Abstract
BACKGROUND:
The optimal level of pedicle ligation during proctectomy for rectal cancer, either at the origin of the inferior mesenteric artery or the superior rectal artery, is still debated.
OBJECTIVE:
The objective was to determine whether superior rectal artery ligation portends equivalent technical or oncologic outcomes.
DESIGN:
This was a retrospective analysis of a rectal cancer database (2007–2017).
SETTINGS:
The study was conducted at 6 tertiary referral centers in the United States (Emory University, University of Michigan, University of Pittsburgh Medical Center, The Ohio State University Wexner Medical Center, Vanderbilt University Medical Center, and Washington University School of Medicine in St. Louis).
PATIENTS:
Patients with primary, nonmetastatic rectal cancer who underwent low anterior resection or abdominoperineal resection were included.
MAIN OUTCOME MEASURES:
Anastomotic leak, lymph node harvest, locoregional recurrence-free survival, recurrence-free survival, and overall survival were measured.
RESULTS:
Of 877 patients, 86% (n = 755) received an inferior mesenteric artery ligation, whereas 14% (n = 122) received a superior rectal artery ligation. A total of 12%, 33%, 24%, and 31% were pathologic stage 0, I, II, and III. Median follow-up was 31 months. Superior rectal artery ligation was associated with a similar anastomotic leak rate compared with inferior mesenteric artery ligation (9% vs 8%; p = 1.0). The median number of lymph nodes removed was identical (15 vs 15; p = 0.38). On multivariable analysis accounting for relevant clinicopathologic factors, superior rectal artery ligation was not associated with increased anastomotic leak rate, worse lymph node harvest, or worse locoregional recurrence-free survival, recurrence-free survival, or overall survival (all p values >0.1).
LIMITATIONS:
This was a retrospective design.
CONCLUSIONS:
Compared with inferior mesenteric artery ligation, superior rectal artery ligation is not associated with either worse technical or oncologic outcomes. Given the potential risks of inadequate blood flow to the proximal limb of the anastomosis and autonomic nerve injury, we advocate for increased use of superior rectal artery ligation. See Video Abstract at http://links.lww.com/DCR/B646.
Keywords: Inferior mesenteric artery, Ligation, Rectal cancer
Abstract
ANTECEDENTES:
el nivel óptimo de la ligadura del pedículo en la proctectomía para el cáncer de recto, ya sea en el origen de la arteria mesentérica inferior o en la arteria rectal superior aún no esta definido.
OBJETIVO:
El objetivo era determinar si la ligadura de la arteria rectal superior pronostica resultados técnicos u oncológicos similares.
DISEÑO:
Análisis retrospectivo de una base de datos de cáncer de recto (2007-2017).
ESCENARIO:
el estudio se realizó en seis centros de referencia de tercer nivel en los Estados Unidos (Universidad de Emory, Universidad de Michigan, Centro médico de la Universidad de Pittsburgh, Centro médico Wexner de la Universidad Estatal de Ohio, Centro médico de la Universidad de Vanderbilt y Escuela de Medicina de la Universidad de Washington en St. Louis).
PACIENTES:
Se incluyeron pacientes con cáncer de recto primario no metastásico que se sometieron a resección anterior baja o resección abdominoperineal.
PRINCIPALES VARIABLES ANALIZADAS:
Se midió la fuga anastomótica, los ganglios linfáticos recuperados, la sobrevida sin recidiva locorregional, la sobrevida sin recidiva y la sobrevida global.
RESULTADOS:
De 877 pacientes, en el 86% (n = 755) se realizó una ligadura de la arteria mesentérica inferior, y en el 14% (n = 122) se realizó una ligadura de la arteria rectal superior. El 12%, 33%, 24% y 31% estaban en estadio patológico 0, I, II y III respectivamente. La mediana de seguimiento fue de 31 meses. La ligadura de la arteria rectal superior se asoció con una tasa de fuga anastomótica similar a la ligadura de la arteria mesentérica inferior (9 vs 8%, p = 1,0). La mediana del número de ganglios linfáticos extirpados fue idéntica (15 contra 15, p = 0,38). En el análisis multivariado que tiene en cuenta los factores clínico-patológicos relevantes, la ligadura de la arteria rectal superior no se asoció con una mayor tasa de fuga anastomótica, una peor cosecha de ganglios linfáticos o una peor sobrevida libre de recurrencia locorregional, sobrevida libre de recurrencia o sobrevida global (todos p> 0,1).
LIMITACIONES:
Diseño retrospectivo.
CONCLUSIONES:
En comparación con la ligadura de la arteria mesentérica inferior, la ligadura de la arteria rectal superior no se asocia a peores resultados técnicos ni oncológicos. Debido a los riesgos potenciales de un flujo sanguíneo inadecuado del muñon proximal de la anastomosis y la lesión de los nervios autonómicos, proponemos una mayor realización de la ligadura de la arteria rectal superior. Consulte Video Resumen en http://links.lww.com/DCR/B646. (Traducción—Dr Lisbeth Alarcon-Bernes)
Colorectal cancer is the second most common cause of cancer-related deaths in the United States, with rectal cancer representing 23% to 37% of cases, depending on age.1 For locally advanced disease, curative-intent treatment is a total mesorectal excision (TME) via a low anterior resection (LAR) or abdominoperineal resection (APR) to ensure adequate removal of the tumor and lymph nodes along its associated blood supply.
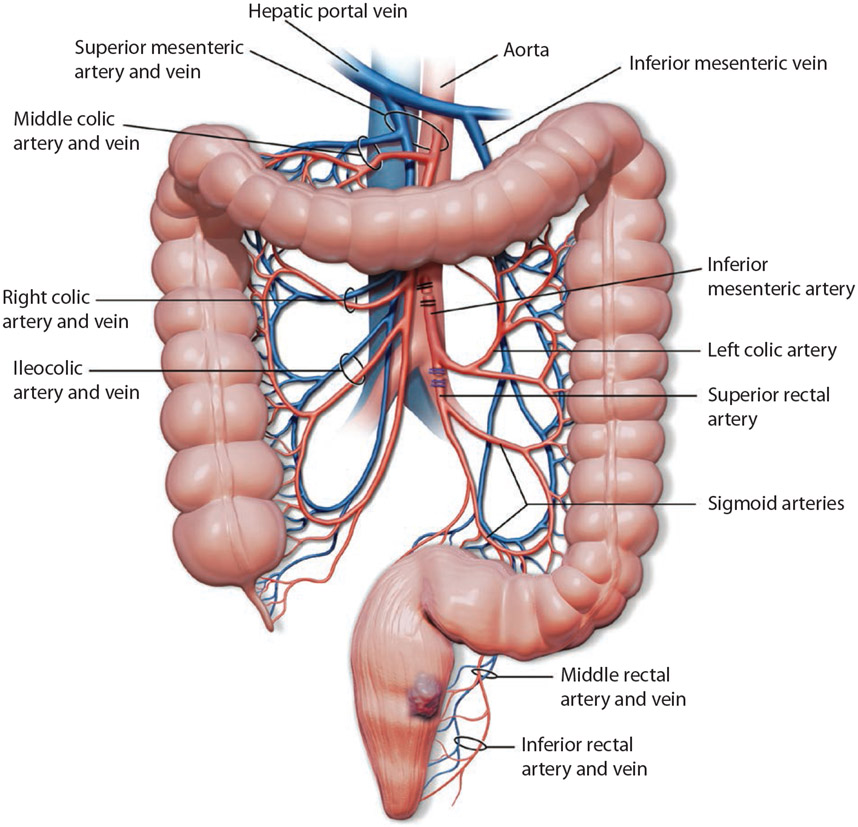
Approaching the TME plane and regional lymphadenectomy is achieved with either an inferior mesenteric artery (IMA) or superior rectal artery (SRA) ligation. The IMA is traditionally ligated within 2 cm from its origin off of the aorta (high ligation; Fig. 1).2 This technique may be used in situations to allow for improved lymph node retrieval, which is critical for pathologic staging and provides additional colonic length for a tension-free anastomosis.3 In contrast, after identifying the IMA and the left colic artery, SRA ligation just distal to the takeoff of the left colic artery offers another approach (low ligation). SRA ligation ensures sufficient blood flow to the left colon, potentially decreasing the risk for anastomotic leak and autonomic nerve injury. Thus far, the optimal approach for vascular ligation remains debated, and ligation of the IMA is routinely used. We aimed to assess the necessity and validity of this practice. Our hypothesis was that SRA ligation was oncologically equivalent with a lower incidence of anastomotic leaks when compared with IMA ligation.
