Abstract
Methadone maintenance treatment has proven effectiveness in the treatment of opioid use disorder, but significant barriers remain to treatment retention. In a randomized clinical trial, 300 newly-admitted methadone patients were randomly assigned to patient-centered methadone (PCM) v. treatment-as-usual (TAU). In PCM, participants were treated under revised program rules which permitted voluntary attendance at counseling and other changes focused on reducing involuntary discharge, and different staff roles which shifted disciplinary responsibility from the participant's counselor to the supervisor. The study found no significant differences in treatment retention, measures of opioid use, or other patient outcomes. This paper employs an activity-based costing approach to estimate the cost and cost-effectiveness of the two study conditions. We found that service use and costs were similar between PCM and TAU. Specifically, the average cost for PCM patients was $2396 compared to $2292 for standard methadone, while the average length of stay was 2 weeks longer for PCM patients. Incremental cost-effectiveness ratios (ICER) for self-reported heroin use, opioid positive urine screens, and meeting DSM-IV criteria for opioid dependence were mixed, with TAU achieving non-significantly better outcomes at lower treatment episode costs (i.e., economically dominating) for opioid positive urine screens. PCM patients reported slightly more days abstinent from heroin and fewer meet the opioid dependence criteria. While these differences are small and not statistically significant, we can still examine the cost-effectiveness implications. For days, abstinent from heroin, the ICER was $242 for one additional day of abstinence, however, there was notable uncertainty around this estimate. For opioid dependence criteria, the ICER was $1160 for a one-percentage point increase in the probability that a participant no longer met criteria for opioid dependence at follow-up. This economic study finds that patient choice concepts can be introduced into methadone treatment without significant impacts on costs or patient outcomes.
Keywords: Opioid use disorder, Methadone treatment, Cost, Cost-effectiveness, Substance use
1. Introduction
The misuse of prescription opioids and use of heroin is a significant public health problem in the United States. In 2016, prescription opioid misuse and heroin abuse resulted in over 40,000 opioid-related overdose deaths, approximately 66% of all drug overdoses for that year (Hedegaard, Warner, & Miniño, 2017). Overdose deaths and opioid misuse create substantial economic costs for the United States ranging from about $80 billion (2015$; Florence, Zhou, Luo, & Xu, 2016) to $500 billion (2015$; The Council of Economic Advisors, 2017).
To address the growing economic and human costs of opioid misuse, providing effective substance use treatment is essential. The effectiveness of methadone maintenance in reducing illicit opioid use is well established (Mattick, Breen, Kimber, & Davoli, 2009). Nonetheless, barriers to treatment retention remain, as a substantial proportion of patients discontinue methadone treatment within the first year (Magura, Nwakeze, & Demsky, 1998; Deck & Carlson, 2005; Reisinger et al., 2009; Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016). Patients report that factors such as disagreement over program rules, conflict with counselors, not paying fees, and schedule conflicts are common reasons for involuntary discharge (Reisinger et al., 2009). Overcoming these barriers to retention is of first-order importance as patients who discontinue treatment are at elevated risk of relapse (Bart, 2012) and overdose death (Woody, Kane, Lewis, & Thompson, 2007).
Introducing patient-centered elements into the delivery of methadone treatment is one avenue through which retention could potentially be improved. Patient-centered care concepts have played a significant role in improving the quality of general medical practices, yet these have only begun to impact mental health and substance use treatment (Annuals of Family Medicine, 2014; IOM, 2001; Pincus et al., 2007). The core concepts of patient-centered care stress that care should be respectful and responsive to a patient's preferences, values, and needs, and that a patient's values guide treatment decisions (Barry & Edgman-Levitan, 2012; Epstein & Street, 2011; IOM, 2001). These core concepts are commonly implemented through a shared-decision making model when caregivers and patients jointly choose treatment options. Many studies have shown that the shared-decision making model increases patient knowledge, understanding of risks, decisions consistent with patients' values, and reduced internal conflict for patients (Stacey et al., 2011).
As patient-centered care concepts are relatively new to opioid agonist treatment, there are few studies of how patient-centered care impacts substance use treatment outcomes. Korthuis' qualitative study of patients choosing office-based buprenorphine treatment over standard opioid treatment programs (OTPs) suggested that one reason patients preferred office-based treatment because they perceived it as offering a more patient-centered approach to care (Korthuis et al., 2010).
More recently, Schwartz et al. (2014) compared 12-month outcomes of patient-centered methadone (PCM) vs. methadone treatment-as-usual (TAU) in a randomized clinical trial among 300 newly-admitted methadone patients in two OTPs in Baltimore, MD. PCM differed from TAU in two principal ways. First, PCM revised the rules of the programs pertaining to the PCM participants to increase participant involvement in treatment decision making and to reduce the likelihood of involuntary “administrative discharge” due to non-adherence to the rules and disciplinary issues. Thus, PCM participants were permitted to choose whether to attend regularly scheduled counseling sessions required in TAU, how much counseling to attend, and whether to attend individual, group counseling or both. Participants were not to be discharged for rule infractions (e.g., positive urine drug screens). Second, PCM revised the roles of the staff such that the PCM participants' counselors were not involved in any disciplinary actions against the participant. Instead, any disciplinary action was taken by a clinical supervisor. The study found no significant differences between conditions in terms of treatment retention, opioid and cocaine use, HIV risk, and global quality of life.
In this study, we built upon the Re-engineering Methadone outcome study (Schwartz et al., 2017) to estimate the costs of PCM and TAU. We then conducted a cost-effectiveness analysis of PCM relative to TAU across self-reported heroin use, opioid-positive urine screening test results, and meeting DSM-IV opioid dependence criteria at 12-month follow-up. First, we estimated the costs of delivering PCM and TAU. Second, we calculated the incremental cost, incremental effectiveness, and incremental cost-effectiveness ratios by comparing the difference in cost and outcomes, if any, between patients randomly assigned to PCM and those receiving TAU. Finally, as described under methods and results, we evaluated the probability of either PCM or TAU as the optimal treatment choice by interpreting the findings from calculated cost-effectiveness acceptability curves (CEAC).
2. Study design
Two methadone treatment programs (MTPs) in Baltimore, Maryland, participated in the study. One MTP was university-affiliated and the other was a part of a not-for-profit organization. Across the two sites, 300 patients were enrolled over a 30-month period with 295 participants evaluated (5 were withdrawn shortly after enrollment for a variety of reasons). All patients were newly-admitted adults, aged 18 and over, not pregnant, had no unstable medical/psychiatric conditions, and provided informed consent. Eligible patients were randomized using block randomization such that for a successive block of 4 patients, 2 were assigned to each condition: PCM or TAU. Following enrollment in the study, patients were followed in their assigned study condition for up to one year, during which time services received from the clinic were tracked. Each condition was led by a senior counselor with a separate team of counselors to provide PCM or TAU. Schwartz et al. (2014) provides a full description of the PCM and TAU conditions and the study's overall design.
