Abstract
A large body of research has shown the presence of a complex pathway of communications between the gut and the brain. It is now recognized that, through this pathway, the microbiota can influence brain homeostasis and plasticity under normal and pathological conditions.
This review aims at providing an overview of preclinical and clinical pieces of evidence supporting the possible role of gut-brain axis modulation in physiological aging, in a neurodevelopmental disorder, the autism spectrum disorders and in a substance abuse disorder, the alcohol addiction.
Since the normalization of gut flora can prevent changes in the behavior, we postulate that the gut-brain axis might represent a possible target for pharmacological and dietary strategies aimed at improving not only intestinal but also mental health. The present review also reports some regulatory considerations regarding the use of probiotics, illustrating the most debated issues about the possibility of considering probiotics not only as a food supplement but also as a “full” medicinal product.
Keywords: Gut-brain axis, aging, alcohol, autism, brain disorders, preclinical and clinical studies, regulatory aspects
1. INTRODUCTION
Since its origins, medicine has paid attention to the relationship between the state of health or disease and the conditions of the digestive tract. For centuries the so-called “mood theory” has been the foundation of diagnosis and treatment of diseases since “moods” are a consequence of the good or bad functioning of the digestive system. The humoral theory, conceived by Hippocrates of Kos, represents the oldest attempt, in the western world, to provide an etiological explanation of the onset of diseases, overcoming the superstitious, magical or religious conception [1].
Despite this, while the idea that the brain can alter the intestinal function has been documented and accepted, the idea that signs from the gut can affect mood, behavior, and cognitive function is less common.
The mutual impact of the gastrointestinal tract on brain function is well summarized in the concept of the “gut-brain axis”, which provides a bidirectional homeostatic route of communication between the “little” and the “big” brain. The increasing knowledge of this complex system has highlighted the multiplicity and the varied nature of the different components of the axis. In particular, experimental studies during the past years have shown that the neuronal circuits have a pivotal but not unique role in the connections between the gut and the brain. Hormonal and immunological routes, as well as the enteric microbiota, integrate bidirectional information, building a complex network, whose dysfunction can have pathophysiological consequences, not only influencing gastrointestinal efficiency but also modulating central functions.
In the present review, we focus our attention on the influence of the gut-brain axis in the physiological brain decline (aging) and specific mental disorders, including autism spectrum disorders and alcohol addiction. We summarize preclinical and clinical pieces of evidence on the potential use of probiotics in the prevention or management of these conditions, with a particular emphasis on regulatory aspects.
2. ANATOMICAL AND PHYSIOLOGICAL ASPECTS OF THE GUT-BRAIN AXIS: AN OVERVIEW
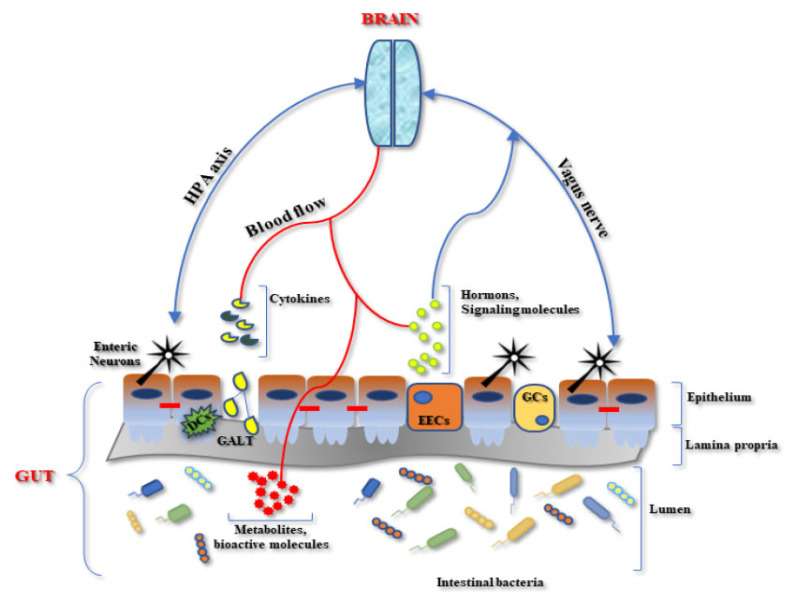
The brain-gut axis consists of a complex network of bidirectional interconnections in which components of different nature are involved (Fig. 1).
Fig. (1).
Schematic representation of the microbiota-gut-brain axis interplay. DCs= Dendritic Cells, EECs= EnteroEndocrine cells, GCs = Globet cells; GALT= Gut-Associated Lymphoid Tissue, HPA= Hypothalamus -Pituitary-Adrenal axis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Neuronal Control
The gastrointestinal neuronal connections involve both extrinsic innervation that consists of fibers belonging to the central and the autonomic nervous systems (CNS and ANS, respectively) and intrinsic innervation that represents the enteric nervous system (ENS).
The ENS consists of a network of hundreds of millions of neurons and glial cells clustered in small ganglia, which are connected by nerve bundles and organized in two major layers: the myenteric plexus (or Auerbach’s plexus) and the submucosal plexus (or Meissner’s plexus). The ENS controls motor functions, regulate local blood flow, mucosal transport and secretions, and interacts with the immune and endocrine systems of the gut. Studies on the anatomy and physiology of the ENS have shown, at the beginning of the last century, that the peristaltic reflex (i.e. , the propulsive activity of the intestine induced by pressure) is a local nervous mechanism that occurs in the absence of external nerve inputs. For this autonomy and for its complexity Michael D. Gershon [2] compared the ENS to a second brain. The control of these functions is not, of course, completely independent from the CNS. Through networks with the central and peripheral nervous system, the ENS (the “gut’s brain”) control digestive functions, such as the motor activity of the alimentary canal, secretions, bowel absorption of nutrients and the splanchnic circle [3]. Sensory afferent fibers conduct visceral sensory stimuli that originate in the nerve endings found in the wall of the alimentary canal. In particular, vagal fibers have a pivotal role in the relationship between the intestine and the brain (in particular with the hypothalamus) [4]. The vagus nerve is the tenth of the twelve pairs of cranial nerves and has the nucleus in the bulb called the nucleus ambiguous. It has a parasympathetic component that controls part of the smooth muscle and spinal nerves that regulate some viscera. The sympathetic system primarily exerts an inhibitory influence on the gut, decreasing intestinal motor function and secretion via the release of neurotransmitters such as noradrenaline. The autonomic input from the gut is connected to the limbic system. Communications between the limbic and autonomic systems provide the neural circuitry underlying the strong link between behavior and gut function in health (such as stomach 'butterflies') and disease (such as irritable bowel syndrome) [5].
Enteric neurons use more than 50 neurotransmitters in synaptic communication, some of which are also present in the central and autonomous nervous system (e.g., acetylcholine; serotonin). Nerve endings can also release peptidergic neurotransmitters (e.g., calcitonin gene-related peptide; somatostatin, substance P, vasoactive intestinal peptide, nociceptin, pancreatic polypeptide, gastrin, cholecystokinin, neurotensin and bombesin), or gas as nitric oxide [6-14]. The interaction of these neurotransmitters with their specific receptors generates excitatory or inhibitory responses.
Dietary changes and disturbances of the intestinal microbiome, with its metabolites and neuroactive compounds, impact the functioning of the ENS and its connections with the CNS since they may modify the mucosal permeability and the secretion of hormones and immune cells. In addition, enteric neurons are vulnerable to aging-related degeneration, as well as during pathological conditions.
2.2. Humoral Control
The humoral components of the gut-brain axis are mainly regulated by the hypothalamic-pituitary-adrenal (HPA) axis and are represented by the enteroendocrine system and the mucosal immune system.
The HPA axis is responsible for stress responses, resulting in the release of cortisol, adrenaline, and noradrenaline. From a physiological point of view, the stress relationship immediately leads to a reduction in energy consumption in various areas of the body in favor of the musculoskeletal system and the CNS, and in general a greater ability to use energy substrates, with the activation of gluconeogenesis, lipolysis, and metabolic proteolysis [9]. Chronic psychological stress is associated with a perturbation in the HPA axis and is related to enhanced abdominal pain and altered intestinal barrier function [5, 15]. Postnatal alterations of the HPA axis due to different emotional events can cause not only pathophysiological modifications of the behavior and the neuroendocrine system but also of the homeostasis of the gastrointestinal tract in adults [16]. Maternal separation is a typical experimental model that induces, during the first period after birth, chronic stress in the offspring. The effect of this condition could cause in adulthood anxious and depressive behavior, and predispose to visceral sensitivity, abdominal pain and altered intestinal motility, all typical symptoms of irritable bowel syndrome in humans [17]. Actually, moderate stress (that could be considered positive stimulatory behavior) during the postnatal period induces, on the contrary, a protective effect on the progeny, reducing the possibility to develop colitis [18, 19]. Altogether, this evidence show that the modulation of the HPA axis is a critical point that can “disturb” the homeostatic balance with acute but also long-term effects. Peripheral corticotrophin-releasing factor (CRF) signaling also participates in the stress-related alterations of the intestinal homeostasis. In particular, CRF-receptor 1 (CRFR1) in the colon increases intestinal permeability through mast cell degranulation-dependent mechanism and CRF-CRFR1 pathway enhances propulsive motor functions and induces visceral hyperalgesia both in animals and humans, all features of stress-related intestinal disorders [20]. Furthermore, the stress-linked release of CRF does not only act via the HPA axis, as hypothalamic and amygdala-derived CRF may also activate mucosal mast cells to increase TNF-α, which can change gut permeability [21].
The enteroendocrine cells (EECs) are the primary “gut sensors”; these cells are dispersed throughout the gastrointestinal mucosa and secrete hormones and amines in response to the three macronutrient types - carbohydrates, proteins, and lipids [22]. Under appropriate stimulation, they are capable of sensing luminal content, modulating the physiological and homeostatic gastrointestinal functions, and producing and releasing a plethora of hormones and signaling molecules (i.e. histamine, somatostatin, gastrin, and serotonin). Interestingly, 95% of the total amount of serotonin is produced in the gastrointestinal tract. Serotonin is a polyfunctional signaling molecule, playing a major role in promoting intestinal motility through a combination of neuronal and mucosal mechanisms [23]. In the CNS, serotonin plays an important role in regulating mood, sleep, body temperature, sexuality, and appetite [24]. Quite interestingly, since the microbiota, microbiome and disruption of the gut-brain axis were linked to various metabolic, immunological, physiological, neurodevelopmental, and neuropsychiatric diseases it has been suggested that intestinal serotonin, produced by intestinal enterochromaffin cells, picked up and accumulated by circulating platelets, participates and has a crucial role in the regulation of membrane permeability in the intestine, brain, and other organs [25]. Indeed, intestinal serotonin may act as a hormone-like continuous regulatory signal for the whole body, including the brain. This regulatory signal function is mediated by platelets and is primarily dependent on and reflects the intestine's actual health condition [25]. Such recent postulation could explain why gut dysbiosis could be associated with several human disorders as well as neurodevelopmental and neuropsychiatric disruptions [25].
Secretory products can act locally in the mucosa, reach distant targets through their release into the bloodstream or act directly on nerve endings close to the site of release. In this regard, direct or indirect communication of EECs with nerves is a major mechanism underlying EEC function in the gut mucosa through peptide release. Hormones released by EECs act on receptors located on the vagal and spinal neurons and influence neuronal pathways in the bidirectional brain-gut communication [26]. This information sends positive or negative feedback to the CNS, which modulates functions of the gastrointestinal tract, glucose homeostasis, and satiety.