FIGURE 1.
Illustration of ligation techniques: IMA ligation (black clips) and SRA ligation (blue clips). IMA = inferior mesenteric artery; SRA = superior rectal artery.
PATIENTS AND METHODS
Data Source and Cohort Selection
In this retrospective cohort study, the US Rectal Cancer Consortium (USRCC) database, a multi-institutional consortium of 6 high-volume tertiary referral centers including Emory University, University of Michigan, University of Pittsburgh Medical Center, The Ohio State University Wexner Medical Center, Vanderbilt University Medical Center, and Washington University School of Medicine in St. Louis was queried. The USRCC prospectively determined a series of objectives and designed a standardized data form along with a data dictionary. The data were then collected from each institution and merged into a common database. This study was the result of one of those objectives. All adult patients with primary, nonmetastatic rectal adenocarcinoma who underwent treatment with LAR or APR were included from 2007 to 2017. Patients with unknown vessel ligation status, those who underwent an emergent or palliative operation, and those who received an R2 resection margin were excluded. Institutional review board approval was obtained at each participant site before data collection.
Study Variables
Demographic, preoperative, operative, postoperative, pathologic, and long-term outcomes were collected via a review of patient electronic medical charts. Clinical and pathologic staging was based on the American Joint Committee on Cancer, eighth edition. Lymph node harvest was categorized as a dichotomous variable <12 or ≥12, which is the acceptable standard for adequate staging.4 Neoadjuvant treatment was defined as receiving chemotherapy (as a part of a total neoadjuvant therapy program), radiation therapy, or chemoradiation before surgery. A minimally invasive operative approach was considered laparoscopic, robotic, or laparoscopic hand-assisted surgery.
The primary outcomes were technical, including anastomotic leak, defined by clinical leak in the electronic medical chart diagnosed at the discretion of the individual institution, and lymph node harvest. Patients who received an APR or diverting loop ileostomy were excluded from the analyses for anastomotic leak. Secondary outcomes were oncologic, including locoregional recurrence-free survival (LRFS), recurrence-free survival (RFS), and overall survival (OS).
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software (IBM Inc, Armonk, NY). Descriptive statistics for each variable were reported. Statistical significance was defined as a significance level (α) of 0.05. A χ2 test or Fisher exact test was used for discrete variables, whereas a Student t test or Mann–Whitney test was performed for continuous variables. Univariate binary and multivariable logistic regression analyses were performed to determine the association of clinicopathologic variables and anastomotic leak and lymph node harvest. Kaplan–Meier analyses, log-rank tests, and univariate/multivariable Cox regression were performed to assess associations between the level of vessel ligation and recurrence and survival. Clinically relevant and statistically significant covariates on univariate analyses were selected for inclusion in the multivariable models.
RESULTS
Study Cohort Characteristics
Of the 1881 patients in the USRCC, 877 met the specified inclusion criteria. Seventy-eight patients were excluded for recurrent disease, 207 for metastatic disease, 13 for undergoing palliative-intent surgery, 160 for non-LAR/APR procedures, 8 for an R2 margin, 260 for missing SRA/IMA ligation data, and 278 for missing histopathologic/staging data. A total of 479 patients who received a diverting loop ileostomy were excluded from the analyses for anastomotic leak. The demographic, preoperative, operative, postoperative, and long-term outcomes data are presented in Table 1. Eighty-six percent (n = 755) received an IMA ligation, and 14% (n = 122) received an SRA ligation. The median age at diagnosis was 59 years (interquartile range (IQR), 52–67 y). Sixty-two percent (n = 541) were men. A minority of patients had diabetes (16%, n = 135) or a history of smoking (25%, n = 228). The majority were classified as ASA class II (45%, n = 371) or class III (53%, n = 439). Seventy-seven percent of patients received neoadjuvant therapy (77%, n = 582). Seventy-one percent (n = 620) underwent an LAR, and 29% (n = 257) underwent an APR. The median number of lymph nodes retrieved was 15 (IQR, 12–19). The majority of tumors were considered moderately differentiated (86%, n = 591). The median anastomosis distance from the anal verge was 4 cm (IQR, 3–6 cm). Only a minority of patients had a postoperative course complicated by anastomotic leak (9%, n = 38).
TABLE 1.
