Abstract
Objectives
To evaluate concentrations of procalcitonin (PCT) in transplant recipients receiving immunosuppressive therapy compared with nonimmunosuppressed patients.
Methods
We analyzed a data set of 9,500 inpatient encounters to compare levels of PCT and other biomarkers of infection (C-reactive protein [CRP], WBC count, and absolute neutrophil count [ANC]) between immunosuppressed and nonimmunosuppressed cohorts. We also assessed the correlation between PCT and clinical variables in immunosuppressed patients.
Results
Patients receiving immunosuppressive drugs had significantly higher levels of maximal and minimal PCT compared with the nonimmunosuppressed patients (P < .0001 and P = .0019, respectively). However, CRP levels, WBC count, and ANC were significantly lower in immunosuppressed patients compared with the nonimmunosuppressed patients (P = .0003, P < .0019, and P = .0001, respectively).
Conclusions
Our results from real-world data demonstrated that PCT dynamics remain intact despite immunosuppressive therapy, in contrast to other biomarkers such as CRP, WBC, and ANC. In addition, higher PCT levels are associated with systemic infections and reflect disease severity.
Keywords: Procalcitonin, Immunosuppressed hosts, Antibiotic stewardship
Key Points.
• Procalcitonin (PCT) is used as a biomarker to guide antimicrobial therapy, but limited data are available with regard to the use of PCT in transplant-related immunosuppressed patients.
• PCT dynamics in transplant-related immunosuppressed patients remain intact, in contrast to the attenuation of other traditional biomarkers (C-reactive protein, leukocyte, and absolute neutrophil count).
• Highest PCT levels are found in patients with bacteremia compared with patients with localized infections in transplant-related immunosuppressed patients.
The number of immunocompromised patients has increased over the past few decades due to improved management of immunodeficiency disorders and longer survival of organ transplant recipients.1 Infection is a major cause of morbidity and mortality in immunocompromised patients who are at a significantly higher risk of infection compared with immunocompetent individuals. In addition, the spectrum of potential infections for immunocompromised individuals includes opportunistic pathogens such as commensal bacteria, fungi, viruses, and parasites that rarely cause clinically significant infections in immunocompetent individuals.
Due to a higher risk of adverse outcomes in this vulnerable group, use of broad-spectrum antibiotics is commonplace. As a result, the selective pressure for multidrug-resistant (MDR) organisms is high, further intensifying the risk of health care–associated infections such as Clostridium difficile, vancomycin-resistant Enterococcus, and organisms that harbor extended-spectrum β-lactamases.2 Balancing thoughtful antibiotic stewardship with appropriately targeted therapy is challenging. Moreover, the telltale signs and symptoms of infection are often muted in immunocompromised patients compared with patients with normal immune function. Use of traditional biomarkers such as fever, leukocyte count, and C-reactive protein (CRP) to distinguish acute bacterial infections is limited in this population due to the attenuation of their dynamics by immunosuppressive agents.3 In addition, fever or other classical signs of infections can also be caused by noninfectious mechanisms related to the primary disease or underlying conditions such as malignancy (disease-fever), allograft rejection, use of antithymocyte globulin, engraftment syndrome, and graft-vs-host disease.4 Therefore, bacterial infections in these patients are more difficult to recognize and require a heightened index of suspicion.1
Procalcitonin (PCT), a 116–amino acid residue prohormone, which is normally synthesized by the C cells of the thyroid gland, is a promising biomarker for early detection of bacterial infections.5 In the presence of a bacterial infection, the systemic production of PCT can be increased up to 100- to 1,000-fold by endotoxins6 and/or cytokines (eg, interleukin 6, tumor necrosis factor α).7 It is the specificity of PCT for bacterial infections5 as well as the favorable kinetics of PCT, with an increase in PCT level seen within 6 to 12 hours after initiation of bacterial infection and a half-life of 24 hours,8 that has propelled PCT utilization for recognizing bacterial infection and antibiotics stewardship.
However, limited data are available with regard to the use of PCT in immunosuppressed patients,9 and this group of patients is underrepresented in clinical trials. Given the limitations of existing biomarkers for identifying bacterial infection in immunosuppressed patients, we investigated whether PCT dynamics were similarly attenuated by immunosuppressive medications. In this study, we report results from real-world clinical data of PCT usage in patients receiving immunosuppressive therapy and discuss the advantage of PCT for detecting bacterial infection.
