Abstract
Introduction
The role of digoxin (cardiac glycoside) in controlling the heart rate (HR) for the treatment of atrial fibrillation (AF) patients has not been explored in depth.
Methods
To contribute to the limited data, our team conducted retrospective analysis of the clinical records of 1444 AF patients. We divided the AF patients into two groups, wherein group 1 patients were treated with beta-blockers (BB), low-dose digoxin, and an anticoagulant (vitamin K antagonist/factor-IIa inhibitor/factor-Xa inhibitor), and group 2 patients were treated with just BB and an anticoagulant. Our objectives were to compare the impact of combination therapy of BB and digoxin on the resting HR in patients with permanent AF and the patients’ quality of life (QOL) at periodic intervals.
Results
The findings of our study showed a better control of the resting HR rate (<110bpm) and an improved QOL among the group 1 patients when compared with group 2 patients.
Conclusion
Our findings are indicative of the favorable clinical outcomes that resulted from the addition of a low-dose of digoxin to the AF treatment regimen. However, larger studies/trials elucidating the outcomes of AF patients treated with the dual rate control therapy are required, to clarify the role of digoxin, guide the choice of agents, and standardize the AF treatment protocol.
Keywords: atrial fibrillation, digoxin, beta-blockers, dual rate control, mortality, quality of life
Introduction
Atrial fibrillation (AF) is considered the most common cardiac arrhythmia. It can lead to severe neurological deficits due to its high potential of causing stroke and it may even cause death. Approximately 1% of the adult population above the age of 60 years have AF. The incidence rate of AF increases with the age.1–3 The condition mostly affects the elderly but can also be seen in the younger population, albeit with increased severity and lower incidence rates in the latter group. AF is a highly heterogeneous disease in terms of its epidemiology and pathophysiology, and its treatment plans tend to vary from patient to patient. 4 Overall, AF limits the level of activity and decreases the quality of life (QOL) in the affected individuals.3,4
Genetic factors, hypertension, hyperthyroidism, valvular heart disease, smoking, alcohol consumption, etc., are factors that are commonly implicated in the occurrence of AF in the young, reflecting the multifactorial etiology of this condition.3,5–8 Priority should be given to tracing the presence of an underlying disease in its early stages to treat the disease and prevent its complications.8,9 Scrutinizing the potential hidden causes of idiopathic AF would encourage further research regarding AF pathophysiology and its potential effective treatment. 9
The non-stroke outcomes of AF may include myocardial infarction, heart failure, cognitive impairment/dementia, chronic kidney disease progression, and an increased risk of mortality.10–13 When AF occurs as a complication of cardiac surgery, it is known as postoperative atrial fibrillation (POAF). The increased risk of POAF is believed to be associated with a postoperative overexpression of monoamine oxidase.4–6,14–16
The overall risk of stroke increases by 142% in patients with AF. The risk of ischemic stroke increases by 133% in patients with AF as compared with the individuals without AF. 17 AF accounts for almost one-third of all stroke cases caused by thromboembolic events (TE), and the mortality rate in such cases is higher when compared with strokes of other etiologies.18,19 Furthermore, the severity of stroke is higher and the degree of debilitation in the AF patients is much worse than the non-AF stroke patients.20,21 Silent cerebral infarctions (SCI’s) are a common feature in AF patients, putting them at a greater risk of developing cognitive impairment, disabilities, and stroke events in the future. The rate of SCIs occurrence is even higher in post-AF ablation procedures.18,22
Post-stroke pain (PSP) is experienced in many patients irrespective of the underlying cause of stroke, compromising the overall quality of life (QOL). In many countries, the patient’s QOL is evaluated based on pre-established questionnaire(s). The following five parameters are used to assess the QOL; anxiety, depression, fatigue, cognitive function and physical function.23,24 Periodic evaluations of these parameters can further help the physicians to provide supportive therapies and care for a better QOL.24,25
