Summary
Background
Adverse childhood experiences (ACEs) are associated with increased health risks across the life course. We aimed to estimate the annual health and financial burden of ACEs for 28 European countries.
Methods
In this systematic review and meta-analysis, we searched MEDLINE, CINAHL, PsycINFO, Applied Social Sciences Index and Abstracts, Criminal Justice Databases, and Education Resources Information Center for quantitative studies (published Jan 1, 1990, to Sept 8, 2020) that reported prevalence of ACEs and risks of health outcomes associated with ACEs. Pooled relative risks were calculated for associations between ACEs and harmful alcohol use, smoking, illicit drug use, high body-mass index, depression, anxiety, interpersonal violence, cancer, type 2 diabetes, cardiovascular disease, stroke, and respiratory disease. Country-level ACE prevalence was calculated using available data. Country-level population attributable fractions (PAFs) due to ACEs were generated and applied to 2019 estimates of disability-adjusted life-years. Financial costs (US$ in 2019) were estimated using an adapted human capital approach.
Findings
In most countries, interpersonal violence had the largest PAFs due to ACEs (range 14·7–53·5%), followed by harmful alcohol use (15·7–45·0%), illicit drug use (15·2–44·9%), and anxiety (13·9%–44·8%). Harmful alcohol use, smoking, and cancer had the highest ACE-attributable costs in many countries. Total ACE-attributable costs ranged from $0·1 billion (Montenegro) to $129·4 billion (Germany) and were equivalent to between 1·1% (Sweden and Turkey) and 6·0% (Ukraine) of nations’ gross domestic products.
Interpretation
Availability of ACE data varies widely between countries and country-level estimates cannot be directly compared. However, findings suggest ACEs are associated with major health and financial costs across European countries. The cost of not investing to prevent ACEs must be recognised, particularly as countries look to recover from the COVID-19 pandemic, which interrupted services and education, and potentially increased risk factors for ACEs.
Funding
WHO Regional Office for Europe.
Introduction
Awareness of the harms associated with adverse childhood experiences (ACEs) has increased substantially over the past two decades, supported by a proliferation of literature on the prevalence of ACEs and their relationships with poor life-course health.1 The term ACEs refers to various intensive stressors that can affect children while growing up, such as suffering maltreatment, witnessing violence in the home or community, and living with family difficulties (eg, parental substance abuse).2 Exposure to such stressors can influence children's neurological, biological, and social development and increase their susceptibility to social difficulties (eg, low educational attainment), health-harming behaviours (eg, smoking), and mental and physical illness across the life course.3, 4 Increased awareness of the links between ACEs and multi-agency priorities has driven the development of policy and practice aimed at preventing ACEs and supporting those affected by them.5, 6 However, as global attention and resources have diverted to addressing COVID–19, there are concerns that responses to the pandemic might have increased exposure to ACEs and exacerbated their effects.7, 8 Restrictions imposed to manage the pandemic have confined children and families within homes, closed essential social networks and support structures, and increased risk factors for ACEs such as unemployment and parental stress. Nations must now address the challenge of recovery, with investment choices being made in the face of a potential recession and competing priorities for funding. To support sound economic decision making, the costs of not investing in childhood must be recognised.
Research in context.
Evidence before this study
A rapidly expanding evidence base links adverse childhood experiences (ACEs) to poorer health and wellbeing across the life course. Exposure to ACEs can influence neurological and physiological development and increase vulnerability to the adoption of health-harming behaviours and development of mental illness and chronic disease. Such health effects impose major costs on both individuals and society. Our previous systematic reviews have examined the associations between ACEs and health outcomes and estimated the health and financial costs of ACEs at a regional level. Thus, the financial costs of ACEs across ten health risks and causes of ill health in 2017 were estimated to be US$581 billion in Europe and $748 billion in North America; equivalent to 2·7% of Europe's and 3·5% of North America's regional gross domestic products (GDPs).
Added value of this study
With few country-level estimates of the health and financial cost of ACEs available, this study calculated such estimates for each of 28 European countries. We updated previous searches to identify additional literature on the prevalence of ACEs in country samples and their associations with health outcomes in European populations. We used meta-analyses to generate pooled relative risks for 12 health outcomes. ACE prevalence estimates were generated for each of 28 European countries using available data and were used to calculate country-level population attributable fractions for each outcome. ACE-attributable disability-adjusted life-years for 2019 were calculated and equivalent financial costs estimated. Estimated annual costs attributable to ACEs were equivalent to between 1·1% and 6·0% of countries' GDPs.
Implications of all the available evidence
ACEs are consistently related to many leading risk factors for, and causes of, ill health and estimates suggest that ACEs can impose major health and financial burdens on European nations. The extent and quality of data available to estimate costs varies widely across countries; thus, our findings here are not comparable between countries and estimates could be improved in most countries by improved data collection. Importantly, ACEs can be prevented and their costs avoided by investing in evidence-based early years, family, and resilience-building programmes. The COVID-19 pandemic has disrupted many such services and increased risk factors for ACEs. Equally, the links between ACEs, health-harming behaviours, and chronic conditions mean ACEs might increase an individual's susceptibility to COVID-19 and to the potential negative effects of restrictions imposed to control the virus. Investment choices made by governments towards pandemic recovery and future pandemic preparedness must recognise the importance of ACE prevention and support services in creating resilient societies.
Until recently, little data were available on the economic costs of ACEs, with most studies focusing on individual adversity types such as child maltreatment.9 However, a 2019 study estimated the annual burden of ACE exposure across ten risk factors for, and causes of, ill health to be US$581 billion in Europe and $748 billion in North America; equivalent to 2·7% of Europe's and 3·5% of North America's gross domestic product (GDP).10 Although these figures expose the immense costs associated with ACEs, more nuanced country-level data are required to support policy development and decision making at a national level. Thus, estimates have since been published for some countries and regions. For example, in California, USA, the annual cost of ACEs across eight health risks and causes has been estimated to exceed $100 billion,11 while in England and Wales annual costs across 13 health risks and causes of ill health have been estimated at £43 billion.12 However, to our knowledge no cost estimates are available for other European countries.
Estimating the national costs of ACEs requires a measure of the prevalence of ACEs within a country's population. In the past few years, the number of studies measuring ACEs has burgeoned with a bibliographic review finding that almost half of studies on ACEs published between 1998 and 2018 had been published since 2017.1 Although much research focuses on North American populations, studies are emerging from an increasing number of countries around the world, including many European countries. Thus, here we developed the approach used to produce continent-level estimates for Europe and North America,10 to estimate the annual costs associated with ACEs in individual European countries. Using data from European studies, we calculated country-level population attributable fractions (PAFs) for one and multiple ACEs across 12 health outcomes, including four risk factors (harmful alcohol use, smoking, illicit drug use, high body-mass index [BMI]; defined as ≥25 kg/m2 in all included studies) and eight causes of ill health (depression, anxiety, interpersonal violence, cancer, type 2 diabetes, cardiovascular disease, stroke, and respiratory disease). We calculated the health and financial burden of ACEs for each of 28 countries using an adapted human capital method. The study does not intend to provide comparable estimates of ACE prevalence or ACE burden between countries, but rather to develop the best estimates for each country using available data.