Materials and Methods
Study Design and Data Set
We conducted a retrospective chart review of all patient encounters meeting the following criteria: (1) patient admitted to UCSD Health hospitals between February 2017 and October 2019, (2) at least one PCT measurement during the admission, and (3) patient 18 years or older. According to the eligibility criteria, 9,500 encounters (from 7,320 patients) were identified. This study was reviewed and approved by the UCSD Human Research Protection Program Institutional Review Board (Protocol #181656XL).
Definition of Immunosuppression
Patients in our study population with tacrolimus and sirolimus levels measured during the same hospitalization as the PCT measurement were considered “immunosuppressed.” Among 9,500 encounters (from 7,320 eligible patients), 446 (4.7%) encounters (from 329 patients) from transplant-related immunosuppressed patients were identified. To validate the suitability of the definition of immunosuppression applied in this study, 200 patients were randomly selected from the nonimmunosuppressed patient group, and their medical records were assessed for a history of organ transplant. Only one patient was identified to have had a previous hematopoietic stem cell transplant, and based on this internal validation, the predictive accuracy of the classification scheme was high (>99%) and considered adequate.
Definition of Antibiotic Therapy and Microbiologically Documented Infection
Antibiotic therapy was identified from the medication administration record. Only systemic antibiotics administered by the oral or intravenous route were included. We excluded antibiotics targeting infections not likely to affect PCT levels, such as fungal or viral infections, C difficile, and antibiotics used specifically as part of an allergy desensitization protocol.
Microbiologically documented infection was based on positive culture data retrieved from the electronic medical record (EMR) database. Culture-positive results were classified according to the site of infection: blood, respiratory tract (eg, sputum, bronchial wash, bronchoalveolar lavage), urinary tract, peritoneal fluid, cerebrospinal fluid, and other body fluids. We excluded organisms that were believed to be potential contaminants or atypical bacteria (eg, Mycoplasma, Mycobacterium tuberculosis), viruses, and other fungal pathogens (eg, Candida species).10
Data Collection and Subcategorization of the Immunosuppressed Patient Group
The medical records of the immunosuppressed patients were reviewed, and the following variables were analyzed: posttransplantation period, site of admission (intensive care unit [ICU] or ward), and presence of rejection during the encounter. The immunosuppressed patient group was further categorized by different definitions of infection with respect to microbiologic and/or clinical criteria. Group I included patients with no evidence of infection, group II was defined by clinical criteria only, and group III was defined by both clinical and microbiologic criteria. Patients with infections (in groups II and III) were also classified according to the following subgroups: infection without systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock.
Procalcitonin, CRP, and CBC Measurement
PCT was measured with the Elecsys BRAHMS PCT assay (Roche Diagnostics) according to the manufacturer’s specifications. CRP levels were measured on a cobas c702 platform using the Tina-quant CRP assay (Roche Diagnostics). The within-laboratory coefficient of variation (CV) for the PCT assay was 2.8% and 2.0% for the low- and high-quality controls at mean concentrations of 0.54 ng/mL and 9.3 ng/mL, respectively. Also, the within-laboratory CV for the CRP assay was 2.8% and 2.2% for the low- and high-quality controls at mean concentrations of 0.78 mg/dL and 4.34 mg/dL, respectively. The maximal PCT, CRP, WBC count, and absolute neutrophil count (ANC) data were retrieved using an automated query of the EMR.
Statistical Analysis
Categorical data are presented as frequency and percentage, and continuous data are presented as median values and interquartile ranges for nonnormally distributed data. Normality was assessed using the D’Agostino-Pearson normality test, and since most laboratory data did not follow a normal distribution, medians of observed values were used for comparison between the immunosuppressed patient and nonimmunosuppressed patient cohorts. The χ 2 analysis, Mann-Whitney test, and Kruskal-Wallis test were used to compare categorical variables and continuous variables, respectively. Statistical analyses were performed using MedCalc 19.1.3 (MedCalc Software), and a two-tailed P value of .05 or less was considered statistically significant.
