Abstract
Neurology and associated nanotherapeutics are a complex field in terms of therapeutics and neurological disorder complexity. The brain is an intricate appendage and requires more precise embattled treatment for the particular diseases and hence it is a broad scale for developing more targeted drug deliveries. The brain is one of the most inaccessible tissues of the body due to the existence of the blood-brain barrier (BBB), thus delivery of drugs inside the brain is a striking dare and it is also tricky to treat central nervous system (CNS) complications pharmacologically. The therapeutic aspiration is to accomplish the lowest drug meditation in the brain tissues so as to gain favoured therapeutic results. To devastate this obstacle, nanotechnology is engaged in the field of targeted brain drug delivery and neuropathology targeting. These carriers hold myriad abilities as they may augment the drug delivery into the brain by shielding them from degradation and prolonging their transmission in the blood, as well as promoting their transport through the BBB. Nanopharmaceuticals are quickly sprouting as a new avenue that is engaged with the drug-loaded nanocarriers to demonstrate unique physicochemical properties and tiny size range for penetrating the central nervous system. The enchantment behind their therapeutic achievement is the condensed drug dose and inferior toxicity, whereby restricting the therapeutic compound to the specific site. Therefore, in this article, we have tried to recapitulate the advances of the novel scopes for the brain targeted drug delivery for complex neurological disorders.
Keywords: Brain barriers, brain tumour barriers, neuropathology, neuro-nano therapeutics, nanocarriers, blood brain barrier
1. INTRODUCTION
The brain is the most occupied organ in the regulation of the various physiological functions of the body protected under the skull and covered throughout with the tightly arranged endothelial fluid barrier. The microvascular endothelial cells of these barriers defend the brain from exogenous substances. These include blood-brain barrier (BBB), brain-cerebrospinal fluid barrier (CSFB), meningeal barrier and Circumventricular organs barrier (CVO), such protection is mainly based upon the cellular structure of the brain capillary endothelial cells (BCECs). Permeation through the brain barriers is regulated by a variety of complicated transport machineries located at the membrane of the BCECs, which are considered to be responsible for selective barrier functions. The BCECs are stated to have diverse functionalities by association with astrocyte and pericytes [1]. P-glycoprotein (P-gp) efflux transport is a membrane bound protein and important component of the brain barrier, present in huge concentration on the apical surface of BCECs. The efflux transport is mediated ATP dependent hydrolysis. These barriers also limit the entrance of the drug molecules that
have therapeutic significance in the treatment of the challenging brain diseases, such as vascular dementia, parkinsonism, epilepsy, Alzheimer's, encephalopathy, glioblastoma, neurosyphilis and neuro HIV [2, 3]. The most difficult task is the delivery of drugs into the brain in the presence of such natural brain effective protection mechanisms [4].
The targeted drug delivery seeks the attention on the concentration of the drug molecules to be delivered into the brain, then reducing the relative concentration of the drug absorbing into other periphery tissues. This can lead to the reduction of the concentration of the drug and can also reduce the side effects. Targeting delivery of drugs to receptors or tissues into the brain is a tricky task and can be claimed with novel drug delivery systems. The favourable distribution of drugs for targeted delivery would migrate to the rest of the body in the absence of specific targeted delivery systems. Novel advancements in nanotechnology open a wide scope of novel nanotherapeutics, which can significantly decrease the overall toxicity and improve the therapeutic benefits into CNS disorders [5]. Nanomedicine, including the site-specific or targeted delivery of drugs, is definitely a very emerging and promising area of research because it provides one of the most excellent prospective ways to enhance the therapeutic index of the drugs [6]. Novel approaches towards specific drug deliveries specifically to brain targeting, enchant the better understanding in regards to the drug transport mechanism through brain barriers, and physicochemical properties of drugs [7].
Nanomedicine is one of the novels approaching branch of the medicine which applies the knowledge and tools of nanotechnology into the prevention, diagnosis and treatment of the diseases. Nanomedicine is usually practised in medical imaging and novel targeted delivery systems. Novel targeted delivery systems can raise the intracellular concentration of a drug by encapsulating it with different nanocarriers that will avoid the efflux pumps. Novel targeted systems in anti-cancer therapy like bio-molecule assisted drug administration approaches can boost the concentration of anticancer agents into the site specific tumor and can improve their passage times [8]. For the development of brain targeted innovative nanocarriers, numerous nano scaled drug delivery and targeting approaches have been emerged to maximize the site-specific delivery of anticancer drugs with minimal side effects [1]. Moreover, nanocarriers are also able to devastate the problem of crossing the cellular membrane, as they can cross the brain barriers and can improve the effective drug concentration into the brain [9]. This novel and promising discipline is based on the application of nanomaterials, including nanotechnologies for enormous neuro therapeutics. The implication of the envisioned bio distribution of nanocarriers is estimated by an aura of blood- or body fluid derived proteins rather than the intended surface [10]. This opsonization of the nanoparticles and thus resulting protein corona particularly the accompaniments usually initiate the recognition by the RES (reticuloendothelial system). Since the last few decades, the potential of nanomedicine to overcome the difficulties of conventionally administered anti-tumor drugs has been viewed as one of the paramount capable tools and has been expansively discovered [11].
2. PHYSIOLOGICAL BARRIERS AS OBSTACLES AND CHALLENGES IN TARGETED BRAIN DELIVERY
It is well recognized that vasculature inside the brain capillaries is created of exclusive obstacle mint of brain barriers, which quarantine the brain from malign substances while retarding the delivery of therapeutics into the brain. The hedges of brain barriers restrict the transport of the drug, in effective pharmacological therapy, through the blood-brain barrier in various neurological disorders. The obstacles in delivering therapeutic molecules into the brain could only be conquest by the intensive struggle in understanding the physiology of brain barriers and its permeability under different pathological conditions. This realization suggests to understand the relationship between the cellular transport mechanisms, both inward and outward at the brain barriers, which are next described briefly. This concept may provide a better understanding of the cellular transport mechanisms, including both inward and outward transverse of substances at the brain barriers.
2.1. Introduction to Brain Barriers
The brain is undoubtedly one of the least reachable organs for the delivery of the drugs, due to brain’s natural protective mechanisms implicate to protect the brain and restrict the entry of therapeutic substances in the normal physiological function of various systems in our body, the capillaries of the endothelial wall are permeable and allow movement of non-electrolytic substances and ions to the macromolecular size of albumin from blood to body intestinal fluid. On the other side, in the central nervous system (CNS), cerebral capillaries play a different role in the routine transport mechanism. The transport mechanism through CNS is selective and very difficult due to the presence of cerebral capillaries of the endothelial cells, which restrict the passage of molecules to the brain, and it fails to achieve equilibrate brain fluid even under steady state situation and also in neuropathologies. This phenomenon explains the complexity of the CNS physiology of brain barriers [4]. The brain is protected peripherally by four major barriers, (A) Meningeal barrier, four layer compacted coat around the brain (dura, pia, arachnoid mater and glial limitans) [9], (B) Blood-brain barrier (BBB) exists larger surface area about 20 m2 creating an inimitable environment between extracellular fluid of the brain and spinal cord [10], (C) Blood-cerebrospinal fluid barrier (BCSFB) contains sone sub-barrier such as an arachnoid epithelial which separates the blood from the sub-arachnoid cerebrospinal fluid (CSF) [11] and (D) Circumventricular organs (CVOs). (Fig. 1) demonstrates a schematic comparison between the barriers of the brain.
Fig. (1).
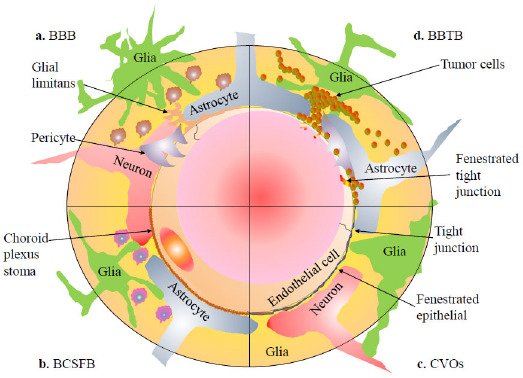
Schematic comparison between barriers of the brain.
2.1.1. Meningeal Barrier
It is the structurally most complex barrier located between the components of meninges, arachnoid, dura and pia mater. The three layers of meningeal cells form a barrier with the outer layer of the arachnoid membrane forma tight junction to separate the outer CSF between the superficial Dural layers and subarachnoid space (SAS). It has similar barrier characteristics as cerebral blood vessels, although it lacks the surrounding pericytes and astrocytic end-feet [12]. The outer layer of the meningeal barrier is about 1 mm thick and composed of dense fibrous tissue of dura mater. The Dural capillaries are more permeable, which allow larger and extensive molecules passage as compared to the BBB. The dura is almost beside the arachnoid mater, which makes the outer BCSFB of the SAS. The trabeculae structure crosses the SAS to the pia mater and to the leptomeningeal arteries and veins. The arachnoid mater is around 200 mm in thickness and not allowing fluids inside and bounds to permeate into the inner membrane of the dura, forming a boundary layer of cells connected by tight junctions and initiate efflux pumps, same as the BBB. The arachnoid mater is the ascertain barrier between the dura mater and the CSF-drained subarachnoid space and therefore, it is a factual blood–CSF barrier. A layer of pia mater is about one cell thick; sheath surrounding the outer surfaceof the brain and is only
allowed to permeate solutes and immune cells but is restrictedto erythrocytes. It limits the surface of the CNS to protect arteries and veins in the SAS and splits the SAS from the CNSand perivascular compartments. The glia limitans forms a barrier between the surface of the brain parenchyma and surrounding blood vessels. It mostly consists of compressed astrocyte foot progressions to the overlying parenchymal basement membrane. It is permeable to the fluid and lower molecular weight solutes but restricted to the limited T cells [13].
2.1.2. Blood-Brain Barrier (BBB)
Paul Ehrlich was the first who originally described the BBB as a barrier primarily composed of non-fenestrated brain micro-vessel endothelial cells categorized by the tight junctions which form almost continuous impermeable barrier that controls traffic of immune macrophages, some endogenous compounds and xenobiotics [14]. (Fig. 1a) shows a physical barrier of the epithelial layer comprising basal membrane surrounded by brain cells like glial cells, astrocytes and pericytes. It forms a selective and semi-permeable barrier that defends the brain from a noxious and infectious agent; at the same time, it supplies selective essential biologically active molecules to the brain. On the other side, it restricts the transfer of the drug (therapeutics agents and parasites) to the brain; it produces the enzymatic blockade by mortifying enzymes present inside the endothelial cells. Contrariwise, it is commonly the rate-limiting aspect to determine the infiltration of drugs into the brain [10].
The straight implication of this restriction is adjuvant to intercellular junctions that have an important functional effect of allowing the quite a few transporters within individual cells to activate over the large surface of the barrier interfaces, deprived of this permeability restriction inward and outward transport mechanisms would be useless [12]. Epithelial layer surrounding the cerebral ventricles comprises three different types of glial cells (1) Astrocytes make the mechanical framework around neurons and control their biochemical atmosphere, which is extensive and bordering one another to compress the capillaries thoroughly associated with the blood vessels to create the BBB; (2) Oligodendrocytes are responsible for the expansion and protection of the myelin sheath, and shelter the axons and remain fundamental for the better transmission of action potentials by saltatory conductivity; (3) Microglia stand for blood-derived mononuclear macrophages. The tight junctions (TJ) between the endothelial layer lead to a dreadfully high trans-endothelial electrical resistance of 1500-2000Ω.cm2 compared to 3-33Ω.cm2 of different tissues, which decrease the aqueous based para-cellular circulation that is normally observed in other body organs [15-19].