Demographic and clinicopathologic factors of the study population
| Variable | All patients (N = 877) | IMA ligation (N = 755), 86% | SRA ligation (N = 122), 14% | p |
|---|---|---|---|---|
| Age at diagnosis, median (IQR), y | 59 (52–67) | 59 (52–67) | 61 (51–68) | 0.2 |
| Sex, n (%) | ||||
| Men | 541 (62) | 454 (60) | 87 (71) | 0.02* |
| Women | 336 (38) | 301 (40) | 35 (29) | |
| Race, n (%) | ||||
| White | 682 (90) | 682 (90) | 98 (81) | <0.01* |
| Black | 59 (8) | 59 (8) | 15 (12) | |
| Other | 13 (2) | 13 (2) | 8 (7) | |
| Diabetes, n (%) | ||||
| No | 732 (84) | 623 (84) | 109 (90) | 0.17 |
| Yes | 135 (16) | 123 (16) | 12 (10) | |
| Smoking history, n (%) | ||||
| No | 644 (74) | 553 (74) | 91 (75) | 0.87 |
| Yes | 228 (26) | 198 (26) | 30 (25) | |
| Neoadjuvant therapy, n (%) | ||||
| No | 202 (23) | 171 (23) | 31 (25) | 0.59 |
| Yes | 673 (77) | 582 (77) | 91 (75) | |
| Functional status, n (%) | ||||
| Independent | 811 (98) | 697 (98) | 114 (98) | 0.88 |
| Partially dependent | 19 (2) | 17 (2) | 2 (2) | |
| ASA class, n (%) | ||||
| I | 6 (<1) | 5 (1) | 1 (1) | 0.14 |
| II | 371 (45) | 323 (45) | 48 (41) | |
| III | 439 (53) | 377 (53) | 62 (54) | |
| IV | 13 (2) | 8 (1) | 5 (4) | |
| Operation type, n (%) | ||||
| LAR | 620 (71) | 541 (72) | 79 (65) | 0.13 |
| APR | 257 (29) | 214 (28) | 43 (35) | |
| Approach, n (%) | ||||
| Open | 294 (34) | 223 (30) | 71 (58) | <0.01* |
| Minimally invasive | 582 (66) | 531 (70) | 51 (42) | |
| Small bowel resection(s), n (%) | ||||
| No | 605 (98) | 516 (99) | 89 (97) | 0.46 |
| Yes | 11 (2) | 8 (1) | 3 (3) | |
| Splenic flexure mobilization, n (%) | ||||
| No | 193 (24) | 170 (24) | 12 (21) | 0.61 |
| Yes | 628 (76) | 524 (76) | 86 (79) | |
| Lymph nodes retrieved, median (IQR) | 15 (12–19) | 15 (12–19) | 15 (13–20) | 0.38 |
| Lymph harvest, n (%) | ||||
| <12 lymph nodes | 190 (22) | 174 (23) | 16 (13) | 0.02* |
| ≥12 lymph nodes | 685 (78) | 580 (77) | 105 (87) | |
| Level of tumor from sphincters, mean, median (IQR), cm | 2.0, 1 (0–3) | 2.1, 1 (0–3) | 1.9, 1 (0–4) | 0.89 |
| Level of anastomosis from anal verge, mean, median (IQR), cm | 4.4, 4 (3–6) | 4.3, 4 (3–6) | 4.6, 5 (3–6) | 0.45 |
| Tumor differentiation, n (%) | ||||
| Well | 33 (5) | 30 (5) | 3 (3) | 0.08 |
| Moderate | 591 (86) | 498 (85) | 93 (94) | |
| Poor | 56 (8) | 53 (9) | 3 (3) | |
| Undifferentiated | 8 (1) | 8 (1) | 0 (0) | |
| Pathologic T stage (AJCC eighth edition), n (%) | ||||
| T0 | 110 (13) | 99 (13) | 11 (9) | <0.02* |
| Tis | 4 (<1) | 4 (<1) | 0 (0) | |
| T1 | 85 (10) | 71 (10) | 14 (12) | |
| T2 | 274 (31) | 227 (30) | 47 (39) | |
| T3 | 343 (39) | 308 (41) | 35 (29) | |
| T4a | 14 (2) | 11 (2) | 3 (2) | |
| T4b | 42 (5) | 31 (4) | 11 (9) | |
| Pathologic N stage (AJCC eighth edition), n (%) | ||||
| N0 | 592 (68) | 524 (70) | 68 (56) | 0.12 |
| N1a | 111 (13) | 93 (12) | 18 (15) | |
| N1b | 61 (7) | 50 (7) | 11 (9) | |
| N1c | 37 (4) | 29 (4) | 8 (7) | |
| N2a | 39 (4) | 31 (4) | 8 (7) | |
| N2b | 34 (4) | 27 (3) | 7 (6) | |
| Pathologic stage (AJCC eighth edition), n (%) | ||||
| 0 | 101 (12) | 91 (12) | 10 (9) | <0.01* |
| I | 281 (33) | 241 (33) | 40 (34) | |
| II | 206 (24) | 189 (26) | 17 (14) | |
| III | 269 (31) | 218 (30) | 51 (43) | |
| Final margin status, n (%) | ||||
| R0 | 833 (95) | 717 (95) | 116 (95) | 1.00 |
| R1 | 44 (5) | 38 (5) | 6 (5) | |
| Any complication, n (%) | ||||
| No | 403 (49) | 349 (49) | 54 (49) | 1.00 |
| Yes | 421 (51) | 364 (51) | 57 (51) | |
| Anastomotic leak, n (%) | ||||
| No | 393 (91) | 306 (91) | 87 (92) | |
| Yes | 38 (9) | 30 (9) | 8 (8) | 1.00 |
| Follow-up, median (IQR), mo | 31 (13–55) | 30 (13–55) | 36 (18–51) | 0.05* |
IQR = interquartile range; LAR = low anterior resection; APR = abdominoperineal resection; IMA = inferior mesenteric artery; SRA = superior rectal artery; AJCC = American Joint Committee on Cancer.
P value is significant.
Patients who received an IMA ligation were more likely to be women (40% vs 29%, p = 0.02) and White (90% vs 81%; p < 0.01). A higher proportion of patients who received an IMA ligation underwent a minimally invasive (MIS) approach compared with those receiving an SRA ligation (70% vs 42%; p < 0.01). Among MIS patients, 91% (n = 531) received an IMA ligation compared with 9% (n=51) who received an SRA ligation. Among patients who underwent an open operation, 76% (n = 223) received an IMA ligation compared with 24% (n = 71) who received an SRA ligation. For IMA ligation patients, a smaller proportion had a lymph node harvest of ≥12 lymph nodes (77% vs 87%; p = 0.02). Among SRA ligation patients, a higher proportion were pathologic stage III (43% vs 30%; p < 0.01). Median follow-up for IMA and SRA ligation patients was 30 months (IQR, 13–55 mo) and 36 months (IQR, 18–51 mo; p = 0.05).
Technical Outcomes
The ligation method was not associated with anastomotic leak (OR = 1.82 (95% CI, 0.48–6.92); p = 0.38). Factors associated with an increased odds for anastomotic leak on univariate analysis include female sex and smoking history (all p < 0.05; Table 2). On multivariable logistic regression, accounting for sex, ligation method, and operative approach, smoking history (OR = 3.87 (95% CI, 1.07–14.03); p = 0.04) remained associated with an increased odds of a postoperative anastomotic leak.
TABLE 2.
