Abstract
Anomalous aortic origin of a coronary artery (AAOCA) is a rare pathology that may cause episodic ischemia owing to possible vessel compression during systolic expansion of the aortic root. This anomaly can lead to myocardial infarction, malignant arrhythmias and sudden cardiac death (SCD). Several surgical techniques have been described; however, there are no defined guidelines regarding the treatment of AAOCA. We report the case of a 47-year-old woman with ectopic origin of the right coronary artery (RCA) from the left sinus of Valsalva, with an interarterial course of the proximal segment of the artery, running between the aorta and the pulmonary trunk. Revascularization was accomplished by harvesting the right internal mammary artery (RIMA) and anastomosing it to the anomalous RCA, given the small portion of the RCA following an intramural course and our familiarity with the procedure. The RCA was ligated proximal to the anastomosis to avoid the string sign phenomenon. This procedure is safe and fast and can be considered an alternative to coronary reconstruction.
Keywords: Anomalous coronary artery, congenital heart disease, cardiac surgery, coronary artery bypass graft, internal mammary artery, aorta
Introduction
Anomalous aortic origin of a coronary artery (AAOCA) occurs in 1% 1 of the general population and is reported to cause 5% to 35% of sudden deaths in the young 2 and almost 19% of sudden deaths in young athletes. 3 The prevalence of a right coronary artery (RCA) originating from the left sinus of Valsalva varies in the literature but is estimated to be 0.02% to 0.9%.4–6
An ectopic RCA can have different possible courses, which may be clinically benign or malignant: 1) retrocardiac, or RCA running in the posterior atrioventricular groove; 2) retroaortic; 3) interarterial, between the aorta and the pulmonary artery; 4) intraseptal; and 5) precardiac, anterior to the right ventricular outflow tract (RVOT).
Rigatelli et al. 7 classified AAOCA into five categories according to the clinical significance, which depends on the route of the coronary artery relative to the great vessels and the presence of concomitant coronary artery disease, offering a new tool in decision-making for both surgical and interventional management. According to the aforementioned classification (Table 1), an ectopic origin of the RCA from the left sinus of Valsalva has Class III clinical relevance (severe). This is because an ectopic origin of the RCA from the left sinus of Valsalva with the artery running between the aorta and pulmonary trunk may cause episodic ischemia owing to possible vessel compression during systolic expansion of the aortic root, leading to myocardial infarction and sudden cardiac death (SCD). Moreover, this anomaly is associated with a higher risk of malignant ventricular arrhythmias. 7
Table 1.
Rigatelli classification.
| I-Benign | • Ectopic origin of the LCx from the RS• Separate origin of the LCx and LAD• Ectopic origin of the LCx from the RCA• Ectopic coronary origin from the AO• Dual LAD type I to IV• Myocardial bridge (score: ≤5) • Intercoronary circulation |
| II-Relevant | • Coronary artery fistula• Single coronary artery R/L, I/II/III, A/P• Ectopic origin of the LCA from the PA• Atretic coronary artery• Hypoplastic coronary artery |
| III-Severe | • Ectopic origin of the LCA from the RS• Ectopic origin of the RCA form the LS• Ectopic origin of the RCA from the PA• Single coronary artery R/L, I/II/III B• Myocardial bridge (score: 5) |
| IV-Critical | • Class II and superimposed CAD• Class III and superimposed CAD |
AO, ascending aorta; CAD, coronary artery disease; L, left; LAD, left anterior descending coronary artery; LCA, left coronary artery; LCx, left circumflex coronary artery; LS, left sinus; PA, pulmonary artery; R, right; RCA, right coronary artery; RS, right sinus.
We present the case and our treatment approach for a 47-year-old woman with an anomalous RCA originating from the left sinus of Valsalva that followed an interarterial course.