Some key features of the BBB endure its truncated and selective permeability for molecules which are recognized with distinguishing biological properties. These include:
Endothelial mitochondria with wide diversity and volume, but few pinocytotic vesicles and lack of arrangement.
The Tight Junction (TJ) cells formed by an active coalition of cytosol accent protein and transmembrane proteins, which are merged in the cytosol. The TJs are enclosed and protected by pericytes and astrocytes in the brain endothelial cells.
The expression of Glucose Transporter 1 (GLUT1) along with assorted transporters such as glucose carrier, insulin receptors, transferrin receptors, large neutral amino acid carrier (LAT1), lipoprotein receptors, and p-glycoproteins and multidrug resistance proteins, a class of ATP family transporters, which aids the efflux of drugs into the brain while others prevent.
The lack of lymphatic drainage and histocompatibility complex (MHC) antigens in CNS offers convincing protection to neuronal activity induced by immunological momentary response.
The BBB is confined by a rigid boundary for the transport of immune cells, especially lymphocytes and their endothelial immune barrier associated with perivascular macrophages and mast cells, which are additionally enclosed by local microglial cells [4, 14, 20].
2.1.3. Blood Cerebrospinal Fluid Barrier (BCSFB)
The barrier which separates the blood from the cerebrospinal fluid lies into the ventricle of the brain, called as a blood-cerebrospinal fluid barrier (BCSFB), which is responsible for the drug resistance travelling into the CNS [21]. This barrier is still not documented as the main route for the uptake of drugs because its surface area is 5000-fold smaller than the BBB. CSF can exchange molecules through the interstitial fluid of the brain parenchyma; the passage of blood-borne molecules into the CSF, which is sensibly controlled by the BCSFB. Physiologically, the BCSFB is located into the epithelium of the choroid’s plexus stoma, as shown in (Fig. 1b), which is structured in a manner to constrain the passage of molecules and cells into the CSF [22, 23]. On the exterior surface of the brain, the ependymal cells fold on them to create a double-layered structure stuck between the dura and pia, which is coined as the arachnoid membrane. It is profusely impermeable for the hydrophilic substances within the development of the BCSFB. Both choroid plexus and arachnoid membrane act together between the blood and CSF [24]. In humans, the total volume of CSF is about 140 ml, which is replaced four to five times daily. The main purpose of CSF is to provide a drainage system for the brain recognized as the sink effect in which metabolized products of higher molecular weight hydrophilic compounds are diluted and consequently eradicated. At the similarity of choroid plexus, epithelial cells with the BBB, polarized face of numerous receptors, ion channels and transporters have been reported [25]. The choroid plexus is made up of tremendously vascularised, “cauliflower-like” tissues of pia mater dip into pockets made by ependymal cells. The majority of choroid plexus is extended through the fourth ventricle close to the base of the brain and in the lateral ventricles inside the right and left cerebral hemispheres. The ependymal cells, which blot the ventricles, make a continuous sheet around the choroid plexus. Although the capillaries of the choroid plexus are relocated, non-continuous and having holes between the endothelial cells, allowing free movement of small molecules, the adjacent choroidal epithelial cells make tight junction avoiding most macromolecules from efficiently passing into the CSF from the blood. Nevertheless, these epithelial cells have lower resistance as compared to the cerebral endothelial cells, about 200 Ω.cm2, between blood and CSF [26, 27].
In addition, the upgraded information has renowned the significance of the choroid plexus in brain function, which has opened new opportunities for the management and prevention of neurological disorders. Choroid plexus function deviates with the disease, which elaborates its significance for understanding CNS pathologies, such as Alzheimer’s, Parkinson’s and disorders of CSF circulation [28].
2.1.4. Circumventricular Organs (CVOs)
Few regions of the brain where the BBB capillary and endothelial tight junctions are non-continuous called circumventricular organs (CVOs). As illustrated in (Fig. 1c), these are located near the ventricles of the brain. These brain areas are extremely vascularized as compared to other brain regions and lack of BBB comprises the fenestrated endothelial cells rather than tight junction and capillary system supplying CVOs. Such regions include pineal gland, choroid plexus, neurohypophysis, lamina terminalis, median eminence, chemoreceptor trigger zone (CTZ) and nucleus tractus solitarius (NTS). These regions regulate the composition of blood and respond consequently by intimate close interaction. The capillaries of CVOs have relatively very less surface area as about 5000:1 as compared to the area of the tight of BBB capillaries that allows CVOs not to enable the significant diffusion of substances into the brain [29].
2.1.5. Metastatic Brain Barriers (Blood-Brain Tumor Barriers BBTB)
In the pathologies of tumors, we often found that the vasculogenic barriers created by tumor cells are morphologically changed, which restrict the neurotransmission and neuronal firing for neurotransmission to the different parts of the brain. The blood-brain tumor barrier (BBTB), likely to the blood-brain barrier (BBB) as illustrated in (Fig. 1d), has situated between brain tumor tissues and micro-vessels made by highly specified endothelial cells (ECs), preventing the paracellular delivery of most hydrophilic molecules to tumor tissue. The BBTB is made by brain tumor capillaries and involves a barrier which is highly discrete from the BBB. Brain tumor capillaries can show the over-expression of receptors that mediate ligand-dependent drug deliveries, which can be selectively used to enhance drug delivery to tumor tissues. The BBTB can be comprised of three different microvessels like continuous and non-fenestrated capillaries, continuous and fenestrated capillaries, and capillaries containing inter-endothelial gaps. Within the enlargement of brain tumors, normal brain tissues are surrounded by the invading tumor cells. A certain extent of tumor cell clusters rises to the region with the BBB and then it damaged to form the BBTB. The permeability of the blood-tumor barrier (BTB) is usually more than the BBTB of a similar type of malignant solid tumors growing surrounded by peripheral tissues within the brain. The reported physiologic upper limit of pore size within the BTB of the malignant solid tumor found in orthotopic RG-2 rat gliomas microvasculature is about 12 nm [29]. Intracranial drug delivery is usually more difficult to target the CNS tumors. The BBB has a clinical significance of its presence in the CNS tumor microenvironment, while both primary and secondary systemic tumors respond to chemotherapeutic agents. Intracranial metastases often continue to grow while chemotherapeutic agents are delivered by the cardiovascular route. In CNS malignancies, mostly the BBB is highly compromised, while a variety of physiological barriers which are common to all solid tumors inhibit drug delivery via the cardiovascular system [4, 10]. The functions of BBB are altered under pathological conditions, like multiple sclerosis, stroke, epilepsy, dementia, neuro-autoimmune deficiency syndrome (Neuro-AIDS) and brain cancer. Significantly, the modifications in the barrier induced by tumors in the brain are not linked with tumor size, tumor type, the location of a tumor and are patchy within any single neoplasm. In low-grade gliomas, the normal BBTB vascularization and physiology remain mostly intact and look like the BBB. In high-grade gliomas, leaky BBTB are easily observed with the contrast MRI due to alteration of vascular function resulting in disruption. The magnitude of this disruption is sufficient to permit drug diffusion in reasonable quantities and taking into brain tissues with the invasion commonly not been enhanced during contrast MRI. The actively metabolic stresses of high-grade glioma make hypoxic areas that trigger the overexpression of Vascular Endothelial Growth Factor (VEGF) and angiogenesis, leading to the development of anomalous vessels and a dysfunctional BBTB [30]. It is notable that, intrusive nature of high-grade glioma causes extensive proliferation of tumor cells outside areas of the disrupted BBTB and inside areas of the normal brain, where the physiology of the barrier is quite intact. Normally, these areas do not exhibit gadolinium enhancement on T1 by weighted MRI. As a concern of both low-grade and high-grade glioma, the BBB and the BBTB form a major hindrance in brain tumor therapy by preventing the delivery of adequate amounts of potentially effective antitumor drugs [30].
2.2. Cellular Transport Systems Across the Brain Barriers
Various transportation routes of drug delivery to the brain can be recognized by which solute molecules transport across the brain barriers. Paracellular and transcellular transport is the type of diffusion of the molecules into the brain. As illustrated in (Fig. 2), transport mechanism available for molecular traffic through a brain barrier, hydrophilic small molecules can transport through the TJ but not to a great extent. Small hydrophobic molecules can easily penetrate by lipid plasma membrane [31].
Fig. (2).
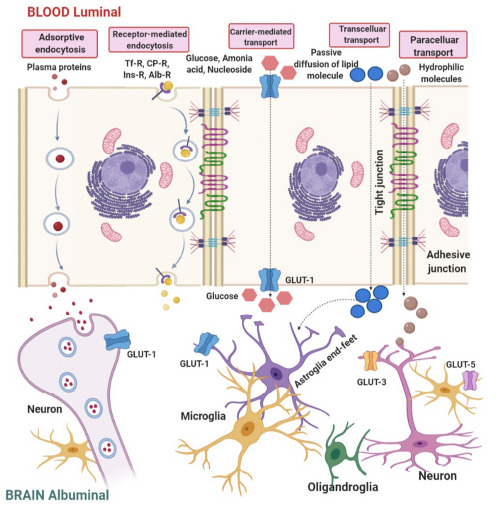
Transport mechanisms available for molecular traffic through a BBB.
In carrier-mediated transport system, fastening of a solute such as glucose and amino acid to a protein molecule on the outer side of the membrane causes the changes in the conformational in the protein structure, resulting in the transport of the substances according to the concentration gradient [1]. In case of molecules moving against a concentration gradient, ATP may provide the energy to facilitate the process. Efflux pumps such as ATP binding cassette (ABC) transport, P- glycoprotein and multidrug resistant protein (MRP) are responsible for extruding from the brain. Clinical trials reveal that inhibition of P-glycoprotein improves the penetration of the paclitaxel into the brain [32].
The most significant approaches of cellular transport system across the brain barriers for nanoparticles are endocytosis and transcytosis. Endothelial cells containing receptors for uptakes of many different types of ligands, include growth factors, enzymes, and plasma protein. Those receptors which are specialized in plasma membrane known as coated pits or endosomes and form vesicles after binding with ligands such as insulin. After acidifications of these endosomes, the ligands dissociate and spread across the membrane. Various receptors like transferrin receptors (TfR), insulin receptors, lipoprotein receptors, glutathione receptors, and diphtheria toxin receptors are extensively studied in brain targeting [33].
Adsorptive mediated transcytosis, also termed as pinocytosis route, is activated by electrostatic interaction between positive charges of peptides such as cationic protein or cell- penetrating peptides and negative charges of plasma membrane surfaces such as heparin sulphate proteoglycans. Cell- mediated transcytosis is a recently identified route for drugs across the BBB, a well-recognized mechanism for entry of HIV virus and pathogens like Cryptococcus neoformans and Cryptococcus gattii into the brain, outlined as “Trojan horse” model. This route depends on the host immunity and different immune cells, such as monocytes or macrophages, to cross the intact BBB [22]. Due to the unique properties of TJs, transport of hydrophilic drugs by paracellular routes is restricted and they transport via transcellular routes by passive diffusion.
The parameters considered for the transport of hydrophilic drugs by passive diffusion across the brain barriers are:
The compound should be unionized form.
Molecular weight should be less than 500 Da.
Lop P value should be near to 2.
Cumulative hydrogen bonding should not go beyond 10.
Besides these direct deliveries to the brain, many efforts have been made to develop approaches which can have advantages like non-invasive drug delivery and bypass the BBB using the olfactory nerves as a non-conventional administration route of drug delivery, which is known as Intranasal drug delivery. Here, the drugs absorbed to nasal mucosa can be used to target the brain directly. The intranasal drug delivery is characterized by increased patient compliance, high safety, self-administration, rapid onset of action, higher bioavailability, avoid hepatic first pass metabolism and minimized systemic exposure. To understand this concept, there is a need to understand the anatomy of the nasal cavity and the cellular and molecular mechanisms for nasal administration and drug penetration to the brain [34, 35].
