Abstract
This study presents the effects of OspA vaccination on two-step testing for Borrelia burgdorferi antibodies. Although vaccinees developed enzyme-linked immunosorbent assay reactivity, immunoblots did not fulfill Centers for Disease Control and Prevention criteria for positivity. Furthermore, OspA reactivity did not interfere with interpretation of immunoblots with sera from patients who developed early Lyme disease despite vaccination.
In December 1998, the Food and Drug Administration approved a recombinant outer surface protein A (OspA) vaccine preparation for prevention of Lyme disease (2). It has been suggested that widespread use of this vaccine will affect the performance of serologic testing for Lyme disease because the OspA antigen is a component of the whole-cell sonicate of Borrelia burgdorferi, which is used in most commercially available enzyme-linked immunosorbent assays (ELISAs) (11). This study evaluates the potential impact of OspA vaccination on two-stage B. burgdorferi serologic testing.
Serum samples.
Sequential serum specimens were obtained from 17 healthy adults participating in a phase 2 safety and immunogenicity study sponsored by Connaught Laboratories, Inc. (Swiftwater, Pa.). Serum specimens were collected prior to receipt of the first 30-μg intramuscular dose of the OspA vaccine (pre), at day 30, when a second 30-μg OspA vaccine dose was administered, and at days 60, 90, and 180 after the first vaccination. Serum samples were stored at −70°C until time of testing. Linkage was removed before testing.
To illustrate vaccine effects further, serum samples from selected individuals receiving the same OspA vaccine preparation in an efficacy study (12), including patients who developed early Lyme disease with erythema migrans despite vaccination, were studied. In the efficacy study, three 30-μg doses of vaccine were given to the volunteers at time 0, day 30, and 1 year.
ELISA.
Specimens were tested in an immunoglobulin M (IgM)-IgG ELISA (Lyme Stat; Whittaker M. A. Bioproducts Inc., Walkersville, Md.) in accordance with the manufacturer's instructions.
Immunoblots.
Separate IgM and IgG immunoblots (MarDx Diagnostics, Inc., Carlsbad, Calif.) were used to test all the serum specimens according to the manufacturer's instructions. The Centers for Disease Control and Prevention-Association of State and Territorial Public Health Laboratory Directors (CDC/ASTPHLD) criteria were used for blot interpretation (3).
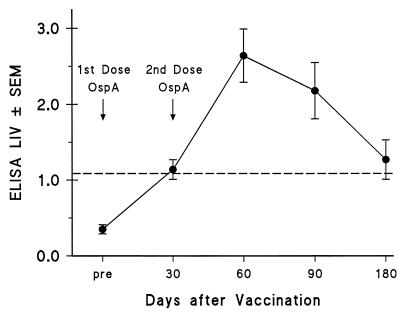
None of the 17 volunteers enrolled in the phase 2 trial had a positive ELISA or immunoblot result prior to receiving vaccination. Nine (53%) had a positive ELISA by 30 days after receipt of the first vaccine dose, and all 17 (100%) were reactive by ELISA by 30 days following the second dose (day 60) (Table 1). Six (35%) still had a positive ELISA by day 180. The highest mean Lyme index value (LIV) by ELISA was observed at day 60 (Fig. 1).
TABLE 1.
Reactivity by ELISA and IgM and IgG immunoblot tests after OspA vaccination in 17 individuals participating in phase 2 of the Connaught Lyme vaccine trial
| Day after vaccinationa | % of samples with:
|
||
|---|---|---|---|
| Positive ELISA | Positive IgM immunoblotb | Positive IgG immunoblotb | |
| Pre | 0 | 0 | 0 |
| 30c | 53 | 0 | 0 |
| 60d | 100 | 0 | 0 |
| 90 | 76 | Not done | Not done |
| 180 | 35 | 0 | 0 |
See text for details.
Based on CDC/ASTPHLD criteria (3).
Thirty days after receipt of the first 30-μg intramuscular dose of a recombinant OspA vaccine preparation.
Sixty days after receipt of the first 30-μg dose of the recombinant OspA vaccine preparation.
FIG. 1.
LIVs determined by ELISA (± standard errors of the means) are shown at different times postvaccination in 17 individuals receiving two vaccine doses. The dashed line indicates the cut-off LIV for a positive ELISA (LIV ≥ 1.09). The first vaccination dose was given after collecting the pre serum specimen, and the second dose was administered before the 30-day serum specimen was collected.
