Abstract
Autophagy is a strictly regulated process that degrades and recycles long-lived or misfolded proteins and damaged organelles for the maintenance of energy and function homeostasis of cells. Insufficient oxygen and glucose supply caused by cerebral ischemia lead to a higher ratio of AMP/ATP, which will activate the AMPK pathway to initiate the process of autophagy. Accumulating evidence shows that autophagy participates in the pathogenesis of ischemic stroke as a double-edged sword. However, the exact role of autophagy in the pathogenesis of ischemic stroke is controversial and yet to be elucidated. In this review, the autophagy pathway, both in physiological conditions and in ischemic stroke, is expounded. The focus was also on discussing the double-edged sword effect of autophagy in brain ischemia and its underlying mechanisms. In addition, potential therapeutic strategies for ischemic stroke targeting autophagy pathway were also reviewed.
Keywords: Autolysosome, autophagy, double-edge sword, ischemic stroke, pathogenesis, therapeutic strategies
1. INTRODUCTION
Stroke is assumed to be the leading cause of death and disability globally [1-3]. It hits 15 million people globally annually, with 5 million deaths and another 5 million left permanently disabled, causing a heavy economic burden on family and society [4]. Stroke is a neurological disease caused by insufficient cerebral circulation because of infarction or hemorrhage of cerebral vessels. According to the pathological changes of cerebral vessels, a stroke could be an ischemic stroke (IS) or a hemorrhagic stroke (intracerebral hemorrhage and subarachnoid hemorrhage) [3].
IS is initiated by the infarction of cerebral vessels because of thromboembolism and thrombus formation. Cerebral vessel occlusion leads to insufficient oxygen and glucose supply for brain parenchyma, causing cascade responses, including blood-brain barrier dysfunction [5], neuroinflammation [6], oxidative stress [7], etc. Such responses are biological defense mechanisms under IS, yet can be deleterious to brain parenchyma when activated intensively and continuously.
Autophagy is a natural, regulated process which helps cells to eliminate unnecessary or dysfunctional components, including long-lived proteins, insoluble proteins, and even whole organelles [8, 9]. Autophagy is composed of microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA) according to the way of cargo delivery to the lysosomes. Microautophagy is a non-selective lysosomal degradative process that engulfs cytoplasmic constituents
via the invagination of the lysosomal membrane [10, 11]. Through engulfment of long-lived and membrane proteins, microautophagy exerts the functions of maintaining organellar size, membrane homeostasis, and cell survival under nitrogen restriction [11]. Macroautophagy is the most prevalent form of autophagy that forms a double-membrane compartment known as phagophore enfolding the cargo, which then matures into an autophagosome [12, 13]. The combination of autophagosome and lysosome generates autolysosome, in which the cargo is degraded and the degradation products are released into the cytoplasm for reuse [12, 14, 15]. CMA is the only selective lysosomal degradative process, which targets the substrates by heat shock cognate 70 (HSC70) selective binding [13, 16]. Proteins bound by chaperone then are delivered to the lysosomes, and the transportation into lysosomes is mediated by the specific recognition and combination of the lysosome-associated membrane protein type 2a (LAMP2a) receptor and the chaperone-bound proteins [13, 17]. Through the three mechanisms mentioned above, autophagy is the main regulatory catabolic process to degrade long-lived proteins and damaged organelles in eukaryotic cells [18].
Autophagy also exerts a neuroprotective effect in IS by controlling neural survival and death through variable mechanisms [19]. Many efforts have been made to understand the pathogenesis of IS since the first identification hemorrhagic and IS [20]. However, it remains obscure and unthorough, and thrombolytic therapy is the only drug treatment recommended by the American Heart Association/American Stroke Association, which includes only two drugs (alteplase and tenecteplase) with a limitation of short therapeutic time window [21, 22]. Accumulating evidence has supported that autophagy plays a crucial role in IS. Therefore, understanding the role of autophagy in the pathogenesis of IS can provide a new perspective in the research and contribute to the development of new treatment in the clinic. Multiple researches have demonstrated that drugs aimed at autophagy have therapeutic potential in IS. For example, a mitochondrial fusion protein, mitofusin 2, which is capable of enhancing the formation of autophagosomes and facilitate the fusion of autophagosomes and lysosomes, is suggested to alleviate ischemia-reperfusion induced damage [23]. Hamartin, a product of the tuberous sclerosis complex 1 gene, is demonstrated to confer neuroprotection against IS via induction of autophagy [24]. Melatonin, an endogenous hormone, is suggested to be neuroprotective against IS by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signaling pathways [25]. In this study, a comprehensive overview of the role of autophagy in the pathogenesis of IS is provided, and an attempt is made to shed new light on the design of new strategies against IS.
2. AUTOPHAGY PATHWAY UNDER A NORMAL PHYSIOLOGICAL SITUATION
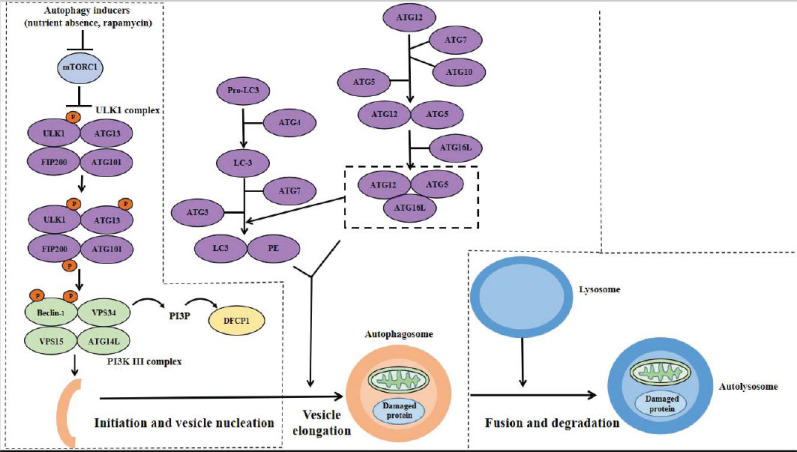
The autophagy pathway is strictly controlled by variable regulatory constituents. Currently, the autophagy pathway is divided into four steps, including initiation, vesicle nucleation, vesicle elongation, fusion and degradation (Fig. 1) [26]. Through these processes, autophagy accomplishes the metabolic and renewal needs of cells, degrading and recycling the long-lived or misfolded proteins and damaged organelles.
Fig. (1).