3. TECHNIQUE FOR QUANTITATIVE ESTIMATION OF DRUG TRANSPORT INTO CNS
Brain-drug uptake summary and drug pharmacokinetics across the BBB are the understanding of the ability of the drugs to cross the brain barriers. To understand the drug delivery system and drug delivery to the brain, one must have to understand the brain drug uptake ratio and mechanism. The brain uptake index (BUI) represents the uptake of a drug as compared to reference substances. The reference substance should be freely diffusible across the brain barrier such as 14C- butanol and the test compound should also be radio-labelled with 3H. Buffer containing a small volume of both test and reference compound should be rapidly injected into the common carotid artery of the anesthetized rat. The bolus passes through the brain is in less than 2 sec. After 5 to 15 sec, the brain should be isolated and radioactivity in brain tissue and the injected buffer must be determined. The BUI can be calculated using the given equation [36].

The BUI can be expressed as a percentage. The BUI represents the net uptake of the drug normalized by the net uptake of the reference compound. It is direct functioning of the extraction if the extraction of references is known; the extraction of the drug can be calculated.

The benefit of the BUI technique is that it is quick, while disadvantages are low sensitivity and not suitable for slowly up taken drugs. Permeability is better-defined measures of brain uptake that expresses either by permeability surface area product (PS) or permeability coefficient (PC), measures the drug profile in arterial blood by intravenous injections and in-situ vascular perfusion techniques measure the rate of transport. Perfusion techniques minimized back transport and biological degradation. Brain uptake can be positively correlated with lipid solubility and negatively correlated with hydrogen bonding. Higher the hydrogen bonding potential, lower the brain uptake [10]. By using this technique and proven mechanisms, we can invade the targeted drug delivery systems for particular CNS pathologies.
4. RECENT STRATEGIES OF DRUG TRANSPORT ACROSS THE BRAIN BARRIERS
Several newer approaches have been revealed for the direct delivery of neurotherapeutics to the CNS. The advantages of these approaches are delivering a higher concentration of drugs to the targeted region and subsequent reducing peripheral accumulation. Various factors have been monitored during drug delivery, such as the site of drug administration, rate of CSF production, lipophilicity and transient of the barrier, pathological condition, volume of drug distribution, permeability of drugs, physicochemical properties of drugs and rate of clearance. (Fig. 3) narrates several techniques have been investigated for direct delivery of macromolecules such as drugs, genes, viruses and peptides into CNS.
Fig. (3).

Modern methods for transporting drugs across the BBB. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.1. Lipophilic Analogue
Lipophilicity of barriers, lower ionization at physiological pH and lower molecular weight of drugs are highly favoured for the drug penetration inside the brain [37]. Due to poor lipid solubility of compounds, the delivery to the brain requires some novel approaches to getting through the barrier. When a drug is delivered via the circulatory system for the treatment of the CNS diseases, a slight equilibrium is required between cerebro-vascular permeability and plasma solubility. The drug molecules should have an optimal partition coefficient (Log PO/W) value about 1.5 to 2.5 for being efficient when administered via the parenteral route, hence the possible way to transport a drug is through lipophilic precursors. Drug’s lipophilicity also strongly correlates with cerebro-vascular permeability, hydrophobic analogue of the hydrophilic drug ought to more eagerly penetrate the brain barrier. These strategies have frequently used, but outcomes have been dissatisfied [25]. The series of the lipophilic analogues of nitrosourea where a quantitative structural activity relationship (QSAR) studies indicated the anti-cancer activity was inversely proportional to their lipophilicity. Usually, lipophilic drugs form protein binding very quickly, so less amount of drug administration is required to achieve the Tmax and Cmax and its diffusion state into the CNS to achieve desired anti-cancer activity and decrease the dose related toxicity. Lipophilic drugs formed protein binding very quickly, so less amount of drug administration is required to achieve the Tmax and Cmax and diffusion into the CNS, which increases anti-cancer activity and decreases the dose related toxicity [38].
The second strategy for improvement or increasing lipophilicity of hydrophilic drugs is to encapsulate with lipid nano-carriers or merged with a sphere of lipid in the form of liposomes. Liposomes are small unilamellar vesicles, which do not undergo significant transport through the BBB. Another major problem with liposomes is the similarity of their structures to the biological membrane, which is rapidly cleared by reticulo-endothelial system after intravenous administration. These dual problems of BBB mediated transport and peripheral clearance overcome by combination approach of chimeric peptide and PEGylation of liposomes, respectively. The novel multifunctional monoclonal antibody coated PEGylated immunoliposomes were prepared by maleimide and 1,2- distearoylphosphotidylethanolamine (DSPE) conjugation, which are enough to permeate through the BBB in vivo [39, 40].
4.2. Prodrug
Prodrugs are therapeutically inert substances transient into biologically active molecules after enzymatic, hydrolytic and hepatic metabolism. They consist of a drug covalently attached to an unrelated chemical moiety, which improves drug’s lipophilicity, pharmacokinetic properties and enhances permeation through the brain barrier. For example, esterification or amidation of -amino, -carboxylic and -hydroxy containing groups could improve lipid solubility. Morphine apparently does not cross the brain barrier due to hydrophilicity [41]. Acetylation of both hydroxyl groups via latentiation produces heroin like hallucinogenic drug, which can easily permeate the barrier and subsequent hydrolysis of the acetyl groups produces a high concentration of morphine stuck inside the brain. While increasing lipophilicity also tends to uptake of certain drugs and pro-drugs into other tissue. The target-specific drug delivery is especially problematic when potent drugs such as steroids and cytotoxic agents could produce toxicity at non-target sites [42].
4.3. Receptor/Vector Mediated Drug Delivery
In this novel drug delivery approach to the brain, the non-transportable drug molecules associated with chimeric peptide or vectors are permeating through the brain barrier. This modified peptide or receptor mediated specific antibody vector latter undergoes receptor mediated transcytosis in-vivo. Conjugation of the drug transport vector is attached with chemical linker, avidin-biotin technology, chimeric technology, PEG linker and liposomes [43]. Chimeric peptide technology has been established for the multiple classes of therapeutics, such as vasoactive technology, neurotrophins, antisense therapeutic peptide, including peptide nucleic acids (PNAs) and small molecules encapsulated liposomes. Few drugs might not be pharmacologically active af-
ter attachment to the BBB transport vector, but the cleavage of the drug from the vector at a disulphide linkage makes the drugs pharmacologically active. There is also a demerit of using a disulphide linker as it reduces disulphide activity, which persistently remains within the cytosol. Therefore, the chimeric peptide must undergo endosomal release following endocytosis by targeted brain cells [44].
The receptor mediated transcytosis (RMT) of peptides through the brain barriers was initiated in the mid-1980s, receptor mediated endocytosis of insulin growth factors (IGFs) in-vitro into endothelial brain capillary and in-vivo transcytosis of insulin through the brain barriers were done [45]. Adsorptive mediated transcytosis (AME), facilitated by electrostatic interactions of the luminal plasma membrane with the anionic site or by specific interaction with glucose molecules, was performed. In respect to established structural specificity at the barrier, molecular sizes, basicity, hydrophilicities and carboxyl-terminal structures were compared with bovine endothelial cells primary culture, which play an important role in identifying factors of uptake by AME at the BBB site [46]. The transferrin receptor (TfR) is the most recognized RMT for brain targeting. Zhang et al. prepared paclitaxel loaded polyphosphoester hybrid transferrin-modified micelles, which revealed the brain targeting efficacies with high glioblastoma activity and increased mean survival rate of rat bearing U87-MG glioma in-vivo and in- vitro [47]. Angiopep-2, derived from aprotinin, a low-density lipoprotein as a brain transport vector coupled with liposome, was evaluated for its barrier transport ability using the in-vitro model and in-situ brain perfusion. The parenchymal accumulation and transcytosis across the bovine brain barrier endothelial cell were 10-fold higher than of transferrin conjugation. After treatment with angiopep-2-immunoliposome formulation, the mean survival time was significantly increased in glioma tumor bearing rat [48, 49]. Nicotinic acetylcholine receptors (nAChRs) are widely expressed in BCECs. When binding AChRs with 16 amino acid CDX, loop-II of snake neurotoxin candoxin shows high binding affinity and selectivity as well. It can be facilitated barrier transport and being a promising approach for intracranial transport of the drug delivery when conjugated with micelles, significantly increases the mean survival time of intracranial-glioma bearing mice [50]. Cai L et al. described the cellular uptake of dendrigraft poly-L-lysine (DGL)- based siRNA and D peptide (Dp) functionalized nanoparticles specifically recognized by TfR receptor on the BBB endothelial, enhanced the penetration through the receptor mediated endocytosis and deposited in AD abrasion. in vitro BBB transcytosis model and in vivo fluorescence imaging studies demonstrated that T7 and Tef1 peptides were specifically targeted TfR receptor linked to DGL by acid-cleavable PEG which could quickly escape from endo/lysosomal systems, acquiring effective transcytosis of nanoparticles to neurons in brain parenchyma in AD treatment [51]. Ruan S. et al. developed doxorubicin loaded DGL nanoparticles decorated with T7 peptide and P-aminophenyl-α-D-mannopyranoside (MAN), which undergo acid-responsive cleavage of T7 following detached of MAN decorated DOX-DGL escaped from reticulo endo/lysosomal system. Further exocytosis of DOX-DGL into the brain via GLUT transport located on the abluminal endothelial membrane, significantly enhanced the accumulation and targeting of doxorubicin at glioma tumor. In vivo fluorescence and Photo-aquatic imaging studies indicated that doxorubicin loaded DGL nanoparticles could precisely target glioma tumor after I.V. administration [52].
4.4. Molecular Packing
The transport of peptides like enkephalin, kyotorphin and thyrotropin releasing hormone (TRH) through brain barriers is an even more complex due to enzymatic hydrolysis by ubiquitous peptidases. These issues can be resolved by three approaches, which are (i) enhancing passive transport and by increasing lipophilicity, (ii) stabilized enzymatic activity to avoid premature degradation and (iii) lock-in mechanism to provide targeting delivery. These complex mechanisms are known as molecular packaging, where the peptide molecules act as bulky units and unrecognized by peptidases through which they penetrate directly inside the BBB. The brain targeted package delivery system contains different units of carrier specific molecules like redox targeter (T), a spacer molecule (S), a bulky lipophilic moiety, (L) conjugated to the ester bond via carboxyl terminal adjuster (A) to enhance lipid solubility and finally the peptide itself (P) tactically used amino acid to confirm timely removal of the charged target from the peptide [53, 54].
The first reported successful delivery of peptide package was Tyr-D-Ala-Gly-Phe-D-Leu (DADL), an analogue of leucine enkephalin, a naturally occurring pentapeptide that binds to the opioid receptor [55]. A similar kind of strategy was used for the delivery of TRH analogue to the CNS for the management of Alzheimer’s like neurodegenerative diseases [56].
4.5. Dual Targeting Drug Delivery Approach
As described above, the brain barriers are yet the hindrance for the treatment of brain tumors, particularly for a low-grade brain tumor and invading piece of the high-grade brain tumors. Conventional targeting delivery systems were not efficient enough for the treatment of brain tumors as a result of a few reasons 1) Transportation of drugs across the brain barriers is very critical when brain tumor cells are constrained in one or more areas rather than spread through the brain; 2) Neurotoxicity and side effects of the drugs diffuse in the normal brain regions, which could be barely tolerant by the patient; 3) Resection of brain tumor cells is often confined and leads to tumor relapse. To conquer this problem, dual targeting drug delivery techniques were proposed. The dual targeting delivery systems could encourage the permeation of drugs across the brain barriers and then specific guiding of drugs to the brain tumor cells [57, 58].
