Abstract
Tetrahydrobipterin (BH4) is a pivotal enzymatic cofactor required for the synthesis of serotonin, dopamine and nitric oxide. BH4 is essential for numerous physiological processes at periphery and central levels, such as vascularization, inflammation, glucose homeostasis, regulation of oxidative stress and neurotransmission. BH4 de novo synthesis involves the sequential activation of three enzymes, the major controlling point being GTP cyclohydrolase I (GCH1). Complementary salvage and recycling pathways ensure that BH4 levels are tightly kept within a physiological range in the body. Even if the way of transport of BH4 and its ability to enter the brain after peripheral administration is still controversial, data showed increased levels in the brain after BH4 treatment. Available evidence shows that GCH1 expression and BH4 synthesis are stimulated by immunological factors, notably pro-inflammatory cytokines. Once produced, BH4 can act as an anti-inflammatory molecule and scavenger of free radicals protecting against oxidative stress. At the same time, BH4 is prone to autoxidation, leading to the release of superoxide radicals contributing to inflammatory processes, and to the production of BH2, an inactive form of BH4, reducing its bioavailability. Alterations in BH4 levels have been documented in many pathological situations, including Alzheimer's disease, Parkinson's disease and depression, in which increased oxidative stress, inflammation and alterations in monoaminergic function are described. This review aims at providing an update of the knowledge about metabolism and the role of BH4 in brain function, from preclinical to clinical studies, addressing some therapeutic implications.
Keywords: tetrahydrobiopterin (BH4), guanosine-triphosphate-cyclohydrolase-1 (GCH1), cofactor, monoamines, depression, Alzhemier, Parkinson
1. INTRODUCTION
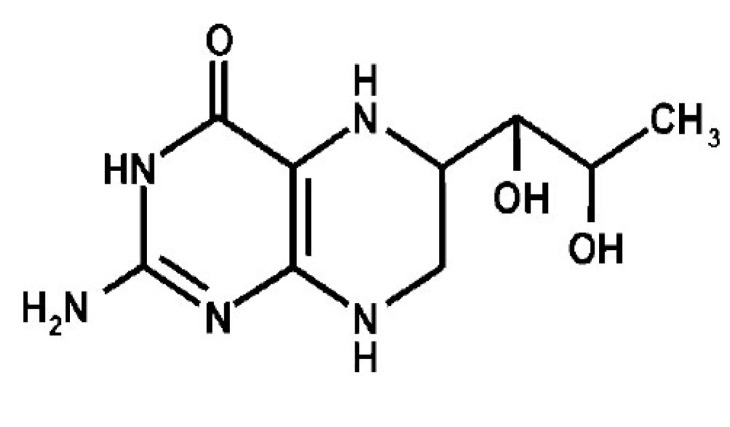
Tetrahydrobiopterin (BH4) belongs to the chemical group of pteridines, a widely distributed class of natural heterocyclic compounds (Fig. 1). Pteridine chemical ring systems can exist in several oxidation states, i.e., in the fully oxidized, dihydro (BH2; dihydrobiopterin), and tetrahydro (BH4) forms, but only the fully reduced form BH4 exhibits biological activity. All forms are synthesized from guanosine tri-phosphate (GTP) in the majority of prokaryotes and eukaryotes cell types [1]. They were originally discovered in butterfly wing pigments and derived their names from the Greek “pteron” meaning wing.
Fig. (1).
Chemical structure of BH4.
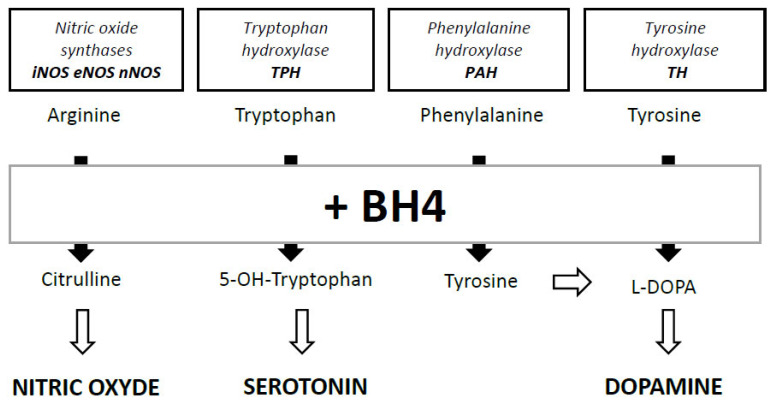
BH4 was identified in 1958 as an essential cofactor for phenylalanine hydroxylase (PAH), the enzyme that converts phenylalanine (Phe) into tyrosine (Tyr) [2, 3]. Ten years later, its role was shown as a cofactor for enzymatic reactions catalyzed by two other enzymes involved in the hydroxylation of aromatic amino acids, i.e., tyrosine hydroxylase (TH) [4] converting Tyr to 3,4-dihydroxyphenylalanine (L-DOPA) and tryptophan 5-hydroxylase (TPH) converting tryptophan to serotonin [5] (Fig. 2). BH4 is also necessary for the function of alkylglycerol monooxygenase (AGMO) [6]. Its role as a cofactor for nitric oxide synthases (NOS) for the production of nitric oxide (NO) was demonstrated in the 1990s [7, 8]. Due to its role as a cofactor for PAH and TH, BH4 is also needed in the production of norepinephrine (NE) and adrenaline (A) that are synthesized from dopamine (DA). The role of BH4 was particularly studied in the context of phenylketonuria, a rare disease caused by functional abnormalities of PAH, and leading to a deleterious accumulation of Phe in the body (hyperphenylalaninemia). An atypical phenylketonuria subtype caused by mutations in the enzymes responsible for BH4 synthesis has also been identified [9, 10]. The 1970s saw the emergence of the first synthetic form of BH4, also called sapropterin, whose uptake allows recovery normalization of circulating Phe levels in patients [11-13]. With regard to human disease, several pathological situations, including Alzheimer's disease (AD), Parkinson's disease (PD) and depression, have been suggested to be a consequence of restricted BH4 levels. This review aims at providing an update of the knowledge about metabolism and the role of BH4 in brain function, from preclinical to clinical studies, addressing some therapeutic implications.
Fig. (2).
Main roles of BH4 as enzymatic cofactor in the synthesis of nitric oxyde, serotonin, tyrosine and dopamine.
2. METABOLISM OF BH4 AND MAINTAIN OF HOMEOSTASIS
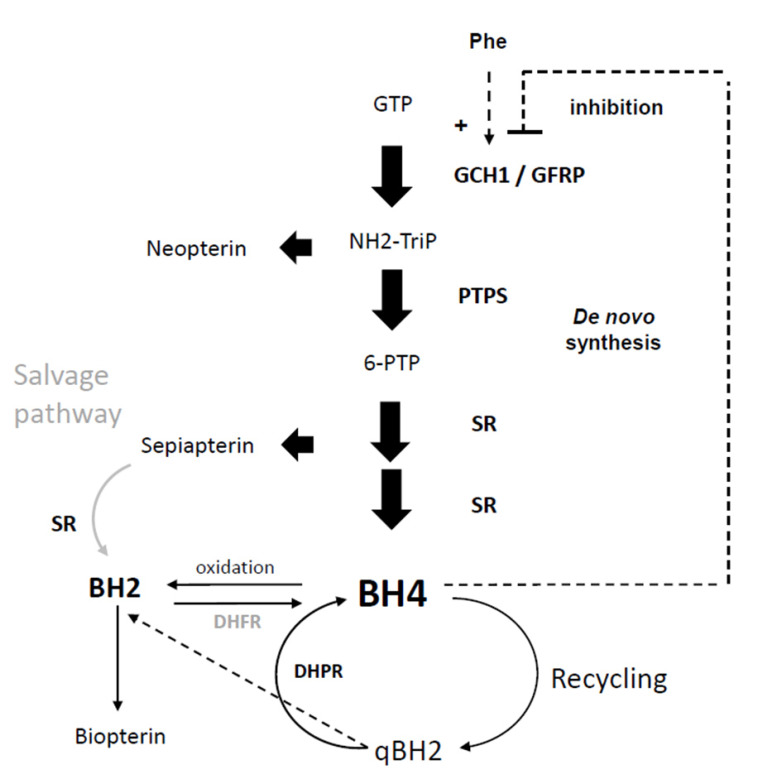
The availability of BH4 depends on its synthesis, its enzymatic use and its regeneration after use. Two major synthetic routes have been identified: the “de novo” synthesis and the “salvage” routes. A so-called “recycling” pathway also enables BH4 to be regenerated from BH2, the oxidized and inactive form of BH4 (Fig. 3) [1, 14].
Fig. (3).
Synthetic pathways for the de novo synthesis, salvage and recycling pathways of BH4. Dotted lines are the non-enzymatic reactions. Neopterin and biopterin are degradation products that are eliminated by the body but cannot be re-transformed into BH4.
Guanosine triphosphate (GTP), GTP cyclohydrolase 1 (GCH1), GTPCH feedback regulatory protein (GFRP), dihydroneopterin triphosphate (NH2-triP), 6-pyruvoyltetrahydrobiopterin synthase (PTPS), Phe, phenylalanine; 6-pyruvoyltetrahydrobiopterin (6-PTP), sepiapterin reductase (SR), tetrahydrobiopterin (BH4), dihydrofolate reductase (DHFR), dihydropteridine reductase (DHPR), dihydrobiopterin (BH2), dihydrobiopterin quinoid (qBH2). Adapted from [1].
2.1. Synthesis
At the peripheral and central levels, BH4 synthesis proceeds via the de novo pathway, starting from guanosine triphosphate (GTP) and involving a system of three consecutive enzymes: GTP cyclohydrolase 1 (GCH1), 6-pyruvoyltetrahydrobiopterin synthase (PTPS) and sepiapterin reductase (SR) (Fig. 2). GCH1 catalyzes the first step transforming GTP to 7-8 dihydroneopterin triphosphate (NH2-triP) [15, 16]. This last compound is then converted by PTPS to 6-pyruvoyltetrahydrobiopterin (6-PTP) [17, 18] that will form BH4 via a three-fold successive reductions reaction managed by SR [19, 20]. Aldoses and carbonyls reductases can also catalyze one or more of the reductions provided by SR, thus creating multiple alternative de novo pathways in the periphery, but not in the brain [1, 21]. Patients suffering from a genetic deficiency of the SR, therefore, have neurological dysfunctions without displaying peripheral hyperphenylalaninemia [22, 23]. In humans, GCH1 activity can be increased up to 100-fold under stimulation by interferon-gamma (IFγ) or interleukin 1beta (IL1β), for example, while PTPS and SR remain slightly increased [24, 25]. Consequently, PTPS becomes the rate-limiting enzyme in the pathway, favoring neopterin formation that signs the cell-mediated immune activation [26, 27].
In rats, the highest GCH1 activity, which is correlated to biopterin levels, was measured in the liver, followed by adrenal glands and kidneys [28, 29]. It is suggested that BH4 in these organs is supplied not only by intracellular de novo biosynthesis but also by uptake from the blood when BH4 is supplied exogenously [30]. GCH1 enzyme activity was also measured in different brain regions, the highest levels in rat being found in striatum and hypothalamus [29]. BH4, a product of GCH1 activity, is highly distributed in a tissue-specific way in mammals, suggesting the presence of a tissue-specific regulatory mechanism to maintain cellular BH4 homeostasis [31]. Interestingly, it has been shown that the intestinal microbiota also contains bacteria capable of generating BH4, being a possible source for the body [32, 33].
The final step of the de novo synthesis of BH4 is the conversion of BH2 to BH4 by the dihydrofolate reductase (DHFR). It occurs notably when the last reduction of 6-PTP transformation does not take place, resulting in the formation of 1-oxo-2-hydroxypropyl-BH4. This compound will be rearranged into sepiapterin, which can be converted by SR into dihydrobiopterin (BH2) and then reduced by DHFR to reform BH4 [34, 35]. The role of DHFR is then important in the synthesis of BH4, and to maintain BH4 / BH2 ratio [36] throughout the transformation of the oxidized inactive BH2 into BH4. This alternative salvage pathway contributes to BH4 synthesis in the case of SR deficiency, in patients or in KO mice [23, 37].
2.2. Recycling Route
The recycling pathway occurs after the use of BH4 by aromatic amino acid hydroxylases (AAAHs) for the synthesis of monoamines and NO. Then BH4 is regenerated in two reactions: the hydroxy-4a derivative formed can be rapidly transformed into qBH2 via the removal of a water molecule and then regenerated to BH4 by dihydropteridine reductase (DHPR) [38]. In the absence or reduction of DHPR activity, qBH2 will rapidly be rearranged into BH2, which will be further reduced into BH4 through the salvage pathway. However, it has been shown in patients with DHPR mutations that regeneration of BH4 using DHFR is not as effective as DHPR [39].
2.3. Synthesis Inhibition and Degradation
In the presence of high levels of BH4, GCH1-feedback regulatory protein (GFRP) binds to GCH1, leading to inhibition of its activity (Fig. 3) [34, 40]. This post-translational regulation is well known at the periphery but is less described in the brain where GFRP is poorly expressed [41]. Many proteins interact with GCH1 as partners, differentially modulating its activity and according to organs such as kidney, liver, heart or brain. To date, no mechanism of active degradation of BH4 has been demonstrated, probably due to its instability at physiological pH and its rapid oxidation to BH2 and biopterin [42].
2.4. BH4 Transport
Transport of BH4 and its ability to enter the brain after peripheral administration is still controversial. It must first be noted that the bioavailability of BH4 following oral intake is low, due to an important renal elimination [43, 44]. Thus, the pool of circulating BH4 available for the brain remains limited at any given time point. It is thus not surprising that early studies showed that very few or no peripherally administered radiolabeled BH4 was found in the brain [30, 45, 46]. It was also postulated that BH4 did not cross the blood-brain barrier (BBB) because of its hydrophilic structure. However, others reported a significant increase in BH4 brain levels after peripheral administration in rats [44, 47] or mice [46, 48-52]. We recently showed that peripheral acute administration of 50 mg/kg BH4 to mice induced a significant increase in BH4 levels in the brain, 3 hours after injection [53]. Most studies in humans and primates have shown that oral administration of BH4 or sapropterin leads to an increase in BH4 in the cerebrospinal fluid, suggesting its entry into the brain compartment [54-57]. Using in-situ brain perfusion, we found that the brain uptake clearance (Clup) of BH4 was approximately 0.08 μl.g-1.sec-1, consistent with a modest rate of transfer across the BBB [53]. Overall, this suggests that BH4 access to the brain is limited more by its rapid elimination from the blood than by an inability to cross the BBB.