Results
A total of 446 encounters from 329 immunosuppressed patients were identified. The demographic and clinical characteristics of the transplant-related immunosuppressed patients are shown in Table 1. No statistically significant associations were observed between the maximal PCT values and patients’ age or sex. The type of the transplanted organ did not have an impact on the PCT levels (P = .08). The different combinations of immunosuppressive drugs that were used to treat these patients are shown in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Immunosuppressed Patient Cohort and Median Maximum PCT Values for Each Subgroup
| Characteristic | Immunosuppressed Cohort | PCT, Median (IQR), ng/mL | P Value |
|---|---|---|---|
| No. of encounters | 446 | ||
| Age, median (95% CI), y | 59 (57-62) | .5415 | |
| Male | 298 | 0.31 (0.12-1.16) | .2133 |
| Female | 148 | 0.28 (0.11-1.02) | |
| Organ transplanted, No. (%) | |||
| Single-organ transplant | |||
| Kidney | 48 (23) | 0.59 (0.24-3.82) | .0832 |
| Liver | 35 (17) | 0.26 (0.11-0.92) | |
| Heart | 30 (15) | 0.28 (0.12-1.54) | |
| Lung | 45 (22) | 0.23 (0.09-0.53) | |
| HSCT | 47 (23) | 0.33 (0.17-2.56) | |
| Multiple-organ transplant | |||
| Kidney/pancreas | 2 (1) | ||
| Heart/kidney | 8 (2) | ||
| Heart/liver | 2 (1) | ||
| Heart/lung | 3 (1) | ||
| Kidney/liver | 11 (3) | ||
| Kidney/lung | 3 (1) | ||
| Heart/kidney/lung | 1 | ||
| Immunosuppressive drugs, No. (%) | |||
| Tacrolimus | 322 (85) | ||
| Cyclosporine | 14 (4) | ||
| Sirolimus | 86 (23) | ||
| Azathioprine | 6 (2) | ||
| Mycophenolate mofetil | 138 (37) | ||
| Prednisone | 277 (73) |
HSCT, hematopoietic stem cell transplant; IQR, interquartile range; PCT, procalcitonin.
Effect of Immunosuppression on PCT, CRP, WBC, and ANC Levels
The comparison of biomarkers of bacterial infection in the nonimmunosuppressed and the immunosuppressed patients is shown in Table 2. Of note, antimicrobial therapy was more frequently administered in encounters from immunosuppressed patients compared with the nonimmunosuppressed patients (P < .0001).
Table 2.
Comparison of the Biomarker Levels Between the Nonimmunosuppressed and the Immunosuppressed Cohorts
| Characteristic | Nonimmunosuppressed Cohort | Immunosuppressed Cohort | P Value |
|---|---|---|---|
| No. of encounters | 9,054 | 446 | |
| No. of patients | 6,991 | 329 | |
| PCT per encounter, No. (%) | |||
| 1 | 6,239 (69) | 285 (64) | |
| 2 | 1,523 (17) | 70 (16) | |
| 3 | 751 (8) | 44 (10) | |
| 4 | 280 (3) | 18 (4) | |
| 5 | 120 (1) | 10 (2) | |
| PCT, ng/mL | |||
| Maximum PCT, median (IQR) | 0.22 (0.09-0.91) | 0.31 (0.12-1.20) | .0001 |
| Minimum PCT, median (IQR) | 0.17 (0.08-0.59) | 0.22 (0.10-0.62) | .0019 |
| High PCT (>0.5 ng/mL), No. | 3,959 | 237 | .0001 |
| Low PCT (<0.25 ng/mL), No. | 5,095 | 209 | |
| CRP, mg/L | |||
| Maximum CRP, median (IQR) | 4.80 (1.40-11.10) | 2.52 (0.70-7.40) | .0003 |
| High CRP (>5 mg/L), No. | 982 | 45 | .0007 |
| Low CRP (≤5 mg/L), No. | 1,057 | 91 | |
| WBC, × 103 cells/μL | |||
| Maximum, median (IQR) | 10.1 (7.1-14.3) | 8.25 (5.60-12.00) | <.0001 |
| High WBC (>10 × 103 cells/μL), No. | 4,585 | 169 | <.0001 |
| Low WBC (≤10 × 103 cells/μL), No. | 4,442 | 277 | |
| ANC, × 103 cells/μL | |||
| Maximum, median (IQR) | 9.0 (5.10-13.80) | 7.1 (3.80-12.78) | .0001 |
| High ANC (>7.7 × 103 cells/μL), No. | 2,981 | 140 | .0001 |
| Low ANC (≤7.7 × 103 cells/μL), No. | 2,218 | 167 | |
| Antimicrobial therapy, No. | |||
| Yes | 7,870 | 417 | <.0001 |
| No | 1,184 | 29 |
ANC, absolute neutrophil count; CRP, C-reactive protein; IQR, interquartile range; PCT, procalcitonin.