Case report
A 47-year-old woman was referred to our hospital for recurrent palpitations during exercise, with worsening symptoms in the previous 3 months. Her medical history was unremarkable except for transient, pregnancy-related hyperthyroidism and a smoking habit. Her physical examination findings were also unremarkable, with normal blood pressure, normal jugular venous pressure, and normal heart sounds, while her resting electrocardiogram (ECG) showed sinus rhythm with isolated premature ventricular contractions (PVCs). Echocardiography demonstrated absence of valvular disease, preserved ejection fraction (EF: 66%), and mild hypokinesis of the basal and medial segments of the left ventricle.
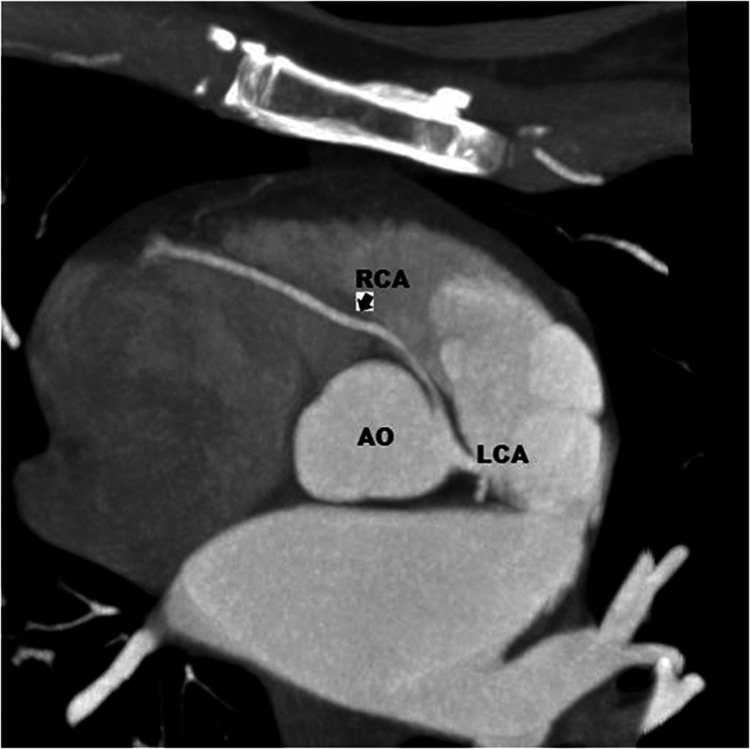
Cardiac computed tomography (CT) was performed and revealed an ectopic origin of the RCA from the left sinus of Valsalva, with an interarterial course of the proximal segment of the artery, running between the aorta and the pulmonary trunk [Figure 1 and Figure 2]. The posterior descending artery and the posterolateral artery originated from the RCA. There were no atherosclerotic lesions in either the RCA or the left coronary artery (LCA). CT also revealed the presence of a 12-mm lung nodule.
Figure 1.
Preoperative heart CT scan. The CT image shows the origin of the RCA from the left coronary sinus with an interarterial course (arrow).
CT, computed tomography; AO, aorta; LCA, left coronary artery; RCA, right coronary artery.