Ruan S. et al. developed doxorubicin loaded gold nanoparticles functionalized angiopep-2 and acid-responsive PEG linker. In vivo glioma and tissue distribution studies indicate that Angiopep-2 mediated nanoparticles permeated through brain barriers targeting glioma [59]. Kumari S et al. developed dual targeting temozolamide loaded lactoferrin fucntionlized nanoparticles against brain tumors [138].
4.6. Disturbing the Blood-Brain-Barrier
In spite of recent advances for drug delivery to CNS, BBB permeation is still challenging for the treatment of various neurological disorders. This invasive strategy for enhanced drug delivery to the CNS involves the systemic administration of drugs in combination with transient BBB disruption (BBBD). The systemic administration of drugs could undergo cerebral endothelial with enhanced extravasation rate, leading to increased parenchymal drug administration. A number of techniques have been investigated that transient disrupt the BBB; though, many of them are physiologically interested but not acceptable due to their toxicity. These include an infusion of solvents such as dimethyl sulfoxide or ethanol and metal such as aluminium; X-irradiation; and the induction of pathological condition such as ischemia or hypoxia, hypercapnia and hypertension. Similar techniques are safer and involve systemic administration of anticonvulsant drugs Metrazol, which transiently increases the BBB permeation at the same time resulting in atypical seizures [60]. When concurrent administration pentobarbital minimizes the seizure while permitting BBB disruption [61]. The systemic administration of some antineoplastic drugs such as cisplatin, fluorouracil, etoposide and VP-16 could also be responsible for BBB disruption [62, 63].
4.6.1. Osmotic BBBD
Intracranial infusion of a mannitol or arabinose solution directly into an artery of the target area of the brain is the most applied clinical technique for achieving BBBD and localized drug delivery [64]. In previous studies, it was investigated that acute dehydration of endothelial cells after mannitol solution infusion through cerebral capillaries results in cell shrinkage, which broadens tight junctions. Following rehydration of normal plasma takes to complete restoration of the BBB in about 4 h following the treatment [65]. Glial tumors have compromised endothelial barrier due to altered production of the barrier-inducing factor. Brain drug delivery of cytotoxic drugs such as cisplatin conjunction with an osmotic opening may give promising signature over traditional chemotherapy [50]. Osmotic brain disruption has also been suggested for the delivery of adenoviral gene vector transfer to intracerebral tumors [66]. MRI based diagnosis of brain diseases using iron oxide nanoparticle conjugates thought here are complications which must be overcome before the tedious clinical uses [66, 67]. Osmotic BBB disruption has also been used for the delivery of macromolecules such as monoclonal antibodies, viruses and nanoparticles [68, 69]. However, this process breaks down the self-defence mechanism of the brain and increases the susceptibility to infection or damage from all circulating toxins and chemicals. The risk includes alteration in glucose uptake, heat shock protein expression, permeation of plasma proteins, neuronal dysfunction and micro embolism [70].
4.6.2. Biochemical BBBD
The biochemical techniques are safe and selectively disrupt the intratumorally BBB without or minimal altering the normal BBB. Intracranial infusion of leukotriene C4 was accomplished by the selective opening of brain tumor capillaries without alteration of adjacent BBB [71]. In-vivo studies also demonstrated that infusion of histamine, bradykinin and synthetic bradykinin analogue Receptor mediate permeabilizer–7 (RMP-7), also selectively opens the BBB. Although, the biochemical mechanism is yet not elucidated, it has been recognized that the effect of RMP-7 is specifically mediated by bradykinin B2 receptor. Moreover, the mean survival time in glioma tumor bearing rats and brain drug delivery have shown enriched with RMP-7 [72]. These positive impacts are so promising to be used in clinical trials of anti-tumor drugs. The abundance of g-glutamyl transpeptidase (g-GTP) in normal brain capillaries was also related to the biochemical mechanism. The expression of the g-GTP enzyme requires glial inductive influence, which down-regulates in tumors, resulting in a reduction of enzymatic barriers in tumors [23].
4.6.3. Ultrasound BBBD
A newer Focused Ultrasound (FUS) strategy can offer reversible BBB disruption and enhanced permeability by focusing acoustic energy to a deep focal point, which could be used for target specific brain drug delivery. Gas containing microbubbles in combination with FUS improve the BBB disruption and less damage to periphery brain tissues. Gas microbubbles have about less than 50 µm in diameter containing a lipidic or galactose shell with gas core, when they are introduced with ultrasound serve as cavitation nuclei to focus and transform acoustic energy into mechanical energy [73]. The microbubbles not only restrict an acoustic effect to the vascular system but also decrease the energy applied to the exposed BBB and aid ultrasound to be imposed through the intact skull, as a result, the BBB disrupted temporally for drug permeation [74]. When a combination of FUS with a diagnostic system such as MRI was approached, it has been employed in anatomical targeting of therapeutics and monitoring regional temperature elevated by ultrasound [36, 75].
The FUS strategy has been utilized to enhance the brain delivery of drugs and genes by nanotechnology approaches [76]. Etame AB. et al. [77] evaluated the contract based imaging of brain tumors using gold-NPs. The delivery of gold-NPs in the brain hemisphere while only one hemisphere was tackled with ultrasound. The accumulation of gold-NPs in the sonicated hemisphere was observed 3.36-fold higher than the normal hemisphere. Liposomes encapsulated with doxorubicin enhanced the median survival time of the brain tumor bearing mice about 27 days and 35 days with and without FUS, respectively [78]. Accumulation of brain tumor targeting magnetic SPIONs loaded with doxorubicin was enhanced 7.6 times higher when combining microbubble-FUS treatment [79].
5. RECENT ADVANCEMENTS USING BDDS IN NANONEUROTHERAPEUTICS
5.1. Signature Targeted Delivery Systems
Following points exhibit the progress towards novel nanoneurotherapeutics:
Development of Non-Viral Nanoparticles for targeted brain delivery.
Exosomes based targeted delivery.
Using Brain Permeability Enhancers.
Targeted Delivery Through Active Transporters via the BBB.
Targeted Delivery Through Permeable BBB site in Pathologic Conditions.
Non-Invasive Techniques to enhance drug uptake in the brain via the BBB.
Use of Nano formulations for Particular Diagnostics & Brain Imaging.
Ultra sound guided (USG) nanocarrier drug delivery directly to the site.
Use of Hybrid Nanogels for compact prompt targeted brain delivery.
Peptide mediated drug delivery across the brain barriers.
The brain is a complex entity and covered with specific barriers for transportation into it. Hence, it is exceptionally selective to target the drugs into the brain for meticulous brain disease. Brain targeted novel drug delivery systems are potentially difficult due to multiple anatomical and pathological barriers of the brain and natural protective mechanism of the brain. Recent advances in nanotechnology present opportunities to defeat such boundaries and to transport the drug to the brain. Nano pharmacotherapy and nano drug delivery are the comparatively novel area that engaged “Nanomaterial with more surface area and increased therapeutic potency” with unique physicochemical as well as therapeutic qualities due to their diminutive size. (Fig. 3) shows differentiated approaches for conventional and novel targeted CNS drug deliveries in neuropathology. Viral vectors have a natural ability to infect cells with nucleic acids. The applications of viral vectors for gene targeted to patients associated with neurological disorders have been investigated for two decades. Targeted nanotherapeutics has shown improved embattled delivery with a variety of routes of administration for a variety of neurological complications. Lentivirus, herpes simplex virus, adenovirus and adeno-associated virus (AAV) vectors have achieved gene transduction in the brain [80]. Due to high gene transfection efficiency, the nanocarriers can be targeted with the greater release of drugs in specific neuropathologies like chronic neuroinflammation, epilepsy, narcolepsy and more [81]. Due to the potential ability to cross the BBB, exosomes are the novel nanocarriers for targeted brain delivery [82]. Glatiramer Acetate, as a nanocarrier, is being used for multiple sclerosis treatment [83]. Paclitaxel covalently linked to SLN is being used for Glioblastoma treatment as a nanocarrier in progressive brain cancer treatment [80]. An Amino silane-coated superparamagnetic iron oxide (15 nm) nanoparticle is being used for local or typical glioblastoma treatment directly into tumor injection (Intra tumoral Injection) [81]. Morphine sulphate nanocrystals are used as a psycho stimulant through the oral route. Amphotericin B liposome is being used to treat cryptococcal meningitis nowadays and it is potentially useful for better treatment and outcome than other alternative therapeutics [84]. Dexmethylphenidate HCl nanocrystals and Methylphenidate HCl nanocrystals are used for the potential treatment for the ADHD and also taken orally [85]. These nanomedicines have shown a prompt change in the therapeutic efficacy of ADHD. Paliperidone nanocarriers are used as the positive psychotherapy of schizophrenia. Cationic liposomes with anti-transferrin antibody have shown significant advances and potent efficacy in Phase-II trials of glioblastoma treatment. Silica nanoparticles with a fluorophore PEG-coated nanocarrier for brain imaging diagnostics are the Phase-II clinical trials [86]. Daunorubicin liposome for paediatric brain tumors is in clinical trial Phase-I [87]. In addition, nanoparticles are functionalized amid ligands or have unambiguous surfaces to elicit receptor-mediated transcytosis otherwise carrier-mediated transport, which conveys drugs across the blood-brain barrier. The affection of ligands similar to lactoferrin, transferrin, insulin facilitated receptor-mediated convey [88]. Cationized ligands and peptides similar to albumin cross through receptor-mediated absorptive transfer [89]. Nanoparticles plane can be tailored to exploit active transport arrangement comprising multidrug-resistant proteins, nucleoside transporter, P-glycoproteins, ionic transporter, L-transporters to facilitate shifting of the molecules which has keen on the brain by overwhelming adenosine triphosphate (ATP) [90, 91]. The smaller particle size contains a large surface area and requires a lower dosage to produce more effective drug efficacy. Various drug loaded nano-compositions are being used as therapeutics in many neurological and psychological brain pathologies. The nano formulations are being selected according to the drug bio-availability and possible neuropathology. In recent years, various nano compositions have been administered by different routes of administration for potential desired effects in particular targeted diseases. It is not only beneficial but also reduces the cost of therapeutics. Polylactide-co-glycolide (PLGA) nanoparticles of oestradiol are nowadays used for Alzheimer’s disease treatment [91]. Chitosan nanoparticle loaded Diadanosinedideoxyinosine (dd), Rivastigmine and Venlafaxine nasal sprays are also used as the targeted treatment for Alzheimer’s disease [89, 92, 93]. Intra nasal preparation of venlafaxine with chitosan loaded nanoparticles is used in depression, major depression and anxiety like cognitive decline therapeutics [94]. Chitosan nanoparticle loaded bromocriptine intra nasal preparation is used as the effective therapeutic treatment of Parkinson’s disease [95]. Sumatriptan and Zolmitriptan’s micellar nanocarriers with less than 20 nm particle size are used in migraine therapy with precise targeting [96]. Moreover, nanotherapeutics has developed remarkable achievements as potential therapeutics towards the fatal life-threatening neurological complications like epilepsy, cerebrovascular stroke, severe to moderate Alzheimer’s disease, progressive glioma and glioblastoma, malignant glioma, intra cranial glioblastoma, glial-glial neuroinflammation and subarachnoid haemorrhages. Intravenous valproic acid nanoparticles are used for re-current and atypical epileptic attacks’ treatment [97]. PEG modified cellulose loaded cabazitaxel nano particles (24-68 nm) and Peptide modified Cationic loaded docetaxel liposomes are used as a straight intravenous and intra-tumor targeted delivery for the glioma and glioblastoma treatment [98-100]. Nanoparticles of PEG-Chitosan loaded OX-26 antibody (Monoclonal antibody (OX26)) are a signature novel targeted therapeutics in recurrent cerebral ischemia or stroke [101]. Moreover, Ultrasound guided FE3O4/SPAnH nanoparticles for malignant glioma are the targeted drug therapeutics and signature novel advancements of nanotherapeutics [102]. PLGA loaded dexamethasone nanoparticles in alginate hydrogels are the novel nano-formulations for the targeted delivery in glial-glial neuroinflammation, any brain injury related neuroinflammation through the neural probes is the signature achievement of neuro nano drug therapeutics [103].
