Abstract
Targets:
Diabetes increases the risk for cognitive impairment and doubles the rate of cognitive decline after diagnosis. In turn, cognitive dysfunction makes diabetes self-management more difficult. Nurses who help manage these conditions are focused on identifying patients who are at risk of complications, promoting symptom management, and preventing further decline. Therefore, the purpose of this study was to develop and pilot test a nurse-led comprehensive cognitive training intervention for persons with type 2 diabetes.
Intervention Description:
The 8-week intervention combined in-person classes and online computer training. Development included (1) adaptation of established, tested interventions; (2) interviews with stakeholders; (3) integration of course content; and (4) pilot testing of the intervention in a one-group, pre-test/post-test design (n=19).
Mechanisms of Action:
We expected that participants who completed the intervention would show improved cognitive function, which would result in improved self-management adherence followed by better glycemic control.
Outcomes:
Post-intervention scores improved in all areas; improvements were statistically significant for diet adherence (t(18) = −2.41, p <0.05), memory ability (t(18) = 5.54, p <0.01), and executive function (t(18) = 3.11, p <0.01). Fifty-eight percent of participants stated the intervention helped their diabetes self-management and 74% said they wanted to continue using cognitive strategies learned in the intervention.
Introduction
People with type 2 diabetes (T2DM) are at higher risk for developing cognitive dysfunction than the general population and about 50% of older adults with T2DM will have some form of cognitive impairment (Munshi, 2017). The underlying mechanism of cognitive changes in T2DM is not clear, but hyperglycemia and hypoglycemia, oxidative stress, and insulin resistance are all thought to contribute to detrimental changes. Additionaly, prior research has revealed that people with type 2 diabetes are not routinely told that cognitive dysfunction is considered a complication of T2DM (Authors, 2017). This is unfortunate because T2DM has a large self-management component that relies on understanding complex information about diet, exercise, medications, and glucose monitoring. If cognitive function is impaired these tasks may be more difficult and interventions to improve glucose control may be less effective. Despite this potential problem, few studies have investigated how self-management relates to cognitive function and even fewer have examined interventions designed to improve cognitive function in T2DM. Studies that have investigated these variables have been limited and included measurement of non-diabetes-specific activities of daily living and/or focused only on one aspect of T2DM self-management (e.g., diet) or one cognitive domain (e.g., memory) (Camp et al., 2015; Whitelock et al., 2015).
The primary objective of this study was to develop a cognitive function intervention and implement it in a multiethnic sample diagnosed with T2DM. The specific aims of the project were to:
Refine a current cognitive training intervention and tailor it for persons with T2DM by using the existing literature and qualitative data from participants with T2DM.
Conduct a pilot study of the adapted intervention for participants with T2DM.
Current Cognitive Training Approaches
This project built on prior work examining the relationships of perceived memory, cognitive ability, T2DM self-management, and glycemic control (Authors, 2017). The goal of this intervention was to foster self-efficacy for compensatory cognitive skills with in-person classes and individual home-based computer-assisted practice exercises related to those cognitive domains. In the case of cognitive impairment, there are few single treatments that have more than an unsustained symptomatic effect (Rebok et al., 2014). Treatments for other chronic illnesses such as cancer or cardiovascular disease have been improved though the use of combination therapies (Park, Jung, Kim, & Bae, 2017; Pressler et al., 2015; Towe, Patel, & Meade, 2017). Thus, therapies to optimize cognition in people with T2DM should address as many of the components as possible. Cognitive training uses theoretically driven strategies and skills as well as “guided practice” on various cognitive tasks (Mowszowski, Batchelor, & Naismith, 2010). It includes the teaching of techniques and strategies to augment strengths and adapt to weaknesses as well as computerized games and cognitive strategies taught in-person targeting different cognitive domains (Mowszowski et al, 2010). Using a combination of in-person class sessions focusing on lifestyle management for cognitive health and online programs for cognitive training may have a positive synergistic effect on cognitive function by optimizing diet, reducing stress, increasing physical activity, and stimulating the brain—all components of T2DM self-management and cognitive health (Sigmundsdottir, Longley, & Tate, 2016).
Providing cognitive training that incorporates a widely available and increasingly acceptable technology is an innovative way to improve T2DM self-management. Nurse-led diabetes self-management education has been shown to help patients lower A1C and increase self-efficacy, but no other studies could be identified that have used a comprehensive lifestyle-based cognitive training as an intervention in T2DM (Azami, 2018; Authors, 2016; Tshianaga et al., 2012). This study helps to fill that gap by adding the component of cognition to the study of T2DM self-management—a novel and important focus. The inclusion of the online format with traditional group training has the potential to increase engagement and encourage persons with T2DM to make crucial changes in self-management behavior.