Association of ligation method with technical outcomes
|
Univariate regression
|
Multivariable regression
|
|||
|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Anastomotic leak | ||||
| Age at diagnosis, median (IQR) | 0.98 (0.95–1.02) | 0.41 | ||
| Sex | ||||
| Men | Reference | Reference | ||
| Women | 5.22 (1.01–24.72) | 0.04* | 4.34 (0.88–21.27) | 0.07 |
| Diabetes | ||||
| No | Reference | |||
| Yes | 0.86 (0.17–4.26) | 0.85 | ||
| Smoking history | ||||
| No | Reference | Reference | ||
| Yes | 4.63 (1.36–15.69) | 0.01* | 3.87 (1.07–14.03) | 0.04* |
| Neoadjuvant therapy | ||||
| No | Reference | |||
| Yes | 1.88 (0.59–5.98) | 0.29 | ||
| Functional status | ||||
| Independent | Reference | |||
| Partially dependent | 2.10 (0.20–21.77) | 0.53 | ||
| Ligation method | ||||
| IMA | Reference | Reference | ||
| SRA | 2.43 (0.72–9.24) | 0.15 | 1.82 (0.48–6.92) | 0.38 |
| Approach | ||||
| Open | Reference | Reference | ||
| Minimally invasive | 0.97 (0.28–3.38) | 0.96 | 1.30 (0.32–5.23) | 0.71 |
| Splenic flexure mobilization | ||||
| No | Reference | |||
| Yes | 1.73 (0.20–14.71) | 0.61 | ||
| Lymph nodes retrieved | 1.00 (0.94–1.07) | 0.98 | ||
| Lymph node harvest | ||||
| <12 lymph nodes | Reference | |||
| ≥12 lymph nodes | 0.54 (0.13–2.24) | 0.39 | ||
| Level of tumor from sphincters, cm | 0.95 (0.62–1.46) | 0.83 | ||
| Level of anastomosis from anal verge | 0.43 (0.11–1.70) | 0.23 | ||
| Pathologic T stage (AJCC eighth edition) | ||||
| T0 | Reference | |||
| Tis | 0.80 (0.04–17.20 | 0.89 | ||
| T1 | 0.70 (0.06–7.94) | 0.77 | ||
| T2 | 0.65 (0.06–6.71) | 0.72 | ||
| T3 | – | |||
| T4a | – | |||
| T4b | – | |||
| Pathologic N stage (AJCC eighth edition) | ||||
| N1a | Reference | |||
| N1b | 1.65 (0.38–7.22) | 0.50 | ||
| N1c | 1.23 (0.13–12.31) | 0.83 | ||
| N2a | – | |||
| N2b | – | |||
| N1a | – | |||
| Lymph node harvest (≥ 12) | ||||
| Age at diagnosis, median (IQR) | 0.98 (0.97–0.99) | <0.01* | 0.97 (0.95–0.99) | 0.02* |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.71 (0.50–0.99) | 0.05* | 1.13 (0.64–1.97) | 0.68 |
| Race | ||||
| White | Reference | |||
| Black | 1.35 (0.73–2.52) | 0.34 | ||
| Other | 2.74 (0.63–11.86) | 0.18 | ||
| Diabetes | ||||
| No | Reference | |||
| Yes | 0.93 (0.59–1.44) | 0.73 | ||
| Smoking history | ||||
| No | Reference | |||
| Yes | 0.82 (0.57–1.18) | 0.28 | ||
| Neoadjuvant therapy | ||||
| No | Reference | Reference | ||
| Yes | 0.32 (0.19–0.53) | <0.01* | 0.47 (0.22–1.01) | 0.05 |
| Functional status | ||||
| Independent | Reference | |||
| Partially dependent | 0.43 (0.16–1.13) | 0.09 | ||
| ASA class | ||||
| I | Reference | |||
| II | 0.75 (0.09–6.52) | 0.80 | ||
| III | 0.69 (0.08–5.99) | 0.74 | ||
| IV | 0.67 (0.05–8.16) | 0.75 | ||
| Ligation method | ||||
| IMA | Reference | Reference | ||
| SRA | 1.97 (1.13–3.42) | 0.02* | 3.77 (0.86–16.58) | 0.08 |
| Operation type | ||||
| LAR | Reference | Reference | ||
| APR | 1.71 (1.00–2.92) | 0.05* | 1.40 (0.80–2.45) | 0.24 |
| Approach | ||||
| Open | Reference | Reference | ||
| Minimally invasive | 1.02 (0.72–1.43) | 0.93 | – | – |
| Small bowel resection(s) | ||||
| No | Reference | |||
| Yes | 1.08 (0.23–5.12) | 0.92 | ||
| Splenic flexure mobilization | ||||
| No | Reference | |||
| Yes | 1.44 (0.99–2.08) | 0.06 | ||
| Level of anastomosis from anal verge | 1.28 (0.87–1.88) | 0.21 | ||
| Tumor differentiation | ||||
| Well | Reference | |||
| Moderate | 1.40 (0.63–3.08) | 0.41 | ||
| Poor | 1.96 (0.69–5.58) | 0.21 | ||
| Undifferentiated | 2.63 (0.28–24.44) | 0.40 | ||
| Pathologic T stage (AJCC eighth edition) | ||||
| T0 | Reference | |||
| Tis | 0.31 (0.04–2.34) | 0.26 | ||
| T1 | 1.02 (0.52–1.98) | 0.96 | ||
| T2 | 0.97 (0.58–1.63) | 0.90 | ||
| T3 | 1.32 (0.79–2.20) | 0.30 | ||
| T4a | 4.07 (0.51–32.63) | 0.19 | ||
| T4b | 1.52 (0.60–3.84) | 0.37 | ||
| Pathologic N stage (AJCC eighth edition) | Reference | |||
| N1a | 1.27 (0.77–2.10) | 0.36 | ||
| N1b | 2.08 (0.96–4.47) | 0.06 | ||
| N1c | 0.82 (0.38–1.73) | 0.59 | ||
| N2a | 2.13 (0.82–5.55) | 0.12 | ||
| N2b | 5.01 (1.19–21.18) | 0.03* | ||
| N1a | – | |||
| Pathologic stage (AJCC eighth edition) | ||||
| 0 | Reference | |||
| I | 0.90 (0.53–1.53) | 0.70 | ||
| II | 1.20 (0.68–2.11) | 0.54 | ||
| III | 1.61 (0.92–2.82) | 0.10 | ||
| Final margin status | ||||
| R0 | Reference | |||
| R1 | 1.80 (0.75–4.33) | 0.19 | ||
| Anastomotic leak | ||||
| No | Reference | |||
| Yes | 0.81 (0.38–1.73) | 0.59 | ||
IQR = interquartile range; LAR = low anterior resection; APR = abdominoperineal resection; IMA = inferior mesenteric artery; SRA = superior rectal artery; AJCC = American Joint Committee on Cancer.
P value is significant.
For the outcome of lymph node harvest, age, female sex, and receipt of neoadjuvant therapy were associated with a decreased odds for harvesting ≥12 lymph nodes, whereas SRA ligation, APR, and 4 to 6 positive regional lymph nodes were associated with an increased odds for harvesting ≥12 lymph nodes (Table 2). When accounting for age, sex, neoadjuvant therapy, operation type, and operative approach in the multivariable model, there was no difference in adequate lymph node harvest with respect to level of ligation (OR = 3.77 (95% CI, 0.86–16.58); p = 0.08).
Oncologic Outcomes
Locoregional Recurrence-Free Survival
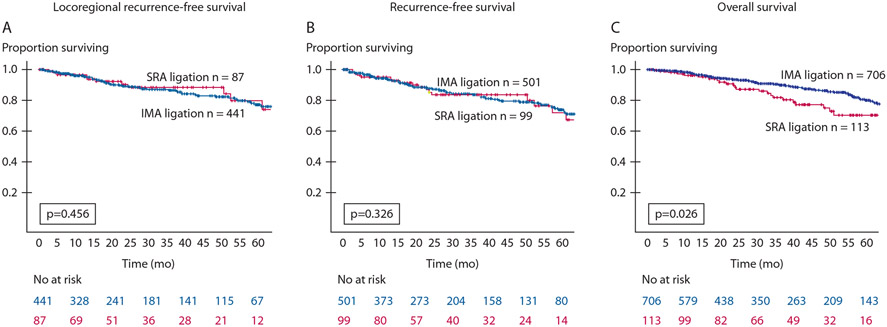
IMA and SRA ligation methods had equivalent 5-year LRFS (87% vs 90%; p = 0.456; Fig. 2A). Black race, more advanced pathologic stage, and an R1 resection were associated with worse LRFS on univariate Cox regression (Table 3). An MIS approach was associated with improved LRFS (HR = 0.53 (95% CI, 0.30–0.93); p = 0.03). Accounting for ligation method and operative approach on multivariable analysis, Black race, more advanced pathologic stage, and an R1 resection margin were associated with worse LRFS. SRA ligation method was not associated with worse LRFS (HR = 1.51 (95% CI, 0.78–2.92); p = 0.22).