2.1. Data collection
2.1.1. Patient outcomes
Patient baseline demographics were collected at enrollment, service use was tracked throughout the study, and patient outcomes were collected at 3, 6, and 12 months following enrollment. For this study, we focus on 12-month outcomes of heroin use, opioid use, and opioid dependence. Specifically, outcome measures include self-reported heroin use in the past 30 days using the Addiction Severity Index (ASI), research assistant-administered urine screening tests for opioid use, and opioid dependence diagnoses based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria (APA, 2000; McLellan et al., 1992).
Service utilization was tracked with service receipt logs that recorded the number of weeks in methadone treatment and medical and counseling sessions attended. All patients had complete service records while they remained in the study. Follow-up data were collected separately from each patient, and 82.4% of participating patients completed a 12-month follow-up assessment.
2.1.2. Cost data
The cost study component used the Substance Abuse Services Cost Analysis Program (SASCAP; Zarkin, Dunlap, & Homsi, 2004) for methadone treatment programs to collect data on service-level resource use and associated costs. The SASCAP is a validated instrument that collects program expenditures for resource categories, such as labor, building space, contracted services, and supplies and materials, as well as staff labor hours spent on the provision of direct treatment services and administrative activities typically performed in methadone maintenance programs (Zarkin et al., 2004).
Both sites completed the SASCAP for the TAU condition which provided a baseline cost of each service provided. These data were checked and revised as needed during a follow-up phone interview with each site's clinic director. For activities under PCM, we collected changes in resource use in a separate phone interview with each site's clinic director and clinical supervisor. During the interview, they were asked to describe how resource use had changed for each activity under PCM. If an activity changed, we asked staff to describe the change in resources used for the activity (e.g., staff labor hours, supplies, materials). Any changes in resource use for a specific activity was made to the baseline SASCAP collected for TAU. This process allowed us to collect the resource use and cost differences due to PCM relative to TAU.
3. Methods
3.1. Costs
Using the cost data collected for both PCM and TAU, we employed an activity-based costing approach to compute patient-level resource use associated with the PCM and TAU conditions. An activity-based costing approach identifies the key activities performed and/or services delivered by an organization and collects resource use data and costs at the activity level so that estimates of the cost of each activity or service can be calculated (Zarkin et al., 2004). Resource use was calculated from the provider perspective, as it is the most relevant for providers looking to implement similar treatment protocols in real-world practice. Therefore, we only included the labor and nonlabor resources used by the treatment sites to provide services under the PCM and TAU approaches. We did not include the value of the patient's time or other costs that they may have incurred to undergo methadone treatment (e.g., travel costs to/from treatment, treatment fees, insurance co-pays). In addition, we did not include non-treatment activities related to implementing the clinical research trial such as costs associated with randomization of study participants and conducting follow-up assessments because these costs would not be incurred in real-world clinical practice. All costs were converted to 2015 dollars using the Bureau of Labor Statistics' Consumer Price Index (http://www.bls.gov/cpi/).
To estimate the average unit cost for specific treatment services, we used information on the total costs incurred by the treatment site and the staff's labor allocation across specified services. To derive direct labor costs for each service, we identified all staff types that performed each service. Using the salary and staff type information provided by the site, we estimated the average hourly wage for each staff type. This average wage includes the cost of fringe benefits and employer taxes. For each service, we multiplied the weekly hours of service provided by each staff type by their appropriate average hourly wage. Summing these products across staff types for each service yields the total weekly labor cost of providing each service.
The cost of nonlabor resources includes the costs for building space, contracted services, supplies and materials, and miscellaneous resources. We do not have information on how nonlabor resources were allocated across service categories. Therefore, we developed an algorithm to allocate nonlabor costs proportionate to the amount of time each site spent on each service in a typical week. One exception to this allocation rule was the drug cost of methadone, which was allocated solely to methadone dosing. We calculated the weekly nonlabor cost by dividing the annual nonlabor cost by 52. Then, based on hours of service provided, we allocated the weekly nonlabor costs proportionally to the appropriate services.
The total weekly cost for a specific service was simply the sum of the weekly direct labor costs and the weekly nonlabor costs for that service. Hourly costs for key treatment service activities, including initial patient assessments, medical assessments, psychiatric appointments, and individual and group counseling, were derived by dividing the total weekly service cost by the reported hours per week that the staff spent providing the service. We then multiplied the hourly cost of the service by the average session length reported by each site to produce session costs. Costs for group counseling sessions were calculated at the per-patient level by dividing the session cost by the average number of group attendees reported by each site. The remaining five direct service activities, methadone dosing, case management, patient education, ongoing medical services, and any other patient services were not tracked directly by the service logs. These services often have an array of different activities, which do not have easily identifiable sessions. Therefore, for each of these services, we estimated the clinic's weekly cost per patient by dividing the total weekly service cost by the clinic's average daily census.
To calculate costs per patient, service-level cost data were combined with data on the services received by participating patients during the study's intervention period. These costs were calculated for each patient during the period in which the patient remained in the study, up to one year. Any treatment and costs incurred after a patient stopped study treatment or completed a year of treatment were not included.
3.2. Outcomes
The outcomes of interest for this study included days of self-reported heroin use in the past 30 days, opioid-positive urine screening test results, and whether the participants met DSM-IV opioid dependence diagnostic criteria over the previous 12 months, all of which were reported during the 12-month follow-up assessment interview.1 Heroin use was measured based on self-reported days abstinent from heroin use in the past 30 days. A dichotomous measure of opioid use was based on whether the patient had a negative opioid urine screen at the 12-month follow-up assessment. We chose to include both measures of abstinence-based effectiveness in our economic analysis for two reasons. First, this approach aligns with the results presented in the primary outcomes paper as each outcome was viewed as important by the clinical team (Schwartz et al., 2014). Second, self-reported abstinence and urine testing results are different. For example, self-reported use may be subject to recall bias and societal pressure not to report substance use. However, self-reported days of use are more common in the literature and its inclusion in this analysis allows for comparison with findings in the literature. Our final effectiveness measure was opioid dependence, and it was measured based on a structured assessment of DSM-IV criteria. Measures were collected at 3-, 9-, and 12-months post-intervention, and Schwartz et al. (2014) did not find significant differences in outcomes across these time points.
Approximately 20% of the 12-month follow-up assessments were missing. Consistent with our primary outcome paper (Schwartz et al., 2014), we assumed, conservatively that participants lost to follow-up had poor outcomes on missing metrics. Thus, for days abstinent from heroin in the past 30 days and opioid dependence, missing 12-month values were replaced by observed baseline variables. For missing opioid urine screening test results at 12-month follow-up, a positive test result was assumed.
