Abstract
Sheeppox and goatpox (SGP) are transboundary, highly contagious diseases affecting sheep and goats with characteristic clinical signs. SGP affect populations of small ruminants in Africa, Asia and the Middle East and, as a result, threaten farmers’ livelihoods. Despite their importance, studies looking at factors that increase the risk of sheeppox-virus (SPPV) and goatpox-virus (GTPV) exposure and infection are limited. A cross-sectional study was conducted in three states of Northern Nigeria (Bauchi, Kaduna and Plateau) to determine the sero-prevalence and spatial patterns of SGP, and identify risk factors for SPPV/GTPV exposure at animal and household level. Sera samples were collected from 1,800 small ruminants from 300 households. Data on putative risk factors were collected using a standardised questionnaire. Twenty-nine small ruminants were sero-positive to SGP - apparent weighted sero-prevalence 2.0 %; 95 % C.I. 1.1–.3.0 %. Sero-positive animals came from 19 (6.3 %) households. Analysis of the questionnaire showed that a fifth (20.3 %) of farmers claimed to have experienced SGP outbreaks previously in their flocks, with 33 (1.8 %) of the individual animals sampled in this study reported to have had clinical signs. At animal level, the odds of being sero-positive were higher in older animals (>24months; OR = 8.0, p = 0.008 vs ≤24 months) and small ruminants with a history of clinical SGP (OR = 16.9, p = 0.01). Bringing new small ruminants into the household and having a history of SGP in the flock were the main factors identified at household level. Households were less likely to be sero-positive if the time between bringing animals into the household and sampling was over a year (PR = 0.31, p = 0.05), while households with a history of SGP were more likely to be sero-positive regardless of the timeframe. Important spatial heterogeneity was found. The Bayes smooth rate ranged from 0.06 to 4.10 % across local government areas (LGA), with LGA in the north-east or north-west of the study area identified as hot-spots for SGP exposure. Results from this study shed new light on the understanding of SGP epidemiology and provide key inputs to design risk-based surveillance and intervention programmes in the area.
Keywords: Sheeppox, Goatpox, Sero-prevalence, Risk factors, Subsistence farmers, Nigeria
1. Introduction
Sheeppox and goatpox (SGP) are transboundary, highly contagious, viral diseases caused by sheeppox viruses (SPPV) and goatpox virus (GTPV), both within the genus capripoxvirus. The host preference of SPPV and GPPV varies, with some isolates of SPPV and GPPV causing disease only in sheep and goats respectively, while other isolates of SPPV and GTPV cause disease in both species (Rao and Bandyopadhyay, 2001; Babiuk et al., 2008; Tuppurainen et al., 2017).
SGP is widely distributed in Africa, the Middle East, and Asia (including China) affecting a considerable number of small ruminants mainly kept by subsistence producers. Similar to other poxviral diseases, SGP have highly characteristic clinical signs of multifocal cutaneous papules, pustules and nodules (Tuppurainen et al., 2017). Affected animals also exhibit weight loss, oral and nasal secretions, lethargy, fever and decreased wool and cashmere production, therefore having an indirect negative impact on farmers’ livelihoods. Furthermore, international trade is hampered in endemic countries. Morbidity and mortality can be up to 90 % and 50 % respectively in countries where SGP is endemic, with important variations depending on the circulating strain (Babiuk et al., 2008; Tuppurainen et al., 2017).
Previous studies suggest that SPPV and GPPV transmission occurs through aerosol and direct contact, with infected sheep and goats shedding the virus from skin nodules, as well as oral, nasal and ocular secretions (Babiuk et al., 2008; Tuppurainen et al., 2017), suggesting that management practices that lead to close contact between animals increases the risk of exposure and infection However, current knowledge has been generated mainly from studies under controlled or experimental conditions and field estimates are lacking (Bowden et al., 2008; Boshra et al., 2015; Boumart et al., 2016).
Similarly, only a few studies have identified factors that increase (or decrease) the risk of exposure and/or infection. Among those studies, an important variation in the risk of exposure has been reported across agroecological zones, with arid areas presenting a higher risk (Bhanuprakash et al., 2005; Kardjadj, 2017; Pham et al., 2020). Herd size, free range systems and transhumance systems have also been reported as management practices that increase the risk of exposure and infection (Kardjadj, 2017; Pham et al., 2020). At the animal level, females and young animals have been reported to have higher risk of infection in endemic areas (Fentie et al., 2017; Bolajoko et al., 2019; Limon et al., 2020; Pham et al., 2020). Vaccination has been identified as an effective control measure (Kardjadj, 2017).
With an estimated combined population of over 115 million sheep and goats, Nigeria has one of the largest populations of small ruminants in Africa (Bolajoko et al., 2019). These animals serve as important sources of livelihood and preservation of wealth for rural poor communities in Nigeria (Bolajoko et al., 2019; FAO, 2019). Previous studies in northern Nigeria have showed that outbreaks of SGP caused immediate and long-lasting economic impacts, reporting prevalence and case fatality rates of up to 34 % and 53 % respectively with lower mortality in adults (Gambo et al., 2018; Bolajoko et al., 2019; Limon et al., 2020). Outbreaks of SGP were reported in Bauchi and Kaduna States in 2016 and for both 2016 and 2017 in Plateau State, with different GPPV strains isolated (Adedeji et al., 2019; Bolajoko et al., 2019). Moreover, empirical observations by veterinarians and animal health workers suggests that SGP is endemic in the region. However, the extent to which SGP is present in the region is unknown and factors that might increase the risk of exposure have not been identified in the country. Understanding the distribution of animal disease and identifying potential risk factors are key to designing effective surveillance programmes and disease control interventions, especially in settings with limited resources.
The objectives of this study were (i) to determine the sero-prevalence of SGP in backyard small ruminants in selected States of northern Nigeria, (ii) to identify factors associated with SGPV antibody detection at animal and farm level and (iii) to identify geographical patterns for SGPV exposure.