Treatment of AF with or without stroke is important and challenging, and can substantially decrease mortality if instituted accurately. Overall, the mainstay of the treatment includes converting the rhythm back to normal sinus rhythm and achieving rate control and preventing stroke.26–28 In specialized units, ablation therapy is also used on a large scale to destroy the abnormal foci leading to AF, but it is not feasible for all patients worldwide due to limited resources and scarcity of cardiovascular surgeons.26,28–30 Left atrial appendage obliteration, if and when possible can also be used to reduce the risk of the stroke event. 29
Most often pharmacological therapy is initiated to prevent TEs and strokes, especially, when surgical procedures are not possible due to various reasons.26,27 Although the use of anticoagulation agents reduce the risk of TEs and strokes, they tend to increase the chances of bleeding in some patients, and therefore, before the initiation of the anticoagulation therapy and periodically thereafter, the patients need to be evaluated for bleeding tendencies, using the HAS-BLED score as an assessment tool. 29 Furthermore, the choice of agent is based on individuals’ preferences, risks versus potential benefits, and cost.26,27,29,31
The drug classes that are used for controlling the heart rate in AF patients are beta-blockers, non-dihydropyridine calcium channel blockers/antagonists (CCB; diltiazem or verapamil), and cardiac glycosides. 32 Among these, beta-blockers are widely prescribed due to their effectiveness in maintaining sinus rhythm and controlling ventricular rate during AF and decreasing mortality.33,34 Although the use of cardiac glycosides (e.g., digoxin) alone in AF patients has been controversial, citing their benefits many physicians still prefer their use in combination with beta-blockers, especially for heart failure with low ejection fraction that can be worsened by a high ventricular rate.35,36 Nevertheless, many studies have highlighted the limitations of digoxin use due to its narrow therapeutic index and constant need for serum levels monitoring. Furthermore, some studies have shown the association of digoxin with increased all-cause mortality in AF patients, with or without HF.33,35 The paucity of clinical data on the potential benefits of the digoxin use as the sole rate-controlling agent in AF patients has led to its restrictive use by clinicians. However, many studies have highlighted that the combination therapy of digoxin and beta-blockers decreases mortality.35,37 On the other hand, CCBs may also be used in AF patients, but they are contraindicated in HF patients with systolic dysfunction. 36 Additionally, digoxin can be of tremendous help in situations of hypotension or when beta-blockers are absolutely contraindicated due to the presence of underlying pathologies or sedentarism in patients; nonetheless, it is not the first line of treatment to control HR in AF patients32,35,37.
Aim
Our primary objective was to compare the extent of rate control (lenient rate control (<110 bpm)) between the two groups of patients with permanent AF, wherein group 1 received the triple therapy of low-dose digoxin (0.125 mg/day), beta-blockers, and anticoagulants and group 2 received the dual therapy of beta-blockers and anticoagulants. We also aimed at assessing the patients’ quality of life in both the groups and of the patients who had transient ischemic attacks during the study period.
Method
In our retrospective study, we extracted and analyzed the records of patients with permanent AF. These patients were admitted multiple times for follow-ups/complications/acute-episodes during the period of 2015–2019, to the Municipal Emergency University Hospital, Timisoara, Romania. We excluded the following patients; newly diagnosed cases between the period of January 2017 to December 2019, patients who underwent ablation or electrical cardioversion, patients who received beta-blockers alone (without anticoagulants due to low CHA2DS2-VASc score <2) or other treatment options, and patients with incomplete documentation. We obtained ethical consent from concerned regulatory authorities before initiating this study.
The baseline parameter taken into consideration is shown in (Table 1). Mortality rate was compared as well. Patients receiving triple therapy (beta-blockers + digoxin + anticoagulants) were denoted as group 1, while those receiving dual therapy (beta-blockers and anticoagulants) were referred to as group 2. The prescribed dose of beta-blockers was either bisoprolol 10 mg/zi, or nebivolol 5 mg/zi, or metoprolol succinate 200 mg/zi. The patients were followed-up every 6 months for the assessment of their QOL (patient-reported), based on the MMSE exam (Mini Mental State Exam), and SCL-90-R checklist. To assess the QOL (patient reported), these patients were evaluated based on five primary parameters: anxiety, depression, fatigue, cognitive function, and physical function.
Table 1.