3.3. Cost-effectiveness
For each of the three outcome measures, our cost-effectiveness analysis compared PCM to TAU by calculating an incremental cost-effectiveness ratio (ICER). The ICER is calculated as the difference in average cost (C) divided by the difference in average effectiveness (E) (Drummond, Sculpher, Claxton, Stoddart, & Torrance, 2015). When neither condition is strictly dominated, the cost-effectiveness ratio is calculated regardless of statistical significance.
Choosing the optimal treatment option after strictly dominated options are removed depends on a decision maker's willingness to pay (WTP). WTP refers to the value that a decision maker is willing to pay to achieve a given outcome. While actual WTP estimates for this study's outcomes have not been calculated, we can use economic theory to help identify the probability that an intervention is the economically optimal choice (i.e., cost-effective) for a given outcome across a range of WTP values. Economic theory suggests that the optimal intervention is the one with the greatest ICER that is not more than the decision maker's WTP for an additional unit of the outcome (Drummond et al., 2015). Therefore, we also calculated a CEAC to show the probability that a treatment condition is the cost-effective option as a function of the decision maker's WTP for each of the clinical outcomes. The CEAC incorporates the inherent variability of the cost and effectiveness estimates and allows us to better capture the variability in our cost-effectiveness analysis in lieu of confidence intervals for the ICERs (Fenwick, Claxton, & Sculpher, 2001; Fenwick, Marshall, Levy, & Nichol, 2006). We used a nonparametric bootstrap method to calculate the CEAC that compares the PCM condition relative to TAU across patient outcomes with a calculated ICER.
4. Results
The patient's baseline characteristics and services received are presented Table 1. Patient characteristics were very similar between the TAU and PCM conditions, and services received were also similar between the conditions. PCM patients had slightly longer lengths of stay, averaging 36.7 weeks compared to 34.3 weeks in TAU. One-year retention rates were similar, with 49% of patients in the PCM condition and 46% in the TAU condition still enrolled in the original OTP at 12-month follow-up.
Table 1.
Patient baseline characteristics and treatment experience.
| Total | TAU | PCM | |
|---|---|---|---|
| Number of patients | 295 | 146 | 149 |
| Age, mean (SE) | 43 (0.6) | 42 (0.8) | 44 (0.9) |
| Percent White (SE) | 41% (2.9) | 44% (4.1) | 38% (4.0) |
| Percent African American (SE) | 58% (2.9) | 54% (4.1) | 62% (4.0) |
| Percent Hispanic (SE) | < 1% (0.5) | 1% (0.0) | 0% (0.0) |
| Percent Currently Married (SE) | 20% (2.3) | 20% (3.3) | 19% (3.3) |
| Years of Education completed (SE) | 11.3 (0.1) | 11.4 (0.2) | 11.3 (0.2) |
| Percent employed in the past 30 days (SE) | 37% (2.8) | 41% (4.0) | 34% (3.9) |
| Average number of Medical Assessments (SE) | 1.3 (0.2) | 1.5 (0.2) | 1.2 (0.3) |
| Average Individual Counseling sessionsb (SE) | 8.5 (0.4) | 8.0 (0.4) | 9.0 (0.6) |
| Average Group Counseling sessions (SE)a | 5.1 (0.5) | 6.3 (0.7) | 3.8 (0.7) |
| Average Length of stay (weeks in program) (SE) | 35.5 (1.1) | 34.3 (1.6) | 36.7 (1.6) |
| Retention (percent remaining in treatment for one year) (SE) | 48% (2.9) | 46% (4.1) | 49% (4.2) |
Standard errors (SE) of the mean are presented in parenthesis.
Statistically significant difference between TAU and PCM at p < 0.05 based on both double-sided t-tests and Wilcoxon rank-sum tests. This analysis differs from the analysis described in Schwartz et al. (2017).
Includes individual Psychiatrist appointments.
Unit and weekly costs for services are presented in Table 2. These unit costs are the same across TAU and PCM as the price to provide a unit of a given service did not vary between the two conditions. Rather, differences in overall average treatment costs per patient were driven solely by different service utilization rates and lengths of stay in treatment (average of 36.7 weeks of treatment for PCM compared to 34.3 weeks of treatment for TAU).2 Overall, PCM patients had slightly higher average treatment costs of $2395, compared to $2292 in TAU (Table 3), although these differences were not found to be statistically significant.
Table 2.
Costs.
| Average unit or weekly costs per patient (SE) |
TAU | PCM | p-Value for any differencea |
|
|---|---|---|---|---|
| N = 149 | N = 146 | |||
| Average total costs per patient (SE) |
Average total costs per patient (SE) |
|||
| Initial patient assessmentb | $197 (80) | $197 (7) | $197 (7) | 0.86 |
| Medical assessmentsc | $91 (38) | $168 (31) | $123 (31) | 0.31 |
| Individual counseling | $46 (3) | $368 (20) | $406 (25) | 0.30 |
| Group counselingd | $8 (2) | $47 (6) | $31 (7) | 0.08 |
| Weekly servicese | $44 (7) | $1512 (75) | $1641 (75) | 0.23 |
| Total | – | $2292 (108) | $2396 (103) | 0.49 |
| – | (108) | (103) |
p-Value shown for a two-tailed test with a null hypothesis of a difference of zero the average mean cost of TAU and PCM services. A p-value of 0.05 or less would indict a non-zero significant difference.
Differences in average patient costs are due to site level cost differences and rounding.
Includes case management, methadone dosing, patient education, ongoing medical services and any other patient services.
Medical assessment records were not available for three patients in TAU and two patients in PCM.
Costs are calculated on a per patient basis where the session cost of group counseling is divided by the average group attendance.
Table 3.
Cost-effectiveness results for re-engineered methadone (2015 $).
| Treatment Arm | N | Average total cost of treatment per participant episode |
Past 30 days abstinent from heroin in 30 days prior to 12-month follow-up (self- reported) |
Percentage of participants with a negative opioid urine screen at 12-month follow-up |
Percentage of participants not meeting DSM-IV criteria for opioid dependence at 12-month follow-up |
|||
|---|---|---|---|---|---|---|---|---|
| Average cost (SE) |
Mean effectiveness ± (SE) |
Incremental CE ratio (ΔC/ΔE) |
Mean effectiveness ± (SE) |
Incremental CE ratio (ΔC/ΔE) |
Mean effectiveness ± (SE) |
Imputed incremental CE ratio (ΔC/ΔE) |
||
| TAU | 149 | $2292 | 20.2 | 40% | – | 31% | ||
| (108.26) | (1.01) | (4.02) | – | (3.80) | ||||
| PCM | 146 | $2396 | 20.6 | $242 | 39% | Economically dominated | 40% | $1138 |
| (102.64) | (1.00) | (4.05) | – | (4.06) | ||||
Note: Standard errors (SE) are presented in parenthesis.
