Abstract
Cytomegalovirus (CMV) is associated with significant morbidity and mortality in allogeneic hematopoietic cell transplantation (HCT) patients. We evaluated the efficacy of letermovir as primary and secondary prophylaxis in 53 CMV-seropositive hematopoietic stem cell transplant recipients. 70% of patients were at high risk for CMV reactivation and disease (primarily ex vivo T-cell–depleted HCT [n = 18; 34%] or haploidentical T-replete HCT [n = 12; 23%]). This was a retrospective, single-center study which identified patients transplanted between January 2018 and June 2018. Patients were followed through September 2018. The primary outcome was the incidence of clinically significant CMV infection (CMV viremia requiring preemptive treatment or CMV disease). Primary letermovir prophylaxis started at a median of 7 days (range, 7-40) after allo-HCT. The median duration of primary letermovir prophylaxis was 116 days (range, 12-221). With primary prophylaxis in 39 patients, the observed CMV reactivation rate was 5.1%. Twenty-nine patients continued primary prophylaxis beyond 14 weeks with a reactivation rate of 3.4%. No recurrent reactivation was seen with secondary prophylaxis of an additional 14 patients. Our experience demonstrates the efficacy of letermovir in a real-world setting for CMV prevention for the first 14 weeks and continued efficacy when given longer than 14 weeks after allogeneic stem cell transplantation or as secondary prophylaxis.
Keywords: allogeneic hematopoietic cell transplantation, cytomegalovirus, letermovir, primary prophylaxis, secondary prophylaxis
1 |. INTRODUCTION
Cytomegalovirus (CMV) is the most common clinically significant viral infection in allogeneic hematopoietic cell transplantation (allo-HCT) recipients and is associated with significant morbidity and mortality.1,2 Over the past three decades, most centers adopted a preemptive strategy (PET) where CMV surveillance and detection in blood triggers antiviral therapy to prevent CMV end-organ disease while minimizing toxicity of these antivirals (eg, ganciclovir, valganciclovir, foscarnet).3,4 Approximately 60% of patients require PET within the first year of allo-HCT.5Despite PET’s effectiveness, CMV adversely affects transplant outcomes.1,2,5
Letermovir was approved by the US Food and Drug Administration in November 2017 for primary CMV prevention in CMV-seropositive recipients (CMV R+) based upon the phase 3 trial showing significantly reduced CMV infection through week 24 after allo-HCT in comparison with placebo (18.9% vs 44.3%, P< .001).6 Given toxicities of antivirals used in PET, there is also interest in utilizing letermovir as secondary prophylaxis. We report our singlecenter experience with letermovir as both primary and secondary prophylaxis for CMV in allo-HCT.
2 |. METHODS
The study was reviewed and approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center and granted a waiver of authorization. The study cohort consisted of CMV R + adult (≥18 years) recipients of allo-HCT between January 2018 and June 2018 who received letermovir for CMV prevention. Representative myeloablative and reduced intensity conditioning transplant protocols have been previously described.7–9 Ex vivo T-cell depletion by CD34 selection was performed by the CliniMACS CD34 Reagent System (Miltenyi Biotech). Cord blood recipients were excluded from this analysis as preliminary results have been presented separately.10 Supportive care was provided by institutional standards of care (eg, acyclovir for herpes simplex virus and varicella zoster virus prevention).11 Plasma CMV viral load (VL) was monitored by quantitative CMV PCR (COBAS® AmpliPrep/COBAS® TaqMan® CMV Test, Roche Diagnostics), starting on day + 5 and continued weekly until day + 100, then per immune recovery for unmodified allo-HCT (non-CD34-selected) or every 1-2 weeks for months 3-6 for other transplant types.
Primary letermovir prophylaxis was started by day + 7 in high-risk patients (eg, T-cell–depleted graft, mismatched, or haploidentical donor) and by day + 28 in low-risk patients. Letermovir was switched to systemic antiviral treatment when there were >2 consecutive values of CMV VL >300 IU/mL. Patients ineligible for primary letermovir prophylaxis but achieved virologic suppression (defined as undetectable VL or <137 IU/mL) on systemic antiviral therapy for CMV could be switched to letermovir for secondary prophylaxis. The primary outcome was the proportion of patients who developed clinically significant CMV reactivation (CMV viremia requiring PET or CMV disease). Patients were followed through September 2018 or death, whichever occurred first.