Schematic illustration of the autophagy pathway. Autophagy is strictly controlled through multiple pathways. Autophagy inducer, such as nutrient absence or rapamycin, activates the ULK1 complex through phosphorylating ULK1, which leads to the activation of PI3K III complex. Activated PI3K III complex produces phosphatidylinositol 3-phosphate by VPS34. Phosphatidylinositol 3-phosphate then recruits DFCP1 to accelerate the process of vesicle nucleation. ATG12 is conjugated to ATG7, ATG10, and ATG5 in order, forming the ATG12-ATG5 conjugate with the help of ATG7 and ATG10. Combined with ATG16L, the ATG12-ATG5-ATG16L conjugate is formed. Mediated by ATG4, Pro-LC3 is converted to LC3, which is conjugated to PE by ATG3, ATG7, and ATG12-ATG5-ATG16L conjugate, forming LC3-PE conjugate. Both ATG12-ATG5-ATG16L conjugate and LC3-PE conjugate participate in the elongation of autophagosomes. After elongation, autophagosomes are fused with lysosomes forming autolysosomes, which is mediated by SNARE syntaxin 17, PLEKHM1, and Atg14. Cargos in autolysosomes are digested by lysosomal enzymes. Nutrients generated by digestion act as activators and inhibitors for mTOR and AMP-activated protein kinase (AMPK), respectively, which lead to the inactivation of ULK1 complex and termination of autophagy. Abbreviation: mTOR, mammalian target of rapamycin; ULK1, Unc-51-like kinase 1; ATG, autophagy related proteins; FIP, focal adhesion kinase family interacting protein; VPS, vacuolar protein sorting; PI3P, phosphatidylinositol-3-phosphate. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Initiation of Autophagy
Autophagy is initiated by autophagy inducers (nutrients absence, rapamycin, etc.), which dephosphorylate the mammalian target of rapamycin (mTOR) mTORC1 and cause the disassociation of mTORC1 and the serine–threonine protein kinase, Unc-51-like kinase 1 (ULK1) [18]. The ULK1 complex comprises of ULK1, the autophagy-related proteins 13 (ATG13), ATG101, and focal adhesion kinase family interacting protein (FIP200) [19, 27-29]. Following the separation from mTOR, ULK1 then undergoes autophosphorylation and activates ATG13 and FIP200 through phosphorylation, thus initiating autophagy [18].
2.2. Vesicle Nucleation
The class III phosphatidylinositol 3-kinase (PI3K III) complex, including Beclin-1, vacuolar protein sorting 34 (VPS34), VPS15, and ATG14L, is responsible for vesicle nucleation [19, 30]. After the initiation of autophagy, the PI3K III complex is recruited to the location of autophagosome formation by the activated ULK1 complex [31]. The ULK1 kinase targets and activates the PI3K III complex by phosphorylating Beclin-1 [32], which facilitates VPS34 to produce phosphatidylinositol 3-phophate [33-37]. Phosphatidylinositol 3-phosphate then recruits the DFCP1 to accelerate the process of vesicle nucleation [37].
2.3. Vesicle Elongation
The vesicle elongation is mediated by two ubiquitin-like conjugates, ATG12-ATG5-ATG16L and ATG8 (LC3)-phosphatidylethanolamine (PE) [38]. ATG12 is conjugated to ATG7, ATG10, and ATG5 in order, forming the ATG12-ATG5 conjugate with the help of ATG7 and ATG10 [39, 40]. Integrated with ATG16-like (ATG16L), the ATG12-ATG5 conjugate becomes the ATG12-ATG5-ATG16L conjugate, which promotes cargo recruitment and vesicle elongation [41]. The conversion of Pro-LC3 to LC3 is mediated by ATG4. LC3 then conjugates with PE with the help of ATG3, ATG7, and ATG12-ATG5-ATG16L conjugate, forming LC3-PE conjugate that is responsible for the elongation of autophagosomes [42, 43].
2.4. Fusion and Degradation
Autophagy receptor or adaptor proteins, including SNARE syntaxin 17 [44], PLEKHM1 [45], and Atg14 [46], mediate the fusion of autophagosomes and lysosomes for the formation of autolysosomes where target proteins or organelles are digested by lysosomal enzymes. Through such a process, substances like amino acids, lipids, and nucleosides, are produced for reuse by the cells [22]. Recently, it was discovered that some proteins (e.g. autophagy related proteins 14) are required not only for the initiation of autophagy but also for the fusion of autophagosomes with lysosomes [46].
2.5. Termination of Autophagy
Nutrients generated by autolysosomes act as activators for mTOR [38, 47, 48], leading to the inactivation of ULK1 complex and the termination of autophagy. Moreover, reactivated mTOR leads to the regeneration of lysosomes by generating proto-lysosomal tubules and vesicles, which are extruded from autolysosomes [38, 47, 48].
Except for mTOR reactivation, autophagy can achieve self-inhibition regardless of stress conditions. Such a mechanism is mediated by CULLINs, the E3 ubiquitin ligases, which participate in the degradation of autophagy proteins [48]. Through the ubiquitin-proteasome pathway, CULLINs mediate the degradation of members of ULK and VSP34 complex and lead to autophagy termination eventually [48].
3. AUTOPHAGY PATHWAY IN ISCHEMIC STROKE
Occlusion of cerebral arterial circulation leads to diminished oxygen and glucose supply to the brain, causing various subsequent metabolic changes. Following infarction, cells in the brain are exposed to nutrient-depleted conditions, manifesting higher ratio of AMP/ATP, which is a major stimulus for AMPK activation [49]. Activated AMPK initiates the autophagy pathway by activating the ULK1 kinase complex through direct and indirect pathways (Fig. 2) [50].
Fig. (2).
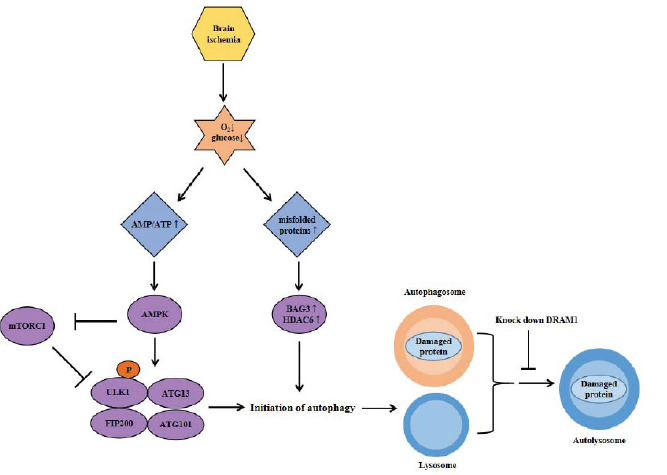
Regulation of autophagy pathway in ischemic stroke. Brain ischemia leads to diminished oxygen and glucose supply to the brain, causing multiple metabolic changes. Exposed in nutrient-depleted condition, the ratio of AMP/ATP in brain cells becomes higher, which is a major stimulus for AMPK activation. Activated AMPK initiates autophagy through a direct and indirect pathway. In a direct pathway, AMPK activates the ULK1 complex by phosphorylating ULK1 at several serine residues, while in indirect pathway, AMPK activates it by inhibiting mTORC1. In IS, accumulated misfolded proteins disrupt the balance between the ubiquitin-proteasome and autophagy-lysosome pathway. Increased BAG3 and HDAC6 initiate the conversion of ubiquitin-proteasome pathway to autophagy-lysosome pathway. A study shows that knockdown DRAM1 inactivates the autophagy pathway through blocking the fusion of autophagosome and lysosome, indicating the role of DRAM1 in the autophagy pathway. Abbreviation: mTOR, mammalian target of rapamycin; AMPK, 5’-Adenosine monophosphate-activated protein kinase; ULK1 Unc-51-like kinase 1; ATG, autophagy related proteins; FIP, focal adhesion kinase family interacting protein; DRAM1, DNA damage-regulated autophagy modulator protein 1. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In a direct pathway, AMPK activates the ULK1 kinase complex by phosphorylating ULK1 at several serine residues, while in an indirect pathway, AMPK activates it by inhibiting mTORC1 [50]. Several studies suggest that the activation of AMPK mediates the autophagy-related neuroprotection [51-53]. In addition, the number of misfolded proteins surges in IS, which overloads the ubiquitin-proteasome pathway and disrupts the balance between the ubiquitin-proteasome and autophagy pathway [9]. Increased BAG3 and HDAC6 initiate the conversion from the ubiquitin-proteasome pathway to the autophagy pathway [54], which leads to the initiation of the autophagy pathway in IS (Fig. 2). There is a study demonstrating that the autophagy pathway is activated by the expression of DNA damage-regulated autophagy modulator protein 1 (DRAM1), which is expressed in IS [55]. And knockdown of DRAM1 inactivates the autophagy pathway through blocking the fusion of autophagosome and lysosome and intensifies the cell death in IS [55]. In conclusion, autophagy inducers (such as nutrient deprivation and misfolded proteins accumulation) initiate autophagy through various mechanisms.
