that citicoline was an effective adjuvant to citalopram in the treatment of major depressive disorder. 26 Comparing HAM-D scores of patients treated with citalopram plus citicoline and patients treated with citalopram plus placebo, the clinical trial observed a significantly greater rate of remission in the citalopram plus citicoline group. 26 The clinical trial also studied the antidepressant effect of its metabolite cytidine and the citicoline therapeutic potential in bipolar disorder (observing a reduction of manic symptoms) and in patients with substance abuse disorder (observing a reduction of craving and of depressive symptoms). 26 Findings suggest that the effect of citicoline on depressive symptoms may be mediated by its effect on brain metabolism and by its modulation of a number of neurotransmitters (increasing acetylcholine, noradrenaline, serotonin and dopamine concentration). 27
This current study had several limitations. First, being retrospective did not allow for observations of the effects on patients treated with an antidepressant therapy in combination with nootropic drugs. This will be considered in future prospective studies, as well as the importance of randomly assigning patients to each group, matching them for demographic characteristics (e.g. age and sex) and clinical condition, after the calculation of an adequate sample size. Thus, this study should be considered as explorative as it was not able to detect smaller yet clinically relevant differences. Secondly, the sample size was small and the follow-up short. Future studies should test the potential of citicoline on a larger patient number with longer follow-up to better evaluate the long-term effects of nootropic treatment.
In conclusion, this finding suggests that the administration of nootropic drugs could be a valid therapeutic strategy to manage post-stroke patients with mild–moderate anxiety or anxious-depressive syndrome, which is very common because depression, in many cases, occurs as a comorbidity with anxiety. 28 In the future it would be interesting to investigate the use of other drugs that have the potential to be effective in the treatment of emotional disturbances following a stroke.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Francesco Corallo https://orcid.org/0000-0003-4862-3832
Michele Torrisi https://orcid.org/0000-0003-4738-9191
Caterina Formica https://orcid.org/0000-0001-7591-3080
Maria Cristina De Cola https://orcid.org/0000-0002-7509-3833
References
- 1.Kapoor A, Lanctôt KL, Bayley M, et al. “Good Outcome” Isn’t Good Enough: Cognitive Impairment, Depressive Symptoms, and Social Restrictions in Physically Recovered Stroke Patients. Stroke 2017; 48: 1688–1690. [DOI] [PubMed] [Google Scholar]
- 2.Espárrago Llorca G, Castilla-Guerra L, Fernández Moreno MC, et al . Post-stroke depression: an update. Neurología 2015; 30: 23–31. [DOI] [PubMed] [Google Scholar]
- 3.Xu XM, Zou DZ, Shen LY, et al. Efficacy and feasibility of antidepressant treatment in patients with post-stroke depression. Medicine (Baltimore) 2016; 95: e5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khaku AS, Tadi P. Cerebrovascular Disease. 2021 Sep 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Jan. PMID: 28613677.
- 5.Tu J, Wang LX, Wen HF, et al. The association of different types of cerebral infarction with post-stroke depression and cognitive impairment. Medicine (Baltimore) 2018; 97: e10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke Quinlan E, Dodakian L, See J, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol 2015; 77: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer Hollender K. . Screening, diagnosis, and treatment of post-stroke depression. J Neurosci Nurs 2014; 46: 135–141. [DOI] [PubMed] [Google Scholar]
- 8.Göthe F, Enache D, Wahlund LO, et al . Cerebrovascular diseases and depression: epidemiology, mechanisms and treatment. Panminerva Med 2012; 54: 161–170. [PubMed] [Google Scholar]
- 9.Provinciali L, Coccia M. Post-stroke and vascular depression: a critical review. Neurol Sci 2002; 22: 417–428. [DOI] [PubMed] [Google Scholar]
- 10.Campbell Burton CA, Murray J, Holmes J, et al. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int J Stroke 2013; 8: 545–559. [DOI] [PubMed] [Google Scholar]
- 11.Qin B, Chen H, Gao W, et al. Efficacy, acceptability, and tolerability of antidepressant treatments for patients with post-stroke depression: a network meta-analysis. Braz J Med Biol Res 2018; 51: e7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng L, Sun X, Qiu S, et al. Interventions for management of post-stroke depression: A Bayesian network meta-analysis of 23 randomized controlled trials. Sci Rep 2017; 7: 16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert Opin Pharmacother 2017; 18: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Ling S, Yang Y, et al. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett 2014; 35: 104–109. [PubMed] [Google Scholar]
- 15.Mortensen JK, Andersen G. Safety of selective serotonin reuptake inhibitor treatment in recovering stroke patients. Expert Opin Drug Saf 2015; 14: 911–919. [DOI] [PubMed] [Google Scholar]
- 16.Corallo F, Scarfì C, Arcadi FA, et al. Role of functional pharmacological therapy in post-stroke depression: a narrative review. J Int Med Res 2020; 48:300060520950557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santangelo G, Siciliano M, Pedone R, et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci 2015; 36: 585–591. [DOI] [PubMed] [Google Scholar]
- 18.Snaith RP, Taylor CM. Rating scales for depression and anxiety: a current perspective. Br J Clin Pharmacol 1985; 19: 17S–20S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Secades JJ. Probably role of citicoline in stroke rehabilitation: review of the literature. Rev Neurol 2012; 54: 173–179. [PubMed] [Google Scholar]
- 20.Álvarez-Sabín J, Román GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke 2011; 42: S40–S43. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Xiang Y, Yang Y, et al. Depression after minor stroke: Prevalence and predictors. J Psychosom Res 2015; 79: 143–147. [DOI] [PubMed] [Google Scholar]
- 22.Hurtado O, Cárdenas A, Pradillo JM, et al. A chronic treatment with CDP-choline improves functional recovery and increases neuronal plasticity after experimental stroke. Neurobiol Dis 2007; 26: 105–111. [DOI] [PubMed] [Google Scholar]
- 23.Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clin Interv Aging 2006; 1: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasielski P, Piędel F, Piwek M, et al. Application of Citicoline in Neurological Disorders: A Systematic Review. Nutrients 2020; 12: 3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gareri P, Castagna A, Cotroneo AM, et al. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging 2015; 10: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roohi-Azizi M, Arabzadeh S, Amidfar M, et al. Citicoline combination therapy for major depressive disorder: a randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol 2017; 40: 1–5. [DOI] [PubMed] [Google Scholar]
- 27.Adibhatla RM, Hatcher JF, Dempsey RJ. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem 2002; 80: 12–23. [DOI] [PubMed] [Google Scholar]
- 28.Torrisi M, De Cola MC, Buda A, et al. Self-Efficacy, Poststroke Depression, and Rehabilitation Outcomes: Is There a Correlation? J Stroke Cerebrovasc Dis 2018; 27: 3208–3211. [DOI] [PubMed] [Google Scholar]