Methods
Search strategy and selection criteria
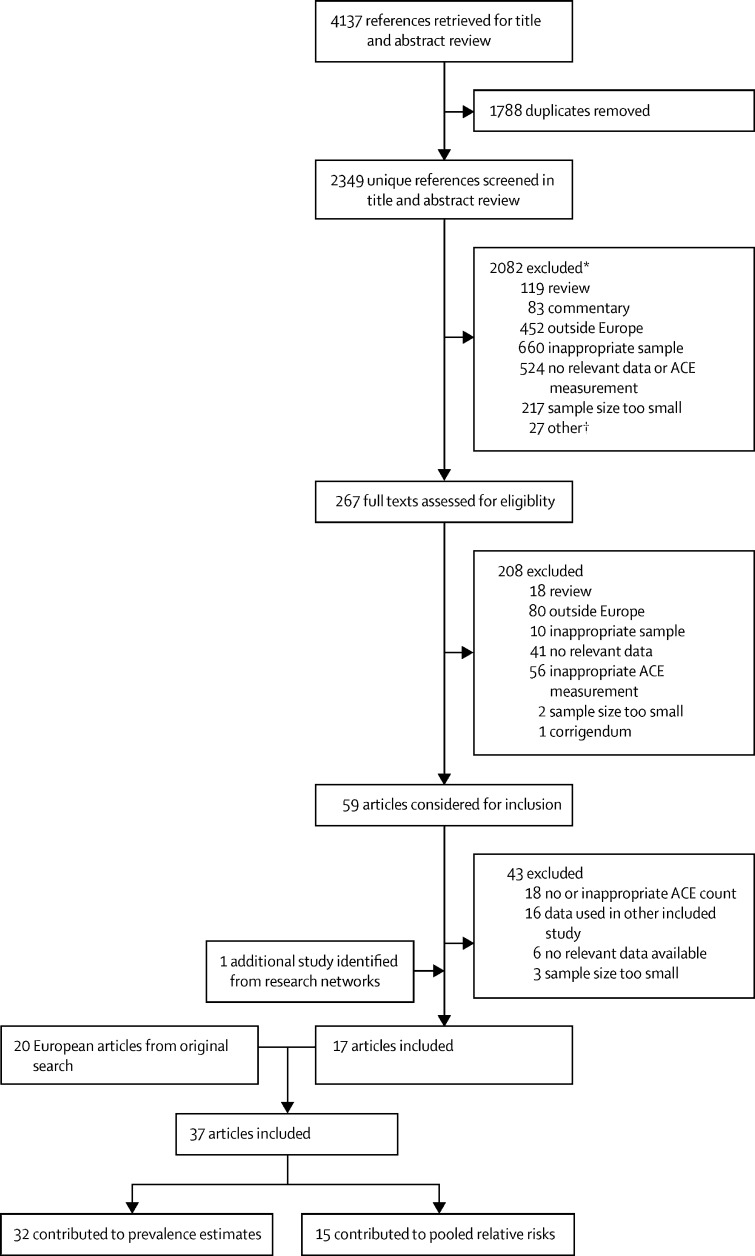
In this systematic review and meta-analysis, we updated searches done by Bellis and colleagues10 in 2019 (covering Jan 1, 1990, to July 11, 2018) to identify additional quantitative studies (published July 11, 2018, to Sept 8, 2020; figure) that reported (1) prevalence of cumulative ACEs (ie, number of ACEs) and (2) risks of health outcomes associated with cumulative ACEs. We aimed to identify studies providing data that enabled an initial estimation of the cost of ACEs in various European countries. Six electronic databases (MEDLINE, CINAHL, PsycINFO, Applied Social Sciences Index and Abstracts, Criminal Justice Databases, and Education Resources Information Center) were searched with the terms “adverse childhood experience*”, “adverse childhood event*”, and “childhood adversit*” in title or abstract fields. Searches were done by FG, limited to journal articles published from July 11, 2018, with no language restrictions applied. Search results were extracted into Microsoft Excel. Searches were supplemented by use of author research networks. Title and abstract screening was undertaken by two reviewers (FG and EH) and full-text copies of relevant articles were obtained and screened by two reviewers (FG, EH, and KH) independently (figure).
Figure.
Study selection flowchart
The original search covered Jan 1, 1990, to July 11, 2018, and the updated search July 11, 2018, to Sept 8, 2020. ACE=adverse childhood experience. *Denotes the primary reason given but other reasons could apply. †Included case studies, corrections, and animal studies.
Selected studies met the following criteria: reported on samples from WHO European region countries; focused on adults; used general population or non-high-risk samples; had a sample size of at least 1000 individuals; used a cumulative measure of at least three ACEs that included both direct (eg, maltreatment) and indirect (eg, household difficulties) types (table 1); provided prevalence data for 0 ACEs, one ACE, and multiple ACEs (in categories permitting synthesis into a category of ≥2 ACEs); and, for outcome studies, presented odds ratios (ORs), relative risks (RRs), or hazard ratios for one ACE and multiple ACEs (in categories permitting synthesis to a category of ≥2 ACEs) compared with those without ACEs. We contacted study authors for additional data to enable inclusion when possible. Studies considered for inclusion in Bellis and colleagues’ 2019 study10 were reconsidered for inclusion if they had contributed to European pooled risk ratios or met the inclusion criteria. Studies were selected for inclusion in prevalence and outcome estimates separately. For each estimate type, when more than one study reported the same data for the same sample, a single study was selected on the basis of larger sample size or data suitability. 12 health outcomes were included for which data were available from at least three studies. No specific definitions were used for outcomes (appendix p 1).
Table 1.
ACE types measured by included studies
| Studies, n | |
|---|---|
| Household substance abuse | 30 |
| Household mental illness | 29 |
| Parental separation or divorce | 25 |
| Physical abuse | 24 |
| Sexual abuse | 21 |
| Household member incarcerated or criminality | 20 |
| Exposure to domestic violence | 17 |
| Emotional, psychological, or verbal abuse | 17 |
| Parental death | 15 |
| Child welfare intervention (eg, out-of-home care) | 14 |
| Neglect | 12 |
| Family welfare or financial difficulties | 10 |
| Serious childhood illness or injury | 5 |
Questions used to determine ACEs varied between studies. Other ACE types (measured in <3 studies) included childhood maltreatment, frequent fear of a family member, bullying, peer violence, victim of serious physical attack, frightening experience, family conflict or discord, parental affectionless control, parental dissatisfaction, parental illness or disability, separation from mother, other parental loss, sent away from home, death of a sibling, low parental concern for child's education, child labour, hunger, or residential instability. ACE=adverse childhood experience.