4. ROLES OF AUTOPHAGY IN THE PATHOGENESIS OF ISCHEMIC STROKE
Accumulating data have indicated that autophagy is involved in the pathogenesis of IS, and regulating the activity of autophagy can change the outcomes of IS. However, whether autophagy is beneficial or detrimental for the survival of neuronal cells in IS is controversial, and currently, it is thought of as a double-edge sword [56, 57]. After searching in PubMed and other resources, most of the current in vivo and in vitro studies are listed in Table 1.
Table 1. In vivo and In vitro Role of Autophagy in the Pathogenesis of Ischemic Stroke.
| Animals | Model | Phenotypes | Role of Autophagy in Ischemic Stroke | References |
|---|---|---|---|---|
| Adult male Wistar rats | 4VO | Hamartin induced autophagy | Protective | [24] |
| Adult male SD rats | tMCAO | Melatonin inhibited autophagy | Harmful | [25] |
| - | pMCAO | Metformin induced autophagy | Protective | [52] |
| - | 4VO | Rapamycin induced autophagy | Protective | [58] |
| - | pMCAOand OGD/R* | 3-MA or Wort inhibited autophagy | Harmful | [59] |
| - | pMCAO and tMCAO | Carnosine inhibited autophagy | Harmful | [60] |
| - | pMCAO | Compound C inhibited autophagy | Protective | [61] |
| - | pMCAO | 3-MA inhibited autophagy | Harmful | [62] |
| - | dMCAO | 3-MA or Becn1-shRNA inhibited autophagy | Harmful | [63] |
| - | pMCAO | 3-MA or bafliomycin A1 inhibitedautophagy | Harmful | [64] |
| - | tMCAO and OGD/R* | Eugenol induced autophagy | Protective | [65] |
| Adult SD rats | tMCAO | Homocysteine induced autophagy | Harmful | [66] |
| Adult male C57BL/6 J mice | tMCAO and OGD/R* | IL-17A induced autophagy | Harmful | [67] |
| - | pMCAO | Silibinin inhibited autophagy | Harmful | [68] |
| - | pMCAO | N-acetylserotonin inhibited autophagy | Harmful | [69] |
| - | tMCAO and OGD/R* | Schizandrin inhibited autophagy | Harmful | [70] |
| Adult male C57BL/6 mice | tMCAO | circHECTD1 knockdown inhibited autophagy | Harmful | [71] |
| - | tMCAO | 3-MA inhibited autophagy | Harmful | [72] |
| - | OGD/R* | Rapamycin induced autophagy | Protective | [73] |
| GPR30-/- mice | tMCAO | GPR30 knockout inhibited autophagy | Protective | [74] |
| IL-21-/- mice | tMCAO | IL-21 knockout inhibited autophagy | Harmful | [75] |
| GPR37-/- mice | tMCAO | GPR37 knockout induced autophagy | Harmful | [76] |
| TIGAR-/- mice and TIGAR-transgenic mice | tMCAO and OGD/R* | TIGAR knockout induced autophagy and TIGAR transgene inhibited autophagy | Harmful | [77] |
| Male arrb1−/− and arrb2−/− mice | tMCAO | ARRB1 knockout inhibited autophagy | Protective | [78] |
| ATF6 knock-in mice | tMCAO | ATF6 knock-in induced autophagy | Protective | [79] |
| Irgm1-/- mice | pMCAO | IRGM1 knockout inhibited autophagy | Protective | [80] |
| Neonatal Wistar rats | ischemia-hypoxia | Lithium inhibited autophagy | Harmful | [81] |
Abbreviations: SD, Sprague Dawley; 4VOs, 4 vessels occlusion; tMCAO/pMCAO/dMCAO, transient/permanent/distal middle cerebral artery occlusion;OGD/R, oxygen glucose deprivation/reperfusion; * means in vitro.
4.1. Detrimental Role of Autophagy in Ischemic Stroke
Studies, including in vivo and in vitro findings, suggest that autophagy may exhibit a detrimental influence on the outcome of IS. Autophagy inhibitors, 3-methyladenine (3- MA) or wortmannin (Wort), reduced infarction volume in rats of middle cerebral artery occlusion (MCAO) model [59, 62-64, 72]. Atg5 is an indispensable component in the vesicle elongation process of autophagy and showed a neuroprotective effect of astrocytes. Atg5 gene knockout mice had less cortical injury [59]. TP53-induced glycolysis and apoptosis regulator (TIGAR), a regulator of the autophagy pathway, was shown to ameliorate cerebral ischemia/reperfusion (I/R)-induced neuronal injury [77]. In TIGAR-/- mice, I/R-induced injury is partly prevented by 3-MA, an autophagy inhibitor [77]. While in TIGAR+/+ mice, the neuroprotective effect of TIGAR was partly abolished by rapamycin, an autophagy activator [77]. IL-21 contributed to the neuronal injury after IS and IL-21 promoted autophagy expression in neuronal cells after hypoxia/ischemia, suggesting that autophagy might exert harmful impact on IS [75]. GPR37 knockout mice showed increased infarction and autophagic cell death compared with wild-type mice after IS, which suggested that autophagy participates in the pathogenesis of IS and its effect is detrimental [76]. Moreover, multiple agents, including carnosine [60], melatonin [25], lithium [81], schizandrin [70], silibinin [68], homocysteine [66], bafilomycin [64], N-acetylserotonin [69], were reported to either exert neuroprotection by inhibition of autophagy or aggravate ischemic brain damage by its’ activation.
4.2. Beneficial Role of Autophagy in Ischemic Stroke
Many studies also suggested that autophagy may have a neuroprotective effect on IS. In a 4 vessel occlusion (4VO) model of mice, administration of autophagy activator rapamycin showed less infarction volume, neuronal injury, and motor deficits [58]. Such a result was also found in an in vitro oxygen glucose deprivation/reperfusion (OGD/R) model [73]. Metformin, a famous first-line diabetes drug, initiates autophagy by activating AMPK, thus exerting a protective role in IS [52]. IRGM1 knockout mice were reported to have more severe brain damage in IS and lower level of autophagy activity than wild type mice [80]. Other genetically engineered models of mice, such as ATF6 knock-in mice [79], ARRB1 knockout mice [78], and GPR30 knockout mice [74], also demonstrated that autophagy has a protective role in IS. Various agents, including hamartin [24], compound C [61], and eugenol [65], were reported to be either neuroprotective by activation or deleterious by inhibition of autophagy.