5.2. Unconventional Targeted Nano Neuro Delivery Advancements
Straight delivery (conventional enhanced delivery (CED), of a therapeutic agent to the target brain barriers, can be bypassed by injecting the drug straight into the tissues using a catheter [104]. Many pre-clinical studies tailored CED to impart nano-formulations straight into the brain for advanced drug delivery and therapeutics. Directly, administering the drug into the carotid artery provides an unconventional key to undeviating delivery. This straight systemic delivery requires a catheter which injects drugs into the bloodstream. In previous reports, the efficacy of direct systemic delivery was reported almost twice to that of CED in terms of brain damage [105]. Tumor targeted in-vivo drug therapeutic approaches are an enhanced mode of drug delivery and therapeutics. Polylactic acid (PLA) and poly-diethylaminomethyl methacrylate (PDMAEMA) were used to synthesize amphiphilic star-branched co-polymeric nanoparticles for intra-tumor delivery of the drugs for treating brain tumors [106]. This kind of novel tumor targeted approach is the advanced route of glioma, glioblastoma or malignant glioma like brain tumor targeted drug therapeutics. Intrathecal deliveries of nano carriers or nano formulations are most commonly used for the delivery in the CSF for aesthetes and neurotic pain [107, 108]. Although this route is still in the experimental phase, it gives promising signs of a better therapeutic approach and better therapeutic scaffolds. Table (1) summarises the key investigations were done using nanocarriers in CNS specific delivery.
Table 1. List of key investigations for the use of nanocarriers in CNS specific delivery.
| Carrier System | Polymeric Materials | Drug(s) Delivered | Major Findings and Comments | Refs. |
|---|---|---|---|---|
| Polymeric nanoparticle | PBCA | Methotrexate | Smaller sized nanoparticles increased the drug concentration in the brain and CSF. | [109] |
| - | - | Temozolomide | Tween-80 coated PBCA enhances BBB uptake of temozolomide both in vitro and in vivo. | [110] |
| - | MMA-SPM; PCBA | Lamivudine, zidovudine | Enhanced BBB permeability of Lamivudine and Zidovudine by 10 and 20 times respectively. | [111] |
| - | PLGA/ alginate | Dexamethasone | Extended drug release up to 2 weeks and reduced inflammation in glial cells. | [112] |
| - | PLA/ PEG | Vasoactive intestinalPeptide | Drug level in the brain increased by 5.6 to 7.7-fold. | [113] |
| - | PLGA | SuperoxideDismutase | Reduced infarct volume up to 65%, regain vital neurological functions in ischemic perfused rat. | [114] |
| - | PLA-PEG blocks polymer coated with Tween-80 | Amphotericin B | Increases the permeability across the BBB in mice brain. | [115] |
| - | PLGA, Surface modification with the peptide | Loperamide | Effective carrier of Loperamide for brain targeting when administered intravenously. | [116] |
| - | Magnetic iron oxide nanoparticles coated with gum Arabic | Iron oxide | Effective in intracellular drug delivery and greater ability to tumor targeting. | [117] |
| - | Chitosan | Oestradiol | Prominent blood and CSF concentration after intranasal administration. | [118] |
| - | PLA-PEG co-polymer with acylation | Nimodipine | Enhanced concentrations in blood, cerebrospinal fluid and brain tissues by direct nose-brain transport. | [119] |
| Solid lipid nanoparticle(SLN) | Soya phosphatidyl-choline 95% | Doxorubicin | Stearic acid associated PEGylation of nanoparticles improves in brain accumulation and circulation time. | [120] |
| - | Stearic acid/ soy beanLecithin | Camptothecin | Highest drug concentration observed in the brain when orally administered, and release drug up to a week. | [121] |
| - | Stearyl amine | Paclitaxel | Higher brain drug concentration and serum concentration increases 3 times when administered orally. | [122] |
| - | Magnetic silica-Pluronic F-127 | Doxorubicin with Transferrin | Enhanced drug permeability across the BBB and satisfactory efficacy was achieved against glioblastoma. | [123] |
| Lipid nanocapsule | Triglycerides of capric and caprylic acids, Solution | Indomethacin | Lipid nanocapsule formulation of indomethacin showed lower LD 50 in C6, and U318-MG glioma cell lines. | [124] |
| - | - | Paclitaxel and CpG-ODN | Immunopotency of CpG-ODN and anti-tumor activity of Paclitaxel was enhanced by tumor microenvironment delivery in glioblastoma. | [125] |
| Liposome | Phospholipids andCholesterol | curcumin | Reduced epilepsy by local action reduced pentylenetetrazol induced epilepsy. | [126] |
| - | - | Cisplatin | Increased accumulation of the drug in intracranial glioma and enhanced anticancer effect. | [127] |
| - | Low density lipoprotein with T7 peptide | Vincristine | Localization of vincristine in glioma enhanced the anti-glioma efficacy in-vivo and in-vitro | [128] |
| Micelle | Pluronic P85 | Biphalin, Enkephalin, Morphine | P85 enhanced their analgesic profile both above and below the critical micelle concentration. | [129] |
| - | D-Retro enantiomer of Quorum-Sensing Peptide | Paclitaxel | QS-peptide conjugation enhanced glioma tumor targeting of paclitaxel exhibited longer median survival time. | [130] |
| - | Labrasol-Pluronic F68 | Myricetin & Quercetin | Oral administration of nano-micelle loaded with flavonoids enhanced anti-tumor activity in vivo with no significant effect of cytotoxicity to Caco-2 cells. | [131] |
| Dendrimer | PEGOL-60 | - | PEGOL-60 transient impaired CNS barrier and stated influential anti-inflammatory and anti-oxidant activity in inflamed microglia in-vitro. | [132] |
| - | PAMAM | Streptavidin | Peptide conjugated dendrimers uptake by transcytosis process to astrocytes and neurons showed neuroprotective activity. | [133] |
| - | Folic acid-PAMAM | Doxorubicin | Increased the BBB permeation of Doxorubicin and median survival time of xenografted brain tumor in rats. | [134] |
5.3. Nanomaterials Modification for Brain Tumor Theranostics
Glioblastoma multiforme [GBM] a primary brain tumor arising from the most aggressive astrocytic cells to form intracranial malignancies. At the initial stages of tumors, cells depend on the normal brain barrier to supply nutrients and get benefits from the trans-endothelial electrical resistance (TEER), which restricts the drug transport. Though in advances of malignancies, tumor cells kill the normal tissue, and neo-vasculatures form continuous fenestrate microvascular tolerate molecules less than 12 nm to pass through [135]. The current therapy and detection of glioblastoma smuggle with a significant reduction in the death rate. The tumor heterogenicity, diagnosis failure in the primary stage and vague drug delivery are the accountable reasons for inefficient treatment. The combination of targeting therapy and earlier diagnosis procedures defined as theranostics, has been established as a novel strategy to improve glioblastoma treatment. Hence, the strategies for targeting therapeutic agents inside the brain mostly involve functionalized dual targeting, a single multimodal nanoparticle system gives promising signs for tumor specificity and neovasculature [136]. Table (2) enumerates the modification of nanomaterials for brain tumor theranostics.
Table 2. Modifications of nanomaterials for brain tumor theranostics.
| Nanomaterial | Modifications for Glioma Theranostics | Refs. |
|---|---|---|
| Polymericnanoparticles | Coated with IL-13 peptide binding with glioma specific IL13Ra-2 receptor and RGD peptide binding with avb3 which is overexpressed on neovascular endothelial cells. | [137] |
| Coated with lactoferrin (lactoferrin receptor is overexpressed in brain endothelial cells and glioma cells) for delivery of temozolomide. | [138] | |
| Coated with peptide-22 (special binding efficiency with LDL receptor highly expressed at the BBB) for exposure of tumor microenvironment. | [139] | |
| Magnetic nanoparticles | USPIOs conjugated with HIV-Tat peptide and dextran to facilitate crossing the BBB and enhance biocompatibility and glioma specificity. | [140] |
| Conjugating USPIOs with scorpion venom derived peptide Chlorotoxin to glioma specificity and diagnosis. | [141] | |
| SPIONs conjugated with Lactoferrin for specific MRI contrast agent for glioma diagnosis in-vivo. | [142] | |
| Liposomes | Liposomes Air-filled bubbles are efficient reflectors of sound. Lipid coated micro-bubbles (liposomes) have been used as contrast agents for the rapid detection of tumors through neuro-sonography. | [143] |
| Quantum dots | QDs were incorporated within the core of PEG-PLA nanoparticles conjugated with Lectin protein to enhance uptake by lymphocytes and aid in transport across the BBB for contrast-based glioma imaging. | [144] |
| - | CdTe QDs conjugated with transferrin since tumor cells highly overexpressed transferrin receptor for targeting glioma theranostics. | [145] |
| Goldnanoparticles | Exploiting the natural tendency of elements with a higher atomic number to efficiently absorb X-rays, GNPs have been used as contrast agents for glioma imaging. | [146] |
CONCLUSION
Brain pathology embattled drug delivery is a tricky matter because of anatomic and pathophysiological brain obstacles (all brain barriers). The existing advances in nanotechnology endow with an elucidation in the form of nanopharmaceuticals, drug containing nanocarriers towards brain pathology targeted drug delivery. Nano drug delivery or nanomedicine to the brain has proven the possible feasible, effective therapeutics with fewer side effects. This makes it more significant for future aspects. The idea for delivering the drug directly into the brain with less drug concentration and maximum therapeutic effect and less side effects impacts it as future medicine. Moreover, the drugs which cannot target directly to the brain by any routes of administration, such drugs can target with the help of different nanocarriers like polymeric nanoparticles, solid lipid nanoparticles, liposomes, dendrimers, micelles, nanoemulsions, nanogels, quantum dots, etc. and can take the help of current advanced unconventional delivery systems like ultrasound guided or radio imaging guided drug delivery to the pathological site, ligand based active targeting, advances of biotechnology and sophisticated imaging techniques to identify novel targets, fusion of proteins and peptides, a combination approach of intranasal administration and diseased brain cell targeting, etc. In this review, we have tried to describe the obstacles to brain delivery, advancements in brain targeted nanomedicine and its broad scope as promising future targeted brain delivery for the treatment of complex neuropathologies. The recent advancements of brain delivery via nano-neuro targeted brain drug delivery give us a great hope to fight against the complex life-threatening neuropathologies like AD, PD, vascular dementia, cognitive decline and psychiatric complications which cannot be easily overcome or treated via conventional drug delivery systems by focusing and overcoming various challenges like physiological factors of the brain, toxicity nature of the system, drug concentration and its release profile, biocompatibility, stability issues etc., which may lead to successful strategies after recapitulating the success of matching of preclinical with clinical studies.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Omidi Y., Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bioimpacts. 2012;2(1):5–22. doi: 10.5681/bi.2012.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayant R.D., Tiwari S., Atluri V., Kaushik A., Tomitaka A., Yndart A., Colon-Perez L., Febo M., Nair M. Multifunctional Nanotherapeutics for the treatment of neuroAIDS in drug abusers. Sci. Rep. 2018;8(1):12991. doi: 10.1038/s41598-018-31285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahajan S.D., Aalinkeel R., Law W.C., Reynolds J.L., Nair B.B., Sykes D.E., Yong K.T., Roy I., Prasad P.N., Schwartz S.A. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int. J. Nanomed. 2012;7(10):5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwibhashyam V.S., Nagappa A.N. Strategies for enhanced drug delivery to the central nervous system. Indian J. Pharm. Sci. 2008;70(2):145–153. doi: 10.4103/0250-474X.41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srikanth M., Kessler J.A. Nanotechnology-novel therapeutics for CNS disorders. Nat. Rev. Neurol. 2012;8(6):307–318.5. doi: 10.1038/nrneurol.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra A.K., Agrahari V., Mandal A., Cholkar K., Natarajan C., Shah S., Joseph M., Trinh H.M., Vaishya R., Yang X., Hao Y., Khurana V., Pal D. Novel delivery approaches for cancer therapeutics. J. Control. Release. 2015;219:248–268. doi: 10.1016/j.jconrel.2015.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali I.U., Chen X. Penetrating blood-brain barrier: promise of novel nanoplatforms and delivery vehicles. ACS Nano. 2015;9(10):9470–9474. doi: 10.1021/acsnano.5b05341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prajapati J., Patel H., Agrawal Y.K. Targeted drug delivery for central nervous system: a review. Int. J. Pharm. Pharm. Sci. 2012;4(3):32–38. [Google Scholar]