In physiological conditions, the human gut contains more immune cells than the rest of the body. This is a reflection of the fact that intestinal mucosa is continuously exposed to a myriad of antigens derived from the diet and luminal flora. Activation of the immune system can occur in various structures of the gut-associated lymphoid tissue, including isolated follicles distributed throughout the small intestine and colon or Peyer’s patches in the small intestine, which contain different secondary follicles, each consisting of a germinal center B cells surrounded by T lymphocytes. The epithelium overlying these structures contains highly specialized epithelial cells, called M cells, aimed at transporting luminal antigens into the dome area of the follicle, where antigen-presenting cells, such as dendritic cells (DCs), promote the activation of T lymphocytes and B lymphocytes. Lamina propria DCs can extend protrusions directly into the gut lumen for uptake, both commensals and pathogenic bacteria [27]. Therefore, the intestinal epithelial barrier represents a highly dynamic structure that limits but does not exclude, antigens from entering the tissues. Indeed, the gut lamina propria is a site of active surveillance, in which various immune cells contribute to maintain the immunological homeostasis (tolerance towards symbiotic bacteria and dietary antigens) as well as ensure prompt response against pathogens [28]. The ongoing mucosal immune response causes no tissue damage since the effector cell responses are tightly controlled by counter-regulatory mechanisms (i.e. , the action of regulatory DC and T cells, T cell apoptosis) [29]. In the gut, there is also an active cross-talk between the immune cells and nervous systems, which occurs through a complex set of neurotransmitters, cytokines and hormones and undoubtedly plays a crucial role in the regulation of an immune response [22]. Inflammatory mediators released locally can activate sensory nerves and send signals to the nervous system. Through the induction of the so-called “inflammatory reflex,” efferent nerves also convey signals from the nervous system to the periphery where the release of neural mediators affects immune responses and inflammation [4]. High level of circulating LPS increases gut permeability via Toll-like receptors (TLRs), a critical pathway for intestinal homeostasis [30]. Notably, bacterial associated-TLRs activation impacts not only on gastrointestinal functions but also on distant districts as the CNS, through neuronal and circulating connections [31].
3. THE GUT MICROBIOTA
The intestine is the most ancient apparatus of the organism and has evolved in a world dominated by bacteria creating a condition not only of coexistence with bacteria but also of mutual benefit, known as symbiosis. This compromise has allowed the intestine and bacterial colonies to evolve together and to penetrate so much that the relationship between them is indissoluble.
The gastrointestinal tract hosts 1014 bacterial cells (with their genomes) differently distributed in the entire apparatus, but to date, only 30% of total microorganisms have been identified by using the classical technique of microbiology. The concept that the fetus develops in a sterile environment has been accepted for many years (known as “sterile womb paradigm”), although new bacterial identification techniques are opening the possibility that the first bacterial colonization may occur in utero [32]. Interesting, different mode of childbirth (natural or cesarean) changes the composition of the intestinal microbiota [33]. Breast-fed and formula-fed infants present a different relative abundance of some phyla of bacteria [34]. The microbiota of breast-fed babies is more heterogeneous than that of formula-fed babies and contains a higher taxonomic diversity [35]. Mammary microbiota derives from the entero-mammary pathway through which maternal gut commensal bacteria across the intestinal barrier to the lymph/blood circulation, reaching the mammary gland epithelium. The bacteria, together with the breast skin microbiota are ingested through the infant suction. The sequential system of the microbiota lasts a few weeks with the gradual introduction of different bacterial species. Gut microbiota shows a spatial and temporal distribution in humans [36] and rodents [37]. During the weaning, bacterial colonization further changes into adulthood. Actually, even the adult bacterial ecosystem may vary, influenced by various factors, as the location where we leave. In this regard, a study has investigated and compared human intestinal microbiota from children characterized by a modern western diet and a rural diet, showing how the relative abundance of some phyla differs between the two groups [38]. In addition, at the beginning of life, the microbiota also undergoes significant changes toward the old age. These alterations are due to the various physiological changes that the elderly go through, such as modifications in lifestyle, nutritional behavior, increase in infection rates and inflammatory diseases, (requiring more medication). All of these issues certainly affect also the composition and activity of the microbiota, even if the course and mechanisms behind these changes are not yet completely understood. As well as probiotics and dietary effects on microbiome composition, it should be noted that the nutraceutical, sodium butyrate, not only maintains the gut barrier integrity and modulate immune responses, but can enhance the butyrate-producing bacteria [39]. Such evidences disclose a direct link between the gut and the brain. For example, an increase in gut permeability and decreased butyrate may contribute to the elevation in proinflammatory cytokines, which via induction of indoleamine 2,3-dioxygenase may potentiate neuro-regulatory kynurenines, such as kynurenic acid and quinolinic acid, thereby altering brain functions [40]. Another crucial aspect that links gut and brain functioning is the impact of the gut on mitochondrial function, both in immune and CNS cells, with an emphasis on the consequences for transdiagnostic processes across psychiatry, but with relevance to wider medical conditions [41].
The microorganisms have a symbiotic connection with the host and do many essential health-promoting functions. The gut microbiota plays essential roles in protecting against pathogens, metabolizing dietary nutrients and drugs, production of vitamins, immune responses and influence the absorption and distribution of dietary fat [42].
3.1. Role in the Brain Homeostasis
The idea that the microbiome can have a role in the physiological homeostasis of the brain is becoming more evident. It is progressively recognized that the gastrointestinal microbiota contributes substantially to determining the development of the CNS. The novel conceptual model of a “microbiota-gut-brain axis” confers to the commensal microorganisms a key role, not only in the control of metabolic, immune and trophic functions of the intestine but also for the proper physiology of the CNS. The parallel development of the CNS and the microbiota in early-life and the bi-directional communication between these organs puts the bacterial components in a position where they may exert substantial influence over the developing brain [43]. Different studies conducted in animals with altered commensal flora suggest that rodent behavioral responses are impaired when the bacterial status of the gut is altered because of inflammation, infection or drugs [44]. Concerning cognition, it was originally supposed to be exclusively controlled by the CNS, with mechanisms contributing to the formation and storage of memories, but nowadays, other systems, including the immune system and the intestinal microbiome may also be involved. Studies conducted in germ-free mice have shown that these animals have a deficit in non-spatial memory and an impaired working memory, accompanied by decrease in brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) and c-FOS, proteins playing a crucial role in hippocampal-dependent memory formation [45-47]. Cognitive impairment is not only a geriatric related disorder but, on the contrary, is often correlated to metabolic syndromes (type 2 diabetes, obesity) [48-50], in which microbiota composition seems to play a role in attributing cognitive traits [51]. In humans, impaired cognitive function in patients with hepatic encephalopathy is associated with alterations in the intestinal microbiome [52].
The microbiome can interact with the brain through the vagal afferent nerves that convey sensory information from viscera to the CNS [53]. This represents one of the possible mechanisms that allow the gut microbiota to modulate the brain-gut axis. Gut bacteria can produce neurotransmitters (serotonin, γ-aminobutyric acid) [54], that through the systemic circulation, potentially impact brain development. In early-life, serotonin is considered as an essential signaling molecule that regulates the development of many systems throughout the body.
4. GUT-BRAIN AXIS DEREGULATION
4.1. Role in Aging
Aging is a physiological process that progressively affects the entire organism in several ways, from a cellular to a functional level. Changes associated with age manifest themselves as a decline in several abilities, including sensory, motor, metabolic, and higher cognitive functions [55].
It is widely recognized that the evolutionary dynamics and fluctuations of gut microbiota undergo profound modifications during aging. Indeed, several studies have ascertained that the main alterations occurring in the pattern of microbiota throughout life derive both from external factors (nutrition, stress, lifestyle, antibiotic exposure and infection) and from the individual features of the host [56-58]. The host factors influencing the composition and the functionality of the gut microbiota are mainly characterized by genetic factors, intestinal senescence processes and immunosenescence mechanisms, which are amplified from 65 years of age [59-61]. One of the main events strictly related to aging is represented by reduced intestinal motility and following constipation [62], which in turn induces a decrease in bacterial excretion and an enhancement in the breakdown of pancreatic enzymes that negatively affects the gut homeostasis [63]. Furthermore, it is important to underline that within the intestinal tract of a young/adult individual, numerous structures (such as Peyer’s patches, cryptopatches and lymphoid follicles) cooperate synergistically in order to ensure immune tolerance to symbiotic and commensal microorganism as well as to food protein, establishing an important barrier against pathogens and intestinal homeostasis and ensuring the correct functionality of the inflammatory and immune response of the digestive system [64, 65]. During aging, however, there is a progressive decrease in Peyer’s patches, associated with a defective action of T and B cells, chronic activation of macrophages in the intestinal lumen and an increase in the growth of pathogenic bacteria [66-68]. These morpho-functional modifications compromise the innate and adaptive immune processes, which are the basis of lower antigen-specific antibody titers, lower tolerance to harmless antigen and to chronic inflammation processes, a phenomenon termed “inflammaging” [69, 70]. Recently it has been hypothesized a direct correlation between “inflammaging” and the structure and composition of the microbiome gut [71]. In healthy adults, Bacteroidetes (mostly the genus Bacteroides) and Firmicutes (largely the genus Eubacteria) represent the taxa dominant (95% and 93%, respectively), while Actinobacteria [including genus Bifidobacterium], Proteobacteria and Verrucomicrobia are present only in a very small fraction [72, 73]. In the elderly, the characterization of the different taxa present in the gut microbiome does not always show unique characteristics, as considerable variability depending on dietary habits, antibiotic treatment and presence of chronic pathologies were disclosed [74, 75]. However, recent studies have shown that during aging numerous Firmicutes subgroups decrease in number with a corresponding increase in Bacteroidetes [76]. Numerous authors have also shown a significant decrease in Bifidobacteria, which play a primary role in maintaining correct intestinal function and protecting it from pathogenic infections, while other studies found enrichment in microbiota known to trigger an inflammatory response (Escherichia, Enterobacteriaceae, Clostridium difficile, etc. .) [77-79]. Remarkably, the gut of centenarians does not show significant differences when compared to other adult populations, suggesting that a healthy microbiome might represent a key factor to maintain the longevity [77, 79]. A further correlation between “inflammaging” and gut microbiota derives from the study by Biagi et al., who described a significant enhancement of the pro-inflammatory cytokines IL-6 and IL-8 in the elderly population; this event was strictly linked with an increase in Protobacteria levels, a decrease in bacterial species able to produce butyrate and an elevation in the general inflammatory status [77]. However, this series of information does not clearly demonstrate whether or not the intestinal flora is capable of inducing age-dependent changes in the “inflammaging” phenomena or whether it is profoundly influenced by the systemic inflammatory state.
As for the cause-effect relationship between the “inflammaging” processes and the modification of the intestinal bacterial flora, it is now widely recognized that alteration of gut microbiota diversity and stability during aging induces a profound gut barrier dysfunction, with a consequent increased intestinal permeability (leaky gut), enhancement of the systemic circulation of proinflammatory cytokines and bacteria-derived products, as well as blood-brain barrier weakening [80]. Indeed, it has been postulated that the consequence of the functional impairment of both barriers has the potential to accelerate the “inflammaging” mechanisms in the brain, triggering a chronic inflammatory state and microglia activation [81]. Currently, several mechanisms have been identified on how gut bacteria could affect the CNS, by the gut-brain axis communication. For instance, the aged-related chronic intestinal inflammatory process triggers the overproduction of pro-oxidant and pro-inflammatory metabolites, such as nitric oxide, chemokines, prostaglandins and cytokines which diffuse by the “leaky gut” to the systemic blood circulation, reaching the brain where negatively disrupt the neuronal homeostasis and survival [82, 83]. Remarkably, it has been demonstrated that circulating cytokines (TNF-α and IL-6) induce a modification of the blood-brain barrier’s permeability, facilitating the easy influx in the brain of peripheral immune cells and inflammatory markers, which in turn might promote neuronal damage [83, 84]. During aging, cognitive decline represents a typical manifestation of physiological neurodegeneration. Age-related cognitive decay is due to a progressive impairment of the underlying brain cell processes due to neuroinflammation, oxidative stress, reduced synaptic plasticity and neurogenesis, thus leading to a consequent and irreversible neuronal loss, shrinkage of neurons, reduction of synaptic spines and lower numbers of synapses [85, 86]. The basic cognitive functions most affected by age are attention and memory. Nevertheless, higher-level cognitive functions, such as language processing and decision-making may also be affected by age. Hippocampal-dependent cognitive functions rely on the construction of new neurons and maintenance of dendritic structures to elicit the synaptic plasticity needed for learning and the formation of new memories. Although the mechanism leading to impaired cognitive functions in the elderly is complex, persistent oxidative stress, impaired neurogenesis and aberrations of the immune system likely play an important role. Age-related changes in long-term potentiation (LTP) and long-term depression (LTD), the two major forms of synaptic plasticity strictly linked to learning and memory [87, 88], result from neuroinflammatory changes, oxidative stress, and a decrease in N-methyl-D-aspartate receptor function and expression [89]. The hippocampal structure represents the main brain region responsible for the formation and consolidation of new memories, with the pivotal contribution of adult hippocampal neurogenesis, which provide a continuous supply of new neurons and neuroplasticity throughout life [90, 91]. Early inflammatory responses strongly reduce adult neurogenesis and hippocampal homeostasis, with detrimental effects on learning and memory processes [92]. The recent discovery of the presence of adult neurogenesis in the human dentate gyrus and its consistent decline in aging prevention [93, 94], open very interesting challenges on the role of a virtuous lifestyle aimed at stimulating human adult neurogenesis and slowing its decay.
