Abstract
Introduction:
Oncoplastic breast-conserving surgery (OBCS) is considered a cornerstone in the management of locally invasive breast cancer. We evaluated patient-reported outcomes of OBCS with contralateral balancing breast reduction mammoplasty and reviewed its oncologic outcomes and complications.
Methods:
This is mixed method study design using retrospective chart review and prospective cohort study. Patient demographics were reviewed. Outcome measures included clinicopathologic characteristics, complications, margin status, local recurrence, tumor histopathologies, duration of follow-up, patient satisfaction, self-esteem, event-related stress, and quality of life.
Results:
A total of 48 patients were included in this study. Complete excision with negative margins was obtained in 42 (87.5%) patients, positive margins in 6 (12.5%) patients, all who had re-excision with repeat lumpectomy. Thirteen patients developed minor complications, defined as being managed as an outpatient. No patients developed major complications requiring inpatient admission. These complications did not delay commencement of chemotherapy or radiotherapy. Postsurgery BREAST-QTM26 scores demonstrated no statistical difference in satisfaction with breasts, nipples, and sexual well-being. There was high satisfaction with overall outcome with average score of 80.8%. For the Rosenberg self-esteem scale, the results were similar for 3- and 12-month post-operative indicating maintenance of normal self-esteem post-operatively. The Impact of Events Scale showed statistically significant difference at 12-month post-operative (25.1) when compared with preoperative scores indicating that patients had lower event-related stress. There was no significant change in Hospital Anxiety and Depression Scale.
Conclusion:
Our study has shown that the patient who undergo OBCS have high patient-reported outcomes with acceptable oncologic outcomes and complication rates.
Keywords: breast cancer, breast-conserving surgery, breast reconstruction, oncoplastic breast-conserving surgery, oncoplastic reconstruction
Abstract
Introduction:
La chirurgie oncoplastique de conservation mammaire (COCM) est considérée comme un pilier de la prise en charge du cancer du sein localement invasif. Les chercheurs ont évalué le pronostic de COCM déclaré par les patientes après une mammoplastie de réduction avec symétrie controlatérale et ont analysé les résultats oncologiques et les complications.
Méthodologie:
Dans la présente étude à méthodologie mixte, les chercheurs ont privilégié une analyse rétrospective de dossiers et une étude de cohorte prospective. Les chercheurs ont examiné la démographie des patients. Les mesures de résultat incluaient les caractéristiques clinicopathologiques, les complications, l’état des marges, les récurrences locales, l’histopathologie des tumeurs, la durée du suivi, la satisfaction des patientes, l’estime de soi, le stress lié à l’événement et la qualité de vie.
Résultats:
Au total, 48 patientes ont participé à l’étude. De ce nombre, 42 (87,5 %) ont subi une excision complète aux marges négatives, et six (12,5 %), une excision complète aux marges positives et toutes ont subi réexcision avec une reprise de la lumpectomie. Treize patientes ont développé des complications mineures, définies comme prises en charge dans un contexte ambulatoire. Aucune patiente n’a développé de complications majeures exigeant une hospitalisation. Les complications n’ont pas retardé le début de la chimiothérapie ou de la radiothérapie. Les scores BREAST-QTM26 après l’opération n’ont démontré aucune différence statistique pour ce qui est de la satisfaction envers les seins, les mamelons et le bien-être sexuel. Les patientes avaient un taux de satisfaction élevé à l’égard des résultats globaux, pour un score moyen de 80,8 %. Sur l’échelle d’estime de soi de Rosenberg, le maintien de l’estime de soi normal était semblable trois et 12 mois après l’opération. L’échelle d’effet des événements a révélé une différence statistiquement significative 12 mois après l’opération (25,1) par rapport aux scores avant l’opération, indiquant que les patientes ressentaient moins de stress envers l’événement. Il n’y avait pas de changement significatif dans l’échelle d’anxiété et de dépression à l’hôpital.
Conclusion:
La présente étude a révélé que la patiente qui subit une COCM déclare des résultats très positifs, dont les pronostics oncologiques et le taux de complications sont acceptables.