3 |. RESULTS
Baseline characteristics of 53 patients who received letermovir are shown in Table 1. Thirty-nine (74%) and 14 (26%) patients received letermovir for primary and secondary prophylaxis, respectively. During the observational period, all patients eligible to receive letermovir for primary prophylaxis were treated. Prescription coverage screening was done at a pre-admission visit, and the pharmacists obtained prior authorizations, wrote appeal letters, and/or applied for manufacturer patient assistance as needed. The median age was 55 years (range, 20-74), and 57% (30/53) were men. Thirty-seven of 53 (70%) were characterized as high risk for CMV reactivation, including 18 (34%) T-cell–depleted and 12 (23%) haploidentical T-cell replete transplants. Most patients received myeloablative conditioning (64%), and antithymocyte globulin was given to 19 patients (36%).
TABLE 1.
Characteristics of patients receiving primary or secondary letermovir prophylaxis
| Characteristic | All patients (N = 53) | Primary prophylaxis (N = 39) | Secondary prophylaxis (N = 14) |
|---|---|---|---|
| Median age, years (range) | 55 (20-74) | 59 (20-74) | 54 (28-72) |
| Male, N (%) | 30 (57) | 21 (54) | 9 (64) |
| Underlying cancer diagnosis, N (%) | |||
| Acute myeloid leukemia | 16 (30) | 12 (31) | 4 (29) |
| Myelodysplastic syndrome/myeloproliferative neoplasm | 15 (28) | 13 (33) | 2 (14) |
| Non-Hodgkin’s lymphoma | 7 (13) | 5 (13) | 2 (14) |
| Multiple myeloma | 5 (9) | 2 (5) | 3 (21) |
| Acute lymphocytic leukemia | 4 (8) | 3 (8) | 1 (7) |
| Other disease | 6 (11) | 4 (10) | 2 (14) |
| HLA matching and donor type, N (%) | |||
| Matched unrelated | 24 (45) | 16 (41) | 8 (57) |
| Matched related | 11 (21) | 9 (23) | 2 (14) |
| Haploidentical related donor | 12 (23) | 9 (23) | 3 (21) |
| Other mismatched related | 2 (4) | 2 (5) | -- |
| Mismatched unrelated | 4 (8) | 3 (8) | 1 (7) |
| Stem cell source, N (%) | |||
| Peripheral blood | 45 (85) | 32 (82) | 13 (93) |
| Bone marrow | 8 (15) | 7 (18) | 1(7) |
| Myeloablative conditioning regimen, N (%) | 34 (64) | 28 (72) | 7 (50) |
| Antithymocyte globulin use, N (%) | 19 (36) | 13 (33) | 7 (50) |
| Ex vivo T-cell depletion, N (%) | 18 (34) | 14 (36) | 5 (36) |
| Risk of CMV disease, N (%) | |||
| High risk | 37 (70) | 27 (69) | 10 (71) |
| Low risk | 16 (30) | 12 (31) | 4 (27) |
| CMV reactivation, N (%) | 2 (4) | 2 (5) | -- |
Note: Abbreviations: CMV, cytomegalovirus; N, number.
All others who did not meet the definition of being at high risk for CMV reactivation were considered low risk.
(Primary prophylaxis) High risk was defined as meeting one or more of the following criteria: having a related donor with at least one mismatch at one of the specified three HLA gene loci (HLA-A, B, or DR); having an unrelated donor with at least one mismatch at one of the specified four HLA gene loci (HLA-A, B, C, and DRB1); having a haploidentical donor; the use of ex vivo T-cell–depleted grafts; having graft-versus-host disease of grade 2 or greater that led to the use of prednisone (or its equivalent) at a dose of 1 mg or more per kilogram of body weight per day.6
Primary letermovir prophylaxis started at a median of 7 days (range, 7-40) after allo-HCT. Upon letermovir initiation, 35 patients had an undetectable CMV VL, and four patients had a detectable CMV VL <137 IU/mL. The median duration of primary letermovir prophylaxis was 116 days (range, 12-221) with primary prophylaxis continuing beyond 14 weeks after allo-HCT in 29 patients (74%). Of these 29 patients, 20 were high risk for CMV reactivation and took letermovir for a median of 131 days (range, 84-221), whereas 9 low-risk patients took letermovir for a median of 110 days (range, 84-151).