Assessment of baseline parameters.
| Variable | Group 1 (triple therapy) (n = 298) | Group 2 (dual therapy) (n = 466) |
p value |
|---|---|---|---|
| Sex (male) | 151 (50.5%) | 217 (46.5%) | 0.198 |
| Diabetes mellitus type II | 98 (33.0%) | 135 (28.9%) | 0.158 |
| COPD* | 62 (20.9%) | 101 (21.6%) | 0.786 |
| Hypertension | 254 (85.3%) | 389 (83.4%) | 0.440 |
| Obesity | 97 (32.5%) | 149 (31.9%) | 0.858 |
| Ischemic cardiomyopathy | 174 (58.4%) | 286 (61.4%) | 0.304 |
| Angina pectoralis | 114 (38.2%) | 178 (38.2%) | 0.808 |
| Asthma | 32 (10.6%) | 46 (9.9%) | 0.756 |
| Hypertriglyceridemia | 147 (49.2%) | 210 (45.1%) | 0.188 |
| Increased low density lipoprotein | 172 (57.7%) | 245 (52.6%) | 0.099 |
| Dyslipidemia | 140 (46.9%) | 206 (44.1%) | 0.374 |
| Atherosclerosis | 60 (20.1%) | 100 (21.4%) | 0.643 |
*Chronic obstructive pulmonary disease.
Statistical analysis
Statistical analysis was performed with SPSS software (version 17, SPSS Inc., Chicago, USA). The data were electronically filed using Microsoft Excel (version 2013, MS Corp., Redmond, Washington, USA). Pharmacological treatment plans were statistically analyzed to compare the degree of rate control (lenient rate control (110 bpm) and QOL between the groups. Additionally, QOL of a subset of patients within group 1 was noted and assessed.
Results
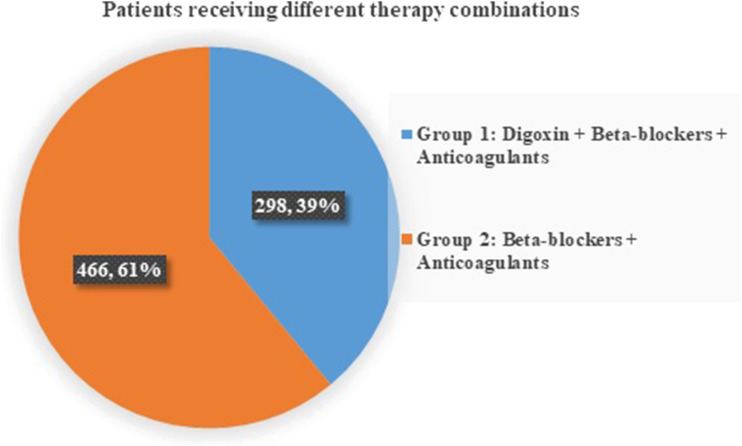
A total number of 1444 cases having a diagnosis of AF or its complications were admitted. Out of which 501 (34.69%) patients had paroxysmal AF while the remaining 943 (65.30%) had the permanent type of AF. 87.5% presented to the emergency room (ER) and were then shifted to the medical wards after initially stabilizing the acute condition, while only 12.5% of cases were admitted for a regular pre-planned follow-up of the medical condition. Out of 943 cases, 938 cases (99.46%) were above the age of 45 years, while only five patients were below 45 years. Based on the inclusion criteria, only 764 out of 943 cases of permanent AF were included in our study. Out of these 764 cases, 298 patients (39%) received the prescribed triple therapy and were part of group 1, while the remaining 466 patients (60.99%) received the dual therapy (beta-blockers and anticoagulants) were assigned to group 2 (Figure 1). There were no significant differences in the baseline characteristics between the two groups.
Figure 1.
Pie chart showing the percentage of patients receiving different therapy combinations. Group 1 patients (61%) who were on digoxin, beta-blockers, and anticoagulants. Group 2 patients (39%) who had just beta-blockers and anticoagulants on their treatment plan.