Current standard cognitive training is delivered in person in a clinic or lab by a trained researcher or educator (Becker, Henneghan, Volker, & Mikan, 2017). The combination of group and individual online training has the advantage of enabling self-paced work with quick feedback as well as improving convenience, replicability, and accessibility. Other key benefits are: (1) consistency in intervention administration, (2) ability to generate multiple forms of testing with progressively challenging activities, (3) decreased cost of administration, and (4) increased access for a broader population, which can lead to greater diversity among participants. Online cognitive training is in the beginning stages of research, but it has been effective for improving core cognitive abilities in memory, reasoning, and executive function (Author et al., 2012; Ferguson, Ahles, & Saykin, 2007). For example, Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE), which used an earlier version of the online exercises (BrainHQ) used in this study, showed that such training increased engagement and improved long-term retention in the elderly (Rebok et al., 2014). The ACTIVE randomized clinical trial (RCT) was also one of the first trials to show significant transfer effects at 5 and 10 years post-intervention. At 10-years post-intervention participants in the intervention groups showed less decline in instrumental activities of daily living than the control group (Rebok et al., 2014).
A systematic review of seven cognitive training RCTs demonstrated that at 2 years follow-up persistent protective effects and transfer of those effects were reported (Valenzuela & Sachdev, 2009). Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT), the largest RCT to examine whether a cognitive training program, BrainHQ, can improve cognitive abilities in adults, demonstrated improvements in memory and processing speed, as well as an increase in self-reported positive changes in everyday lives (Smith, House, Yaffe, Ruff, Kennison et al., 2009). In 2013, the Iowa Healthy and Active Minds Study (IHAMS) showed that participants using the BrainHQ cognitive training program had greater improvement in cognitive capabilities than did those trained on other games (Wolinsky, VanderWeg, Howren, Jones, & Dotson, 2013). The Metabolic Enhancement for Neurogenerative Disorders (MEND) study used a comprehensive program that involved diet, exercise, and online cognitive training (Bredesen, 2014). The study found that participants with mild cognitive impairment showed the most impact and may be successful in delaying progression to dementia. Additionally, cognitive training can give individuals a greater sense of control over cognitive changes as well as have a beneficial effect on quality of life (Buckley, Saling, Frommann, Wolfsgruber, & Wagner, 2015). This research is not complete; large studies have yet to be conducted to show how improvement on neuropsychological tests due to cognitive training influences T2DM self-management.
Method
Study Design
Prior to the start of the study, approval was obtained from The University of Texas at Austin Institutional Review Board. This was a sequential exploratory mixed methods pilot study with two phases. First, the current Memory Attention and Problem Solving Skills in Persons with Multiple Sclerosis (MAPSS-MS) cognitive training intervention was modified (content and format) based on input from interviews with 10 people diagnosed with T2DM. Second, the feasibility of using this adapted intervention was determined using a one-group, pre-test/post-test design with 19 participants. The first phase took place over three months; and the second phase took six months. The convenience sample of participants for both phases was recruited by distributing flyers at a multisite endocrinology clinic located in Central Texas. The flyer listed key points about involvement in the study as well as directions on how to contact the principal investigator (PI). Trained research staff at the clinic also identified potential participants and assisted in the screening process.
Inclusion criteria required participants to be 40 to 70 years old and diagnosed with T2DM for at least 2 years. Having T2DM for a period of two years or longer provided participants with the experience of living with T2DM and self-management requirements. The age range was set at 40 to 70 years due to the higher prevalence of T2DM in middle to late middle age and the likelihood of differences in intervention preferences and responses between older and younger adults with diabetes. Furthermore, those with type 1 diabetes typically present at a younger age and may require different treatments and self-management strategies. Participants also had to have subjective concerns about their cognitive function and assessed via the Perceived Deficits Questionnaire (PDQ). Those scoring at least 10 on the PDQ were eligible to participate. Other inclusion criteria were that participants speak, read, and respond in English and have access to transportation and telephone and Internet services. Exclusion criteria were having: (1) vision that is not corrected to 20/70 (in order to work the computer screen), (2) other medical causes of dementia or disorders that may affect cognition such as depression, or (3) other limitations that precluded study activities such as stroke, a physical disability, or geographic distance from the study site.
In phase 1 of the project semi-structured interviews were conducted with 10 people with T2DM and perceived cognitive problems (Cuevas & Stuifbergen, 2017). An additional 19 participants in phase 2 of the intervention completed pre- and post-test measures of diabetes self-management adherence, and perceived cognitive function. At post-test, an additional survey about intervention logistics, satisfaction/usefulness of the intervention, and perceived changes in diabetes self-management was administered.