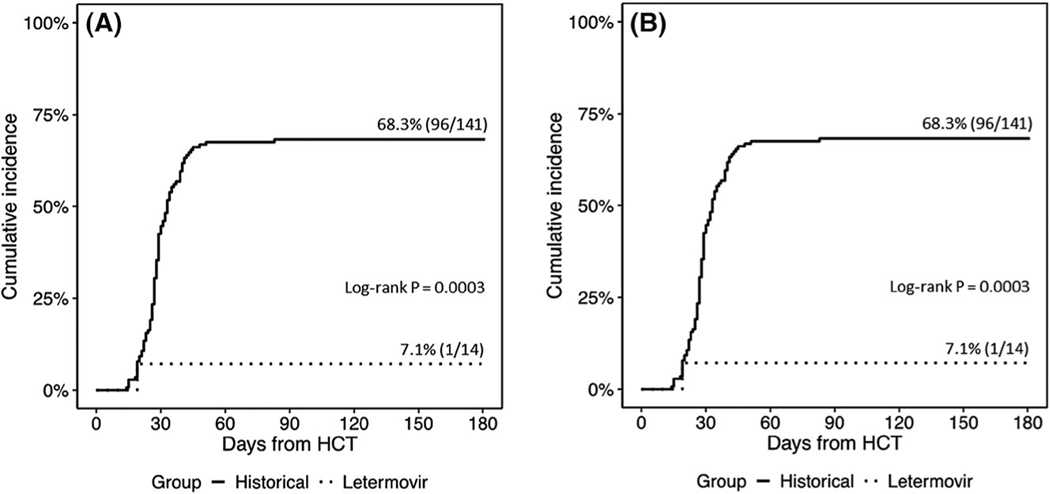
Clinically significant CMV reactivation without disease occurred in 2 of 39 (5%) patients, including only 1 of 39 patients (2.5%) at 14 weeks after allo-HCT. None of the four patients with a CMV VL <137 IU/mL developed a clinically significant CMV reactivation. One recipient of a T-cell–depleted allo-HCT was treated with valganciclovir for persisting CMV <137 IU/mL and then was switched back to letermovir for secondary prophylaxis. The other, a recipient of a second allo-HCT, developed breakthrough CMV viremia after brief medication non-adherence around day + 100 and subsequently developed a documented mutation in UL56 at site C325Y. This patient’s CMV viremia was successfully treated with valganciclovir. This was the only case of clinically significant CMV reactivation in the 29 patients (3.4%) who received extended primary prophylaxis with letermovir. The incidence of CMV viremia by D + 180 and CMV disease in T-cell–depleted allo-HCT compared to contemporary controls are shown in Figure 1A,B, respectively. The contemporary historical cohort included 141 CMV-seropositive T-cell–depleted HCT recipients transplanted from 6/2010-12/2014. The conditioning regimens utilized and underlying hematologic malignancies were the same as the T-cell–depleted group in this current observational study. The contemporary cohort also received routine CMV monitoring and preemptive management.11
FIGURE 1.
A, Cumulative incidence of CMV viremia by D + 180 in CMV R + TCD allo-HCT. B, Cumulative incidence of CMV end-organ disease by one year in CMV R + TCD allo-HCT
Secondary prophylaxis commenced upon completion of at least one course of CMV PET. These 14 patients received a median of one treatment course (range, 1-2) requiring 1-3 CMV-directed antivirals before initiation of letermovir. At the time of letermovir initiation, 11 patients had an undetectable CMV VL, and three had CMV VL <137 IU/mL. The median duration of secondary letermovir prophylaxis was 125 days (range, 18-270) with no recurrent reactivation.