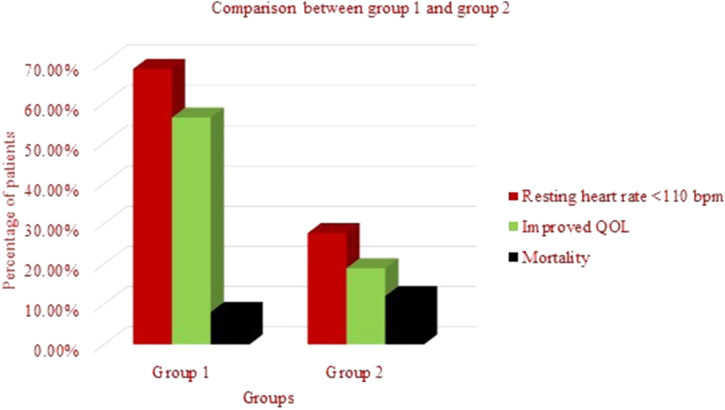
The results of the periodic (every 6 months) assessments of the patients showed that in 204 (68.45%) patients from group 1 had better rate control (lenient HR <110 bpm), while only 129 out of 466 patient (27.68%) from group 2 patients had similar findings (Figure 2).
Figure 2.
Comparison between groups 1 and 2 shown by a clustered column chart on the basis of the following three parameters, heart rate <110 bpm, better quality of life, and mortality.
A significantly more number of patients (56.37%) in group 1 reported better QOL versus the patients in group 2 (18.02%). Within group 1, the reported self-assessed QOL was marginally better among the less active older patients with compromised cardiac output. Furthermore, among the group 1 patients who had transient ischemic attack (TIA) during the period (2015–2019) also expressed satisfaction with their post event QOL when compared with group 2 patients who had TIA.
During the follow-up period of 2015–2019, the overall mortality in both groups was 10.47%. However, mortality in group 1 [8.04% (24 cases)] was lower than the group 2, wherein the mortality was higher by 4% [12.01% (56 cases)] (Figure 2).
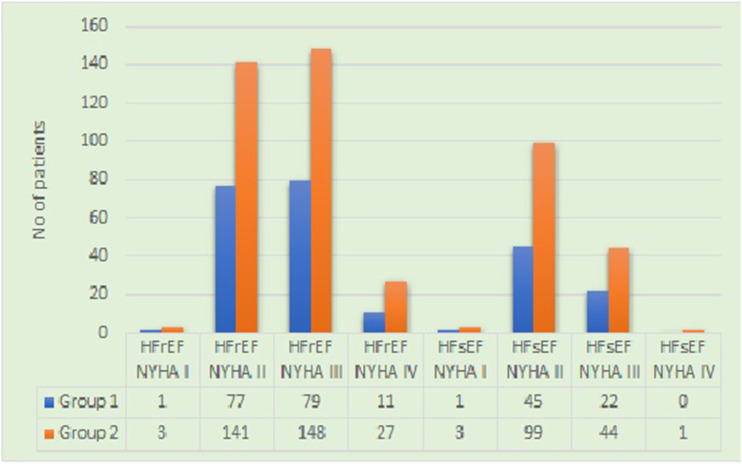
On comparing the patients from both the groups having heart failure with different grades of NYHA (New York Heart Failure) classification with reduced (HFrEF) or sustained ejection fraction (HFsEF), it was noted that group 2 patients were in more advanced stage of heart failure compared to the group receiving digoxin (Figure 3).
Figure 3.
Comparison between groups 1 and 2 based on the New York Heart Failure classification of severity of heart failure with reduced or sustained ejection fraction.
Discussion
Management of AF entails anticoagulation for stroke prevention, selection of patients for sinus rhythm restoration, and the control of HR. In contrast to other management strategies, the rate control therapy has a poor evidence base38,39. Citing this deficiency European society of cardiology (ESC) and guidelines from the National Institute for Health and Care Excellence 41 have mandated rate control-oriented research39–41.