Data extraction and analysis
Included studies were independently quality assessed (by KF, FG, and EH) using criteria covering sampling approach, bias, measurement of ACEs, response rate, refuser characteristics, sample description and, for outcome studies, data analysis (appendix p 3). Data on country, study type, participants, ACE measurements, ACE prevalence, and outcome measurements were extracted independently by two reviewers and checked by a third (KH, KF, FG, and EH; appendix pp 2–3).
To calculate pooled risk estimates, RRs or ORs (adjusted for sociodemographic data when available) were extracted from new outcome articles for each available ACE count level. ORs were transformed into RRs using RR=OR/(1 − p0 + (p0 × OR)), where p0 is the baseline risk in the absence of ACEs.13 Two articles presented data from ACE studies using students in multiple countries;14, 15 raw data were available from these student studies and thus each study was included as a separate sample with RRs calculated using generalised linear models. Raw data were also available for five UK studies combined in one article,12 and here study-level RRs were calculated by the authors when not included in previous estimates. For studies presenting multiple ACEs in more than one category (eg, 2–3 ACEs, ≥4 ACEs), these data were combined to an RR for at least two ACEs using a weighted mean method. Adjusted positive and negative counts by outcome and ACE category were calculated for use in meta-analyses. Existing calculations of positive and negative counts were used when studies had contributed to previous pooled RRs.10 For each outcome, pooled RRs (95% CIs) were generated through random effects models. Heterogeneity between studies was measured using I2 statistic (table 2).16 Risk of publication bias was explored using Begg-Mazumdar and Egger tests and visual inspection of funnel plots when sufficient samples (>10)16 were available.
Table 2.
Pooled relative risks for risk factors and causes of ill health
| Samples, n* | Individuals, n |
1 ACE |
≥2 ACEs |
|||
|---|---|---|---|---|---|---|
| Pooled relative risk (95% CI) | Heterogeneity, I2 (95% CI) | Pooled relative risk (95% CI) | Heterogeneity, I2 (95% CI) | |||
| Risk factors | ||||||
| Harmful alcohol use | 15 | 29 013 | 1·64 (1·43–1·88) | 51·6% (0–71·8) | 2·60 (2·07–3·27) | 87·8% (81·8–91·1) |
| Smoking | 17 | 32 173 | 1·27 (1·18–1·35) | 31·7% (0–61·0) | 1·74 (1·55–1·95) | 83·1% (73·9–88·1) |
| Illicit drug use | 17 | 32 513 | 1·59 (1·44–1·76) | 48·6% (0–69·4) | 2·63 (2·27–3·05) | 84·0% (75·4–88·6) |
| High BMI† | 5 | 12 586 | 1·02 (0·97–1·06) | 0% (0–64·1) | 1·07 (1·02–1·12) | 36·2% (0–75·7) |
| Causes of ill health | ||||||
| Depression | 6 | 1 473 863 | 1·44 (1·34–1·56) | 86·1% (69·2–91·8) | 2·19 (1·92–2·51) | 95·3% (92·7–96·6) |
| Anxiety | 3 | 6744 | 1·49 (1·27–1·74) | 0% (0–72·9) | 2·71 (1·89–3·89) | 85·1% (24·9–93·3) |
| Interpersonal violence | 5 | 15 267 | 1·31 (0·93–1·86) | 50·3% (0–79·9) | 3·72 (2·79–4·97) | 65·6% (0–84·7) |
| Cancer | 6 | 25 035 | 1·13 (0·95–1·33) | 30% (0–71·7) | 1·51 (1·28–1·78) | 18% (0–67·6) |
| Type 2 diabetes | 5 | 19 178 | 1·15 (0·89–1·48) | 65·5% (0–84·7) | 1·45 (1·08–1·94) | 75·7% (11·6–88·2) |
| Cardiovascular disease | 6 | 20 909 | 1·08 (0·99–1·19) | 0% (0–61) | 1·46 (1·25–1·69) | 36·9% (0–73·9) |
| Stroke | 4 | 12 769 | 1·28 (0·84–1·96) | 0% (0–67·9) | 1·76 (1·22–2·52) | 0% (0–67·9) |
| Respiratory disease | 5 | 19 215 | 1·32 (1·09–1·59) | 0% (0–64·1) | 2·26 (1·88–2·72) | 0% (0–64·1) |
ACE=adverse childhood experience. BMI=body-mass index.
See appendix (p 2) for included studies.
Defined in included studies as BMI ≥25 kg/m2.
Country-level ACE prevalence was calculated for countries with sufficient prevalence data available (≥1000 individuals; table 3). To provide a level of correction for countries’ data coverage (ie, how representative available data were at a population level), country-level estimated marginal means and 95% CIs were calculated for each ACE category (0 ACE, 1 ACE, ≥2 ACEs) using a generalised linear model; weighting for sample size and incorporating data on sample country, sample age, sample representativeness, and number of ACEs measured. Estimated marginal means were adjusted towards samples that included all adult ages (vs younger [approximately <30 years], mid-age [approximately 30–49 years], or older samples [approximately ≥50 years]), represented their target population demographics (vs non-representative samples); and measured more ACEs (≥8 vs <8).
Table 3.