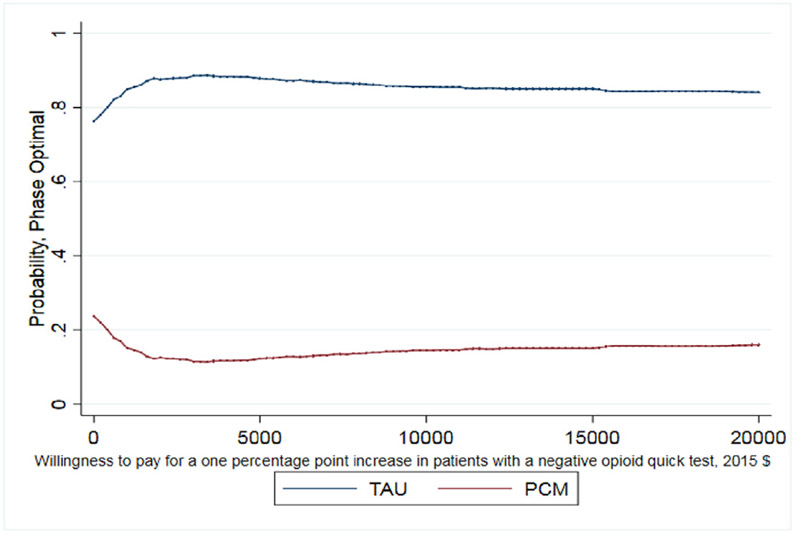
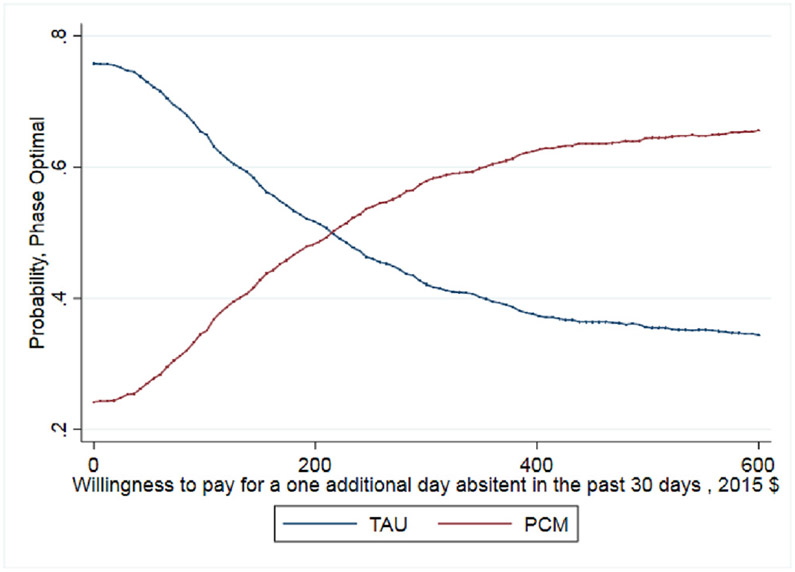
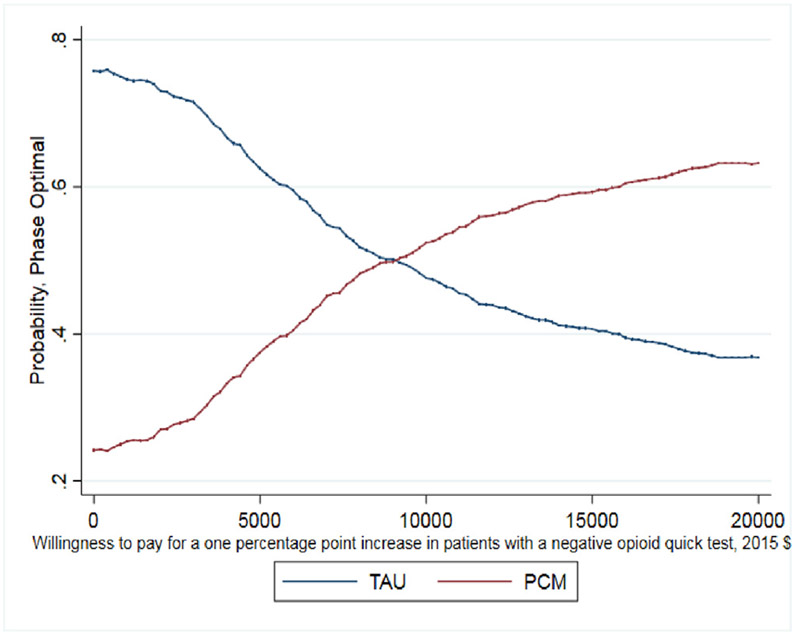
Small, statistically insignificant differences were found between the point-estimates of the mean for patient outcomes between PCM and TAU (Table 3). While these differences do not indicate that PCM or TAU produced superior outcomes, economic analysis can provide further information. On average, TAU patients reported being abstinent from heroin for 20.2 days (out of 30 days) while PCM patients reported 20.6 days abstinent from heroin. The ICER shows that achieving one additional day abstinent from heroin costs $242. Regarding patient's negative opioid urine screening test results, TAU and PCM patients show comparable results with 40% of TAU patients having a negative urine screening test result at the 12-month assessment compared to 39% of patients in PCM. Here TAU economically dominates PCM with a lower average cost and similar average outcome, but again these differences are small.
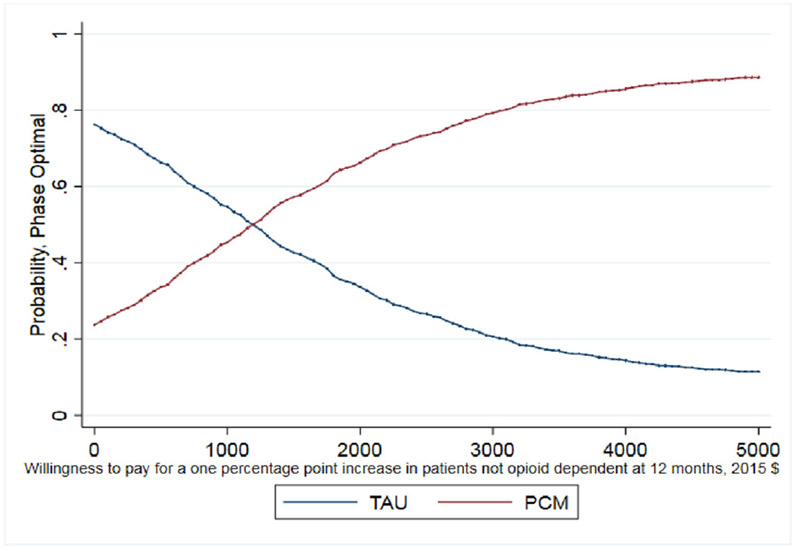
The third patient outcome is the percentage of patients meeting the DSM-IV criteria for opioid dependence. Here, the PCM condition had a higher average than TAU though the difference was not statistically significant. On average, 40% of PCM patients did not meet the DSM-IV criteria for opioid dependence compared to only 31% of TAU patients. This suggests that a greater percentage of PCM patients are less likely to be opioid dependent at 12 months following treatment entry. The resulting ICER shows that a one percentage point increase in the percentage of patients not meeting the dependence criteria costs $1138.