2. Material and methods
2.1. Study design
2.1.1. Study area
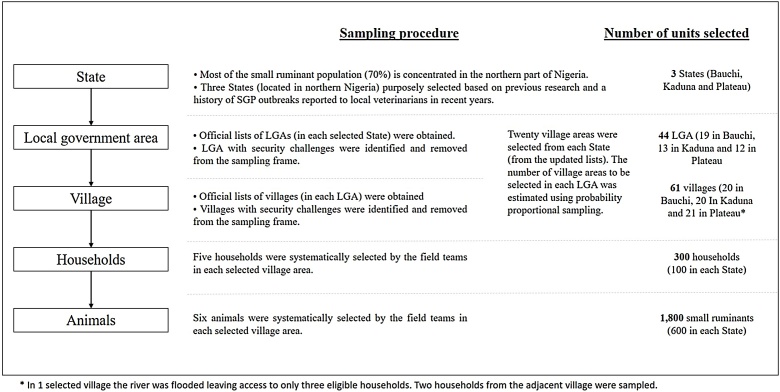
Nigeria is located in West Africa (Fig. 1a). The country is divided into 36 States (Fig. 1b). Each State is divided into local governments areas (LGA) which are further divided into village areas (smaller official divisions). Most of the small ruminant population (70 %) is concentrated in the northern part of Nigeria and are mainly kept by either backyard (sedentary) producers or transhumance (nomadic) farmers. Official registries of individual animals or households raising animals do not exist in Nigeria, so a cross-sectional approach with a multi-stage sampling method was used in this study. In the first stage, three States in northern Nigeria were purposely selected (Bauchi, Plateau and Kaduna – Fig. 1b) based on previous research showing that SGP was present in the area (Adedeji et al., 2019; Ifende et al., 2019; Limon et al., 2020) and a history of SGP outbreaks reported to local veterinarians in recent years. In each State, an official list of LGAs was obtained and within each LGA, the list of village areas and, where available, their village heads. Local government officers were contacted, the study objectives and protocol explained, and their support requested through contacting their respective village heads.
Fig. 1.
(a) Geographic location of Nigeria (grey) in West Africa and (b) The 36 States of Nigeria with the study area identified in grey.
In each State, LGA and villages with security challenges (based on the knowledge and judgement of local field staff) were identified and removed from the sampling frame. Twenty village areas were then randomly selected from each State from the updated list. The number of village areas to be selected in each LGA was estimated using probability proportional sampling. For this, the proportion of villages in each LGA was estimated by dividing the number of villages in the LGA by the total number of villages in the sampling frame, then the proportion estimated was multiplied by the total number of villages to be selected (n = 20) to obtain the number of villages to be selected in each LGA. In each selected village area, the village head was contacted and approval requested to conduct the study. A list of households in each village and number of animals per household were not available prior to sampling. Therefore, five households were systematically selected by the field teams in each selected village area. To select the households, the sampling interval was determined by dividing the estimated number of households in the village area by five. In each selected household, the aim of the study was explained and the farmer’s consent to take part in the study was sought. Six small ruminants (three sheep and three goats) were systematically selected in each selected household and sampled. Six animals were considered a realistic number based on herds and flock sizes recorded in previous research in the study area. If the farmer did not give consent or had fewer than six small ruminants, another household in the same village area was selected. If fewer than three sheep or goats were found in a herd, the species that had fewer than three animals were all sampled and the remaining animals were sampled from the other species to complete the required total of six animals. Deworming of animals in selected households were offered as an incentive to take part of the study. Each field team consisted of four people and included at least one qualified veterinarian and one person that could speak the local language.
A total of 100 households and 600 small ruminants were selected and sampled (once) in each State using the same sample frame (described above) in all the States (Fig. 2). Sample and data collection was conducted between September and November 2019. The number of small ruminants to be sampled was calculated to estimate the proportion of sero-positive animals in each state with 95 % confidence and 6.5 % precision. There were no previous studies estimating sero-prevalence of SGP in Nigeria, therefore a 15 % expected sero-prevalence was assumed in each State based on results reported in a neighbouring country with similar production system (Fentie et al., 2017). In order to take into account the clustering of small ruminants within village areas and households, the resulting number of animal samples was adjusted for a design effect (intra-cluster correlation coefficient [ICC]) at each stage, which were assumed to be 0.05 at village area level and 0.12 at household level.
Fig. 2.
Sampling procedure.
2.1.2. Data and sample collection
Herd and flock sizes, data on management practices, biosecurity measures, past history of SGP (i.e. animals with clinical signs) and household coordinates were collected in each selected household using a standardised questionnaire. In addition, animal characteristics (sex, age, breed and birthplace) were collected for each animal sampled. Past history of SGP was determined with the aid of pictures of small ruminants with highly characteristic SGP lesions (i.e. multifocal cutaneous skin nodules) in order to facilitate recognition of the diseases. Each household was given a unique ID number which was linked to each individual animal sampled.
Five millilitres of whole blood were collected from the jugular vein of each selected animal by a qualified veterinarian using pre-labelled plain vacutainer tubes. Once the animal was sampled it was identified with a line on the ear using a non-toxic colour marker to avoid double sampling. Separation of sera samples was carried out using standard protocol and sera samples stored at -20℃ until testing. Sera samples were tested at National Veterinary Research Institute, Vom, Nigeria for SPPV and GTPV antibodies.
Ethical approval was obtained from the NVRI Animal Ethics Committee (AEC/03/72/19), the Animal Welfare Ethical Review Board of the Pirbright Institute and the Social Science Research Ethical Review Board at the Royal Veterinary College (URN SR2020-0150).
2.1.3. Laboratory analysis
Sera samples were tested using a commercially available ELISA kit (ID Screen® Capripox Double Antigen Multi-species ELISA kit CPVDA, IDvet, Grabels, France) following the manufacturer’s instructions. The ELISA kit can detect antibodies against lumpy skin disease virus, SPPV and GTPV. Samples were tested singularly in microplates with the sample/positive ratio (S/P) ≥30 % used to determine positive samples.
It is not currently possible to distinguish between infection with SPPV or GTPV using serological methods. Host specificity of SPPV and GTPV is not absolute as some strains of sheeppox virus can cause disease in goats, and some strains of goatpox virus can cause disease in sheep. Therefore, sero-positive results in the study were considered to be positive to either SPPV or GTPV.