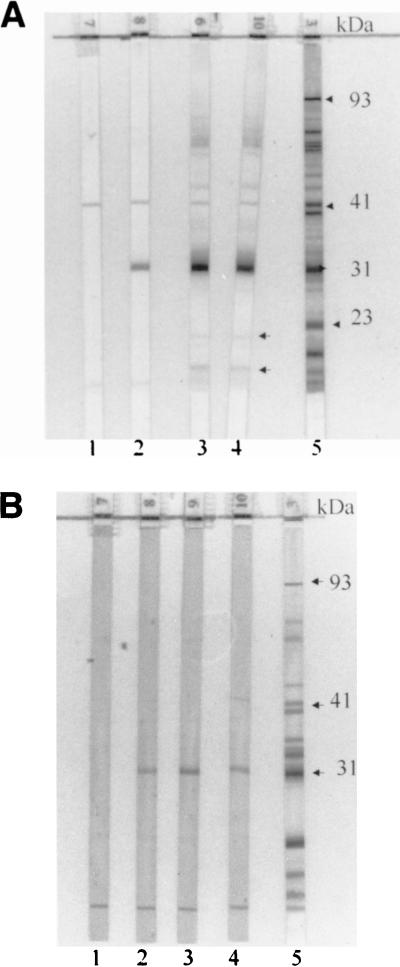
Both IgM and IgG antibodies to OspA were observed by immunoblot tests of serum samples from 65% of vaccine recipients by day 30. While IgM reactivity to OspA at day 60 was nearly identical to that at day 30, the IgG reactivity to OspA had risen to 93%. By day 180, 88% still showed IgG reactivity to OspA whereas only 18% had IgM reactivity. Of interest, despite the high rate of OspA reactivity by IgG immunoblot on day 180, only 35% were still reactive by ELISA. Importantly, none of the IgM or IgG immunoblot reactivities at any time point fulfilled the CDC/ASTPHLD criteria for a positive immunoblot result (Fig. 2).
FIG. 2.
IgG (A) and IgM (B) immunoblot series of serum samples obtained from a vaccinated individual participating in the phase 2 trial at different times postvaccination. Strip numbers are located at the bottom of each strip. Results with pre (strips 1), day 30 (strips 2), day 90 (strips 3), and day 180 (strips 4) samples are shown. Positive controls are shown in strips 5, with arrows indicating a few relevant bands. Those specimens showing the most intense reactivity to OspA in IgG blots (strips 3 and 4) also show reactivity to two other bands below 23 kDa (OspC) (arrows positioned next to strip 4) and diffuse darkening in the upper region of the blot. IgM reactivity to OspA was less intense than IgG reactivity.
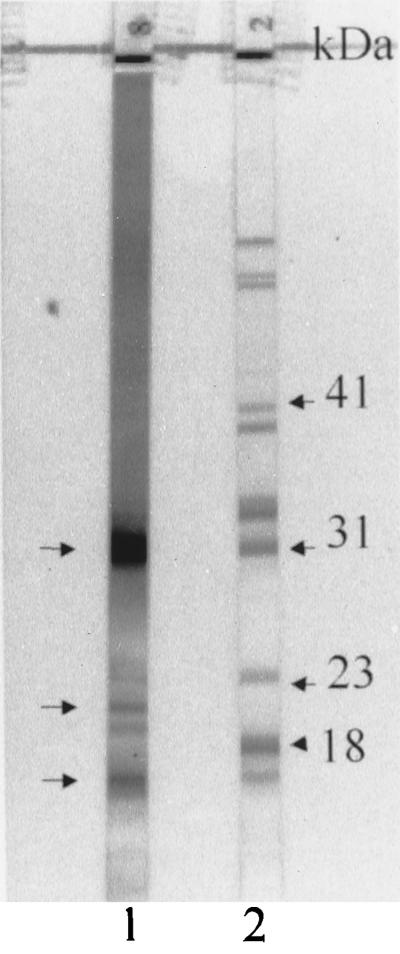
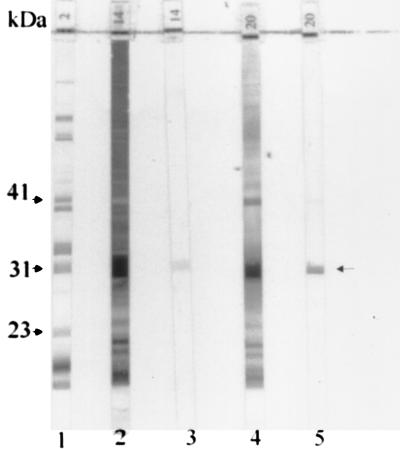
Although none of the immunoblots was interpreted as positive, several noteworthy effects on immunoblot reactivity were observed. In addition to the expected reactivity at the 31-kDa band (OspA), those serum samples with intense IgG OspA reactivity regularly had several bands of smaller molecular sizes, including bands of approximately 22 and 16 kDa, particularly in individuals receiving three vaccine doses (Fig. 3). The reason for the production of these additional bands is not known, but they may represent reactivity to degradation products of OspA or to other smaller-molecular-weight proteins which have amino acid sequences in common with epitopes of OspA. The presence of these bands might lead to diagnostic confusion if they are interpreted as indicating reactivity to the 18-kDa protein, which is a band of diagnostic importance for IgG immunoblot interpretation. Sera with OspA antibodies were also associated with darkening of the immunoblot extending down to the 31-kDa band and somewhat beyond. Reactivity of high-titer OspA antibodies to small amounts of residual OspA protein that failed to migrate to the 31-kDa position is a possible explanation for this. The darkening may make it difficult to visualize bands above 31 kDa or to judge their intensity accurately, including the six significant IgG bands with molecular sizes greater than 31 kDa (3). The darkening may be even more pronounced with serum samples from individuals after receipt of a third dose of recombinant OspA vaccine at the 12-month time point (Fig. 3, 4, and 5B). OspA antibody titers after three doses of vaccine are substantially greater than those after two doses (data not shown) (7). IgM reactivity to OspA, however, is of lesser intensity than the IgG response (Fig. 4).
FIG. 3.
