Abstract
Aim:
To assess whether treatment with sitagliptin, starting before surgery and continued during the hospital stay, can prevent and reduce the severity of perioperative hyperglycaemia in patients with type 2 diabetes undergoing coronary artery bypass graft (CABG) surgery.
Materials and Methods:
We conducted a double-blinded, placebo-controlled trial in adults with type 2 diabetes randomly assigned to receive sitagliptin or matching placebo starting 1 day prior to surgery and continued during the hospital stay. The primary outcome was difference in the proportion of patients with postoperative hyperglycaemia (blood glucose [BG] > 10 mmol/L [>180 mg/dL]) in the intensive care unit (ICU). Secondary endpoints included differences in mean daily BG in the ICU and after transition to regular wards, hypoglycaemia, hospital complications, length of stay and need of insulin therapy.
Results:
We included 182 participants randomized to receive sitagliptin or placebo (91 per group, age 64 ± 9 years, HbA1c 7.6% ± 1.5% and diabetes duration 10 ± 9 years). There were no differences in the number of patients with postoperative BG greater than 10 mmol/L, mean daily BG in the ICU or after transition to regular wards, hypoglycaemia, hospital complications or length of stay. There were no differences in insulin requirements in the ICU; however, sitagliptin therapy was associated with lower mean daily insulin requirements (21.1 ± 18.4 vs. 32.5 ± 26.3 units, P = .007) after transition to a regular ward compared with placebo.
Conclusion:
The administration of sitagliptin prior to surgery and during the hospital stay did not prevent perioperative hyperglycaemia or complications after CABG. Sitagliptin therapy was associated with lower mean daily insulin requirements after transition to regular wards.
Keywords: cardiac surgery, CABG, DPP-4 inhibitors, hyperglycaemia, stress
1 |. INTRODUCTION
More than 400 000 patients undergo coronary artery bypass graft surgery (CABG) each year in the United States.1 Nearly 40% of patients undergoing cardiac surgery have diabetes.2,3 Hyperglycaemia during the perioperative period is reported in more than 90% of patients with diabetes and in ~80% of patients without a history of diabetes.1,4 Large cohort studies in patients with and without diabetes have identified hyperglycaemia as an independent risk factor for poor outcomes after cardiac surgery compared with patients with normoglycaemia, specifically higher perioperative mortality,5 deep sternal wound infections,5,6 renal failure,1 postoperative strokes,7 longer hospital stays8 and higher healthcare resource utilization.9 Despite an ongoing debate about the optimal glucose target, there is strong agreement that improvement in glycaemic control reduces perioperative complications and inpatient mortality.10,11
Clinical guidelines recommend the use of continuous intravenous insulin infusion (CII) for the treatment of hyperglycaemia (blood glucose [BG] > 10 0 mmol/L [>180 mg/dL]) in cardiac surgery patients with diabetes.12 Although effective and widely utilized,10,11 the use of CII is labour intensive, requiring hourly BG testing and insulin drip adjustment, and is associated with a significant risk of hypoglycaemia, which is reported in 10% to 32% of patients in intensive care units (ICUs).13,14 Recently, the authors and others reported that therapy with dipeptidyl peptidase-4 (DPP-4) inhibitors is an effective strategy to improve glycaemic control in general medicine and surgical patients with type 2 diabetes and mild to moderate hyperglycaemia.15,16 In this randomized controlled trial (RCT), we examined whether starting sitagliptin the day prior to surgery and continued during the hospital stay can prevent and reduce the severity of perioperative hyperglycaemia in cardiac surgery patients with type 2 diabetes.
2 |. METHODS
2.1 |. Study design and population
We performed a single-centre, prospective, double-blinded, randomized placebo-controlled study at four academic hospitals, Emory University Hospital, Emory Midtown Hospital, Emory Saint Joseph’s Hospital and Grady Memorial Hospital in Atlanta, Georgia, from January 2016 to October 2018. The study protocol and consent were approved by the Emory University Institutional Review Board. All participants provided written consent prior to any study procedures and/or interventions. This trial is registered with ClinicalTrials.gov (NCT02556918).
Participants were approached during their presurgical assessment or during hospital admission prior to surgical intervention. We enrolled adult participants aged 18–80 years with a known diagnosis of type 2 diabetes, treated with diet, oral antidiabetic agents, glucagon-like peptide-1 receptor analogues (GLP-1 RAs) or insulin. We excluded patients with type 1 diabetes, a history of diabetic ketoacidosis and/or hyperosmolar hyperglycaemic state, decreased renal function (estimated glomerular filtration rate [eGFR] < 30 mL/min per 1.73m2) or with clinically significant liver disease, history of pancreatitis, gastrointestinal obstruction or adynamic ileus, clinically relevant pancreatic or gallbladder disease or patients treated with oral or injectable corticosteroids, pregnancy, or a mental condition rendering the subject unable to understand the nature, scope and possible consequences of the study.
2.2 |. Randomization and masking
Participants were randomly assigned to sitagliptin or matched placebo once daily. A statistician provided research pharmacists at each institution with a computer-generated randomization table to assign participants (1:1). All medical and/or surgical management decisions except glycaemic management were under the responsibility of the primary surgical care team as per standard of care.