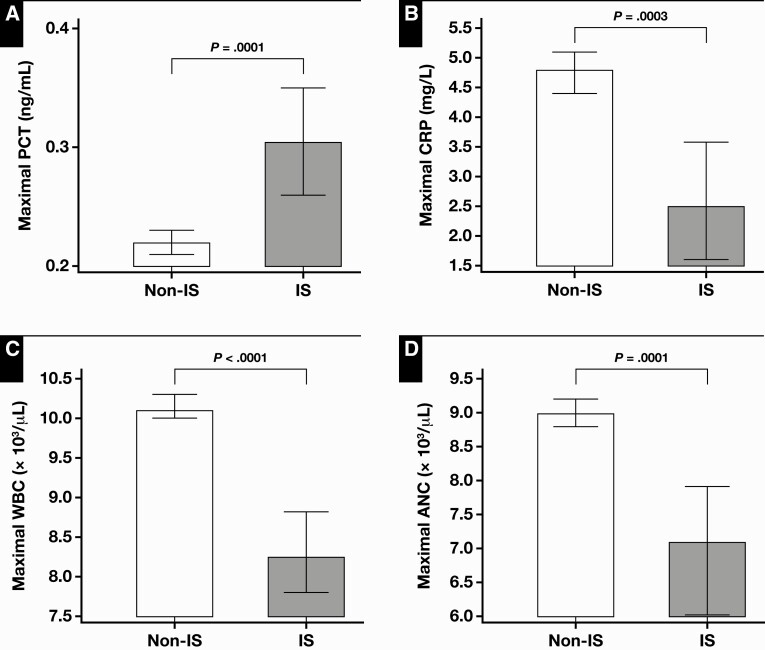
Maximum PCT levels were significantly higher in the immunosuppressed patients (P < .0001) Figure 1A. Most published studies suggest that for patients with sepsis, antibiotic therapy can be withheld at PCT concentrations less than 0.5 ng/mL11; for patients with lower respiratory tract infections, the cut point is 0.25 ng/mL.12,13 When maximal PCT value was compared with the cutoff value of less than 0.5 ng/mL, which represents a high risk of severe sepsis and/or septic shock, a greater proportion of maximal PCT values in the immunosuppressed group was higher than 0.5 ng/mL (53%) compared with the nonimmunosuppressed group (44%) (P = .0001). Compared with the cutoff of less than 0.25 ng/mL, a greater proportion of maximal PCT values was more than 0.25 ng/mL in the immunosuppressed group (55%) compared with the nonimmunosuppressed group (47%) (P = .0004).
Figure 1.
Comparison of median values of maximal procalcitonin (PCT) (A), C-reactive protein (CRP) (B), WBC (C), and absolute neutrophil count (ANC) (D) levels between nonimmunosuppressed (Non-IS) vs immunosuppressed (IS) patients. Error bars represent 95% confidence interval of median.
All other markers of infection that were analyzed (maximal CRP level, WBC count, and ANC) had significantly lower levels in the immunosuppressed patients compared with the nonimmunosuppressed patients (P = .0003, P < .0019, and P = .0001, respectively) Figure 1B, Figure 1C, and Figure 1D. Moreover, a greater proportion of maximal CRP, WBC, and ANC levels was within the reference ranges in the immunosuppressed patient group compared with the nonimmunosuppressed patient group (P = .0007, P < .0001, and P = .0001, respectively).
Subgroup Analysis of the Effect of Immunosuppression on PCT, CRP, WBC, and ANC Levels in Culture-Positive Patients
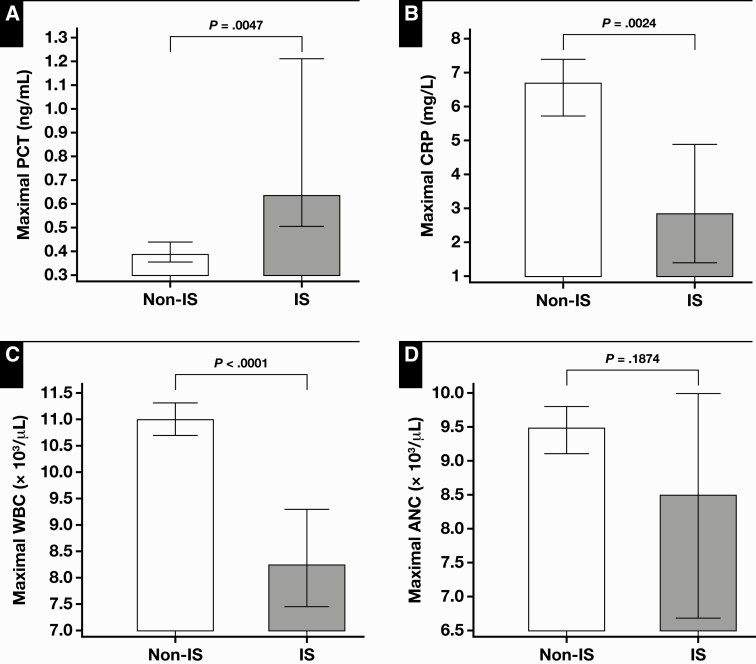
When the comparison of biomarkers of bacterial infection was performed in the subgroup of patients with microbiologically documented infections (ie, culture positive), maximal PCT levels were significantly higher in the immunosuppressed patients (0.64 ng/mL) compared with the nonimmunosuppressed patients (0.39 ng/mL) Table 3, with a statistical significance (P = .0045) Figure 2A.