Number of samples, total sample size, sample types, and resulting adjusted proportion within each ACE group by country
| Samples, n | Sample size, n | Sample type* |
Adjusted prevalence |
|||
|---|---|---|---|---|---|---|
| 0 ACEs | 1 ACE | ≥2 ACEs | ||||
| Albania | 1 | 1395 | Students | 48·4% | 20·3% | 31·2% |
| Belgium | 1 | 2618 | Older adults | 69·9% | 23·4% | 6·8% |
| Czech Republic | 2 | 3327 | Students and older adults | 65·6% | 20·4% | 14·0% |
| Denmark | 3 | 985 987 | General† and older adults | 57·0% | 28·9% | 14·1% |
| Finland | 2 | 32 817 | General† | 30·6% | 30·6% | 38·8% |
| France | 1 | 2174 | Older adults | 65·9% | 25·6% | 8·5% |
| Germany | 3 | 5748 | General†, students, and older adults | 51·8% | 22·6% | 25·6% |
| Greece | 1 | 2776 | Older adults | 77·3% | 18·4% | 4·2% |
| Hungary | 1 | 1174 | General† | 75·0% | 12·0% | 12·9% |
| Ireland | 1 | 6408 | General† | 53·2% | 30·8% | 16·1% |
| Italy | 1 | 2399 | Older adults | 66·8% | 24·9% | 8·4% |
| Latvia | 1 | 1003 | Students | 43·1% | 25·9% | 31·0% |
| Lithuania | 1 | 1398 | Students | 64·0% | 19·7% | 16·3% |
| Moldova | 1 | 1351 | Students | 68·9% | 16·8% | 14·3% |
| Montenegro | 1 | 1084 | Students | 72·6% | 16·5% | 11·0% |
| Netherlands | 3 | 12 228 | General† and older adults | 43·8% | 25·8% | 30·4% |
| North Macedonia | 1 | 1200 | Students | 79·6% | 14·3% | 6·1% |
| Norway | 3 | 23 591 | General† and students | 38·0% | 32·4% | 29·6% |
| Poland | 2 | 3446 | Students and older adults | 63·5% | 22·7% | 13·8% |
| Romania | 1 | 1542 | Students | 62·1% | 20·3% | 17·6% |
| Russia | 1 | 1403 | Students | 72·7% | 15·8% | 11·6% |
| Serbia | 1 | 2074 | Students | 73·2% | 16·7% | 10·0% |
| Spain | 2 | 4216 | General† and older adults | 72·8% | 20·2% | 7·0% |
| Sweden | 3 | 1 002 796 | General† and older adults | 75·7% | 17·2% | 7·1% |
| Switzerland | 3 | 7957 | General† and older adults | 38·3% | 29·0% | 32·7% |
| Turkey | 1 | 1763 | Students | 72·1% | 16·5% | 11·3% |
| Ukraine | 2 | 2997 | General† and students | 50·3% | 28·9% | 20·8% |
| UK | 11 | 64 840 | General† and older adults | 53·9% | 23·0% | 23·0% |
| Overall | 55 | 2 181 712 | .. | 62·3% | 22·6% | 15·2% |
Adjusted ACE prevalence levels were calculated from available study data for the purpose of generating country-level population attributable fractions and are provided for information purposes. Figures should not be assumed to be nationally representative and compared between countries because of differences in data collection methods and sample characteristics (appendix p 2). ACE=adverse childhood experience.
Students includes samples recruited from educational institutions. Older adults comprised individuals predominantly older than 50 years.
Including general population samples and those from primary care (n=1, UK) and public sector (n=1, Finland) studies.
Country-level PAFs were calculated for each outcome and ACE level using pooled RRs and country-level ACE prevalence according to:
where RRACE(exposure) is the pooled RR associated with ACEs and PACE(exposure) the proportion of the sample exposed.17
An adapted human capital methodology was used to calculate annual costs associated with each outcome.9, 10, 18 For each country, 2019 disability-adjusted life-years (DALYs) estimates for matched Global Burden of Disease study outcomes (appendix p 1) were extracted for age categories 15–49 years, 50–69 years, and 70 years or older; and GDP and GDP per capita (2019 US$) were extracted from World Bank data. For each country, one DALY was assumed to equal GDP per capita (which ranged from $3659 in Ukraine to $81 994 in Switzerland); thus, costs for each outcome were calculated using DALYs*GDP per capita. This commonly used calculation reflects a year of life lost to disability or death being unproductive; with GDP per capita being the annual economic production value of each person in a population. Country-level PAFs were applied to total DALYs and costs to estimate the economic value of DALYs lost for each outcome by ACE level. To generate a total cost of ACEs for each country, we excluded DALYs from risk factors that related to included causes to avoid double counting (eg, DALYs for smoking attributed to cancer; for each risk factor, DALYs relating to all included causes were excluded). The value of DALYs lost as a proportion of total national GDP was also calculated. In sensitivity analyses, country-level PAFs for each outcome were generated using upper and lower 95% CIs for pooled RRs, upper and lower estimates for country-level ACE prevalence, and overall ACE prevalence for all studies combined (appendix pp 8–35). Data editing and calculations were done in Microsoft Excel. Generalised linear models were done in SPSS (version 24). Meta-analyses were done using StatsDirect (version 3.1.18).
Role of the funding source
Members of the funding organisation supported identification of relevant studies through partner networks, contributed to manuscript editing, and supported the decision to submit.
Results
2349 unique references were identified. Full-text copies of 267 (11·4%) articles were obtained and screened (figure). After 208 (77·9%) were excluded, 59 (22·1%) articles were considered for inclusion, along with 53 European articles drawn from the 223 articles considered in the 2019 original search. 37 articles (20 from the original search and 17 from the updated search) were selected for inclusion (figure), 15 (40·5%) of which contributed to pooled RRs and 32 (86·5%) to ACE prevalence estimates. Study characteristics are shown in the appendix (pp 2–3). 15 articles used UK samples;12, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 four used multi-country samples;14, 15, 33, 34 two used samples from either Denmark,35, 36 Finland,37, 38 Germany,39, 40 the Netherlands,41, 42 Norway,43, 44 Sweden,45, 46 or Switzerland;47, 48 and one each from Hungary,49 Ireland,50 Spain,51 and Ukraine.52 Study sample sizes ranged from 1059 individuals to 978 647 individuals (appendix pp 2–3). Samples were predominantly drawn from general populations, but student,14, 15, 39, 43 primary care,24 and public sector employee37 samples were also included. The number and variety of ACEs collected by studies varied (table 1; appendix pp 4–5), as did definitions of several outcome measurements (appendix p 1). Outcome studies also varied in the level of adjustment for confounding, although most studies adjusted for demographic factors (appendix pp 2–3).
Table 2 presents pooled RRs for individuals with one ACE and two or more ACEs (vs 0 ACEs) for each outcome, with included sample numbers and sizes (forest plots are provided in the appendix pp 36–53). For all outcomes, pooled RRs increased from one ACE to two or more ACEs (table 2). RRs for one ACE ranged from 1·02 (95% CI 0·97–1·06) for high BMI to 1·64 (1·43–1·88) for harmful alcohol use, and for two or more ACEs ranged from 1·07 (1·02–1·12) for high BMI to 3·72 (2·79–4·97) for interpersonal violence (table 2). The lower boundary for the 95% CI was below 1·0 for high BMI, interpersonal violence, cancer, type 2 diabetes, cardiovascular disease, and stroke with one ACE (table 2). We found considerable heterogeneity (I2 >75%) between estimates for depression with one ACE, and for harmful alcohol use, smoking, illicit drug use, depression, anxiety, and type 2 diabetes with two or more ACEs (table 2). Begg-Mazumdar and Egger tests for bias were done for harmful alcohol use, smoking, and illicit drug use; results were only significant for harmful alcohol use with two or more ACEs (appendix pp 38, 41, 44).
The 32 studies contributing to prevalence estimates provided sufficient data to calculate estimates for 28 countries. Across all studies, adjusted prevalence of any ACE was 37·8% (one ACE, 22·6%; two or more ACEs, 15·2%; n=2 181 712 individuals; table 3). Table 3 shows adjusted ACE prevalence for each country (upper and lower estimates and unadjusted figures are given in the appendix p 6). Adjusted proportions with any ACE ranged from 20·4% (North Macedonia) to 69·4% (Finland), and for two or more ACEs ranged from 4·2% (Greece) to 38·8% (Finland; table 3). Distribution of ACE category among those with ACEs varied by country, with the proportion of individuals with any ACE reporting two or more ACEs ranging from 18·7% in Greece to 60·6% in Albania (table 3).