The MAPSS-DM Intervention Development
The adaptation of the MAPSS-DM intervention was based on the MAPSS-MS intervention developed by the co-PI of the project (Author et al., 2012). It is based on Bandura’s (2001) theory of self-efficacy with the goal to build self-efficacy for completing cognitive strategies in order to improve day-to-day function. The MAPSS-DM altered the goal slightly to include aspects of T2DM self-management as a background for which to practice cognitive strategies. The approach of this intervention included content presentation in a variety of methods (online and in-person) as well as goal-setting and interactive approaches to engage participants. Additionally, semi-structured interviews with 10 adults with T2DM were conducted to obtain their opinion on the proposed intervention content, class logistics, and experiences with cognitive problems. The interview schedule contained questions such as, “What, if any, problems have you noticed with your cognitive function since your diagnosis with diabetes?” and “How do these class topics relate to your life?” The full results of these interviews are published elsewhere (Cuevas, Stufbergen, Brown, & Rock 2017).
All content was developed at an 8th grade reading level. Principles of adult learning were used along with weekly goal-setting by participants. Table 1 describes the content of each session. Phase 2, the final intervention, consisted of four 2-hour classes that met every other week and were led by a registered nurse. Participants were also asked to complete three 45-minute sessions of the online computer training each week. Each educational session built on the didactic information and had activities to improve cognitive health by practicing strategies in class and on the computer. The computer-training program used, BrainHQ, was developed by the Posit Science and was the same computer program used in the original MAPSS-MS intervention (Posit Science, 2016). In prior work, using the MAPSS-MS intervention, participants tested three different online training programs. Participants had specific complaints about two of the online program –the programs were “frustrating,” they disliked the use of lound noises, and opportunities for feedback were limited. The Posit Science program was rated the highest.
Table 1.
Class Content
| Class Content: Cognitive function in the context of type 2 diabetes | |
|---|---|
| Week 1 | • Understanding T2DM, symptoms, complications, and medications • Understanding how cognitive function is related to T2DM • Orientation to computer training • Discussing effective strategies to facilitate better communication with health care providers e.g. understanding instructions or recommendations from health care providers • Strategies to enhance attention and problem solving |
| Week 3 | • Strategies to enhance memory • Addressing resources and barriers to self-management (e.g. planning ahead for meals and organizing medications) that take into account elements of executive functioning • Visuospatial skills required for blood glucose self-monitoring |
| Week 5 | • Addressing ADA dietary recommendations and how they can benefit cognitive health • Discussion of favorite recipes, more healthy food preparation, eating out and emphasis on portion control • Acknowledging and appreciating stress associated with diabetes and cognitive issues. • Providing resources for mental health care services • Strategies to manage stress |
| Week 8 | • Addressing ADA activity recommendations and benefits of following the guidelines on cognitive function • Discussion of practical ways to increase activity • Review of cognitive skills/training and the potential impact on self-management skills including blood glucose monitoring, medication adherence, diet, and exercise. • Addressing resources and barriers to maintaining cognitive function. |
Tracks in the computer component of the intervention include attention, memory, executive function, and processing speed. These are organized so that the most basic skills are addressed first (attention). As the participant moves through the activities, tasks become more difficult and challenging. BrainHQ meets the Institute of Medicine’s five requirements for a brain-training program: (1) transfer of training to other lab tasks that measure the same cognitive construct as does the training task; (2) transfer of training relevant to real-world tasks; (3) evaluation using an active control group whose members have the same expectations of cognitive benefits as do members of the experimental group; (4) retention of trained skills; and (5) benefits of the training product replicated by research groups other than those selling the product. The version used for this project was subscription-based. The PI subscribed to the website service for a fee for each participant ($70) so they had unlimited access that allowed them to log in and complete the exercises from any computer with Internet access. The fee also allowed the research team to monitor and analyze engagement and progress with the games. At the end of the project the participants were made aware that there was a free version, without PI oversight, of the program if they chose to continue to practice.
Data Collection
All study variables and measures are shown in Table 2. Members of the research team had used these measures effectively in past studies. Data were gathered pre- and post-intervention by the research assistant at data collection visits scheduled for each participant. Demographic information, glycosylated hemoglobin A1C (A1C), and body mass index (BMI) were collected and used to describe the sample.
Table 2.