Epidemiological studies support the hypothesis that modifiable lifestyle-related factors are associated with cognitive deterioration, opening new avenues for its prevention [95]. Diet and physical exercise, in particular, have become the object of intense research in relation to cognitive decline [96, 97]. Nutritional factors affect adult neurogenesis and cognitive functions too. It has been demonstrated in aged mice that polyunsaturated fatty acids supplementation enhances hippocampal functionality, improves performance in many hippocampus-dependent memory tasks and provides a plethora of neuroprotective effects both in physiological and pathological conditions [98, 99]. Moreover, the polyphenol-rich extracts from grape, olive and blueberry may potentiate neurotrophic factors activity, inhibit neuroinflammation, attenuate cognitive decline and improve neuronal function in old mice [100-107]. Physical exercise is a recognized potent enhancer of adult hippocampal neurogenesis mediated by neurotrophic factors [108-112], and it has emerged as a potential therapy or an adjunctive therapeutic strategy for cognitive decline [113].
Finally, a recent study has demonstrated that the gut microbiota plays a pivotal role in the bioavailability of antioxidants, unsaturated fatty acids and polyphenols that protect neurons from senescence and apoptosis [114].
The correlation between gut microbiota composition, increased gut barrier permeability and neurological alteration has been recently highlighted by the discovery of the neuroprotective and therapeutic effects exerted by probiotic administration [115]. Indeed, many lines of preclinical evidence have demonstrated that probiotic supplementation can represent a useful approach for increasing gut epithelial integrity, protecting gut barrier leaking, inhibiting proinflammatory processes and counteracting the onset or propagation of neurodegenerative disorders [116, 117]. For example, it has been evidenced that probiotics improve memory deficits, attenuate aging-related disruption of the intestinal barrier and reduce neuroinflammation in a mouse model of accelerated senescence [118].
The phenomena of gut dysbiosis can induce or exacerbate a series of pathologies collectively denominated geriatric syndromes and, in particular, Parkinson’s disease and Alzheimer’s disease. In fact, numerous studies have found a high percentage of metabolic and gastrointestinal pathologies such as obesity, diabetes, microbial dysbiosis, constipation, diarrhea, comorbid with neurodegenerative diseases, suggesting a causal role played by the gut-brain axis in the development of neurodegenerative syndromes [119-121].
The gut-brain axis is regulated by direct and indirect mechanisms through vagus nerve stimulation, immunological changes, gut hormonal system activation and microbiota’s metabolites [122, 123]. In this two-way communication, the brain can impact the gut microbiota through the action of the HPA axis, which regulates the stressful events. Indeed, it has been shown that stress may affect the microbiota profile and diversity, with the reduction of the anti-inflammatory bacteria such as Lactobacillus and the concomitant translocation in the gut of proinflammatory bacteria such as Clostridia [124, 125].
Therefore, the gut inflammatory process might trigger an inflammatory pathway from the gut to the brain by the gut-brain axis and these events might be implicated in the pathophysiology of those neurodegenerative diseases with a major inflammatory component.
4.2. Autism Spectrum Disorders
The human gut microbiota is nowadays considered an important factor that can influence brain physiology and behavior. On the other hand, it is widely recognized that the dysregulation of the gut-brain axis contributes to the development of several brain disorders, including autism spectrum disorders (ASD). Affected ASD subjects suffer from multiple gastrointestinal symptoms (i.e. constipation, abdominal pain, diarrhea, gas, and vomiting) with a higher prevalence than in neurotypical individuals [126, 127]. Notably, an association between the gastrointestinal manifestations and the clinical symptomatology of ASD has also been observed. In fact, autistic features are more frequent and severe in ASD children showing comorbid gastrointestinal problems [128].
The etiology of ASD is not known but the predominant view indicates a multifactorial etiology, including genetic, epigenetic and environmental factors [129, 130]. Importantly, the microbiota is a crucial element at the intersection of genes and environment, as its composition and function are dependent on genetic background and are shaped by multiple environmental factors [127, 131].
The human brain development occurs in parallel with the maturation of the intestinal flora during prenatal and early postnatal stages. Any imbalance in the quantitative and qualitative composition of intestinal microorganisms during critical periods might affect both the intestinal nervous system and the CNS, suggesting that the microbiome-gut-brain axis may represent a predisposing factor in the occurrence of neurodevelopmental disorders later in life.
Brain functions in normal and pathological conditions are influenced by the intestinal microbiota through the endocrine, immune, and autonomic nervous systems. However, the hypothesized gut-brain axis pathways and their relevance to ASD is beyond the scope of this review. We refer the reader to recently published reviews [83, 132, 133].
Several preclinical studies carried out in the last years have provided mechanistic insight in the gut-brain dysregulation in different pathological conditions, including ASD. The impact of distinctive microbiota compositions on early life control of the emotional state, motor activity and cognitive function has been extensively investigated in mice models following manipulation of the microbiota [134-136]. These studies generally suggest that antibiotic-treated or germ-free adult mice show alterations in brain function and structure [83, 137]. Indeed, impairment of synaptic plasticity, learning and memory, along with alterations in social and emotional behaviors are generally associated with these models [44, 127, 138, 139].
Interestingly, a previous report has shown that the bacterial cell wall peptidoglycan can cross the murine placenta and reach the developing fetal brain thereby inducing a neuroproliferative response that correlated with deficits in cognitive behavior in cell wall peptidoglycan-exposed pups following birth [140]. Among the different mechanisms, a recent work has demonstrated that the microbiota profoundly modulates the properties and function of microglial cells [141]. Accordingly, germ-free mice display alterations in the gene expression pattern and in the morphological features of microglia since immature microglial cells have been found in the brain cortex of these mice [141]. These results suggest that gut microbiota plays a role in the maturation stage of naïve microglia in the brain. Importantly, the manipulation of microbiota from prenatal stages can affect microglia in a gender- and time-dependent manner [142]. A recent study indicates that antibiotic-treated or germ-free mice exhibit deficits in fear extinction and display immature-type microglia along with changes in gene expression in excitatory neurons, glia and other cell types [143]. Importantly, these effects were reversed following recolonization of germ-free mice with microbiota from healthy untreated mice immediately after birth, suggesting that during critical periods microbiota-derived chemical signaling is essential in preserving synaptic plasticity and cognitive function [143]. Given the well-established role of microglia in synaptic plasticity [144], it is believed that changes in microglial activity directly affect neuronal function via synaptic pruning alteration [145]. By using diffusion tensor imaging, a recent work revealed that changes in white matter structural integrity occur in a diet-dependent manner via modulation of synaptic pruning [146].
Interestingly, these immune-mediated changes may be associated with developmental modifications in specific brain regions, among which it is worth mentioning the amygdala. Indeed, it has been established that alterations in amygdala functioning is able to influence other brain areas, such as the prefrontal cortex and sensory processing in the cortex and thalamus [147, 148]. In this scenario, non-clinical data have demonstrated a key role for amygdala microglia reactivity in shaping male-typical play behavior [149], as well as driving brain sexual differentiation [150]. Therefore, the gut microbiome might contribute, through the amygdala, to the male-characteristics typically associated with ASD.
The composition and function of the intestinal microbial community are also critical for ensuring proper brain development in humans, especially in the light of the ability of the gut flora to produce several neuroactive compounds and metabolites which are transmitted via afferent fibers of the vagus nerve and are diffused to various parts of the brain [151]. Noteworthy, the trafficking of these molecules is enhanced due to the increased permeability of both intestinal and blood-brain barriers occurring under chronic low-grade inflammation [152]. Increased permeability has also been described in preterm infants and in ASD individuals, further supporting the gut–immune–brain axis theory of ASD [153]. Individuals with ASD also exhibit alterations in the levels of neurotransmitters and hormones, such as catecholamines, serotonin, melatonin, glutamate, γ-aminobutyric acid, oxytocin and arginine-vasopressin, vitamin D, and endogenous opioids [154]. Other neuroactive compounds are elevated in ASD, such as 3-(3 hydroxyphenyl)-3-hydroxypropionic acid. 4-ethylphenylsulfate, p-cresol, and propionic acids [155, 156]. Altogether these factors contribute to the phenotypic heterogeneity typically associated with the complex multifactorial etiology of ASD.
Notably, ASD has been associated with hyperserotonemia in conjunction with reduced levels of N-acetylserotonin (NAS) and melatonin, suggesting that dysregulation of this metabolic pathway is relevant to gut–brain communication possibly via impaired mitochondria functioning [157]. In this frame, also the levels of the short-chain fatty acid including butyrate, propionate and acetate, are altered in ASD patients [158]. Interestingly, among other effects, butyrate acts as a histone deacetylase inhibitor thereby triggering the activation of the mitochondrial melatonergic pathways leading to increased acetyl-CoA levels and ATP production [147, 159].
Previous studies conducted in humans also suggest an interplay between microbiota perturbation and the severity of neuropsychiatric symptomatology [160, 161]. A recent large systematic review and meta-analysis compared the composition of gut microbiota in children with and without ASD [162]. Authors found that the microbiota of ASD individuals displays a greater abundance of Bacteroidetes (Bacteroides and Parabacteroides) and some Firmicutes genera (specifically Clostridium, Faecalibacterium and Phascolarctobacterium) along with reduced levels of Coprococcus and Bifidobacteria. The qualitative and quantitative alterations in microbiota composition were related not only to the comorbid gastrointestinal manifestation but also to the severity of the autistic symptomatology, suggesting that dysbiosis in ASD patients influences the severity of ASD symptomatology, although the causal link remains to be established. Controversial results were found throughout the literature. One limiting factor is that a unique microbiota profile does not exist as each one of us has a distinct identity which is also subject to changes depending on dietary habits, lifestyle and age [163]. Therefore, balance and diversity within the bacterial composition in a dynamic fashion are crucial to ensure appropriate physiological activity of the immune, metabolic, and nervous systems. On the contrary, disruption of the natural balance between good and bad bacteria might lead not only to intestinal but also to extra-intestinal diseases as previously described.
On these premises, maintaining a well-balanced composition of the intestinal microbiota through a healthy lifestyle and a correct diet, as well as considering the use of antibiotics, prebiotics, probiotics, might be effective in the treatment of the gastrointestinal symptomatology and/or the concurrent psychiatric conditions [136].
A previous study investigating the effects of treatment with oral vancomycin in ASD found a short-term improvement in communication and behavior testing [164]. More recently, a randomized controlled trial exploring treatment with either minocycline or placebo in addition to risperidone, showed that this combination therapy was successful in decreasing irritability and hyperactivity/noncompliance score in autistic children, although further studies are necessary to establish the long-term effect of this approach [165].
Although there is low direct causal evidence that the manipulation of microbiota is effective in the treatment of ASD, it is increasingly hypothesized that the use of probiotic strains might improve gastrointestinal symptoms in ASD individuals [166]. Of note, oral treatment with Bacteroides fragilis (a commensal bacterium) in a mouse model displaying features of ASD, normalized the microbial composition, restructured gut permeability caused by dysbiosis, and reversed behavioral abnormalities through the production of neuroactive metabolites [167].
Clinical studies evaluating the use of probiotics in gastrointestinal disorders and neuropsychiatric conditions, including ASD, are ongoing, showing also positive results. It has to be noted that whereas a single bacterial strain provided beneficial results in irritable bowel disorders, on the other hand, the multi-strains probiotic formulation may be more effective in treating both behavioral and gastrointestinal manifestations of ASD [168-170]. In conclusion, probiotics represent a relatively safe option and could be recommended for children with ASD as adjuvant therapy, even though wide-scale randomized controlled trials are crucial to confirm the efficacy of probiotics in ASD.
Finally, fecal microbiota transplantation, which represents the injection of filtrate stools from a healthy donor to a patient, has proven successful in an open-label trial in combination with vancomycin [171]. Indeed, autistic-like features and associated GI symptoms were significantly improved and this effect persisted up to two years following treatment [172]. Notably, the clinical outcome was paralleled by increases in the levels of Bifidobacterium and Prevotella species in the intestinal microbiome.
Certainly, clinical trial design assessing the efficacy of the above-mentioned approaches might suffer from several limitations. Therefore, future studies should consider a thorough diagnostic assessment along with a rigorous description of the phenotypic heterogeneity of ASD patients. Clinical studies should also evaluate lifestyle, diet, intake of probiotics/antibiotics, as well as gastrointestinal history and examination. This approach will possibly lead to the stratification of multiple cohorts of patients rather than one-type ASD. Subgroup analyses (e.g., responders vs non-responders) should also be considered in the planned clinical studies.