Introduction
Breast reconstruction and the preservation of breast appearance post breast cancer treatment correlates with better psychosocial outcomes. 1 -4 Breast-conserving therapy (BCT), which includes wide local excision of the tumor (or lumpectomy) followed by irradiation, has become a standard of care in the management of early-stage invasive breast cancer. 4,5 An important secondary goal is a satisfactory cosmetic outcome as this is associated with both patient satisfaction and improved quality of life. 6,7 Multiple long-term randomized controlled trials have shown survival following BCT to be equivalent to that of mastectomy. 8 -11 Although the standard lumpectomy using breast-conserving techniques may result in very minor asymmetry, they can also result in large defects that leave the breast distorted. Current literature reports incidence of unfavorable aesthetic results following BCT to affect up to 40% of patients and has shown to affect patients’ psychosocial and quality of life. 4,5,12,13 Oncoplastic breast conservation surgery (OBCS) is a type of BCT, which involves combining the latest plastic surgery techniques with breast surgical oncology. When a large lumpectomy is required, the remaining tissue is sculpted to realign the nipple and areola and restore a natural appearance to the breast shape. The contralateral breast is also modified to create symmetry. 14 -16 There are 3 ways this can be achieved, through simple reduction, rearrangement of internal breast parenchymal tissue or replacing by using local or distant flap to reconstruct the defect. Studies in Europe and Asian countries have demonstrated many benefits to immediate oncoplastic reconstruction including single surgery, surgery completion prior to radiation, which decreases the risk of wound healing problems, immediate symmetry of breast after lumpectomy, and relief of symptoms of macromastia. 6,17 -24 Despite these benefits there are limited data on outcomes of oncoplastic reconstruction in North America. 4,25 The goal of our study was to review patient-reported outcomes of OBCS with contralateral balancing breast reduction mammoplasty, complications, oncologic outcomes, and patient satisfaction.
Methods
This was a mixed method study using retrospective chart review and a prospective cohort study. The study population involved patients 18 years of age or older, who underwent OBCS and contralateral balancing breast reduction mammoplasty with a multidisciplinary approach at the Ottawa Hospital during the period of September 2014 to December 2017. Data collection included retrospective chart review from September 2015 to October 2016. Patients were recruited onward and data and outcome measures were collected prospectively. Approval from the Ottawa Health Science Network Research Ethics Board was obtained.
Patient demographics included age, body mass index (BMI), course of disease, past medical history, type of procedure, and reduction technique. Outcome measures included clinicopathologic characteristics, complications, margin status, local recurrence, tumor histopathologies, duration of follow-up, patient satisfaction, self-esteem, event-related stress, and quality of life.
Patient-reported outcomes and satisfaction were collected using questionnaires, which were given preoperatively, then at 3-month and 12-month post-operatively. The questionnaires included BREAST-QTM, 26 Rosenburg Self-Esteem Scale, 27 Impact of Events Scale (IES), 28 and Hospital Anxiety and Depression Scale (HADS). 29
A total scale score of BREAST-QTM was calculated through the QScore software, ranging from 0 to 100, with a higher score meaning better quality of life or higher satisfaction. Rosenburg Self-Esteem Scale was measured using a 10-item scale that measures global self-worth by measuring both positive and negative feelings about the self. All items are answered using a 4-point Likert scale format ranging from strongly agree to strongly disagree, a score less than 15 may indicate problematic low self-esteem. The IES is rated on a 5-point scale ranging from 0 (“not at all”) to 4 (“often”). The IES scale consists of 15 items, 7 of which measure intrusive symptoms (intrusive thoughts, nightmares, intrusive feelings, and imagery), 8 avoidance symptoms (numbing of responsiveness, avoidance of feelings, situations, ideas), and combined, provide a total subjective stress score. The scale is intended to be helpful in detecting the effect of the most severe impact events, and those that can leave patients having post-traumatic stress disorder. Cutoff point of above 26 is considered moderate or severe impact. The HADS is a 14-item scale, 7 of the items relate to anxiety and 7 relate to depression. Each item on the questionnaire is scored from 0 to 3 and this means that a person can score between 0 and 21 for either anxiety or depression.
Statistical Analysis
We characterized the sample using descriptive statistics. Outcome measures at 3 different time points (preoperative, 3 months post-operative and 12 months post-operative) were compared using 1-way analysis of variance with repeated measures and paired sample t test for 2 different time points (3-month and 12-month post-operative). Significance level was set at P < .05. Data were expressed as mean and standard deviation for the entire sample.