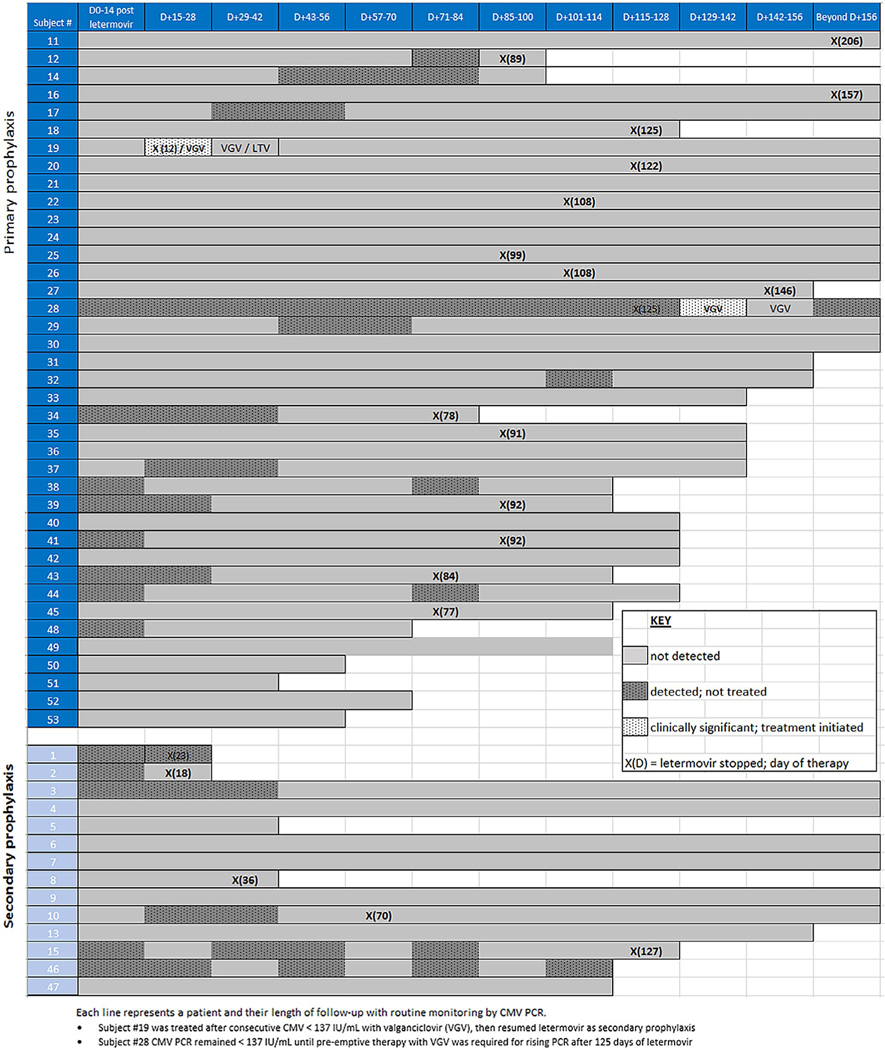
There were no discontinuations of letermovir due to toxicity or intolerance. The duration of letermovir prophylaxis was at the discretion of the treating physician. Given the delayed T-cell recovery seen in T-cell–depleted allo-HCT, our group has aimed to continue letermovir for up to 6 months post-transplant in this and other high-risk subgroups.12 The duration of letermovir prophylaxis was often shorter if insurance coverage was limited to 100 days and/or for patient compliance and pill burden issues. CMV outcomes are summarized in Figure 2. Median follow-up was 117 days (range, 12-270). At the end of follow-up, 6 (11.3%) patients died. Causes of death included relapse or progression of disease (N = 4) and graft-versus-host disease (N = 2). No deaths were attributable to CMV.
FIGURE 2.
CMV outcomes with letermovir
4 |. DISCUSSION
In this first report of real-world experience with letermovir, we show that primary letermovir prophylaxis was efficacious in preventing CMV infection in CMV R + allo-HCT patients. Only 2 (5%) of 39 patients who received primary letermovir prophylaxis subsequently required antiviral therapy for CMV viremia, and no patient (0/14, 0%) experienced recurrent CMV reactivation while on secondary prophylaxis. Letermovir was well tolerated as there were no discontinuations due to treatment-emergent adverse events.
In the letermovir registration trial, prophylaxis was started up to 28 days after allo-HCT. We implemented a risk-stratified approach in which the time to initiation of primary prophylaxis was contingent upon risk of CMV reactivation with allo-HCT patients at high risk started earlier (by day + 7) than those deemed low risk (by day + 28). We found no adverse impact to starting letermovir prophylaxis later in low-risk allo-HCT patients.
The optimal duration of primary prophylaxis has yet to be determined. At 14 weeks post-transplant, our results were similar with a 2.5% incidence of clinically significant CMV reactivation vs 7.7% in the registration trial. However, Marty et al noted a rise in post-prophylactic CMV events starting around week 18 that likely signaled ongoing or new periods of CMV risk beyond day + 100.6 We observed continued efficacy when extending primary letermovir prophylaxis beyond 14 weeks as only 1 of 29 patients reactivated CMV. Our institution reported rates of CMV viremia of 66.3% in a contemporary cohort of T-cell–depleted HCT recipients.0.11 Similarly, a 53%-81% incidence of CMV viremia was reported in haploidentical allo-HCT.13 In our study, clinically significant CMV was detected in only 1 of 30 patients (3%) who received a T-cell–depleted or haploidentical allo-HCT and took letermovir prophylaxis for a median of 122 days (range, 12-270). There is an ongoing randomized clinical trial to evaluate efficacy and safety of letermovir prophylaxis when extended to 200 days post-allo-HCT (NCT03930615). In the interim, our finding suggests that duration of primary prophylaxis should probably be individualized according to the patient’s period of CMV risk.