FIGURE 2.
Kaplan–Meier curves comparing SRA ligation (red) and IMA ligation (blue) for locoregional recurrence-free survival (A), recurrence-free survival (B), and overall survival (C) based on ligation method. IMA = inferior mesenteric artery; SRA = superior rectal artery.
TABLE 3.
Association of ligation method with oncologic outcomes
|
Univariate regression
|
Multivariable regression
|
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Locoregional RFS | ||||
| Age at diagnosis | 0.99 (0.97–1.01) | 0.34 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 1.18 (0.66–2.11) | 0.59 | ||
| Race | ||||
| White | Reference | Reference | ||
| Black | 2.43 (1.173–4.80) | 0.02* | 2.08 (1.00–4.32) | 0.05* |
| Other | 0.72 (0.10–5.27) | 0.73 | 0.36 (0.05–2.82) | 0.33 |
| Diabetes | ||||
| No | Reference | |||
| Yes | 0.84 (0.37–1.86) | 0.66 | ||
| Smoking history | ||||
| No | Reference | |||
| Yes | 1.55 (0.86–2.78) | 0.14 | ||
| Neoadjuvant therapy | ||||
| No | Reference | |||
| Yes | 1.25 (0.61–2.57) | 0.55 | ||
| Functional status | ||||
| Independent | Reference | |||
| Partially dependent | 1.11 (0.15–8.13) | 0.91 | ||
| ASA class | ||||
| I | Reference | |||
| II | 0.19 (0.03–1.44) | 0.11 | ||
| III | 0.17 (0.02–1.30) | 0.09 | ||
| IV | 0.21 (0.13–3.46) | 0.28 | ||
| Ligation method | ||||
| IMA | Reference | Reference | ||
| SRA | 1.74 (0.92–3.27) | 0.09 | 1.51 (0.78–2.92) | 0.22 |
| Operation type | ||||
| LAR | Reference | |||
| APR | 1.12 (0.34–3.76) | 0.85 | ||
| Approach | ||||
| Open | Reference | Reference | ||
| Minimally invasive | 0.53 (0.30–0.93) | 0.03* | 0.59 (0.33–1.03) | 0.06 |
| Lymph nodes retrieved | 0.99 (0.95–1.03) | 0.55 | ||
| Lymph node harvest | ||||
| <12 lymph nodes | Reference | |||
| ≥12 lymph nodes | 0.73 (0.39–1.38) | 0.33 | ||
| Tumor Differentiation | ||||
| Well | Reference | |||
| Moderate | 2.27 (0.31–16.57) | 0.42 | ||
| Poor | 4.04 (0.47–34.62) | 0.20 | ||
| Undifferentiated | ||||
| Pathologic stage (AJCC eighth edition) | ||||
| 0 | Reference | Reference | ||
| I | 3.75 (0.49–28.70) | 0.20 | 3.11 (0.40–23.98) | 0.28 |
| II | 4.73 (0.61–36.53) | 0.14 | 4.06 (0.52–31.61) | 0.18 |
| III | 7.83 (1.06–57.88) | 0.04* | 6.35 (0.85–47.28) | 0.07 |
| Final margin status | ||||
| R0 | Reference | Reference | ||
| R1 | 2.85 (1.13–7.21) | 0.03* | 2.06 (0.80–5.27) | 0.14 |
| RFS | ||||
| Age at diagnosis | 0.99 (0.97–1.00) | 0.06 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 1.16 (0.81–1.68) | 0.42 | ||
| Race | ||||
| White | Reference | |||
| Black | 1.38 (0.79–2.40) | 0.26 | ||
| Other | 0.26 (0.04–1.87) | 0.18 | ||
| Diabetes | ||||
| No | Reference | |||
| Yes | 0.90 (0.56–1.45) | 0.67 | ||
| Smoking history | ||||
| No | Reference | |||
| Yes | 1.25 (0.85–1.82) | 0.26 | ||
| Neoadjuvant therapy | ||||
| No | 1.09 (0.71–1.68) | 0.69 | ||
| Yes | ||||
| Functional status | ||||
| Independent | 1.69 (0.62–4.59) | 0.31 | ||
| Partially dependent | 1.08 (0.50–2.32) | 0.85 | ||
| ASA class | ||||
| I | Reference | |||
| II | 0.40 (0.06–2.90) | 0.36 | ||
| III | 0.35 (0.05–2.52) | 0.30 | ||
| IV | 0.35 (0.03–3.87) | 0.39 | ||
| Ligation method | ||||
| IMA | Reference | Reference | ||
| SRA | 1.12 (0.78–1.88) | 0.39 | 0.93 (0.59–1.48) | 0.93 |
| Operation type | ||||
| LAR | Reference | |||
| APR | 0.79 (0.40–1.50) | 0.45 | ||
| Approach | ||||
| Open | Reference | Reference | ||
| Minimally invasive | 0.63 (0.44–0.89) | <0.01* | 0.69 (0.48–0.99) | 0.05* |
| Lymph nodes retrieved | 0.99 (0.95–1.03) | 0.55 | ||
| Lymph node harvest | ||||
| <12 lymph nodes | Reference | |||
| ≥12 lymph nodes | 0.73 (0.39–1.38) | 0.33 | ||
| Level of anastomosis from anal verge | 1.22 (0.52–2.85) | 0.65 | ||
| Tumor differentiation | ||||
| Well | Reference | |||
| Moderate | 1.05 (0.43–2.60) | 0.91 | ||
| Poor | 1.49 (0.51–4.36) | 0.47 | ||
| Undifferentiated | 2.22 (0.26–19.08) | 0.47 | ||
| Pathologic stage (AJCC eighth edition) | ||||
| 0 | Reference | Reference | ||
| I | 2.92 (0.68–12.44) | 0.15 | 2.56 (0.59–10.93) | 0.21 |
| II | 5.23 (1.25–21.83) | 0.02* | 4.35 (1.04–18.22) | 0.45 |
| III | 8.45 (2.07–34.46) | <0.01* | 6.31 (1.52–26.17) | 0.01* |
| Final margin status | ||||
| R0 | Reference | Reference | ||
| R1 | 2.77 (1.61–4.75) | <0.01* | 2.09 (1.20–3.64) | <0.01* |
| Anastomotic leak | ||||
| No | Reference | |||
| Yes | 1.01 (0.44–2.32) | 0.99 | ||
| Overall survival | ||||
| Age at diagnosis | 1.03 (1.02–1.05) | <0.01* | Reference | |
| Sex | 1.02 (1.00–1.04) | 0.02* | ||
| Male | Reference | |||
| Female | 1.38 (0.95–2.02) | 0.09 | ||
| Race | ||||
| White | Reference | |||
| Black | 1.32 (0.79–2.21) | 0.29 | ||
| Other | 0.80 (0.20–3.24) | 0.75 | ||
| Diabetes | ||||
| No | Reference | |||
| Yes | 1.36 (0.86–2.14) | 0.18 | ||
| Smoking history | ||||
| No | Reference | |||
| Yes | 1.06 (0.73–1.55) | 0.77 | ||
| Neoadjuvant therapy | ||||
| No | Reference | |||
| Yes | 1.00 (0.65–1.56) | 0.99 | ||
| Functional status | ||||
| Independent | Reference | Reference | ||
| Partially dependent | 4.01 (1.86–8.63) | <0.01* | 4.81 (1.97–11.73) | <0.01* |
| Ligation method | ||||
| IMA | Reference | Reference | ||
| SRA | 1.64 (1.06–2.55) | 0.03* | 1.46 (0.76–2.46) | 0.16 |
| Operation type | ||||
| LAR | Reference | |||
| APR | 0.756 (0.39–1.48) | 0.42 | ||
| Approach | ||||
| Open | Reference | Reference | ||