Results
During October 2014 to December 2017, a total of 48 patients underwent OBCS and contralateral balancing reduction mammoplasty. The mean age was 56 (range, 36-83years), with a BMI 29.11 kg/m 2 (range, 19.6-42.3 kg/m 2 ), over a mean follow-up of 72.5 weeks (range, 1-260 weeks; Table 1). Complete excision with negative margins was obtained in 42 (87.5%) patients, positive margins in 6 (12.5%) patients, all who had re-excision with repeat lumpectomy. No patients had local recurrence. The most common tumor histopathologies were invasive ductal carcinoma 28 (58.3%), followed by ductal carcinoma in situ (DCIS) 8 (16.6%), and invasive lobular carcinoma 6 (12.5%).
Table 1.
Demographic and Clinical Characteristics.
| Variables | No. of patients (%) |
|---|---|
| Patients | 48 (100) |
| Age, year | |
| Mean | 56 |
| Range | 36-83 |
| BMI, kg/m 2 | |
| Average | 29.11 |
| Range | 19.6-42.3 |
| Margin status | |
| Positive | 6 (12.5) |
| Negative | 42 (87.5) |
| Smokers | 5 (10.41) |
| Comorbidities | |
| Hypertension | 10 (20.83) |
| Asthma | 3 (6.28) |
| Thyroid disease (hypo/hyperthyroidism, goiter) | 6 (12.50) |
| Hypercholesterolemia | 5 (10.41) |
| Obesity | 15 (31.25) |
| Diabetes mellitus | 2 (4.16) |
| Stage | |
| I | 3 (6.28) |
| II | 17 (35.4) |
| III | 18 (37.5) |
| IV | 2 (4.16) |
| I and II | 1 (2.08) |
| II and III | 1 (2.08) |
| NA | 4 (8.33) |
| Tumor histopathologies | |
| Invasive ductal carcinoma | 28 (58.33) |
| DCIS | 8 (16.66) |
| Invasive lobular carcinoma | 6 (12.50) |
| Invasive mammary carcinoma | 3 (6.25) |
| Benign phyllodes tumor | 1 (2.08) |
| Intraductal papilloma | 1 (2.08) |
| Pleomorphic LCIS | 1 (2.08) |
| Local recurrence | 0 |
| Lumpectomy size—documented | 27 (56.25) |
| Mean | 426 g |
| Range | 50-1090 g |
| Lumpectomy size—NA | 21 (43.75) |
| Reduction size | |
| Mean | 472 g |
| Range | 50.7-1500 g |
Abbreviations: BMI, body mass index; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ; NA, not available.
Grade II and III disease were the most common, 17 (35.4%) and 18 (37.5%), respectively. A total of 43 patients were administered adjuvant radiation, 35 patients were administered hormonal therapy and 24 patients were administered adjuvant chemotherapy (Table 2). Patients began chemotherapy mean of 49.7 days following their surgery (range, 34-72 days). Four (8.3%) patients had reported delays in commencement of chemotherapy. This was due to patient choice or uncertainty about proceeding with chemotherapy in 3 patients and only one patient had delay due to surgical complication with bilateral breast cellulitis. Patients started radiotherapy mean of 114.1 days following surgery (range, 34-231 days). Patients who received chemotherapy began their radiation generally one month following last cycle of chemotherapy. No patients had reported delay in radiation due to surgical complications. Two patients had delays in commencement of radiation due to patient choice and genetic testing.
Table 2.
Summary of Therapy.