Study Measures
| Measure | Description of Measure | |
|---|---|---|
| Physiologic Measures (pre-intervention only) | ||
| A1C | Laboratory: blood samples | The national standard measure of glycemic control over a 3 month period |
| BMI | Weight, height | |
| Self-Report Measures (pre- and post- intervention) | ||
| Demographics | Background information | Age, gender, years with diabetes, ethnicity/race |
| Diabetes Self-Care | Summary of Diabetes Self-Care Activities | 18 items; Participants answer questions regarding how many days in the last week have they performed a certain aspect of diabetes self-management behaviors such as diet, smoking, and physical activity. Inter-item correlations range from r = 0.20 to r = 0.76 for four SDCA subscales; 4-month test-retest reliability ranged from r = −0.05 to 0.78. |
| Self-efficacy | General Self-Efficacy Scale | 10 items; confidence in ability to influence outcomes. Responses are made on a 4-point scale (1 = not true at all, 2= hardly true, 3 = moderately true, 4= exactly true) to items such as “I can always manage to solve difficult problems If I try hard enough.” Cronbach’s alphas range from 0.76 to 0.90. |
| Memory | Multifactorial Memory Questionnaire | 57 items; assesses contentment with one’s memory, subjective memory capability, and use of memory aids. Participants rate their level of agreement with each item on a 5-point scale (strongly agree = 1, agree = 2, undecided = 3, disagree = 4, strongly disagree = 5) for the Contentment subscale; The Ability subscale asks participants to indicate the frequency with which each memory failure has occurred in the past 2 weeks on a 5-point scale (all the time = 1, often =2, sometimes = 3, rarely =4, never =5); The third subscale, Strategy, asks participants to rate the frequency of use of certain memory strategies on a 5-point scale (never =1, rarely =2, sometimes =3, often =4, all the time =5). Cronbach’s alphas for each subscale are 0.95 for Contentment; 0.93 for Ability; 0.83 for Strategy |
| Executive Function | Barkley Deficits in Executive Functioning Scale – Short Form | 20 items assessing the frequency at which participants have exhibited certain behaviors in specific executive functioning areas over the past 6 months. The areas include: self-management to time, self-organization/problem-solving, self-restraint, self-motivation, and self-regulation of emotion. Items are measured using a 4-point Likert scale, ranging from never or rarely (1) to very often (4). Internal consistency for the short-form scale: Cronbach’s alpha of 0.92 |
| Feasibility Measures | ||
| Accessibility of intervention | Recruitment and retention | Logs of recruitment activities |
| Practice of skills | Homework logs | Measures time spent practicing cognitive training skills |
| Satisfaction with intervention | Project team developed | a. Six-item rating scale with responses ranging from 0 strongly disagree to 3 strongly agree on items such as “The activities kept my interest,” and “The computer activities helped improve my cognitive skills.” To be assessed at the end of the intervention. b. Twelve open-ended questions asked in focus groups regarding cognitive strategies tried as well as feedback on class sessions and online games. For example, “What cognitive strategies did you try during the 8 weeks?” and “What could be changed about the classes to make them better?” |
Participants completed the Summary of Diabetes Self-Care Activities (SDSCA) questionnaire as a measure of adherence to T2DM self-management activities in order to examine the potential effect of the intervention on those activities (Toobert, Hampson, & Glasgow, 2000). The General Self-Efficacy Scale (GSES) was used as a measure of confidence in the ability to change outcomes in a variety of situations (Schwarzer & Jerusalem, 1995). The Multifactorial Memory Questionnaire (MMQ) and the Barkley Deficits in Executive Function Scale–Short Form (BDEFS-SF) were used to describe two types of perceived cognitive function most affected in diabetes (Barkley, 2014). Note that higher scores on the BDEFS-SF indicated lower levels of perceived executive function. Since depression and symptoms related to depression are associated with diabetes as well as with working memory, the 10-item Center for Epidemiological Studies Depression Scale (CES-D) was used as a measure of depressive symptoms (Stahl, Lum, Liow, Chan, & Verma, 2003). Additional data were collected at the end of the study using a six-item rating scale with responses ranging from 0 (strongly disagree) to 3 (strongly agree) on items such as “The activities kept my interest” and “The computer activities helped improve my cognitive skills.”
Data Analysis
Results from the measures described above were entered into a computer database for analysis using IBM SPSS Statistics version 23 (IBM Inc., Armonk, NY, USA, 2013). Data were checked for accuracy and evaluated for violations of statistical tests. Reliability estimates (internal consistency) were determined for each instrument, and alphas of more than 0.70 were considered acceptable. Descriptive analyses were performed to obtain a description of the sample on demographic and illness-related variables. Paired t-tests were used to examine pre-test and post-test differences. The significance level was set at 0.05. Correlations were also run to examine the relationships between the variables. For example, the associations between A1C and memory, A1C and executive function, and T2DM self-care and memory were analyzed.
Results
Sample Description
Ninety-three patients with cognitive complaints were recruited during the screening period (Figure 1). Of these, 32 did not meet the PDQ criteria and 19 had a history of dementia. Forty-two others declined after initial screening. Of the remaining patients, 19 consented to participate.
Figure 1.