2.2. Data management and analysis
Questionnaire data were entered into EpiCollect5 (https://five.epicollect.net) by each field team and exported to Microsoft Excel. Inconsistencies across the data were checked and verified by three team members.
Descriptive statistics were generated stratified by State when appropriate. A summary of data collected and description of variables re-categorization for analysis is presented in the supplementary material (Tables S1 and S2).
Weighted apparent sero-prevalence was estimated to take into account the variation in herd sizes between households.
2.2.1. Risk factors
Risk factors analysis was conducted separately at both the animal and household level.
At animal level, associations between potential putative factors and SGP seroconversion were assessed in mixed-effects logistic regression models including household as a random effect to account for clustering within households. Odds ratios (ORs) were calculated as a measure of strength of association.
At household level, association between household management practices and seroprevalence was assessed using Poisson regression with the number of sero-positive animals (in each household) as outcome variable and the number of animals sampled in the household as offset.
For both animal and household level analyses, the relevant univariable models were run, followed by the multivariable models.
For both animal and household level models, variables that were related to the outcome with a p-value <0.20 in the univariate analysis were entered into multivariable models (mixed-effects logistic regression models for animal level and Poisson regression models for household level) following assessment of collinearity between variables. When collinearity was present (≥ 0.8 following Pearson correlation test), only one of the two variables was considered in the multivariable model. Multivariable models were selected using a backward selection process with one variable manually removed each time. A likelihood ratio test was then used to assess which model fit the data best (with or without the variable removed).
2.2.2. Intra-cluster correlation (ICC)
Intra-cluster correlation for sero-positive status of individual animals was estimated using the variance (σ) from mixed-effect models considering State, LG, village or household as random effects (Eq. (1)).
| (1) |
Statistical analysis was performed in R 4.0.2 using packages car, MASS, MuMIn, survey, lme4 and lmtest.
2.2.3. Spatial analysis
A choropleth map of empirical Bayes smoothed rate was generated for sheep and goat pox at the level of LGA using Eqs. (2), (3), (4) (Pfeiffer et al., 2008) as follows: given that yi equalled the number of positive small ruminants observed in the ith LGA, ni the total number of small ruminants sampled in the ith LGA, ri was the proportion of positive small ruminants for the ith LGA and the mean number of animals, then the pooled rate across all LGA () was calculated as:
| (2) |
and the estimate of the population variance of the rate based on a weighted sample of the observed rates () was calculated as:
| (3) |
then , the empirical Bayes-smoothed rate for the ith LGA, was calculated as:
| (4) |
2.2.3.1. Exploring spatial autocorrelation and clustering
Spatial autocorrelation of the smoothed Bayes risk was explored at a global scale using the Moran’s I statistic and at a local scale using the Getis-Ord GI* statistic. The global Moran’s I statistic was used to assess the presence, strength and direction of spatial autocorrelation over the whole study area, using a queen’s contiguity weight matrix and 499 random permutations. A p-value ≤ 0.05 was considered significant. The GI* statistics was used to detect clustering of LGA with similar risk of SGP, and to identify the locations of hot- and/or cold-spots at the LGA level. The GI* statistic returned a z-score for each LGA and for statistically significant positive z-scores, the larger the z-score the more intense the clustering of high values (hot-spot). For statistically significant negative z-scores, the smaller the z- score the more intense the clustering of low values (cold-spot).
All spatial analyses were conducted using tools provided in ArcGIS 10.5.1. (Esri Inc, 2016).
3. Results
3.1. Animal level
Out of the 1,800 animals sampled, the majority (n = 1,366; 75.9 %) were goats and the remainder were sheep (n = 434; 24.1 %). Sampled goats were evenly distributed in the three States with the highest number in Kaduna (n = 505; 37.0 %), followed by Plateau (n = 468; 34.3 %) and Bauchi (n = 393; 28.8 %), while almost half of the sheep sampled were from households in Bauchi (n = 207; 47.7) followed by Plateau (n = 132; 30.4 %) and Kaduna (n = 95; 21.9 %), reflecting the species distribution in these States. Most sampled animals were females (n = 1,488; 82.7 %) regardless of the species (sheep 319/434; 73.5 %; goats 1169/1366; 85.6 %) reflecting the ratio of male/ females kept by subsistence farmers in the study area. The median age of animals sampled was 24 months (1st quartile 12 months; 3rd quartile 42 months), with females being on average older than males (median 36 months for females and 12 months for males), with the same pattern in sheep and goats. The majority were offspring of animals from the same household (n = 1,503; 83.5 %), the rest were acquired from a range of places including livestock markets (n = 184; 10.2 %), neighbours (n = 68; 3.8 %), middleman (n = 17; 0.94 %), inheritance (n = 14; 0.78 %), gift (n = 8; 0.44 %), dowry (n = 2; 0.11 %) or donation from the government (n = 2; 0.11 %), with the remaining two (0.11 %) kept on behalf of a friend or relative, and reared among animals in the household.
Twenty-nine small ruminants were positive for SGP antibodies, giving an apparent weighted sero-prevalence of 2.0 % (95 % C.I. 1.1–3.0%). The majority of the sero-positive animals came from Bauchi (19/29; 65.5 %), followed by Kaduna (7/29; 24.1 %) and Plateau (3/29; 10.3 %). Apparent weighted sero-prevalence was 2.9 % (95 % C.I. 1.9–5.0%) in Bauchi, 0.9 % (95 % C.I. 0.5–2.0 %) in Kaduna and 1.7 % (95 % C.I. 0.7–4.0%) in Plateau. Sex, age, place where animals were acquired, and reported prior clinical signs were strongly associated with sero-positivity in the univariate analysis (Table 1). Twenty-one (72.4 %) of the sero-positive animals were offspring of animals from the same household. The remaining eight (27.6 %) sero-positive animals were acquired outside the household; all came from livestock markets, encompassing eight different livestock markets located in Kaduna (n = 4), Bauchi (n = 3) and Katsina (n = 1) States (supplementary material, Table S3). Thirty-three (1.8 %) of the sampled animals had prior clinical signs of SGP according to the farmer. From these, only five animals were sero-positive, and these five animals had SGP between 6 and 12 months prior to sampling. There was no clear pattern for animals with SGP clinical signs in the past that tested negative, with time between clinical signs and symptoms ranging from 1 to 36 months (Supplementary material, Fig. S1).