2.3 |. Procedures
The study drug, sitagliptin or placebo, was given once daily, starting the day prior to surgery and until hospital discharge, or up to 10 days. Sitagliptin dose was adjusted according to the eGFR as per the manufacturer’s instructions: 100 mg/day if eGFR was 50 mL/min per 1.73m2 or higher, 50 mg/day if eGFR was less than 50 mL/min per 1.73m2, and 25 mg daily if the calculated GFR was less than 30 mL/min per 1.73m2.
2.3.1 |. Prior to surgery
For participants who had consented in the ambulatory setting, written instructions were provided on how to modify home diabetes therapy prior to hospital admission, and a telephone call was made 1–2 days prior to surgery to answer any questions regarding treatment.
For insulin-naive patients, oral agents were discontinued on admission. Daily GLP-1 RAs were held 48 hours prior to surgery, and weekly (exenatide extended-release, dulaglutide) agents were held 1 week before surgery. For participants who were treated with insulin prior to admission, the total daily insulin dose was reduced by 20% (Appendix S1). Half of total daily dose was given once daily as basal insulin (glargine or levemir) and half as prandial insulin (lispro, aspart) divided into three equal doses before meals. The dose of prandial insulin was held in those patients without oral intake.17 Insulin doses were adjusted daily to maintain a fasting and predinner BG of 4.4–10 mmol/L (80–180 mg/dL)17 (Appendix S2). BG levels were assessed by capillary point of care testing before meals and bedtime or every 6 hours in those without oral intake.
2.3.2 |. Perioperative and ICU
Intraoperatively, BG was measured every 30 minutes, and within 2 hours after arrival at the ICU. Patients with BG greater than 10 mmol/L (>180 mg/dL) were treated with CII until haemodynamically stable, able to eat and/or transferred to a non-ICU setting. Insulin regimen was titrated daily to a BG target of 6.1–10 mmol/L (110–180 mg/dL) following standard hospital protocol (Appendix S3).18 Glucose levels in the ICU were assessed by point of care testing every 1–2 hours during CII.
2.3.3 |. Transition from Continuous insulin infusion to subcutaneous insulin
We transitioned participants from CII to subcutaneous (SC) insulin following a prespecified hospital algorithm. Participants treated with insulin prior to admission received basal insulin ~4 hours before discontinuation of CII at the same presurgery dose (Appendix S4).17 Insulin-naive participants who did not require CII in the ICU or who received an insulin infusion rate of less than 1 U/hour were transitioned to sliding scale insulin coverage for BG greater than 10 mmol/L (>180 mg/dL) (Appendix S2). Participants with two consecutive BG readings of more than 10 mmol/L, or an average daily BG of greater than 10 mmol/L, received rescue therapy with SC basal (glargine or detemir) insulin. The starting insulin dose was 0.3 U/kg/day for BG of 7.8–11.1 mmol/L (140–200 mg/dL) or 0.4 U/kg/day for BG of 11.2–22.2 mmol/L (201–400 mg/dL).17 Patients older than 70 years or with impaired renal function (eGFR < 50 mL/min/1.73 m2) were started at a total daily dose of 0.25 U/kg. We adjusted insulin daily according to a target BG of 4–10 mmol/L (80–180 mg/dL) before meals. Glucose levels were assessed by point of care testing before meals and bedtime or every 6 hours in those without oral intake.
2.4 |. Study outcomes
The primary endpoints were differences in the frequency of hyperglycaemia (% patients with BG > 10 mmol/L [>180 mg/dL]) in the ICU after surgery. Secondary outcomes included difference in glycaemic control in the ICU and after discontinuation of insulin infusion between treatment groups, need for CII for treatment, frequency of hypoglycaemic events (<3.9 mmol/L [<70 mg/dL], <3.0 mmol/L [<54 mg/dL] and < 2.2 mmol/L [<40 mg/dL]) and insulin requirements in the ICU and after transition to a regular ward. We also examined differences in a composite of perioperative complications including sternal wound infection (deep and superficial), bacteraemia, pneumonia (infection was confirmed by a positive culture of blood, sputum, urine, pleural or mediastinal fluid and/or incisional discharge), respiratory failure, acute kidney injury (serum creatinine increment level increasing by >50% from baseline) and major adverse cardiovascular events, including acute myocardial infarction, stroke, heart failure and cardiac arrhythmias (significant arrhythmias were those that caused haemodynamic instability and required treatment). Stroke was described as a neurological abnormality resulting in transient or permanent motor deficit that was confirmed by a computerized tomography scan and/or neurologist. Additionally, we compared differences between groups in length of stay in the ICU and the hospital, mortality, hospital readmissions and emergency room visits within 30 days after hospital discharge.
2.5 |. Statistical analysis
This clinical trial was a two-arm, randomized, double-blinded, placebo-controlled clinical trial. The primary outcome was the difference in the frequency of hyperglycaemia greater than 10 mmol/L (>180 mg/dL) in the ICU. Our sample size and power calculations were based on the data from the GLUCO-CABG trial, in which 91% of subjects with diabetes had a BG greater than 10 mmol/L (180 mg/dL) in the ICU.19 For this study, we assumed the same rate of hyperglycaemia in the control (placebo) group. Assuming a reduction in the rate of hyperglycaemia of 20%−25%, using two-sided Fisher’s exact test with an alpha of 0.05, the sample size required for an 80% power to detect the conjectured treatment effect of an OR of 0.264 (i.e. 91% vs. 73%) would be 79 patients per study group. Accounting for an attrition rate of 15%−20%, ~99 patients needed to be recruited to each group.