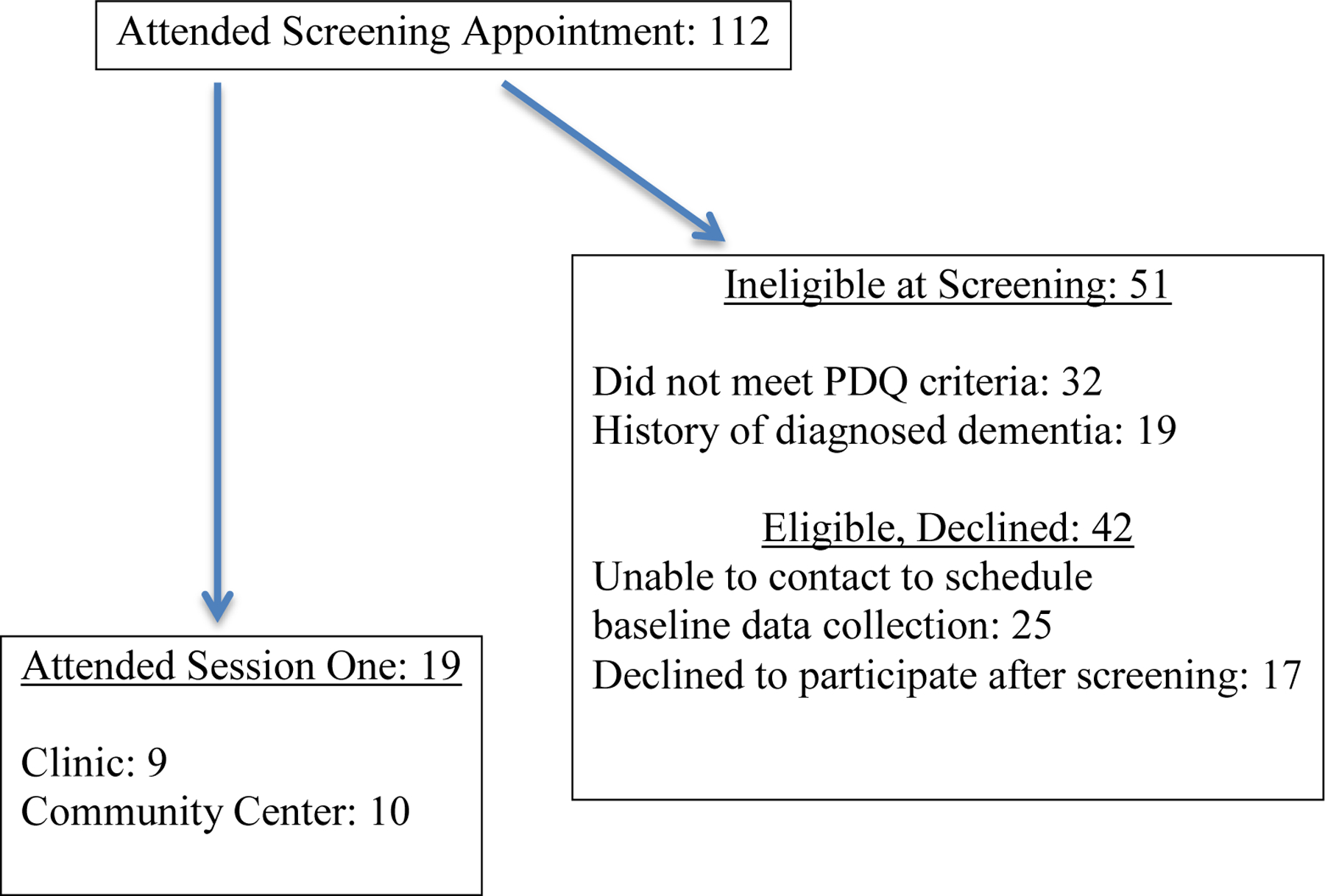
Recruitment Flow Chart
The mean age was 55 years (SD = 10.9), mean time with T2DM was 7 years (SD = 4.8), and mean A1C was 8.3% (SD = 1.8). The sample was diverse; 32% non-Hispanic white, 53% Hispanic, and 15% African American (Table 3).
Table 3.