On these premises, the regulatory approach in ASD is rather complex. Indeed, gut-brain comorbidities (for example, sleep problems; epilepsy and gastrointestinal disorders) are highly prevalent in ASD, with many patients also having a learning disability. Therefore, the development of pharmacological treatments targeting single symptoms in ASD is generally not recommended by regulators (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline- clinical-development-medicinal-products-treatment-autism-spectrum-disorder-asd_en.pdf. Accessed 18 May 2020). However, sponsors can claim an indication for targeting a single symptom if data robustly show that the effect on that symptom is specific to ASD. In general, clinically relevant efficacy should be demonstrated usually in combination with other treatments for ASD (since it is difficult for a single compound to demonstrate efficacy on all characteristics of ASD). Importantly, robust efficacy on one core symptom (investigated with appropriate scales also validated for the studied age range) and clinically relevant improved functioning need to be demonstrated in both short and long term studies (like randomized, double-blind, parallel-group trials) with the maintenance of effect on one core symptom and function established in the long term studies (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline- clinical-development-medicinal-products-treatment-autism-spectrum-disorder-asd_en.pdf. Accessed 18 May 2020). A duration greater than 12 weeks might be necessary if the treatment effect is time-dependent or if tolerance built over time. As for the primary endpoints studied, investigators could focus on one or two of the core symptoms with other core symptoms specified as a key secondary endpoint. Prospective applicants/researchers should approach regulators to obtain scientific advice on their clinical development initiatives/programs so that the data obtained from clinical trials would be robust enough to support a marketing authorization (https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-advice-protocol-assistance). We do want to stress the point that regulators have reviewed all too many studies that were not designed well with primary and secondary endpoints including their estimands not able to demonstrate efficacy and safety in the company’s claimed indication (which is the population that the medicinal product is intended to be used in clinical practice in line with the license). So, regulators encourage both academics and industry to approach them and request scientific advice so that issues are ironed out early in a products’ development.
4.3. Alcohol Use Disorders
A more recent field of investigation addressed the possible role of the gut-brain axis also in the field of alcohol dependence. In the present review, we do focus our attention on alcohol abuse, in particular, the potential role of intestinal environment on the management of alcohol use disorders (AUD)-related psychiatric symptoms (anxiety, depression, craving), and also of physical damages induced by its uncontrolled consumption [173-176].
Concerning the physical damages due to continuous alcohol intake, the main problem regards the increased reactive oxygen species production as consequences of lipid oxidation by ethanol that occurs in the liver. In this regard, the use of antioxidant compounds of natural origin, also as diet supplementation, is considered a useful tool to counteract the deleterious effect of free radicals in the organism [177-179]. Among these, polyphenols, micronutrients widely present in the plant kingdom, show potential beneficial properties for human health. In a preclinical mouse model of alcohol abuse, our research has demonstrated that both polyphenols [101] contained in extra virgin olive oil (as hydroxytyrosol, tyrosol, oleuropein), and resveratrol [180, 181], a non- flavonoid polyphenol mainly present in grapes, may provide powerful protection against ethanol-induced oxidative stress by reducing serum-free oxygen radicals without affecting the free oxygen radicals defense.
In alcoholic men during withdrawal, it has also been shown that the prolonged consumption of a blend of polyphenols containing hydroxytyrosol, tyrosol and also oleuropein modulated serum levels of neurotrophins, known to play subtle roles in the addiction processes. In particular, the increased level of BDNF, but not NGF, was counteracted by the natural compound supplementation, with a time course, suggesting that monitoring serum BDNF and/or NGF in alcoholics during withdrawal could contribute to characterize alcohol dependence profiles and could be important to improve recovery processes throughout antioxidant compounds supplementation [105].
Recently, a link between polyphenols and microbiota modulation has been disclosed. Animal studies have shown that gut microorganisms can influence the activity of flavonoids [182], but little is known about how food flavonoids shape the gut microbiota in humans. By analyzing data from over 240 healthy men, a research conducted by Ivey [183] identified six types of microbial communities associated with the consumption of flavonoids, observing a strong association between the intake of blueberries and tea and specific microbial communities. This study supports the idea that flavonoid intake could modulate the composition of the microbial community. In particular what emerges is that, rather than the total amount of microorganisms in the intestine, the relative abundance of specific phyla of bacteria is a critical point that could be responsible, at least in part, of the imbalance between healthy vs unhealthy state. The prevalence of a phylum may reflect altered metabolic capacity (carbohydrate and protein metabolism), also affecting the normal micronutrient balance, making the organism susceptible to the onset of morbid states.
In experimental models of alcohol addiction, different studies have demonstrated that alcohol consumption induced dysbiosis and bacterial overgrowth [184, 185]. This condition is associated with the development of oxidative stress, altered intestinal permeability, endotoxemia, and steatohepatitis, suggesting that intestinal dysbiosis may potentially contribute to the pathogenesis of liver disease through the production of pro-inflammatory factors and promoting liver pathology.
In a study conducted in germ-free mice fed with ethanol, authors implanted stool from patients with or without alcoholic hepatitis, analyzing the effect on liver damage. Mice that received microbial tools from a patient with severe alcoholic hepatitis developed more severe liver inflammation with an increased number of liver T lymphocyte subsets and Natural Killer T, higher liver necrosis, greater intestinal permeability and higher translocation of bacteria, respect to mice transplanted with a microbial tool from those subjects without alcoholic hepatitis [186].
Alcoholic hepatitis is the most serious form of liver disease. To date, the possibilities of treatment are still limited and the etiopathological mechanisms to be explored. A recent study [187] evaluated the contribution of the microbiota in the transmission and course of alcoholic hepatitis in vivo, also providing preliminary human data and potential therapeutic solutions. The administration of specific bacteriophages for E. faecalis producing the cytolysin exotoxin improved significantly the clinical picture, suggesting its prospective clinical use to manage this pathological condition [187].
Concerning alcohol use disorders, a recent paper illustrates how alcohol consumption changes gut microbiota, in an AUD murine model. Moreover, in the same study, the impact of these alterations was correlated with alcohol withdrawal-induced anxiety and behavioral changes [188].
Recent evidence demonstrates how certain behavioral characteristics can lead to a higher risk of addiction through a link with the microbiome-intestine-brain axis, suggesting the possibility of investigating whether diversity and bacterial composition could be associated with traits related to AUD. A recent study conducted by Jadhav and co [188] show how the predisposition to the development of AUD would be related to the microbiota and altered expression of dopaminergic receptors. This study is among those aimed at identifying biomarkers of vulnerability to the development of alcohol dependence. Through the study of the inter-individual vulnerability of rat models to which dependence was induced, a different bacterial profile and an altered expression of dopaminergic receptors (increased D1 and decreased D2 receptors in the striatum of withdrawal rats) were demonstrated in the group defined as “vulnerable”, characterized by a more marked dependent profile, compared to “resilient” [188].
Some clinical trials have been carried out, based on the possibility to modulate gut microbiota through the consumption of probiotics, to obtain beneficial effects in AUD. In a completed clinical trial (ClinicalTrials.gov Identifier: NCT01501162), Han and co investigated the therapeutic effects of the probiotic mixture composed by Lactobacillus subtilis/Streptococcus faecium (1500 mg/day) in patients with alcoholic hepatitis. In detail, 117 patients were prospectively randomized to receive the 7 days of probiotics or placebo. All patients enrolled were not allowed to consume alcohol for the period of treatment. Liver function test (enzyme activity), serum proinflammatory cytokines, lipopolysaccharide, and colony-forming units by stool culture were examined and compared after therapy. Abstinence is the most imperative behavior for patients with alcoholic hepatitis. In addition, supplementation with cultured L. subtilis/S. faecium was associated with the re-establishment of intestinal flora and improvement of LPS in patients with alcoholic hepatitis.
Concerning the use of probiotics in alcohol-related liver diseases (i.e. alcoholic hepatitis and liver cirrhosis), two other clinical trials are in the phase of patients recruiting. In the first study, coordinated by Aleksander Krag, Odense University Hospital (ClinicalTrials.gov Identifier: NCT03863730) investigators hypothesized that the gut microbiota and its metabolites are major drivers of fibrosis in human liver disease and that modulating the intestinal flora may alter disease progression by reducing the activity of hepatic stellate cells. For this aim patients will consume, twice every day for 24 weeks, Profermin Plus®, a product based on fermented oats, Lactobacillus plantarum, barley malt, lecithin, and thiamin. The primary outcome will be to evaluate an attenuation of liver hepatic stellate cell activity after treatment, and secondarily to measure hepatic inflammation markers and metabolites and the reduction in fibrosis markers. In the second study, coordinated by Mack Mitchell, University of Texas Southwestern Medical Center (ClinicalTrials.gov Identifier: NCT01922895), researchers will evaluate if a diet supplemented with a probiotic nutrient (Lactobacillus rhamnosus daily for 180 days) can improve alcoholic hepatitis and gut complications compared to routine standard care. The MELD (Model for End-Stage Liver Disease) scoring system for assessing the severity of the chronic liver disease will be monitored to assess the validity of the treatment.
Alcohol dependence is defined in ICD-10 (International Statistical Classification of Diseases, Injuries and Causes of Death 10th version) as a primary, chronic disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. The disease is often progressive and fatal. Recent marketing authorizations applications at the level of the European Medicines Agency pursued indications in both alcohol withdrawal syndrome as well as maintenance of alcohol abstinence (https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=uriserv:OJ.C_.20 18.030.01.0010.01.ENG; Accessed 18 May 2020). Sponsors pursuing clinical development programs for these objectives for alcohol withdrawal syndrome should decrease the severity of symptoms, prevent more severe withdrawal clinical manifestations such as seizure and delirium and facilitate the entry of the patient into a treatment program in order to achieve and maintain long-term abstinence from alcohol. While for alcohol abstinence maintenance, continued abstinence should be demonstrated following the active treatment phase (3 to 6 months) through a double-blind withdrawal phase in responders without treatment for 15 months after randomization. According to the European Medicines Agency guideline on the development of medicinal products for use in alcohol dependence, for a product to be licensed for the maintenance of alcohol abstinence, confirmatory efficacy clinical trials should be randomized, double-blind, parallel-group and placebo-controlled and designed to demonstrate superiority vs. placebo. It is also very important to try to minimize methodological issues (relating to the design of the studies) that can arise in pursuing clinical trials in this setting, and these should be risk mitigated a priori (for example considerations of sample size, the appropriate selection of the patient population in this setting, as well as carefully planned post-hoc analyses) to establish efficacy in the applied target indications (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-medicinal-products-treatment-alcohol-dependence_en.pdf. Acces- sed 18 May 2020).
As for gestational alcohol consumption or paternal preconceptual alcohol exposure inducing Fetal Alcohol Spectrum Disorders (FASD) in the newborns, only a few data are available on the relationship heathy-gut vs healthy-brain. FASD are complex malformative conditions of the newborn and the child due to the teratogenic effect of alcohol taken during pregnancy and caused by preconceptual paternal alcohol drinking throughout epigenetics mechanisms [189-193]. FASD is the most common cause of mental retardation developed in childhood, therefore totally avoidable, through the complete abstaining of the pregnant woman from the intake of alcohol [194-196]. The outcomes of alcohol on the fetus range from the absence of damage to abortion, including a spectrum of clinical expressions as neonatal congenital morphological defects and neurological development disruptions [195, 197, 198]. Although FASD is a quite common cause of mental disabilities, there are still no crystal data on its incidence leading to an underestimation of the problem. Indeed, the global prevalence of FASD is about 2-4% in the newborns [199]. A study in the mouse [200] on the influence of prenatal alcohol exposure on the serotoninergic system in the gastrointestinal system disclosed decreased serotonin concentrations in the gut mucosa and longitudinal muscle myenteric plexus (females only). Furthermore, an elevation in mucosal and longitudinal muscle myenteric plexus tryptophan concentration was only observed in prenatally exposed female mice. These findings show that prenatal alcohol exposure may elicit a decrease in the conversion of tryptophan to serotonin in both muscle and mucosa, effect more pronounced in females, changes probably associated with the estrous cycle [200]. Other earlier human and animal model studies clearly demonstrated that prenatal alcohol exposure affects intestine formation/functioning development and nutrients absorption [190, 201-203]. Further lines of investigation deal now with the use of antioxidants’ supplementation to prevent or counteract the deleterious effects of alcohol consumption during gestation [204-211].