Many studies did not take sex as a variable when analyzing the experimental outcomes of IS research. However, accumulating data shows that sex differences play an important role in the regulation of the autophagy pathway and may lead to the different outcomes between males and females [82-84]. There are several mechanisms through which sex differences occur in IS and the most widely accepted are gonadal hormones and chromosomal makeup hypotheses. Gonadal hormones, including androgens and estrogens, modulate the autophagic pathway via their receptors, androgen receptor (AR), estrogen receptor 1 (ESR1), and estrogen receptor 2 (ESR2). Increasing evidence indicates that AR, together with ESR1 and ESR2, is involved in the translational modulation of many autophagy related genes which act in the processes of vesicle nucleation and elongation in humans [82]. By binding to introns of target genes, AR regulates the transcription of ULK2, ATG3, ATG5, BCL2, AMBRA1 (autophagy, and beclin 1 regulator 1), and PIK3C3 (phosphatidylinositol 3-kinase catalytic subunit type 3) [82]. There are 19 autophagy genes regulated by ESR1, including ULK2, ATG5, LC3B (light chain 3 beta), PIK3C3 (phosphatidylinositol 3-kinase catalytic subunit type 3), and SQSTM1, and 12 autophagy genes regulated by ESR2, including ULK2, ATG7, ATG13, UVRAG (UV radiation resistance associated), and AMBRA1 (autophagy and beclin 1 regulator 1) [82]. In addition, accumulating data indicate that many genes located on the X chromosome participate in autophagic pathway or regulate its’ activity, including ATP6AP2 (ATPase H+ transporting accessory protein 2) and LAMP2 (lysosomal associated membrane protein 2) [82]. Such genes located on the X chromosome may explain the sex differences between menopausal female and age- matched male morbidity, fatality rate, and disabling rate in IS.
Recent studies showed that autophagy also participates in the regulation of microglia activation in neuroinflammatory diseases. Shibutani et al. and Deretic et al. found that autophagy suppresses the activation of inflammasomes in microglia through the proteolytic elimination of its adaptor protein ASC or its substrate pro-IL-1β, as well as clearance of damaged mitochondria by mitophagy [22, 85-87]. Accordingly, autophagy is proved to participate in the regulation of microglial inflammation in a rodent model of brain ischemia [22, 88-90]. However, whether autophagy promotes or inhibits inflammatory response after IS is controversial and yet to be elucidated. Apart from the inhibition of inflammasome activation, autophagy can also modulate the phenotypic transformation of microglia via the nuclear factor-κB pathway, which is conducive for the recovery of neural tissue after ischemia [22].
Although the exact role of autophagy in the pathogenesis of IS is ambiguous and inconsistent, it is unanimously believed that moderate activation of autophagy is neuroprotective, while excessive autophagy activation is harmful [22]. The inconsistent results of these studies may be due to several reasons. First, the time of activation or inhibition of autophagy was different among these studies. As a self-protective mechanism, activation of autophagy at the early stage of IS is neuroprotective via degrading misfolded proteins and damaged organelles to maintain the intracellular environment [91]. Interestingly, several studies suggested that autophagy participates in the ischemic preconditioning-inducing neuroprotection [61, 92-94]. In these studies, activation (Sphingosine kinase 2 [92] and conventional protein kinase C γ [93]) or inhibition (compound C [61] and 3-MA [94]) of autophagy, enhanced or diminished the neuroprotective effects of the ischemic preconditioning. Therefore, activation of autophagy at the early stage of IS or before IS is neuroprotective. However, if the ischemic stress continues for a long time, continuously activated autophagy will cause damage. Second, the extent of autophagy activation varied among these studies due to the different models and modulators. As mentioned before, moderate autophagy activation is protective, while excessive autophagy activation is deleterious in IS. Last but not the least, the agents used in some studies are not specific to autophagy. For example, 3-MA is widely used as an inhibitor for autophagy in various studies. However, 3-MA is not specific for targeting the autophagy pathway. 3-MA acts as an autophagy inhibitor [95] through inhibiting PI3K pathway, which participates in other biological processes, including necrosis and apoptosis [96, 97], besides autophagy activation [98, 99]. Rapamycin, an autophagy activator, initiates autophagy via inhibiting mTOR activity. But the mTOR signaling pathway is also suggested to have immunosuppressive and antiproliferative impacts [100, 101]. Therefore, it is hard to tell which signaling pathway, caused by these agents, actually influences the outcome of IS. To solve these problems, standardized protocols of IS models and more specific agents for autophagy need future studies.
5. POTENTIAL THERAPEUTIC STRATEGIES FOR ISCHEMIC STROKE TARGETING AUTOPHAGY PATHWAY
Multiple potential therapeutic agents for IS targets the autophagy pathway (Table 2). These agents modulate different processes of autophagy to exert a neuroprotective effect through variable signaling pathways. The first category is targeting the initiation of autophagy. Rapamycin, an autophagy activator, initiates autophagy by inhibiting mTORC1 to disinhibit the ULK1 complex, which is the key component of autophagy initiation [102]. It has been reported that the administration of rapamycin in rodent models of focal ischemia can diminish infarct volume and augment behavior outcomes [103]. Interestingly, it was found that lower doses of rapamycin were associated with better and improved outcomes, which is potentially due to an optimal level of autophagy activation. Hamartin, a protein encoded by the tuberous sclerosis complex 1 gene (TSC1), has been reported to exert neuroprotection through the mechanism, which is similar to rapamycin [24]. Metformin, the first-line medicine for type 2 diabetes treatment [104, 105], activates AMPK to initiate autophagy. It is suggested that metformin preconditioning is beneficial for the outcomes of cerebral ischemia [52]. Other agents targeting autophagy initiation include fingolimod [106], N-acetyl-serotonin [69], minocycline [107], and carnosine [60]. The second category is targeting vesicle nucleation of autophagy. Melatonin, an endogenous hormone, is demonstrated to alleviate neuronal injury after IS by impeding the expression of Beclin-1 [25, 108], which is critical for vesicle nucleation of autophagy. Interestingly, N-acetyl-serotonin, a precursor of melatonin, is reported to be neuroprotective similar to melatonin but with different mechanisms [69]. N-acetyl-serotonin exerts its effects through inhibiting the initiation of autophagy while melatonin plays its roles through suppressing the vesicle nucleation of autophagy [25, 69, 108]. Bexarotene, an FDA-approved agent, is suggested to have neuroprotective potential in stroke [109]. By suppressing the degradation of the autophagosome, a double-membrane vesicle enfolding substrates needs degradation, bexarotene acts on vesicle elongation to promote autophagy [109]. 3-MA, a classic autophagy inhibitor, inhibits ischemia-induced LC3 expression to repress vesicle elongation, thus diminishes autophagy-induced injury in IS [64].
Table 2. Potential Therapeutic Agents for Ischemic Stroke Targeting Autophagy Pathway.
| Agents | Targeting Autophagy Pathway | Pharmacological Effects | References |
|---|---|---|---|
| Hamartin | Initiation of autophagy | Inhibition of mTORC1 to disinhibit ULK1 complex | [24] |
| Metformin | Initiation of autophagy | Activating ULK1 complex by activating AMPK | [52] |
| Carnosine | Initiation of autophagy | Reversing the inhibited phosphorylated levels of mTOR by ischemia | [60] |
| 3-MA | Vesicle nucleation | Inhibition of ischemia-induced LC3 expression | [64] |
| N-acetyl-serotonin | Initiation of autophagy | Reducing the activation of autophagy | [69] |
| Rapamycin | Initiation of autophagy | Inhibition of mTORC1 to disinhibit ULK1 complex | [102] |
| Fingolimod | Initiation of autophagy | Activating mTOR/p70S6K pathway to suppress autophagy | [106] |
| Minocycline | Initiation of autophagy | Reducing the activation of autophagy | [107] |
| Melatonin | Vesicle nucleation | Impeding the expression of Beclin-1 | [108] |
| Bexarotene | Vesicle elongation | Inhibition of autophagosome degradation | [109] |
Although agents targeting the autophagy pathway have therapeutic potential for IS, there are possible side effects that need to be considered. Long term administration of rapamycin, which activates autophagy pathway through inhibiting mTORC1, may also inhibit mTORC2 and cause immunosuppression and glucose intolerance [110]. Metformin initiates autophagy via activating AMPK. However, many patients can not tolerate adequate amounts of metformin because of its associated gastrointestinal adverse events, including diarrhea, nausea, flatulence, indigestion, vomiting, and abdominal discomfort [111]. As the first-line medicine for type 2 diabetes treatment [104], metformin may cause hypoglycemia when administrated with a high dosage in a short period. Therefore, finding an optimal agent dosage is necessary for maximizing the neuroprotective effect while minimizing the possible side effects.