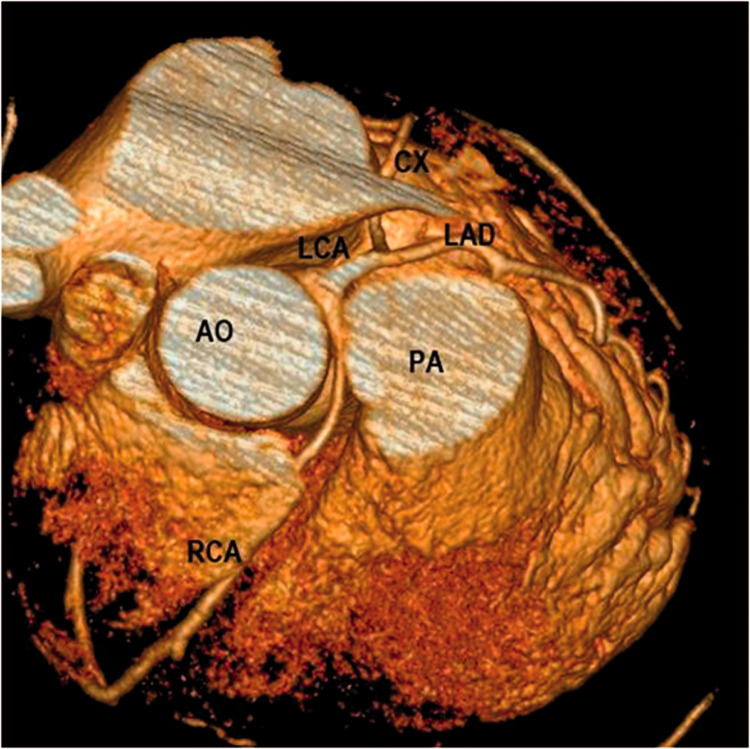
Figure 2.
Preoperative 3D CT reconstruction showing the origin and interarterial course of the RCA in our 47-year-old patient.
3D, three-dimensional; CT, computed tomography; AO, aorta; PA, pulmonary artery; LCA, left coronary artery; LAD, left anterior descending artery; CX, circumflex artery; RCA, right coronary artery.
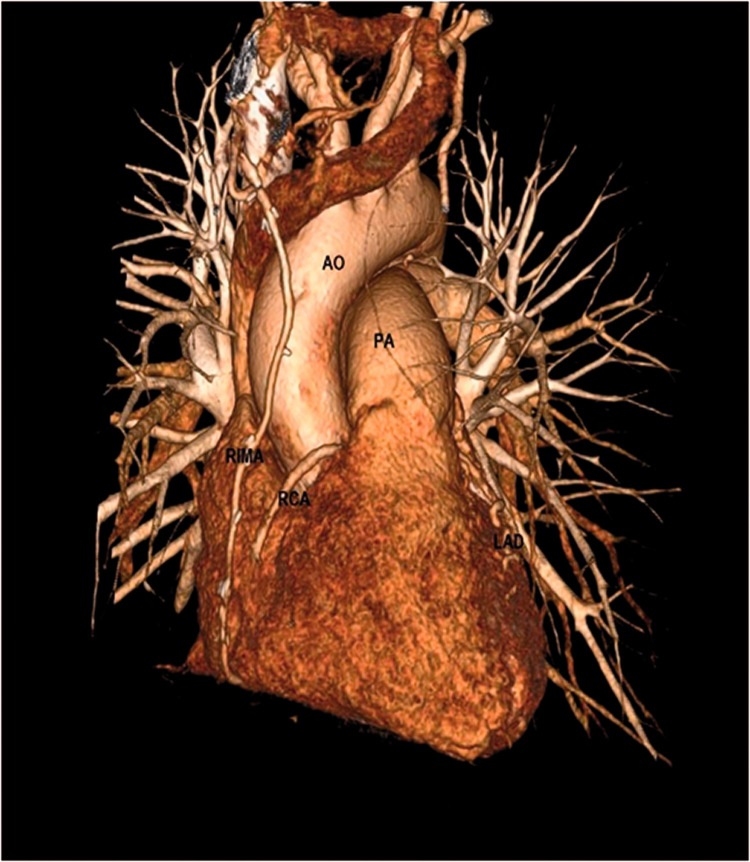
We performed coronary artery bypass grafting (CABG) and concomitant excision of the lung nodule, which proved to be a hamartoma on histological examination. The procedure was performed under cardiopulmonary bypass with aortic cross-clamping and the administration of antegrade cold blood cardioplegia. The right internal mammary artery (RIMA) was harvested, skeletonized, and anastomosed in-situ to the RCA. Subsequently, the RCA was ligated proximal to the anastomosis to prevent coronary steal syndrome and flow competition [Figure 3].