Table 1.
Distribution of potential predictor variables for sheeppox and goatpox sero-positive and sero-negative small ruminants in selected states in northern Nigeria following univariate analysis using mixed-effects logistic regression models with household as a random effect.
| Predictor variable | Sero-negative N (%) | Sero-positive N (%) | OR (95 % C.I) | P-value |
|---|---|---|---|---|
| State | ||||
| Kaduna | 593 (98.8) | 7 (1.2) | Ref | |
| Bauchi | 581 (96.8) | 19 (3.2) | 3.5 (0.34–84.9) | 0.31 |
| Plateau | 597 (99.5) | 3 (0.5) | 0.31 (0.0003–16.2) | 0.57 |
| Species | ||||
| Goats | 1,351 (98.9) | 15 (1.1) | Ref | |
| Sheep | 420 (96.8) | 14 (3.2) | 1.6 (0.5–4.9) | 0.39 |
| Sex | ||||
| Male | 310 (99.0) | 2 (1.0) | Ref | |
| Female | 1461 (98.2) | 27 (1.7) | 6.6 (4.7–8.5) | 0.05 |
| Age | ||||
| Up to 24 months | 955 (99.6) | 4 (0.4) | Ref | |
| ≥24 months | 816 (97.0) | 25 (3.0) | 13.7 (3.6–76.2) | <0.001 |
| Origin of the animal | ||||
| Same farm | 1,482 (98.6) | 21 (1.4) | Ref | |
| Other * | 289 (97.3) | 8 (2.7) | 6.57 (1.6–33.9) | 0.01 |
| SGP clinical signs in the past† (n = 1,508) | ||||
| • No | 1,459 (98.8) | 17 (1.2) | Ref | |
| • Yes | 27 (84.4) | 5 (15.6) | 22.4 (2.83–200.0) | 0.004 |
Unweighted seroprevalence estimates are reported in this table.
all sero-positive animals in the ‘Other’ category were sourced from livestock markets. Each sero-positive animal came from a different market – see supplementary material.
Only considering those that were born in the household or had clinical signs after they were bought. This was done to avoid ambiguities as it was unreliable to know if the animal had SGP clinical signs prior to arriving on the farm.
Only two variables were retained in the final multivariable model: age and presence of clinical signs in the past (Table 2). Older animals (>24months) and small ruminants with SGP clinical signs in the past had higher odds of being sero-positive, OR 8.0 (95 % C.I. 1.9–47.0) and OR 17.0 (95 % C.I. 1.9–170.0) respectively, once adjusted for each other.
Table 2.
Results for multivariable analysis for identification of factors associated with sheeppox and goatpox sero-positive at animal-level using a mixed-effects logistic regression model with household as a random effect.
| Predictor variables | Adjusted OR (95 % C.I.) | P-value |
|---|---|---|
| Age | ||
| Up to 24 months | Ref | |
| Age: > 24 months | 8.0 (1.9–47.0) | 0.008 |
| Clinical signs in the past | ||
| No | Ref | |
| Yes | 17.0 (1.9–170.0) | 0.011 |
ICCHousehold = 0.93.
3.2. Household level
Three hundred households were selected encompassing 3 states, 44 LGA and 61 villages distributed as follows: Bauchi (19 LGA, 20 villages), Plateau (12 LGA, 21 villages) and Kaduna (13 LGA, 20 villages). Most selected households (n = 282; 94.0 %) kept at least one goat, while half of the households had at least one sheep (n = 146; 48.7 %). All selected households accepted to take part of the study. The number of households with less than six small ruminants was not recorded systematically. The median herd size was14 small ruminants (1st quartile 10; 3rd quartile 22 small ruminants) with larger herds, on average, in Bauchi (median 17 animals) and Plateau (median 16 animals) (Supplementary material Fig. S2). Only two herds (0.67 %) had more than 100 small ruminants. Semi-intensive production was the main production system (n = 285; 95 %), with few households classified as intensive (n = 10; 3.3 %) or extensive (n = 5; 1.7 %) systems. Fifty-three household (17.7 %) also owned cattle with a median number of 4 (1 st quartile 2; 3rd quartile 9 cattle).
The majority of farmers reported taking their sheep and/or goats to graze in communal areas (281; 93.7 %) and sharing reproductive males (238; 79.3 %). Similarly, it was common practice for animals to drink water from a communal source (280; 93.3 %) (Table 3). Giving medication (which encompassed treatment and vaccination) was a common practice (195; 65.0 %) and all farmers that reported vaccinating small ruminants was against Peste des petits ruminants (PPR). A fifth of the farmers (61; 20.3 %) reported that their herds had been affected in the past with SGP, with around 40 % of affected households having only goats affected (26/61), 24 out of 61 affected households reporting sheep and goats affected and nearly a fifth (11/61) only sheep affected. Out of the 61 farmers that reported an outbreak in their herd in the past, 40 (65.6 %) were from Bauchi, 17 (27.9 %) from Plateau and 4 (6.5 %) from Kaduna.
Table 3.