6. CONCLUSION AND FUTURE PERSPECTIVES
Autophagy is a strictly controlled multi-stages process and has been suggested to be involved in multiple diseases, including stroke [19, 57], rheumatology [112], cancer [113, 114], neurodegenerative disease [115, 116], cardiovascular disease [117, 118], and so on. As an evolutionarily conserved process, autophagy maintains cellular homeostasis by eliminating misfolded proteins and injured organelles [38]. Canonical autophagy pathway comprises initiation, vesicle nucleation, vesicle elongation, fusion and degradation [38, 119]. While during IS, diminished oxygen and glucose supply leads to the accumulation of AMP, which activates AMPK, thus initiating autophagy [49, 50]. In addition, the accumulation of misfolded proteins during IS causes upregulated BAG3 and HDAC6, leading to the conversion from the ubiquitin-proteasome pathway to the autophagy-lysosome pathway [9, 54]. The research community has reached a consensus that autophagy is responsible for the maintenance of cerebral homeostasis during IS [19]; however, it is still ambiguous about the exact role of autophagy in the pathogenesis of IS. On one hand, some research suggested that autophagy is detrimental to the outcomes of cerebral ischemia [25, 60, 67]. On the other hand, some studies showed a neuroprotective effect of autophagy during IS [24, 52, 58]. Whether autophagy is a friend or a foe in IS is hard to tell. But it is unanimously believed that moderate activation of autophagy is neuroprotective, while excessive autophagy activation is harmful [22]. The incongruous results of these studies may be due to different IS models and agent administration, which lead to different time and extent of modulation of autophagy.
Currently, the only medication that the FDA has approved for IS is limited to thrombolytic treatment [57], whose clinical application value is not promising due to its short therapeutic window and risk for intracerebral hemorrhage. Therefore, studies focused on potential therapeutic strategies for IS are urgently needed. Many researchers have found potential therapeutic strategies for IS, targeting the autophagy pathway [24, 52, 102]. But many agents targeting autophagy are not specific and even genetically engineered models of autophagy genes have limitations since autophagy-related genes also exert autophagy-independent functions [96, 99, 119]. Hence, more specific agents or genetic tools and unified disease models are needed to decipher the role of autophagy in the pathogenesis of IS in future studies. With a more comprehensive and profound understanding of the role of autophagy in the pathogenesis of IS, novel therapeutic strategies for IS are on their way.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 3-MA
3-methyladenine
- AMPK
AMP-Activated Protein Kinase
- AR
Androgen Receptor
- ATG16L
ATG16-like
- DRAM1
Damage-Regulated Autophagy Modulator Protein 1
- ESR1
Estrogen Receptor 1
- ESR2
Estrogen Receptor 2
- IS
Ischemic Stroke
- mTOR
Mammalian Target of Rapamycin
- OGD/R
Oxygen Glucose Deprivation/Reperfusion
- PE
Phosphatidylethanolamine
- PI3K III
Class III Phosphatidylinositol 3-kinase
- TIGAR
TP53-induced Glycolysis and Apoptosis Regulator
- ULK1
Unc-51-like kinase 1; VPS34, vacuolar protein sorting 34
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the National Natural Science Foundation of China grants (81873769).
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haley M.J., Krishnan S., Burrows D., de Hoog L., Thakrar J., Schiessl I., Allan S.M., Lawrence C.B. Acute high-fat feeding leads to disruptions in glucose homeostasis and worsens stroke outcome. J. Cereb. Blood Flow Metab. 2019;39(6):1026–1037. doi: 10.1177/0271678X17744718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankey G.J. Stroke. Lancet. 2017;389(10069):641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 4.WHO Global burden of stroke. http://www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdf
- 5.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabrowska S., Andrzejewska A., Lukomska B., Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflammation. 2019;16(1):178. doi: 10.1186/s12974-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P., Stetler R. A., Leak R. K., Shi Y., Li Y., Yu W., Bennett M. V. L., Chen J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134(Pt B):208–217. doi: 10.1016/j.neuropharm.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky D.J. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4(6):740–743. doi: 10.4161/auto.6398. [DOI] [PubMed] [Google Scholar]
- 9.Chen C., Qin H., Tan J., Hu Z., Zeng L. The Role of Ubiquitin-Proteasome Pathway and Autophagy-Lysosome Pathway in Cerebral Ischemia. Oxid. Med. Cell. Longev. 2020;2020:5457049. doi: 10.1155/2020/5457049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimore G. E., Hutson N. J., Surmacz C. A. Quantitative correlation between proteolysis and macroautophagy and microautophagy in mouse hepatocytes during starvation and refeeding. P Natl Acad Sci-Biol. 1983;80(8):2179–2183. doi: 10.1073/pnas.80.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W.W., Li J., Bao J.K. Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 2012;69(7):1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh R., Pattison J.S. Macroautophagy and Chaperone-Mediated Autophagy in Heart Failure: The Known and the Unknown. Oxid. Med. Cell. Longev. 2018;2018:8602041. doi: 10.1155/2018/8602041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunk U.T., Jones C.B., Sohal R.S. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat. Res. 1992;275(3-6):395–403. doi: 10.1016/0921-8734(92)90042-N. [DOI] [PubMed] [Google Scholar]
- 15.Schulze H., Kolter T., Sandhoff K. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim. Biophys. Acta. 2009;1793(4):674–683. doi: 10.1016/j.bbamcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Dice J.F. Chaperone-mediated autophagy. Autophagy. 2007;3(4):295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 17.Martinet W., De Meyer G.R. Autophagy in atherosclerosis. Curr. Atheroscler. Rep. 2008;10(3):216–223. doi: 10.1007/s11883-008-0034-y. [DOI] [PubMed] [Google Scholar]
- 18.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19(6):349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang P., Shao B.Z., Deng Z., Chen S., Yue Z., Miao C.Y. Autophagy in ischemic stroke. Prog. Neurobiol. 2018;163-164:98–117. doi: 10.1016/j.pneurobio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Thompson J.E. The evolution of surgery for the treatment and prevention of stroke. The Willis Lecture. Stroke. 1996;27(8):1427–1434. doi: 10.1161/01.STR.27.8.1427. [DOI] [PubMed] [Google Scholar]
- 21.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., Jauch E.C., Kidwell C.S., Leslie-Mazwi T.M., Ovbiagele B., Scott P.A., Sheth K.N., Southerland A.M., Summers D.V., Tirschwell D.L. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 22.Mo Y., Sun Y.Y., Liu K.Y. Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 2020;15(8):1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng C., Rao W., Zhang L., Gao F., Hui H., Wang K., Dai S., Yang Y., Luo P., Ma Y., Ma W., Yu X., Fei Z. Mitofusin 2 exerts a protective role in ischemia reperfusion injury through increasing autophagy. Cell. Physiol. Biochem. 2018;46(6):2311–2324. doi: 10.1159/000489621. [DOI] [PubMed] [Google Scholar]