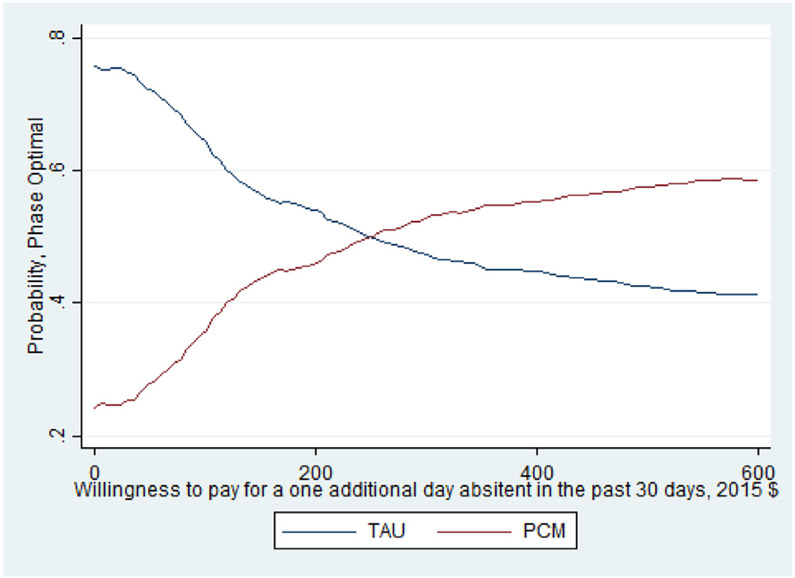
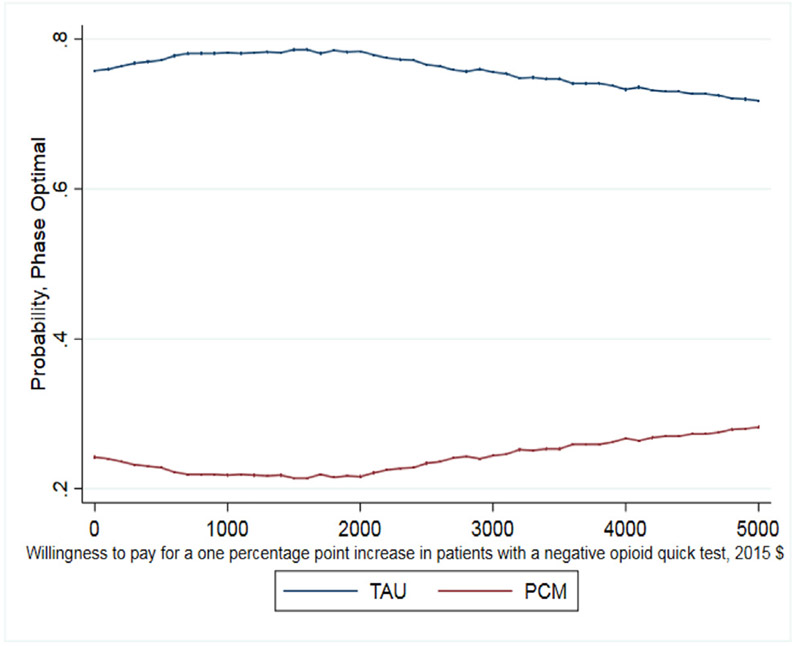
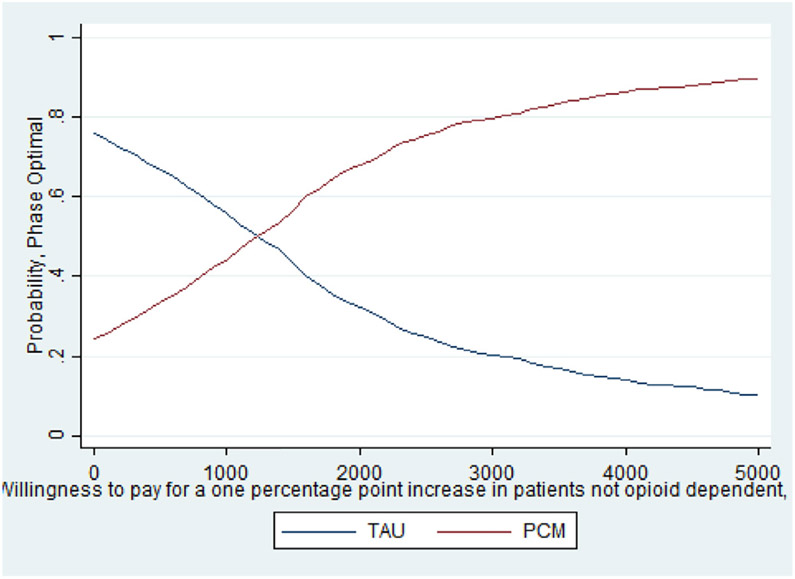
As noted earlier, whether this cost per percentage point increase is a good value depends on the decision makers WTP. The CEAC presented in Fig. 1 helps interpret the ICER for days abstinent from heroin in the past 30 days and illustrates when PCM is more likely to be the optimal treatment choice given a decision maker's WTP for the better outcome. As shown, as the WTP for one day of heroin abstinence increases, the probability that PCM is the optimal choice also increases. For WTP values over $250, the probability that PCM is the optimal, cost-effective choice relative to TAU exceeds 50%. It is important to note that even when hypothetical WTP values reach $600, the probability that PCM is the optimal choice remains just below 60% as the slight differences between costs and days abstinent (20.2 days abstinent for TAU and 20.6 days abstinent for PCM) increase the uncertainty around the ICER calculation. The CEAC presented in Fig. 2 illustrates that TAU economically dominates PCM for the outcome of probability of a negative test at 12-month follow up with the probability of TAU being the optimal choice being > 70% across all values of WTP. Finally, the CEAC presented in Fig. 3 for the probability of a patient not meeting the DSM-IV criteria for opioid dependence illustrates when PCM is more likely to be the optimal treatment choice given society's WTP for the better outcome. As shown, as the WTP for a percentage point improvement in the dependence criteria outcome increases, the probability that PCM is the optimal choice also increases. For WTP values over $1150, the probability that PCM is the optimal, cost-effective choice relative to TAU exceeds 50% with this probability > 80% for WTP values exceeding $3000. While not shown in any of these figures, for these CEACs the probability that PCM or TAU is the optimal, cost effective choice does not reach certainty (100%) for any willingness to pay value as the small, statically insignificant differences in both treatment costs and outcomes limit the certainty we can place in these results.