Chi-square tests or Fisher’s exact tests were used to compare the rate of hyperglycaemia and other categorical variables between the treatment group (sitagliptin) and the control group (matching placebo). Non-parametric Kruskal-Wallis tests were used to compare continuous variables such as length of stay or BG values. P-values less than .05 were considered as statistically significant. The data analyses were performed with SAS 9 4.
3 |. RESULTS
A total of 206 participants with type 2 diabetes provided written consent to participate in the study. One participant was excluded because of surgery cancellation before randomization and three patients withdrew consent before treatment assignment. A total of 202 participants completed enrolment and randomization; 101 (50%) were assigned to each group. Nineteen patients were excluded from the analyses because of surgery cancellation or consent withdrawal before surgery. One patient that died before CABG (no study drug exposure) was also excluded (Figure 1). Thus, our study included 182 randomized participants (91 in each group) undergoing CABG.
FIGURE 1.
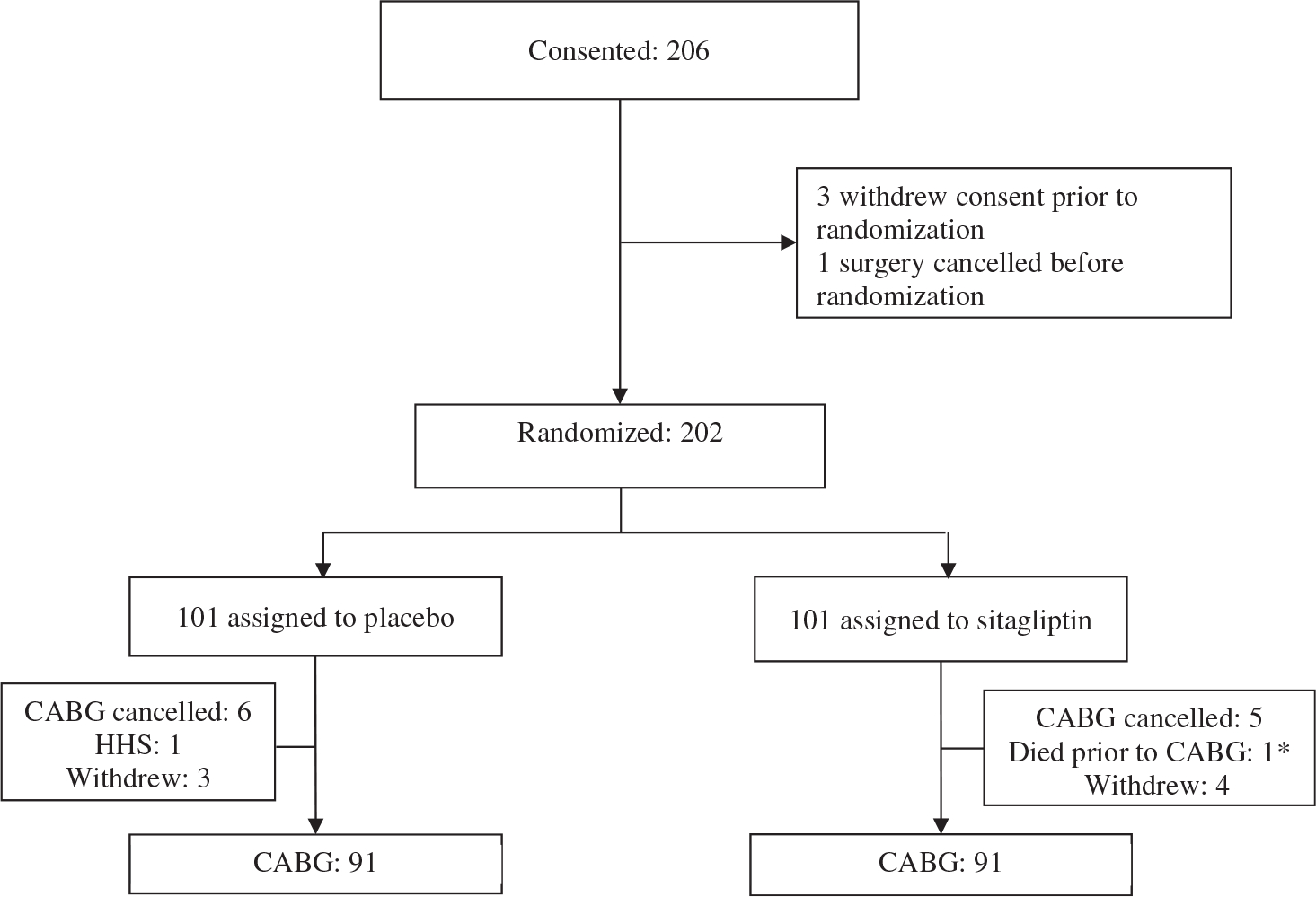
Study flow diagrams. *Randomized, died before receiving study medication
The clinical characteristics of the study participants are provided in Table 1. Both groups had similar characteristics at baseline, with no significant differences in age, gender, weight, body mass index, race, co-morbidities, type of surgery or American Society of Anaesthesiologists physical score classification (Table 1). There were no differences in the mean glucose concentration at admission or randomization, duration of diabetes, HbA1c or outpatient antidiabetic therapy prior to admission (Table 1). The median inpatient stay was 9.0 (6.0, 12.0) days in the sitagliptin group and 7.0 (6.0, 13.0) days in the placebo group (P = .56).
TABLE 1.