- 10.Wong H.L., Wu X.Y., Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012;64(7):686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017;18(2):123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 12.Sharif Y., Jumah F., Coplan L., Krosser A., Sharif K., Tubbs R.S. Blood brain barrier: A review of its anatomy and physiology in health and disease. Clin. Anat. 2018;31(6):812–823. doi: 10.1002/ca.23083. [DOI] [PubMed] [Google Scholar]
- 13.Saunders N.R., Habgood M.D., Møllgård K., Dziegielewska K.M. The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system? F1000 Res. 2016;5:1–15. doi: 10.12688/f1000research.7378.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrlich P. The oxygen-need of the organism: a color-analytical study. Berlin: Hirschwald; 1885. pp. 1–85. [Google Scholar]
- 15.Nakhlband A., Omidi Y. Barrier functionality of porcine and bovine brain capillary endothelial cells. Bioimpacts. 2011;1(3):153–159. doi: 10.5681/bi.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omidi Y., Campbell L., Barar J., Connell D., Akhtar S., Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 2003;990(1-2):95–112. doi: 10.1016/S0006-8993(03)03443-7. [DOI] [PubMed] [Google Scholar]
- 17.Smith M., Omidi Y., Gumbleton M. Primary porcine brain microvascular endothelial cells: biochemical and functional characterisation as a model for drug transport and targeting. J. Drug Target. 2007;15(4):253–268. doi: 10.1080/10611860701288539. [DOI] [PubMed] [Google Scholar]
- 18.Krol S. Challenges in drug delivery to the brain: nature is against us. J. Control. Release. 2012;164(2):145–155. doi: 10.1016/j.jconrel.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Jain S., Mishra V., Singh P., Dubey P.K., Saraf D.K., Vyas S.P. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int. J. Pharm. 2003;261(1-2):43–55. doi: 10.1016/S0378-5173(03)00269-2. [DOI] [PubMed] [Google Scholar]
- 20.Das S., Carnicer-Lombarte A., Fawcett J.W., Bora U. Bio-inspired nano tools for neuroscience. Prog. Neurobiol. 2016;142:1–22. doi: 10.1016/j.pneurobio.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Alam M.I., Beg S., Samad A., Baboota S., Kohli K., Ali J., Ahuja A., Akbar M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010;40(5):385–403. doi: 10.1016/j.ejps.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Pardridge W.M. Non-invasive drug delivery to the human brain using endogenous blood-brain barrier transport systems. Pharm. Sci. Technol. Today. 1999;2(2):49–59. doi: 10.1016/S1461-5347(98)00117-5. [DOI] [PubMed] [Google Scholar]
- 24.de Boer A.G., Gaillard P.J. Strategies to improve drug delivery across the blood-brain barrier. Clin. Pharmacokinet. 2007;46(7):553–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- 25.Nabeshima S., Reese T.S., Landis D.M.D., Brightman M.W. Junctions in the meninges and marginal glia. J. Comp. Neurol. 1975;164(2):127–169. doi: 10.1002/cne.901640202. [DOI] [PubMed] [Google Scholar]
- 26.de Lange E.C.M. Potential role of ABC transporters as a detoxification system at the blood-CSF barrier. Adv. Drug Deliv. Rev. 2004;56(12):1793–1809. doi: 10.1016/j.addr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y., Wright E.M. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J. Physiol. 1983;336(336):635–648. doi: 10.1113/jphysiol.1983.sp014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra A., Ganesh S., Shahiwala A., Shah S.P. Drug delivery to the central nervous system: a review. J. Pharm. Pharm. Sci. 2003;6(2):252–273. [PubMed] [Google Scholar]
- 29.Singh S.B. Novel Approaches for Brain Drug Delivery System-Review. Int J pharma Res Rev. 2013;2(6):36–44. [Google Scholar]
- 30.Hardee M.E., Zagzag D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 2012;181(4):1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei X., Chen X., Ying M., Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm. Sin. B. 2014;4(3):193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Tellingen O., Yetkin-Arik B., de Gooijer M.C., Wesseling P., Wurdinger T., de Vries H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Gao H., Yang Z., Cao S., Xiong Y., Zhang S., Pang Z., Jiang X. Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment. Biomaterials. 2014;35(7):2374–2382. doi: 10.1016/j.biomaterials.2013.11.076. [DOI] [PubMed] [Google Scholar]
- 34.Erdő F., Bors L.A., Farkas D., Bajza Á., Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Nair A.B., Shah J., Chavda V., Shah H., Patel S. Polysaccharide-based nano-biocarrier in drug delivery. Vol. 1. Goyal T, Ghosh B Ed.; CRC Press, Taylor and Francis Group, Florida; 2018. Delivery of biomolecules to the central nervous system using a polysaccharide nanocomposite. pp. 105–128. [DOI] [Google Scholar]
- 36.van Rooy I., Cakir-Tascioglu S., Hennink W.E., Storm G., Schiffelers R.M., Mastrobattista E. in vivo methods to study uptake of nanoparticles into the brain. Pharm. Res. 2011;28(3):456–471. doi: 10.1007/s11095-010-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulvihill J.J., Cunnane E.M., Ross A.M., Duskey J.T., Tosi G., Grabrucker A.M. Drug delivery across the blood-brain barrier: recent advances in the use of nanocarriers. Nanomedicine (Lond.) 2020;15(2):205–214. doi: 10.2217/nnm-2019-0367. [DOI] [PubMed] [Google Scholar]
- 38.Patel C.N., Kumar S.P., Rawal R.M., Patel D.P., Gonzalez F.J., Pandya H.A. A multiparametric organ toxicity predictor for drug discovery. Toxicol. Mech. Methods. 2020;30(3):159–166. doi: 10.1080/15376516.2019.1681044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng L., Zhang Y., Ma L., Jing X., Ke X., Lian J., Zhao Q., Yan B., Zhang J., Yao J., Chen J. Comparison of anti-EGFR-Fab’ conjugated immunoliposomes modified with two different conjugation linkers for siRNa delivery in SMMC-7721 cells. Int. J. Nanomedicine. 2013;8:3271–3283. doi: 10.2147/IJN.S47597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Z., Lin Y., Zhang X., Feng C., Lu Y., Gao Y., Dong C. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: enhanced cellular uptake and improved therapeutic effects. Int. J. Nanomedicine. 2017;12:1941–1958. doi: 10.2147/IJN.S125573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu C.T., Zhao Y.Z., Wong H.L., Cai J., Peng L., Tian X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomedicine. 2014;9(1):2241–2257. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikitsh J.L., Chacko A.M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Medicin. Chem. 2014;6(6):11–24. doi: 10.4137/PMC.S13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pardridge W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020;11:373. doi: 10.3389/fnagi.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Q., Jiang C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm. Sin. B. 2020;10(6):979–986. doi: 10.1016/j.apsb.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galla H.J. Monocultures of primary porcine brain capillary endothelial cells: Still a functional in vitro model for the blood- brain-barrier. J. Control. Release. 2018;285:172–177. doi: 10.1016/j.jconrel.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X., Jin K., Huang Y., Pang Z. In: Brain Targeted Drug Delivery System. Gao H., Gao X., editors. Vol. 1. China: Elsevier; 2018. Brain drug delivery by adsorption-mediated transcytosis. pp. 159–183. [Google Scholar]
- 47.Zhang P., Hu L., Yin Q., Zhang Z., Feng L., Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J. Control. Release. 2012;159(3):429–434. doi: 10.1016/j.jconrel.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Papademetriou I., Vedula E., Charest J., Porter T. Effect of flow on targeting and penetration of angiopep-decorated nanoparticles in a microfluidic model blood-brain barrier. PLos One. 2018;13(10):e0205158. doi: 10.1371/journal.pone.0205158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu F., Pang Z., Zhao J., Jin K., Li H., Pang Q., Zhang L., Pang Z. Angiopep-2-conjugated poly(ethylene glycol)-co- poly(ε-caprolactone) polymersomes for dual-targeting drug delivery to glioma in rats. Int. J. Nanomed. 2017;12:2117–2127. doi: 10.2147/IJN.S123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan C., Li B., Hu L., Wei X., Feng L., Fu W., Lu W. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew. Chem. Int. Ed. Engl. 2011;50(24):5482–5485. doi: 10.1002/anie.201100875. [DOI] [PubMed] [Google Scholar]
- 51.Cai L., et al. Endo/lysosome-escapable delivery depot for improving BBB transcytosis and neuron targeted therapy of Alzheimer’s disease. Adv. Funct. Mater. 2018;28:802227. [Google Scholar]
- 52.Ruan S., et al. Acid-responsive transferrin dissociation and GLUT mediated exocytosis for increased blood-brain barrier transcytosis and programmed glioma targeting therapy. Adv. Funct. Mater. 2020:190999. [Google Scholar]
- 53.Lalatsa A., Schatzlein A.G., Uchegbu I.F. Strategies to deliver peptide drugs to the brain. Mol. Pharm. 2014;11(4):1081–1093. doi: 10.1021/mp400680d. [DOI] [PubMed] [Google Scholar]
- 54.Biabanikhankahdani R., Alitheen N.B.M., Ho K.L., Tan W.S. PH-responsive virus-like nanoparticles with enhanced tumour-targeting ligands for cancer drug delivery. Sci. Rep. 2016;6(1):37891. doi: 10.1038/srep37891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tóth F., Farkas J., Tóth G., Wollemann M., Borsodi A., Benyhe S. Synthesis and binding characteristics of a novel enkephalin analogue, [3H]Tyr-D-Ala-Gly-Phe-D-Nle-Arg-Phe. Peptides. 2003;24(9):1433–1440. doi: 10.1016/j.peptides.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Remesic M., Lee Y.S., Hruby V.J. Cyclic opioid peptides. Curr. Med. Chem. 2016;23(13):1288–1303. doi: 10.2174/0929867323666160427123005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao H., et al. Ligand modified nanoparticles increses cell uptake, alter endocytosis and elevates glioma distrubution and internalization. Sci. Rep. 2013;3:25–34. doi: 10.1038/srep02534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao H. Perspectives on dual targeting delivery systems for brain tumors. J. Neuroimmune Pharmacol. 2017;12(1):6–16. doi: 10.1007/s11481-016-9687-4. [DOI] [PubMed] [Google Scholar]