CONCLUSION
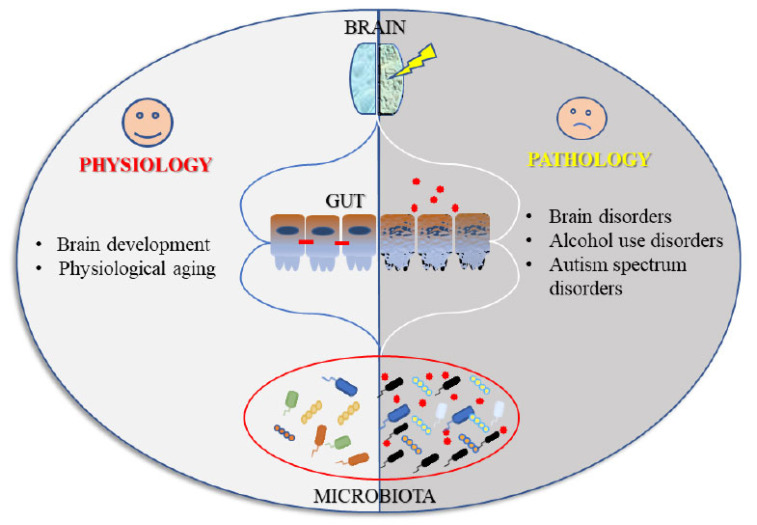
The intense research in recent years on the role of gut-brain axis, has undoubtedly endorsed the hypothesis of a close relationship between gut health and the health of the “higher” functions. As summarized in the present review, central disorders of different types (from physiological aging to alcohol dependence, till autism spectrum disorders) have in common alterations in the bowel district (Fig. 2).
Fig. (2).
An outline illustrating how the microbiota-gut-brain axis could participate in the physiological regulation of brain homeostasis, and in the pathophysiology of different psychiatric disorders. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The evidence that intestinal disturbances are often not only the consequence of altered psychological states, but could contribute, together with other factors, to the onset of central function disorders, opens the way for new fields of investigation and promises new therapeutic strategies. The use of probiotics and/or natural antioxidants represents today a new and interesting strategy to obtain important benefits, without substantial side effects, in the control of many clinical manifestations related to central-type disorders. Certainly, we are still far from being able to replace the current pharmacological protocols for the control of psychiatric disorders due to the lack of answers to questions that can expand the applicability of these supplements to an exclusive and not only prophylactic therapeutic use. Further preclinical and clinical studies are necessary, for example to evaluate the duration over time of the beneficial effects of probiotics and/or natural antioxidants after the end of the supplementation, a critical point for any drug therapy. “A healthy gut for a healthy brain” represents not only a slogan, but an increasingly consolidated concept that undoubtedly deserves further investigation for the understanding of the etiopathogenesis of many central diseases.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Ventegodt S., Thegler S., Andreasen T., Struve F., Jacobsen S., Torp M., Aegedius H., Enevoldsen L., Merrick J. A review and integrative analysis of ancient holistic character medicine systems. Sci. World J. 2007;7:1821–1831. doi: 10.1100/tsw.2007.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershon M. D. The Enteric Nervous System: A Second Brain. Hosp. Pract. 1999;34(7):31–32. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss W.M., Starling E.H. The movements and innervation of the small intestine. J. Physiol. 1899;24(2):99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B., Sinniger V., Pellissier S. The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Front. Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broccardo M., Scaccianoce S., Del Bianco P., Agostini S., Petrella C., Improta G. Nociceptin/orphanin FQ-induced delay in gastric emptying: role of central corticotropin-releasing factor and glucocorticoid receptors. Neurogastroenterol. Motil. 2005;17(6):871–877. doi: 10.1111/j.1365-2982.2005.00717.x. [DOI] [PubMed] [Google Scholar]
- 7.Petrella C., Giuli C., Broccardo M., Eutamene H., Cartier C., Leveque M., Bedini A., Spampinato S., Bueno L., Theodorou V., Improta G., Agostini S. Protective and worsening peripheral nociceptin/orphanin FQ receptor-mediated effect in a rat model of experimental colitis. Pharmacol. Res. 2013;70(1):72–79. doi: 10.1016/j.phrs.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Severini C., Improta G., Falconieri-Erspamer G., Salvadori S., Erspamer V. The tachykinin peptide family. Pharmacol. Rev. 2002;54(2):285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 9.Farzi A., Fröhlich E.E., Holzer P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics. 2018;15(1):5–22. doi: 10.1007/s13311-017-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzer P., Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzer P. Neuropeptides, Microbiota, and Behavior. Int. Rev. Neurobiol. 2016;131:67–89. doi: 10.1016/bs.irn.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Broccardo M., Agostini S., Petrella C., Guerrini R., Improta G. Central and peripheral role of the nociceptin/orphaninFQ system on normal and disturbed colonic motor function and faecal pellet output in the rat. Neurogastroenterol. Motil. 2008;20(8):939–948. doi: 10.1111/j.1365-2982.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 13.Broccardo M., Guerrini R., Petrella C., Improta G. Gastrointestinal effects of intracerebroventricularly injected nociceptin/orphaninFQ in rats. Peptides. 2004;25(6):1013–1020. doi: 10.1016/j.peptides.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Agostini S., Petrella C. The endogenous nociceptin/orphanin FQ-NOP receptor system as a potential therapeutic target for intestinal disorders. Neurogastroenterol. Motil. 2014;26(11):1519–1526. doi: 10.1111/nmo.12460. [DOI] [PubMed] [Google Scholar]
- 15.Agostini S., Goubern M., Tondereau V., Salvador-Cartier C., Bezirard V., Lévèque M., Keränen H., Theodorou V., Bourdu- Naturel S., Goupil-Feuillerat N., Legrain-Raspaud S., Eutamene H. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol. Motil. 2012;24(4):376–e172. doi: 10.1111/j.1365-2982.2011.01865.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Mahony S.M., Clarke G., Dinan T.G., Cryan J.F. Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 17.Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol. Rep. 2013;65(6):1451–1461. doi: 10.1016/S1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- 18.Petrella C., Giuli C., Agostini S., Bacquie V., Zinni M., Theodorou V., Broccardo M., Casolini P., Improta G. Maternal exposure to low levels of corticosterone during lactation protects against experimental inflammatory colitis-induced damage in adult rat offspring. PLoS One. 2014;9(11):e113389. doi: 10.1371/journal.pone.0113389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinni M., Zuena A.R., Marconi V., Petrella C., Fusco I., Giuli C., Canu N., Severini C., Broccardo M., Theodorou V., Lattanzi R., Casolini P. Maternal exposure to low levels of corticosterone during lactation protects adult rat progeny against TNBS-induced colitis: A study on GR-mediated anti-inflammatory effect and prokineticin system. PLoS One. 2017;12(3):e0173484. doi: 10.1371/journal.pone.0173484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tache Y., Larauche M., Yuan P-Q., Million M. Brain and Gut CRF Signaling: Biological Actions and Role in the Gastrointestinal Tract. Curr. Mol. Pharmacol. 2018;11(1):51–71. doi: 10.2174/187446721066617022409574128240194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanuytsel T., van Wanrooy S., Vanheel H., Vanormelingen C., Verschueren S., Houben E., Salim Rasoel S., Tόth J., Holvoet L., Farré R., Van Oudenhove L., Boeckxstaens G., Verbeke K., Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 22.Sharkey K.A., Beck P.L., McKay D.M. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium. Nat. Rev. Gastroenterol. Hepatol. 2018;15(12):765–784. doi: 10.1038/s41575-018-0051-4. [DOI] [PubMed] [Google Scholar]
- 23.Ge X., Pan J., Liu Y., Wang H., Zhou W., Wang X. Intestinal Crosstalk between Microbiota and Serotonin and its Impact on Gut Motility. Curr. Pharm. Biotechnol. 2018;19(3):190–195. doi: 10.2174/1389201019666180528094202. [DOI] [PubMed] [Google Scholar]
- 24.Strasser B., Gostner J.M., Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19(1):55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 25.Szőke H., Kovács Z., Bókkon I., Vagedes J., Szabó A.E., Hegyi G., Sterner M.G., Kiss Á., Kapócs G. Gut dysbiosis and serotonin: intestinal 5-HT as a ubiquitous membrane permeability regulator in host tissues, organs, and the brain. Rev. Neurosci. 2020;31(4):415–425. doi: 10.1515/revneuro-2019-0095. [DOI] [PubMed] [Google Scholar]
- 26.Latorre R., Sternini C., De Giorgio R., Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016;28(5):620–630. doi: 10.1111/nmo.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J.P., Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2(4):361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald T.T., Monteleone I., Fantini M.C., Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140(6):1768–1775. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald T.T., Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307(5717):1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 30.Price A.E., Shamardani K., Lugo K.A., Deguine J., Roberts A.W., Lee B.L., Barton G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity. 2018;49(3):560–575.e6. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roomruangwong C., Kanchanatawan B., Sirivichayakul S., Anderson G., Carvalho A.F., Duleu S., Geffard M., Maes M. IgA/IgM Responses to Gram-Negative Bacteria are not Associated with Perinatal Depression, but with Physio-somatic Symptoms and Activation of the Tryptophan Catabolite Pathway at the End of Term and Postnatal Anxiety. CNS Neurol. Disord. Drug Targets. 2017;16(4):472–483. doi: 10.2174/1871527316666170407145533. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Muñoz M.E., Arrieta M-C., Ramer-Tait A.E., Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brink L.R., Matazel K., Piccolo B.D., Bowlin A.K., Chintapalli S.V., Shankar K., Yeruva L. Neonatal Diet Impacts Bioregional Microbiota Composition in Piglets Fed Human Breast Milk or Infant Formula. J. Nutr. 2019;149(12):2236–2246. doi: 10.1093/jn/nxz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rendina D.N., Lubach G.R., Phillips G.J., Lyte M., Coe C.L. Maternal and Breast Milk Influences on the Infant Gut Microbiome, Enteric Health and Growth Outcomes of Rhesus Monkeys. J. Pediatr. Gastroenterol. Nutr. 2019;69(3):363–369. doi: 10.1097/MPG.0000000000002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016;14(1):20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheth R.U., Li M., Jiang W., Sims P.A., Leong K.W., Wang H.H. Spatial metagenomic characterization of microbial biogeography in the gut. Nat. Biotechnol. 2019;37(8):877–883. doi: 10.1038/s41587-019-0183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov D.O., Evsyukova I.I., Mazzoccoli G., Anderson G., Polyakova V.O., Kvetnoy I.M., Carbone A., Nasyrov R.A. The role of prenatal melatonin in the regulation of childhood obesity. Biology (Basel) 2020;9(4):E72. doi: 10.3390/biology9040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson G., Seo M., Berk M., Carvalho A.F., Maes M. Gut permeability and microbiota in parkinson’s disease: role of depression, tryptophan catabolites, oxidative and nitrosative stress and melatonergic pathways. Curr. Pharm. Des. 2016;22(40):6142–6151. doi: 10.2174/1381612822666160906161513. [DOI] [PubMed] [Google Scholar]
- 41.Anderson G., Maes M. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Suboptimal Mitochondrial Function: Assessment, Treatment and Classification Implications. Curr. Top. Med. Chem. 2020;20(7):524–539. doi: 10.2174/1568026620666200131094445. [DOI] [PubMed] [Google Scholar]
- 42.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stilling R.M., Dinan T.G., Cryan J.F. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13(1):69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 44.Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., Macqueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 45.Manni L., Aloe L., Fiore M. Changes in cognition induced by social isolation in the mouse are restored by electro-acupuncture. Physiol. Behav. 2009;98(5):537–542. doi: 10.1016/j.physbeh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Sornelli F., Fiore M., Chaldakov G.N., Aloe L. Adipose tissue-derived nerve growth factor and brain-derived neurotrophic factor: results from experimental stress and diabetes. Gen. Physiol. Biophys. 2009;28(Spec No):179–183. [PubMed] [Google Scholar]
- 47.Fiore M., Chaldakov G.N., Aloe L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009;20(2):133–145. doi: 10.1515/REVNEURO.2009.20.2.133. [DOI] [PubMed] [Google Scholar]
- 48.Chaldakov G.N., Fiore M., Tonchev A.B., Dimitrov D., Pancheva R., Rancic G., Aloe L. Homo obesus: a metabotrophin-deficient species. Pharmacology and nutrition insight. Curr. Pharm. Des. 2007;13(21):2176–2179. doi: 10.2174/138161207781039616. [DOI] [PubMed] [Google Scholar]
- 49.Chaldakov G.N., Fiore M., Tonchev A.B., Aloe L. Neuroadipology: a novel component of neuroendocrinology. Cell Biol. Int. 2010;34(10):1051–1053. doi: 10.1042/CBI20100509. [DOI] [PubMed] [Google Scholar]