Distribution of potential risk factors for exposure to sheeppox and goatpox in households in selected states in Northern Nigeria following analysis with univariable poisson regression models with number of positive animals as outcome variable and number of animals sampled in the household as offset.
| Predictor variables | All animals sero-negative (%) | At least one animal sero-positive (%) | Prevalence ratio (95 % CI) | P value |
|---|---|---|---|---|
| State where the household is located | ||||
| • Kaduna | 86 (86) | 14 (14) | Ref | |
| • Bauchi | 96 (96) | 4 (4) | 2.7 (1.2–6.9) | 0.02 |
| • Plateau | 99 (99) | 1 (1) | 0.4 (0.1–1.5) | 0.22 |
| Herd size (small ruminants only) | ||||
| • Up to 14 small ruminants | 146 (94.2) | 9 (5.8) | Ref | |
| • More than 14 small ruminants | 135 (93.1) | 10 (6.9) | 1.0 (0.5–2.1) | 0.99 |
| Management system | ||||
| • Intensive | 9 (90) | 1 (10) | Ref | |
| • Semi Intensive & extensive | 272 (93.8) | 18 (6.2) | 0.3 (0.1–1.3) | 0.05 |
| Small ruminants graze in communal area | ||||
| • No | 17 (89.5) | 2 (10.5) | Ref | |
| • Yes | 264 (94.0) | 17 (6.0) | 0.4 (0.2–1.4) | 0.11 |
| Small ruminants drink water from communal source | ||||
| • No | 18 (90) | 2 (10) | Ref | |
| • Yes | 263 (93.9) | 17 (6.1) | 0.4 (0.2–1.5) | 0.13 |
| Small ruminants share shed with cattle | ||||
| • No | 249 (93.3) | 18 (6.7) | Ref | |
| • Yes | 32 (97.0) | 1 (3.0) | 0.3 (0.02–1.3) | 0.22 |
| Share rams | ||||
| • No | 184 (94.8) | 10 (5.2) | Ref | |
| • Yes | 97 (91.5) | 9 (8.5) | 1.1 (0.5–2.3) | 0.77 |
| Share bucks | ||||
| • No | 68 (89.5) | 8 (10.5) | Ref | |
| • Yes | 213 (95.1) | 11 (4.9) | 0.4 (0.2 – 0.8) | 0.006 |
| Share reproductive males (rams and/or bucks) | ||||
| • No | 55 (88.7) | 7 (11.3) | Ref | |
| • Yes | 226 (95.0) | 12 (5.0) | 0.3 (0.2 – 0.7) | 0.002 |
| Brought new sheep or goats into the household | ||||
| • No | 56 (90.3) | 6 (9.7) | Ref | |
| • Yes | 225 (94.5) | 13 (5.5) | 0.6 (0.3–1.3) | 0.17 |
| Last time new sheep or goats were bought into the household | ||||
| • Never | 56 (90.3) | 6 (9.7) | Ref | |
| • Within a year | 131 (97.8) | 3 (2.2) | 1.1 (0.5–2.5) | 0.008 |
| • More than a year | 94 (90.4) | 10 (9.6) | 0.2 (0.06 – 0.6) | 0.89 |
| Common practice to de-worm | ||||
| • No | 110 (96.7) | 5 (4.3) | Ref | |
| • Yes | 171 (92.4) | 14 (7.6) | 2.4 (1.0–6.5) | 0.06 |
| Common practice of tick control | ||||
| • No | 193 (95.5) | 9 (4.5) | Ref | |
| • Yes | 88 (89.8) | 10 (10.2) | 1.3 (0.6–2.6) | 0.55 |
| Spray sheds to control vectors | ||||
| • No | 253 (93.7) | 17 (6.3) | Ref | |
| • Yes | 28 (93.3) | 2 (6.7) | 0.7 (0.1–2.2) | 0.58 |
| Flock affected by SGP in the past | ||||
| • No | 230 (96.2) | 9 (3.8) | Ref | |
| • Yes | 51 (83.6) | 10 (16.4) | 7.44 (3.5–16.7) | <0.001 |
| Last time the flock was affected in the past | ||||
| • Never | 230 (96.2) | 9 (3.8) | Ref | |
| • Within a year | 27 (81.8) | 6 (18.2) | 6.8 (2.6–17.3) | <0.001 |
| • More than a year | 24 (85.7) | 4 (14.3) | 8.0 (3.4–19.1) | <0.001 |
Nineteen households (6.3 %; 95 % C.I. 3.9–9.9) had at least one sero-positive animal, with the majority (n = 14; 73.7 %) in Bauchi and the rest in Kaduna (n = 4; 21.0 %) and Plateau (n = 1; 5.3 %). Most households with at least one positive animal kept sheep and goats (n = 15; 78.9 %); followed by households that held only goats (n = 3; 15.8 %) and households that kept only sheep (n = 1; 5.3 %). Out of the six animals tested per farm, the number of animals positive ranged between 1 and 3. Distribution of putative risk factors and results from the univariate analysis are presented in Table 3. After checking for collinearity (supplementary material Table S4), the following characteristics were taken forward to the multivariable model: State where the household was located, management system, grazing in a communal area, drinking water from a communal source, sharing reproductive males (rams and/or bucks), time since new sheep or goats were brought into the household, de-worm on regular basis and time since the flock or herd was affected with SGP. Table 4 shows the final multivariate model at the farm-level. Bringing new small ruminants into the household and having a history of SGP in the herds were the main factors associated with SGP seroprevalence. Households were less likely to be sero-positive if the time between bringing animals into the household and sampling was over a year (PR = 0.31; 95 % CI 0.08–0.95), while households that had animals affected by SGP in the past were more likely to be sero-positive regardless of the timeframe (PR = 5.58, 95 % CI 2.12–14.23 within a year and PR = 7.47, 95 % CI 3.11–18.21 more than a year).
Table 4.
Results for multivariable analysis for identification of risk factors for SGP sero-positive at household level using a poisson regression model with number of animals positive as outcome variable and number of animals sampled in the household as offset.
| Predictor variables | Adjusted prevalence ratio (95 % C.I.) | P value |
|---|---|---|
| Last time new sheep or goats were bought into the household | ||
| Never | Ref | |
| Within a year | 1.38 (0.61–3.30) | 0.45 |
| More than a year | 0.31 (0.08−0.95) | 0.05 |
| Last time the herd/flock was affected by SGP in the past | ||
| Never | Ref | |
| Within a year | 5.58 (2.12–14.23) | 0.0003 |
| More than a year | 7.47 (3.11–18.21) | <0.001 |
3.3. Intra-cluster correlation (ICC)
The ICC measures the level of similarity (correlation) two individuals have within a cluster (e.g. household, village, LGA, State). ICC values ranged from 0 to 1, with 1 being total correlation (i.e. individuals in a given cluster are identical for the characteristic measured) and 0 being no correlation at all. There were differing degrees of clustering for sero-positive animals at all levels, with the lowest at the State level (ICC = 0.12) and the highest at the household level (ICC = 0.93). LGA and Village level ICC were 0.48 and 0.68 respectively.