| Therapy | No. of patients |
|---|---|
| Neoadjuvant chemotherapy | 8 |
| Adjuvant chemotherapy | 24 |
| Neoadjuvant radiation | 0 |
| Adjuvant radiation | 43 |
| Hormonal therapy | 35 |
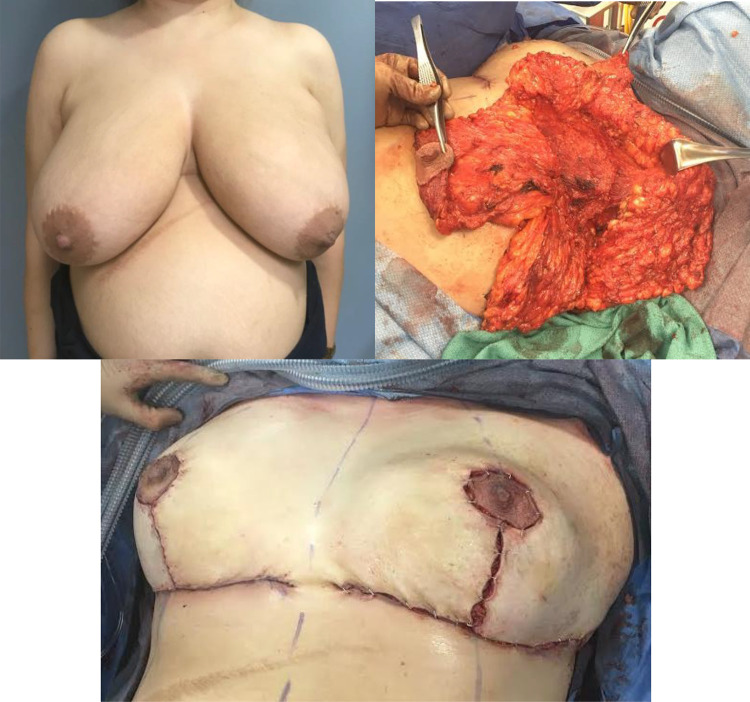
The mean lumpectomy size weighed 426 g and reduction size weighed 472 g with largest size, respectively, weighing 1090 g and 1500 g. Figure 1 demonstrated a large lumpectomy oncoplastic reconstruction. Most common pedicles for nipple areolar complex were superior medial pedicle (35.4%) and inferior pedicle (35.4%) on oncoplastic side and superior medial pedicle on the contralateral side (68.2%).
Figure 1.
Large lumpectomy with oncoplastic reconstruction.
Thirteen patients developed minor complications, defined as being managed as an outpatient. No patients developed major complications requiring inpatient admission. Of the minor complications, 7 (14.5%) were wound infections treated with outpatient antibiotics. Some of these were diagnosed by ED physician or family physician and a smaller number were diagnosed by primary surgeon. Five (10.4%) patients developed seroma that was aspirated as outpatient, and 6 (12.5%) had minor wound dehiscence that were treated with dressings and healed by secondary intention. These complications were immediate post-operative stage. Only one patient had delay in commencement of chemotherapy due to surgical complication, and no patients had delay in their radiation due to these minor complications.
Patient-reported outcomes and satisfaction questionnaires were given to 18 patients. At 3-month post-operative, 18 patients had returned their questionnaires which is a response rate of 100%. At 12-month post-operatively, 16 patients returned their questionnaires, a response rate of 83.8%. Our results for BREAST-QTM26 demonstrate no statistical significant difference in satisfaction with breasts, nipples, and sexual well-being (P = .522). Similarly, there was no statistically significant difference in physical well-being of the chest and psychosocial well-being (P = .117). There was high satisfaction with overall outcome with average score of 80.8%. Women also reported satisfaction with care with average scores of 82.7% for surgeon, 93.7% for medical staff, and 99.4% for office staff (Table 3). Figure 2 demonstrated preoperative and post-operative photos of a patient 6 months following completion of radiotherapy.
Table 3.
BREAST-Q Questionnaire Scores Mean (Standard Deviation).
| Items | Preoperative score | Post- operative 3-month score | Post- operative 12-month score |
|---|---|---|---|
| Satisfaction with breasts | 50.4 (18.4) | 66.4 (25.7) | 71.4 (19.4) |
| Satisfaction with outcome | 78.4 (19.5) | 80.8 (18.6) | |
| Psychosocial well-being | 70.1 (18.0) | 82.2 (20.1) | 76.2 (18.3) |
| Physical well-being: Chest | 74.1 (12.2) | 71.3 (15.6) | 67.2 (20.8) |
| Sexual well-being | 47.9 (28.8) | 63.8 (27.9) | 55.5 (27.6)a |
| Satisfaction with nipples | 75.9 (35.2) | 80.0 (23.3) | |
| Satisfaction with information | 73.1 (18.4) | 73.9 (17.5) | |
| Satisfaction with surgeon | 82.9 (20.2) | 82.7 (21.3) | |
| Satisfaction with medical staff | 91.5 (17.5) | 93.7 (14.9) | |
| Satisfaction with office staff | 97.4 (11.1) | 99.4 (2.3) |
a P < .05.
Figure 2.
Oncoplastic reconstruction with contralateral reduction mammaplasty preoperative (above) and 6 months post radiation (below).