Fig. 1.
CEAC for days abstinent from heroin.
Fig. 2.
CEAC for opioid urine test.
Fig. 3.
CEAC for opioid dependence.
4.1. Sensitivity analysis
The cost results presented here are not sensitive to changes in the cost of labor, building space, or materials, as the same unit costs were applied to both PCM and TAU for most treatment activities. One exception was the patient disciplinary meetings where clinical supervisors met with PCM patients while counselors meet with TAU patients. This difference did not affect costs as the clinical supervisors' hourly wage was only slightly higher than most counselors and disciplinary meetings were equally infrequent in both TAU and PCM.
The method used for imputing missing outcome measures did impact the point estimates of the mean used in our cost-effectiveness analysis. As such, we explored two additional imputation approaches: dropping missing values and using STATA 14's multiple imputation (MI) methods. To impute the missing data, we used STATA 14's multiple imputation (MI) program. A linear regression model was used to impute days abstinent from heroin in the past 30 days while logit models were used to impute results for opioid urine tests and dependence assessment measures. Each model included the following covariates: controls for assigned treatment condition and site; baseline measures for the three primary outcomes; patient demographic characteristics, including age, gender, marital status, and years of education; self-reported days of cocaine use; measures of HIV-risk behaviors related to drug use and sexual behavior, quality of life measure based on the overall score from the WHO Quality of Life BREF, and measures of physical and mental health status based on aggregate scores from the SF-12.
The resulting point estimates of the mean for each method are presented in Table 4. Estimates for the means are very similar between dropping missing values and the MI approach. For days of heroin use in the past 30 days, the means were the same within condition for both approaches (5.3 days for TAU and 6.5 days for PCM) For opioid urine screens, the estimated percentage of a positive urine screen is 49% for both approaches for TAU and 54% and 53% for PCM respectively. For opioid dependence, the estimated percentage of a patient meeting the criteria is 63% and 64% for TAU respectively and 54% and 55% for PCM respectively. However, assuming baseline values and positive results for the opioid urine screening, the estimates are notably different and change the cost-effectiveness results for days using/abstinent from heroin in the past 30 days. This approach estimates 9.8 using days (20.2 abstinent days) for TAU compared to 9.4 using days (20.6 abstinent days) for PCM. For the cost-effectiveness analysis, dropping missing values and using MI, TAU has marginally better outcomes and, as it costs less than PCM, TAU would economically dominate PCM whereas assuming baseline values allowed us to calculate a positive ICER (Table 3) and a CEAC (Fig. 1). The uncertainty around the ICER was discussed in our Results section and the challenge of drawing conclusions given this uncertainty is noted in our Discussion. Our cost-effectiveness analysis results and conclusions for opioid urine screens and dependence at 12 months are unchanged between the three methods for dealing with missing data.
Table 4.
Imputation results.
| Treatment Arm | Approach to imputing missing 12- month follow-up data |
Days using heroin at 12- month follow- up |
Percentage of patients with a positive opioid urine screen at 12-month follow-up |
Percentage of patients meeting DSM-IV criteria for opioid dependence at 12-month follow-up |
|---|---|---|---|---|
| Mean (SE) |
Mean (SE) |
Mean (SE) |
||
| TAU | Drop missing | 5.3 (0.8) | 49% (4.7) | 63% (4.4) |
| Multiple imputation | 5.3 (0.7) | 49% (1.3) | 64% (1.2) | |
| Assume baseline valuesa | 9.8 (1.0) | 60% (4.0) | 69% (3.8) | |
| PCM | Drop missing | 6.5 (0.9) | 54% (4.5) | 54% (4.5) |
| Multiple imputation | 6.6 (0.8) | 53% (1.3) | 55% (1.3) | |
| Assume baseline valuesa | 9.4 (1.0) | 61% (4.1) | 60% (4.1) |
Standard errors (SE) of the mean are presented in parenthesis.
Opioid urine screen at 12-month follow-up missing values were replaced with positive results.
In addition, we estimated outcomes using GEE regression modeling similar to those presented in the main findings paper (Schwartz et al., 2014) to take advantage of the longitudinal data. These models were estimated for the outcomes of days abstinent in past 30 days and probability of a negative opioid urine test at 12-month follow-up. Data for our third outcome, probability of a patient not meeting the DSM-IV criteria for opioid dependence was only collected at two points—baseline and at 12-month follow up—and, therefore, we did not run the GEE model for this outcome. Results using the GEE methods compared to our primary methods were not meaningfully different. Using GEE, we have mean predicted values of days abstinent in the past 30 days of 20 days for the TAU condition and 20.5 for the PCM condition. For the probability of a negative opioid urine test at 12-month follow up, the GEE model yielded mean predicted outcomes of 39% for TAU and 40% for PCM, which is similar to the 40% and 39%, respectively, reported using our primary methods. The main difference for this outcome is that PCM is no longer economically dominated; however, the 1-percentage point difference is not significant. Furthermore, ICERs using the predicted outcomes at 12 months from the GEE models do not notably change the results for days abstinent; however, not surprisingly, results are switched for the probability of negative opioid urine test such that the ICER is now positive but large (over $8000). It was not unexpected that the ICER on the opioid drug test outcome changes to positive as this outcome was sensitive to changes in the model and imputation method used. The cost-effectiveness results using estimates from the GEE model and related CEACs are presented in the Appendix A.
5. Discussion
This study analyzed the cost and the cost-effectiveness of patient-centered methadone treatment. Foremost, our paper shows that adding aspects of patient-centered care to methadone treatment does not significantly affect costs. Patients in PCM attended fewer group counseling sessions but remained in treatment for about two weeks longer, on average, than TAU patients with a small overall cost impact. The approximately $100 difference in costs over the course of treatment between PCM and TAU patients is neither statistically significant nor a meaningful real-world economic difference. We also found small, nonsignificant differences in our primary outcome measures. This combination of small incremental costs and outcome differences produced mixed cost-effectiveness results. TAU patients had better average outcomes for opioid positive urine screens while having lower average number of days in treatment. If reducing opioid positive urine screens is the primary outcome objective, TAU would be preferred over PCM regardless of a decision maker's willingness to pay due to lower costs and similar outcomes.