| Minimally invasive | 0.63 (0.44–0.89) | <0.01* | 0.72 (0.48–1.08) | 0.12 |
| Lymph nodes retrieved | 1.01 (0.98–1.03) | 0.63 | ||
| Lymph node harvest | ||||
| <12 lymph nodes | Reference | |||
| ≥12 lymph nodes | 0.98 (0.60–1.61) | 0.93 | ||
| Level of anastomosis from anal verge | 1.13 (0.82–1.56) | 0.46 | ||
| Tumor differentiation | ||||
| Well | Reference | Reference | ||
| Moderate | 2.60 (0.64–10.57) | 0.18 | 1.92 (0.47–7.93) | 0.37 |
| Poor | 4.70 (1.08–20.44) | 0.04* | 2.49 (0.55–11.18) | 0.24 |
| Undifferentiated | 5.06 (0.45–56.30) | 0.19 | 2.59 (0.23–29.73) | 0.45 |
| Pathologic stage (AJCC eighth edition) | ||||
| 0 | Reference | Reference | ||
| I | 1.57 (0.69–3.58) | 0.29 | 0.80 (0.19–3.48) | 0.77 |
| II | 2.30 (1.01–5.24) | 0.05* | 1.33 (0.31–5.76) | 0.70 |
| III | 3.68 (1.67–8.90) | <0.01* | 1.75 (0.41–7.42) | 0.45 |
| Final margin status | ||||
| R0 | Reference | Reference | ||
| R1 | 3.39 (2.01–5.71) | <0.01* | 3.02 (1.67–5.48) | <0.01* |
| Anastomotic leak | ||||
| No | Reference | |||
| Yes | 1.36 (0.58–3.19) | 0.49 | ||
LAR = low anterior resection; APR = abdominoperineal resection; IMA = inferior mesenteric artery; SRA = superior rectal artery; AJCC = American Joint Committee on Cancer; RFS = recurrence-free survival.
P value is significant.
Recurrence-Free Survival
Similar to LRFS, IMA and SRA ligation methods had nearly equivalent 5-year RFS (85% vs 82%; p = 0.326; Fig. 2B). On univariate Cox regression, an MIS approach was associated with improved RFS (HR = 0.63 (95% CI, 0.44–0.89); p < 0.01; Table 3). More advanced pathologic stage and an R1 resection margin were associated with worse RFS. In the multivariable model, accounting for the ligation method, these associations persisted for MIS approach (HR = 0.69 (95% CI, 0.48–0.99); p = 0.05), a more advanced pathologic stage (HR = 6.31 (95% CI, 1.52–26.17); p = 0.01), and an R1 resection margin (HR = 2.09 (95% CI, 1.20–3.64); p < 0.01). Ligation method was not associated with RFS.
Overall Survival
On Kaplan–Meier analysis, IMA ligation was associated with improved 3-year OS compared with SRA ligation (90% vs 85%; p = 0.026; Fig. 2C). On univariate Cox regression, increased age, partially dependent functional status, SRA ligation, poorly differentiated tumors, higher pathologic stage, and an R1 resection margin were associated with worse 3-year OS (Table 3). An MIS approach was associated with improved 3-year OS. When accounting for operative approach, tumor differentiation, and pathologic stage, SRA ligation was not associated with worse 3-year OS (HR = 1.46 (95% CI, 0.76–2.46); p = 0.16), whereas increasing age, partially dependent functional status, and an R1 resection margin were independent predictors for worse survival.
DISCUSSION
For rectal cancer surgery, the level of arterial ligation is dictated by technical and oncologic considerations. Currently, there is no consensus regarding the optimal level for ligation, and many surgeons advocate for performing an IMA ligation when approaching the TME plane. Existing data are inconclusive and heterogeneous. Our analyses of a large, multi-institutional cohort from the United States demonstrated that SRA ligation was not associated with inferior technical or oncologic outcomes. Specifically, SRA ligation was not associated with increased anastomotic leak rates, inadequate lymph node harvest, or worse LRFS, RFS, and OS. Therefore, the practice of SRA ligation should be considered more often.
Surgeons must balance technical factors such as adequate blood flow and minimal tension to the anastomosis, both universally known to be critical in mitigating the risk of anastomotic leak, with oncologic appropriateness. In an era of minimally invasive surgery, many surgeons advocate for IMA ligation as the preferred method, a trend that is consistent with the 91% (n = 531) of patients who received an IMA ligation compared with 9% (n = 51) who received an SRA ligation in our study cohort.5 This is likely attributable to the greater ease that a high ligation affords after accessing the sigmoid mesentery. With the division of the IMA at its origin, the blood supply to the left colon depends on the blood flow from the superior mesenteric artery via the middle colic artery supplying the marginal artery of Drummond and the arc of Riolan. However, Gourley and Gering6 reported that the marginal artery can be absent in up to 4% to 20% of patients. For individuals with this anatomic variant, there is a theoretical increased risk of inadequate blood flow to the proximal limb of the anastomosis and, consequently, anastomotic leak. The SRA ligation method mitigates this potential problem.
Documented risk factors for anastomotic leak after rectal surgery include diabetes, tobacco use, vascular disease, malnutrition, and a low level of anastomosis.7-9 Similarly, our multivariable model demonstrated smoking history as an independent predictor for anastomotic leak. In addition, data remain inconsistent regarding the association of preoperative radiation and anastomotic leak.10-12 Administration of preoperative radiation was not an independent predictor for anastomotic leak on univariate or multivariable analyses. At this time, there is a paucity of level I evidence to suggest that preoperative therapy impairs healing of an anastomosis.