- 50.Chaldakov G.N., Fiore M., Ghenev P.I., Stankulov I.S., Aloe L. Atherosclerotic Lesions: Possible Interactive Involvement of Intima, Adventitia and Associated Adipose Tissue. Int. Med. J. 2000;7(1):43–49. [Google Scholar]
- 51.Arnoriaga-Rodríguez M., Fernández-Real J.M. Microbiota impacts on chronic inflammation and metabolic syndrome - related cognitive dysfunction. Rev. Endocr. Metab. Disord. 2019;20(4):473–480. doi: 10.1007/s11154-019-09537-5. [DOI] [PubMed] [Google Scholar]
- 52.Bajaj J.S., Ridlon J.M., Hylemon P.B., Thacker L.R., Heuman D.M., Smith S., Sikaroodi M., Gillevet P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302(1):G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 54.O’Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 55.Salthouse T.A. When does age-related cognitive decline begin? Neurobiol. Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R., Angenent L.T., Ley R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biagi E., Candela M., Franceschi C., Brigidi P. The aging gut microbiota: new perspectives. Ageing Res. Rev. 2011;10(4):428–429. doi: 10.1016/j.arr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Magrone T., Jirillo E. The interaction between gut microbiota and age-related changes in immune function and inflammation. Immun. Ageing. 2013;10(1):31. doi: 10.1186/1742-4933-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 60.Ghaisas S., Maher J., Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leung K., Thuret S. Gut Microbiota: A Modulator of Brain Plasticity and Cognitive Function in Ageing. Healthcare (Basel) 2015;3(4):898–916. doi: 10.3390/healthcare3040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gidwaney N.G., Bajpai M., Chokhavatia S.S. Gastrointestinal Dysmotility in the Elderly. J. Clin. Gastroenterol. 2016;50(10):819–827. doi: 10.1097/MCG.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 63.Macfarlane G.T., Cummings J.H., Macfarlane S., Gibson G.R. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989;67(5):520–527. doi: 10.1111/j.1365-2672.1989.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 64.Tomasello E., Bedoui S. Intestinal innate immune cells in gut homeostasis and immunosurveillance. Immunol. Cell Biol. 2013;91(3):201–203. doi: 10.1038/icb.2012.85. [DOI] [PubMed] [Google Scholar]
- 65.Di Santo J.P., Vosshenrich C.A.J., Satoh-Takayama N. A ‘natural’ way to provide innate mucosal immunity. Curr. Opin. Immunol. 2010;22(4):435–441. doi: 10.1016/j.coi.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Mabbott N.A., Kobayashi A., Sehgal A., Bradford B.M., Pattison M., Donaldson D.S. Aging and the mucosal immune system in the intestine. Biogerontology. 2015;16(2):133–145. doi: 10.1007/s10522-014-9498-z. [DOI] [PubMed] [Google Scholar]
- 67.Schmucker D.L., Owen R.L., Outenreath R., Thoreux K. Basis for the age-related decline in intestinal mucosal immunity. Clin. Dev. Immunol. 2003;10(2-4):167–172. doi: 10.1080/10446670310001642168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith P.D., Smythies L.E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4(1):31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Müller-Werdan U., Nuding S., Ost M. Assessing inflammageing. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20(5):346–348. doi: 10.1097/MCO.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 70.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calder P.C., Bosco N., Bourdet-Sicard R., Capuron L., Delzenne N., Doré J., Franceschi C., Lehtinen M.J., Recker T., Salvioli S., Visioli F. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H.B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E.G., Wang J., Guarner F., Pedersen O., de Vos W.M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H.M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K.U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M’rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S.D., Bork P. MetaHIT Consortium. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salazar N., Arboleya S., Valdés L., Stanton C., Ross P., Ruiz L., Gueimonde M., de Los Reyes-Gavilán C.G. The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front. Genet. 2014;5:406. doi: 10.3389/fgene.2014.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 76.Mariat D., Firmesse O., Levenez F., Guimarăes V., Sokol H., Doré J., Corthier G., Furet J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkïla J., Monti D., Satokari R., Franceschi C., Brigidi P., De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng J., Palva A.M., de Vos W.M., Satokari R. Contribution of the intestinal microbiota to human health: from birth to 100 years of age. Curr. Top. Microbiol. Immunol. 2013;358:323–346. doi: 10.1007/82_2011_189. [DOI] [PubMed] [Google Scholar]
- 79.Claesson M.J., Cusack S., O’Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G., Stanton C., van Sinderen D., O’Connor M., Harnedy N., O’Connor K., Henry C., O’Mahony D., Fitzgerald A.P., Shanahan F., Twomey C., Hill C., Ross R.P., O’Toole P.W. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA. 2011;108(Suppl. 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Köhler C.A., Maes M., Slyepchenko A., Berk M., Solmi M., Lanctôt K.L., Carvalho A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016;22(40):6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 81.Dinan T.G., Cryan J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. North Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 82.Serra D., Almeida L.M., Dinis T.C.P. The Impact of Chronic Intestinal Inflammation on Brain Disorders: the Microbiota-Gut-Brain Axis. Mol. Neurobiol. 2019;56(10):6941–6951. doi: 10.1007/s12035-019-1572-8. [DOI] [PubMed] [Google Scholar]
- 83.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sochocka M., Diniz B.S., Leszek J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017;54(10):8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baker D.J., Petersen R.C. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J. Clin. Invest. 2018;128(4):1208–1216. doi: 10.1172/JCI95145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bettio L.E.B., Rajendran L., Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 87.Moser E.I., Krobert K.A., Moser M.B., Morris R.G. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281(5385):2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 88.McNaughton B.L., Barnes C.A., Rao G., Baldwin J., Rasmussen M. Long-term enhancement of hippocampal synaptic transmission and the acquisition of spatial information. J. Neurosci. 1986;6(2):563–571. doi: 10.1523/JNEUROSCI.06-02-00563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenzweig E.S., Barnes C.A. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69(3):143–179. doi: 10.1016/S0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 90.Kempermann G., Song H., Gage F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015;7(9):a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toda T., Gage F.H. Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2018;373(3):693–709. doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 92.Kohman R.A., Rhodes J.S. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 2013;27(1):22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J., Hen R., Mann J.J. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018;22(4):589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreno-Jiménez E.P., Jurado-Arjona J., Ávila J., Llorens- Martín M. The Social Component of Environmental Enrichment Is a Pro-neurogenic Stimulus in Adult c57BL6 Female Mice. Front. Cell Dev. Biol. 2019;7:62. doi: 10.3389/fcell.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Solfrizzi V., Capurso C., D’Introno A., Colacicco A.M., Santamato A., Ranieri M., Fiore P., Capurso A., Panza F. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev. Neurother. 2008;8(1):133–158. doi: 10.1586/14737175.8.1.133. [DOI] [PubMed] [Google Scholar]
- 96.Ma C-L., Ma X-T., Wang J-J., Liu H., Chen Y-F., Yang Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017;317:332–339. doi: 10.1016/j.bbr.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 97.Suo C., Singh M.F., Gates N., Wen W., Sachdev P., Brodaty H., Saigal N., Wilson G.C., Meiklejohn J., Singh N., Baune B.T., Baker M., Foroughi N., Wang Y., Mavros Y., Lampit A., Leung I., Valenzuela M.J. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry. 2016;21(11):1645. doi: 10.1038/mp.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cutuli D., De Bartolo P., Caporali P., Laricchiuta D., Foti F., Ronci M., Rossi C., Neri C., Spalletta G., Caltagirone C., Farioli-Vecchioli S., Petrosini L. n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front. Aging Neurosci. 2014;6:220. doi: 10.3389/fnagi.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cutuli D., Landolfo E., Decandia D., Nobili A., Viscomi M.T., La Barbera L., Sacchetti S., De Bartolo P., Curci A., D’Amelio M., Farioli-Vecchioli S., Petrosini L. Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. Int. J. Mol. Sci. 2020;21(5):E1741. doi: 10.3390/ijms21051741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carito V., Venditti A., Bianco A., Ceccanti M., Serrilli A.M., Chaldakov G., Tarani L., De Nicolò S., Fiore M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014;28(22):1970–1984. doi: 10.1080/14786419.2014.918977. [DOI] [PubMed] [Google Scholar]
- 101.Carito V., Ceccanti M., Cestari V., Natella F., Bello C., Coccurello R., Mancinelli R., Fiore M. Olive polyphenol effects in a mouse model of chronic ethanol addiction. Nutrition. 2017;33:65–69. doi: 10.1016/j.nut.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Carito V., Ceccanti M., Tarani L., Ferraguti G., Chaldakov G.N., Fiore M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016;23(28):3189–3197. doi: 10.2174/0929867323666160627104022. [DOI] [PubMed] [Google Scholar]
- 103.Carito V., Ciafrè S., Tarani L., Ceccanti M., Natella F., Iannitelli A., Tirassa P., Chaldakov G.N., Ceccanti M., Boccardo C., Fiore M. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist. Super. Sanita. 2015;51(4):382–386. doi: 10.4415/ANN-15-04-2126783228. [DOI] [PubMed] [Google Scholar]
- 104.De Nicoló S., Tarani L., Ceccanti M., Maldini M., Natella F., Vania A., Chaldakov G.N., Fiore M. Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition. 2013;29(4):681–687. doi: 10.1016/j.nut.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Ceccanti M., Valentina C., Vitali M., Iannuzzi S., Tarani L., De Nicolo S., Ceccanti M., Ciafre S., Tirassa P., Capriglione I., et al. Serum BDNF and NGF Modulation by Olive Polyphenols in Alcoholics during Withdrawal. J. Alcohol. Drug Depend. 2015;03(04) doi: 10.4172/2329-6488.1000214. [DOI] [Google Scholar]
- 106.Bensalem J., Dudonné S., Gaudout D., Servant L., Calon F., Desjardins Y., Layé S., Lafenetre P., Pallet V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018;7:e19. doi: 10.1017/jns.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Witte A.V., Kerti L., Margulies D.S., Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014;34(23):7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fiore M., Amendola T., Triaca V., Alleva E., Aloe L. Fighting in the aged male mouse increases the expression of TrkA and TrkB in the subventricular zone and in the hippocampus. Behav. Brain Res. 2005;157(2):351–362. doi: 10.1016/j.bbr.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 109.Fiore M., Amendola T., Triaca V., Tirassa P., Alleva E., Aloe L. Agonistic encounters in aged male mouse potentiate the expression of endogenous brain NGF and BDNF: possible implication for brain progenitor cells’ activation. Eur. J. Neurosci. 2003;17(7):1455–1464. doi: 10.1046/j.1460-9568.2003.02573.x. [DOI] [PubMed] [Google Scholar]
- 110.Saraulli D., Costanzi M., Mastrorilli V., Farioli-Vecchioli S. The Long Run: Neuroprotective Effects of Physical Exercise on Adult Neurogenesis from Youth to Old Age. Curr. Neuropharmacol. 2017;15(4):519–533. doi: 10.2174/1570159X14666160412150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Farioli-Vecchioli S., Mattera A., Micheli L., Ceccarelli M., Leonardi L., Saraulli D., Costanzi M., Cestari V., Rouault J-P., Tirone F. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells. 2014;32(7):1968–1982. doi: 10.1002/stem.1679. [DOI] [PubMed] [Google Scholar]