Participant Characteristics
| Characteristic | n | Range | % | M (SD) | M (SD) |
|---|---|---|---|---|---|
| Age in years | 19 | 40–70 | 55.1 (10.9) | ||
| A1C | 19 | 5.4–12 | 8.3 (1.8) | ||
| Length of time with DM in years | 19 | 2 – 21 | 7.1 (4.8) | ||
| Hispanic | 10 | 52.6 | |||
| Non-Hispanic White | 6 | 31.6 | |||
| African American | 3 | 15.8 | |||
| Female | 11 | 57.9 | |||
| SDSCA Variables (n=19) | Pre-test | Post-test | |||
| General diet | 0–7 | 2.9 (1.9) | 4.4 (2.1) | ||
| Specific diet | 0–6 | 3.8 (1.6) | 4.2 (1.5) | ||
| General exercise | 0–6 | 1.8 (1.4) | 2.9 (1.5) | ||
| General glucose testing | 0–7 | 2.3 (1.2) | 3.4 (2.5) | ||
| General foot care | 0–7 | 2.6 (2.5) | 3.6 (2.8) | ||
| Days of smoking in the past week | 0–3 | 0.3 (.8) | 0.2 (0.7) | ||
| Cognitive Variables (n=19) | |||||
| Memory-Contentment | 28–54 | 40.0 (5.8) | 23.5 (6.3) | ||
| Memory-Ability | 31–86 | 45.5 (11.4) | 24.8 (7.3) | ||
| Memory-Strategies | 12–101 | 32.8 (19.6) | 40.0 (3.5) | ||
| Executive Function-Time | 4–14 | 10.0 (2.6) | 9.0 (2.7) | ||
| Executive Function-Self-Organization | 4–12 | 8.5 (2.4) | 6.9 (1.4) | ||
| Executive Function-Restraint | 4–14 | 7.9 (2.9) | 5.6 (1.7) | ||
| Executive Function-Motivation | 4–11 | 6.9 (2.8) | 5.8 (1.7) | ||
| Executive Function-Self-Regulation | 4–14 | 8.2 (2.5) | 5.3 (1.4) | ||
| Executive Function-Total | 24–57 | 41.6 (9.9) | 32.8 (5.1) | ||
| CESD-total | 10–18 | 14.6 (2.1) | 12.3 (1.1) | ||
| GSE-total | 20–40 | 28.2 (3.2) | 32.2 (4.8) |
Seventy percent of the participants attended at least 3 of the 4 sessions. Average time spent per week on the program was 106 minutes (range = 9–120 minutes; SD = 35.4 minutes), which was less than the requested time of 45 minutes, 3 days per week or 135 minutes total.
Baseline adherence to T2DM self-management activities was low (Table 3). Participants completed an average of 2.1 days/week of physical activity and only 1.5 days of specific exercise. Rates of “healthful eating” averaged 2.7 days, and consumption of high-fat foods averaged 4.6 days. Length of time with T2DM was significantly correlated positively with some of the self-management activities: healthful eating in the past month (r = .586, p < .01), healthful eating in the last week (r = .605, p < .01), eating 5 or more servings of fruits and vegetables per day (r = .547, p < .5), participating in 30 minutes of physical activity in the last week (r = .701, p < .01), and participating in a specific exercise in the past week (r = .630, p < .01). General self-efficacy was significantly and positively correlated with diet (r = .50, p < .05), exercise (r = .61, p < .01), and foot care (r = .51, p < .5), and negatively with perceived problems with executive function (r = −.67, p < .1).
Baseline scores on the BDEFS-SF, but not the MMQ, were significantly correlated with self-management activities (general diet r = −.665, p < .01; exercise r = −.725, p < .01; foot care r = −.516, p < .05) and positively correlated with eating high fat foods in the past week (r = .692, p < .01). Rates of self-management adherence with lower perceived executive function were lower for exercise only. A1C was not significantly correlated with the cognitive variables.
Post-intervention scores in all areas improved, but statistically significant improvements were only seen for diet (diet t(18) = −2.41, p <0.05), perceived memory ability (t(18) = 5.54, p <0.01), and perceived executive function (t(18) = 3.11, p <0.01).
Fifty-eight percent of participants felt the intervention helped their T2DM self-management and 74% said they wanted to continue using the cognitive strategies learned in the intervention. However, 32% agreed strongly with the statement, “The amount of time burden for the intervention was high.” Additionally, the cognitive strategies were rated as more helpful than the computer training (53% vs. 47%).
Discussion
Despite the strong association between T2DM and cognitive dysfunction, little has been done to treat both conditions concurrently. One potential solution to this problem is to merge and adapt established, tested interventions to meet the needs of both conditions. Despite a small sample size, the results of this study suggest that a comprehensive cognitive training intervention utilizing both online and in-person training can be successfully adapted for adults with T2DM and helpful in improving facets of self-management. Strengths of this project were the focus on increasing self-efficacy for using compensatory strategies and lifestyle changes that benefitted both T2DM and perceived cognitive dysfunction as well as a focus on self-management. Although only perceived memory ability and executive function improved after the intervention, positive changes were seen in the participants’ reports of cognitive problems and self-management. They reported using more strategies, which may be important in showing what can be done in day-to-day life to improve cognitive abilities. The correlational relationships need to be interpreted with caution due to the small sample size, but the results indicated that these relationships merit further investigation.