For the Rosenberg Self-Esteem 27 Scale, the results were similar for 3- and 12- months post-operative, with an average score of 25.3 and 25.4, respectively; indicating normal and maintenance of self-esteem post-operatively (P > .05; Table 4). The IES 28 showed statistically significant difference at 3 and 12 months post-operative when compared with preoperative scores, preoperative (41.7) versus 3-month post-operative (27.2) and 12-month post-operative (25.1); P < .003. The results indicate that patients had lower event-related stress (Table 4). There was no statistically significant change in HADS (P > .05; Table 4).
Table 4.
Patient-Reported Outcomes.
| Outcome measures | Mean (SD) |
|---|---|
| Rosenberg Self-Esteem Scale | |
| Post-operative 3-month | 25.3 (2.4) |
| Post-operative 12-month | 25.4 (1.8) |
| Impact of Events Scale | |
| Preoperative | 41.7 (16.2) |
| Post-operative 3-month | 27.2 (15.0) |
| Post-operative 12-month | 25.1 (18.7)a |
| Hospital Anxiety and Depression Scale | |
| Preoperative | 27.8 (5.14) |
| Post-operative 3-month | 30.2 (8.7) |
| Post-operative 12-month | 29.8 (6.8) |
Abbreviation: SD, standard deviation.
a P < .05.
Discussion
Breast-conserving therapy is considered a cornerstone in the management of locally invasive breast cancer, aiming to create a breast with a natural shape and symmetry. The patient experience is crucial; key indicators such as patient satisfaction and quality of life have become important outcomes for evaluating the success of OBCS.
In our study, OBCS demonstrated oncological safety comparable to the literature, good patient satisfaction, and psychosocial outcomes post-operatively. Complete excision with negative margins was obtained in 42 (87.5%) patients, positive margins in 6 (12.5%) patients, all who had re-excision with repeat lumpectomy. Breast-conserving therapy has become a standard of care in the management of early-stage invasive breast cancer. Women in our study had lower grade disease with grade II and III disease most common, 17 (35.4%) and 18 (37.5%), respectively. Patients who undergo OBCS generally have day surgery with low rates of complications. Thirteen patients developed minor complications, defined as being managed as an outpatient. No patients developed major complications requiring inpatient admission. Patients avoid the risk of associated with prosthesis or longer recovery with autologous free flap reconstruction.
At our institute, patients are referred to radiation and medical oncology immediately following breast-conserving surgery. Patients who are candidates and elect to proceed with chemotherapy begin this followed by radiation therapy approximately one month after the completion of their chemotherapy. Patients in our study began chemotherapy mean of 49.7 days and radiotherapy a mean of 114.1 days following their surgery. Four (8.3%) patients had reported delays in commencement of chemotherapy. This was due to patient choice or uncertainty about proceeding with chemotherapy in 3 patients and only one patient had delay due to surgical complication with bilateral breast cellulitis. No patients had reported delay in radiation due to surgical complications. Two patients had delays in commencement of radiation due to patient choice and genetic testing.
Cancer Care Ontario reports that the optimal sequencing of chemotherapy and radiotherapy is unknown. 30 They report that it is reasonable to start radiotherapy after the completion of chemotherapy, or concurrently if anthracycline-containing regimens are not used. 30 At our institute, women begin with chemotherapy and proceed with radiation generally one month following their last cycle of chemotherapy. Cancer Care Ontario also recommends women with early stage (stage I and II) breast cancer who have undergone breast-conserving surgery should be offered post-operative breast irradiation and begin this as soon as possible following wound healing. 30 They suggest it be reasonable to start breast irradiation within 12 weeks of definitive surgery, but that the safe interval between surgery and the start of radiotherapy is unknown. 30 The mean time from surgery to radiation was slightly greater than 12 weeks in our study which can be explained by the sequencing of chemotherapy completion prior to initiation of radiation.
Our results for BREAST-QTM26 demonstrate no statistically significant difference in satisfaction with breasts, nipples, and sexual well-being. There was also no statistically significant difference in satisfaction with physical well-being of the chest and psychosocial well-being. This is not surprising as women are at the start of their cancer journey and usually undergo radiation and sometimes chemotherapy following their OBCS. There was high satisfaction with overall outcome with average score of 80.8%. Women also reported satisfaction with care with average scores of 82.7% for surgeon, 93.7% for medical staff, and 99.4% for office staff. The Rosenberg Self-Esteem 27 Scores indicated maintenance and normal self-esteem at 3- and 12-month post-operatively. The IES 28 showed statistically significant difference at 3 and 12 months post-operative when compared with preoperative scores. The results indicate that patients had lower event-related stress the farther they were out from surgery.