IgG immunoblot of serum samples from an individual who received the third dose of the recombinant OspA vaccine preparation 2 weeks prior to collection of this serum specimen (strip 1). Besides the band of strong reactivity to OspA (top left arrow), two other bands below 23 kDa are visible (bottom two left arrows). These two bands are located above and below the 18-kDa band, which is included in the diagnostic criteria for IgG immunoblot interpretation (3). In addition, there is a general darkening of the immunoblot extending from the superior portion to beyond the 31-kDa band. Strip 2 shows a positive control, with arrows indicating a few relevant bands.
FIG. 4.
Representative IgG and IgM immunoblots of serum samples from two individuals who received three doses of the OspA vaccine preparation. Strip numbers are shown at the bottom of each strip. Strip 1 shows a positive control, and the arrows on the left indicate a few relevant bands. Strips 2 and 3 show the IgG and IgM immunoblots, respectively, of a serum sample from one individual collected 6 weeks after the third dose of vaccine. Strips 4 and 5 show the IgG and IgM reactivities of a serum sample collected from another individual 5 weeks following the third vaccine dose.
FIG. 5.
IgM and IgG blots of serial serum specimens from two patients who developed early Lyme disease associated with erythema migrans despite OspA vaccination. Strip numbers are shown at the bottom of each strip. (A) IgM (strips 1 to 4) and IgG immunoblots (strips 6 to 9) of serum samples from a patient who presented with erythema migrans (confirmed by a positive culture for B. burgdorferi) 6 months after the second vaccine dose. Serum specimens were collected prior to the first vaccine dose (strips 1 and 6) and 1 day (strips 2 and 7), 8 days (strips 3 and 8), and 3 weeks (strips 4 and 9) after onset of erythema migrans. A positive control is shown in strip 5, with arrows indicating a few relevant bands. The IgG immunoblots postvaccination show weak reactivity to OspA, and new IgG bands were observed in the acute- and convalescent-phase specimens. IgM immunoblots showed the appearance of the 41- and 23-kDa bands in the specimen collected 8 days postonset of illness, with expansion of reactivity at 3 weeks. (B) IgM (strips 1 to 3) and IgG (strips 5 to 7) immunoblot series of serum samples from a patient presenting with a 2-day history of multiple erythema migrans lesions when the serum specimens shown in strips 3 and 7 were collected. Strips 1 and 5 show the IgM and IgG immunoblots, respectively, of serum specimens collected prior to the first vaccine dose, and strips 2 and 6 show the IgM and IgG immunoblots of serum specimens collected prior to the third vaccine dose. The serum specimens shown in strips 3 and 7 were obtained 12 days after the third vaccine dose. Strip 4 shows a positive control, with arrows indicating a few relevant bands. Note that the IgM bands of diagnostic significance were not affected by the presence of IgM reactivity to OspA (strip 3). Strip 7 shows intense IgG reactivity to OspA and a general darkening of the immunoblot. Arrows located to the right of strip 7 indicate the frequently observed bands after two to three doses of vaccine, at 31 kDa and two bands below OspC.