Clinical characteristics on admission and glycaemic control
| Variable | Placebo | Sitagliptin | P-value |
|---|---|---|---|
| Number of participants | 91 | 91 | |
| Gender | .24 | ||
| Male | 70 (77) | 63 (69) | |
| Female | 21 (23) | 18 (31) | |
| Age, years | 64 ± 9 | 63 ± 8 | .48 |
| Race | .92 | ||
| Caucasian | 68 (75) | 67 (74) | |
| African American | 19 (21) | 21 (23) | |
| Other | 4(4) | 3(3) | |
| Body weight, kg | 97 ± 21 | 96 ± 19 | .96 |
| BMI, kg/m2 | 32 ± 7 | 32 ± 6 | .34 |
| Diabetes history | |||
| Diabetes duration, years | 9±7 | 12 ± 10 | .18 |
| Outpatient diabetes therapy | .32 | ||
| None | 7 (8) | 2 (2) | |
| Diet only | 5 (5) | 3 (3) | |
| OAD only | 52 (57) | 63 (69) | |
| DPP-4 inhibitors | 11 (17) | 20 (27) | |
| Metformin | 62 (89) | 66 (81) | |
| Sulphonylureas | 18 (28) | 24 (32) | |
| Thiazolinediones | 0(0) | 6 (8) | |
| SGLT-2 | 7 (14) | 5 (8) | |
| OAD + insulin | 17 (19) | 15 (16) | |
| GLP-1 | 0 (0) | 2 (2) | |
| GLP-1 + OAD | 2 (2) | 2 (2) | |
| Insulin only | 7 (8) | 3 (3) | |
| Home total insulin dose, units | 50.3 ± 34.4 | 57.1 ± 50.4 | .95 |
| Total insulin dose adjusted by weight | 0.49 ± 0.33 | 0.61 ± 0.51 | .37 |
| Type of surgery | .54 | ||
| Elective/outpatient | 53 (59) | 49 (54) | |
| Emergent | 5(6) | 10 (11) | |
| Transfer from another hospital | 7(8) | 5 (5) | |
| Urgent | 25 (28) | 27 (30) | |
| Type of CABG procedure | .65 | ||
| Open CABG | 76 (84) | 79 (87) | |
| Robotic CABG | 14 (16) | 12 (13) | |
| Length of surgery (minutes) | 311 ± 90 | 310 ± 93 | .85 |
| Number of vessels, median (Q1, Q3) | 3.0 [2.0, 4.0] | 3.0 [2.0,3.0] | .89 |
| ASA | .83 | ||
| III | 12 (13) | 14 (15) | |
| IV | 77 (86) | 77 (85) | |
| V | 1(1) | 0 (0) |
Abbreviations: ASA, American Society of Anaesthesiology; BMI, body mass index; CABG, coronary artery bypass graft surgery; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; ICU, intensive care unit; OAD, oral antidiabetic drug; SGLT-2, sodium-glucose co-transporter-2.
Data are n (%), mean ± standard deviation, or median [IQR]; IQR, interquartile range.
The frequency of hyperglycaemia greater than 10 mmol/L (>180 mg/dL) in the ICU was not significantly different between groups (75% and 84%, P = .14; difference = −9%; 95% CI: −21%, 3%) for patients on sitagliptin and placebo, respectively. The number of participants who developed hyperglycaemia and required continuous insulin therapy in the ICU was similar in both groups (93% vs. 95%, P > .99; Figure 2). The mean BG during surgery (9.0 ± 1.7 vs. 9.2 ± 1.6 mmol/L), ICU stay (8.2 ± 0.9 vs. 8.3 ± 0.9 mmol/L) or after transition to a regular ward (9.3 ± 1.7 vs. 9.2 ± 1.7 mmol/L) was similar in the sitagliptin and placebo groups (all P = NS). We conducted an analysis to assess the response in participants without previous incretin therapy prior to admission. In this group, sitagliptin therapy resulted in a significant reduction in the proportion of patients with hyperglycaemia greater than 10 mmol/L (>180 mg/dL) in the ICU (71% and 85%, P = .041), but not after transition to regular wards. In addition, there was no difference in the proportion of participants with BG greater than 11.1 mmol/L (>200 mg/dL) in the ICU (54% in both groups) or after transition (72% vs. 75%, P = .62) compared with patients treated with insulin prior to admission.
FIGURE 2.