Figure 3.
Postoperative 3D CT reconstruction The right internal mammary artery is anastomosed to the mid-RCA, which is ligated proximally. Postoperative heart CT showing the RIMA grafted on the RCA.
3D, three-dimensional; CT, computed tomography; AO, aorta; PA, pulmonary artery; RIMA, right internal mammary artery; RCA, right coronary artery; LAD, left anterior descending artery.
The patient had an uneventful postoperative course and was discharged home on the 8th postoperative day.
Resting 12-lead ECG, 24-hour Holter monitoring, and exercise ECG (stress test) 3, 6, and 12 months after discharge showed sinus rhythm, and no PVCs were observed. Follow-up echocardiography revealed preserved EF and no wall motion abnormalities. CT re-evaluation 3 months after surgery revealed patency of the RIMA graft.
The reporting of this study conforms to the CARE guidelines. 8
Discussion
SCD is frequently reported in the literature as the first and only presentation of AAOCA in patients with an interarterial course. Considering this, as well as the hypokinesis of the left ventricle, which was suggestive of ischemia, we decided to treat our patient even though she did not present with life-limiting symptomatology.
Embryologically, the coronary circulation originates from proliferation of endothelial cells, vascular smooth muscle cells, and fibroblasts of the capillary plexus, which migrate into the aortic sinuses. These cells penetrate the aortic wall from a capillary ring surrounding the aortic root, giving origin to the coronary circulation. In AAOCA, the cells of the capillary plexus surrounding the aorta fail to reach and/or penetrate the two normal sites on the aorta, the right and left coronary sinus, probably due to vascular endothelial growth factor C (VEGF-C) deficiency. 9
The pathophysiology of SCD is not completely understood; however, a few mechanisms have been proposed.10–14 In patients with an interarterial course, there can be vascular compression of the proximal segment of the RCA, which passes between the aorta and pulmonary artery, because of engorgement of the two vessels during systole. This may reduce blood supply to the myocardium, especially to the right ventricle, causing ischemia and leading to arrhythmias that may have a fatal outcome. Another proposed mechanism may be attributed to the intramural course of the RCA, with possible lumen diameter reduction during systole, secondary to myocardial contraction. An additional mechanism is represented by the angulation of the artery, with proximal stenosis occasionally observed at the origin of the RCA, especially in the presence of a retrocardiac course. In pediatric patients, diagnosis is generally performed through echocardiography, even if CT is necessary to further investigate the coronary tree, especially in patients weighing more than 40 kg. The use of Doppler ultrasonography improves the precision of echocardiographic diagnosis because flow velocity is lower in diastole, whereas systolic compression reduces flow. In adolescents or adults, structural magnetic resonance imaging (s-MRI) is the ideal imaging tool because it allows precise and reliable diagnosis of high-risk coronary artery anomalies, and this imaging modality is readily available in most centers. Cardiac CT (CCT) also offers high-quality imaging. However, intramural aortic course evaluation may be challenging, and exact quantification of stenosis cannot be achieved by s-MRI or CCT. Only cross-sectional imaging perpendicular to the vessel’s direction obtained during both cardiac phases can help quantify the area of stenosis. This imaging can be achieved only with catheter angiography, which is used for definitive imaging of the vessel and stenosis quantification, combined with intravascular ultrasonography (IVUS) evaluation. 15