Figure 5 shows examples of immunoblots of serum samples from participants in an efficacy study (12) who despite OspA vaccination developed Lyme disease. Although such patients generally had weak OspA reactivity, the bands of diagnostic significance on IgM immunoblots were readily visualized.
In our laboratory, similar findings to those presented in this study were observed in serum samples from individuals receiving the Food and Drug Administration-approved Lyme disease vaccine manufactured by SmithKline Beecham (data not shown).
If OspA vaccination becomes widely used, current ELISA testing using B. burgdorferi that contains OspA will become superfluous. Unfortunately, use of immunoblotting alone without a preceding ELISA will reduce specificity and increase costs. In studies to date, the reduction in specificity has ranged from 1.5 to 8% (mean, 4.1%), depending on the particular group of control sera tested and the particular ELISA and immunoblot tests used (4, 5, 9). Even a small reduction in specificity would have a substantial impact on the predictive value of a positive test when testing patients with a low pretest probability of Lyme disease (6).
Further, under the assumptions of an ELISA negativity rate of 75% in clinical practice (unpublished data) and a cost of $52 for an ELISA and $106 for an immunoblot test (8), an additional expenditure of 77 million dollars per year would be incurred by routinely performing immunoblot tests instead of conditional two-stage testing for the estimated 2.8 million serum samples submitted for Lyme disease serologic testing annually in the United States (10).
Pending the introduction of assays without OspA (13), we recommend that serologic testing for Lyme disease be limited to patients with at least a 20% pretest probability of Lyme disease (1) and that initial ELISA testing be omitted for OspA-immunized individuals.
Acknowledgments
We thank Eleanor Bramesco, Donna McKenna, Diane Holmgren, Susan Bittker, Denise Cooper, and Louis Rosenfeld for their assistance. We also thank Daniel Benevento for his expert photographic assistance.
REFERENCES
- 1.American College of Physicians. Guidelines for the laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1106–1108. doi: 10.7326/0003-4819-127-12-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Lyme disease vaccine approved. JAMA. 1999;281:405. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 4.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson B J B, Robbins K E, Bailey R E, Cao B-L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 6.Sivak S, Aguero-Rosenfeld M E, Nowakowski J, Nadelman R B, Wormser G P. Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease. Arch Intern Med. 1996;156:2105–2109. [PubMed] [Google Scholar]
- 7.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukemp S, Busarino C, Krause D S the Lyme Disease Vaccine Study Group. N. Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 8.Strickland G T, Karp A C, Mathews A. Utilization and cost of serologic tests for Lyme disease in Maryland. J Infect Dis. 1997;176:819–821. doi: 10.1086/517311. [DOI] [PubMed] [Google Scholar]
- 9.Trevejo R T, Krause P J, Sikand V K, Schriefer M E, Ryan R, Lapore T, Porter W, Dennis D T. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J Infect Dis. 1999;179:931–938. doi: 10.1086/314663. [DOI] [PubMed] [Google Scholar]
- 10.Tugwell P, Dennis D T, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere A C. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Wormser G P. Prospects for a vaccine to prevent Lyme disease in humans. Clin Infect Dis. 1995;21:1267–1274. doi: 10.1093/clinids/21.5.1267. [DOI] [PubMed] [Google Scholar]
- 12.Wormser G P, Nowakowski J, Nadelman R B, Schwartz I, McKenna D, Holmgren D, Aguero-Rosenfeld M. Efficacy of an OspA vaccine preparation for prevention of Lyme disease in New York State. Infection. 1998;26:208–212. doi: 10.1007/BF02962365. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y-Q, Mathiesen D, Kolbert C P, Anderson J, Schoen R T, Fikrig E, Persing D H. Borrelia burgdorferi enzyme-linked immunosorbent assay for discrimination of OspA vaccination from spirochete infection. J Clin Microbiol. 1997;35:233–238. doi: 10.1128/jcm.35.1.233-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]