More recently, Hasegawa and colleagues showed that intracellular accumulation of BH4 was abolished by the DHFR inhibitor methotrexate, leading to the hypothesis that BH2 is the “transportable” form of BH4, allowing its intracellular accumulation. Intracellular BH2 would then be reduced to BH4 by the rescue route [58-60]. It has also been shown that this transmembranous passage was mediated by the equilibrative nucleoside transporters ENT1 and ENT2 [61], at the periphery and within the brain. Organic anion transporters have also been shown to be carriers of BH4 and /or BH2 in kidneys [62] and brain [63]. However, ex vivo results from rat brain synaptosomes suggest that BH4 crosses passively through membranes [64].
BH4 contents in the brain may be saturated under physiological conditions, whereas it can be increased by peripheral administration in pathological conditions. Such cases will be discussed in part 6 of this review. However, the very rapid and uncontrollable conversion of BH4 to BH2 precludes any definitive conclusion, and studies comparing the cerebral passage of BH4 and BH2 remain to be done.
3. REGULATION OF BH4 SYNTHESIS
Modulation of BH4 synthesis is mainly expressed throughout the regulation of the activity of its limiting-enzyme [31]. Then cellular levels of Phe and BH4 exert an allosteric regulation of GCH1 activity via their binding to GFRP. GFRP induces the activity of GCH1 in the presence of Phe, whereas it leads to inhibition of the enzyme in the presence of BH4 (Fig. 3). GCH1 activity is also post-translationally regulated by phosphorylation, and transcriptionally by proinflammatory mediators. Then available evidence shows that GCH1 expression and BH4 synthesis are stimulated by immunological factors, notably pro-inflammatory cytokines, such as IL-1β, IFNγ, and tumor necrosis factor-alpha (TNFα), in response to pathogen invasion [24, 65-68]. In contrast, glucocorticoids and anti-inflammatory cytokines (IL-4, IL-10, and transforming growth factor [TGF]-beta) inhibit endothelial BH4 synthesis. Induction of GCH1 by pro-inflammatory factors has been observed in many tissues, including the vascular endothelium and the brain, in endothelial cells and astrocytes [65, 69-71]. Immune activation of GCH1 aims to increase BH4 bioavailability, in order to support iNOS function and NO production, contributing in turn to the elimination of the pathogen [72, 73]. GCH1 transcription occurs via activation of the nuclear factor-κB (NFκB), JAK/STAT pathway [66] and the MEK/ERK kinase pathway [69]. Under immune stimulation, the expression of PTPS and SR slightly increases [24, 25, 74], whereas GFRP expression decreases [75]. The extent of GCH1 expression being much higher than that of PTPS, the latter can become limiting in inflammatory conditions and lead to the synthesis of neopterin, a peripheral early marker of immune activation in Human, at the expense of BH4 [24, 27, 76].
Although GCH1 is induced in pro-inflammatory conditions, the question of the pro- or anti-inflammatory role of the resulting BH4 is not clear. Once synthesized, BH4 can act as an anti-inflammatory molecule and scavenger of free radicals protecting against oxidative stress [77-79]. In addition, BH4 is prone to autoxidation in the presence of molecular oxygen, leading to the release of superoxide radicals and therefore contributing to inflammatory processes [80]. Data obtained in murine models of inflammatory and neuropathic pain showed that pharmacological decrease in BH4 synthesis induces analgesia via a reduction of neuronal hyperexcitability and limitation of immune cell proliferation and inflammation, supporting a pro-inflammatory role of BH4 [81, 82]. Lowering BH4 levels using 2,4-Diamino-6-hydroxypyrimidine (DAHP) as a GCH1 inhibitor reduced neuropathic pain in rats following nerve injury and inflammation, confirming the pronociceptive action of excess BH4 production in the somatosensory system [83]. Concomitantly, the sensory responses to non-inflammatory pain (mechanical or thermal) of hph-1 mice deficient in BH4 are unchanged [84]. These results are in agreement with clinical data reporting significant reductions in both pain and inflammation in rheumatoid arthritis and inflammatory bowel disease patients treated with sulfasalazine [81], an inhibitor of SR [85] leading to reduced BH4 levels.
However, other studies have shown that supplementation with BH4 in murine atherosclerotic models reduces vascular inflammation [86-88], leukocyte infiltration, activation of macrophages, and expression of pro-inflammatory cytokines. A downregulation of pro-inflammatory cytokines has been described after BH4 treatment in a murine model of pancreas transplantation [89]. This was associated with a gain in the cell viability of the transplant and prolonged recipient survival. Primary cultures of neurons depleted in BH4 showed increased vulnerability to hypoxia and oxidative damage that lead to exacerbated cell death, illustrating here the antioxidant action of BH4 [90]. Levels of neopterin have been shown to increase after traumatic brain injury or brain infections, indicating a local production by nervous cells, where it is supposed to exert cytoprotective and anti-inflammatory properties [27]. These data rather support here a role for BH4 as an anti-inflammatory molecule.
Overall, the response of BH4 as a pro- or anti-inflammatory molecule is complex and largely depends on the oxidative status of the cell.
4. COFACTOR FUNCTION OF BH4 IN ENZYMATIC REACTIONS
The main role of BH4 is to serve as a cofactor for monooxygenase enzymes, which catalyze the insertion of a single oxygen atom into an organic substrate. Families of monooxygenases requiring BH4 for catalysis, namely the AAAHs and the NOS, are presented in the following sections.
AAAHs include PAH, TH and TPH, which enzymatic functions are strictly dependent on the presence of BH4, oxygen and iron. The reaction proceeds at the active catalytic site of the enzymes comprising a non-heme iron molecule. BH4 allows the activation of oxygen during its binding to iron and the so formed oxo-iron complex allows enzymatic hydroxylation of the substrate, i.e., Tyr, Phe or tryptophan. During these enzymatic reactions, BH4 is oxidized and a pterin 4a-carbinolamine intermediate is formed [91, 92]. This derivative will be converted back to BH4 via the recycling pathway. In the complete absence of regeneration, the reaction is stoechiometric. NOS is a family of catalytic synthases, including neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible stoechiometric NOS (iNOS) that synthesize NO, the smallest known bioactive gas molecule [93, 94]. Basal NOS enzyme activity is due to a residual amount of BH4 bound tightly to protein. NOS use BH4 differently as compared to AAAHs and present greater affinity for the cofactor [95]. Thus, the Km values of BH4 for PAH and NOS are 2-3 μM and 0.02-0.03 μM, respectively. It has been shown that basal BH4 synthesis appears to be adequate to support iNOS activity, whereas BH4 production has to be increased to support PAH activity [96]. In NOS function, BH4 acts as a single electron donor [97, 98], which is used to reduce and activate oxygen and to oxidize L-arginine to L-citrulline and NO. This reaction is not stoechiometric and a single molecule of BH4 allows the production of 15 to 26 molecules of NO [99, 100]. The dimer form is the active one of NOS and the presence of BH4 is necessary for their dimerization [101-103]. Limitation of BH4 availability leads to NOS uncoupling, generating superoxide rather than NO. Moreover, superoxide anions and oxygenated free radicals promote the oxidation of BH4 to BH2, its inactive form [104, 105], contributing to maintain a BH4 deficiency state. It has been shown that BH2 competes with BH4 for NOS binding site making the BH4/BH2 ratio an important determinant in NOS dimerization and function [36, 106, 107]. Thus, oxidation of BH4 is both a cause and a consequence of the monomerization and then uncoupling of NOS, creating a “vicious circle” that leads to the increase of oxidative stress.
5. CENTRAL ROLES OF BH4
BH4 is an essential molecule for numerous well known physiological processes such as proliferative activity, self-protecting factor for NO toxicity, vascularization, inflammation, glucose homeostasis and oxidative status [14]. In this part, we will focus on its role in the central nervous system (CNS) as a key regulator of monoaminergic neurotransmission and related behavior.
5.1. Cerebral Distribution of BH4
The brain distribution of BH4 is heterogeneous with highly enriched areas in regions concentrating on monoaminergic neurons expressing TH and TPH [108]. It has been confirmed that mRNA and protein expression of GCH1 predominates in serotonergic neurons compared to noradrenergic and dopaminergic neurons [109-111]. The expression of enzymes for BH4 synthesis is also observed in the monoaminergic neurons of the Raphe nuclei, the substantia nigra and the ventral tegmental area [112]. Thus, BH4 can be synthesized in all monoaminergic cells and is readily available for enzymes requiring it. Primary or immortalized culture studies also showed that the system for BH4 synthesis is present and functional in cerebral glial cells, with BH4 production by astrocytes and microglia after immune stimulation [65, 113, 114]. However, immunohistochemistry analyses do not provide evidence of colocalization between nNOS and GCH1 [115]. This suggests that NOS cells may obtain BH4 from monoamine-containing processes which terminate in close proximity, or from the circulating pool.
5.2. Synthesis and Release of Neurotransmitters
As a cofactor of PAH, TH and TPH, BH4 is essential for the synthesis of two major neurotransmitters: DA and serotonin (5HT). It is also essential for the synthesis of NO, which, apart from its vascular role, also acts as a modulator of neurotransmission. Measurements of monoamine levels in murine models of partial deficiencies in BH4 have confirmed its importance in monoamine synthesis. Accordingly, reduced levels of 5HT and DA are measured in the brain of hph-1 mouse (hyperphenylalaninemia 1, hph1 model), in which GCH1 is muted and brain BH4 levels decreased by a half [48, 116, 117]. This effect is also observed in models of genetic inactivation of other BH4 synthesis enzymes, i.e., SR (SR (-/-) model) [37, 118, 119] and PTPS (PTPS (-/-) model) [120].
Since the 1990s, many studies have tested the effects of BH4 supplementation on monoamine amounts in the brain of rodents, with divergent conclusions (Table 1). Differences of the BH4 effect on monoamines amounts can be explained by the multiplicity of doses and modes of administration of BH4 but also by assay methods. Regarding DA, in particular, it remains unclear whether BH4-mediated enhancement of release is due to an increase in DA synthesis or to a direct pro-release effect, independently of its cofactor action on TH. Indeed, it was shown that enhancement of DA release in the rat striatum, by local BH4 administration through retro-dialysis, persists at a lower level when DA biosynthesis is blocked by TH inhibition [121-124]. Moreover, we recently showed that increased BH4 brain levels induced by acute administration of BH4 in mice led to a rise in stimulated DA release in the nucleus accumbens, independently of changes in DA transporters DAT or VMAT2 protein expression [53]. Our results rather support that the peripheral administration of BH4 leads to increased synthesis and storage of DA, then ready to be released upon stimulation.
Table 1. Summary of studies investigating the effect of BH4 administration on cerebral monoamines.
| Route of BH4 administration | Brain structure of interest | Species | Observation | References |
|---|---|---|---|---|
| Superfused slices,50 μM | Mesencephalon | Wistar rats | ↗5HT release | [29] |
| i.p., 50 mg/kg | Striatum, hippocampus | Wistar rats | No change of 5HT tissue contents | [47] |
| s.c., 100-1000 μM/kg | Total brain | hph-1 mice | ↗ 5HT and metabolites tissue contents | [48] |
| i.p., 50 mg/kg for7 days | Total brain | SR KO mice | ↗DA tissue contents | [49] |
| Oral, 50 mg/kg for10 days | Total brain | ENU1/2 mice | No change of DA, 5HT or metabolites tissue contents | [51] |
| Oral, 20-100 mg/kg | Total brain | C57Bl6 mice | ↗ metabolites of DA and 5HT | [52] |
| i.p., 50 mg/kg | Nucleus accumbens | C57Bl6/J mice | ↗stimulated-DA release | [53] |
| i.v., 20 mg/kg | CSF | Rhesus monkeys | No change of NA, DA, or 5HT synthesis | [56] |
| i.p., 50 mg/kg | Total brain | New born PTPS ko mice | ↗ DA and 5HT tissue contents | [120] |
| Retrodialysis,0.25-1 mM | Striatum | Wistar rats | ↗ DA release | [121] |
| Slices superfusion,0.1 mM | Striatum | Sprague-Dawley rats | ↗ DA release | [122] |
| Retrodialysis, 1 mM | Prefrontal cortex, striatum | Wistar rats | ↗ DA, 5HT, and glutamate release | [123] |
| ICV 500 μg | Whole brain | Wistar rats | ↗ DA, 5HT and metabolites tissue contents | [125] |
| Retrodialysis,0.25 -1 mM | Striatum | Wistar rats | ↗ DA release | [126] |
| Synaptosome incubation,10-200 μM | Forebrain | Sprague-Dawley rats | No change of 5HT release | [127] |
| Superfused slices,200 μM | Hippocampus | Sprague-Dawley rats | No change of 5HT spontaneous release,↗ stimulated release | [128] |
| Retrodialysis,1.25 -1 mM | Hippocampus | Wistar rats | ↗ DA, 5HT, and Ach release | [129] |
| i.p., 50 mg/kg | Striatum | C57Bl6/J mice | ↗ L-DOPA release | [130] |
Abbreviations: ICV, intracerebroventricular; CSF, cerebrospinal fluid; i.v., intravenous; i.p., intra-peritoneal; Ach, acetylcholine; s.c., subcutaneous.
5.3. Role of BH4 in Behavior: Lessons Learned from Animal Studies
Some clinical conditions are associated with defective in BH4-pathway function, caused by mutations in genes encoding the enzymes involved in its biosynthesis (GCH1, PTPS, and SR) or regeneration. The clinical manifestation of BH4 deficiency largely varies according to the type of mutation; however, motor and/or cognitive dysfunctions and monoamine neurotransmitter deficiency in the brain are repeatedly observed [131]. Preclinical studies have been led thanks to the development of congenital BH4 deficient models.