Studies have shown no difference in anastomotic leak rates when comparing IMA with SRA ligation. A Swedish 2007–2009 retrospective review of 2023 rectal cancer patients demonstrated no difference in anastomotic leak rates between the 2 ligation methods.13 Similarly, in a Japanese prospective, randomized controlled trial of 331 patients from 2006 to 2012 who underwent LAR for rectal cancer, Fujii et al14 reported that the level of ligation did not significantly impact the rate of anastomotic leak, although the trial was halted prematurely for slow enrollment. These results were substantiated in a 2012 systematic review by Cirocchi et al15 when comparing IMA and SRA ligation. Recently, a 2018 meta-analysis by Yang et al16 confirmed that there were no differences in anastomotic leak rates or the total number of lymph nodes harvested for patients undergoing surgery for colorectal cancer. The results from the current study, which, to our knowledge, is the largest series in the United States, corroborate these previous findings. Based on these data, there is clinical equipoise when considering the technical merits of the 2 ligation approaches.
Regarding oncologic outcomes, available data reveal that the method of ligation does not significantly impact long-term oncologic outcomes. A 2019 Korean study of 1213 patients who underwent LAR for stage I to III rectal cancer described similar 5-year locoregional RFS (92% vs 96%; p = 0.20) and 5-year OS (88% vs 93%; p = 0.17) for patients who underwent IMA versus SRA ligation.17 More recently, a 2020 meta-analysis by Hajibandeh et al18 that included 8 randomized controlled trials for patients who underwent rectal cancer surgery reported no difference in 5-year RFS (77% vs 79%; OR = 0.88 (95% CI, 0.56–1.39); p = 0.58) or 5-year OS (84% vs 88%; OR = 0.76 (95% CI, 0.43–1.32); p = 0.32) when comparing high and low ligation, although the sample sizes were relatively small (sample size in high ligation group: 29–164). In our study, RFS and OS were comparable as well (5-year RFS, 85% vs 82%, p = 0.326; 3-year OS, 90% vs 85%, p = 0.026). For all of the oncologic outcomes, predictably, a more advanced pathologic stage and an R1 resection were associated with earlier recurrence and worse survival. There are certain demographic, functional, and technical factors that may be associated with worse outcomes.19,20 Accordingly, our multivariable analyses demonstrated that Black race was associated with worse LRFS, whereas increased age and worse functional status were associated with worse OS. Interestingly, an MIS approach was associated with improved RFS on multivariable analysis, although this is likely a result of a selection bias. A 2014 systematic review of 14 randomized controlled trials comparing laparoscopic versus open surgery for colorectal cancer suggested that each approach had similar local recurrence rates (OR = 0.89 (95% CI, 0.57–1.39)).5 The authors concluded that an MIS approach is feasible and safe. Notably, when accounting for operative approach, our results demonstrate that ligation method is not an independent predictor for recurrence or survival.
Proponents of the IMA ligation method have previously suggested that the technique allows for improved lymph node clearance, thus resulting in improved accuracy of tumor staging.21 However, despite the technical ease of accessing the lymph nodes along the origin of the IMA, more recent studies report no difference in the total number of lymph nodes harvested.22,23 In addition, larger systematic reviews have also debunked this notion.16,18 For low rectal tumors, there is a presumed advantage that a high-ligation approach helps facilitate the mobilization of the left colon down to the pelvis for a tension-free anastomosis. Specifically, an IMA ligation has been reported to allow for ≥10 cm gain in colonic length.24 In the current study, however, there was no difference in the adequacy of lymph node harvest, and the level of the anastomosis from the anal verge was similar in both groups. Thus, an SRA ligation is likely adequate in most instances to still enable a good technical result for a low anastomosis. In instances where added length is needed for a tension-free anastomosis, an IMA ligation is certainly appropriate.
The advantage of an SRA ligation approach is high-lighted in current systematic reviews and meta-analyses. A 2019 systematic review of 8456 patients with colorectal cancer by Yang et al25 demonstrated that SRA ligation was associated with decreased rates of anastomotic leak (OR = 1.23 (95% CI, 1.02–1.48); p = 0.03). In a meta-analysis by Zeng and Su26 that included patients with sigmoid colon and rectal cancer who underwent IMA or SRA ligation, IMA ligation was associated with increased rates of anastomotic leak (OR = 1.33 (95% CI, 1.05–1.68); p = 0.05), although there was no significant difference in the number of retrieved lymph nodes, recurrence rate, or 5-year OS. For patients with rectal cancer who underwent neoadjuvant radiation therapy, Beppu et al27 reported that IMA ligation and undifferentiated tumor type (p < 0.01) were risk factors for anastomotic leak when controlling for hospital stay and size of tumor on multivariable analysis. This evidence highlights the potential advantage of an SRA ligation approach over an IMA ligation approach. In this study, there was no association between SRA ligation and anastomotic leak rates, likely because of equivalency in patient selection and surgical expertise.
Lastly, given that the superior hypogastric nerve plexus lies in a plane posterior to the origin of the IMA, proceeding with an SRA ligation may avoid the unnecessary risk of autonomic dysfunction.28,29 Although a 2017 retrospective review by Kverneng Hultberg et al30 demonstrated no difference in defecatory, urinary, or sexual dysfunction 2 years after LAR for rectal cancer based on ligation method, prospective studies have reported the benefit of a low ligation. For example, in a prospective, randomized controlled trial of 214 patients with rectal cancer who underwent laparoscopic LAR with either high or low ligation, Mari et al31 reported improved genitourinary function preservation with an SRA ligation, emphasizing the advantages of this approach. These data suggest the utility of an SRA ligation method in avoiding sexual and urinary complications by decreasing the risk of nerve injury.
Although a large multi-institutional database allows for improved generalizability, limitations of this study include those inherent to a retrospective design, specifically the exclusion of missing data and absent study variables that can lend itself to potential bias. For example, 260 patients in the database were missing ligation data and were thus excluded. In addition, the level of granularity for variables such as anterior resection (vs LAR) and inferior mesenteric vein resection were not available in the database. Lastly, the decision to pursue an IMA or SRA ligation was left to surgeon preference and was thus subject to potential selection bias.
CONCLUSION
Although obtaining a TME and adequate lymph node clearance can be achieved with either an IMA or SRA ligation approach, analyses from this multi-institutional collaborative demonstrate that SRA ligation is not associated with inferior technical or oncologic outcomes compared with IMA ligation. Recognizing that the ligation technique used depends on multiple surgeon- and patient-specific factors, spanning technical expertise, operative approach, and patient anatomy, given equivalent outcomes and the added potential of decreasing the risk for inadequate blood flow to the anastomosis and autonomic nerve injury, we advocate for increased use of SRA ligation whenever feasible.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Rachel M. Lee, M.D., M.S.P.H. (Division of Surgical Oncology, Winship Cancer Institute, Emory University); Jeffrey Maniko, B.S., and Lillias Maguire, M.D. (Division of Colorectal Surgery, Department of Surgery, University of Michigan); Maryam Mohammed, M.D., and Katherine A. Hrebinko, M.D. (Division of Colon and Rectal Surgery, Department of Surgery, University of Pittsburgh Medical Center); Jason T. Wiseman, M.D., Aslam Ejaz, M.D., and Charles Kimbrough, M.D. (Division of Surgical Oncology, Department of Surgery, The Ohio State University Wexner Medical Center); Kamren Edwards-Hollingsworth, B.A. (Section of Colon and Rectal Surgery, Division of General Surgery, Vanderbilt University Medical Center); and Philip Bauer, M.D. (Section of Colon and Rectal Surgery, Department of Surgery, Washington University School of Medicine) for their contributions. The authors thank Andy Matlock (Emory University) for permission to publish the illustration of ligation techniques included in the article.