Due to limited evidence base, there are wide variations in terms of choice of first-line and subsequent therapy for rate control,39,41–44 resulting in the frequent use of combination therapy by clinicians worldwide. 39 Most guidelines suggest that the treatment of choice should be individualized, based on the presence of ongoing patient symptoms39,41,45. However, the recommendations are predicated on the observational data and low-quality trials with a small number of participants and few weeks’ worth of follow-up.39,46 There are no randomized control trials (RCTs) comparing different long-term HR control options in AF. 39 In AF patients with concomitant HF and reduced ejection fraction (EF), BBs do not reduce all-cause mortality or hospital admissions. 47 Also, comprehensive systematic reviews have shown that the use of digoxin neither increases nor decreases mortality. 48 Studies have demonstrated that digoxin reduces hospital admissions in patients with HF and reduced EF in sinus rhythm.32,39 However, the impact of digoxin on AF patients is unknown, 39 and more studies are required to explore the clinical outcomes. Although BBs have a greater impact on HR during exercise when compared with digoxin, there is no evidence that BBs also increase exercise capacity. 39 A small RCT has also shown that BBs do not improve arrhythmia-related symptoms when compared with CCBs (diltiazem and verapamil). Moreover, the effect of CCBs on HR is more as compared with digoxin alone; however, CCBs are not given to AF patients with reduced EF to avoid adverse outcomes. 39 Only one RCT involving AF patients with concomitant HF has reported improvement in left ventricular EF in patients on a combination of carvedilol and digoxin versus placebo and digoxin.40,49 Most of the current information on the impact of digoxin as a rate-controlling agent in AF is based on the data of observational studies and not randomized trials. 36 Similarly, our retrospective study showed that long-term survival may be increased in patients suffering from permanent atrial fibrillation when cardiac glycosides are added.
Studies such as Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) and Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) that evaluated the addition of rhythm control strategies reported no difference in the clinical outcomes when the AF patients were treated with anti-arrhythmic drugs or direct current cardioversion in addition to the ongoing HR control drug regimen.27,28,39 Many meta-analyses and smaller trials have also highlighted that rhythm control is not superior to control of heart rate alone.39,50–52
Anticoagulation therapy (vitamin K antagonist/FIIa inhibitors/FXa inhibitors) in AF is also crucial to prevent strokes/TIAs. Due to similarities in the pathogenesis of venous thrombosis and AF associated thromboembolic events, some studies even suggest screening patients with recurrent venous thrombosis and positive family history for thrombotic events. These patients should undergo thrombophilia testing (e.g., protein C, protein S, antithrombin, lupus anticoagulant, and activated protein C resistance). 19 However, AF patients on NOACs/DOACs (FIIa and FXa inhibitors) may cause false results, and therefore, such patients need to be carefully assessed. 53
As one of the goals of AF therapy is to prevent deterioration in patients’ QOL, administering pertinent questionnaires can help identify changes in QOL. Many authors encourage the use of such standardized questionnaires to guide the AF treatment plans in patients with or without TIA/stroke. 25
Limitations of the study
Since the QOL assessment is entirely patient response-based, the results might be erroneous. A more concrete method of evaluation of QOL would help future studies. Furthermore, no power analysis was performed for the calculation of sample size.
Conclusion
Rate control is vital in the management of AF, especially in older patients with permanent AF. However, due to the dearth of controlled trial evidence, the patients’ risk stratification and choice of HR control agents are at the discretion of the prescribing clinicians. This lack of standardized treatment protocol necessitates large RCTs on the impact of varying AF therapies on the heart function and disease sequelae. The result of our retrospective study indicate that the addition of small dose digoxin to the treatment protocol may help in better control of the HR (<110 bpm at rest), especially in physically less active older patients with compromised cardiac output, and improve overall QOL. Combination therapy comprising beta-blockers, low-dose digoxin, and anticoagulants may decrease mortality among AF patients to a higher extent in centers where cardiovascular surgeries are not feasible to treat AF.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Prior to the commencement of the study, ethics approval and informed written consent was obtained from all the relevant persons or authorities. The study was approved by the “Comisia de Etica a Cercetarii Stiintifice” (Ethics Committee for Scientific Research) of the University of Medicine and Pharmacy “Victor Babes,” Timisoara (approval nr. 01/16.01.2015), in accordance with the Helsinki declaration—Recommendations Guiding Medical Doctor in Biomedical Research Involving Human Subjects. All the steps of the study were conducted in accordance with the above guidelines, conforming to the standard operational procedures for clinical studies approved for Spitalul Municipal (Municipal Emergency University Hospital, Romania).
Informed consent: This retrospective study was conducted in our University hospital and as a part of routine procedure informed written consent forms stating that the data can be used for future medical research purpose were signed by each patient at the time of admission in the hospital.