There is increasing evidence that OBCS offer patients safe and effective oncological outcomes. In a systematic review, the authors revealed overall low local recurrence, distant recurrence, positive margin rate, re-excision rate, and complication rates, thus endorsing the oncologic safety of OBCS in T1 to T2 invasive breast cancer patients. 31 Chakravorty et al compared the re-excision and local recurrence rates for OBCS with standard BCS and found that OBCS decreased the rates of both oncological outcomes (2.7%). 21 Clough et al found that oncoplastic techniques allow larger resections, and a recurrence rate of 9% was reported. 14 Kaur et al reported a re-excision rate of 16%, 32 Reitjens et al reported local recurrence rate of 3%, 22 Fitoussi et al reported a local recurrence rate of 6.8%, 33 and Chauhan et al reported no recurrence rate when compared with standard BCS. 20
Many studies have agreed that oncoplastic reconstruction has improved patient satisfaction, psychosocial well-being, and quality of life. 5,7,13 A systematic review suggested that patients were satisfied with breast reconstruction regardless of the technique used. 1 A study in United States reported that OBCS increased in the percentage (from 4% to 15%) between 2007 and 2014 of all breast cancer surgeries performed and accounted for more than 33% of all breast conservation surgeries. 34
The first international consensus conference on standardization of OBCS was recently published in 2017. 35 The experts considered OBCS safe and effective for improving aesthetic outcomes. A slim majority believed that OBCS can be used to reduce the rate of positive margins; however, there was consensus that OBCS may increase risk of complications compared to standard BCS. The experts supported the statement that OBCS procedure should be tailored to each individual patient. 35
Timing of contralateral symmetrizing procedures is variable in the literature. In our study, we performed contralateral symmetrizing simultaneously with the breast reconstruction. Similarly, in one study, it was reported that they performed simultaneous contralateral reduction mastopexy in 67% of OBCS. 19
North America is behind in adopting oncoplastic breast surgery and in training its surgeons to perform breast reconstruction when compared with Europe. 3,4,25 Canada has been slow in its clinical uptake of OBCS compared with the rest of the international community. The majority of breast cancer surgery in Ontario is performed by general surgeons in community hospitals (70%). General surgeons with no identified subspecialty perform 69% of breast cancer operations followed by subspecialty breast surgeons and surgical oncologists. A cross-sectional survey of Ontario general surgeons examined the use of oncoplastic techniques in BCS and concluded that lack of training and access to plastic surgeons were considered significant barriers to the adoption of oncoplastic techniques. 4
Conclusion
Our study has shown that the patient who undergo OBCS have high patient-reported outcomes with acceptable oncologic outcomes and complication rates. This is safe, well-tolerated and provides good cosmetic outcomes to be performed with contralateral balancing breast reduction mammoplasty.
Footnotes
Authors’ Note: Ottawa Health Science Network Research Ethics Board (OHSN-REB) protocol #20180004-01H. Approved September 1, 2018. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hannah St Denis-Katz https://orcid.org/0000-0002-8980-2370
Bahareh B. Ghaedi, MSc https://orcid.org/0000-0001-8936-7287
References
- 1. Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast. 2007;16(6):547–567. [DOI] [PubMed] [Google Scholar]
- 2. Kelsall JE, McCulley SJ, Brock L, Akerlund MTE, Macmillan RD. Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: case-matched patient reported outcomes. J Plast Reconstr Aesthet Surg. 2017;70(10):1377–1385. [DOI] [PubMed] [Google Scholar]
- 3. Peiris L, Olson D, Kelly D. Oncoplastic and reconstructive breast surgery in Canada: breaking new ground in general surgical training. Can J Surg. 2018;61(5):294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maxwell J, Roberts A, Cil T, Somogyi R, Osman F. Current practices and barriers to the integration of oncoplastic breast surgery: a Canadian perspective. Ann Surg Oncol. 2016;23(10):3259–3265. [DOI] [PubMed] [Google Scholar]
- 5. Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer (Dove Med Press). 2017;9:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cochrane RA, Valasiadou P, Wilson AR, Al-Ghazal SK, Macmillan RD. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg. 2003;90(12):1505–1509. [DOI] [PubMed] [Google Scholar]