ACE prevalence estimates were used to calculate country-level PAFs due to ACEs for each outcome. PAFs were generated for one ACE and two or more ACEs and summed to provide a total PAF due to ACEs. Total PAFs for each country and outcome are shown in the appendix (p 7). With the highest calculated ACE prevalence, Finland had the highest totalled PAFs due to ACEs across all outcomes, and Greece had the lowest (appendix p 7). In most countries, the largest PAFs due to ACEs were for interpersonal violence (range 14·7–53·5%); followed by harmful alcohol use (15·7–45·0%), illicit drug use (15·2–44·9%), and anxiety (13·9–44·8%; appendix p 7). High BMI had the lowest PAFs due to ACEs in all countries, ranging from 0·6% in Greece and North Macedonia to 3·1% in Finland (appendix p 7).
Country-level PAFs were applied to the equivalent number of DALYs for each outcome for 2019. ACE-attributable DALYs and associated costs for individual outcomes for each country are provided in the appendix (pp 8–35). In many countries, alcohol use, smoking, and cancer had the highest ACE-attributable costs (appendix pp 8–35). Table 4 presents total ACE-attributable DALYs (excluding those for risk factors relating to included causes) and country-level associated costs across all outcomes, and the equivalent proportion of national GDP this cost represents. The estimated number of DALYs attributable to ACEs ranged from 13 000 in Montenegro to 4·3 million in Russia (table 4). ACE-attributable costs ranged from $0·1 billion in Montenegro to $129·4 billion in Germany (table 4). The equivalent proportion of GDP accounted for by ACE-attributable costs ranged from 1·1% in Sweden and Turkey to 6·0% in Ukraine (median proportion 2·6% [IQR 1·5–3·2]; table 4).
Table 4.
Total annual ACE-attributable DALYs and costs calculated for each country
| Population (millions)* | GDP per capita, US$, 2019 | ACE-attributable DALYs (thousands) | ACE-attributable costs (US$ billion) | Equivalent % of GDP | |
|---|---|---|---|---|---|
| Albania | 2·9 | $5352·9 | 79·7 | $0·4 | 2·8% |
| Belgium | 11·5 | $46 116·7 | 162·6 | $7·5 | 1·4% |
| Czech Republic | 10·7 | $23 101·8 | 246·5 | $5·7 | 2·3% |
| Denmark | 5·8 | $59 822·1 | 136·0 | $8·1 | 2·3% |
| Finland | 5·5 | $48 685·9 | 225·2 | $11·0 | 4·1% |
| France | 67·1 | $40 493·9 | 939·4 | $38·0 | 1·4% |
| Germany | 83·1 | $46 258·9 | 2796·6 | $129·4 | 3·4% |
| Greece | 10·7 | $19 582·5 | 123·8 | $2·4 | 1·2% |
| Hungary | 9·8 | $16 475·7 | 239·1 | $3·9 | 2·4% |
| Ireland | 4·9 | $78 661·0 | 97·8 | $7·7 | 2·0% |
| Italy | 60·3 | $33 189·6 | 916·2 | $30·4 | 1·5% |
| Latvia | 1·9 | $17 836·4 | 105·0 | $1·9 | 5·5% |
| Lithuania | 2·8 | $19 455·5 | 93·0 | $1·8 | 3·3% |
| Moldova | 2·7 | $4498·5 | 107·6 | $0·5 | 4·0% |
| Montenegro | 0·6 | $8832·0 | 13·0 | $0·1 | 2·1% |
| Netherlands | 17·3 | $52 447·8 | 536·2 | $28·1 | 3·1% |
| North Macedonia | 2·1 | $6093·1 | 31·6 | $0·2 | 1·5% |
| Norway | 5·3 | $75 419·6 | 145·7 | $11·0 | 2·7% |
| Poland | 38·0 | $15 595·2 | 941·5 | $14·7 | 2·5% |
| Romania | 19·4 | $12 919·5 | 660·5 | $8·5 | 3·4% |
| Russia | 144·4 | $11 585·0 | 4312·4 | $50·0 | 2·9% |
| Serbia | 6·9 | $7402·4 | 191·9 | $1·4 | 2·8% |
| Spain | 47·1 | $29 613·7 | 565·9 | $16·8 | 1·2% |
| Sweden | 10·3 | $51 610·1 | 117·9 | $6·1 | 1·1% |
| Switzerland | 8·6 | $81 993·7 | 250·5 | $20·5 | 2·9% |
| Turkey | 83·4 | $9042·5 | 926·5 | $8·4 | 1·1% |
| Ukraine | 44·4 | $3659·0 | 2538·9 | $9·3 | 6·0% |
| UK | 66·8 | $42 300·3 | 1858·7 | $78·6 | 2·8% |
Findings should not be assumed as comparable between countries and are affected by the characteristics of contributing studies (appendix p 2). ACE=adverse childhood experience. DALY=disability-adjusted life-year. GDP=gross domestic product (current US$).
2019 figures for total population according to World Bank data.
For sensitivity analyses, in the lowest costed model, equivalent proportions of GDP accounted for by ACE-attributable costs ranged from 0·3% of GDP in Greece to 3·1% in Ukraine (appendix pp 8–35). In the upper costed model, these proportions ranged from 1·8% of GDP in Turkey to 8·9% in Ukraine (appendix pp 8–35).
Discussion
This study identifies immense health and financial costs to European nations associated with ACEs and, consequently, highlights the importance of investing in safe and nurturing childhoods. Pooled RRs indicate that adults exposed to ACEs are more likely to engage in health-risk behaviours and develop physical and mental illnesses that reduce their years of healthy and productive life. Although the proportion of DALYs and ACE-associated costs for the 12 health outcomes studied varied between countries, in all countries combined ACE-attributable costs exceeded 1% of national GDP, with the median proportion being 2·6%. This finding is similar to the previous European regional estimate of 2·7% (which used nine of 15 outcome studies included here).10 Highest PAFs due to ACEs were seen for violence, harmful alcohol use, illicit drug use, and mental illness (anxiety and depression). As well as being costly to individuals and society, these outcomes can represent ACEs for the offspring of affected adults, with ACEs known to have intergenerational effects.53 However, despite having lower PAFs, non-communicable diseases such as cancer and cardiovascular disease often had high costs to nations due to the high population burden of these conditions. Thus, in six countries, cancer carried the highest ACE-attributable costs of all conditions (Finland, Germany, Italy, Netherlands, Norway, and Switzerland).