- 59.Ruan S., Yuan M., Zhang L., Hu G., Chen J., Cun X., Zhang Q., Yang Y., He Q., Gao H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–435. doi: 10.1016/j.biomaterials.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Greig N., Hellmann K. Enhanced cerebrovascular permeability by Metrazol: significance for brain metastases. Clin. Exp. Metastasis. 1983;1(1):83–95. doi: 10.1007/BF00118475. [DOI] [PubMed] [Google Scholar]
- 61.Chi O.Z., Mellender S.J., Kiss G.K., Liu X., Weiss H.R. Blood -brain barrier disruption was less under isoflurane than pentobarbital anesthesia via a PI3K/Akt pathway in early cerebral ischemia. Brain Res. Bull. 2017;131:1–6. doi: 10.1016/j.brainresbull.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Fortin D., Desjardins A., Benko A., Niyonsega T., Boudrias M. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience. Cancer. 2005;103(12):2606–2615. doi: 10.1002/cncr.21112. [DOI] [PubMed] [Google Scholar]
- 63.Mainprize T., Lipsman N., Huang Y., Meng Y., Bethune A., Ironside S., Heyn C., Alkins R., Trudeau M., Sahgal A., Perry J., Hynynen K. Blood-brain barrier opening in primary brain tumors with non-invasive mr-guided focused ultrasound: a clinical safety and feasibility study. Sci. Rep. 2019;9(1):321. doi: 10.1038/s41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez A., Tatter S.B., Debinski W. Neurosurgical techniques for disruption of the blood–brain barrier for glioblastoma treatment. Pharmaceutics. 2015;7(3):175–187. doi: 10.3390/pharmaceutics7030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro M.G., Candolfi M., Wilson T.J., Calinescu A., Paran C., Kamran N., Koschmann C., Moreno-Ayala M.A., Assi H., Lowenstein P.R. Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opin. Biol. Ther. 2014;14(9):1241–1257. doi: 10.1517/14712598.2014.915307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu C., Liu G., Janowski M., Bulte J.W.M., Li S., Pearl M., Walczak P. Real-time mri guidance for reproducible hyperosmolar opening of the blood-brain barrier in mice. Front. Neurol. 2018;9:921. doi: 10.3389/fneur.2018.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.On N.H., Kiptoo P., Siahaan T.J., Miller D.W. Modulation of blood-brain barrier permeability in mice using synthetic E-cadherin peptide. Mol. Pharm. 2014;11(3):974–981. doi: 10.1021/mp400624v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavaco M., Gaspar D., Arb Castanho M., Neves V. Antibodies for the treatment of brain metastases, a dream or a reality? Pharmaceutics. 2020;12(1):62. doi: 10.3390/pharmaceutics12010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hersh D.S., Wadajkar A.S., Roberts N., Perez J.G., Connolly N.P., Frenkel V., Winkles J.A., Woodworth G.F., Kim A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016;22(9):1177–1193. doi: 10.2174/1381612822666151221150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gelosa P., Colazzo F., Tremoli E., Sironi L., Castiglioni L. Cysteinyl leukotrienes as potential pharmacological targets for cerebral diseases. Mediators Inflamm. 2017;2017:3454212. doi: 10.1155/2017/3454212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emerich D.F., Dean R.L., Osborn C., Bartus R.T. The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier: from concept to clinical evaluation. Clin. Pharmacokinet. 2001;40(2):105–123. doi: 10.2165/00003088-200140020-00003. [DOI] [PubMed] [Google Scholar]
- 72.Zhang F., Xu C.L., Liu C.M. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des. Devel. Ther. 2015;9:2089–2100. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nainwal N. Recent advances in transcranial focused ultrasound (FUS) triggered brain delivery. Curr. Drug Targets. 2017;18(11):1225–1232. doi: 10.2174/1389450117666161222160025. [DOI] [PubMed] [Google Scholar]
- 74.Le Floc’h J., Lu H.D., Lim T.L., Démoré C., Prud’homme R.K., Hynynen K., Foster F.S. Transcranial photoacoustic detection of blood-brain barrier disruption following focused ultrasound-mediated nanoparticle delivery. Mol. Imaging Biol. 2020;22(2):324–334. doi: 10.1007/s11307-019-01397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovacs Z.I., Kim S., Jikaria N., Qureshi F., Milo B., Lewis B.K., Bresler M., Burks S.R., Frank J.A. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA. 2017;114(1):E75–E84. doi: 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canavese G., Ancona A., Racca L., Canta M., Dumontel B., Barbaresco F., Limongi T., Cauda V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018;340:155–172. doi: 10.1016/j.cej.2018.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Etame A.B., Diaz R.J., O’Reilly M.A., Smith C.A., Mainprize T.G., Hynynen K., Rutka J.T. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine (Lond.) 2012;8(7):1133–1142. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aryal M., Vykhodtseva N., Zhang Y.Z., Park J., McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release. 2013;169(1-2):103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan C.H., Cheng Y.H., Ting C.Y., Ho Y.J., Hsu P.H., Liu H.L., Yeh C.K. Ultrasound/magnetic targeting with SPIO-DOX-microbubble complex for image-guided drug delivery in brain tumors. Theranostics. 2016;6(10):1542–1556. doi: 10.7150/thno.15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jayant R.D., Sosa D., Kaushik A., Atluri V., Vashist A., Tomitaka A., Nair M. Current status of non-viral gene therapy for CNS disorders. Expert Opin. Drug Deliv. 2016;13(10):1433–1445. doi: 10.1080/17425247.2016.1188802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cwetsch A.W., Pinto B., Savardi A., Cancedda L. in vivo methods for acute modulation of gene expression in the central nervous system. Prog. Neurobiol. 2018;168:69–85. doi: 10.1016/j.pneurobio.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang T., Martin P., Fogarty B., Brown A., Schurman K., Phipps R., Yin V.P., Lockman P., Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-based medicines: a review of FDA approved materials and clinical trials to date. Pharm. Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 84.Jadhav M.P., Bamba A., Shinde V.M., Gogtay N., Kshirsagar N.A., Bichile L.S., Mathai D., Sharma A., Varma S., Digumarathi R. Liposomal amphotericin B (Fungisome) for the treatment of cryptococcal meningitis in HIV/AIDS patients in India: a multicentric, randomized controlled trial. J. Postgrad. Med. 2010;56(2):71–75. doi: 10.4103/0022-3859.65276. [DOI] [PubMed] [Google Scholar]
- 85.Malamatari M., Taylor K.M.G., Malamataris S., Douroumis D., Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov. Today. 2018;23(3):534–547. doi: 10.1016/j.drudis.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 86.Gubala V., Giovannini G., Kunc F., et al. Dye-doped silica nanoparticles: synthesis, surface chemistry and bio applications. Cancer Nano; 2020. p. 11. [Google Scholar]
- 87.Lowis S., Lewis I., Elsworth A., Weston C., Doz F., Vassal G., Bellott R., Robert J., Pein F., Ablett S., Pinkerton R., Frappaz D. United Kingdom Children’s Cancer Study Group (UKCCSG) New Agents; Société Française d’Oncologie Pédiatrique (SFOP) Pharmacology Group. A phase I study of intravenous liposomal daunorubicin (DaunoXome) in paediatric patients with relapsed or resistant solid tumours. Br. J. Cancer. 2006;95(5):571–580. doi: 10.1038/sj.bjc.6603288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weissig V., Pettinger T.K., Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomedicine. 2014;9:4357–4373. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saraiva C., Praça C., Ferreira R., Santos T., Ferreira L., Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release. 2016;235(235):34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 90.Micheli M.R., Bova R., Magini A., Polidoro M., Emiliani C. Lipid-based nanocarriers for CNS-targeted drug delivery. Recent Patents CNS Drug Discov. 2012;7(1):71–86. doi: 10.2174/157488912798842241. [DOI] [PubMed] [Google Scholar]
- 91.Mittal G., Sahana D.K., Bhardwaj V., Ravi Kumar M.N.V. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 92.Fazil M., Md S., Haque S., Kumar M., Baboota S., Sahni J.K., Ali J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012;47(1):6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 93.Shah B., Khunt D., Misra M., Padh H. Formulation and in-vivo pharmacokinetic consideration of intranasal microemulsion and mucoadhesive microemulsion of rivastigmine for brain targeting. Pharm. Res. 2018;35(1):8. doi: 10.1007/s11095-017-2279-z. [DOI] [PubMed] [Google Scholar]
- 94.Haque S., Md S., Fazil M., Kumar M., Sahni J.K., Ali J., Baboota S. Venlafaxine loaded chitosan NPs for brain targeting: pharmacokinetic and pharmacodynamic evaluation. Carbohydr. Polym. 2012;89(1):72–79. doi: 10.1016/j.carbpol.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 95.Md S., Khan R.A., Mustafa G., Chuttani K., Baboota S., Sahni J.K., Ali J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013;48(3):393–405. doi: 10.1016/j.ejps.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Jain R., Nabar S., Dandekar P., Hassan P., Aswal V., Talmon Y., Shet T., Borde L., Ray K., Patravale V. Formulation and evaluation of novel micellar nanocarrier for nasal delivery of sumatriptan. Nanomedicine (Lond.) 2010;5(4):575–587. doi: 10.2217/nnm.10.28. [DOI] [PubMed] [Google Scholar]
- 97.Darius J., Meyer F.P., Sabel B.A., Schroeder U. Influence of nanoparticles on the brain-to-serum distribution and the metabolism of valproic acid in mice. J. Pharm. Pharmacol. 2000;52(9):1043–1047. doi: 10.1211/0022357001774958. [DOI] [PubMed] [Google Scholar]
- 98.Bteich J., Ernsting M.J., Mohammed M., Kiyota T., McKee T.D., Trikha M., Lowman H.B., Sokoll K.K. Nanoparticle formulation derived from carboxymethyl cellulose, polyethylene glycol, and cabazitaxel for chemotherapy delivery to the brain. Bioconjug. Chem. 2018;29(6):2009–2020. doi: 10.1021/acs.bioconjchem.8b00220. [DOI] [PubMed] [Google Scholar]
- 99.Jafari B., Pourseif M.M., Barar J., Rafi M.A., Omidi Y. Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors. Expert Opin. Drug Deliv. 2019;16(6):583–605. doi: 10.1080/17425247.2019.1614911. [DOI] [PubMed] [Google Scholar]
- 100.Yang Z.Z., Li J.Q., Wang Z.Z., Dong D.W., Qi X.R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35(19):5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 101.Aktaş Y., Yemisci M., Andrieux K., Gürsoy R.N., Alonso M.J., Fernandez-Megia E., Novoa-Carballal R., Quiñoá E., Riguera R., Sargon M.F., Çelik H.H., Demir A.S., Hincal A.A., Dalkara T., Capan Y., Couvreur P. Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug. Chem. 2005;16(6):1503–1511. doi: 10.1021/bc050217o. [DOI] [PubMed] [Google Scholar]
- 102.Silva A.C., Oliveira T.R., Mamani J.B., Malheiros S.M., Malavolta L., Pavon L.F., Sibov T.T., Amaro E., Jr, Tannús A., Vidoto E.L., Martins M.J., Santos R.S., Gamarra L.F. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int. J. Nanomedicine. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim D.H., Martin D.C. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27(15):3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 104.Allard E., Jarnet D., Vessières A., Vinchon-Petit S., Jaouen G., Benoit J.P., Passirani C. Local delivery of ferrociphenol lipid nanocapsules followed by external radiotherapy as a synergistic treatment against intracranial 9L glioma xenograft. Pharm. Res. 2010;27(1):56–64. doi: 10.1007/s11095-009-0006-0. [DOI] [PubMed] [Google Scholar]