Another strength was use of a nurse diabetes educator. Nurses who help manage chronic conditions such as diabtes are ideally positioned to provide expertise, monitoring, and feedback to help provide individualized care (Burke, Sherr, & Lipman, 2014). Nurses who are diabetes educators use theory to address the complexities of behavior change and have been successful in promoting behavior change, decrease diabetes related stress, and increase empowerment (Siminerio, Ruppert, & Gabbay, 2013; Duncan et al., 2011). Although this study was focused on increasing self-efficacy for use of cognitive strategies, it was centered in aspects of diabetes self-management and the RN educator was skilled in helping participants become effective self-managers.
The closed ended intervention satisfaction questions also lend support for the potential efficacy of the intervention. Most participants felt the time burden was present, but manageable, and all reported an increase in knowledge at the end of the intervention and had new ideas for self-management skills to maintain glycemic control and cognitive health. The comments also demonstrated that the overall design and format of the intervention was helpful in reinforcing the content. However, while attendance at the class sessions was better than participation in the online games, most participants recommended moving it to a completely online format (webinar instead of in-person classes) to reduce the time and travel burden. Ninety percent thought the session length was appropriate and that 4 sessions was the correct number of sessions. However, we could improve recruitment and retention if we could address the time burden associated with use of the online program by adjusting the recommended time to 20 minutes, 7 days a week (total 135 minutes) per participant recommendations.
Other comments from participants offered insights as to why they felt the intervention was beneficial. Relating the cognitive strategies to diabetes self-management was thought to be helpful in applying the strategies to everyday life. Having portions of the training at home and online was perceived as convenient and eased completion of many of the assignments. Participants were almost unanimous in saying they would refer others to this type of training because it focused on both T2DM and cognitive function–and cognitive function was an area they were concerned about but had little information on the underlying pathophysiology or how to prevent or treat it. The most frequent negative comment (and reason for missing a class session) was related to the travel time needed to attend the in-person meetings.
Limitations
Interpretation of the findings from this project is limited by the small sample size and lack of a comparison group. The inclusion of a sample with over 50% from underrepresented minority populations is a strength. Additionally, the participants may have been more motivated for behavioral change as they volunteered for a time-consuming intervention and expressed concern with cognitive problems during the screening process of the study. This group also had good access to health care and the Internet and also had relatively well controlled glucose levels. Therefore this sample was somewhat biased towards a higher educated, better-controlled group of persons with T2DM. Conversely, this could be considered a strength as these individuals could be considered “experts” in T2DM self-management and represent some of the best sources of information to inform the design of future interventions. Also, the study relied on self-reported data, which might include answers influenced by social desirability. In future studies, researchers might want to add neuropsychological tests in which participants are observed as they perform activities for comparison.
Expectations of change in cognitive function may have also influenced results. In other words, those who participate in cognitive training believe it will lead to improvement and lead towards more effort in post-tests. Boot et al. (2013) controlled for these expectations in two survey studies with 400 healthy adults and found that those effects could be explained apart from the true treatment effects. The results of this type of study emphasize the need for future projects to test for those differential expectations.
Another limitation is the multi-compnent nature of the intervention design. Due to the small sample size the ability to determine if one or both of the components is responsible for the outcomes and the power to find statistically significant effects is limited. The research team is currently proposing a larger RCT to address these issues.
Conclusion
The MAPSS-DM is a promising intervention. In a time when rates of co-morbid T2DM and cognitive dysfunction are rising, it is important to explore how interventions can become more sensitive to both conditions. The MAPSS-DM provides the first evaluation of a comprehensive cognitive training intervention for people with T2DM. Thus, despite difficulties with recruitment, further testing of comprehensive cognitive training interventions for people with T2DM is recommended. A collaborative, multidisciplinary intervention can help provide a guide for feasible studies that contribute to more tailored approaches to management of T2DM and cognitive problems. Additionally, documentation of study processes will allow others to replicate the study and understand the contextual differences important to future dissemination. In the future, efficacy testing will build knowledge specific to promoting the cognitive health of people with T2DM.
Contributor Information
Heather E. Cuevas, The University of Texas at Austin, School of Nursing, 1710 Red River, Austin, Texas 78701.
Alexa K. Stuifbergen, James R. Dougherty, Jr. Centennial Professor in Nursing, The University of Texas at Austin, School of Nursing, 1710 Red River, Austin, Texas 78701.
Sharon A. Brown, Joseph H. Blades Centennial Memorial Professorship in Nursing, The University of Texas at Austin, School of Nursing, 1710 Red River, Austin, Texas 78701.
Catherine Ward, HCA Physicians Services Group, Austin, TX.