However, PCM does have a positive incremental cost-effectiveness ratio for the number of self-reported days abstinent from heroin and of meeting DSM-IV opioid dependence criteria at 12 months following treatment entry. This result could be considered particularly valuable from a patient-functioning approach. Our cost-effectiveness analysis shows that a one percentage point increase in the percentage of patients who are not opioid dependent costs about $1150. In more concrete terms, preventing 3 patients from meeting dependence criteria 12 months after treatment entry costs about $2300 (i.e., 3 patients equal approximately 2% increase). The results from our CEAC show that PCM has about an 80% probability of being the optimal choice – alternatively – there is only a 20% probability that TAU might be the optimal choice even at WTP values of $3000.
Our result on days abstinent from heroin, showing that the cost of an additional day of abstinence from heroin is less than $250 might suggest that PCM is effective. Between TAU and PCM, patients used an average of 9.6 days out of 30 at 12 months; the estimated cost of eliminating these using days would be $2323 per patient. The results from our CEAC show that PCM has about a 60% probability of being the optimal choice – alternatively – there is a 40% probability that TAU might be the optimal choice even at WTP values of $600.
It should be noted that the results presented here are comparable to other findings in the literature. For example, Olmstead and Petry (2009) reported costs of about $280 and $220 (in 2015$) for one additional week of abstinence from cocaine or heroin using voucher-based and prize-based contingency management interventions, respectively, compared to standard outpatient treatment. Schumacher, Mennemeyer, Milby, Wallace, and Nolan (2002) estimated costs of two addiction interventions compared to standard treatment to be about $1400 and $1740 (2015$) for an additional week of drug abstinence among homeless persons. Prah, Abdallah, Luekens, and Cottler (2012) examined cocaine and alcohol abuse among women and estimated ICERS of about $3760 to achieve cocaine abstinence at 12 months and $7540 to achieve alcohol abstinence at 12 months comparing a standard intervention combined with a Well Woman Exam to the standard intervention alone.
Overall, the results suggest that PCM and the patient-centered concepts that underpin it did not substantially raise costs or produce worse patient outcomes. These findings provide some evidence that reducing the requirements around methadone treatment while increasing the use of patient-centered concepts, especially patient choice, could be a viable approach to increasing treatment retention, increasing opioid abstinence, and for meeting DSM-IV opioid dependence criteria.
There are significant, real world limits, however, on how far methadone treatment can be modified while still meeting regulations required in the certification process. This reality does limit the practical application of these findings while suggesting the need for further research on patient-centered approaches to methadone treatment. It is likely that OTPs in the United States and throughout the world have a wide range of patient rules regarding involuntary discharge from care. In many parts of the world, methadone is provided through primary care without the OTP structure (Cousins et al., 2016; Carrieri et al., 2014). Thus, there are a diverse range of approaches to treatment that merit study and comparison. Another limit on implementing patient-centered care principles in this study and in real-world applications is maintaining fidelity in implementing patient-centered principles. Allowing participant choice of counseling sessions in PCM did reflect a part of shared-decision making but other aspects such as allowing the patient's values to impact treatment decisions were not studied. Further, patient choice was limited to counseling sessions and while PCM participants clearly exercised their choice in treatment by not attending as many group counseling sessions as TAU participants, other aspects, such as how counselors handled disciplinary actions yielded no tangible cost differences between conditions. Additionally, PCM only targeted counselors and their supervisors in implementing PCM; the nurses and physicians involved in methadone dosing provided the same treatment across both conditions. While limited in its implementation and fidelity to the full patient-centered care model, the present study still introduced and tested how patient choice may impact methadone treatment outcomes and provides a foundation for future study.
Our cost and cost-effectiveness study also includes four primary limitations. First, the cost analysis used the judgement of the principal investigators and clinic managers and supervisors to determine which activities are primarily research-related and which would be used in best clinical practice. To reduce this limitation, we relied on the methadone costing literature and the consensus of those implementing the study to obtain agreement around what activities for best clinical practice would be included. Further, as each activity across conditions had the same unit cost any errors here will have no differential effect across the conditions and will not affect the cost-effectiveness analysis.
Second, at least one of our outcome measures as well as several covariates used in this study were based on patient self-reports. However, most of these measures were captured for a 30-day period to help reduce recall bias. Third, our cost-effectiveness analysis is limited to the interventions used in the Re-engineering Methadone study, PCM and TAU. An alternative set of interventions or the addition of more study conditions could produce different results. Fourth, we used imputed outcome measures for missing outcome data. As discussed in the Sensitivity Analysis, the imputation method used does impact the means and our results around the days abstinent from heroin. We used three approaches to more fully understand how these imputation methods impacted our results and help control for their impact on our results.
Despite these limitations, our study does provide a first look at how patient-centered principles affect costs and costs-effectiveness in methadone treatment. Importantly, our study does not find statistically significant higher costs for PCM. While the cost-effectiveness results are mixed, our study suggests there may be benefits for PCM from a participant functioning perspective as opioid dependence diagnoses decreased at a greater rate among PCM patients. The literature on patient-centered care argues that incorporating patient-centered care may have further benefits beyond improved outcomes such as increased patient understanding of treatment and satisfaction with care. While our cost and cost-effectiveness study suggests that integrating elements of patient choice into methadone treatment does not significantly impact costs and has limited impact on cost-effective outcomes, future studies may use these findings to explore two primary issues. First, other studies may choose to focus less on traditional patient outcomes (e.g., days abstinent) and instead focus more on patient's experience and satisfaction with care to more fully motivate patient-centered care. Second, studies may focus on how patient-centered care might impact specific barriers to treatment retention faced by disadvantaged groups (e.g., women with dependent children, racial minorities, HIV positive). Here, the possible benefits incurred by implementing patient-centered principles may be greater and more valuable to decision makers.
6. Conclusion
The findings from this study suggest that patient choice concepts that allow patients more control over certain treatment aspects can be introduced into standard methadone treatment without significant impacts or costs or patient outcomes. This should be a consideration for policy makers and providers as interventions to increase patients' treatment engagement are explored.
Funding
This work was supported by the National Institute on Drug Abuse (Grant No. R01 A015842 06A1).
± Differences are not statistically significant based on the analysis described in Schwartz et al. (2014).
Appendix
Appendix A
Table A-1.
Service utilization.
| TAU = 149 | PCM N = 146 |
|||
|---|---|---|---|---|
| Average number of sessions or weeks in treatment per patient (SE) |
Average number of service hours per patienta |
Average number of sessions or weeks in treatment per patient (SE) |
Average number of service hours per patienta |
|
| Initial patient assessment (sessions) | 1 – | 2.7 | 1 – | 2.7 |
| Medical assessments (sessions) | 1.5 (0.2) | 1.5 | 1.2 (0.3) | 1.2 |
| Individual counseling (sessions) | 8.0 (0.4) | 5.0 | 9.0 (0.6) | 5.6 |
| Group counseling (sessions)b | 6.3 (0.7) | 1.3 | 3.8 (0.7) | 0.5 |
| Weekly services (weeks)c | 34.3 (1.6) | 18.5 | 36.7 (1.6) | 19.8 |
Does not include additional administrative hours.