The hph-1 mouse model of dominantly inherited GCH1 deficiency is characterized by a great decrease in GCH1 activity and BH4 biosynthesis in liver and plasma and a 50% decrease in brain BH4 levels [84, 116, 132]. Whereas physical, sensorimotor reflexes and locomotor activity are identical to that of the wild-type control mice, hph-1 mice exhibit anxiety and depressive-like behavior. These behaviors are strongly dependent on serotonergic transmission [133, 134], consistent with the decrease in 5HT levels observed in these animals [135]. However, DA-dependent behaviors such as locomotion or motivation seem little affected and remain poorly studied. SR(-/-) mice display quite different behavioral phenotypes from hph-1 mice despite an equivalent decrease in BH4 brain levels [37, 119], with important locomotor disorders in adulthood [49]. SR(-/-) mice also displayed severe growth retardation, and loss of BH4 and monoamines levels in the brain, particularly due to reduced TH protein levels [49, 118]. These mice have also been proposed as an interesting model to study Parkinson disease (PD) since, associated with the dramatic fall of TH protein in the brain, they showed a tremor-like phenotype after weaning [136]. Homozygous KO mice for the PTPS with no BH4 biosynthesis die after birth, emphasizing the crucial role of BH4.
Limited data are available on the effect of exogenous BH4 administration on animal behavior. Asami and Kuribara [137] described an increased hyper-locomotion induced by methamphetamine challenge after peripheral BH4 injection in mice (100 mg/kg). This pro-locomotor effect of repeated BH4 administration was also reported in a rat model of the neonatal serotoninergic lesion [138]. Latini et al. [139] showed that BH4 administered i.c.v. enhanced aversive memory in mice and rats through the activation of glutamatergic neurotransmission and cell threshold reduction triggering long-term potentiation (LTP). We recently showed that acute peripheral injection of BH4 induced an enhanced amphetamine-stimulated DA release in the nucleus accumbens, associated with the improved performance of a motivational task [53]. Other studies also reported beneficial effects of BH4 administration in congenital BH4-deficiency models. Thus, BH4-deficient mice lacking SR showed persistently elevated BH4 and DA levels in the brain and fully restored loss of TH protein after subchronic BH4 administration in infants, whereas supply during adulthood was less efficient [49].
Altogether, these data highlight the role of BH4 in central functions, notably because of its action as a cofactor for AAAHs and NOS activity. Moreover, BH4 administration is suggested to be beneficial in some behavioral conditions, particularly in case of the reduced endogenous pool of monoamines.
6. INVOLVEMENT OF BH4 IN NEUROPSYCHIATRIC AND NEURODEGENERATIVE DISEASES
Recent years have seen the emergence of literature defining BH4 deficiency as a factor causing or aggravating symptoms related to monoaminergic neurotransmission disruptions, or to oxidative damage, due to its role as a coenzyme for AAAHs and NOS. Supporting this assumption, decreases in peripheral and/or central levels of BH4 have been observed in several neuropsychiatric and neurodegenerative diseases in which oxidative stress and / or alterations of monoaminergic function are described. Based on these data, the BH4 pathway is emerging as an important regulator for a number of clinical conditions associated with the overproduction of inflammatory mediators. Here, we will focus on a possible role of BH4 in the onset or progression of depression, Parkinson and Alzheimer diseases.
6.1. Depression
Previous clinical studies reported low postmortem BH4 levels in the brain of subjects with a history of severe depression [140]. Conversely, higher urine and plasma total biopterin levels were measured in depressed patients [28, 141-144], possibly due to altered BH4 metabolism. Moreover, patients with dopa-responsive dystonia caused by an inherited defect in GCH1 show an increased prevalence of depressive disorders and anxiety [145, 146]. These data highlight the potential involvement of reduced bioavailability of BH4 in the development and/or progression of the pathology. Increases in neopterin plasma levels were also reported in depression [147-149]. Neopterin, a byproduct of BH4 (Fig 2), reflects the activation of the cellular immune system, which is important in the pathogenesis and progression of depression. Then, an increasing amount of data, including our, suggest a prominent role of inflammation in mood disorders and particularly in depression [150-154]. A high prevalence of depressive symptoms has thus been described in patients treated with IFN-α, in correlation with increased levels of IL-6 in the CSF of patients [155, 156]. Evidence of reduced BH4 activity has also been shown in the same patients throughout a higher peripheral blood Phe/Tyr ratio, which in turn correlates with decreased CSF DA and its major metabolite homovanillic acid (HVA). Moreover, it is well known that inflammatory stimulation activates iNOS, which considerably increases the use of BH4 for optimal enzymatic activity, and induces the formation of large amounts of oxygen radicals that, in turn, contribute to the oxidative loss of BH4 [157]. Increased use of BH4 driven by a chronic inflammatory state may lead to a depletion altering the function of BH4-dependent enzymes, especially AAAH, and then compromise the biosynthesis of monoamines, which may contribute to the development of mood disorders [155, 158]. Then, it is known that innate immune activation and the release of inflammatory cytokines preferentially affect reward circuitry and DA in the basal ganglia and contribute to reduced motivation and motor slowing, core symptoms of depression [159, 160].
Administration of BH4 has been tested in depressed patients with contrasting results, leading either to an improvement in depressive symptoms [161, 162] or no effect [163]. However, it should be noted that most of these studies have been carried out on small cohorts. It has also been shown that the Phe/Tyr ratio, used as a marker of GCH1 activity, declines in depressive patient responders to electroconvulsive therapy [164], in accordance with an increase in PAH activity. However, very few data are available concerning the possibility of targeting the BH4-pathway to improve the therapeutic antidepressant response. One study described in a Japanese population of depressive patients that GCH1 was associated with antidepressant therapeutic response and may predict response to this class of antidepressant [165]. Moreover, a particular SR promoter haplotype, involved in the final step of BH4 synthesis, was associated with the occurrence of bipolar disorder and a better therapeutic response to selective serotonin reuptake inhibitors (SSRI) [166]. However, these data remain patchy and further clinical investigations in larger cohort samples are needed. A preclinical study showed that antidepressant treatment reduced BH4 levels in the frontal cortex of mice under stress conditions, in parallel to a reduced DA and 5HT turnover [167]. These data raise the possibility that a defect in the BH4 pathway can underlie certain depressive syndromes and impact the response to antidepressant treatment.
6.2. Parkinson Disease
Inherited disorder of BH4-related enzymes produces a phenotype of PD-like movement disorders, including dystonia alleviated following treatment with L-DOPA [168, 169]. Loss-of-function mutations in GCH1 result in striatal DA depletion and DOPA-responsive dystonia but also to nigrostriatal cell loss [170]. Accordingly, GCH1 variants are associated with an increased risk for PD [171, 172]. An early study reported a lower BH4 concentration in the CSF of PD patients [173, 174]. It was confirmed in the following years that levels of BH4 and activity of GCH1 were markedly decreased in the substantia nigra and striatum of PD patients [175, 176]. Depleted concentrations of both DA and 5HT in the caudate-putamen (~98 and ~50%, respectively) are observed in neuropathologically-confirmed PD cases [177]. By contrast, postmortem mRNA levels of SR were reported to be increased in the cerebellum of PD patients [178]. The increase in the expression of the SR gene may suggest a compensatory effect in PD brain in order to mobilize the salvage pathway for BH4 synthesis, and involvement of SR in PD pathogenesis. The loss of monoaminergic BH4-containing neurons in PD stands as an obvious explanation for this BH4 deficiency state. However, a suboptimal availability of the cofactor may also be an early and significant contribution to the events leading to neuronal damages associated with PD. Thus, BH4 has been shown to exert toxicity on DA-producing cell lines by oxidative stress, and that may represent a mechanism by which selective degeneration of dopaminergic terminals and neurons occurs [179-182]. Data showed that BH4 could induce cyclo-oxygenase (COX-2) expression, which in turn is responsible for DA oxidation, leading to the preferential vulnerability of dopaminergic cells in PD [183]. Moreover, preclinical studies showed that intranigral and intrastriatal BH4 injection causes a loss of TH immunoreactivity and decreases DA content in rats, by apoptotic and mitochondrial dysfunction mechanisms [184-186]. Intranigral infusion of iron generates behavioral PD model, and causes increases in production of both highly oxidable DA and BH4, which in turn compete to generate oxidative stress leading to a degeneration of the nigrostriatal pathway [187]. Very recently, the perturbation in BH4 metabolism was shown to be involved in early and persistent DA depletion in the striatum of MPTP-treated mice, a rodent model of PD, whereas administration of BH4 restored DA contents and TH activity [50]. The therapeutic efficacy of BH4 for the treatment of PD patients was suggested decades ago, but clinical studies reported minimal effects [188, 189].
Timing of BH4 supplementation across the progression of the disease is crucial, the earliest the more efficient, when a substantial proportion of dopaminergic neurons remain. Gene therapy assays with genes involved in the production of DA, namely, TH, aromatic L-amino acid decarboxylase (AADC) and also GCH1, as limiting enzyme for the synthesis of BH4, are in progress in preclinical and clinical trials [190-192]. Significant behavioral improvement in movement disorders was observed to last months or years in different models of PD [193-195]. Initial TH gene therapy experiments in animal models showed that a parallel source of BH4 was required to achieve sufficient L-DOPA levels in the brain [193, 196]. Optimizing transgene configuration has been developed to maximize DA production [197, 198]. Therefore, while its role in pathophysiology or in compensatory mechanisms remains unclear, BH4 remains to this day a key element to consider in PD and in its treatment.
6.3. Alzheimer Disease
Case-control studies have reported decreased levels of BH4 and/or its metabolites (BH2 and biopterin) in plasma [199], or in brain regions of AD patients [173, 200-202]. A post-mortem study reported impaired brain synthesis capacity for BH4 without alteration of the recycling pathway in the brain of patients with AD [200]. Increases in neopterin serum levels from AD patients have also been observed, indicative of a pro-inflammatory immune status and/or concomitant reduction of BH4 formation [203-205]. Together, these studies suggest that BH4 is oxidized in patients suffering from AD, and less available for its use as an enzymatic cofactor. This parallel decrease in BH4 levels and an increase in neopterin is also described in normal aging [152, 206]. While these changes might be consequences of the disease, a decrease in BH4 bioavailability could also contribute to increase the risk of developing the neurodegenerative disease with age, particularly by promoting oxidative stress [173]. The reduced availability of BH4 in AD could contribute to alter monoaminergic neurotransmission, known to play a role in AD symptoms. Many clinical studies found reductions of 5HT, along with its metabolites and receptors in AD postmortem brain tissue [207]. The dopaminergic system is also altered in AD, with a reduction of DA, main metabolites, and dopamine receptors [208, 209].
To our knowledge, no clinical study has tested the potential therapeutic effect of BH4 administration in AD. Preclinical studies led to animal models of AD confirmed the involvement of BH4 oxidation in the pathogenesis of the disease. For example, d’Uscio et al. [210] showed reduced levels of BH4 in the aorta of transgenic Tg2576 mice overexpressing mutated human Amiloyd β precorsor protein (APP), caused by increased oxidation and reduced GCH1 activity. It has been proposed that this reduced bioavailability of BH4 is a major mechanism responsible for the pathogenesis of endothelial dysfunction in cerebral microvessels of the AD mice, causing increased oxidative stress that contributes to catabolize BH4 in a vicious cycle [211]. However, the administration of PPARδ (peroxisome proliferator-activated receptor delta) receptor agonist normalizes GCH1 activity and restores the amount of BH4, allowing recovery of normal endothelial function [210]. It has been recently shown that BH4 administration improves memory acquisition, consolidation and hippocampal plasticity in mice [139]. Furthermore, BH4 supplementation in individuals with phenylketonuria improves working memory and neuronal activity in the prefrontal cortex [212]. This evidence leads to consider BH4 as a potential co-treatment for memory deficits, particularly in pathological conditions such as AD [173]. The role of BH4 as adjunctive treatment in AD is particularly interesting in view of its anti-inflammatory and anti-oxidant properties. Neuroinflammation and oxidative stress are key components and neuropathological features of AD [213-215]. Oxidative stress and the immune response participate all together in a close relationship in the molecular, cellular, and behavioral effects of AD, leading to cognitive impairment. For instance, the pro-inflammatory cytokine IL-1β is upregulated in the human AD brain and plasma [216-218] and genetic polymorphism of IL-1β is associated with a higher risk of developing AD [219]. Studies in mouse models of AD have shown that IL-1β is involved in Aβ production and aggregation [220, 221], which in turn up-regulates IL-1β production through the NLRP3 inflammasome activation [222, 223]. Oxidative stress can be either causative or consecutive, to protein aggregation that occurs in AD [224]. Besides its action on monoaminergic transmission, supplying BH4 to reduce inflammation and oxidative stress could then be a promising strategy to prevent AD and its symptoms.
Interestingly, BH4 supplementation could also improve peripheral metabolic disorder that is associated with AD such as glucose intolerance and insulin resistance [225, 226]. Indeed, the beneficial effect of BH4 administration has already been observed in various mouse models of metabolic disturbances [227-229]. Particularly, BH4 treatment ameliorates glucose tolerance and insulin resistance by suppressing hepatic gluconeogenesis [228]. We showed that insulin challenge could reverse the high-fat diet (HFD)-induced metabolic disturbances and cognitive alterations in the 3xTg murine model of AD [230]. Thus, given its ability to regulate metabolism, oxidative stress and inflammation, BH4 could be useful for AD and related dementias associated with metabolic impairments.