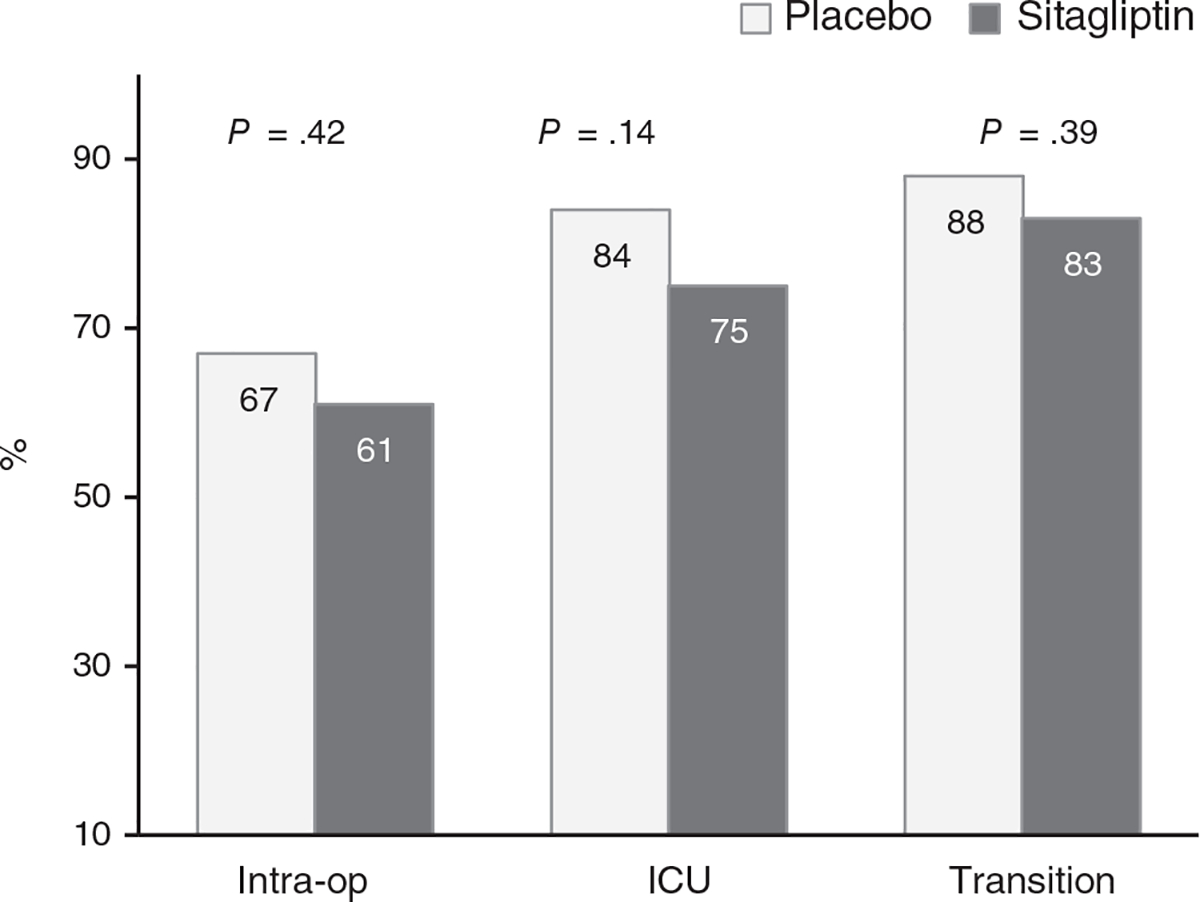
Participants with perioperative hyperglycaemia BG > 10 mmol/L (>180 mg/dL)
The duration of insulin infusion in the ICU was similar in patients on sitagliptin and placebo (27.7 ± 27.6 vs. 27.7 ± 28.0 hours, P > .99). After discontinuation of intravenous (IV) insulin and transition to SC insulin, the numbers of participants requiring basal insulin treatment were similar in both groups (85% vs. 91%, P = .35; Table 2). However, after transition from IV to SC insulin, participants exposed to sitagliptin had lower mean daily insulin doses during their hospital stay (21.1 ± 18.4 vs. 32.5 ± 26.3 U/day, P = .007), with lower mean daily requirements for basal insulin (19.0 ± 9.2 vs. 27.4 ± 14.5 U/day, P = .002) and similar prandial insulin (12.8 ± 8.6 vs. 15.9 ± 11.4 U/day, P = .18) in the sitagliptin compared with the placebo group (Figure 3).
TABLE 2.
Glycaemic control and insulin therapy during hospital admission
| Glycaemic control | Placebo | Sitagliptin | P-value |
|---|---|---|---|
| Admission HbA1c, % | 7.7 ± 1.7 | 7.4 ± 1.3 | .18 |
| Randomization BG, mmol/L | 8.9 ± 3.3 | 8.8 ± 3.5 | .55 |
| Admission BG, mmol/L | 9.2 ± 3.7 | 8.7 ± 3.0 | .38 |
| Preoperative BG, mmol/L | 8.6 ± 2.3 | 8.4 ± 2.7 | .15 |
| Postoperative BG, mmol/L | 8.7 ± 2.0 | 8.6 ± 1.6 | .93 |
| BG during surgery, mmol/L | 9.2 ± 1.6 | 9.0 ± 1.7 | .44 |
| BG during ICU stay, mmol/L | 8.2 ± 0.9 | 8.2 ± 0.9 | .64 |
| BG after transition, mmol/L | 9.2 ± 1.7 | 9.3 ± 1.7 | .94 |
| Hyperglycaemia BG > 10 mmol/L (>180 mg/dL) | |||
| Participants with BG >10 mmol/L in OR | 60 (67) | 56 (61) | .42 |
| Participants with BG >10 mmol/L in the ICU | 76 (84) | 68 (75) | .14 |
| Participants with BG >10 mmol/L after transition | 76 (88) | 72 (83) | .39 |
| Hyperglycaemia BG > 111 mmol/L (>200 mg/dL) | |||
| Participants with BG >111 mmol/L in OR | 36 (40) | 36 (39) | .90 |
| Participants with BG >111 mmol/L in the ICU | 46 (51) | 49 (54) | .66 |
| Participants with BG >111 mmol/L after transition | 66 (77) | 63 (72) | .51 |
| Hypoglycaemia BG < 3 9 mmol/L (<70 mg/dL) | |||
| Participant with BG <3 9 mmol/L in OR | 0(0) | 0(0) | - |
| Participants with BG <3 9 mmol/L in the ICU | 8 (9) | 9 (10) | >.99 |