Table 3.
Comparison of the Biomarker Levels Between the Nonimmunosuppressed and the Immunosuppressed Cohorts in the Subgroup of Patients With Microbiologically Documented Infections
| Characteristic | Nonimmunosuppressed Cohort | Immunosuppressed Cohort | P Value |
|---|---|---|---|
| No. of encounters | 2,677 | 150 | |
| PCT | 2,677 | 150 | |
| Maximum PCT, median (IQR) | 0.39 (0.12-2.15) | 0.64 (0.18-3.19) | .0047 |
| High PCT (>0.5 ng/mL), No. (%) | 1227 (46) | 87 (58) | .0037 |
| Low PCT (<0.5 ng/mL), No. (%) | 1450 (54) | 63 (42) | |
| CRP, No. | 673 | 54 | |
| Maximum CRP, median (IQR) | 6.70 (2.10-13.72) | 2.85 (1.00-8.50) | .0024 |
| High CRP (>5 mg/L), No. (%) | 378 (56) | 18 (33) | .0012 |
| Low CRP (≤5 mg/L), No. (%) | 295 (44) | 36 (67) | |
| WBCs, No. | 2676 | 150 | |
| Maximum, median (IQR) | 11.0 (7.6-15.4) | 8.3 (5.6-12.9) | <.0001 |
| High WBCs (>10 × 103 cells/μL), No. (%) | 1,506 (56) | 56 (37) | <.0001 |
| Low WBCs (≤10 × 103 cells/μL), No. (%) | 1,170 (44) | 94 (63) | |
| ANC, No. | 1,841 | 110 | |
| Maximum, median (IQR) | 9.5 (5.7-14.2) | 8.5 (4.3-15.2) | .1874 |
| High ANC (>7.7 × 103 cells/μL), No. (%) | 1,110 (60) | 61 (55) | .3144 |
| Low ANC (≤7.7 × 103 cells/μL), No. (%) | 731 (40) | 49 (45) |
ANC, absolute neutrophil count; CRP, C-reactive protein; PCT, procalcitonin.
Figure 2.
Comparison of median values of maximal procalcitonin (PCT) (A), C-reactive protein (CRP) (B), WBC (C), and absolute neutrophil count (ANC) (D) levels between culture-positive, nonimmunosuppressed (Non-IS) patients and culture-positive immunosuppressed (IS) patients. Error bars represent 95% confidence interval of median.
However, maximal CRP and maximal WBC levels were significantly lower in the immunosuppressed patients compared with the nonimmunosuppressed patients (P = .0024, and P < .0001) Figure 2B and Figure 2C. The ANC was not different in these patient groups (P = .2) Figure 2D.
Influence of Clinical Variables on the Level of PCT in the Immunosuppressed Cohort
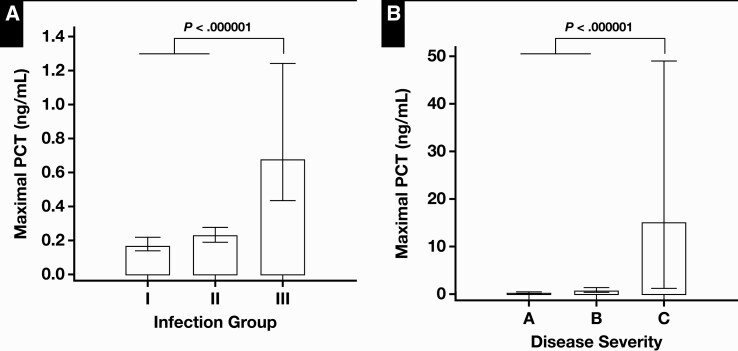
Of 381 encounters, 165 had less than a 1-year posttransplantation period, and their maximal PCT levels were significantly increased compared with those with a posttransplantation period of more than 1 year (0.33 vs 0.22 ng/mL, P = .0022) Table 4. PCT levels were not different between patients with rejection and those without rejection. Patients initially admitted to the ICU had significantly increased maximal PCT levels compared with those admitted to non-ICU wards (0.88 vs 0.22 ng/mL, P < .0001). The maximal PCT levels in patients with clinical and microbiologically proven bacterial infection (group III, 0.68 ng/mL) were significantly increased compared with the levels in both patients with only clinically defined infection (group II, 0.23 ng/mL) and patients with no evidence of infection (group I, 0.17 ng/mL) Figure 3A. In addition, among patients with infection (groups II and III), PCT levels were significantly increased with increasing severity, infection without SIRS, sepsis, severe sepsis, and septic shock (P = .000001) Figure 3B.