CONCLUDING REMARKS / THERAPEUTIC STRATEGIES FOR FUTURE
Data presented in this review showed that despite the critical role of BH4 for the synthesis of DA and 5HT is well known, the effect of exogenous BH4 administration on monoaminergic neurotransmission and related behaviors is poorly characterized. Alterations in BH4 levels are observed in many pathological situations. However, very few studies have characterized the effects of BH4 supplementation in animal models and clinical trials are needed. Some data suggest that BH4 administration represents a potential and complementary approach, helping to improve some cognitive or affective alterations associated with specific neuropsychiatric or neurodegenerative diseases. However, relatively high costs of BH4 synthesis, associated with a limited CNS bioavailability, would likely require the administration of large doses to reach therapeutic effect. A complementary strategy would be to increase BH4 synthesis or limit its oxidative degradation. As an example, folic acid (vitamin B), and in particular its active metabolites 5-methyltetrahydrofolate, may reduce BH4 oxidation and increase the recycling of BH2 to BH4 by increasing DHFR activity [231-234]. Clinical trials have also demonstrated the efficacy of L-methylfolate in the treatment of residual symptoms in depressed patients treated by SSRI [235]. These molecules seem promising to increase BH4 concentrations in key tissues while overcoming the constraints related to its pharmacological administration. Still, additional preclinical data are needed to better design clinical trials using BH4 supplementation in various potential pathological conditions. Increased knowledge is also needed about BH4 reciprocal interaction with biological systems and its various regulators, to be able to adjust BH4 supply according to the inflammation and/or the redox state of the cell and to obtain beneficial physiological effects.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- A
Adrenaline
- Ach
Acetylcholine
- AADC
Aromatic L-Amino Acid Decarboxylase
- AD
Alzheimer Disease
- AGMO
Alkylglycerol Monooxygenase
- AAAHs
Aromatic Amino Acid Hydroxylases
- BBB
Blood Brain Barrier
- BH2
Dihydrobiopterin
- BH4
Tetrahydrobiopterin
- COX-2
Cyclooxygenase-2
- CSF
Cerebrospinal Fluid
- DA
Dopamine
- DAHP 2
4-Diamino-6-hydroxypyrimidine
- DAT
Dopamine Transporter
- DHFR
Dihydrofolate Reductase
- DHPR
Dihydropterin Reductase
- eNOS
Endothelial Nitric Oxide Synthase
- ENT
Equilibrative Nucleoside Transporters
- GCH1
Guanosnine Triphosphate Cyclohydrolase-1
- GFRP
GTP Cyclohydrolase Feedback Regulatory Protein
- GTP
Guanosine Triphosphate
- HVA
Homovanillic Acid
- HFD
High Fat Diet
- hph-1
Hyperphenylalaninemia 1 hph1 Model
- 5HT
5-hydroxytryptamine, Serotonin
- i.c.v.
Intracerebroventricular
- IFγ
Interferon γ
- IL
Interleukin
- iNOS
Inducible Nitric Oxide Synthase
- i.p.
Intraperitoneal
- i.v.
Intravenous
- KO
Knock Out
- L-DOPA
3, 4-dihydroxyphenylalanine
- NE
Norepinephrin
- NFκB
Nuclear Factor-kappa B
- NH2-triP
Dihydroneopterin Triphosphate
- nNOS
Neuronal Nitric Oxide Synthase
- NO
Nitric Oxyde
- NOS
Nitric Oxide Synthase
- PAH
Phenylalanine Hydroxylase
- PD
Parkinson Disease
- Phe
Phenylalanine
- PPAR
Peroxisome Proliferator-Activated Receptor
- PTPS
6-pyruvoyltetrahydrobiopterin Synthase
- qBH2
Dihydrobiopterin Quinoid
- s.c.
Subcutaneous
- SR
Sepiapterin Reductase
- SSRI
Selective Serotonin Reuptake Inhibitor
- TH
Tyrosine Hydroxylase
- TNFα
Tumor Necrosis Factor α
- TPH
Tryptophane Hydroxylase
- Tyr
Tyrosine
- VMAT2
Vesicular Monoamine Transporter 2
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Werner E.R., Blau N., Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem. J. 2011;438(3):397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman S. Phenylalanine hydroxylation cofactor in phenylketonuria. Science. 1958;128(3337):1506–1508. doi: 10.1126/science.128.3337.1506. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman S. The structure of the phenylalanine-hydroxylation cofactor. Proc. Natl. Acad. Sci. USA. 1963;50:1085–1093. doi: 10.1073/pnas.50.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd T., Weiner N. Isolation and characterization of a tyrosine hydroxylase cofactor from bovine adrenal medulla. Mol. Pharmacol. 1971;7(6):569–580. [PubMed] [Google Scholar]
- 5.Storm C.B., Kaufman S. The effect of variation of cofactor and substrate structure on the action of phenylalanine hydroxylase. Biochem. Biophys. Res. Commun. 1968;32(5):788–793. doi: 10.1016/0006-291X(68)90309-4. [DOI] [PubMed] [Google Scholar]
- 6.Tietz A., Lindberg M., Kennedy E.P. A NEW PTERIDINE-REQUIRING ENZYME SYSTEM FOR THE OXIDATION OF GLYCERYL ETHERS. J. Biol. Chem. 1964;239:4081–4090. [PubMed] [Google Scholar]
- 7.Kwon N.S., Nathan C.F., Stuehr D.J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J. Biol. Chem. 1989;264(34):20496–20501. [PubMed] [Google Scholar]
- 8.Tayeh M.A., Marletta M.A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J. Biol. Chem. 1989;264(33):19654–19658. [PubMed] [Google Scholar]
- 9.Kaufman S., Berlow S., Summer G.K., Milstien S., Schulman J.D., Orloff S., Spielberg S., Pueschel S. Hyperphenylalaninemia due to a deficiency of biopterin. A variant form of phenylketonuria. N. Engl. J. Med. 1978;299(13):673–679. doi: 10.1056/NEJM197809282991301. [DOI] [PubMed] [Google Scholar]
- 10.Rey F., Harpey J.P., Leeming R.J., Blair J.A., Aicardi J., Rey J. [Hyperphenylalaninaemia with normal phenylalanine-hydroxylase activity and a deficiency of tetrahydrobiopterin and dihydropteridine reductase]. Arch. Fr. Pediatr. 1977;34(7) Suppl.:CIX–CXX. [Hyperphenylalaninaemia with normal phenylalanine-hydroxylase activity and a deficiency of tetrahydrobiopterin and dihydropteridine reductase]. [PubMed] [Google Scholar]
- 11.Schaub J., Däumling S., Curtius H.C., Niederwieser A., Bartholomé K., Viscontini M., Schircks B., Bieri J.H. Tetrahydrobiopterin therapy of atypical phenylketonuria due to defective dihydrobiopterin biosynthesis. Arch. Dis. Child. 1978;53(8):674–676. doi: 10.1136/adc.53.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heintz C., Cotton R.G.H., Blau N. Tetrahydrobiopterin, its mode of action on phenylalanine hydroxylase, and importance of genotypes for pharmacological therapy of phenylketonuria. Hum. Mutat. 2013;34(7):927–936. doi: 10.1002/humu.22320. [DOI] [PubMed] [Google Scholar]
- 13.Wasim M., Awan F.R., Khan H.N., Ayesha H. An Overview of Traditional and Novel Therapeutic Options for the Management of Phenylketonuria. Crit. Rev. Eukaryot. Gene Expr. 2018;28(2):177–185. doi: 10.1615/CritRevEukaryotGeneExpr.2018023073. [DOI] [PubMed] [Google Scholar]
- 14.Thöny B., Auerbach G., Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000;347(Pt 1):1–16. doi: 10.1042/bj3470001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burg A.W., Brown G.M. The biosynthesis of folic acid. 8. Purification and properties of the enzyme that catalyzes the production of formate from carbon atom 8 of guanosine triphosphate. J. Biol. Chem. 1968;243(9):2349–2358. [PubMed] [Google Scholar]
- 16.Niederwieser A., Staudenmann W., Wetzel E. High-performance liquid chromatography with column switching for the analysis of biogenic amine metabolites and pterins. J. Chromatogr. A. 1984;290:237–246. doi: 10.1016/S0021-9673(01)93579-4. [DOI] [PubMed] [Google Scholar]
- 17.Heintel D., Ghisla S., Curtius H.C., Niederwieser A., Levine R.A. Biosynthesis of tetrahydrobiopterin: possible involvement of tetrahydropterin intermediates. Neurochem. Int. 1984;6(1):141–155. doi: 10.1016/0197-0186(84)90039-1. [DOI] [PubMed] [Google Scholar]
- 18.Milstien S., Kaufman S. Tetrahydro-sepiapterin is an intermediate in tetrahydrobiopterin biosynthesis. Biochem. Biophys. Res. Commun. 1983;115(3):888–893. doi: 10.1016/S0006-291X(83)80018-7. [DOI] [PubMed] [Google Scholar]
- 19.Levine R.A., Kapatos G., Kaufman S., Milstien S. Immunological evidence for the requirement of sepiapterin reductase for tetrahydrobiopterin biosynthesis in brain. J. Neurochem. 1990;54(4):1218–1224. doi: 10.1111/j.1471-4159.1990.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith G.K. On the role of sepiapterin reductase in the biosynthesis of tetrahydrobiopterin. Arch. Biochem. Biophys. 1987;255(2):254–266. doi: 10.1016/0003-9861(87)90392-4. [DOI] [PubMed] [Google Scholar]
- 21.Kapatos G. The neurobiology of tetrahydrobiopterin biosynthesis: a model for regulation of GTP cyclohydrolase I gene transcription within nigrostriatal dopamine neurons. IUBMB Life. 2013;65(4):323–333. doi: 10.1002/iub.1140. [DOI] [PubMed] [Google Scholar]
- 22.Blau N., Bonafé L., Thöny B. Tetrahydrobiopterin deficiencies without hyperphenylalaninemia: diagnosis and genetics of dopa-responsive dystonia and sepiapterin reductase deficiency. Mol. Genet. Metab. 2001;74(1-2):172–185. doi: 10.1006/mgme.2001.3213. [DOI] [PubMed] [Google Scholar]
- 23.Bonafé L., Thöny B., Penzien J.M., Czarnecki B., Blau N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent monoamine-neurotransmitter deficiency without hyperphenylalaninemia. Am. J. Hum. Genet. 2001;69(2):269–277. doi: 10.1086/321970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner E.R., Werner-Felmayer G., Fuchs D., Hausen A., Reibnegger G., Yim J.J., Pfleiderer W., Wachter H. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J. Biol. Chem. 1990;265(6):3189–3192. [PubMed] [Google Scholar]
- 25.Werner-Felmayer G., Prast H., Werner E.R., Philippu A., Wachter H. Induction of GTP cyclohydrolase I by bacterial lipopolysaccharide in the rat. FEBS Lett. 1993;322(3):223–226. doi: 10.1016/0014-5793(93)81574-J. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs D., Spira T.J., Hausen A., Reibnegger G., Werner E.R., Felmayer G.W., Wachter H. Neopterin as a predictive marker for disease progression in human immunodeficiency virus type 1 infection. Clin. Chem. 1989;35(8):1746–1749. doi: 10.1093/clinchem/35.8.1746. [DOI] [PubMed] [Google Scholar]
- 27.Ghisoni K., Martins R. de P., Barbeito L., Latini A. Neopterin as a potential cytoprotective brain molecule. J. Psychiatr. Res. 2015;71:134–139. doi: 10.1016/j.jpsychires.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Duch D.S., Woolf J.H., Nichol C.A., Davidson J.R., Garbutt J.C. Urinary excretion of biopterin and neopterin in psychiatric disorders. Psychiatry Res. 1984;11(2):83–89. doi: 10.1016/0165-1781(84)90090-8. [DOI] [PubMed] [Google Scholar]
- 29.Sawada M., Horikoshi T., Masada M., Akino M., Sugimoto T., Matsuura S., Nagatsu T. A sensitive assay of GTP cyclohydrolase I activity in rat and human tissues using radioimmunoassay of neopterin. Anal. Biochem. 1986;154(1):361–366. doi: 10.1016/0003-2697(86)90537-3. [DOI] [PubMed] [Google Scholar]
- 30.Hoshiga M., Hatakeyama K., Watanabe M., Shimada M., Kagamiyama H. Autoradiographic distribution of [14C]tetrahydrobiopterin and its developmental change in mice. J. Pharmacol. Exp. Ther. 1993;267(2):971–978. [PubMed] [Google Scholar]
- 31.Kim H-L., Park Y.S. Maintenance of cellular tetrahydrobiopterin homeostasis. BMB Rep. 2010;43(9):584–592. doi: 10.5483/BMBRep.2010.43.9.584. [DOI] [PubMed] [Google Scholar]
- 32.Belik J., Shifrin Y., Arning E., Bottiglieri T., Pan J., Daigneault M.C., Allen-Vercoe E. Intestinal microbiota as a tetrahydrobiopterin exogenous source in hph-1 mice. Sci. Rep. 2017;7:39854. doi: 10.1038/srep39854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong J.S., Kang J-Y., Kim H.L., Kwon O-S., Lee K.H., Park Y.S. 6-Pyruvoyltetrahydropterin synthase orthologs of either a single or dual domain structure are responsible for tetrahydrobiopterin synthesis in bacteria. FEBS Lett. 2006;580(20):4900–4904. doi: 10.1016/j.febslet.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Harada T., Kagamiyama H., Hatakeyama K. Feedback regulation mechanisms for the control of GTP cyclohydrolase I activity. Science. 1993;260(5113):1507–1510. doi: 10.1126/science.8502995. [DOI] [PubMed] [Google Scholar]
- 35.Stone K.J. The role of tetrahydrofolate dehydrogenase in the hepatic supply of tetrahydrobiopterin in rats. Biochem. J. 1976;157(1):105–109. doi: 10.1042/bj1570105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crabtree M.J., Tatham A.L., Hale A.B., Alp N.J., Channon K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009;284(41):28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang S., Lee Y.J., Kim J-M., Park S., Peris J., Laipis P., Park Y.S., Chung J.H., Oh S.P. A murine model for human sepiapterin-reductase deficiency. Am. J. Hum. Genet. 2006;78(4):575–587. doi: 10.1086/501372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armarego W.L., Randles D., Waring P. Dihydropteridine reductase (DHPR), its cofactors, and its mode of action. Med. Res. Rev. 1984;4(3):267–321. doi: 10.1002/med.2610040302. [DOI] [PubMed] [Google Scholar]