- 105.Huynh N.T., Passirani C., Allard-Vannier E., Lemaire L., Roux J., Garcion E., Vessieres A., Benoit J.P. Administration-dependent efficacy of ferrociphenol lipid nanocapsules for the treatment of intracranial 9L rat gliosarcoma. Int. J. Pharm. 2012;423(1):55–62. doi: 10.1016/j.ijpharm.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 106.Qian X., Long L., Shi Z., Liu C., Qiu M., Sheng J., Pu P., Yuan X., Ren Y., Kang C. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials. 2014;35(7):2322–2335. doi: 10.1016/j.biomaterials.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 107.Guerin C., Olivi A., Weingart J.D., Lawson H.C., Brem H. Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest. New Drugs. 2004;22(1):27–37. doi: 10.1023/B:DRUG.0000006172.65135.3e. [DOI] [PubMed] [Google Scholar]
- 108.Yang H.W., Hua M.Y., Hwang T.L., Lin K.J., Huang C.Y., Tsai R.Y., Ma C.C., Hsu P.H., Wey S.P., Hsu P.W., Chen P.Y., Huang Y.C., Lu Y.J., Yen T.C., Feng L.Y., Lin C.W., Liu H.L., Wei K.C. Non-invasive synergistic treatment of brain tumors by targeted chemotherapeutic delivery and amplified focused ultrasound-hyperthermia using magnetic nanographene oxide. Adv. Mater. 2013;25(26):3605–3611. doi: 10.1002/adma.201301046. [DOI] [PubMed] [Google Scholar]
- 109.Gao K., Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int. J. Pharm. 2006;310(1-2):213–219. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 110.Tian X.H., Lin X.N., Wei F., Feng W., Huang Z.C., Wang P., Ren L., Diao Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomed. 2011;6:445–452. doi: 10.2147/IJN.S16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuo Y.C., Chen H.H. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Int. J. Pharm. 2006;327(1-2):160–169. doi: 10.1016/j.ijpharm.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 112.Eroğlu H., Kaş H.S., Oner L., Türkoğlu O.F., Akalan N., Sargon M.F., Ozer N. The in-vitro and in-vivo characterization of PLGA:L-PLA microspheres containing dexamethasone sodium phosphate. J. Microencapsul. 2001;18(5):603–612. doi: 10.1080/02652040010019587. [DOI] [PubMed] [Google Scholar]
- 113.Gao X., Wu B., Zhang Q., Chen J., Zhu J., Zhang W., Rong Z., Chen H., Jiang X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release. 2007;121(3):156–167. doi: 10.1016/j.jconrel.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 114.Reddy M.K., Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009;23(5):1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 115.Palma E., Pasqua A., Gagliardi A., Britti D., Fresta M., Cosco D. Antileishmanial activity of amphotericin B-loaded-PLGA nanoparticles: An overview. Materials (Basel) 2018;11(7):E1167. doi: 10.3390/ma11071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai Q., Wang L., Deng G., Liu J., Chen Q., Chen Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016;8(2):749–764. [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L., Yu F., Cole A.J., Chertok B., David A.E., Wang J., Yang V.C. Gum arabic-coated magnetic nanoparticles for potential application in simultaneous magnetic targeting and tumor imaging. AAPS J. 2009;11(4):693–699. doi: 10.1208/s12248-009-9151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang X., Guan F. Cham: Springer; 2019. Therapeutic Intranasal Delivery for Alzheimer’s Disease. Therapeutic Intranasal Delivery for Stroke and Neurological Disorders. Springer Series in Translational Stroke Research. pp. 117–133. [DOI] [Google Scholar]
- 119.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B. 2016;6(4):268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arana L., Bayón-Cordero L., Sarasola L.I., Berasategi M., Ruiz S., Alkorta I. Solid lipid nanoparticles surface modification modulates cell internalization and improves chemotoxic treatment in an oral carcinoma cell line. Nanomaterials (Basel) 2019;9(3):464–480. doi: 10.3390/nano9030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martins S., Tho I., Reimold I., Fricker G., Souto E., Ferreira D., Brandl M. Brain delivery of camptothecin by means of solid lipid nanoparticles: formulation design, in vitro and in vivo studies. Int. J. Pharm. 2012;439(1-2):49–62. doi: 10.1016/j.ijpharm.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 122.Chirio D., Gallarate M., Peira E., Battaglia L., Muntoni E., Riganti C., Biasibetti E., Capucchio M.T., Valazza A., Panciani P., Lanotte M., Annovazzi L., Caldera V., Mellai M., Filice G., Corona S., Schiffer D. Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment. Eur. J. Pharm. Biopharm. 2014;88(3):746–758. doi: 10.1016/j.ejpb.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 123.Heggannavar G.B., Hiremath C.G., Achari D.D., Pangarkar V.G., Kariduraganavar M.Y. Development of Doxorubicin-Loaded Magnetic Silica-Pluronic F-127 Nanocarriers Conjugated with Transferrin for Treating Glioblastoma across the Blood-Brain Barrier Using an in vitro Model. ACS Omega. 2018;3(7):8017–8026. doi: 10.1021/acsomega.8b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernardi A., Frozza R.L., Hoppe J.B., Salbego C., Pohlmann A.R., Battastini A.M., Guterres S.S. The antiproliferative effect of indomethacin-loaded lipid-core nanocapsules in glioma cells is mediated by cell cycle regulation, differentiation, and the inhibition of survival pathways. Int. J. Nanomed. 2013;8:711–728. doi: 10.2147/IJN.S40284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lollo G., Vincent M., Ullio-Gamboa G., Lemaire L., Franconi F., Couez D., Benoit J.P. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int. J. Pharm. 2015;495(2):972–980. doi: 10.1016/j.ijpharm.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 126.Agarwal N.B., Jain S., Nagpal D., Agarwal N.K., Mediratta P.K., Sharma K.K. Liposomal formulation of curcumin attenuates seizures in different experimental models of epilepsy in mice. Fundam. Clin. Pharmacol. 2013;27(2):169–172. doi: 10.1111/j.1472-8206.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 127.Natalia N., Sergey S., Tatiana A., et al. Targeted delivery of cisplatin-loaded liposomes to intracranial gliomas. Front Bioeng Biotechnol.; 10th World Biomaterials Congress; 2016. p. 4. [Google Scholar]
- 128.Liang M., Gao C., Wang Y., Gong W., Fu S., Cui L., Zhou Z., Chu X., Zhang Y., Liu Q., Zhao X., Zhao B., Yang M., Li Z., Yang C., Xie X., Yang Y., Gao C. Enhanced blood-brain barrier penetration and glioma therapy mediated by T7 peptide-modified low-density lipoprotein particles. Drug Deliv. 2018;25(1):1652–1663. doi: 10.1080/10717544.2018.1494223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y., Lefever M.R., Muthu D., Bidlack J.M., Bilsky E.J., Polt R. Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins. Future Med. Chem. 2012;4(2):205–226. doi: 10.4155/fmc.11.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ran D., Mao J., Zhan C., Xie C., Ruan H., Ying M., Zhou J., Lu W.L., Lu W. d-Retroenantiomer of quorum-sensing peptide-modified polymeric micelles for brain tumor-targeted drug delivery. ACS Appl. Mater. Interfaces. 2017;9(31):25672–25682. doi: 10.1021/acsami.7b03518. [DOI] [PubMed] [Google Scholar]
- 131.Wang G., Wang J., Guan R. Novel phospholipid-based Labrasol nanomicelles loaded with flavonoids for oral delivery with enhanced penetration and anti-brain tumor efficiency. Curr. Drug Deliv. 2020;17(3):229–245. doi: 10.2174/1567201817666200210120950. [DOI] [PubMed] [Google Scholar]
- 132.Sharma A., Sharma R., Zhang Z., Liaw K., Kambhampati S.P., Porterfield J.E., Lin K.C., DeRidder L.B., Kannan S., Kannan R.M. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 2020;6(4):eaay8514. doi: 10.1126/sciadv.aay8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moscariello P., Ng D.Y.W., Jansen M., Weil T., Luhmann H.J., Hedrich J. Brain delivery of multifunctional dendrimer protein bioconjugates. Adv. Sci. (Weinh.) 2018;5(5):1700897. doi: 10.1002/advs.201700897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu X., Li J., Han S., Tao C., Fang L., Sun Y., Zhu J., Liang Z., Li F. A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration. Eur. J. Pharm. Sci. 2016;88:178–190. doi: 10.1016/j.ejps.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 135.Lin L., Cai J., Jiang C. Recent advances in targeted therapy for glioma. Curr. Med. Chem. 2017;24(13):1365–1381. doi: 10.2174/0929867323666161223150242. [DOI] [PubMed] [Google Scholar]
- 136.Mendes M., Sousa J.J., Pais A., Vitorino C. Targeted theranostic nanoparticles for brain tumor treatment. Pharmaceutics. 2018;10(4):1–47. doi: 10.3390/pharmaceutics10040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao H., Xiong Y., Zhang S., Yang Z., Cao S., Jiang X. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol. Pharm. 2014;11(3):1042–1052. doi: 10.1021/mp400751g. [DOI] [PubMed] [Google Scholar]
- 138.Kumari S., Ahsan S.M., Kumar J.M., Kondapi A.K., Rao N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017;7(1):6602. doi: 10.1038/s41598-017-06888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang B., Sun X., Mei H., Wang Y., Liao Z., Chen J., Zhang Q., Hu Y., Pang Z., Jiang X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials. 2013;34(36):9171–9182. doi: 10.1016/j.biomaterials.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 140.Zhao X., Shang T., Zhang X., Ye T., Wang D., Rei L. Passage of Magnetic Tat-Conjugated Fe3O4@SiO2 Nanoparticles Across in vitro Blood-Brain Barrier. Nanoscale Res. Lett. 2016;11(1):451. doi: 10.1186/s11671-016-1676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hyder F., Manjura Hoque S. Brain Tumor Diagnostics and Therapeutics with Superparamagnetic Ferrite Nanoparticles. Contrast Media Mol. Imaging. 2017;2017:6387217. doi: 10.1155/2017/6387217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie H., Zhu Y., Jiang W., Zhou Q., Yang H., Gu N., Zhang Y., Xu H., Xu H., Yang X. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials. 2011;32(2):495–502. doi: 10.1016/j.biomaterials.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 143.Park S.H., Yoon Y.I., Moon H., Lee G.H., Lee B.H., Yoon T.J., Lee H.J. Development of a novel microbubble-liposome complex conjugated with peptide ligands targeting IL4R on brain tumor cells. Oncol. Rep. 2016;36(1):131–136. doi: 10.3892/or.2016.4836. [DOI] [PubMed] [Google Scholar]
- 144.Zhu Y., Hong H., Xu Z.P., Li Z., Cai W. Quantum dot-based nanoprobes for in vivo targeted imaging. Curr. Mol. Med. 2013;13(10):1549–1567. doi: 10.2174/1566524013666131111121733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cabral Filho P.E., Cardoso A.L.C., Pereira M.I.A., Ramos A.P., Hallwass F., Castro M.M., Geraldes C.F., Santos B.S., Pedroso de Lima M.C., Pereira G.A., Fontes A. CdTe quantum dots as fluorescent probes to study transferrin receptors in glioblastoma cells. Biochim. Biophys. Acta. 2016;1860(1 Pt A):28–35. doi: 10.1016/j.bbagen.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 146.Meola A., Rao J., Chaudhary N., Sharma M., Chang S.D. Gold nanoparticles for brain tumor imaging: A systematic review. Front. Neurol. 2018;9(328):328. doi: 10.3389/fneur.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]