Table 4.
Median PCT Values of the Immunosuppressed Cohort According to the Clinical Variables
| Characteristic | Immunosuppressed Cohort, No. | Median PCT (IQR), ng/mL | P Value |
|---|---|---|---|
| Posttransplant period | |||
| 0-12 mo | 165 | 0.33 (0.14-1.46) | .0022 |
| >12 mo | 216 | 0.22 (0.10-0.64) | |
| Rejection | |||
| Yes | 49 | 0.21 (0.09-0.44) | .0591 |
| No | 328 | 0.28 (0.12-1.06) | |
| Admission | |||
| Ward | 294 | 0.22 (0.10-0.58) | <.0001 |
| ICU | 84 | 0.88 (0.27-3.63) | |
| Infectiona | |||
| Group I | 132 | 0.17 (0.09-0.55) | <.000001 |
| Group II | 138 | 0.23 (0.11-0.62) | |
| Group III | 150 | 0.64 (0.18-3.19) | |
| Severity of infection | |||
| Infection without SIRS | 166 | 0.25 (0.12-0.70) | <.000001 |
| Sepsis, severe sepsis | 64 | 0.76 (0.23-2.45) | |
| Septic shock | 16 | 15.10 (1.26-53.60) | |
| Culture-positive encounter,b No. | 150 | ||
| Blood, No. (%) | 51 (34) | 3.19 (1.24-21.78) | <.000001c |
| Urine, No. (%) | 36 (24) | 0.29 (0.12-0.63) | |
| Respiratory, No. (%) | 47 (31) | 0.33 (0.15-1.35) | |
| Peritoneal, No. (%) | 2 | NA | |
| CSF, No. (%) | 0 | ||
| Body fluid, No. (%) | 3 (2) | 0.08 (0.07-0.16) | |
| Multiple sites, No. (%) | 11 (7) | 0.58 (0.41-14.74) |
CSF, cerebrospinal fluid; ICU, intensive care unit; IQR, interquartile range; NA, not applicable; PCT, procalcitonin; SIRS, systemic inflammatory response syndrome.
aGroup I, patients with no evidence of infection; group II, patients who only met clinical criteria of infection; group III, patients who met both clinical and microbiologic criteria of infection.
bSame patient subgroup as group III.
cKruskal-Wallis test performed among blood, urine, and respiratory tract culture-positive encounters.
Figure 3.
Comparison of median values of maximal procalcitonin (PCT) according to the type of infections (A) and the severity (B) in the immunosuppressed patients. A, Group I, patients with no evidence of infection; group II, patients who only met clinical criteria of infection; group III, patients who met both clinical and microbiologic criteria of infection. A, infection without systemic inflammatory response syndrome; B, sepsis and severe sepsis; C, septic shock.
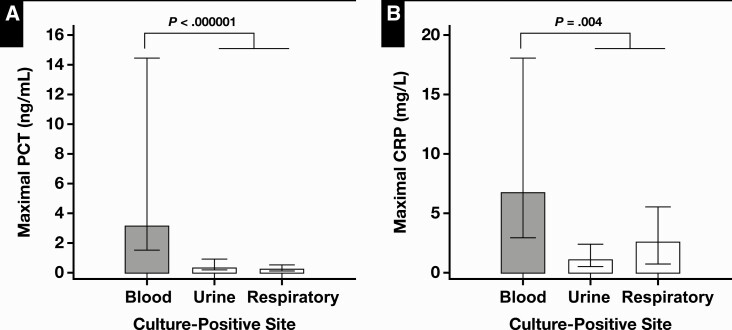
Of 288 encounters with available culture data in the immunosuppressed cohort, 150 (52%) encounters had positive culture results (Table 4). The effect of the culture-positive site on the level of maximal PCT was significant (P < .000001) by the Kruskal-Wallis test. Also, on post hoc analysis Figure 4A, PCT levels associated with bacteremia (median, 3.19 ng/mL) were significantly increased compared with culture-positive encounters associated with the urinary tract and the respiratory tract.