- 39.Thöny B., Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum. Mutat. 2006;27(9):870–878. doi: 10.1002/humu.20366. [DOI] [PubMed] [Google Scholar]
- 40.Gesierich A., Niroomand F., Tiefenbacher C.P. Role of human GTP cyclohydrolase I and its regulatory protein in tetrahydrobiopterin metabolism. Basic Res. Cardiol. 2003;98(2):69–75. doi: 10.1007/s00395-003-0394-y. [DOI] [PubMed] [Google Scholar]
- 41.Du J., Teng R-J., Lawrence M., Guan T., Xu H., Ge Y., Shi Y. The protein partners of GTP cyclohydrolase I in rat organs. PLoS One. 2012;7(3):e33991. doi: 10.1371/journal.pone.0033991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heales S.J., Blair J.A., Meinschad C., Ziegler I. Inhibition of monocyte luminol-dependent chemiluminescence by tetrahydrobiopterin, and the free radical oxidation of tetrahydrobiopterin, dihydrobiopterin and dihydroneopterin. Cell Biochem. Funct. 1988;6(3):191–195. doi: 10.1002/cbf.290060307. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi A., Suetake Y., Saeki Y., Harada T., Aizawa S., Hasegawa H. Rapid clearance of supplemented tetrahydrobiopterin is driven by high-capacity transporters in the kidney. Mol. Genet. Metab. 2012;105(4):575–581. doi: 10.1016/j.ymgme.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Ohashi A., Saeki Y., Harada T., Naito M., Takahashi T., Aizawa S., Hasegawa H. Tetrahydrobiopterin Supplementation: Elevation of Tissue Biopterin Levels Accompanied by a Relative Increase in Dihydrobiopterin in the Blood and the Role of Probenecid-Sensitive Uptake in Scavenging Dihydrobiopterin in the Liver and Kidney of Rats. PLoS One. 2016;11(10):e0164305. doi: 10.1371/journal.pone.0164305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gal E.M., Hanson G., Sherman A. Biopterin : I. Profile and quantitation in rat brain. Neurochem. Res. 1976;1(5):511–523. doi: 10.1007/BF00964212. [DOI] [PubMed] [Google Scholar]
- 46.Kapatos G., Kaufman S. Peripherally administered reduced pterins do enter the brain. Science. 1981;212(4497):955–956. doi: 10.1126/science.7233193. [DOI] [PubMed] [Google Scholar]
- 47.Levine R.A., Zoephel G.P., Niederwieser A., Curtius H.C. Entrance of tetrahydropterin derivatives in brain after peripheral administration: effect on biogenic amine metabolism. J. Pharmacol. Exp. Ther. 1987;242(2):514–522. [PubMed] [Google Scholar]
- 48.Brand M.P., Hyland K., Engle T., Smith I., Heales S.J. Neurochemical effects following peripheral administration of tetrahydropterin derivatives to the hph-1 mouse. J. Neurochem. 1996;66(3):1150–1156. doi: 10.1046/j.1471-4159.1996.66031150.x. [DOI] [PubMed] [Google Scholar]
- 49.Homma D., Katoh S., Tokuoka H., Ichinose H. The role of tetrahydrobiopterin and catecholamines in the developmental regulation of tyrosine hydroxylase level in the brain. J. Neurochem. 2013;126(1):70–81. doi: 10.1111/jnc.12287. [DOI] [PubMed] [Google Scholar]
- 50.Kurosaki H., Yamaguchi K., Man-Yoshi K., Muramatsu S-I., Hara S., Ichinose H. Administration of tetrahydrobiopterin restored the decline of dopamine in the striatum induced by an acute action of MPTP. Neurochem. Int. 2019;125:16–24. doi: 10.1016/j.neuint.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Scherer T., Allegri G., Sarkissian C.N., Ying M., Grisch-Chan H.M., Rassi A., Winn S.R., Harding C.O., Martinez A., Thöny B. Tetrahydrobiopterin treatment reduces brain L-Phe but only partially improves serotonin in hyperphenylalaninemic ENU1/2 mice. J. Inherit. Metab. Dis. 2018;41(4):709–718. doi: 10.1007/s10545-018-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winn S.R., Scherer T., Thöny B., Harding C.O. High dose sapropterin dihydrochloride therapy improves monoamine neurotransmitter turnover in murine phenylketonuria (PKU). Mol. Genet. Metab. 2016;117(1):5–11. doi: 10.1016/j.ymgme.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanet H., Ducrocq F., Tournissac M., Oummadi A., Lo A., Bourrassa P., De Smedt-Peyrusse V., Azzougen B., Capuron L., Layé S., Moussa F., Trifilieff P., Calon F., Vancassel S. Tetrahydrobiopterin administration facilitates amphetamine-induced dopamine release and motivation in mice. Behav. Brain Res. 2020;379:112348. doi: 10.1016/j.bbr.2019.112348. [DOI] [PubMed] [Google Scholar]
- 54.al Aqeel A., Ozand P.T., Gascon G.G., Hughes H., Reynolds C.T., Subramanyam S.B. Response of 6-pyruvoyl-tetrahydropterin synthase deficiency to tetrahydrobiopterin. J. Child Neurol. 1992;7(Suppl.):S26–S30. doi: 10.1177/08830738920070010511. [DOI] [PubMed] [Google Scholar]
- 55.Fernell E., Watanabe Y., Adolfsson I., Tani Y., Bergström M., Hartvig P., Lilja A., von Knorring A.L., Gillberg C., Långström B. Possible effects of tetrahydrobiopterin treatment in six children with autism--clinical and positron emission tomography data: a pilot study. Dev. Med. Child Neurol. 1997;39(5):313–318. doi: 10.1111/j.1469-8749.1997.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 56.Miller L., Insel T., Scheinin M., Aloi J., Murphy D.L., Linnoila M., Lovenberg W. Tetrahydrobiopterin administration to rhesus macaques. Its appearance in CSF and effect on neurotransmitter synthesis. Neurochem. Res. 1986;11(2):291–298. doi: 10.1007/BF00967976. [DOI] [PubMed] [Google Scholar]
- 57.Shintaku H. Disorders of tetrahydrobiopterin metabolism and their treatment. Curr. Drug Metab. 2002;3(2):123–131. doi: 10.2174/1389200024605145. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa H., Sawabe K., Nakanishi N., Wakasugi O.K. Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4. Mol. Genet. Metab. 2005;86(Suppl. 1):S2–S10. doi: 10.1016/j.ymgme.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Sawabe K., Wakasugi K.O., Hasegawa H. Tetrahydrobiopterin uptake in supplemental administration: elevation of tissue tetrahydrobiopterin in mice following uptake of the exogenously oxidized product 7,8-dihydrobiopterin and subsequent reduction by an anti-folate-sensitive process. J. Pharmacol. Sci. 2004;96(2):124–133. doi: 10.1254/jphs.FP0040280. [DOI] [PubMed] [Google Scholar]
- 60.Sawabe K., Yamamoto K., Harada Y., Ohashi A., Sugawara Y., Matsuoka H., Hasegawa H. Cellular uptake of sepiapterin and push-pull accumulation of tetrahydrobiopterin. Mol. Genet. Metab. 2008;94(4):410–416. doi: 10.1016/j.ymgme.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 61.Ohashi A., Sugawara Y., Mamada K., Harada Y., Sumi T., Anzai N., Aizawa S., Hasegawa H. Membrane transport of sepiapterin and dihydrobiopterin by equilibrative nucleoside transporters: a plausible gateway for the salvage pathway of tetrahydrobiopterin biosynthesis. Mol. Genet. Metab. 2011;102(1):18–28. doi: 10.1016/j.ymgme.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Ohashi A., Mamada K., Harada T., Naito M., Takahashi T., Aizawa S., Hasegawa H. Organic anion transporters, OAT1 and OAT3, are crucial biopterin transporters involved in bodily distribution of tetrahydrobiopterin and exclusion of its excess. Mol. Cell. Biochem. 2017;435(1-2):97–108. doi: 10.1007/s11010-017-3060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farthing C.A., Sweet D.H. Expression and function of organic cation and anion transporters (SLC22 family) in the CNS. Curr. Pharm. Des. 2014;20(10):1472–1486. doi: 10.2174/13816128113199990456. [DOI] [PubMed] [Google Scholar]
- 64.Anastasiadis P.Z., Kuhn D.M., Levine R.A. Tetrahydrobiopterin uptake into rat brain synaptosomes, cultured PC12 cells, and rat striatum. Brain Res. 1994;665(1):77–84. doi: 10.1016/0006-8993(94)91154-1. [DOI] [PubMed] [Google Scholar]
- 65.Chiarini A., Armato U., Pacchiana R., Dal Pra I. Proteomic analysis of GTP cyclohydrolase 1 multiprotein complexes in cultured normal adult human astrocytes under both basal and cytokine-activated conditions. Proteomics. 2009;9(7):1850–1860. doi: 10.1002/pmic.200800561. [DOI] [PubMed] [Google Scholar]
- 66.Huang A., Zhang Y-Y., Chen K., Hatakeyama K., Keaney J.F., Jr Cytokine-stimulated GTP cyclohydrolase I expression in endothelial cells requires coordinated activation of nuclear factor-kappaB and Stat1/Stat3. Circ. Res. 2005;96(2):164–171. doi: 10.1161/01.RES.0000153669.24827.DF. [DOI] [PubMed] [Google Scholar]
- 67.Plüss C., Werner E.R., Blau N., Wachter H., Pfeilschifter J. Interleukin 1 beta and cAMP trigger the expression of GTP cyclohydrolase I in rat renal mesangial cells. Biochem. J. 1996;318(Pt 2):665–671. doi: 10.1042/bj3180665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakai N., Kaufman S., Milstien S. Parallel induction of nitric oxide and tetrahydrobiopterin synthesis by cytokines in rat glial cells. J. Neurochem. 1995;65(2):895–902. doi: 10.1046/j.1471-4159.1995.65020895.x. [DOI] [PubMed] [Google Scholar]
- 69.Chiarini A., Dal Pra I., Gottardo R., Bortolotti F., Whitfield J.F., Armato U. BH(4) (tetrahydrobiopterin)-dependent activation, but not the expression, of inducible NOS (nitric oxide synthase)-2 in proinflammatory cytokine-stimulated, cultured normal human astrocytes is mediated by MEK-ERK kinases. J. Cell. Biochem. 2005;94(4):731–743. doi: 10.1002/jcb.20334. [DOI] [PubMed] [Google Scholar]
- 70.Kaneko Y.S., Mori K., Nakashima A., Nagatsu I., Ota A. Peripheral administration of lipopolysaccharide enhances the expression of guanosine triphosphate cyclohydrolase I mRNA in murine locus coeruleus. Neuroscience. 2003;116(1):7–12. doi: 10.1016/S0306-4522(02)00579-1. [DOI] [PubMed] [Google Scholar]
- 71.Ota A., Kaneko Y.S., Mori K., Nakashima A., Nagatsu I., Nagatsu T. Effect of peripherally administered lipopolysaccharide (LPS) on GTP cyclohydrolase I, tetrahydrobiopterin and norepinephrine in the locus coeruleus in mice. Stress. 2007;10(2):131–136. doi: 10.1080/10253890701350511. [DOI] [PubMed] [Google Scholar]
- 72.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Nathan C.F., Hibbs J.B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991;3(1):65–70. doi: 10.1016/0952-7915(91)90079-G. [DOI] [PubMed] [Google Scholar]
- 74.Franscini N., Blau N., Walter R.B., Schaffner A., Schoedon G. Critical role of interleukin-1beta for transcriptional regulation of endothelial 6-pyruvoyltetrahydropterin synthase. Arterioscler. Thromb. Vasc. Biol. 2003;23(11):e50–e53. doi: 10.1161/01.ATV.0000099785.65848.F1. [DOI] [PubMed] [Google Scholar]
- 75.Werner E.R., Bahrami S., Heller R., Werner-Felmayer G. Bacterial lipopolysaccharide down-regulates expression of GTP cyclohydrolase I feedback regulatory protein. J. Biol. Chem. 2002;277(12):10129–10133. doi: 10.1074/jbc.M107326200. [DOI] [PubMed] [Google Scholar]
- 76.Schott K., Gütlich M., Ziegler I. Induction of GTP-cyclohydrolase I mRNA expression by lectin activation and interferon-gamma treatment in human cells associated with the immune response. J. Cell. Physiol. 1993;156(1):12–16. doi: 10.1002/jcp.1041560103. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura K., Bindokas V.P., Kowlessur D., Elas M., Milstien S., Marks J.D., Halpern H.J., Kang U.J. Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J. Biol. Chem. 2001;276(37):34402–34407. doi: 10.1074/jbc.M103766200. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu S., Ishii M., Kawakami Y., Momose K., Yamamoto T. Protective effects of tetrahydrobiopterin against nitric oxide-induced endothelial cell death. Life Sci. 1998;63(18):1585–1592. doi: 10.1016/S0024-3205(98)00427-5. [DOI] [PubMed] [Google Scholar]
- 79.Vásquez-Vivar J., Whitsett J., Martásek P., Hogg N., Kalyanaraman B. Reaction of tetrahydrobiopterin with superoxide: EPR-kinetic analysis and characterization of the pteridine radical. Free Radic. Biol. Med. 2001;31(8):975–985. doi: 10.1016/S0891-5849(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 80.Kirsch M., Korth H-G., Stenert V., Sustmann R., de Groot H. The autoxidation of tetrahydrobiopterin revisited. Proof of superoxide formation from reaction of tetrahydrobiopterin with molecular oxygen. J. Biol. Chem. 2003;278(27):24481–24490. doi: 10.1074/jbc.M211779200. [DOI] [PubMed] [Google Scholar]
- 81.Costigan M., Latremoliere A., Woolf C.J. Analgesia by inhibiting tetrahydrobiopterin synthesis. Curr. Opin. Pharmacol. 2012;12(1):92–99. doi: 10.1016/j.coph.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Latremoliere A., Latini A., Andrews N., Cronin S.J., Fujita M., Gorska K., Hovius R., Romero C., Chuaiphichai S., Painter M., Miracca G., Babaniyi O., Remor A.P., Duong K., Riva P., Barrett L.B., Ferreirós N., Naylor A., Penninger J.M., Tegeder I., Zhong J., Blagg J., Channon K.M., Johnsson K., Costigan M., Woolf C.J. Reduction of Neuropathic and Inflammatory Pain through Inhibition of the Tetrahydrobiopterin Pathway. Neuron. 2015;86(6):1393–1406. doi: 10.1016/j.neuron.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tegeder I., Costigan M., Griffin R.S., Abele A., Belfer I., Schmidt H., Ehnert C., Nejim J., Marian C., Scholz J., Wu T., Allchorne A., Diatchenko L., Binshtok A.M., Goldman D., Adolph J., Sama S., Atlas S.J., Carlezon W.A., Parsegian A., Lötsch J., Fillingim R.B., Maixner W., Geisslinger G., Max M.B., Woolf C.J. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat. Med. 2006;12(11):1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 84.Nasser A., Bjerrum O.J., Heegaard A-M., Møller A.T., Larsen M., Dalbøge L.S., Dupont E., Jensen T.S., Møller L.B. Impaired behavioural pain responses in hph-1 mice with inherited deficiency in GTP cyclohydrolase 1 in models of inflammatory pain. Mol. Pain. 2013;9:5. doi: 10.1186/1744-8069-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haruki H., Pedersen M.G., Gorska K.I., Pojer F., Johnsson K. Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs. Science. 2013;340(6135):987–991. doi: 10.1126/science.1232972. [DOI] [PubMed] [Google Scholar]