Several surgical techniques have been proposed to repair AAOCA. However, no comparison between techniques has been reported; therefore, there is no evidence supporting superiority regarding mortality, safety, and long-term outcomes among the described procedures. Unroofing the RCA to treat this anomaly is often proposed in the literature,16,17 which provides excellent midterm results, and is mostly used when the intramural length of the anomalous vessel is conspicuous. Another applicable technique is reimplantation of the RCA,18,19 which consists of dissecting and mobilizing the proximal portion of the RCA, transecting the artery after aortic cross-clamping, and reimplanting the RCA into the non-coronary sinus. Other techniques, not applicable to our patient, are ostioplasty,20,21 which is the creation of a neo-ostium from the normal ostium to the ectopic artery, with pericardial patch augmentation of the vascular wall, as well as percutaneous coronary intervention (PCI) stenting. Another technique is pulmonary artery translocation,22,23 where the distal main pulmonary artery is transected and then relocated to the left pulmonary artery to prevent compression of the anomalous coronary artery. Alternatively, transection of the right pulmonary artery22,23 at its origin and anastomosis to the pulmonary trunk anterior to the aorta, with patch augmentation, can be performed. Each strategy has pitfalls, and the choice should be based on individual patient anatomy and the center’s surgical experience. For example, possible pitfalls of coronary unroofing are the possibility of coronary injury and, in almost 15% of the cases, 24 the necessity of aortic valve resuspension, with the consequent risk of aortic regurgitation, albeit minimal. In comparison, one of the most important concerns regarding the revascularization strategy is ligating the coronary artery because the flow in the anastomosed mammary artery in the early stages may not adequately sustain myocardial perfusion of the right ventricle (RV). This is because there are no actual anatomical stenoses in the native coronary artery; 25 therefore, some authors limit CABG to patients with concomitant coronary artery disease or as a bailout procedure for failed anatomical repair. Because AAOCA is rare, not all centers have adequate experience with all of the aforementioned techniques. However, surgical risk in published series is very low, with excellent intermediate-term survival,26,27 despite concerns about the long-term impact of coronary surgery in pediatric patients related to scarring or accelerated atherosclerosis. 28
In our patient, revascularization was accomplished by harvesting the RIMA and anastomosing it to the anomalous RCA, as reported by Reul et al. 16 This approach was chosen because the patient was an adult, and RIMA dimensions were adequate; because of the small portion of the RCA following an intramural course; and finally, because of our familiarity with the procedure. The RCA was subsequently ligated proximal to the anastomosis, as described by Gaudino et al., 29 to avoid the string sign phenomenon. 30 Competitive flow predisposes the internal mammary arteries to the string sign phenomenon, which is especially prevalent in revascularization of the right coronary artery when the vessel is not severely stenosed or completely occluded. This is why we strongly suggest ligating the coronary artery when using the aforementioned method, even though this is an admittedly bold choice, as it removes the “safety net” represented by native coronary flow with a failed anastomosis.
There are no defined guidelines regarding the treatment of AAOCA. The clinical indication for intervention is based on the calculated risk of SCD, considering both the patient’s anatomical features and clinical presentation, as well as the patient’s associated comorbidities. However, an initial algorithm for the treatment of anomalies of the coronary arteries has been proposed in association with the Rigatelli classification, where Class I, or benign coronary artery anomalies, may not require treatment, Class II anomalies require careful follow-up, and Class III and IV generally require surgical correction, as stated in the consensus statement in the Guidelines for Management of Adults with Congenital Heart Disease. 30 Age is an important determinant for treatment choice because pediatric patients aged 10 years or older are referred for surgical intervention owing to the increased risk of SCD during exercise. 31 , 32
Follow-up for AAOCA patients depends on the chosen management plan, even though all patients must receive lifelong cardiac check-ups. Close periodic evaluation in patients who do not undergo surgical correction of the anomaly is of utmost importance, while in surgical patients, the time between each cardiac assessment may be extended after the first year. Generally, complete follow-up evaluation requires echocardiography, ECG at rest, a stress test, and 24-hour Holter ECG monitoring.