Funding/Support:
This study is supported in part by the Katz Foundation and the National Center for Advancing Translational Science (grant/award No. UL1TR002378/TL1TR002382).
Footnotes
Financial Disclosure: None reported.
Poster presentations at the American Society for Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, California, January 22 to 25, 2020, and the Society of Surgical Oncology, Boston, Massachusetts, August 17 to 20, 2020.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 2.Nano M, Dal Corso H, Ferronato M, Solej M, Hornung JP, Dei Poli M. Ligation of the inferior mesenteric artery in the surgery of rectal cancer: anatomical considerations. Dig Surg. 2004;21:123–126. [DOI] [PubMed] [Google Scholar]
- 3.Chin CC, Yeh CY, Tang R, Changchien CR, Huang WS, Wang JY. The oncologic benefit of high ligation of the inferior mesenteric artery in the surgical treatment of rectal or sigmoid colon cancer. Int J Colorectal Dis. 2008;23:783–788. [DOI] [PubMed] [Google Scholar]
- 4.Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum. 2017;60:999–1017. [DOI] [PubMed] [Google Scholar]
- 5.Vennix S, Pelzers L, Bouvy N, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database SystRev. 2014;(4):CD005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gourley EJ, Gering SA. The meandering mesenteric artery: a historic review and surgical implications. Dis Colon Rectum. 2005;48:996–1000. [DOI] [PubMed] [Google Scholar]
- 7.Warschkow R, Steffen T, Thierbach J, Bruckner T, Lange J, Tarantino I. Risk factors for anastomotic leakage after rectal cancer resection and reconstruction with colorectostomy: a retrospective study with bootstrap analysis. Ann Surg Oncol. 2011;18:2772–2782. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Gu J. Risk factors for symptomatic anastomotic leakage after low anterior resection for rectal cancer with 30 Gy/10 f/2 w preoperative radiotherapy. World J Surg. 2010;34:1080–1085. [DOI] [PubMed] [Google Scholar]
- 9.Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. 2017;19:288–298. [DOI] [PubMed] [Google Scholar]
- 10.Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K. Do we really know why colorectal anastomoses leak? J Gastrointest Surg. 2013;17:1698–1707. [DOI] [PubMed] [Google Scholar]
- 11.Milgrom SA, Goodman KA, Nash GM, et al. Neoadjuvant radiation therapy prior to total mesorectal excision for rectal cancer is not associated with postoperative complications using current techniques. Ann Surg Oncol. 2014;21:2295–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden DM, Mora Pinzon MC, Francescatti AB, Saclarides TJ. Patient factors may predict anastomotic complications after rectal cancer surgery: anastomotic complications in rectal cancer. Ann Med Surg (Lond). 2015;4:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutegård M, Hemmingsson O, Matthiessen P, Rutegård J. High tie in anterior resection for rectal cancer confers no increased risk of anastomotic leakage. Br J Surg. 2012;99:127–132. [DOI] [PubMed] [Google Scholar]
- 14.Fujii S, Ishibe A, Ota M, et al. Randomized clinical trial of high versus low inferior mesenteric artery ligation during anterior resection for rectal cancer. BJS Open. 2018;2:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirocchi R, Trastulli S, Farinella E, et al. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a RCT is needed. Surg Oncol. 2012;21:e111–e123. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Wang G, He J, Zhang J, Xi J, Wang F. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a meta-analysis. Int J Surg. 2018;52:20–24. [DOI] [PubMed] [Google Scholar]
- 17.AlSuhaimi MA, Yang SY, Kang JH, AlSabilah JF, Hur H, Kim NK. Operative safety and oncologic outcomes in rectal cancer based on the level of inferior mesenteric artery ligation: a stratified analysis of a large Korean cohort. Ann Surg Treat Res. 2019;97:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajibandeh S, Hajibandeh S, Maw A. Meta-analysis and trial sequential analysis of randomized controlled trials comparing high and low ligation of the inferior mesenteric artery in rectal cancer surgery. Dis Colon Rectum. 2020;63:988–999. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel E, Attwood K, Al-Sukhni E, Erwin D, Boland P, Nurkin S. Age-related rates of colorectal cancer and the factors associated with overall survival. J Gastrointest Oncol. 2018;9:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boakye D, Jansen L, Schneider M, Chang-Claude J, Hoffmeister M, Brenner H. Personalizing the prediction of colorectal cancer prognosis by incorporating comorbidities and functional status into prognostic nomograms. Cancers (Basel). 2019;11:E1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titu LV, Tweedle E, Rooney PS. High tie of the inferior mesenteric artery in curative surgery for left colonic and rectal cancers: a systematic review. Dig Surg. 2008;25:148–157. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Zhang S, Huang J, et al. Accurate low ligation of inferior mesenteric artery and root lymph node dissection according to different vascular typing in laparoscopic radical resection of rectal cancer [in Chinese]. Zhonghua Wei Chang Wai KeZa Zhi. 2018;21:46–52. [PubMed] [Google Scholar]
- 23.You X, Liu Q, Wu J, et al. High versus low ligation of inferior mesenteric artery during laparoscopic radical resection of rectal cancer: a retrospective cohort study. Medicine (Baltimore). 2020;99:e19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnet S, Berger A, Hentati N, et al. High tie versus low tie vascular ligation of the inferior mesenteric artery in colorectal cancer surgery: impact on the gain in colon length and implications on the feasibility of anastomoses. Dis Colon Rectum. 2012;55:515–521. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Ma P, Zhang X, et al. Preservation versus non-preservation of left colic artery in colorectal cancer surgery: an updated systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng J, Su G. High ligation of the inferior mesenteric artery during sigmoid colon and rectal cancer surgery increases the risk of anastomotic leakage: a meta-analysis. World J Surg Oncol. 2018;16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beppu N, Matsubara N, Noda M, et al. A’high tieconfers an increased risk of anastomotic leakage for lower rectal cancer surgery in patients treated with preoperative radiotherapy. Surg Today. 2015;45:600–605. [DOI] [PubMed] [Google Scholar]
- 28.Aqar HI, Kuzu MA. Important points for protection of the autonomic nerves during total mesorectal excision. Dis Colon Rectum. 2012;55:907–912. [DOI] [PubMed] [Google Scholar]
- 29.Moszkowicz D, Alsaid B, Bessede T, et al. Where does pelvic nerve injury occur during rectal surgery for cancer? Colorectal Dis. 2011;13:1326–1334. [DOI] [PubMed] [Google Scholar]
- 30.Kverneng Hultberg D, Afshar AA, Rutegard J, et al. Level of vascular tie and its effect on functional outcome 2 years after anterior resection for rectal cancer. Colorectal Dis. 2017;19:987–995. [DOI] [PubMed] [Google Scholar]
- 31.Mari GM, Crippa J, Cocozza E, et al. Low ligation of inferior mesenteric artery in laparoscopic anterior resection for rectal cancer reduces genitourinary dysfunction: results from a randomized controlled trial (HIGHLOW Trial). Ann Surg. 2019;269:1018–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.