- 86.Hattori Y., Hattori S., Wang X., Satoh H., Nakanishi N., Kasai K. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2007;27(4):865–870. doi: 10.1161/01.ATV.0000258946.55438.0e. [DOI] [PubMed] [Google Scholar]
- 87.Li L., Chen W., Rezvan A., Jo H., Harrison D.G. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler. Thromb. Vasc. Biol. 2011;31(7):1547–1554. doi: 10.1161/ATVBAHA.111.226456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt T.S., McNeill E., Douglas G., Crabtree M.J., Hale A.B., Khoo J., O’Neill C.A., Cheng A., Channon K.M., Alp N.J. Tetrahydrobiopterin supplementation reduces atherosclerosis and vascular inflammation in apolipoprotein E-knockout mice. Clin. Sci. (Lond.) 2010;119(3):131–142. doi: 10.1042/CS20090559. [DOI] [PubMed] [Google Scholar]
- 89.Oberhuber R., Ritschl P., Fabritius C., Nguyen A-V., Hermann M., Obrist P., Werner E.R., Maglione M., Flörchinger B., Ebner S., Resch T., Pratschke J., Kotsch K. Treatment with tetrahydrobiopterin overcomes brain death-associated injury in a murine model of pancreas transplantation. Am. J. Transplant. 2015;15(11):2865–2876. doi: 10.1111/ajt.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delgado-Esteban M., Almeida A., Medina J.M. Tetrahydrobiopterin deficiency increases neuronal vulnerability to hypoxia. J. Neurochem. 2002;82(5):1148–1159. doi: 10.1046/j.1471-4159.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 91.Andersen O.A., Flatmark T., Hough E. Crystal structure of the ternary complex of the catalytic domain of human phenylalanine hydroxylase with tetrahydrobiopterin and 3-(2-thienyl)-L-alanine, and its implications for the mechanism of catalysis and substrate activation. J. Mol. Biol. 2002;320(5):1095–1108. doi: 10.1016/S0022-2836(02)00560-0. [DOI] [PubMed] [Google Scholar]
- 92.Davis M.D., Kaufman S. Evidence for the formation of the 4a-carbinolamine during the tyrosine-dependent oxidation of tetrahydrobiopterin by rat liver phenylalanine hydroxylase. J. Biol. Chem. 1989;264(15):8585–8596. [PubMed] [Google Scholar]
- 93.Alderton W.K., Cooper C.E., Knowles R.G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357(Pt 3):593–615. doi: 10.1042/bj3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Förstermann U., Sessa W. C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Teigen K., McKinney J.A., Haavik J., Martínez A. Selectivity and affinity determinants for ligand binding to the aromatic amino acid hydroxylases. Curr. Med. Chem. 2007;14(4):455–467. doi: 10.2174/092986707779941023. [DOI] [PubMed] [Google Scholar]
- 96.Pastor C.M., Williams D., Yoneyama T., Hatakeyama K., Singleton S., Naylor E., Billiar T.R. Competition for tetrahydrobiopterin between phenylalanine hydroxylase and nitric oxide synthase in rat liver. J. Biol. Chem. 1996;271(40):24534–24538. doi: 10.1074/jbc.271.40.24534. [DOI] [PubMed] [Google Scholar]
- 97.Bec N., Gorren A.C., Voelker C., Mayer B., Lange R. Reaction of neuronal nitric-oxide synthase with oxygen at low temperature. Evidence for reductive activation of the oxy-ferrous complex by tetrahydrobiopterin. J. Biol. Chem. 1998;273(22):13502–13508. doi: 10.1074/jbc.273.22.13502. [DOI] [PubMed] [Google Scholar]
- 98.Ramasamy S., Haque M.M., Gangoda M., Stuehr D.J. Tetrahydrobiopterin redox cycling in nitric oxide synthase: evidence supports a through-heme electron delivery. FEBS J. 2016;283(24):4491–4501. doi: 10.1111/febs.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giovanelli J., Campos K.L., Kaufman S. Tetrahydrobiopterin, a cofactor for rat cerebellar nitric oxide synthase, does not function as a reactant in the oxygenation of arginine. Proc. Natl. Acad. Sci. USA. 1991;88(16):7091–7095. doi: 10.1073/pnas.88.16.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mayer B., John M., Heinzel B., Werner E.R., Wachter H., Schultz G., Böhme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett. 1991;288(1-2):187–191. doi: 10.1016/0014-5793(91)81031-3. [DOI] [PubMed] [Google Scholar]
- 101.Heine C.L., Kolesnik B., Schmidt R., Werner E.R., Mayer B., Gorren A.C.F. Interaction between neuronal nitric-oxide synthase and tetrahydrobiopterin revisited: studies on the nature and mechanism of tight pterin binding. Biochemistry. 2014;53(8):1284–1295. doi: 10.1021/bi401307r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horn M., Nienhaus K., Nienhaus G.U. Kinetic Study of Ligand Binding and Conformational Changes in Inducible Nitric Oxide Synthase. J. Phys. Chem. B. 2018;122(49):11048–11057. doi: 10.1021/acs.jpcb.8b05137. [DOI] [PubMed] [Google Scholar]
- 103.Krzyaniak M.D., Cruce A.A., Vennam P., Lockart M., Berka V., Tsai A-L., Bowman M.K. The tetrahydrobiopterin radical interacting with high- and low-spin heme in neuronal nitric oxide synthase - A new indicator of the extent of NOS coupling. Free Radic. Biol. Med. 2016;101:367–377. doi: 10.1016/j.freeradbiomed.2016.10.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuzkaya N., Weissmann N., Harrison D.G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278(25):22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 105.Milstien S., Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999;263(3):681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt K., Kolesnik B., Gorren A.C.F., Werner E.R., Mayer B. Cell type-specific recycling of tetrahydrobiopterin by dihydrofolate reductase explains differential effects of 7,8-dihydrobiopterin on endothelial nitric oxide synthase uncoupling. Biochem. Pharmacol. 2014;90(3):246–253. doi: 10.1016/j.bcp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vásquez-Vivar J., Kalyanaraman B., Martásek P., Hogg N., Masters B.S., Karoui H., Tordo P., Pritchard K.A., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. USA. 1998;95(16):9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levine R.A., Kuhn D.M., Lovenberg W. The regional distribution of hydroxylase cofactor in rat brain. J. Neurochem. 1979;32(5):1575–1578. doi: 10.1111/j.1471-4159.1979.tb11101.x. [DOI] [PubMed] [Google Scholar]
- 109.Dassesse D., Hemmens B., Cuvelier L., Résibois A. GTP-cyclohydrolase-I like immunoreactivity in rat brain. Brain Res. 1997;777(1-2):187–201. doi: 10.1016/S0006-8993(97)01111-6. [DOI] [PubMed] [Google Scholar]
- 110.Lentz S.I., Kapatos G. Tetrahydrobiopterin biosynthesis in the rat brain: heterogeneity of GTP cyclohydrolase I mRNA expression in monoamine-containing neurons. Neurochem. Int. 1996;28(5-6):569–582. doi: 10.1016/0197-0186(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 111.Nagatsu I., Ichinose H., Sakai M., Titani K., Suzuki M., Nagatsu T. Immunocytochemical localization of GTP cyclohydrolase I in the brain, adrenal gland, and liver of mice. J. Neural Transm. (Vienna) 1995;102(3):175–188. doi: 10.1007/BF01281153. [DOI] [PubMed] [Google Scholar]
- 112.Wyler S.C., Donovan L.J., Yeager M., Deneris E. Pet-1 Controls Tetrahydrobiopterin Pathway and Slc22a3 Transporter Genes in Serotonin Neurons. ACS Chem. Neurosci. 2015;6(7):1198–1205. doi: 10.1021/cn500331z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ding M., St Pierre B.A., Parkinson J.F., Medberry P., Wong J.L., Rogers N.E., Ignarro L.J., Merrill J.E. Inducible nitric-oxide synthase and nitric oxide production in human fetal astrocytes and microglia. A kinetic analysis. J. Biol. Chem. 1997;272(17):11327–11335. doi: 10.1074/jbc.272.17.11327. [DOI] [PubMed] [Google Scholar]
- 114.D’Sa C., Hirayama K., West A., Hahn M., Zhu M., Kapatos G. Tetrahydrobiopterin biosynthesis in C6 glioma cells: induction of GTP cyclohydrolase I gene expression by lipopolysaccharide and cytokine treatment. Brain Res. Mol. Brain Res. 1996;41(1-2):105–110. doi: 10.1016/0169-328X(96)00073-3. [DOI] [PubMed] [Google Scholar]
- 115.Hwang O., Baker H., Gross S., Joh T.H. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998;28(2):140–153. doi: 10.1002/(SICI)1098-2396(199802)28:2<140::AID-SYN4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 116.Nasser A., Møller L.B., Olesen J.H., Konradsen Refsgaard L., Andreasen J.T., Andreasen J.T. Anxiety- and depression-like phenotype of hph-1 mice deficient in tetrahydrobiopterin. Neurosci. Res. 2014;89:44–53. doi: 10.1016/j.neures.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 117.Zeng B-Y., Heales S.J.R., Canevari L., Rose S., Jenner P. Alterations in expression of dopamine receptors and neuropeptides in the striatum of GTP cyclohydrolase-deficient mice. Exp. Neurol. 2004;190(2):515–524. doi: 10.1016/j.expneurol.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 118.Homma D., Sumi-Ichinose C., Tokuoka H., Ikemoto K., Nomura T., Kondo K., Katoh S., Ichinose H. Partial biopterin deficiency disturbs postnatal development of the dopaminergic system in the brain. J. Biol. Chem. 2011;286(2):1445–1452. doi: 10.1074/jbc.M110.159426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwak S.S., Jeong M., Choi J.H., Kim D., Min H., Yoon Y., Hwang O., Meadows G.G., Joe C.O. Amelioration of behavioral abnormalities in BH(4)-deficient mice by dietary supplementation of tyrosine. PLoS One. 2013;8(4):e60803. doi: 10.1371/journal.pone.0060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sumi-Ichinose C., Urano F., Kuroda R., Ohye T., Kojima M., Tazawa M., Shiraishi H., Hagino Y., Nagatsu T., Nomura T., Ichinose H. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin. A study from 6-pyruvoyltetrahydropterin synthase knockout mice. J. Biol. Chem. 2001;276(44):41150–41160. doi: 10.1074/jbc.M102237200. [DOI] [PubMed] [Google Scholar]
- 121.Koshimura K., Miwa S., Watanabe Y. Dopamine-releasing action of 6R-L-erythro-tetrahydrobiopterin: analysis of its action site using sepiapterin. J. Neurochem. 1994;63(2):649–654. doi: 10.1046/j.1471-4159.1994.63020649.x. [DOI] [PubMed] [Google Scholar]
- 122.Liang L.P., Kaufman S. The regulation of dopamine release from striatum slices by tetrahydrobiopterin and L-arginine-derived nitric oxide. Brain Res. 1998;800(2):181–186. doi: 10.1016/S0006-8993(98)00452-1. [DOI] [PubMed] [Google Scholar]
- 123.Mataga N., Imamura K., Watanabe Y. 6R-tetrahydrobiopterin perfusion enhances dopamine, serotonin, and glutamate outputs in dialysate from rat striatum and frontal cortex. Brain Res. 1991;551(1-2):64–71. doi: 10.1016/0006-8993(91)90914-H. [DOI] [PubMed] [Google Scholar]
- 124.Koshimura K., Takagi Y., Miwa S., Kido T., Watanabe Y., Murakami Y., Kato Y., Masaki T. Characterization of a dopamine-releasing action of 6R-L-erythro-tetrahydrobiopterin: comparison with a 6S-form. J. Neurochem. 1995;65(2):827–830. doi: 10.1046/j.1471-4159.1995.65020827.x. [DOI] [PubMed] [Google Scholar]
- 125.Miwa S., Watanabe Y., Hayaishi O. 6R-L-erythro-5,6,7,8-tetrahydrobiopterin as a regulator of dopamine and serotonin biosynthesis in the rat brain. Arch. Biochem. Biophys. 1985;239(1):234–241. doi: 10.1016/0003-9861(85)90831-8. [DOI] [PubMed] [Google Scholar]
- 126.Koshimura K., Miwa S., Lee K., Fujiwara M., Watanabe Y. Enhancement of dopamine release in vivo from the rat striatum by dialytic perfusion of 6R-L-erythro-5,6,7,8-tetrahydrobiopterin. J. Neurochem. 1990;54(4):1391–1397. doi: 10.1111/j.1471-4159.1990.tb01974.x. [DOI] [PubMed] [Google Scholar]
- 127.Wolf W.A., Anastasiadis P.Z., Kuhn D.M., Levine R.A. Influence of tetrahydrobiopterin on serotonin synthesis, metabolism and release in synaptosomes. Neurochem. Int. 1990;16(3):335–340. doi: 10.1016/0197-0186(90)90111-6. [DOI] [PubMed] [Google Scholar]
- 128.Wolf W.A., Ziaja E., Arthur R.A., Jr, Anastasiadis P.Z., Levine R.A., Kuhn D.M. Effect of tetrahydrobiopterin on serotonin synthesis, release, and metabolism in superfused hippocampal slices. J. Neurochem. 1991;57(4):1191–1197. doi: 10.1111/j.1471-4159.1991.tb08279.x. [DOI] [PubMed] [Google Scholar]
- 129.Ohue T., Koshimura K., Akiyama Y., Watanabe Y., Miwa S. Monoamine-mediated enhancement of acetylcholine release in rat hippocampus by 6R-L-erythro-5,6,7,8-tetrahydrobiopterin. Brain Res. 1992;570(1-2):173–179. doi: 10.1016/0006-8993(92)90579-X. [DOI] [PubMed] [Google Scholar]