- 24.Papadakis M., Hadley G., Xilouri M., Hoyte L.C., Nagel S., McMenamin M.M., Tsaknakis G., Watt S.M., Drakesmith C.W., Chen R., Wood M.J., Zhao Z., Kessler B., Vekrellis K., Buchan A.M. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat. Med. 2013;19(3):351–357. doi: 10.1038/nm.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng D., Wang B., Wang L., Abraham N., Tao K., Huang L., Shi W., Dong Y., Qu Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017;62(3) doi: 10.1111/jpi.12395. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 27.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., Guan J.L., Oshiro N., Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 2009;20(7):1981–1991. doi: 10.1091/mbc.e08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung C.H., Jun C.B., Ro S.H., Kim Y.M., Otto N.M., Cao J., Kundu M., Kim D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.e08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y.Y., Neufeld T.P. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol. Biol. Cell. 2009;20(7):2004–2014. doi: 10.1091/mbc.e08-12-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itakura E., Kishi C., Inoue K., Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell. 2008;19(12):5360–5372. doi: 10.1091/mbc.e08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Bartolomeo S., Corazzari M., Nazio F., Oliverio S., Lisi G., Antonioli M., Pagliarini V., Matteoni S., Fuoco C., Giunta L., D’Amelio M., Nardacci R., Romagnoli A., Piacentini M., Cecconi F., Fimia G.M. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Cell Biol. 2010;191(1):155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell R.C., Tian Y., Yuan H., Park H.W., Chang Y.Y., Kim J., Kim H., Neufeld T.P., Dillin A., Guan K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15(7):741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bento C.F., Renna M., Ghislat G., Puri C., Ashkenazi A., Vicinanza M., Menzies F.M., Rubinsztein D.C. Mammalian autophagy: how does it work? Annu. Rev. Biochem. 2016;85:685–713, 685-713. doi: 10.1146/annurev-biochem-060815-014556. [DOI] [PubMed] [Google Scholar]
- 34.Park J.M., Jung C.H., Seo M., Otto N.M., Grunwald D., Kim K.H., Moriarity B., Kim Y.M., Starker C., Nho R.S., Voytas D., Kim D.H. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy. 2016;12(3):547–564. doi: 10.1080/15548627.2016.1140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H., Liu W. PIK3C3/VPS34 control by acetylation. Autophagy. 2018;14(6):1086–1087. doi: 10.1080/15548627.2017.1385676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taguchi-Atarashi N., Hamasaki M., Matsunaga K., Omori H., Ktistakis N.T., Yoshimori T., Noda T. Modulation of local PtdIns3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy. Traffic. 2010;11(4):468–478. doi: 10.1111/j.1600-0854.2010.01034.x. [DOI] [PubMed] [Google Scholar]
- 37.Axe E.L., Walker S.A., Manifava M., Chandra P., Roderick H.L., Habermann A., Griffiths G., Ktistakis N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182(4):685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim K.H., Lee M.S. Autophagy-a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014;10(6):322–337. doi: 10.1038/nrendo.2014.35. [DOI] [PubMed] [Google Scholar]
- 39.Mizushima N., Sugita H., Yoshimori T., Ohsumi Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J. Biol. Chem. 1998;273(51):33889–33892. doi: 10.1074/jbc.273.51.33889. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., George M.D., Klionsky D.J., Ohsumi M., Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 41.Kuma A., Mizushima N., Ishihara N., Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J. Biol. Chem. 2002;277(21):18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- 42.Ichimura Y., Kirisako T., Takao T., Satomi Y., Shimonishi Y., Ishihara N., Mizushima N., Tanida I., Kominami E., Ohsumi M., Noda T., Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 43.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell. 2008;19(5):2092–2100. doi: 10.1091/mbc.e07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itakura E., Kishi-Itakura C., Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151(6):1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 45.McEwan D.G., Popovic D., Gubas A., Terawaki S., Suzuki H., Stadel D., Coxon F.P., Miranda de Stegmann D., Bhogaraju S., Maddi K., Kirchof A., Gatti E., Helfrich M.H., Wakatsuki S., Behrends C., Pierre P., Dikic I. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell. 2015;57(1):39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Diao J., Liu R., Rong Y., Zhao M., Zhang J., Lai Y., Zhou Q., Wilz L.M., Li J., Vivona S., Pfuetzner R.A., Brunger A.T., Zhong Q. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520(7548):563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu L., McPhee C.K., Zheng L., Mardones G.A., Rong Y., Peng J., Mi N., Zhao Y., Liu Z., Wan F., Hailey D.W., Oorschot V., Klumperman J., Baehrecke E.H., Lenardo M.J. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonioli M., Di Rienzo M., Piacentini M., Fimia G.M. Emerging Mechanisms in Initiating and Terminating Autophagy. Trends Biochem. Sci. 2017;42(1):28–41. doi: 10.1016/j.tibs.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Jiang S., Li T., Ji T., Yi W., Yang Z., Wang S., Yang Y., Gu C. AMPK: Potential therapeutic target for ischemic stroke. Theranostics. 2018;8(16):4535–4551. doi: 10.7150/thno.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W., Vasquez D.S., Joshi A., Gwinn D.M., Taylor R., Asara J.M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R.J. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabryel B., Kost A., Kasprowska D., Liber S., Machnik G., Wiaderkiewicz R., Łabuzek K. AMP-activated protein kinase is involved in induction of protective autophagy in astrocytes exposed to oxygen-glucose deprivation. Cell Biol. Int. 2017;41(8):928–931. doi: 10.1002/cbin.10793. [DOI] [PubMed] [Google Scholar]
- 52.Jiang T., Yu J.T., Zhu X.C., Wang H.F., Tan M.S., Cao L., Zhang Q.Q., Gao L., Shi J.Q., Zhang Y.D., Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br. J. Pharmacol. 2014;171(13):3146–3157. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L.T., Chen B.L., Wu C.T., Huang K.H., Chiang C.K., Hwa Liu S. Protective role of AMP-activated protein kinase-evoked autophagy on an in vitro model of ischemia/reperfusion-induced renal tubular cell injury. PLoS One. 2013;8(11):e79814. doi: 10.1371/journal.pone.0079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X., Yamashita T., Shang J., Shi X., Morihara R., Huang Y., Sato K., Takemoto M., Hishikawa N., Ohta Y., Abe K. Molecular switching from ubiquitin-proteasome to autophagy pathways in mice stroke model. J. Cereb. Blood Flow Metab. 2020;40(1):214–224. doi: 10.1177/0271678X18810617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu M., Jiang Y., Feng Q., Ouyang Y., Gan J. DRAM1 protects neuroblastoma cells from oxygen-glucose deprivation/reperfusion-induced injury via autophagy. Int. J. Mol. Sci. 2014;15(10):19253–19264. doi: 10.3390/ijms151019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei K., Wang P., Miao C.Y. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci. Ther. 2012;18(11):879–886. doi: 10.1111/cns.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nabavi S.F., Sureda A., Sanches-Silva A., Pandima Devi K., Ahmed T., Shahid M., Sobarzo-Sánchez E., Dacrema M., Daglia M., Braidy N., Vacca R.A., Berindan-Neagoe I., Gulei D., Barreca D., Banach M., Nabavi S.M., Dehpour A.R., Shirooie S. Novel therapeutic strategies for stroke: The role of autophagy. Crit. Rev. Clin. Lab. Sci. 2019;56(3):182–199. doi: 10.1080/10408363.2019.1575333. [DOI] [PubMed] [Google Scholar]