3.4. Spatial patterns
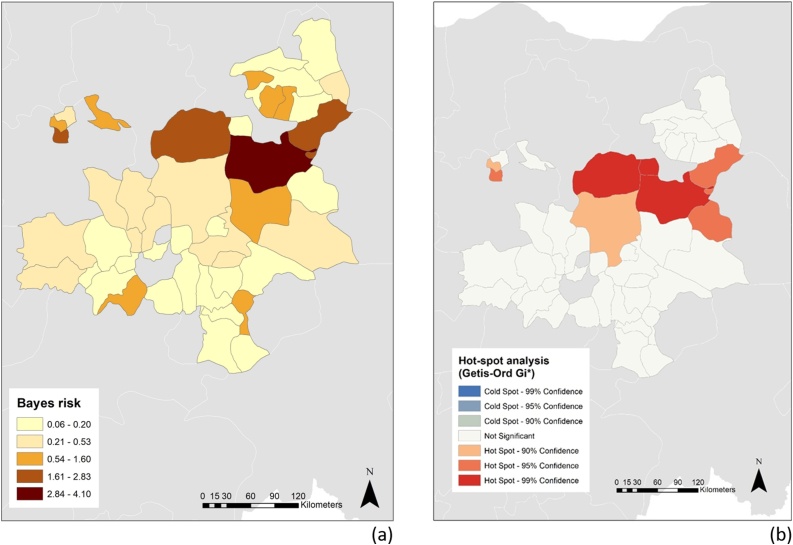
Bayesian approaches allow disease rates to be adjusted through combining the observed rate for an area with rates observed in the surrounding area. Bayes smoothed rate varied across LGA ranging from 0.06 to 4.10 %. Generally increasing from south to north in roughly horizontal bands across the study area, Bayes rate was highest in Ganjuwa (4.1 %) followed by Zaria (2.8 %), Ningi (2.3 %) and Darazo (1.9 %). In contrast, Bayes rate was lowest in the southern and far north-western LGAs (Fig. 3a).
Fig. 3.
Choropleth maps showing (a) Bayes smoothed rate and (b) Getis-Ord Gi* hot-spots of sheeppox and goatpox for the 44 local government areas (LGA) included in the study.
There was significant positive spatial autocorrelation of Bayes smoothed rate at the level of LGA (I = 0.2, p = 0.005) indicating nearby observations were more similar on average than distant ones. Nine of the 44 (20 %) sampled LGA were identified as hot-spots (high risk areas) for SGP antibodies, although with varying degrees of confidence. All hot-spots were in the north-east or north-west of the study area and included Ningi, Warji and Ganjuwa (all in Bauchi State; 99 % confidence), Zaria (Kaduna state), Darazo (Bauchi state), Kirfi (Bauchi State) (95 % confidence), Toro (Bauchi state) and Sabon Gari (Kadina state) (90 % confidence; Fig. 1b). There were no identified cold-spots (low-risk areas).
4. Discussion
This manuscript describes a study conducted in three States of northern Nigeria to understand factors associated with SGP exposure and infection at animal and household levels, using a modified probabilistic sampling. The research is an advance on previous epidemiological studies which were based on convenience sampling and covered small areas within a single State. Importantly, we identified spatial patterns of sero-prevalence, shedding new light on the understanding of SGP epidemiology in this endemic setting.
Apparent weighted sero-prevalence at the animal level was 2.0 % (95 % C.I. 1.1–3.0%), while 33 (1.8 %) of the animals sampled were reported to have SGP clinical signs in the past. True prevalence could not be estimated since sensitivity reported as part of the ELISA test validation was calculated using mainly cattle under controlled conditions, and reports of the test detecting antibodies to sheeppox and goatpox have only been done in an experimental study (Wolff et al., 2020). Sero-positive animals came from a small number of households (19 out of 300), reflected in the high ICC found at household level, which was higher than previously assumed. Moreover, important spatial heterogeneity was found across the study area, which was supported by a significant positive spatial autocorrelation at the LGA level (Moran’s I = 0.2, p = 0.005). Once adjusted for the observed prevalence in the surrounding areas, a cluster of high-risk (hot-spot) LGA was identified in north-east Bauchi suggesting that the probability of exposure in small ruminants kept in backyard/sedentary settings is driven, to a large extent, by the location where animals were kept. It is important to note that some LGAs in parts of Kaduna and Plateau States as well as some villages in Bauchi had to be taken out of the sampling frame due to security reasons; therefore, no information could be collected and the sero-prevalence in these areas remains unknown. SGP seroprevalence might be higher in these areas with limited access and potentially reduced veterinary assistance. In addition, the modified sampling strategy used in this study might have resulted in a sample size with low power. Although we increased our sample size to adjust for the design effect, the parameters used were educated assumptions based on the authors experience given the lack of empirical data, and results from this study showed higher levels of clustering than those used in the sample size calculation. Nonetheless, the approach used in this study was feasible reflecting field conditions and limited data available, allowing us to get an initial understanding on the spatial heterogenicities on SGP sero-prevalence across the study area.
Goats were mainly kept in north west Plateau State due to rustling of sheep and cattle, while sheep are mainly kept in the northern part of Bauchi State due to the religious importance of these animals in this area. Arid and dry regions have been previously identified as agroecological characteristics that increase the risk of SGP infection (Bhanuprakash et al., 2005; Kardjadj, 2017). Similarly, incidence and severity have been reported to be higher during the dry season in Nigeria (Bolajoko et al., 2019). Our results are aligned with these findings as north-eastern Bauchi (the main high-risk area identified in this study) is characterised by semi-desert vegetation (Sahel savannah), whereas more diverse vegetation is found in Kaduna and Plateau where Sudan Savanna and northern Guinea Savanna are found respectively. The reasons for this potential link are not clear. Further studies should be conducted to better understand the degree to which the higher prevalence in arid areas is related to virus survival in this environmental conditions, higher distribution of the host in these agroecological zones, sociological and cultural factors or a combination.