- 130.Nagatsu T., Nakahara D., Kobayashi K., Morita S., Sawada H., Mizuguchi T., Kiuchi K. Peripherally administered (6R)-tetrahydrobiopterin increases in vivo tyrosine hydroxylase activity in the striatum measured by microdialysis both in normal mice and in transgenic mice carrying human tyrosine hydroxylase. Neurosci. Lett. 1994;182(1):44–46. doi: 10.1016/0304-3940(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 131.Longo N. Disorders of biopterin metabolism. J. Inherit. Metab. Dis. 2009;32(3):333–342. doi: 10.1007/s10545-009-1067-2. [DOI] [PubMed] [Google Scholar]
- 132.Tatham A.L., Crabtree M.J., Warrick N., Cai S., Alp N.J., Channon K.M. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of GTP cyclohydrolase feedback regulatory protein expression. J. Biol. Chem. 2009;284(20):13660–13668. doi: 10.1074/jbc.M807959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hayashi E., Kuratani K., Kinoshita M., Hara H. Pharmacologically distinctive behaviors other than burying marbles during the marble burying test in mice. Pharmacology. 2010;86(5-6):293–296. doi: 10.1159/000321190. [DOI] [PubMed] [Google Scholar]
- 134.Li X., Morrow D., Witkin J.M. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci. 2006;78(17):1933–1939. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 135.Hyland K., Gunasekera R.S., Engle T., Arnold L.A. Tetrahydrobiopterin and biogenic amine metabolism in the hph-1 mouse. J. Neurochem. 1996;67(2):752–759. doi: 10.1046/j.1471-4159.1996.67020752.x. [DOI] [PubMed] [Google Scholar]
- 136.Takazawa C., Fujimoto K., Homma D., Sumi-Ichinose C., Nomura T., Ichinose H., Katoh S. A brain-specific decrease of the tyrosine hydroxylase protein in sepiapterin reductase-null mice--as a mouse model for Parkinson’s disease. Biochem. Biophys. Res. Commun. 2008;367(4):787–792. doi: 10.1016/j.bbrc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 137.Asami T., Kuribara H. Enhancement of ambulation-increasing effect of methamphetamine by peripherally-administered 6R-L-erythro-5,6,7,8-tetrahydrobiopterin (R-THBP) in mice. Jpn. J. Pharmacol. 1989;50(2):175–184. doi: 10.1254/jjp.50.175. [DOI] [PubMed] [Google Scholar]
- 138.Mizuma H., Mizutani M., Nozaki S., Iizuka H., Tohyama H., Nishimura N., Watanabe Y., Kohashi R. Improvement by repeated administration of 6R-tetrahydrobiopterin of 5,7-dihydroxytryptamine-induced abnormal behaviors in immature rats. Biochem. Biophys. Res. Commun. 2003;302(1):156–161. doi: 10.1016/S0006-291X(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 139.Latini A., de Bortoli da Silva L., da Luz Scheffer D., Pires A.C.S., de Matos F.J., Nesi R.T., Ghisoni K., de Paula Martins R., de Oliveira P.A., Prediger R.D., Ghersi M., Gabach L., Pérez M.F., Rubiales-Barioglio S., Raisman-Vozari R., Mongeau R., Lanfumey L., Aguiar A.S. Tetrahydrobiopterin improves hippocampal nitric oxide-linked long-term memory. Mol. Genet. Metab. 2018;125(1-2):104–111. doi: 10.1016/j.ymgme.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 140.Blair J.A., Barford P.A., Morar C., Pheasant A.E., Hamon C.G., Whitburn S.B., Leeming R.J., Reynolds G.P., Coppen A. Tetrahydrobiopterin metabolism in depression. Lancet. 1984;2(8395):163. doi: 10.1016/S0140-6736(84)91075-4. [DOI] [PubMed] [Google Scholar]
- 141.Abou-Saleh M.T., Anderson D.N., Collins J., Hughes K., Cattell R.J., Hamon C.G., Blair J.A. The role of pterins in depression and the effects of antidepressive therapy. Biol. Psychiatry. 1995;38(7):458–463. doi: 10.1016/0006-3223(94)00323-U. [DOI] [PubMed] [Google Scholar]
- 142.Garbutt J.C., Duch D.S., Nichol C.A., Woolf J.H. Urinary biopterin and neopterin excretion and pituitary-adrenal activity in psychiatric patients. Psychiatry Res. 1985;16(3):181–187. doi: 10.1016/0165-1781(85)90105-2. [DOI] [PubMed] [Google Scholar]
- 143.Hashimoto R., Mizutani M., Ohta T., Nakazawa K., Nagatsu T. Changes in plasma tetrahydrobiopterin levels of depressives in depressive and remission phases: reconfirmed by measurement with an internal standard. Neuropsychobiology. 1994;29(2):57–60. doi: 10.1159/000119064. [DOI] [PubMed] [Google Scholar]
- 144.Knapp S., Irwin M. Plasma levels of tetrahydrobiopterin and folate in major depression. Biol. Psychiatry. 1989;26(2):156–162. doi: 10.1016/0006-3223(89)90019-X. [DOI] [PubMed] [Google Scholar]
- 145.Trender-Gerhard I., Sweeney M.G., Schwingenschuh P., Mir P., Edwards M.J., Gerhard A., Polke J.M., Hanna M.G., Davis M.B., Wood N.W., Bhatia K.P. Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J. Neurol. Neurosurg. Psychiatry. 2009;80(8):839–845. doi: 10.1136/jnnp.2008.155861. [DOI] [PubMed] [Google Scholar]
- 146.Van Hove J.L.K., Steyaert J., Matthijs G., Legius E., Theys P., Wevers R., Romstad A., Møller L.B., Hedrich K., Goriounov D., Blau N., Klein C., Casaer P. Expanded motor and psychiatric phenotype in autosomal dominant Segawa syndrome due to GTP cyclohydrolase deficiency. J. Neurol. Neurosurg. Psychiatry. 2006;77(1):18–23. doi: 10.1136/jnnp.2004.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Celik C., Erdem M., Cayci T., Ozdemir B., Ozgur Akgul E., Kurt Y.G., Yaman H., Isintas M., Ozgen F., Ozsahin A. The association between serum levels of neopterin and number of depressive episodes of major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(2):372–375. doi: 10.1016/j.pnpbp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 148.Maes M., Scharpé S., Meltzer H.Y., Okayli G., Bosmans E., D’Hondt P., Vanden Bossche B.V., Cosyns P. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry Res. 1994;54(2):143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 149.Tang C-Z., Zhang Y-L., Wang W-S., Li W-G., Shi J-P. Elevated Serum Levels of Neopterin at Admission Predicts Depression After Acute Ischemic Stroke: a 6-Month Follow-Up Study. Mol. Neurobiol. 2016;53(5):3194–3204. doi: 10.1007/s12035-015-9220-4. [DOI] [PubMed] [Google Scholar]
- 150.Benedetti F., Aggio V., Pratesi M.L., Greco G., Furlan R. Neuroinflammation in Bipolar Depression. Front. Psychiatry. 2020;11:71. doi: 10.3389/fpsyt.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Capuron L., Castanon N. Role of Inflammation in the Development of Neuropsychiatric Symptom Domains: Evidence and Mechanisms. Curr. Top. Behav. Neurosci. 2017;31:31–44. doi: 10.1007/7854_2016_14. [DOI] [PubMed] [Google Scholar]
- 152.Capuron L., Geisler S., Kurz K., Leblhuber F., Sperner-Unterweger B., Fuchs D. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Curr. Pharm. Des. 2014;20(38):6048–6057. doi: 10.2174/1381612820666140317110217. [DOI] [PubMed] [Google Scholar]
- 153.Vancassel S., Capuron L., Castanon N. Brain kynurenine and BH4 pathways: relevance to the pathophysiology and treatment of inflammation-driven depressive symptoms. Front. Neurosci. 2018;12:499. doi: 10.3389/fnins.2018.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao G., Liu X. Neuroimmune advance in depressive disorder. Adv. Exp. Med. Biol. 2019;1180:85–98. doi: 10.1007/978-981-32-9271-0_4. [DOI] [PubMed] [Google Scholar]
- 155.Felger J.C., Li L., Marvar P.J., Woolwine B.J., Harrison D.G., Raison C.L., Miller A.H. Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 2013;31:153–160. doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zoller H., Schloegl A., Schroecksnadel S., Vogel W., Fuchs D. Interferon-alpha therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J. Interferon Cytokine Res. 2012;32(5):216–220. doi: 10.1089/jir.2011.0093. [DOI] [PubMed] [Google Scholar]
- 157.Werner E.R., Gorren A.C.F., Heller R., Werner-Felmayer G., Mayer B. Tetrahydrobiopterin and nitric oxide: mechanistic and pharmacological aspects. Exp. Biol. Med. (Maywood) 2003;228(11):1291–1302. doi: 10.1177/153537020322801108. [DOI] [PubMed] [Google Scholar]
- 158.Neurauter G., Schröcksnadel K., Scholl-Bürgi S., Sperner-Unterweger B., Schubert C., Ledochowski M., Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 2008;9(7):622–627. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- 159.Capuron L., Pagnoni G., Drake D.F., Woolwine B.J., Spivey J.R., Crowe R.J., Votaw J.R., Goodman M.M., Miller A.H. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatry. 2012;69(10):1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Felger J.C. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr. Neuropharmacol. 2018;16(5):533–558. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Curtius H.C., Niederwieser A., Levine R.A., Lovenberg W., Woggon B., Angst J. Successful treatment of depression with tetrahydrobiopterin. Lancet. 1983;1(8325):657–658. doi: 10.1016/S0140-6736(83)91837-8. [DOI] [PubMed] [Google Scholar]
- 162.Pan L., McKain B.W., Madan-Khetarpal S., Mcguire M., Diler R.S., Perel J.M., Vockley J., Brent D.A. GTP-cyclohydrolase deficiency responsive to sapropterin and 5-HTP supplementation: relief of treatment-refractory depression and suicidal behaviour. BMJ Case Rep. 2011;2011:bcr0320113927. doi: 10.1136/bcr.03.2011.3927.corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Woggon B., Angst J., Curtius H.C., Niederwieser A. Unsuccessful treatment of depression with tetrahydrobiopterin. Lancet. 1984;2(8417-8418):1463. doi: 10.1016/S0140-6736(84)91648-9. [DOI] [PubMed] [Google Scholar]
- 164.Anderson D.N., Wilkinson A.M., Abou-Saleh M.T., Blair J.A. Recovery from depression after electroconvulsive therapy is accompanied by evidence of increased tetrahydrobiopterin-dependent hydroxylation. Acta Psychiatr. Scand. 1994;90(1):10–13. doi: 10.1111/j.1600-0447.1994.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 165.Kishi T., Ichinose H., Yoshimura R., Fukuo Y., Kitajima T., Inada T., Kunugi H., Kato T., Yoshikawa T., Ujike H., Musso G.M., Umene-Nakano W., Nakamura J., Ozaki N., Iwata N. GTP cyclohydrolase 1 gene haplotypes as predictors of SSRI response in Japanese patients with major depressive disorder. J. Affect. Disord. 2012;142(1-3):315–322. doi: 10.1016/j.jad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 166.McHugh P.C., Joyce P.R., Kennedy M.R. Polymorphisms of sepiapterin reductase gene alter promoter activity and may influence risk of bipolar disorder. Pharmacogenet. Genomics. 2009;19(5):330–337. doi: 10.1097/FPC.0b013e328328f82c. [DOI] [PubMed] [Google Scholar]
- 167.Miura H., Kitagami T., Ozaki N. Suppressive effect of paroxetine, a selective serotonin uptake inhibitor, on tetrahydrobiopterin levels and dopamine as well as serotonin turnover in the mesoprefrontal system of mice. Synapse. 2007;61(9):698–706. doi: 10.1002/syn.20407. [DOI] [PubMed] [Google Scholar]
- 168.Doummar D., Moussa F., Nougues M-C., Ravelli C., Louha M., Whalen S., Burglen L., Rodriguez D., Billette de Villemeur T. Monoamine neurotransmitters and movement disorders in children and adults. Rev. Neurol. (Paris) 2018;174(9):581–588. doi: 10.1016/j.neurol.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 169.Segawa M. [Segawa disease (hereditary progressive dystonia with marked diurnal fluctuation-HPD) and abnormalities in pteridin metabolism]. Rinsho Shinkeigaku. 1996;36(12):1322–1323. [Segawa disease (hereditary progressive dystonia with marked diurnal fluctuation-HPD) and abnormalities in pteridin metabolism]. [PubMed] [Google Scholar]
- 170.Mencacci N.E., Isaias I.U., Reich M.M., Ganos C., Plagnol V., Polke J.M., Bras J., Hersheson J., Stamelou M., Pittman A.M., Noyce A.J., Mok K.Y., Opladen T., Kunstmann E., Hodecker S., Münchau A., Volkmann J., Samnick S., Sidle K., Nanji T., Sweeney M.G., Houlden H., Batla A., Zecchinelli A.L., Pezzoli G., Marotta G., Lees A., Alegria P., Krack P., Cormier-Dequaire F., Lesage S., Brice A., Heutink P., Gasser T., Lubbe S.J., Morris H.R., Taba P., Koks S., Majounie E., Raphael Gibbs J., Singleton A., Hardy J., Klebe S., Bhatia K.P., Wood N.W. International Parkinson’s Disease Genomics Consortium and UCL-exomes consortium. Parkinson’s disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137(Pt 9):2480–2492. doi: 10.1093/brain/awu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rudakou U., Ouled Amar Bencheikh B., Ruskey J.A., Krohn L., Laurent S.B., Spiegelman D., Liong C., Fahn S., Waters C., Monchi O., Fon E.A., Dauvilliers Y., Alcalay R.N., Dupré N., Gan-Or Z. Common and rare GCH1 variants are associated with Parkinson’s disease. Neurobiol. Aging. 2019;73:231.e1–231.e6. doi: 10.1016/j.neurobiolaging.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yan Y-P., Zhang B., Shen T., Si X-L., Guo Z-Y., Tian J., Xu C-Y., Zhang B-R. Study of GCH1 and TH genes in Chinese patients with Parkinson’s disease. Neurobiol. Aging. 2018;68:159.e3–159.e6. doi: 10.1016/j.neurobiolaging.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 173.Foxton R.H., Land J.M., Heales S.J.R. Tetrahydrobiopterin availability in Parkinson’s and Alzheimer’s disease; potential pathogenic mechanisms. Neurochem. Res. 2007;32(4-5):751–756. doi: 10.1007/s11064-006-9201-0. [DOI] [PubMed] [Google Scholar]