Despite covering many leading health risks and causes of ill health in Europe, the actual burden of ACEs might be higher than indicated by our estimates. ACEs can stifle quality of life throughout the life course and forfeit the social benefits of healthy and resilient populations. As well as health conditions, ACEs are associated with other major societal costs, including low educational attainment, unemployment, crime, and social deprivation.54, 55 For example, a UK study found each additional ACE was associated with a 9% earnings penalty, a 25% increased risk of welfare dependency, and a 27% increased risk of subjective poverty at age 55 years.56 Equally, the harms associated with ACEs are not only realised in adulthood but can be seen from the earliest stages of life. Along with acute physical and emotional effects, children that have ACEs can show reduced cognitive and social development, reduced school engagement, early adoption of health-harming behaviours, and increased risk of health conditions and juvenile offending.55, 57, 58 Thus, the health, social, and economic benefits of effective action to prevent and respond to ACEs would emerge far sooner than would the adult health conditions examined here.
The importance of nurturing childhoods has increasingly dominated global health policy,59 with preventing violence against children, supporting families, and providing quality education being core features of the Sustainable Development Goals. The estimates reported here relate to a period before the COVID-19 pandemic and related restrictions. The pandemic might have increased conditions conducive to ACEs in families and reduced resilience-building opportunities if affected children are isolated in traumatic home settings and cut off from sources of support. The pandemic has also disrupted and diverted resources away from many services and programmes60 that help prevent ACEs, such as parenting programmes, socioeconomic development programmes, and youth support services. There is a concern that the resumption of such services will be deprioritised in the drive to catch up on clinical treatment and rebuild economic opportunity. Further, individuals who have had ACEs might have been particularly affected by the pandemic because of their increased risk both of chronic conditions that increase susceptibility to severe COVID–19 symptoms (eg, respiratory disease61) and of broader health harms associated with the pandemic (eg, poor mental health62). The differential impact of the pandemic on individuals with and without ACEs is yet to be quantified. However, preventing ACEs should contribute to reducing the health-harming behaviours and health conditions that can increase a population's susceptibility to infection and thus potentially reduce health risks from future pandemics.
Our study had several limitations. Nationally representative studies measuring cumulative ACEs and associations with health were not available for most countries, and although ACE prevalence data were identified for samples in 28 countries, in many these data were restricted to students or older adults (mainly aged ≥50 years). Study methodologies varied, as did the number and types of ACEs measured. Although we adjusted ACE prevalence calculations to provide some correction for data coverage, additional nationally representative data would strengthen country estimates. However, even where such data are available, further work is required to understand how accurately studies capture ACE exposure. Many included studies used retrospective, self-reported ACE measurement, which can be affected by recall and willingness to report, whereas those using prospectively measured ACEs or administrative data might miss ACEs that were unreported or unrecorded. Consequently, estimates are likely to be more reliable in countries with multiple studies using large representative samples and various methodological approaches, such as Denmark, Sweden and the UK.
Our study relied on ACE count measurements, which do not account for the timing, severity, or length of ACE exposure and assume each ACE type carries the same weight in influencing health.63 Further, although PAFs assume causal effects on outcomes, most included studies were cross-sectional and unable to measure causal relationships. Data from 18 countries contributed to pooled RRs, yet for several outcomes (eg, high BMI and violence) data were only available for UK samples and for others (eg, smoking) all non-UK studies used student populations. Although we used the same set of pooled RRs in calculations for each country, differences might exist between countries in associations between ACEs and outcomes. We also found differences in definitions of outcome measurements (appendix p 1) and levels of adjustment for confounding. Thus, although most outcome studies adjusted for demographic factors, including some level of socioeconomic measurement (appendix pp 2–3), such measurements varied and key demographic factors such as ethnicity were not always included. All these factors might have affected pooled estimates and heterogeneity seen between estimates. Thus, findings should not be compared across countries and figures should be viewed as estimates that are based on the best available data. Despite these limitations, our study provides a methodology for estimating the national burden of ACEs that could be enhanced in many countries by improved data collection and replicated elsewhere by collecting ACE data. Future work would benefit from subgroup analyses to account for differences in ACE prevalence and associations between ACEs and outcomes in different population groups.
To inform prevention and its effects, improved ACE measurement within countries and increased methodological consistency are necessary. WHO has already provided leadership towards such work, which in Europe has supported implementation of ACE studies among students in 13 countries.14, 15 Elsewhere, ACE tools have been incorporated into routine population health surveys (eg, in the USA54 and Scotland21). However, existing ACE tools have received criticism for issues such as measuring a restricted range of ACEs and using simplistic scoring approaches.63 Greater availability and comparability of epidemiological evidence on ACEs and their effects could be central to obtaining political commitment to invest in prevention. Thus, greater consensus is required on the ACEs that population studies should consider, how they should be measured, and among which populations.
ACEs are associated with major health and financial costs at local, national, and international levels. The initial annual estimates calculated here quantify ACE-attributable costs for various leading health risks and causes of ill health across different European countries. These estimates could be enhanced in many countries by improved data availability. However, the burden associated with ACEs goes well beyond those directly affected by poor health, affecting education, social, and criminal justice resources. The COVID-19 pandemic could have increased risk factors for ACEs, exacerbated inequalities, and led to potential economic recessions around the world.64, 65 Effective recovery from the pandemic will require nations to make investment choices that drive sustainable economic growth, increase health equity, and build population resilience to future pandemics. This study indicates the costs that individuals and societies pay when nations do not ensure a safe and nurturing beginning for all children. Deficits in child and family support created by COVID-19 must be urgently addressed. However, after the pandemic we should go further, investing in ACE-free childhoods that propel individuals on to healthy, social, and prosperous life courses that also leave individuals more physically and mentally resilient to future pandemics.
Data sharing
Study data are available from the corresponding author on reasonable request.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The study was funded by the WHO Regional Office for Europe. We are grateful to Matt Bolt and Gergó Baranyi for providing additional data to support the study.
Contributors
MAB, KH, and JP designed the study. KH, FG, EH, and KF undertook review activities including searches, study selection, data extraction, and quality assessment. MAB developed the statistical modelling. KH and KF did meta-analyses and undertook data calculations. KH wrote the manuscript with contributions from KF and MAB. KH and KF accessed and verified the data used and all authors had access to the underlying data. All authors reviewed the study findings and read and approved the final version before submission.