| Participants with BG <3 9 mmol/L after transition | 8 (9) | 8(9) | >.99 |
| Hypoglycaemia BG < 3 0 mmol/L (<54 mg/dL) | |||
| Participants with BG <3 0 mmol/L in OR | 0 (0) | 0 (0) | - |
| Participants with BG <3 0 mmol/L in the ICU | 1 (1) | 4 (4) | .37 |
| Participants with BG <3 0 mmol/L after transition | 0 (0) | 3 (3) | .25 |
| Hypoglycaemia BG < 2 2 mmol/L (<40 mg/dL) | |||
| Participants with BG <2 2 mmol/L in OR | 0 (0) | 0 (0) | - |
| Participants with BG <2 2 mmol/L in the ICU | 0 (0) | 1 (1) | >.99 |
| Participants with BG <2 2 mmol/L after transition | 0 (0) | 0 (0) | - |
| Insulin therapy | |||
| Participants treated with CII in OR | 76 (84) | 74 (80) | .48 |
| Duration of CII in OR, hours | 3.3 ± 2.2 | 3.4 ± 2.5 | .53 |
| Total insulin dose in OR, units | 13.2 ± 13.1 | 14.1 ± 16.1 | .97 |
| Participants treated with CII in ICU | 86 (95) | 85 (93) | >.99 |
| Duration of CII, hours (ICU first 48 hours) | 21.4 ± 13.9 | 22.0 ± 14.3 | .77 |
| Total insulin dose in the ICU (first 48 hours), units | 73.7 ± 69.9 | 80.4 ± 79.8 | .48 |
| Participants treated with SC basal bolus regimen after discontinuation of CII | 78 (91) | 74 (85) | .35 |
Abbreviations: BG, blood glucose; CII, continuous insulin infusion; ICU, intensive care unit; LOS, length of stay; OR, operating room; SC, subcutaneous; SSI, sliding scale insulin.
Data are n (%), mean ± standard deviation, or median [IQR]; IQR, interquartile range.
To convert HbA1c value in % to mmol/mol = 10.93 × %HbA1c value −23.5), BG value in mg/dL to mmol/L = BG mg/dL value/18.
FIGURE 3.
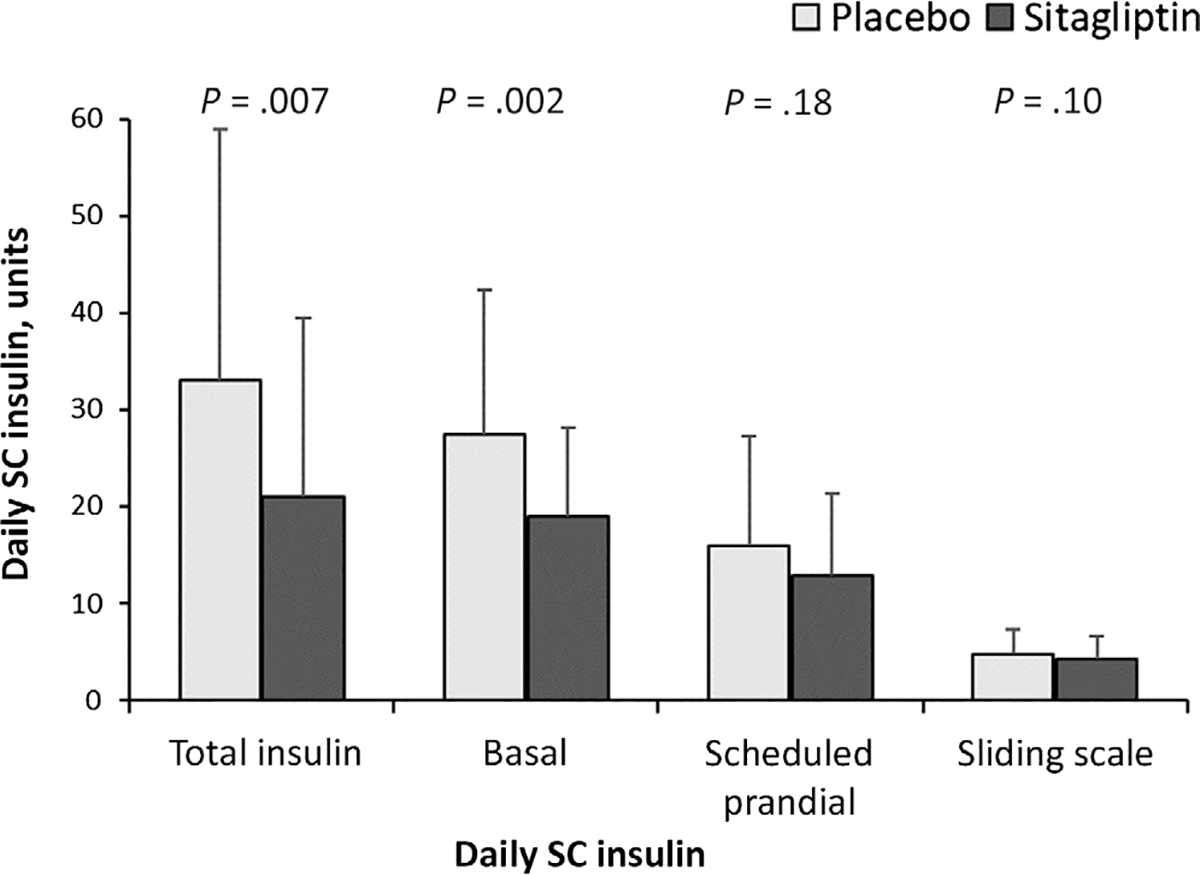
Total SC insulin requirement after transition from insulin
We did not observe differences in the frequency of hypoglycaemia or in mean daily glucose during the hospital stay between treatment groups. There were no differences in the duration of surgery, duration of ICU or hospital length of stay, need for vasopressors, perioperative complications, surgical reinterventions or readmissions after hospital discharge between treatment groups. In addition, there were no differences in the 30-day readmissions or emergency room visits between groups (Appendix S5).