ORCID iDs
Ciprian Ilie Rosca https://orcid.org/0000-0002-8619-0479
Nilima Rajpal Kundnani https://orcid.org/0000-0002-2824-7182
Gabriela Otiman https://orcid.org/0000-0003-3172-9630
Abhinav Sharma https://orcid.org/0000-0002-0865-0054
References
- 1.Lam A, Goulouti E, Roten L. (2017) The search for atrial fibrillation and its impact on public health. Swiss medical weekly 147: w14447. [DOI] [PubMed] [Google Scholar]
- 2.Heeringa J, et al. (2006) Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 27(8): 949–953. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal N, et al. (2015) Atrial fibrillation in the young: a neurologist's nightmare. Neurol Res Int 2015: 374352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sankaranarayanan R, et al. (2013) Comparison of atrial fibrillation in the young versus that in the elderly: a review. Cardiol Res Pract 2013: 976976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain AM, et al. (2011) Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm 8(8): 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pignatelli P, et al. (2015) Relationship between Mediterranean diet and time in therapeutic range in atrial fibrillation patients taking vitamin K antagonists. Europace 17(8): 1223–1228. [DOI] [PubMed] [Google Scholar]
- 7.Germaine S, et al. (2012) The influence of cigarette smoking on endothelial function. J Food Agric Environ 10(1): 25–28. [Google Scholar]
- 8.Balint GS, Borza C, Cristescu C, Andoni M, Simu GM, Malita D, et al. (2011) Endogenous and exogenous antioxidant protection for endothelial dysfunction. Rev Chim (Bucharest) 62: 680–683. [Google Scholar]
- 9.Weijs B, Schotten U, Crijns HJ. (2015) Pathophysiology of idiopathic atrial fibrillation-prognostic and treatment implications. Curr Pharmaceut Des 21(5): 551–572. [DOI] [PubMed] [Google Scholar]
- 10.Bansal N, et al. (2013) Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 127(5): 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conen D, et al. (2011) Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. Jama 305(20): 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalantarian S, et al. (2013) Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med 158(5 Pt 1): 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neal WT, et al. (2015) Atrial fibrillation and incident end-stage renal disease: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Int J Cardiol 185: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson EJ, et al. (2014) Monoamine oxidase is a major determinant of redox balance in human atrial myocardium and is associated with postoperative atrial fibrillation. J Am Heart Assoc 3(1): e000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muntean DM, et al. (2016) The Role of Mitochondrial Reactive Oxygen Species in Cardiovascular Injury and Protective Strategies. Oxid Med Cell Longev, 2016: 8254942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duicu OM, et al. (2016) Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxid Med Cell Longev 2016: 8470394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odutayo A, et al. (2016) Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. Br Med J 354: i4482. [DOI] [PubMed] [Google Scholar]
- 18.Hahne K, Mönnig G, Samol A. (2016) Atrial fibrillation and silent stroke: links, risks, and challenges. Vasc Health Risk Manag 12: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maris MI, et al. (2019) Are inherited combined thrombophilia mutations a causative or an additive factor in recurrent venous thrombotic accidents? Clin Lab 1–65. [DOI] [PubMed] [Google Scholar]
- 20.Andrew NE, Thrift AG, Cadilhac DA. (2013) The prevalence, impact and economic implications of atrial fibrillation in stroke: what progress has been made?. Neuroepidemiology 40(4): 227–239. [DOI] [PubMed] [Google Scholar]
- 21.Hannon N, et al. (2010) Stroke associated with atrial fibrillation--incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis 29(1): 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalantarian S, et al. (2014) Association between atrial fibrillation and silent cerebral infarctions: a systematic review and meta-analysis. Ann Intern Med 161(9): 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahesh PKB, et al. (2020) Post-stroke quality of life index: a quality of life tool for stroke survivors from Sri Lanka. Health Qual Life Outcomes 18(1): 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spertus J, et al. (2011) Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 4(1): 15–25. [DOI] [PubMed] [Google Scholar]