- 112.Nicolis di Robilant V., Scardigli R., Strimpakos G., Tirone F., Middei S., Scopa C., De Bardi M., Battistini L., Saraulli D., Farioli Vecchioli S. Running-activated neural stem cells enhance subventricular neurogenesis and improve olfactory behavior in p21 knockout mice. Mol. Neurobiol. 2019;56(11):7534–7556. doi: 10.1007/s12035-019-1590-6. [DOI] [PubMed] [Google Scholar]
- 113.Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat. Rev. Neurosci. 2017;18(6):335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caracciolo B., Xu W., Collins S., Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech. Ageing Dev. 2014;136-137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 115.Mancuso C., Santangelo R. Alzheimer’s disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018;129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 116.Leblhuber F., Steiner K., Schuetz B., Fuchs D., Gostner J.M. Probiotic Supplementation in Patients with Alzheimer’s Dementia - An Explorative Intervention Study. Curr. Alzheimer Res. 2018;15(12):1106–1113. doi: 10.2174/1389200219666180813144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Plaza-Díaz J., Ruiz-Ojeda F.J., Vilchez-Padial L.M., Gil A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients. 2017;9(6):E555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang X., Yu D., Xue L., Li H., Du J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B. 2020;10(3):475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alam M.Z., Alam Q., Kamal M.A., Abuzenadah A.M., Haque A. A possible link of gut microbiota alteration in type 2 diabetes and Alzheimer’s disease pathogenicity: an update. CNS Neurol. Disord. Drug Targets. 2014;13(3):383–390. doi: 10.2174/18715273113126660151. [DOI] [PubMed] [Google Scholar]
- 120.Naseer M.I., Bibi F., Alqahtani M.H., Chaudhary A.G., Azhar E.I., Kamal M.A., Yasir M. Role of gut microbiota in obesity, type 2 diabetes and Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2014;13(2):305–311. doi: 10.2174/18715273113126660147. [DOI] [PubMed] [Google Scholar]
- 121.Rao M., Gershon M.D. The bowel and beyond: the enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016;13(9):517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bauer K.C., Huus K.E., Finlay B.B. Microbes and the mind: emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 2016;18(5):632–644. doi: 10.1111/cmi.12585. [DOI] [PubMed] [Google Scholar]
- 123.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., Guzzetta K.E., Jaggar M., Long- Smith C.M., Lyte J.M., Martin J.A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O’Connor R., Cruz-Pereira J.S., Peterson V.L., Rea K., Ritz N.L., Sherwin E., Spichak S., Teichman E.M., van de Wouw M., Ventura-Silva A.P., Wallace-Fitzsimons S.E., Hyland N., Clarke G., Dinan T.G. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 124.Scott L.V., Clarke G., Dinan T.G. The Brain-Gut Axis: A Target for Treating Stress-Related Disorders. Inflammation in Psychiatry. 2013;28:90–99. doi: 10.1159/000343971. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marshall J., Hill R.J., Ziviani J., Dodrill P. Features of feeding difficulty in children with Autism Spectrum Disorder. Int. J. Speech Lang. Pathol. 2014;16(2):151–158. doi: 10.3109/17549507.2013.808700. [DOI] [PubMed] [Google Scholar]
- 127.Vuong H.E., Hsiao E.Y. Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry. 2017;81(5):411–423. doi: 10.1016/j.biopsych.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adams J.B., Johansen L.J., Powell L.D., Quig D., Rubin R.A. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schaevitz L.R., Berger-Sweeney J.E. Gene-environment interactions and epigenetic pathways in autism: the importance of one- carbon metabolism. ILAR J. 2012;53(3-4):322–340. doi: 10.1093/ilar.53.3-4.322. [DOI] [PubMed] [Google Scholar]
- 130.Shaw C.A., Sheth S., Li D. T. L. Etiology of Autism Spectrum Disorders: Genes, Environment, or Both? OA Autism. 2014;10(2(2)):11. [Google Scholar]
- 131.Nelson M.B., Chase A.B., Martiny J.B.H., Stocker R., Nguyen J., Lloyd K., Oshiro R.T., Kearns D.B., Schneider J.P., Ringel P.D., Basler M., Olson C.A., Vuong H.E., Hsiao E.Y., Roller B.R., Ackermann M., Smillie C., Chien D., Alm E., Jermy A.J. The Microbial Olympics 2016. Nat. Microbiol. 2016;1(8):16122. doi: 10.1038/nmicrobiol.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Srikantha P., Mohajeri M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019;20(9):E2115. doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lasheras I., Seral P., Latorre E., Barroso E., Gracia-García P., Santabárbara J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatr. 2020;47:101874. doi: 10.1016/j.ajp.2019.101874. [DOI] [PubMed] [Google Scholar]
- 134.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 135.Desbonnet L., Clarke G., Shanahan F., Dinan T. G., Cryan J. F. Microbiota Is Essential for Social Development in the Mouse. Molecular psychiatry. England. 2014:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eltokhi A., Janmaat I.E., Genedi M., Haarman B.C.M., Sommer I.E.C. Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J. Neurosci. Res. 2020;98(7):1335–1369. doi: 10.1002/jnr.24616. [DOI] [PubMed] [Google Scholar]
- 137.Dinan T.G., Cryan J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017;79(8):920–926. doi: 10.1097/PSY.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 138.Smith A.K., Kilaru V., Klengel T., Mercer K.B., Bradley B., Conneely K.N., Ressler K.J., Binder E.B. DNA Extracted from Saliva for Methylation Studies of Psychiatric Traits: Evidence Tissue Specificity and Relatedness to Brain. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015;168(1):36–44. doi: 10.1002/ajmg.b.3227825355443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mayer E.A., Tillisch K., Gupta A. Gut/brain axis and the microbiota. J. Clin. Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Humann J., Mann B., Gao G., Moresco P., Ramahi J., Loh L.N., Farr A., Hu Y., Durick-Eder K., Fillon S.A., Smeyne R.J., Tuomanen E.I. Bacterial Peptidoglycan Traverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host Microbe. 2016;19(3):388–399. doi: 10.1016/j.chom.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thion M.S., Low D., Silvin A., Chen J., Grisel P., Schulte-Schrepping J., Blecher R., Ulas T., Squarzoni P., Hoeffel G., Coulpier F., Siopi E., David F.S., Scholz C., Shihui F., Lum J., Amoyo A.A., Larbi A., Poidinger M., Buttgereit A., Lledo P.M., Greter M., Chan J.K.Y., Amit I., Beyer M., Schultze J.L., Schlitzer A., Pettersson S., Ginhoux F., Garel S. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell. 2018;172(3):500–516.e16. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chu C., Murdock M.H., Jing D., Won T.H., Chung H., Kressel A.M., Tsaava T., Addorisio M.E., Putzel G.G., Zhou L., Bessman N.J., Yang R., Moriyama S., Parkhurst C.N., Li A., Meyer H.C., Teng F., Chavan S.S., Tracey K.J., Regev A., Schroeder F.C., Lee F.S., Liston C., Artis D. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574(7779):543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nisticò R., Salter E., Nicolas C., Feligioni M., Mango D., Bortolotto Z.A., Gressens P., Collingridge G.L., Peineau S. Synaptoimmunology - roles in health and disease. Mol. Brain. 2017;10(1):26. doi: 10.1186/s13041-017-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tognini P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front. Cell. Neurosci. 2017;11:25. doi: 10.3389/fncel.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ong I.M., Gonzalez J.G., McIlwain S.J., Sawin E.A., Schoen A.J., Adluru N., Alexander A.L., Yu J.J. Gut microbiome populations are associated with structure-specific changes in white matter architecture. Transl. Psychiatry. 2018;8(1):6. doi: 10.1038/s41398-017-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seo M., Anderson G. Gut-Amygdala Interactions in Autism Spectrum Disorders: Developmental Roles via regulating Mitochondria, Exosomes, Immunity and microRNAs. Curr. Pharm. Des. 2019;25(41):4344–4356. doi: 10.2174/1381612825666191105102545. [DOI] [PubMed] [Google Scholar]
- 148.Gao W., Salzwedel A.P., Carlson A.L., Xia K., Azcarate-Peril M.A., Styner M.A., Thompson A.L., Geng X., Goldman B.D., Gilmore J.H., Knickmeyer R.C. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl.) 2019;236(5):1641–1651. doi: 10.1007/s00213-018-5161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.VanRyzin J.W., Marquardt A.E., Argue K.J., Vecchiarelli H.A., Ashton S.E., Arambula S.E., Hill M.N., McCarthy M.M. Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron. 2019;102(2):435–449.e6. doi: 10.1016/j.neuron.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.VanRyzin J.W., Pickett L.A., McCarthy M.M. Microglia: Driving critical periods and sexual differentiation of the brain. Dev. Neurobiol. 2018;78(6):580–592. doi: 10.1002/dneu.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wall R., Cryan J.F., Ross R.P., Fitzgerald G.F., Dinan T.G., Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 152.Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sajdel-Sulkowska E.M., Makowska-Zubrycka M., Czarzasta K., Kasarello K., Aggarwal V., Bialy M., Szczepanska-Sadowska E., Cudnoch-Jedrzejewska A. Common Genetic Variants Link the Abnormalities in the Gut-Brain Axis in Prematurity and Autism. Cerebellum. 2019;18(2):255–265. doi: 10.1007/s12311-018-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marotta R., Risoleo M.C., Messina G., Parisi L., Carotenuto M., Vetri L., Roccella M. The Neurochemistry of Autism. Brain Sci. 2020;10(3):E163. doi: 10.3390/brainsci10030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012;57(8):2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 156.De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D.I., Cristofori F., Guerzoni M.E., Gobbetti M., Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8(10):e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maes M., Anderson G., Betancort Medina S.R., Seo M., Ojala J.O. Integrating autism spectrum disorder pathophysiology: mitochondria, vitamin A, CD38, oxytocin, serotonin and melatonergic alterations in the placenta and gut. Curr. Pharm. Des. 2019;25(41):4405–4420. doi: 10.2174/1381612825666191102165459. [DOI] [PubMed] [Google Scholar]
- 158.Liu S., Li E., Sun Z., Fu D., Duan G., Jiang M., Yu Y., Mei L., Yang P., Tang Y., Zheng P. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019;9(1):287. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pagan C., Goubran-Botros H., Delorme R., Benabou M., Lemière N., Murray K., Amsellem F., Callebert J., Chaste P., Jamain S., Fauchereau F., Huguet G., Maronde E., Leboyer M., Launay J.M., Bourgeron T. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017;7(1):2096. doi: 10.1038/s41598-017-02152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dinan T.G., Cryan J.F. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18(6):552–558. doi: 10.1097/MCO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 161.Rogers G.B., Keating D.J., Young R.L., Wong M-L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry. 2016;21(6):738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Iglesias-Vázquez L., Van Ginkel Riba G., Arija V., Canals J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients. 2020;12(3):E792. doi: 10.3390/nu12030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berding K., Donovan S.M. Diet Can Impact Microbiota Composition in Children With Autism Spectrum Disorder. Front. Neurosci. 2018;12:515. doi: 10.3389/fnins.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sandler R.H., Finegold S.M., Bolte E.R., Buchanan C.P., Maxwell A.P., Väisänen M.L., Nelson M.N., Wexler H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000;15(7):429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 165.Ghaleiha A., Alikhani R., Kazemi M-R., Mohammadi M-R., Mohammadinejad P., Zeinoddini A., Hamedi M., Shahriari M., Keshavarzi Z., Akhondzadeh S. Minocycline as Adjunctive Treatment to Risperidone in Children with Autistic Disorder: A Randomized, Double-Blind Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol. 2016;26(9):784–791. doi: 10.1089/cap.2015.0175. [DOI] [PubMed] [Google Scholar]