References
- Bandura A (2001). Social Cognitive Theory: An agentic perspective. Annual Review of Psychology, 52, 1–26. [DOI] [PubMed] [Google Scholar]
- Barkley RA (2014). The assessment of executive functioning using the Barkley Deficits in Executive Functioning Scale. In Goldstein S, Naglieri JA (Eds). Handbook of executive functioning, 245–263. New York: Springer Science. [Google Scholar]
- Becker H, Henneghan AM, Volker DL, & Mikan SQ (2017). A pilot study of a cognitive behavioral intervention for breast cancer survivors. Oncology Nursing Forum, 44, 255–264. [DOI] [PubMed] [Google Scholar]
- Bredesen DE (2014). Reversal of cognitive decline: A novel therapeutic approach. AGING, 6, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckely RF, Saling MM, Frommann I, Wolfsgruber S, & Wagner M(2015). Subjective cognitive decline from a phenomenological perspective: A review of the qualitative literature. Journal of Alzheimer’s Disease, 48(Suppl 1), S125. [DOI] [PubMed] [Google Scholar]
- Camp CJ Fox K, Skrajner MJ, Antenucci V, & Haberman J (2015). Creating effective self-management for older adults with type 2 diabetes and cognitive impairment. Advances in Aging Research, 4, 33–41. [Google Scholar]
- Cuevas H & Stuifbergen A (2017). Perceived cognitive deficits are associated with diabetes self-management in a multi-ethnic sample. Journal of Diabetes and Metabolic Disorders 16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas H, Stuifbergen A, Brown SA, & Rock J (2017). Thinking about cognitive function: Perceptions of cognitive changes in people with type 2 diabetes. The Diabetes Educator 43, 486–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenbergy CT, Cole BF, & Mott LA (2007). Cognitive behavioral management of chemotherapy related cognitive change. Psychooncology,16, 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2013). IBM SPSS Statistics for Windows, Version 22.0 Armonk, NY: IBM Corp. [Google Scholar]
- Munshi N (2017). Cognitive dysfunction in older adults with diabetes: What a clinician needs to know. Diabetes Care, 40, 461–467. [DOI] [PubMed] [Google Scholar]
- Park JH, Jung YS, Kim KS, & Bae SH (2017). Effects of compensatory training intervention for breast cancer patients undergoing chemotherapy: a pilot study. Supportive Care Cancer, 25, 1887–1896. [DOI] [PubMed] [Google Scholar]
- Posit Science (2017). Brain HQ https://www.brainhq.com
- Pressler SJ, Titler M, Koelling T,M, Riley PL, Jung M, Hoyland Domenico, L., …Giordani B (2015). Nurse-enhanced computerized cognitive training increases serum brain-derived neurotropic factor levels and improves working memory in heart failure. Journal of Cardiac Failure, 21, 630–641. [DOI] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim K, King JW…Willis SL (2014). Ten-year effects of the Advanced Cognitive Training for Independent andVital Elderly (ACTIVE) cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer R & Jerusalem M (1995). Generalized self-efficacy scale. In Weinman J, S., Wright S, & Johnston M Measures in health psychology: A user’s portfolio. Causal and control beliefs (pp. 35–37). Windsor, UK:NFER-NELSON. [Google Scholar]
- Sigmundsdottir L, Longley WA, & Tate RL (2016). Computerised cognitive training in acquired brain injury: A systematic review of outcomes using theInternational Classification of Functioning (ICF). Neuropsychological Rehabilitation, 26, 673–741. [DOI] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, & Zelinski EM (2009). A cognitive training program based on principles of brain plasticity: Results from the Improvement in Memory with Plasticity based Adaptive Cognitive Training (IMPACT) study. Journal of the American Geriatrics Society, 57, 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D, Sum CF, Lum SS, Liow PH, Chan YH, Verma S (2003). Screening for depressive symptoms: Validation of the Center for Epidemiologic Studies Depression scale (CES-D) in a multiethnic group of patients with diabetes. Diabetes Care, 31, 118–1119. [DOI] [PubMed] [Google Scholar]
- Author et al. (2012). A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clinical Rehabilitation, 26, 882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toobert DJ, Hampson SE, & Glasgow RE (2000). The summary of diabetes selfcare activities measure: Results from 7 studies and a revised scale. Diabetes Care, 23, 943–950. [DOI] [PubMed] [Google Scholar]
- Towe SL, Patel P & Meade CS (2017). The acceptability and potential utility of cognitive training to improve working memory in persons living with HIV: Apreliminary randomized trial. Journal of the Association of Nurses in AIDS Care, 28, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ & Sachdev P (2009). Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow up. American Journal of Geriatric Psychiatry, 17, 179–187. [DOI] [PubMed] [Google Scholar]
- Whitelock V, Nouwen A, Houben K, van Akker O, Miller IN, Narendan P, Rosenthal M, & Higgs S (2017). Does neurocognitive training have the potential to improve dietary self-care in type 2 diabetes? Study protocol of a double-blind randomized controlled trial. BMC Nutrition, 1,11 [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, & Dotson MM (2013). A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLOSone, 8:e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]