- 25.Aliot E, et al. (2014) Quality of life in patients with atrial fibrillation: how to assess it and how to improve it. Europace 16(6): 787–796. [DOI] [PubMed] [Google Scholar]
- 26.Zamani P, Verdino RJ. (2015) Management of Atrial Fibrillation. J Intensive Care Med 30(8): 484–498. [DOI] [PubMed] [Google Scholar]
- 27.Wyse DG, et al. (2002) A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 347(23): 1825–1833. [DOI] [PubMed] [Google Scholar]
- 28.Van Gelder IC, et al. (2002) A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 347(23): 1834–1840. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez C, Blanchard DG. (2016) Diagnosis and treatment of atrial fibrillation. Am Fam Physician 94(6): 442–452. [PubMed] [Google Scholar]
- 30.Westerman S, Wenger N. (2019) Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev 15(2): 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kundnani NR, et al. (2021) Selecting the right anticoagulant for stroke prevention in atrial fibrillation. Eur Rev Med Pharmacol Sci 25(13): 4499–4505. [DOI] [PubMed] [Google Scholar]
- 32.The effect of digoxin on mortality and morbidity in patients with heart failure . N Engl J Med, 1997. 336(8): p. 525-533. [DOI] [PubMed] [Google Scholar]
- 33.Wang ZQ, et al. (2015) Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol 66(3): 270–275. [DOI] [PubMed] [Google Scholar]
- 34.Kühlkamp V, et al. (2002) Use of beta-blockers in atrial fibrillation. Am J Cardiovasc Drugs 2(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 35.Scalese MJ, Salvatore DJ. (2017) Role of digoxin in atrial fibrillation. J Pharm Pract 30(4): 434–440. [DOI] [PubMed] [Google Scholar]
- 36.Fauchier L, et al. (2016) Beta-blockers or digoxin for atrial fibrillation and heart failure? Card Fail Rev 2(1): 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washam JB, et al. (2015) Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Lancet 385(9985): 2363–2370. [DOI] [PubMed] [Google Scholar]
- 38.Kirchhof P, et al. (2016) A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace 18(1): 37–50. [DOI] [PubMed] [Google Scholar]
- 39.Kotecha D, et al. (2017) A review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial. BMJ Open. 7(7): e015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atrial fibrillation: management. NICE, 180. http://www.nice.org.uk/guidance/cg180/. [Google Scholar]
- 41.Kirchhof P, et al. (2016) ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 50(5): e1–e88. [DOI] [PubMed] [Google Scholar]
- 42.Lip GY, et al. (2014) A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace 16(3): 308–319. [DOI] [PubMed] [Google Scholar]
- 43.Nabauer M, et al. (2009) The registry of the german competence network on atrial fibrillation: patient characteristics and initial management. Europace 11(4): 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steg PG, et al. (2012) Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart 98(3): 195–201. [DOI] [PubMed] [Google Scholar]
- 45.January CT, et al. (2014) AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64(21): e1–76. [DOI] [PubMed] [Google Scholar]
- 46.Segal JB, et al. (2000) The evidence regarding the drugs used for ventricular rate control. J Fam Pract 49(1): 47–59. [PubMed] [Google Scholar]
- 47.Kotecha D, et al. (2014) Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 384(9961): 2235–2243. [DOI] [PubMed] [Google Scholar]
- 48.Ziff OJ, et al. (2015) Safety and efficacy of Digoxin: Systematic Review and Meta-Analysis of Observational and Controlled Trial Data, 351, p. h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khand AU, et al. (2003) Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol 42(11): 1944–1951. [DOI] [PubMed] [Google Scholar]
- 50.Al-Khatib SM, et al. (2014) Rate-and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med 160(11): 760–773. [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee S, et al. (2013) Pharmacologic rate versus rhythm-control strategies in atrial fibrillation: an updated comprehensive review and meta-analysis. Pacing Clin Electrophysiol 36(1): 122–133. [DOI] [PubMed] [Google Scholar]
- 52.de Denus S, et al. (2005) Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med 165(3): 258–262. [DOI] [PubMed] [Google Scholar]
- 53.Favresse J, et al. (2018) Evaluation of the DOAC-stop® procedure to overcome the effect of doacs on several thrombophilia screening tests. TH Open 2(2): e202–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]