Hours are calculated on a per-patient basis where the session length of group counseling is divided by the average group attendance.
Includes Case Management, Methadone Dosing, Patient Education, Ongoing Medical Services and Any Other Patient Services.
Fig. A-1.
CEAC for days abstinent – multiple imputation method.
Fig. A-2.
CEAC for opioid urine test – multiple imputation method.
Fig. A-3.
CEAC for opioid dependence – multiple imputation method.
Table A-2.
Cost-effectiveness results using GEE modeling.
| Treatment Arm |
N | Average total cost of treatment per participant episode (2015$) |
Past 30 days abstinent from heroin in 30 days prior to 12-month follow-up (self-reported) |
Percentage of participants with a negative opioid urine screen at 12- month follow-up |
||
|---|---|---|---|---|---|---|
| Average cost (SE) |
Mean effectiveness ± (SE) |
Incremental CE ratio (ΔC/ΔE) |
Mean effectiveness ± (SE) |
Incremental CE ratio (ΔC/ΔE) |
||
| TAU | 149 | $2292 | 20.0 | – | 39% | – |
| (108.26) | – | – | ||||
| PCM | 146 | $2396 | 20.5 | $196 | 40% | $8317 |
| (102.64) | ||||||
Note: Standard errors (SE) are presented in parenthesis.
Fig. A-4.
CEAC for days abstinent – GEE model.
Fig. A-5.
CEAC for opioid urine test – GEE model.
Footnotes
Although quality of life was assessed, the study was not designed to allow estimation of quality-adjusted life years. Rather, we focused on measures of substance use and dependence which are common in the economic literature for the substance use field.
See Table A-1 in Appendix A for more detailed information on service utilization.
References
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author text rev. [Google Scholar]
- Annuals of Family Medicine (2014). Joint principles: Integrating behavioral health care into the patient-centered medical home. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MJ, & Edgman-Levitan S (2012). Shared decision making—The pinnacle of patient-centered care. New England Journal of Medicine, 366(9), 780–781. [DOI] [PubMed] [Google Scholar]
- Bart G (2012). Maintenance medication for opiate addiction: The foundation of recovery. Journal of Addictive Diseases, 31(3), 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri PM, Michel L, Lions C, Cohen J, Vray M, Mora M, … Methaville Study Group (2014). Methadone induction in primary care for opioid dependence: A pragmatic randomized trial (ANRS Methaville). PLoS ONE, 9(11), e112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins G, Boland F, Courtney B, Barry J, Lyons S, & Fahey T (2016). Risk of mortality on and off methadone substitution treatment in primary care: A national cohort study. Addiction, 111(1), 73–82. [DOI] [PubMed] [Google Scholar]
- Deck D, & Carlson MJ (2005). Retention in publicly funded methadone maintenance treatment in two western states. The Journal of Behavioral Health Services & Research, 32(1), 43–60. [DOI] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, & Torrance GW (2015). Methods for the economic evaluation of health care programmes (4th edition). Oxford University Press. [Google Scholar]
- Epstein RM, & Street RL (2011). The values and value of patient-centered care. The Annals of Family Medicine, 9(2), 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E, Claxton K, & Sculpher M (2001). Representing uncertainty: The role of cost-effectiveness acceptability curves. Health Economics, 10(8), 779–787 [DOI] [PubMed] [Google Scholar]
- Fenwick E, Marshall DA, Levy AR, & Nichol G (2006). Using and interpreting cost-effectiveness acceptability curves: An example using data from a trial of management strategies for atrial fibrillation. BMC Health Services Research, 6(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, & Xu L (2016). The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Medical Care, 54(10), 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, & Miniño AM (2017). Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief, no 294 Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Institute of Medicine (2001). Crossing the quality chasm: A new health system for the 21st century. [PubMed] [Google Scholar]
- Korthuis PT, Gregg J, Rogers WE, McCarty D, Nicolaidis C, & Boverman J (2010). Patients' reasons for choosing office-based buprenorphine: Preference for patient-centered care. Journal of Addiction Medicine, 4(4), 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, & Demsky SY (1998). Research report: Pre-and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction, 93(1), 51–60. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2009). Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews(3), CD002209. 10.1002/14651858.CD002209.pub. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Angeriou M (1992). The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9, 199–213. [DOI] [PubMed] [Google Scholar]
- Olmstead TA, & Petry NM (2009). The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid dependent outpatients. Drug and Alcohol Dependence, 102, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus HA, Page AE, Druss B, Appelbaum PS, Gottlieb G, & England MJ (2007). Can psychiatry cross the quality chasm? Improving the quality of health care for mental and substance use conditions. American Journal of Psychiatry, 164(5), 712–719. [DOI] [PubMed] [Google Scholar]
- Prah RJ, Abdallah AB, Luekens C, & Cottler L (2012). Cost-effectiveness of peer-delivered interventions for cocaine and alcohol abuse among women: A randomized controlled trial. PLoS ONE, 7(3), e33594. 10.1371/journal.pone.0033594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, & O'Grady KE (2009). Premature discharge from methadone treatment: Patient perspectives. Journal of Psychoactive Drugs, 41, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JE, Mennemeyer ST, Milby JB, Wallace D, & Nolan K (2002). Costs and effectiveness of substance abuse treatments for homeless persons. Journal of Mental Health Policy and Economics, 5(1), 33–42. [PubMed] [Google Scholar]
- Schwartz RP, Alexandre PK, Kelly SM, O'Grady KE, Gryczynski J, & Jaffe JH (2014). Interim versus standard methadone treatment: A benefit–cost analysis. Journal of Substance Abuse Treatment, 46(3), 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, Mitchell SG, Gryczynski J, O'Grady KE, Gandhi D, Olsen Y, & Jaffe JH (2017). Patient-centered methadone treatment: a randomized clinical trial. Addiction, 112(3), 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, … Thomson R (2011). Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 10(10). [DOI] [PubMed] [Google Scholar]
- The Council of Economic Advisors (2017). The underestimated cost of the opioid crisis. Report prepared by The Council of Economic Advisors. Accessed at https://www.whitehouse.gov/sites/whitehouse.gov/files/images/The%20Underestimated%20Cost%20of%20the%200pioid%20Crisis.pdf. [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases, 35(1), 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Kane V, Lewis K, & Thompson R (2007). Premature deaths after discharge from methadone maintenance: A replication. Journal of Addictive Medicine, 1(4), 180–185. 10.1097/ADM.0b013e318155980e. [DOI] [PubMed] [Google Scholar]
- Zarkin GA, Dunlap LJ, & Homsi G (2004). The substance abuse services cost analysis program (SASCAP): A new method for estimating drug treatment services costs. Evaluation and Program Planning 27(1), 35–43. [Google Scholar]