Supplementary Material
References
- 1.Struck S, Stewart-Tufescu A, Asmundson AJN, Asmundson GGJ, Afifi TO. Adverse childhood experiences (ACEs) research: a bibliometric analysis of publication trends over the first 20 years. Child Abuse Negl. 2021;112 doi: 10.1016/j.chiabu.2020.104895. [DOI] [PubMed] [Google Scholar]
- 2.Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39:93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Berens AE, Jensen SKG, Nelson CA., 3rd Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:135. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes K, Bellis MA, Hardcastle KA. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 5.Spratt T, Kennedy M. Adverse childhood experiences: developments in trauma and resilience aware services. Br J Soc Work. 2021;51:999–1017. [Google Scholar]
- 6.Bhushan D, Kotz K, McCall J. Office of the California Surgeon General; Sacramento, CA: 2020. Roadmap for resilience: the California Surgeon General's Report on adverse childhood experiences, toxic stress, and health. [Google Scholar]
- 7.Bryant DJ, Oo M, Damian AJ. The rise of adverse childhood experiences during the COVID-19 pandemic. Psychol Trauma. 2020;12:S193–S194. doi: 10.1037/tra0000711. [DOI] [PubMed] [Google Scholar]
- 8.Kaukinen C. When stay-at-home orders leave victims unsafe at home: exploring the risk and consequences of intimate partner violence during the COVID–19 pandemic. Am J Crim Justice. 2020;45:1–12. doi: 10.1007/s12103-020-09533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang X, Fry DA, Brown DS. The burden of child maltreatment in the East Asia and Pacific region. Child Abuse Negl. 2015;42:146–162. doi: 10.1016/j.chiabu.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4:e517–e528. doi: 10.1016/S2468-2667(19)30145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller TR, Waehrer GM, Oh DL. Adult health burden and costs in California during 2013 associated with prior adverse childhood experiences. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes K, Ford K, Kadel R, Sharp CA, Bellis MA. Health and financial burden of adverse childhood experiences in England and Wales: a combined primary data study of five surveys. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348 doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 14.Bellis MA, Hughes K, Leckenby N. Adverse childhood experiences and associations with health-harming behaviours in young adults: surveys in eight eastern European countries. Bull World Health Organ. 2014;92:641–655. doi: 10.2471/BLT.13.129247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes K, Bellis MA, Sethi D. Adverse childhood experiences, childhood relationships and associated substance use and mental health in young Europeans. Eur J Public Health. 2019;29:741–747. doi: 10.1093/eurpub/ckz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.2. 2021. https://www.training.cochrane.org/handbook
- 17.Rückinger S, von Kries R, Toschke AM. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol. 2009;9:7. doi: 10.1186/1471-2288-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DW. Economic value of disability-adjusted life years lost to violence: estimates for WHO Member States. Rev Panam Salud Publica. 2008;24:203–209. doi: 10.1590/s1020-49892008000900007. [DOI] [PubMed] [Google Scholar]
- 19.Bellis MA, Hughes K, Leckenby N, Perkins C, Lowey H. National household survey of adverse childhood experiences and their relationship with resilience to health-harming behaviors in England. BMC Med. 2014;12:72. doi: 10.1186/1741-7015-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly-Irving M, Lepage B, Dedieu D. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol. 2013;28:721–734. doi: 10.1007/s10654-013-9832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudsen L. In: The Scottish Health Survey. 2019 edn. McLean J, Wilson V, editors. Scottish Government; Edinburgh: 2020. Adverse childhood experiences. [Google Scholar]
- 22.McLafferty M, Armour C, McKenna A, O'Neill S, Murphy S, Bunting B. Childhood adversity profiles and adult psychopathology in a representative Northern Ireland study. J Anxiety Disord. 2015;35:42–48. doi: 10.1016/j.janxdis.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Robson E, Norris T, Wulaningsih W, Hamer M, Hardy R, Johnson W. The relationship of early–life adversity with adulthood weight and cardiometabolic health status in the 1946 National Survey of Health and Development. Psychosom Med. 2020;82:82–89. doi: 10.1097/PSY.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 24.Wainwright NWJ, Surtees PG, Wareham NJ, Harrison BDW. Psychosocial factors and asthma in a community sample of older adults. J Psychosom Res. 2007;62:357–361. doi: 10.1016/j.jpsychores.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J Public Health. 2015;37:445–454. doi: 10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellis MA, Lowey H, Leckenby N, Hughes K, Harrison D. Adverse childhood experiences: retrospective study to determine their impact on adult health behaviours and health outcomes in a UK population. J Public Health. 2014;36:81–91. doi: 10.1093/pubmed/fdt038. [DOI] [PubMed] [Google Scholar]
- 27.Bellis M, Ashton K, Hughes K, Ford K, Bishop JSP. Public Health Wales; Cardiff: 2016. Adverse childhood experiences and their impact on health–harming behaviours in the Welsh population. [Google Scholar]
- 28.Cosco TD, Hardy R, Howe LD, Richards M. Early-life adversity, later-life mental health, and resilience resources: a longitudinal population-based birth cohort analysis. Int Psychogeriatr. 2018;31:1–10. doi: 10.1017/S1041610218001795. [DOI] [PubMed] [Google Scholar]
- 29.Demakakos P, Lewer D, Jackson SE, Hayward AC. Lifetime prevalence of homelessness in housed people aged 55–79 years in England: its childhood correlates and association with mortality over 10 years of follow-up. Public Health. 2020;182:131–138. doi: 10.1016/j.puhe.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford K, Butler N, Hughes K, Quigg Z, Bellis M. Liverpool John Moores University; Liverpool: 2016. Adverse childhood experiences in Hertfordshire, Luton and Northamptonshire. [Google Scholar]
- 31.Hughes K, Ford K, Davies A, Homolova L, Bellis M. Public Health Wales; Wrexham: 2018. Sources of resilience and their moderating relationships with harms from adverse childhood experiences. [Google Scholar]
- 32.Kelly-Irving M, Lepage B, Dedieu D. Childhood adversity as a risk for cancer: findings from the 1958 British birth cohort study. BMC Public Health. 2013;13:767. doi: 10.1186/1471-2458-13-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranyi G, Sieber S, Pearce J. A longitudinal study of neighbourhood conditions and depression in ageing European adults: do the associations vary by exposure to childhood stressors? Prev Med. 2019;126 doi: 10.1016/j.ypmed.2019.105764. [DOI] [PubMed] [Google Scholar]
- 34.Boisgontier MP, Orsholits D, von Arx M. Adverse childhood experiences, depressive symptoms, functional dependence, and physical activity: a moderated mediation model. J Phys Act Health. 2020;17:1–10. doi: 10.1123/jpah.2019-0133. [DOI] [PubMed] [Google Scholar]