Figure 4.
Comparison of median values of maximal procalcitonin (PCT) (A) and maximal C-reactive protein (CRP) (B) according to the culture-positive site.
Similarly, there was a significant difference in CRP levels according to the culture-positive sites by Kruskal-Wallis test. By post hoc analysis, CRP levels associated with encounters with bacteremia (median, 6.80 mg/L) were higher compared with other culture-positive sites (P = .004) Figure 4B. WBC and ANC levels were not different among culture-positive sites.
Discussion
Infection is a major cause of morbidity and mortality in transplant-related immunosuppressed patients, and there is an inclination toward initiating broad-spectrum antibiotics at the earliest signs of infection in this patient group. However, unnecessary broad-spectrum antibiotic therapy can be detrimental to the graft as well as the patient, and de-escalation of antibiotic therapy is necessary to reduce preventable drug toxicity, and development of MDR organisms, C difficile, and/or fungal infections.
PCT is a recommended marker to guide antibiotic therapy in patients with lower respiratory tract infections14-16 and sepsis.17 However, the reliability of PCT levels in the immunosuppressed patients has been questioned.18 In this regard, our real-world data from a cohort of immunosuppressed patients with diverse transplanted organs demonstrated that the magnitude of the PCT response is not suppressed by immunosuppressive medications. Moreover, even when the PCT levels were compared within the subgroup of patients with microbiologically documented infections, PCT concentrations were greater in the immunosuppressed patients compared with the nonimmunosuppressed patients. Our data also replicate findings from a previous report that showed elevations of PCT in lung transplant recipients with culture-proven bacterial infections, while other markers of inflammation/infection such as CRP, WBC, and ANC were decreased in this setting.3 This is most likely due to the effect of the immunosuppressants such as calcineurin inhibitors, antiproliferative agents, and steroids on CRP concentrations and leukocyte counts.19 In comparison, PCT release is not influenced by conventional maintenance immunosuppression and is independent of leukocyte production, making PCT a more reliable marker for early and rapid detection of bacterial infection in this group of patients.
Our findings show that the maximum PCT in transplant-related immunosuppressed patients is higher than in nonimmunosuppressed patients. Also, in line with previous studies,20,21 PCT was not increased in patients with concomitant episodes of rejection. Therefore, our study result supports that PCT levels can be used to differentiate acute allograft rejection from bacterial infections in the immunosuppressed patients. However, there seems to be an elevation in baseline PCT levels in the early posttransplantation period. PCT levels were higher in patients within a year of transplantation, and this could not be explained by other variables known to increase PCT levels, including bacterial infections and disease severity. This increase may be the effect of surgical procedures during transplantation, implantation of devices, and use of induction immunosuppressive therapy, even in the absence of infection.9
Importantly, our data clearly demonstrated that PCT levels in immunosuppressed patients reflect the progression and severity of bacterial infections. Higher PCT levels differentiated microbiologically defined bacterial infections from both clinically defined infections and noninfectious etiologies, and PCT levels also reflected disease severity and levels increased from infections without SIRS, sepsis, and septic shock. Also, PCT levels were significantly higher in those with bacteremia compared with the other sites of infections. Previous reports showing that the levels of PCT correlate with the severity of the disease have been limited by the small number of patients and the specificity of the organ transplanted.3,22 Our findings from a large number of immunosuppressed patients with diverse transplanted organs demonstrate that PCT levels could be used to guide antimicrobial stewardship in transplant-related immunosuppressed patients.
Our retrospective data analysis had several limitations. First, the definition of immunosuppressed patients was narrow and only included patients with tacrolimus- and sirolimus-level measurement. Further analysis to assess whether our findings extend to patients on corticosteroids or newer immunomodulators would be of interest. Second, since this was not a prospectively designed study, the kinetics of PCT concentration could not be analyzed, which would ideally be based on a unified number of consecutive PCT measurements at defined time points. Therefore, a maximal PCT level of each encounter used a single representative PCT value. However, because the kinetics of PCT has significantly different interpatient variability,23 a maximal PCT concentration can still be regarded as an appropriate representative measure. Last, although the presence of a bacterial infection was confirmed by culture results, clinically documented infections could not be defined with certainty, and this hindered the designation and comparison with an infection negative control group. This study was also performed at a single site and had a relatively small sample size of immunosuppressed patients relative to the nonimmunosuppressed group.