Sero-prevalence of capripoxvirus (CPPV) antibodies in sheep and goats in northern Nigeria was much lower than previously reported in Ethiopia (Fenti et al., 2017). The study in Ethiopia examined the presence of neutralising antibodies to CPPV in sera from 672 small ruminants and found 104 (15.5 %) of animals sero-positive, with a higher proportion of animals sero-positive in particular districts. The animal-level risk factors identified in the Ethiopian study were sex and age, with females and young animals more likely to be sero-positive. Our study identified age and previous disease status (i.e. had had clinical signs of SGP) as the main factors in the final multivariable model. However, older animals, rather than younger animals, were more likely of being sero-positive. Potential reasons for differences between the findings of the two studies include the type of antibodies measured and sampling frame used as part of the study design. The ELISA test (used in this study) detects antibodies against specific immunogenic CPPV antigens, whereas VNT detects antibodies with the ability to neutralise CPPV and is more sensitive than ELISA. Furthermore, the study in Ethiopia sampled small ruminants in an area with a history of CPPV vaccination, therefore sero-positivity could be due to either vaccination or previous infection. In contrast, SGP vaccination has never been conducted in Nigeria, removing this uncertainty from our study. Classification of young animals was different between the two studies, with our study using 1–24 months to define young animals, and Fenti et al. (2017) study using 5–18 months. Finally, differences in farming systems from which the sample frames were drawn (extensive farming in Ethiopia and backyard/sedentary in Nigeria) might have played a role in differing the probability of exposure.
Given that it is not currently possible to distinguish between infection with SPPV or GTPV using serological methods, sero-positive results in the study were considered to be positive to either SPPV or GTPV, with the implicit assumption that risk factors for sero-positivity are the same (and in the same direction) for sheep and goats. However, it is possible that the risk factors would be different if a SPPV or GTPV strain with a strong host preference for either sheep or goats was present.
The majority of the animals with a known history of clinical SGP were sero-negative (28 out of 33; 84.8 % small ruminants); while those small ruminants that were sero-positive (with previous history of SGP) (5 out of 33; 15.2 %) were reported to have been affected between 6 and 12 months prior to sampling. This suggests that there is a specific timeframe post-disease when antibodies are detectable by the ELISA test. However, it is important to note that our study is only snap-shot in time that relies on farmers’ memory of disease history as health parameters are not normally recorded systematically by subsistence farmers. It is also possible that SGP affected animals had died before the study visit. Further longitudinal studies with serial sampling following infection are needed to better understand the immune response to SPPV/GPPV following infection in endemic settings.
Similarly, at the herd level 20 % (61/300) of farmers reported having a SGP outbreak in their flock in the past suggesting SGP was a common problem in the study area, especially in Bauchi where 40 out of 100 households across LGA reported being previously affected. Nine out of 61 household (14.7 %) with previous SGP history had at least one sero-positive animal. In contrast, 9/239 (3.8 %) of farmers that had not experienced an outbreak in their flock in the past had at least one animal sero- positive. Some of these animals might have had mild disease and went unnoticed, or some animals could have had the disease before joining the flock. These differences reflect the characteristics and dynamics of local farming, with households frequently selling disease animals to reduce losses and buying animals without knowing their disease status, resulting in high turnover of small ruminants.
All sero-positive animals that had not been born in the household came from livestock markets. Previous studies in northern Nigeria found that animals with SGP lesions were frequently sold in livestock markets (Bolajoko et al., 2019; Limon et al., 2020), with the markets acting as hubs of infection for SGP, and potentially for a range of other transboundary diseases. The low number of sero-positive animals in this study and large number of livestock markets in the area precluded more detailed analysis. Further studies should be conducted to better understand the role of livestock markets on SGP transmission (and other potential infectious diseases) in Nigeria.
At the household level, the main risk factors identified were past history of a SGP outbreak in the flock and introduction of new small ruminants into the flock, both common risk factors for many infectious diseases. However, there were important differences depending on the time animals were brought into the household prior to sampling. Higher prevalence ratio was found if animals had been introduced within the past year and a lower prevalence ratio if more than a year had passed. This may be related to antibody levels waning over time, reducing the ability to be detected. In contrast, households with history of clinical disease in their flock were more likely of being sero-positive regardless of the time frame. It is important to note that the putative confounders considered were not an exhaustive list and so other confounders that were not recorded in this study might also play a role.
The wide confidence intervals in the animal and household level models reflect the small number of sero-positive animals clustered in few households. Therefore, slight changes on risk factors categories might have an impact on the observed results, especially the strength of association. Similarly, further validation of the ELISA test for small ruminants might result in different classification of positive animals, highlighting the importance of conducting field studies to validate laboratory test.
A limitation of this study is that only backyard/sedentary farmers were studied, and some management practices were fairly homogenous across these farmers. This might not have been the case if different types of farmers (e.g. transhumance and/or commercial farmers) with potentially different management practices had been included in the study. Nonetheless, given the lack of field epidemiological studies in endemic settings, the results and parameters estimated here provide inputs that can be used to parametrise economic analyses in Nigeria and other settings with similar characteristics, and can be used to plan further epidemiological and interventions studies.
Our results suggest that monitoring exposure and infection of SGP in this part of Nigeria would be more reliable at the household rather than animal level. Importantly, combining farmers’ information on previous outbreaks in their flocks with risk-based serological surveillance could be an efficient approach for disease monitoring in northern Nigeria.
Results from this study confirm that SGP is endemic in the study area. Once the overall impact is quantified, SGP might be considered as a priority disease among other transboundary animal diseases known to be present such as foot-and-mouth disease (FMD) and peste des petits ruminants (PPR). Integrated control policies for transboundary animal diseases could be a cost-effective approach that should be considered in Nigeria.
5. Conclusions
In conclusion, our results showed SGP was present in the study area with important geographic heterogeneity on the probability of exposure and infection. Results from this study can be used to plan and design risk-based surveillance and intervention programmes (such as vaccination) to reduce and control SGP in the area.