- 35.Dahl SK, Larsen JT, Petersen L. Early adversity and risk for moderate to severe unipolar depressive disorder in adolescence and adulthood: a register-based study of 978,647 individuals. J Affect Disord. 2017;214:122–129. doi: 10.1016/j.jad.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Dich N, Hansen ÅM, Avlund K. Early life adversity potentiates the effects of later life stress on cumulative physiological dysregulation. Anxiety Stress Coping. 2015;28:372–390. doi: 10.1080/10615806.2014.969720. [DOI] [PubMed] [Google Scholar]
- 37.Halonen JI, Vahtera J, Kivimäki M, Pentti J, Kawachi I, Subramanian SV. Adverse experiences in childhood, adulthood neighbourhood disadvantage and health behaviours. J Epidemiol Community Health. 2014;68:741–746. doi: 10.1136/jech-2013-203441. [DOI] [PubMed] [Google Scholar]
- 38.Pulkki-Råback L, Elovainio M, Virtanen M. Job demands and job control as predictors of depressive symptoms: moderating effects of negative childhood socioemotional experiences. Stress Health. 2016;32:383–394. doi: 10.1002/smi.2632. [DOI] [PubMed] [Google Scholar]
- 39.Wiehn J, Hornberg C, Fischer F. How adverse childhood experiences relate to single and multiple health risk behaviours in German public university students: a cross-sectional analysis. BMC Public Health. 2018;18 doi: 10.1186/s12889-018-5926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witt A, Sachser C, Plener PL, Brähler E, Fegert JM. The prevalence and consequences of adverse childhood experiences in the German population. Dtsch Arztebl Int. 2019;116:635–642. doi: 10.3238/arztebl.2019.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussemakers C, Kraaykamp G, Tolsma J. Co-occurrence of adverse childhood experiences and its association with family characteristics. A latent class analysis with Dutch population data. Child Abuse Negl. 2019;98 doi: 10.1016/j.chiabu.2019.104185. [DOI] [PubMed] [Google Scholar]
- 42.Enns MW, Cox BJ, Afifi TO, De Graaf R, Ten Have M, Sareen J. Childhood adversities and risk for suicidal ideation and attempts: a longitudinal population-based study. Psychol Med. 2006;36:1769–1778. doi: 10.1017/S0033291706008646. [DOI] [PubMed] [Google Scholar]
- 43.Huang L, Mossige S. Resilience and poly-victimization among two cohorts of Norwegian youth. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheikh MA. Retrospectively reported childhood adversity is associated with asthma and chronic bronchitis, independent of mental health. J Psychosom Res. 2018;114:50–57. doi: 10.1016/j.jpsychores.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Björkenstam E, Vinnerljung B, Hjern A. Impact of childhood adversities on depression in early adulthood: a longitudinal cohort study of 478,141 individuals in Sweden. J Affect Disord. 2017;223:95–100. doi: 10.1016/j.jad.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Björkenstam E, Hjern A, Vinnerljung B. Adverse childhood experiences and disability pension in early midlife: results from a Swedish National Cohort Study. Eur J Public Health. 2017;27:472–477. doi: 10.1093/eurpub/ckw233. [DOI] [PubMed] [Google Scholar]
- 47.Gebreab SZ, Vandeleur CL, Rudaz D. Psychosocial stress over the lifespan, psychological factors, and cardiometabolic risk in the community. Psychosom Med. 2018;80:628–639. doi: 10.1097/PSY.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 48.Henchoz Y, Seematter-Bagnoud L, Nanchen D. Childhood adversity: a gateway to multimorbidity in older age? Arch Gerontol Geriatr. 2019;80:31–37. doi: 10.1016/j.archger.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Ujhelyi Nagy A, Kuritár Szabó I, Hann E, Kósa K. Measuring the prevalence of adverse childhood experiences by survey research methods. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCrory C, Dooley C, Layte R, Kenny RA. The lasting legacy of childhood adversity for disease risk in later life. Health Psychol. 2015;34:687–696. doi: 10.1037/hea0000147. [DOI] [PubMed] [Google Scholar]
- 51.Perales J, Olaya B, Fernandez A. Association of childhood adversities with the first onset of mental disorders in Spain: results from the ESEMeD project. Soc Psychiatry Psychiatr Epidemiol. 2013;48:371–384. doi: 10.1007/s00127-012-0550-5. [DOI] [PubMed] [Google Scholar]
- 52.Fowler C, Homandberg L, Steele C. Adult correlates of adverse childhood experiences in Ukraine. Child Abuse Negl. 2020;107 doi: 10.1016/j.chiabu.2020.104617. [DOI] [PubMed] [Google Scholar]
- 53.Narayan AJ, Lieberman AF, Masten AS. Intergenerational transmission and prevention of adverse childhood experiences (ACEs) Clin Psychol Rev. 2021;85 doi: 10.1016/j.cpr.2021.101997. [DOI] [PubMed] [Google Scholar]
- 54.Metzler M, Merrick MT, Klevens J, Ports KA, Ford DC. Adverse childhood experiences and life opportunities: shifting the narrative. Child Youth Serv Rev. 2017;72:141–149. doi: 10.1016/j.childyouth.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graf GHJ, Chihuri S, Blow M, Li G. Adverse childhood experiences and justice system contact: a systematic review. Pediatrics. 2021;147:1–15. doi: 10.1542/peds.2020-021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schurer S, Trajkovski K, Hariharan T. Understanding the mechanisms through which adverse childhood experiences affect lifetime economic outcomes. Labour Econ. 2019;61 [Google Scholar]
- 57.Jimenez ME, Wade R, Jr, Lin Y, Morrow LM, Reichman NE. Adverse experiences in early childhood and kindergarten outcomes. Pediatrics. 2016;137 doi: 10.1542/peds.2015-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houtepen LC, Heron J, Suderman MJ, Fraser A, Chittleborough CR, Howe LD. Associations of adverse childhood experiences with educational attainment and adolescent health and the role of family and socioeconomic factors: a prospective cohort study in the UK. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter LM, Darmstadt GL, Daelmans B. Advancing early childhood development: from science to scale. Oct 4, 2016. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb-assets/Lancet/stories/series/ecd/Lancet_ECD_Executive_Summary-1507044811487.pdf
- 60.Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: a systematic review and meta-analysis. Respir Med. 2020;171 doi: 10.1016/j.rmed.2020.106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daly M, Sutin AR, Robinson E. Longitudinal changes in mental health and the COVID-19 pandemic: evidence from the UK Household Longitudinal Study. Psychol Med. 2020 doi: 10.1017/S0033291720004432. published online Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hrynick TA, Ripoll Lorenzo S, Carter SE. COVID-19 response: mitigating negative impacts on other areas of health. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLennan JD, MacMillan HL, Afifi TO. Questioning the use of adverse childhood experiences (ACEs) questionnaires. Child Abuse Negl. 2020;101 doi: 10.1016/j.chiabu.2019.104331. [DOI] [PubMed] [Google Scholar]
- 64.Goldblatt P, Shriwise A, Yang L, Brown C. World Health Organization Regional Office for Europe; Copenhagen: 2020. Health inequity and the effects of COVID–19: assessing, responding to and mitigating the socioeconomic impact on health to build a better future. [Google Scholar]
- 65.Sachs JD, Abdool Karim S, Aknin L. Lancet COVID-19 Commission Statement on the occasion of the 75th session of the UN General Assembly. Lancet. 2020;396:1102–1124. doi: 10.1016/S0140-6736(20)31927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data are available from the corresponding author on reasonable request.