- 58.Hwang J.Y., Gertner M., Pontarelli F., Court-Vazquez B., Bennett M.V., Ofengeim D., Zukin R.S. Global ischemia induces lysosomal-mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ. 2017;24(2):317–329. doi: 10.1038/cdd.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou X.Y., Luo Y., Zhu Y.M., Liu Z.H., Kent T.A., Rong J.G., Li W., Qiao S.G., Li M., Ni Y., Ishidoh K., Zhang H.L. Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 2017;8(2):e2618. doi: 10.1038/cddis.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baek S.H., Noh A.R., Kim K.A., Akram M., Shin Y.J., Kim E.S., Yu S.W., Majid A., Bae O.N. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014;45(8):2438–2443. doi: 10.1161/STROKEAHA.114.005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang T., Yu J.T., Zhu X.C., Zhang Q.Q., Tan M.S., Cao L., Wang H.F., Shi J.Q., Gao L., Qin H., Zhang Y.D., Tan L. Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Mol. Neurobiol. 2015;51(1):220–229. doi: 10.1007/s12035-014-8725-6. [DOI] [PubMed] [Google Scholar]
- 62.Qin A.P., Liu C.F., Qin Y.Y., Hong L.Z., Xu M., Yang L., Liu J., Qin Z.H., Zhang H.L. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6(6):738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 63.Xing S., Zhang Y., Li J., Zhang J., Li Y., Dang C., Li C., Fan Y., Yu J., Pei Z., Zeng J. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy. 2012;8(1):63–76. doi: 10.4161/auto.8.1.18217. [DOI] [PubMed] [Google Scholar]
- 64.Wen Y.D., Sheng R., Zhang L.S., Han R., Zhang X., Zhang X.D., Han F., Fukunaga K., Qin Z.H. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4(6):762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 65.Sun X., Wang D., Zhang T., Lu X., Duan F., Ju L., Zhuang X., Jiang X. Eugenol Attenuates Cerebral Ischemia-Reperfusion Injury by Enhancing Autophagy via AMPK-mTOR-P70S6K Pathway. Front. Pharmacol. 2020;11:84. doi: 10.3389/fphar.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M., Liang X., Cheng M., Yang L., Liu H., Wang X., Sai N., Zhang X. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell Death Dis. 2019;10(8):561. doi: 10.1038/s41419-019-1798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu T., Han S., Dai Q., Zheng J., Liu C., Li S., Li J. IL-17A- mediated excessive autophagy aggravated neuronal ischemic injuries via Src-PP2B-mTOR pathway. Front. Immunol. 2019;10:2952. doi: 10.3389/fimmu.2019.02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M., Li Y.J., Ding Y., Zhang H.N., Sun T., Zhang K., Yang L., Guo Y.Y., Liu S.B., Zhao M.G., Wu Y.M. Silibinin prevents autophagic cell death upon oxidative stress in cortical neurons and cerebral ischemia-reperfusion injury. Mol. Neurobiol. 2016;53(2):932–943. doi: 10.1007/s12035-014-9062-5. [DOI] [PubMed] [Google Scholar]
- 69.Zhou H., Wang J., Jiang J., Stavrovskaya I.G., Li M., Li W., Wu Q., Zhang X., Luo C., Zhou S., Sirianni A.C., Sarkar S., Kristal B.S., Friedlander R.M., Wang X. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J. Neurosci. 2014;34(8):2967–2978. doi: 10.1523/JNEUROSCI.1948-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang G., Wang T., Zhang Y., Li F., Yu B., Kou J. Schizandrin protects against OGD/R-induced neuronal injury by suppressing autophagy: involvement of the AMPK/mTOR pathway. Molecules. 2019;24(19):E3624. doi: 10.3390/molecules24193624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han B., Zhang Y., Zhang Y., Bai Y., Chen X., Huang R., Wu F., Leng S., Chao J., Zhang J.H., Hu G., Yao H. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14(7):1164–1184. doi: 10.1080/15548627.2018.1458173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L., Chen J., Sun S., Zhao J., Dong X., Wang J. Effects of Estradiol on Autophagy and Nrf-2/ARE Signals after Cerebral Ischemia. Cell. Physiol. Biochem. 2017;41(5):2027–2036. doi: 10.1159/000475433. [DOI] [PubMed] [Google Scholar]
- 73.Tripathi M., Zhang C.W., Singh B.K., Sinha R.A., Moe K.T., DeSilva D.A., Yen P.M. Hyperhomocysteinemia causes ER stress and impaired autophagy that is reversed by Vitamin B supplementation. Cell Death Dis. 2016;7(12):e2513. doi: 10.1038/cddis.2016.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X.S., Yue J., Hu L.N., Tian Z., Zhang K., Yang L., Zhang H.N., Guo Y.Y., Feng B., Liu H.Y., Wu Y.M., Zhao M.G., Liu S.B. Activation of G protein-coupled receptor 30 protects neurons by regulating autophagy in astrocytes. Glia. 2020;68(1):27–43. doi: 10.1002/glia.23697. [DOI] [PubMed] [Google Scholar]
- 75.Clarkson B.D., Ling C., Shi Y., Harris M.G., Rayasam A., Sun D., Salamat M.S., Kuchroo V., Lambris J.D., Sandor M., Fabry Z. T cell-derived interleukin (IL)-21 promotes brain injury following stroke in mice. J. Exp. Med. 2014;211(4):595–604. doi: 10.1084/jem.20131377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCrary M.R., Jiang M.Q., Giddens M.M., Zhang J.Y., Owino S., Wei Z.Z., Zhong W., Gu X., Xin H., Hall R.A., Wei L., Yu S.P. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J. 2019;33(10):10680–10691. doi: 10.1096/fj.201900070R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang D.M., Zhang T., Wang M.M., Wang X.X., Qin Y.Y., Wu J., Han R., Sheng R., Wang Y., Chen Z., Han F., Ding Y., Li M., Qin Z.H. TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic. Biol. Med. 2019;137:13–23. doi: 10.1016/j.freeradbiomed.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Wang P., Xu T.Y., Wei K., Guan Y.F., Wang X., Xu H., Su D.F., Pei G., Miao C.Y. ARRB1/β-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy. 2014;10(9):1535–1548. doi: 10.4161/auto.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu Z., Sheng H., Liu S., Zhao S., Glembotski C.C., Warner D.S., Paschen W., Yang W. Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J. Cereb. Blood Flow Metab. 2017;37(3):1069–1079. doi: 10.1177/0271678X16650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He S., Wang C., Dong H., Xia F., Zhou H., Jiang X., Pei C., Ren H., Li H., Li R., Xu H. Immune-related GTPase M (IRGM1) regulates neuronal autophagy in a mouse model of stroke. Autophagy. 2012;8(11):1621–1627. doi: 10.4161/auto.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Q., Li H., Roughton K., Wang X., Kroemer G., Blomgren K., Zhu C. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death Dis. 2010;1:e56. doi: 10.1038/cddis.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shang D., Wang L., Klionsky D.J., Cheng H., Zhou R. Sex differences in autophagy-mediated diseases: toward precision medicine. Autophagy. 2020:1–12. doi: 10.1080/15548627.2020.1752511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliván S., Calvo A.C., Manzano R., Zaragoza P., Osta R. Sex differences in constitutive autophagy. BioMed. Res. Int. 2014;2014:652817. doi: 10.1155/2014/652817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azcoitia I., Barreto G.E., Garcia-Segura L.M. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front. Neuroendocrinol. 2019;55:100787. doi: 10.1016/j.yfrne.2019.100787. [DOI] [PubMed] [Google Scholar]