Conclusion
AAOCA represents a rare pathology, which, especially in the adult population, can prove to be a therapeutic challenge. Consensus on AAOCA treatment is limited, likely owing to its rarity; however, it is widely accepted that patients with clinically malignant anomalies must undergo surgical treatment to avoid catastrophic complications secondary to ischemic events. While techniques such as coronary unroofing, ostium reimplantation, or neo-ostium creation are valid options, in adults, CABG is a fast, effective, and safe strategy. Using CABG requires creating an occlusion proximal to the anastomosis, to avoid the string sign phenomenon. Careful consideration of the treatment approach is fundamental to choose the correct technique for an individual patient, as each technique requires a tailored methodology.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211054438 for Setting things “right”: right internal mammary artery on anomalous right coronary artery - a case report by D’Abramo M, Saltarocchi S, Saade W, Chourda E, De Orchi P and Miraldi F in Journal of International Medical Research
Footnotes
Ethics statement: This study was a descriptive case report, and ethics committee approval was not required. The patient participating in this study provided written informed consent.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: S Saltarocchi https://orcid.org/0000-0002-5052-1205
References
- 1.Angelini P. Coronary artery anomalies–current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J 2002; 29: 271–278. [PMC free article] [PubMed] [Google Scholar]
- 2.Basso C, Corrado D, Thiene G. Congenital coronary artery anomalies as an important cause of sudden death in the young. Cardiol Rev 2001; 9: 312–317. [DOI] [PubMed] [Google Scholar]
- 3.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 2002; 105: 2449–2454. [DOI] [PubMed] [Google Scholar]
- 4.Benge W, Martins JB, Funk DC. Morbidity associated with anomalous origin of the right coronary artery from the left sinus of Valsalva. Am Heart J 1980; 99: 96–100. [DOI] [PubMed] [Google Scholar]
- 5.Bekedam MA, Vliegen HW, Doornbos J, et al. Diagnosis and management of anomalous origin of the right coronary artery from the left coronary sinus. Int J Cardiovasc Imaging 1999; 15: 253–258. [DOI] [PubMed] [Google Scholar]
- 6.Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation 2007; 115: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 7.Rigatelli G. Coronary artery anomalies: what we know and what we have to learn. A proposal for a new clinical classification. Ital Heart J 2003; 4: 305–310. [PubMed] [Google Scholar]
- 8.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 9.Tomanek R, Angelini P. Embryology of coronary arteries and anatomy/pathophysiology of coronary anomalies. A comprehensive update. Int J Cardiol 2019; 281: 28–34. doi: 10.1016/j.ijcard.2018.11.135 [DOI] [PubMed] [Google Scholar]
- 10.Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J 1986; 111: 941–963. [DOI] [PubMed] [Google Scholar]
- 11.Roberts WC. Congenital coronary arterial anomalies unassociated with major anomalies of the heart and great vessels. In Roberts WC. (ed.) Adult Congenital Heart Diseases. Philadelphia: FA Davis, 1987, pp.583–630. [Google Scholar]
- 12.Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med 1996; 334: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol 1992; 20: 640–647. [DOI] [PubMed] [Google Scholar]
- 14.Basso C, Maron BJ, Corrado D, et al. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 2000; 35: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 15.Angelini P. Imaging Approaches for Coronary Artery Anomalies: Purpose and Techniques. Curr Cardiol Rep 2019; 21: 101. Published 2019 Jul 29. doi: 10.1007/s11886-019-1188-7 [DOI] [PubMed] [Google Scholar]