Funding
This study was funded by National Veterinary Research Institute in Nigeria and the Biotechnology and Biological Sciences Research Council (BBSRC) responsive mode research grant BB/R008833/1, as well as strategic funding from the BBSRC to the Pirbright Institute (BBS/E/I/00007036) and the Roslin Institute (BBS/E/D/20002173).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors wish to acknowledge the contributions of the following persons during data collection and samples testing: Dyek Yohana, Dyek Adrian Maguda, Abbas Waziri and Ibrahim Jakawa (National Veterinary Research Institute, Vom, Nigeria). The authors particularly thank farmers for their invaluable support.
Finally, thanks to Dr Simon Gubbins for advice on the Bayes smooth rate equations.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.prevetmed.2021.105473.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adedeji A.J., Moller J., Meseko C.A., Adole J.A., Tekki I.S., Shamaki D., Hoffmann B. Molecular characterization of Capripox viruses obtained from field outbreaks in Nigeria between 2000 and 2016. Transbound. Emerg. Dis. 2019:1–11. doi: 10.1111/tbed.13197. [DOI] [PubMed] [Google Scholar]
- Babiuk S., Bowden T.R., Boyle D.B., Wallace D.B., Kitching R.P. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound. Emerg. Dis. 2008;55:263–272. doi: 10.1111/j.1865-1682.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- Bhanuprakash V., Moorthy A.R.S., Krishnappa G., Srinivasa Gowda R.N., Indrani B.K. An epidemiologiacal study of sheep pox infection in Karnataka state, India. Rev. Sci. Tech. Off. Int. Epiz. 2005;24:909–920. [PubMed] [Google Scholar]
- Bolajoko M.B., Adedeji A.J., Dashe G.D., Òsemeke O.H., Luka P.D. Molecular epidemiology and economic impact of goat pox on small holder sheep and goats farmers in north central Nigeria. Small Rumin. Res. 2019;179:75–78. [Google Scholar]
- Boshra H., Truong T., Nfon C., Rowden T.R., Gerdts V., Tikoo S., Babiuk L.A., Kara P., Mather A., Wallace D.B., Babiuk S. A lumpy skin disease virus deficient of an IL-10 gene homologue provides protective immunity against virulent capripoxvirus challenge in sheep and goats. Antiviral Res. 2015;123:39–49. doi: 10.1016/j.antiviral.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Boumart Z., Daouam S., Belkourati I., Rafi L., Tuppurainen E., Omari Tadlaoui K., El Harrak M. Comparative innocuity and efficacy of live and inactivated sheeppox vaccines. BMC Vet. Res. 2016;12 doi: 10.1186/s12917-016-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden T.R., Babiuk S.L., Parkyn G.R., Copps J.S., Boyle D.B. Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology. 2008;371:380–393. doi: 10.1016/j.virol.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esri Inc . 2016. ArcMap (version 10.5.1). Software. Redlands, CA. [Google Scholar]
- FAO . Food and Agriculure Organization of the United Nations; Rome, Italy: 2019. The Future of Livestock in Nigeria. Opportunities and Challenges in the Face of Uncertainty. [Google Scholar]
- Fentie T., Fenta N., Leta S., Molla W., Ayele B., Teshome Y., Nigatu S., Assefa A. Sero-prevalence, risk factors and distribution of sheep and goat pox in Amhara Region, Ethiopia. BMC Vet. Res. 2017;13:385. doi: 10.1186/s12917-017-1312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambo P., Maguda A.S., Adole J.A., Dyek D.Y., Ifende V.I., Bot C., Adedeji A.J. A survey of viral diseases of livestock characterized by skin lesions in Kanam local government area of plateau State, Nigeria. Niger. Vet. J. 2018;39:250–262. [Google Scholar]
- Ifende V.I., Maurice N.A., Abbas Y., Agu C., Bolajoko M.B., Jambol A., Adole J.A., Asala O., Wungak Y.S., Maguda A., Umeh E., Adedeji A.J. A retrospective study of viral skin diseases of cattle, sheep and goats in Plateau State, Nigeria. Sokoto J. Vet. Sci. 2019;17:49–55. [Google Scholar]
- Kardjadj M. Prevalence, distribution, and risk factor for sheep pox and goat pox (SPGP) in Algeria. Trop. Anim. Health Prod. 2017;49:649–652. doi: 10.1007/s11250-017-1220-0. [DOI] [PubMed] [Google Scholar]
- Limon G., Gamawa A.A., Ahmed A.I., Lyons N.A., Beard P.M. Epidemiological characteristics and economic impact of lumpy skin disease, sheeppox and goatpox among subsistence farmers in northeast Nigeria. Front. Vet. Sci. 2020 doi: 10.3389/fvets.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D.U., Robinson T.P., Stevenson M., Stevens K.B., Rogers D.J., Clements A.C.A. Oxford University Press; Oxford: 2008. Spatial Variation in Risk. [Google Scholar]
- Pham T.H., Lila M.A.M., Rahaman N.Y.A., Lai H.L.T., Nguyen L.T., Van Do K., Noordin M.M. Epidemiology and clinico-pathological characteristics of current goat pox outbreak in Northern Vietnam. BMC Vet. Res. 2020;16 doi: 10.1186/s12917-020-02345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T., Bandyopadhyay S. A comprehensive review of goat pox and sheep pox and their diagnosis. Anim. Health Res. Rev. 2001;1:127–136. doi: 10.1017/s1466252300000116. [DOI] [PubMed] [Google Scholar]
- Tuppurainen E.S.M., Venter E.H., Shisler J.L., Gari G., Mekonnen G.A., Juleff N., Lyons N.A., De Clercq K., Upton C., Bowden T.R., Babiuk S., Babiuk L.A. Review: capripoxvirus diseases: current status and opportunities for control. Transbound. Emerg. Dis. 2017;64:729–745. doi: 10.1111/tbed.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., King J., Moritz T., Pohlmann A., Hoffmann D., Beer M., Hoffmann B. Experimental infection and genetic characterization of two different capripox virus isolates in small ruminants. Viruses. 2020;12:1098. doi: 10.3390/v12101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.