4 |. DISCUSSION
The results of several observational and randomized controlled studies have shown that perioperative hyperglycaemia after cardiac surgery is associated with higher rates of hospital complications, longer hospital stay and higher healthcare resource utilization compared with patients with normoglycaemia.9,20,21 In these patients, improved glycaemic control has been shown to reduce perioperative complications.10,11,22 Therefore, we conducted a randomized, double-blinded, placebo-controlled trial to determine whether the administration of sitagliptin, 1 day prior to surgery and continued during the hospital stay, could prevent perioperative hyperglycaemia and reduce the use of insulin and postoperative complications. We report that the use of sitagliptin did not prevent the development of perioperative hyperglycaemia in patients with type 2 diabetes undergoing CABG surgery. There were no differences in the number of patients requiring insulin therapy in the ICU or after transition to a regular ward, length of hospital stay, or in the rate of perioperative complications. Sitagliptin therapy, however, was associated with lower mean daily insulin requirements after transition from IV to SC insulin.
Clinical guidelines from professional organizations recommend the use of insulin to manage perioperative hyperglycaemia in cardiac surgery patients.23,24 Although effective in improving glycaemic control,10,11 its use is labour intensive and is associated with a higher risk of hypoglycaemia,13,14 which has been reported to be an independent factor for an increased risk of complications and increased mortality.25,26 In addition to iatrogenic hypoglycaemia, the significant workload associated with continuous insulin infusion and multiple daily insulin injections has led to an increased use of non-insulin agents to manage patients with diabetes in hospital.27 As an alternative to intensified insulin regimens, our group and others have reported on the use of DPP-4 inhibitors for the management of inpatients with diabetes.15,16,27,28 Recent RCTs have reported that treatment with DPP-4 inhibitors alone or in combination with basal insulin in general surgery patients results in improved glycaemic control with a lower risk of hypoglycaemia compared with a basal bolus insulin regimen.15,16,28 Similarly, two previous studies with sitagliptin and one with saxagliptin in general medicine patients15,28,29 also reported no differences in glycaemic control and resulted in lower insulin requirements.29 The results of these RCTs led us to investigate if the use of DPP-4 inhibitors can facilitate the management of patients with type 2 diabetes undergoing CABG surgery.
In the current study, treatment with sitagliptin failed to prevent hyperglycaemia or improve glycaemic control in the ICU or regular ward. Several factors may explain the limited effect of DPP-4 inhibitors after cardiac surgery, including the use of inotropes and vasopressors, the higher level of surgical stress compared with general surgery patients and its mild increase in endogenous GLP-1 concentration. Results of the GLOBE trial, a multicentre clinical trial in 278 patients (14% with diabetes) randomized to liraglutide or placebo before cardiac surgery, have recently been reported. The primary outcome was insulin administration for BG greater than 8.0 mmol/L (144 mg/dL) in the operating theatre. A lower proportion of patients in the liraglutide group required additional insulin compared with placebo (43% vs. 61%, absolute difference: 18%, P = .003).30 Liraglutide, a DPP-4-resistant GLP-1 analogue, is a more potent agent than sitagliptin and may explain the differences between ours and the former study (30).
We acknowledge some limitations to this trial, including the comparatively small number of randomized participants. Because we did not have preliminary data on the effects of perioperative sitagliptin exposure, we estimated a potential 20% reduction in the incidence of postoperative hyperglycaemia of greater than 10 mmol/L, thus this study was underpowered to observe smaller differences. In addition, we included patients with preadmission treatment with diet, oral and low-dose insulin therapy, with long duration of diabetes (average 10 years) and different levels of glycaemic control. A more homogeneous population of patients with a mild and/or more recent history of hyperglycaemia/diabetes may have experienced a better response to therapy. Although we observed significant differences in the total insulin dose requirements after transition from IV to SC insulin in the sitagliptin group, these differences are unlikely to be clinically significant to thus recommend the use of DPP-4 inhibitors to improve perioperative glycaemic control in patients with type 2 diabetes after CABG surgery. These results, however, confirm the safety of sitagliptin in hospital and suggest it may be continued during the perioperative period of patients already treated with this agent.
In conclusion, our findings indicate that the use of sitagliptin, prior to surgery and continued during hospital stay, did not reduce the frequency of perioperative hyperglycaemia or hospital complications in patients with type 2 diabetes after cardiac surgery.
Supplementary Material
ACKNOWLEDGEMENTS
Cardio-Thoracic Study Team collaborators: Omar Lattouf, Bradley G. Leshnower, Jeffrey Miller, John D. Vega, Katherine Carssow, N. Renee Cook, Michele Fielding and Sonya Mathewson. Partial data from this trial were presented at the American Diabetes Association meeting in June 2019. This investigator-initiated study was supported by a clinical research grant from Merck Inc. who also provided sitagliptin and placebo medications. The supporters of the study were not involved in the study design, data collection, analysis or interpretation of the results, or preparation of the manuscript.