- 85.Shibutani S.T., Saitoh T., Nowag H., Münz C., Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015;16(10):1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 86.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13(10):722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plaza-Zabala A., Sierra-Torre V., Sierra A. Autophagy and Microglia: Novel Partners in Neurodegeneration and Aging. Int. J. Mol. Sci. 2017;18(3):E598. doi: 10.3390/ijms18030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Z., Zhong L., Zhong S., Xian R., Yuan B. Hypoxia induces microglia autophagy and neural inflammation injury in focal cerebral ischemia model. Exp. Mol. Pathol. 2015;98(2):219–224. doi: 10.1016/j.yexmp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang Y., Zhu J., Wu L., Xu G., Dai J., Liu X. Tetracycline inhibits local inflammation induced by cerebral ischemia via modulating autophagy. PLoS One. 2012;7(11):e48672. doi: 10.1371/journal.pone.0048672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W., Kang J., Li H., Su J., Wu J., Xu Y., Yu H., Xiang X., Yi H., Lu Y., Sun L. Regulation of endoplasmic reticulum stress in rat cortex by p62/ZIP through the Keap1-Nrf2-ARE signalling pathway after transient focal cerebral ischaemia. Brain Inj. 2013;27(7-8):924–933. doi: 10.3109/02699052.2013.793397. [DOI] [PubMed] [Google Scholar]
- 92.Song D.D., Zhang T.T., Chen J.L., Xia Y.F., Qin Z.H., Waeber C., Sheng R. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death Dis. 2017;8(7):e2912. doi: 10.1038/cddis.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei H., Li Y., Han S., Liu S., Zhang N., Zhao L., Li S., Li J. cPKCγ-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke Through Akt-mTOR Pathway. Transl. Stroke Res. 2016;7(6):497–511. doi: 10.1007/s12975-016-0484-4. [DOI] [PubMed] [Google Scholar]
- 94.Park H.K., Chu K., Jung K.H., Lee S.T., Bahn J.J., Kim M., Lee S.K., Roh J.K. Autophagy is involved in the ischemic preconditioning. Neurosci. Lett. 2009;451(1):16–19. doi: 10.1016/j.neulet.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 95.Klionsky D.J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007;8(11):931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 96.Barré B., Perkins N.D. The Skp2 promoter integrates signaling through the NF-kappaB, p53, and Akt/GSK3beta pathways to regulate autophagy and apoptosis. Mol. Cell. 2010;38(4):524–538. doi: 10.1016/j.molcel.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 97.Qin L., Wang Z., Tao L., Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6(2):239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 98.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 99.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008;415(3):333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 100.Ma X.M., Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 101.Menzies F.M., Rubinsztein D.C. Broadening the therapeutic scope for rapamycin treatment. Autophagy. 2010;6(2):286–287. doi: 10.4161/auto.6.2.11078. [DOI] [PubMed] [Google Scholar]
- 102.Hadley G., Beard D.J., Couch Y., Neuhaus A.A., Adriaanse B.A., DeLuca G.C., Sutherland B.A., Buchan A.M. Rapamycin in ischemic stroke: Old drug, new tricks? J. Cereb. Blood Flow Metab. 2019;39(1):20–35. doi: 10.1177/0271678X18807309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beard D.J., Hadley G., Thurley N., Howells D.W., Sutherland B.A., Buchan A.M. The effect of rapamycin treatment on cerebral ischemia: A systematic review and meta-analysis of animal model studies. Int. J. Stroke. 2019;14(2):137–145. doi: 10.1177/1747493018816503. [DOI] [PubMed] [Google Scholar]
- 104.Maruthur N.M., Tseng E., Hutfless S., Wilson L.M., Suarez-Cuervo C., Berger Z., Chu Y., Iyoha E., Segal J.B., Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med. 2016;164(11):740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 105.Arbeláez-Quintero I., Palacios M. To Use or Not to Use Metformin in Cerebral Ischemia: A Review of the Application of Metformin in Stroke Rodents. Stroke Res. Treat. 2017;2017:9756429. doi: 10.1155/2017/9756429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li X., Wang M.H., Qin C., Fan W.H., Tian D.S., Liu J.L. Fingolimod suppresses neuronal autophagy through the mTOR/p70S6K pathway and alleviates ischemic brain damage in mice. PLoS One. 2017;12(11):e0188748. doi: 10.1371/journal.pone.0188748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Z., Zou X., Zhu W., Mao Y., Chen L., Zhao F. Minocycline is effective in intracerebral hemorrhage by inhibition of apoptosis and autophagy. J. Neurol. Sci. 2016;371:88–95. doi: 10.1016/j.jns.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 108.Zheng Y., Hou J., Liu J., Yao M., Li L., Zhang B., Zhu H., Wang Z. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J. Pharmacol. Sci. 2014;124(3):354–364. doi: 10.1254/jphs.13220FP. [DOI] [PubMed] [Google Scholar]
- 109.Huuskonen M.T., Loppi S., Dhungana H., Keksa-Goldsteine V., Lemarchant S., Korhonen P., Wojciechowski S., Pollari E., Valonen P., Koponen J., Takashima A., Landreth G., Goldsteins G., Malm T., Koistinaho J., Kanninen K.M. Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci. Rep. 2016;6:33176. doi: 10.1038/srep33176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arriola Apelo S.I., Lamming D.W. Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(7):841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonnet F., Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes. Metab. 2017;19(4):473–481. doi: 10.1111/dom.12854. [DOI] [PubMed] [Google Scholar]
- 112.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 113.Mariotto E., Viola G., Zanon C., Aveic S. A BAG’s life: Every connection matters in cancer. Pharmacol. Ther. 2020;209:107498. doi: 10.1016/j.pharmthera.2020.107498. [DOI] [PubMed] [Google Scholar]
- 114.Amaravadi R.K., Kimmelman A.C., Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9(9):1167–1181. doi: 10.1158/2159-8290.CD-19-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wallings R.L., Humble S.W., Ward M.E., Wade-Martins R. Lysosomal dysfunction at the centre of parkinson’s disease and frontotemporal dementia/amyotrophic lateral sclerosis. Trends Neurosci. 2019;42(12):899–912. doi: 10.1016/j.tins.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Di Meco A., Curtis M.E., Lauretti E., Pratico D. Autophagy dysfunction in alzheimer’s disease: mechanistic insights and new therapeutic opportunities. Biol. Psychiatry. 2020;87(9):797–807. doi: 10.1016/j.biopsych.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Phadwal K., Feng D., Zhu D., MacRae V.E. Autophagy as a novel therapeutic target in vascular calcification. Pharmacol. Ther. 2020;206:107430. doi: 10.1016/j.pharmthera.2019.107430. [DOI] [PubMed] [Google Scholar]
- 118.Del Re D.P., Amgalan D., Linkermann A., Liu Q., Kitsis R.N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019;99(4):1765–1817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Galluzzi L., Green D.R. Autophagy-Independent Functions of the Autophagy Machinery. Cell. 2019;177(7):1682–1699. doi: 10.1016/j.cell.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]