In conclusion, we have shown that the ability of PCT to become elevated remains intact despite immunosuppressive therapy, in contrast to other commonly used biomarkers such as CRP, WBC, and ANC. Also, we have shown that PCT levels in immunosuppressed patients increase most significantly in patients with bacteremia and reflect the severity of bacterial infection. These results indicate that PCT could potentially be used for antibiotic stewardship in transplant-related immunosuppressed patients. Larger, multicenter prospective studies will be necessary to determine a threshold value and develop a PCT-guided algorithm to guide antibiotics stewardship efforts in transplant-related immunosuppressed patients.
Acknowledgments: The project described was partially supported by the National Institutes of Health (grant UL1TR001442) of Clinical and Translational Science Awards funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Gregory B. Seymann and Nicholas Bevinshave received speaking fees from ThermoFisher. Robert L. Fitzgerald has received advisory board fees from Roche Diagnostics and speaking fees from ThermoFisher.
References
- 1. Pizzo PA. Fever in immunocompromised patients. N Engl J Med. 1999;341:893-900. [DOI] [PubMed] [Google Scholar]
- 2. Cervera C, van Delden C, Gavalda J, et al. Multidrug-resistant bacteria in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(suppl 7):49-73. [DOI] [PubMed] [Google Scholar]
- 3. Suberviola B, Castellanos-Ortega A, Ballesteros MA, et al. Early identification of infectious complications in lung transplant recipients using procalcitonin. Transpl Infect Dis. 2012;14:461-467. [DOI] [PubMed] [Google Scholar]
- 4. Halder R, Seth T, Chaturvedi PK, et al. Comparison of CRP and procalcitonin for etiological diagnosis of fever during febrile neutropenia in hematology patients- an experience from a tertiary care center in northern India. Blood Cells Mol Dis. 2020;84:102445. [DOI] [PubMed] [Google Scholar]
- 5. Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605-1608. [DOI] [PubMed] [Google Scholar]
- 7. Whang KT, Vath SD, Becker KL, et al. Procalcitonin and proinflammatory cytokine interactions in sepsis. Shock. 2000;14:73-78. [DOI] [PubMed] [Google Scholar]
- 8. Meisner M, Schmidt J, Huttner H, et al. The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive Care Med. 2000;26(suppl 2):S212-S216. [DOI] [PubMed] [Google Scholar]
- 9. Sandkovsky U, Kalil AC, Florescu DF. The use and value of procalcitonin in solid organ transplantation. Clin Transplant. 2015;29:689-696. [DOI] [PubMed] [Google Scholar]
- 10. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3:e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wirz Y, Meier MA, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95-107. [DOI] [PubMed] [Google Scholar]
- 13. Schuetz P, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group . Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059-1066. [DOI] [PubMed] [Google Scholar]
- 14. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600-607. [DOI] [PubMed] [Google Scholar]
- 15. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84-93. [DOI] [PubMed] [Google Scholar]
- 16. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168:2000-2007. [DOI] [PubMed] [Google Scholar]
- 17. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498-505. [DOI] [PubMed] [Google Scholar]
- 18. Smith SE, Muir J, Kalabalik-Hoganson J. Procalcitonin in special patient populations: guidance for antimicrobial therapy. Am J Health Syst Pharm. 2020;77:745-758. [DOI] [PubMed] [Google Scholar]
- 19. de Kruif MD, Lemaire LC, Giebelen IA, et al. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008;34:518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammer S, Fraunberger P, Meiser B, et al. Procalcitonin, a new indicator for non-viral infections in heart, lung or liver transplant patients. Ann Transplant. 1999;4:5-9. [PubMed] [Google Scholar]
- 21. Prieto B, Llorente E, González-Pinto I, et al. Plasma procalcitonin measured by time-resolved amplified cryptate emission (TRACE) in liver transplant patients: a prognosis marker of early infectious and non-infectious postoperative complications. Clin Chem Lab Med. 2008;46:660-666. [DOI] [PubMed] [Google Scholar]
- 22. Staehler M, Hammer C, Meiser B, et al. Procalcitonin: a new marker for differential diagnosis of acute rejection and bacterial infection in heart transplantation. Transplant Proc. 1997;29:584-585. [DOI] [PubMed] [Google Scholar]
- 23. Desmard M, Benbara A, Boudinet S, et al. Post-operative kinetics of procalcitonin after lung transplantation. J Heart Lung Transplant. 2015;34:189-194. [DOI] [PubMed] [Google Scholar]