We note that 1 of 39 (3%) patients taking primary prophylaxis developed breakthrough viremia with a confirmed UL56 mutation following a transient period of sporadic dosing. Since experimental data suggest letermovir may possess a low resistance barrier, close clinical and laboratory monitoring are essential.14
Treatment of CMV infection with currently available drugs can lead to significant therapy-related toxicities (eg, myelosuppression, nephrotoxicity). Given the effectiveness and safety profile of letermovir, it is an attractive candidate for secondary prophylaxis in patients who are unable to tolerate ganciclovir or foscarnet over the long-term. Our data support the utility of letermovir as secondary prophylaxis, as none of the patients developed recurrent CMV viremia.
Our study has several limitations inherent to its retrospective and observational nature. Our sample size is relatively small. Since this was a real-world study, we relied on patient reports of adherence. Furthermore, when patients transferred follow-up care to their local oncologists, CMV monitoring was not standardized (eg, different monitoring frequencies or different assays used). While acknowledging these limitations, our data provides real-world data from a major tertiary cancer center performing allo-HCT at high risk for CMV. Ex vivo T-cell depleted and haploidentical HCT comprised 34% and 23% of our cohort, respectively. These HCT types were underrepresented in the letermovir registration study (2.4% and 16% of the study population, respectively).6 Approximately 70% of ex vivo T-cell–depleted HCT recipients require PET for CMV, often requiring an extended duration due to prolonged immunosuppression and resulting in substantial healthcare utilization. Up to 12.5% of T-cell–depleted HCT with CMV viremia develop CMV disease by 1-year post HCT, which has been associated with decreased overall survival.11 Additional studies are needed to quantify the impact of letermovir on the reduction of days of PET, readmissions, hospital length of stay, and overall long-term survival in high-risk patients.
In summary, our data support the efficacy of letermovir for CMV prevention for the first 14 weeks and continued efficacy when given longer than 14 weeks after allo-HCT or as secondary prophylaxis. Larger studies are required to confirm these findings and to address the optimal duration of prophylaxis.
ACKNOWLEDGEMENTS
This research was supported in part by National Institutes of Health award P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
Dr Papanicolaou serves as a Consultant for Merck, Astellas, Chimerix, Clinigen, Ideogen, Shionogi, ADMA, and Amplyx. She has received research funding and/or grant support from Merck, Astellas, Chimerix, and Shire. Dr Perales reports honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite (Gilead), and Miltenyi Biotec. He serves in a volunteer capacity as a member of the Board of Directors of American Society of Transplantation and Cellular Therapy (ASTCT) and Be the Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Advisory Committee and Cellular Immunotherapy Data Resource (CIDR) Oversight Committee.
Footnotes
CONFLICT OF INTERESTS
All other authors have no relevant conflicts of interests to disclose.
REFERENCES
- 1.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2014;59:473–481. [DOI] [PubMed] [Google Scholar]
- 2.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325:1601–1607. [DOI] [PubMed] [Google Scholar]
- 5.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3:e119–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–2444. [DOI] [PubMed] [Google Scholar]
- 7.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 9.Pingali SR, Milton D, di Stasi A, et al. Haploidentical transplantation for advanced hematologic malignancies using melphalan-based conditioning - Mature results from a single center. Biol Blood Marrow Transplant. 2014;20:S40–S41. [Google Scholar]
- 10.Lau C, Politikos I, Maloy M, et al. Letermovir prophylaxis demonstrates high efficacy in adult cytomegalovirus (CMV) seropositive cord blood transplant (CBT) recipients: a comparison with pre-letermovir era CBT controls. Biol Blood Marrow Transplant. 2019;25:S94–S95. [Google Scholar]
- 11.Huang YT, Su Y, Kim SJ, et al. Cytomegalovirus infection in allogeneic hematopoietic cell transplantation managed by the preemptive approach: estimating the impact on healthcare resource utilization and outcomes. Biol Blood Marrow Transplant. 2019;25:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg JD, Junting Z, Ratan R, et al. Early recovery of T-cell function predicts improved survival after T-cell depleted allogeneic transplant. Leuk Lymphoma. 2017;58:1859–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammerstrom AE, Lombardi LR, Pingali SR, et al. Prevention of Cytomegalovirus Reactivation in Haploidentical Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24: 353–358. [DOI] [PubMed] [Google Scholar]
- 14.Chou S. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother. 2015;59:6588–6593. [DOI] [PMC free article] [PubMed] [Google Scholar]