- 166.Navarro F., Liu Y., Rhoads J.M. Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 2016;22(46):10093–10102. doi: 10.3748/wjg.v22.i46.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., Patterson P.H., Mazmanian S.K. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arnold L.E., Luna R.A., Williams K., Chan J., Parker R.A., Wu Q., Hollway J.A., Jeffs A., Lu F., Coury D.L., Hayes C., Savidge T. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child Adolesc. Psychopharmacol. 2019;29(9):659–669. doi: 10.1089/cap.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sanctuary M.R., Kain J.N., Chen S.Y., Kalanetra K., Lemay D.G., Rose D.R., Yang H.T., Tancredi D.J., German J.B., Slupsky C.M., Ashwood P., Mills D.A., Smilowitz J.T., Angkustsiri K. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. 2019;14(1):e0210064. doi: 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shaaban S.Y., El Gendy Y.G., Mehanna N.S., El-Senousy W.M., El-Feki H.S.A., Saad K., El-Asheer O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018;21(9):676–681. doi: 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 171.Kang D-W., Adams J.B., Gregory A.C., Borody T., Chittick L., Fasano A., Khoruts A., Geis E., Maldonado J., McDonough-Means S., Pollard E.L., Roux S., Sadowsky M.J., Lipson K.S., Sullivan M.B., Caporaso J.G., Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kang D-W., Adams J.B., Coleman D.M., Pollard E.L., Maldonado J., McDonough-Means S., Caporaso J.G., Krajmalnik-Brown R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019;9(1):5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ceccanti M., Coriale G., Hamilton D.A., Carito V., Coccurello R., Scalese B., Ciafrè S., Codazzo C., Messina M.P., Chaldakov G.N., Fiore M. Virtual Morris task responses in individuals in an abstinence phase from alcohol. Can. J. Physiol. Pharmacol. 2018;96(2):128–136. doi: 10.1139/cjpp-2017-0013. [DOI] [PubMed] [Google Scholar]
- 174.Ceccanti M., Hamilton D., Coriale G., Carito V., Aloe L., Chaldakov G., Romeo M., Ceccanti M., Iannitelli A., Fiore M. Spatial learning in men undergoing alcohol detoxification. Physiol. Behav. 2015;149:324–330. doi: 10.1016/j.physbeh.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 175.Ledda R., Battagliese G., Attilia F., Rotondo C., Pisciotta F., Gencarelli S., Greco A., Fiore M., Ceccanti M., Attilia M.L. Drop-out, relapse and abstinence in a cohort of alcoholic people under detoxification. Physiol. Behav. 2019;198:67–75. doi: 10.1016/j.physbeh.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 176.Ceccanti M., Iannitelli A., Fiore M. Italian Guidelines for the treatment of alcohol dependence. Riv. Psichiatr. 2018;53(3):105–106. doi: 10.1708/2925.2941029912210. [DOI] [PubMed] [Google Scholar]
- 177.Xia N., Daiber A., Förstermann U., Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017;174(12):1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mukherjee S., Dudley J.I., Das D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response. 2010;8(4):478–500. doi: 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Csiszar A., Labinskyy N., Podlutsky A., Kaminski P.M., Wolin M.S., Zhang C., Mukhopadhyay P., Pacher P., Hu F., de Cabo R., Ballabh P., Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am. J. Physiol. Heart Circ. Physiol. 2008;294(6):H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tiwari V., Chopra K. Resveratrol abrogates alcohol-induced cognitive deficits by attenuating oxidative-nitrosative stress and inflammatory cascade in the adult rat brain. Neurochem. Int. 2013;62(6):861–869. doi: 10.1016/j.neuint.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 181.Petrella C., Carito V., Carere C., Ferraguti G., Ciafrè S., Natella F., Bello C., Greco A., Ralli M., Mancinelli R., et al. Oxidative Stress Inhibition by Resveratrol in Alcohol Dependent Mice. Nutrition. 2020 doi: 10.1016/j.nut.2020.110783. [DOI] [PubMed] [Google Scholar]
- 182.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ivey K.L., Chan A.T., Izard J., Cassidy A., Rogers G.B., Rimm E.B. Role of dietary flavonoid compounds in driving patterns of microbial community assembly. MBio. 2019;10(5):e01205-19. doi: 10.1128/mBio.01205-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yan A.W., Fouts D.E., Brandl J., Stärkel P., Torralba M., Schott E., Tsukamoto H., Nelson K.E., Brenner D.A., Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mutlu E., Keshavarzian A., Engen P., Forsyth C.B., Sikaroodi M., Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol. Clin. Exp. Res. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Llopis M., Cassard A.M., Wrzosek L., Boschat L., Bruneau A., Ferrere G., Puchois V., Martin J.C., Lepage P., Le Roy T., Lefèvre L., Langelier B., Cailleux F., González-Castro A.M., Rabot S., Gaudin F., Agostini H., Prévot S., Berrebi D., Ciocan D., Jousse C., Naveau S., Gérard P., Perlemuter G. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 187.Duan Y., Llorente C., Lang S., Brandl K., Chu H., Jiang L., White R.C., Clarke T.H., Nguyen K., Torralba M., Shao Y., Liu J., Hernandez-Morales A., Lessor L., Rahman I.R., Miyamoto Y., Ly M., Gao B., Sun W., Kiesel R., Hutmacher F., Lee S., Ventura-Cots M., Bosques-Padilla F., Verna E.C., Abraldes J.G., Brown R.S., Jr, Vargas V., Altamirano J., Caballería J., Shawcross D.L., Ho S.B., Louvet A., Lucey M.R., Mathurin P., Garcia-Tsao G., Bataller R., Tu X.M., Eckmann L., van der Donk W.A., Young R., Lawley T.D., Stärkel P., Pride D., Fouts D.E., Schnabl B. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jadhav K.S., Peterson V.L., Halfon O., Ahern G., Fouhy F., Stanton C., Dinan T.G., Cryan J.F., Boutrel B. Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology. 2018;141:249–259. doi: 10.1016/j.neuropharm.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 189.Rompala G.R., Finegersh A., Homanics G.E. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol. 2016;53:19–25. doi: 10.1016/j.alcohol.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Estrada G., Del Rio J.A., García-Valero J., López-Tejero M.D. Ethanol in utero induces epithelial cell damage and altered kinetics in the developing rat intestine. Teratology. 1996;54(5):245–254. doi: 10.1002/(SICI)1096-9926(199611)54:5<245::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 191.Bekdash R., Zhang C., Sarkar D. Fetal alcohol programming of hypothalamic proopiomelanocortin system by epigenetic mechanisms and later life vulnerability to stress. Alcohol. Clin. Exp. Res. 2014;38(9):2323–2330. doi: 10.1111/acer.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ciafrè S., Carito V., Ferraguti G., Greco A., Chaldakov G.N., Fiore M., Ceccanti M., Ciafrè S., Carito V., Ferraguti G., et al. How alcohol drinking affects our genes: an epigenetic point of view. Biochem. Cell Biol. 2019;97(4):345–356. doi: 10.1139/bcb-2018-0248. [DOI] [PubMed] [Google Scholar]
- 193.Chater-Diehl E. J., Laufer B. I., Singh S. M. Changes to Histone Modifications Following Prenatal Alcohol Exposure: An Emerging Picture. Alcohol. 2017:41–52. doi: 10.1016/j.alcohol.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 194.Coriale G., Fiorentino D., Di Lauro F., Marchitelli R., Scalese B., Fiore M., Maviglia M., Ceccanti M. Fetal Alcohol Spectrum Disorder (FASD): neurobehavioral profile, indications for diagnosis and treatment. Riv. Psichiatr. 2013;48(5):359–369. doi: 10.1708/1356.15062. [DOI] [PubMed] [Google Scholar]
- 195.Ferraguti G., Merlino L., Battagliese G., Piccioni M.G., Barbaro G., Carito V., Messina M.P., Scalese B., Coriale G., Fiore M., et al. Fetus Morphology Changes by Second-Trimester Ultrasound in Pregnant Women Drinking Alcohol. Addict. Biol. 2019 doi: 10.1111/adb.12724. [DOI] [PubMed] [Google Scholar]
- 196.Ferraguti G., Ciolli P., Carito V., Battagliese G., Mancinelli R., Ciafrè S., Tirassa P., Ciccarelli R., Cipriani A., Messina M.P., Fiore M., Ceccanti M. Ethylglucuronide in the urine as a marker of alcohol consumption during pregnancy: Comparison with four alcohol screening questionnaires. Toxicol. Lett. 2017;275:49–56. doi: 10.1016/j.toxlet.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 197.Messina M.P., D’Angelo A., Battagliese G., Coriale G., Tarani L., Pichini S., Rasio D., Parlapiano G., Fiore M., Petrella C., Vitali M., Ferraguti G., Ceccanti M. FASD Study Group. Fetal alcohol spectrum disorders awareness in health professionals: implications for psychiatry. Riv. Psichiatr. 2020;55(2):79–89. doi: 10.1708/3333.3302232202545. [DOI] [PubMed] [Google Scholar]
- 198.Tarani L., Micangeli G., Rasio D., Ottombrino S., Liberati N., De Angelis D., Carito V., Greco A., Ceccanti M., Fiore M. Clinical and Genetic Approach to the Dysmorphic Child. Biomed. Rev. 2018;29(0):37–46. doi: 10.14748/bmr.v29.5848. [DOI] [Google Scholar]
- 199.Senturias Y., Asamoah A. Fetal alcohol spectrum disorders: guidance for recognition, diagnosis, differential diagnosis and referral. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44(4):88–95. doi: 10.1016/j.cppeds.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 200.Dylag K.A., Fidalgo S.V.S., Gard P.R., Patel B.A. Prenatal alcohol exposure reduces 5-HT concentration in mouse intestinal muscle and mucosa. Environ. Toxicol. Pharmacol. 2018;61:24–29. doi: 10.1016/j.etap.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 201.Tavares E., Gomez-Tubio A., Murillo M.L., Carreras O. Folic acid intestinal absorption in newborn rats at 21 day postpartum: effects of maternal ethanol consumption. Life Sci. 1999;64(22):2001–2010. doi: 10.1016/S0024-3205(99)00147-2. [DOI] [PubMed] [Google Scholar]
- 202.Miller M.W. Effect of prenatal exposure to ethanol on glutamate and GABA immunoreactivity in macaque somatosensory and motor cortices: critical timing of exposure. Neuroscience. 2006;138(1):97–107. doi: 10.1016/j.neuroscience.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 203.Través C., Coll O., Cararach V., Gual A., de Tejada B.M., López-Tejero M.D. Clinical approach to intestinal maturation in neonates prenatally exposed to alcohol. Alcohol Alcohol. 2007;42(5):407–412. doi: 10.1093/alcalc/agm005. [DOI] [PubMed] [Google Scholar]
- 204.Ceccanti M., De Nicolò S., Mancinelli R., Chaldakov G., Carito V., Ceccanti M., Laviola G., Tirassa P., Fiore M. NGF and BDNF long-term variations in the thyroid, testis and adrenal glands of a mouse model of fetal alcohol spectrum disorders. Ann. Ist. Super. Sanita. 2013;49(4):383–390. doi: 10.4415/ANN-13-04-1124334784. [DOI] [PubMed] [Google Scholar]
- 205.De Nicolò S., Carito V., Fiore M., Laviola G. Aberrant Behavioral and Neurobiologic Profiles in Rodents Exposed to Ethanol or Red Wine Early in Development. Curr. Dev. Disord. Rep. 2014;1(3):173–180. doi: 10.1007/s40474-014-0023-5. [DOI] [Google Scholar]
- 206.Fiore M., Laviola G., Aloe L., di Fausto V., Mancinelli R., Ceccanti M. Early exposure to ethanol but not red wine at the same alcohol concentration induces behavioral and brain neurotrophin alterations in young and adult mice. Neurotoxicology. 2009;30(1):59–71. doi: 10.1016/j.neuro.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 207.Fiore M., Mancinelli R., Aloe L., Laviola G., Sornelli F., Vitali M., Ceccanti M. Hepatocyte growth factor, vascular endothelial growth factor, glial cell-derived neurotrophic factor and nerve growth factor are differentially affected by early chronic ethanol or red wine intake. Toxicol. Lett. 2009;188(3):208–213. doi: 10.1016/j.toxlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 208.Ceccanti M., Mancinelli R., Tirassa P., Laviola G., Rossi S., Romeo M., Fiore M. Early exposure to ethanol or red wine and long-lasting effects in aged mice. A study on nerve growth factor, brain-derived neurotrophic factor, hepatocyte growth factor, and vascular endothelial growth factor. Neurobiol. Aging. 2012;33(2):359–367. doi: 10.1016/j.neurobiolaging.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 209.Nogales F., Ojeda M.L., Delgado M.J., Jotty K., Diaz Castro J., Murillo M.L., Carreras O. Effects of antioxidant supplementation on duodenal Se-Met absorption in ethanol-exposed rat offspring in vivo. J. Reprod. Dev. 2011;57(6):708–714. doi: 10.1262/jrd.11-049K. [DOI] [PubMed] [Google Scholar]
- 210.Joya X., Garcia-Algar O., Salat-Batlle J., Pujades C., Vall O. Advances in the development of novel antioxidant therapies as an approach for fetal alcohol syndrome prevention. Birth Defects Res. A Clin. Mol. Teratol. 2015;103(3):163–177. doi: 10.1002/bdra.23290. [DOI] [PubMed] [Google Scholar]
- 211.Miller E.A., Manning S.E., Rasmussen S.A., Reefhuis J., Honein M.A. National Birth Defects Prevention Study. Maternal exposure to tobacco smoke, alcohol and caffeine, and risk of anorectal atresia: National Birth Defects Prevention Study 1997-2003. Paediatr. Perinat. Epidemiol. 2009;23(1):9–17. doi: 10.1111/j.1365-3016.2008.00976.x. [DOI] [PubMed] [Google Scholar]