- 7. Pirro O, Mestak O, Vindigni V, et al. Comparison of patient-reported outcomes after implant versus autologous tissue breast reconstruction using the BREAST-Q. Plast Reconstr Surg Glob Open. 2017;5(1):e1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332(14):907–911. [DOI] [PubMed] [Google Scholar]
- 9. Lichter AS, Lippman ME, Danforth DN, Jr, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the national cancer institute. J Clin Oncol. 1992;10(6):976–983. [DOI] [PubMed] [Google Scholar]
- 10. Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the national cancer institute randomized trial. Cancer. 2003;98(4):697–702. [DOI] [PubMed] [Google Scholar]
- 11. Straus K, Lichter A, Lippman M, et al. Results of the national cancer institute early breast cancer trial. J Natl Cancer Inst Monogr. 1992;(11):27–32. [PubMed] [Google Scholar]
- 12. Haloua MH, Krekel NM, Winters HA, et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg. 2013;257(4):609–620. [DOI] [PubMed] [Google Scholar]
- 13. Thiessen FEF, Tjalma WAA, Tondu T. Breast reconstruction after breast conservation therapy for breast cancer. Eur J Obstet Gynecol Reprod Biol. 2018;230:233–238. [DOI] [PubMed] [Google Scholar]
- 14. Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003;237(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baildam AD. Oncoplastic surgery of the breast. Br J Surg. 2002;89(5):532–533. [DOI] [PubMed] [Google Scholar]
- 16. de Andrade Urban C. New classification for oncoplastic procedures in surgical practice. Breast. 2008;17(4):321–322. [DOI] [PubMed] [Google Scholar]
- 17. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–1150. [DOI] [PubMed] [Google Scholar]
- 18. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. [DOI] [PubMed] [Google Scholar]
- 19. De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77. [DOI] [PubMed] [Google Scholar]
- 20. Chauhan A, Sharma MM, Kumar K. Evaluation of surgical outcomes of oncoplasty breast surgery in locally advanced breast cancer and comparison with conventional breast conservation surgery. Indian J Surg Oncol. 2016;7(4):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398. [DOI] [PubMed] [Google Scholar]
- 22. Rietjens M, Urban CA, Rey PC, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast. 2007;16(4):387–395. [DOI] [PubMed] [Google Scholar]
- 23. Shekhawat L, Busheri L, Dixit S, Patel C, Dhar U, Koppiker C. Patient-reported outcomes following breast reconstruction surgery and therapeutic mammoplasty: prospective evaluation 1 year post-surgery with BREAST-Q questionnaire. Indian J Surg Oncol. 2015;6(4):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meretoja TJ, Svarvar C, Jahkola TA. Outcome of oncoplastic breast surgery in 90 prospective patients. Am J Surg. 2010;200(2):224–228. [DOI] [PubMed] [Google Scholar]
- 25. Khayat E, Brackstone M, Maxwell J, et al. Training Canadian surgeons in oncoplastic breast surgery: where do we stand? Can J Surg. 2017;60(6):369–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg M. Society and the Adolescent Self-Image. Princeton University Press; 1965. [Google Scholar]
- 28. Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–218. [DOI] [PubMed] [Google Scholar]
- 29. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 30. Whelan TJ, Rumble B, Lada B, et al. Breast irradiation in women with early stage invasive breast cancer following breast conserving surgery. Fletcher GG, Dayes IS, reviewers. Cancer Care Ontario; 2011 Sept 15 [Ed & Info 2016 October 11]. Program in Evidence-based Care Evidence-Based Series No.: 1-2 Version 2 Education and Information 2016. [Google Scholar]
- 31. De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann Surg Oncol. 2016;23(10):3247–3258. [DOI] [PubMed] [Google Scholar]
- 32. Kaur N, Petit JY, Rietjens M, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2005;12(7):539–545. [DOI] [PubMed] [Google Scholar]
- 33. Fitoussi AD, Berry MG, Fama F, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases [outcomes article]. Plast Reconstr Surg. 2010;125(2):454–462. [DOI] [PubMed] [Google Scholar]
- 34. Carter SA, Lyons GR, Kuerer HM, et al. Operative and oncologic outcomes in 9861 patients with operable breast cancer: single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol. 2016;23(10):3190–3198. [DOI] [PubMed] [Google Scholar]
- 35. Weber WP, Soysal SD, El-Tamer M, et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res Treat. 2017;165(1):139–149. [DOI] [PubMed] [Google Scholar]