- 16.Reul RM, Cooley DA, Hallman GL, et al. Surgical treatment of coronary artery anomalies: report of a 37 1/2-year experience at the Texas Heart Institute. Tex Heart Inst J 2002; 29: 299–307. [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudino M, Robinson NB, Hameed I, et al. Coronary Bypass With the Free Internal Thoracic Artery to Treat Anomalous Right Coronary Artery. Ann Thorac Surg 2020; 109: e371–e373. doi: 10.1016/j.athoracsur.2019.11.063. Epub 2020 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villareal RP, Mathur VS. The string phenomenon: an important cause of internal mammary artery graft failure. Tex Heart Inst J 2000; 27: 346–349. [PMC free article] [PubMed] [Google Scholar]
- 19.Poynter JA, Bondarenko I, Austin EH, et al. Repair of anomalous aortic origin of a coronary artery in 113 patients: a Congenital Heart Surgeons' Society report. World J Pediatr Congenit Heart Surg 2014; 5: 507–514. [DOI] [PubMed] [Google Scholar]
- 20.Mustafa I, Gula G, Radley-Smith R, et al. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death. Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg 1981; 82: 297–300. [PubMed] [Google Scholar]
- 21.Rogers SO, Jr, Leacche M, Mihaljevic T, et al. Surgery for anomalous origin of the right coronary artery from the left aortic sinus. Ann Thorac Surg 2004; 78: 1829–1831. [DOI] [PubMed] [Google Scholar]
- 22.Lee MK, Choi JB, Kim KH, et al. Surgery for anomalous origin of the left main coronary artery from the right sinus of Valsalva, in association with left main stenosis. Tex Heart Inst J 2009; 36: 309–312. [PMC free article] [PubMed] [Google Scholar]
- 23.Han WS, Park PW, Cho SH. Neo-ostium formation in anomalous origin of the left coronary artery. Korean J Thorac Cardiovasc Surg 2011; 44: 355–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alphonso N, Anagnostopoulos PV, Nolke L, et al. Anomalous coronary artery from the opposite sinus of Valsalva: a physiologic repair strategy. Ann Thorac Surg 2007; 83: 1472–1476. [DOI] [PubMed] [Google Scholar]
- 25.Mainwaring RD, Reddy VM, Reinhartz O, et al. Anomalous aortic origin of a coronary artery: medium term results after surgical repair in 50 patients. Ann Thorac Surg 2011; 92: 691–697. [DOI] [PubMed] [Google Scholar]
- 26.Mainwaring RD, Reddy VM, Reinhartz O, et al. Surgical repair of anomalous aortic origin of a coronary artery. Eur J Cardiothorac Surg 2014; 46: 20–26. [DOI] [PubMed] [Google Scholar]
- 27.Romp RL, Herlong JR, Landolfo CK, et al. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg 2003; 76: 589–596. doi: 10.1016/s0003-4975(03)00436-3. [DOI] [PubMed] [Google Scholar]
- 28.Karangelis D, Mylonas KS, Loggos S, et al. Surgical repair of anomalous aortic origin of coronary artery in adults Asian Cardiovasc Thorac Ann 2021; 29: 51–58. doi: 10.1177/0218492320957818. [DOI] [PubMed] [Google Scholar]
- 29.Turner II, Turek JW, Jaggers J, et al. Anomalous aortic origin of a coronary artery: preoperative diagnosis and surgical planning. World J Pediatr Congenit Heart Surg 2011; 2: 340–345. [DOI] [PubMed] [Google Scholar]
- 30.Brothers JA, Frommelt MA, Jaquiss RDB, et al. Expert consensus guidelines: anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg 2017; 153: 1440–1457. [DOI] [PubMed] [Google Scholar]
- 31.Poynter JA, Williams WG, McIntyre S, et al. Anomalous aortic origin of a coronary artery: a report from the Congenital Heart Surgeons Society Registry. World J Pediatr Congenit Heart Surg 2014; 5: 22–30. [DOI] [PubMed] [Google Scholar]
- 32.Osaki M, McCrindle BW, Van Arsdell G, et al. Anomalous origin of a coronary artery from the opposite sinus of Valsalva with an interarterial course: clinical profile and approach to management in the pediatric population. Pediatr Cardiol 2008; 29: 24–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211054438 for Setting things “right”: right internal mammary artery on anomalous right coronary artery - a case report by D’Abramo M, Saltarocchi S, Saade W, Chourda E, De Orchi P and Miraldi F in Journal of International Medical Research