CONFLICT OF INTEREST
GEU is partly supported by research grants from the NIH/NATS UL1 TR002378 from the Clinical and Translational Science Award programme, and 1P30DK111024–01 from NIH and National Centre for Research Resources. FJP, GMD and PV are supported by NIH grants: 1K23GM128221–01A1, 1K23DK122199–01A1 and 3K12HD085850–03S1. RJG is partially supported by research grants from NIH/NIDDK P30DK11102 and 1K23DK123384–01. GEU has also received unrestricted research support for research studies (to Emory University) from Merck, Novo Nordisk and Dexcom Inc. FJP received consulting fees from Merck, Boehringer Ingelheim, Lilly, AstraZeneca and Sanofi, and research support from Merck and Dexcom Inc. PV has received consulting fees from Merck and Boehringer Ingelheim. RJG has received unrestricted research support for studies (to Emory University) from Novo Nordisk and consulting fees from Abbott Diabetes Care, Sanofi, Valeritas, Eli Lilly and Novo Nordisk. No other potential conflict of interest relevant to this article was reported. SC, KT, SJ, MF, MH, LP, RAG, BA, ALM, WBK and SM declare no conflicts of interest.
Funding information
This investigator-initiated study was supported by a clinical research grant from Merck Inc. who also provided sitagliptin and placebo medications.
Footnotes
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14241.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Schmeltz LR, DeSantis AJ, Thiyagarajan V, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30(4):823–828. [DOI] [PubMed] [Google Scholar]
- 2.Whang W, Bigger JT Jr. Diabetes and outcomes of coronary artery bypass graft surgery in patients with severe left ventricular dysfunction: results from the CABG patch trial database. The CABG patch trial investigators and coordinators. J Am Coll Cardiol. 2000;36(4): 1166–1172. [DOI] [PubMed] [Google Scholar]
- 3.Szabo Z, Hakanson E, Svedjeholm R. Early postoperative outcome and medium-term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2002;74(3):712–719. [DOI] [PubMed] [Google Scholar]
- 4.McAlister FA, Man J, Bistritz L, Amad H, Tandon P. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26(5):1518–1524. [DOI] [PubMed] [Google Scholar]
- 5.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40(3):418–423. [DOI] [PubMed] [Google Scholar]
- 7.Bucerius J, Gummert JF, Borger MA, et al. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75(2):472–478. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub WS, Stein B, Kosinski A, et al. Outcome of coronary bypass surgery versus coronary angioplasty in diabetic patients with multivessel coronary artery disease. J Am Coll Cardiol. 1998;31(1): 10–19. [DOI] [PubMed] [Google Scholar]
- 9.Cardona S, Pasquel FJ, Fayfman M, et al. Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. J Diabetes Complications. 2017;31(4):742–747. [DOI] [PubMed] [Google Scholar]
- 10.Kitabchi AE, Freire AX, Umpierrez GE. Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients. Metabolism. 2008;57(1):116–120. [DOI] [PubMed] [Google Scholar]
- 11.Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med. 2006;34(3):612–616. [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. [DOI] [PubMed] [Google Scholar]
- 13.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. [DOI] [PubMed] [Google Scholar]
- 14.Preiser JC, Brunkhorst F. Tight glucose control and hypoglycemia. Crit Care Med. 2008;36(4):1391–1392. [DOI] [PubMed] [Google Scholar]
- 15.Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5(2):125–133. [DOI] [PubMed] [Google Scholar]
- 16.Vellanki P, Rasouli N, Baldwin D, et al. Glycaemic efficacy and safety of linagliptin compared to basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: a multicenter randomized clinical trial. Diabetes Obes Metab 2019;21(4):837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galindo RJ, Fayfman M, Umpierrez GE. Perioperative management of hyperglycemia and diabetes in cardiac surgery patients. Endocrinol Metab Clin North Am. 2018;47(1):203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care. 2015;38(9):1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szekely A, Levin J, Miao Y, et al. Impact of hyperglycemia on perioperative mortality after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2011;142(2):430–437. [DOI] [PubMed] [Google Scholar]
- 21.Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2): 663–669. [DOI] [PubMed] [Google Scholar]
- 23.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seley JJ, D’Hondt N, Longo R, et al. Position statement: inpatient glycemic control. Diabetes Educator. 2009;35(Suppl 3):65–69. [Google Scholar]
- 25.D’Ancona G, Bertuzzi F, Sacchi L, et al. Iatrogenic hypoglycemia secondary to tight glucose control is an independent determinant for mortality and cardiac morbidity. Eur J Cardio-Thoracic Surg. 2011;40 (2):360–366. [DOI] [PubMed] [Google Scholar]
- 26.Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367(12):1108–1118. [DOI] [PubMed] [Google Scholar]
- 27.Pasquel FJ, Fayfman M, Umpierrez GE. Debate on insulin vs non-insulin use in the hospital setting-is it time to revise the guidelines for the Management of Inpatient Diabetes? Curr Diab Rep. 2019;19 (9):65. [DOI] [PubMed] [Google Scholar]
- 28.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care. 2013;36(11):3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diabetes Res Care. 2017;5(1):e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulst AH, Visscher MJ, Godfried MB, et al. Liraglutide for perioperative management of hyperglycaemia in cardiac surgery patients: a multicentre randomized superiority trial. Diabetes Obes Metab. 2020; 22(4):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