- 174.Lovenberg W., Levine R.A., Robinson D.S., Ebert M., Williams A.C., Calne D.B. Hydroxylase cofactor activity in cerebrospinal fluid of normal subjects and patients with Parkinson’s disease. Science. 1979;204(4393):624–626. doi: 10.1126/science.432666. [DOI] [PubMed] [Google Scholar]
- 175.Nagatsu T., Sawada M. Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J. Neural Transm. Suppl. 2007;(72):113–120. doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- 176.Nagatsu T., Nagatsu I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): historical overview and future prospects. J. Neural Transm. (Vienna) 2016;123(11):1255–1278. doi: 10.1007/s00702-016-1596-4. [DOI] [PubMed] [Google Scholar]
- 177.Calon F., Morissette M., Rajput A.H., Hornykiewicz O., Bédard P.J., Di Paolo T. Changes of GABA receptors and dopamine turnover in the postmortem brains of parkinsonians with levodopa-induced motor complications. Mov. Disord. 2003;18(3):241–253. doi: 10.1002/mds.10343. [DOI] [PubMed] [Google Scholar]
- 178.Tobin J.E., Cui J., Wilk J.B., Latourelle J.C., Laramie J.M., McKee A.C., Guttman M., Karamohamed S., DeStefano A.L., Myers R.H. Sepiapterin reductase expression is increased in Parkinson’s disease brain tissue. Brain Res. 2007;1139:42–47. doi: 10.1016/j.brainres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi H.J., Jang Y.J., Kim H.J., Hwang O. Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol. Pharmacol. 2000;58(3):633–640. doi: 10.1124/mol.58.3.633. [DOI] [PubMed] [Google Scholar]
- 180.Choi H.J., Kim S.W., Lee S.Y., Hwang O. Dopamine-dependent cytotoxicity of tetrahydrobiopterin: a possible mechanism for selective neurodegeneration in Parkinson’s disease. J. Neurochem. 2003;86(1):143–152. doi: 10.1046/j.1471-4159.2003.01808.x. [DOI] [PubMed] [Google Scholar]
- 181.Enzinger C., Wirleitner B., Spöttl N., Böck G., Fuchs D., Baier-Bitterlich G. Reduced pteridine derivatives induce apoptosis in PC12 cells. Neurochem. Int. 2002;41(1):71–78. doi: 10.1016/S0197-0186(01)00134-6. [DOI] [PubMed] [Google Scholar]
- 182.Lee S.Y., Moon Y., Hee Choi D., Jin Choi H., Hwang O. Particular vulnerability of rat mesencephalic dopaminergic neurons to tetrahydrobiopterin: Relevance to Parkinson’s disease. Neurobiol. Dis. 2007;25(1):112–120. doi: 10.1016/j.nbd.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 183.Chae S-W., Bang Y.J., Kim K-M., Lee K.Y., Kang B.Y., Kim E.M., Inoue H., Hwang O., Choi H.J. Role of cyclooxygenase-2 in tetrahydrobiopterin-induced dopamine oxidation. Biochem. Biophys. Res. Commun. 2007;359(3):735–741. doi: 10.1016/j.bbrc.2007.05.190. [DOI] [PubMed] [Google Scholar]
- 184.Choi H.J., Kim S.W., Lee S.Y., Moon Y.W., Hwang O. Involvement of apoptosis and calcium mobilization in tetrahydrobiopterin-induced dopaminergic cell death. Exp. Neurol. 2003;181(2):281–290. doi: 10.1016/S0014-4886(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 185.Choi H.J., Lee S.Y., Cho Y., No H., Kim S.W., Hwang O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: implications for Parkinson’s disease. Neurochem. Int. 2006;48(4):255–262. doi: 10.1016/j.neuint.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 186.Kim S.W., Jang Y.J., Chang J.W., Hwang O. Degeneration of the nigrostriatal pathway and induction of motor deficit by tetrahydrobiopterin: an in vivo model relevant to Parkinson’s disease. Neurobiol. Dis. 2003;13(2):167–176. doi: 10.1016/S0969-9961(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 187.Aryal B., Lee J-K., Kim H.R., Kim H-G. Alteration of striatal tetrahydrobiopterin in iron-induced unilateral model of Parkinson’s disease. Korean J. Physiol. Pharmacol. 2014;18(2):129–134. doi: 10.4196/kjpp.2014.18.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Curtius H.C., Niederwieser A., Levine R., Müldner H. Therapeutic efficacy of tetrahydrobiopterin in Parkinson’s disease. Adv. Neurol. 1984;40:463–466. [PubMed] [Google Scholar]
- 189.LeWitt P.A., Miller L.P., Newman R.P., Burns R.S., Insel T., Levine R.A., Lovenberg W., Calne D.B. Tyrosine hydroxylase cofactor (tetrahydrobiopterin) in parkinsonism. Adv. Neurol. 1984;40:459–462. [PubMed] [Google Scholar]
- 190.Jarraya B., Boulet S., Ralph G.S., Jan C., Bonvento G., Azzouz M., Miskin J.E., Shin M., Delzescaux T., Drouot X., Hérard A-S., Day D.M., Brouillet E., Kingsman S.M., Hantraye P., Mitrophanous K.A., Mazarakis N.D., Palfi S. Dopamine gene therapy for Parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci. Transl. Med. 2009;1(2):2ra4. doi: 10.1126/scitranslmed.3000130. [DOI] [PubMed] [Google Scholar]
- 191.Palfi S., Gurruchaga J.M., Ralph G.S., Lepetit H., Lavisse S., Buttery P.C., Watts C., Miskin J., Kelleher M., Deeley S., Iwamuro H., Lefaucheur J.P., Thiriez C., Fenelon G., Lucas C., Brugières P., Gabriel I., Abhay K., Drouot X., Tani N., Kas A., Ghaleh B., Le Corvoisier P., Dolphin P., Breen D.P., Mason S., Guzman N.V., Mazarakis N.D., Radcliffe P.A., Harrop R., Kingsman S.M., Rascol O., Naylor S., Barker R.A., Hantraye P., Remy P., Cesaro P., Mitrophanous K.A. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 192.Palfi S., Gurruchaga J.M., Lepetit H., Howard K., Ralph G.S., Mason S., Gouello G., Domenech P., Buttery P.C., Hantraye P., Tuckwell N.J., Barker R.A., Mitrophanous K.A. Long-Term Follow-Up of a Phase I/II Study of ProSavin, a Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 2018;29(3):148–155. doi: 10.1089/humc.2018.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kirik D., Georgievska B., Burger C., Winkler C., Muzyczka N., Mandel R.J., Bjorklund A. Reversal of motor impairments in parkinsonian rats by continuous intrastriatal delivery of L-dopa using rAAV-mediated gene transfer. Proc. Natl. Acad. Sci. USA. 2002;99(7):4708–4713. doi: 10.1073/pnas.062047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muramatsu S., Fujimoto K., Ikeguchi K., Shizuma N., Kawasaki K., Ono F., Shen Y., Wang L., Mizukami H., Kume A., Matsumura M., Nagatsu I., Urano F., Ichinose H., Nagatsu T., Terao K., Nakano I., Ozawa K. Behavioral recovery in a primate model of Parkinson’s disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum. Gene Ther. 2002;13(3):345–354. doi: 10.1089/10430340252792486. [DOI] [PubMed] [Google Scholar]
- 195.Shen Y., Muramatsu S.I., Ikeguchi K., Fujimoto K.I., Fan D.S., Ogawa M., Mizukami H., Urabe M., Kume A., Nagatsu I., Urano F., Suzuki T., Ichinose H., Nagatsu T., Monahan J., Nakano I., Ozawa K. Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson’s disease. Hum. Gene Ther. 2000;11(11):1509–1519. doi: 10.1089/10430340050083243. [DOI] [PubMed] [Google Scholar]
- 196.Mandel R.J., Rendahl K.G., Snyder R.O., Leff S.E. Progress in direct striatal delivery of L-dopa via gene therapy for treatment of Parkinson’s disease using recombinant adeno-associated viral vectors. Exp. Neurol. 1999;159(1):47–64. doi: 10.1006/exnr.1999.7159. [DOI] [PubMed] [Google Scholar]
- 197.Badin R.A., Binley K., Van Camp N., Jan C., Gourlay J., Robert C., Gipchtein P., Fayard A., Stewart H., Ralph G.S., Lad Y., Kelleher M., Loader J., Hosomi K., Palfi S., Mitrophanous K.A., Hantraye P. Gene Therapy for Parkinson’s Disease: Preclinical Evaluation of Optimally Configured TH:CH1 Fusion for Maximal Dopamine Synthesis. Mol. Ther. Methods Clin. Dev. 2019;14:206–216. doi: 10.1016/j.omtm.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stewart H.J., Ralph G.S., Fong-Wong L., Strickland I., McCloskey L., Barnes L., Blount I., Wells O., Truran C.J.M., Kingsman A.J., Palfi S., Mitrophanous K.A. Optimizing Transgene Configuration and Protein Fusions to Maximize Dopamine Production for the Gene Therapy of Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 2016;27(3):100–110. doi: 10.1089/humc.2016.056. [DOI] [PubMed] [Google Scholar]
- 199.Aziz A.A., Leeming R.J., Blair J.A. Tetrahydrobiopterin metabolism in senile dementia of Alzheimer type. J. Neurol. Neurosurg. Psychiatry. 1983;46(5):410–413. doi: 10.1136/jnnp.46.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barford P.A., Blair J.A., Eggar C., Hamon C., Morar C., Whitburn S.B. Tetrahydrobiopterin metabolism in the temporal lobe of patients dying with senile dementia of Alzheimer type. J. Neurol. Neurosurg. Psychiatry. 1984;47(7):736–738. doi: 10.1136/jnnp.47.7.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kay A.D., Milstien S., Kaufman S., Creasey H., Haxby J.V., Cutler N.R., Rapoport S.I. Cerebrospinal fluid biopterin is decreased in Alzheimer’s disease. Arch. Neurol. 1986;43(10):996–999. doi: 10.1001/archneur.1986.00520100018008. [DOI] [PubMed] [Google Scholar]
- 202.Sawada M., Hirata Y., Arai H., Iizuka R., Nagatsu T. Tyrosine hydroxylase, tryptophan hydroxylase, biopterin, and neopterin in the brains of normal controls and patients with senile dementia of Alzheimer type. J. Neurochem. 1987;48(3):760–764. doi: 10.1111/j.1471-4159.1987.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 203.Hull M., Pasinetti G.M., Aisen P.S. Elevated plasma neopterin levels in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2000;14(4):228–230. doi: 10.1097/00002093-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 204.Leblhuber F., Walli J., Demel U., Tilz G.P., Widner B., Fuchs D. Increased serum neopterin concentrations in patients with Alzheimer’s disease. Clin. Chem. Lab. Med. 1999;37(4):429–431. doi: 10.1515/CCLM.1999.070. [DOI] [PubMed] [Google Scholar]
- 205.Parker D.C., Mielke M.M., Yu Q., Rosenberg P.B., Jain A., Lyketsos C.G., Fedarko N.S., Oh E.S. Plasma neopterin level as a marker of peripheral immune activation in amnestic mild cognitive impairment and Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2013;28(2):149–154. doi: 10.1002/gps.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pierce G.L., Jablonski K.L., Walker A.E., Seibert S.M., DeVan A.E., Black S.M., Sharma S., Seals D.R. Tetrahydrobiopterin supplementation enhances carotid artery compliance in healthy older men: a pilot study. Am. J. Hypertens. 2012;25(10):1050–1054. doi: 10.1038/ajh.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Šimić G., Babić Leko M., Wray S., Harrington C.R., Delalle I., Jovanov-Milošević N., Bažadona D., Buée L., de Silva R., Di Giovanni G., Wischik C.M., Hof P.R. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017;151:101–138. doi: 10.1016/j.pneurobio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Krashia P., Nobili A., D’Amelio M. Unifying Hypothesis of Dopamine Neuron Loss in Neurodegenerative Diseases: Focusing on Alzheimer’s Disease. Front. Mol. Neurosci. 2019;12:123. doi: 10.3389/fnmol.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nobili A., Latagliata E.C., Viscomi M.T., Cavallucci V., Cutuli D., Giacovazzo G., Krashia P., Rizzo F.R., Marino R., Federici M., De Bartolo P., Aversa D., Dell’Acqua M.C., Cordella A., Sancandi M., Keller F., Petrosini L., Puglisi-Allegra S., Mercuri N.B., Coccurello R., Berretta N., D’Amelio M. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017;8:14727. doi: 10.1038/ncomms14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.d’Uscio L.V., Das P., Santhanam A.V.R., He T., Younkin S.G., Katusic Z.S. Activation of PPARδ prevents endothelial dysfunction induced by overexpression of amyloid-β precursor protein. Cardiovasc. Res. 2012;96(3):504–512. doi: 10.1093/cvr/cvs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Santhanam A.V.R., d’Uscio L.V., He T., Das P., Younkin S.G., Katusic Z.S. Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice. J. Neurochem. 2015;134(6):1129–1138. doi: 10.1111/jnc.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Christ S.E., Moffitt A.J., Peck D., White D.A. The effects of tetrahydrobiopterin (BH4) treatment on brain function in individuals with phenylketonuria. Neuroimage Clin. 2013;3:539–547. doi: 10.1016/j.nicl.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 214.Calsolaro V., Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016;12(6):719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 215.Bello-Medina P.C., González-Franco D.A., Vargas-Rodríguez I., Díaz-Cintra S. Oxidative stress, the immune response, synaptic plasticity, and cognition in transgenic models of Alzheimer disease. Neurologia. 2019;•••:S0213-4853(19)30109-4. doi: 10.1016/j.nrl.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 216.Griffin W.S., Stanley L.C., Ling C., White L., MacLeod V., Perrot L.J., White C.L., III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dursun E., Gezen-Ak D., Hanağası H., Bilgiç B., Lohmann E., Ertan S., Atasoy İ.L., Alaylıoğlu M., Araz Ö.S., Önal B., Gündüz A., Apaydın H., Kızıltan G., Ulutin T., Gürvit H., Yılmazer S. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J. Neuroimmunol. 2015;283:50–57. doi: 10.1016/j.jneuroim.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 218.Brosseron F., Krauthausen M., Kummer M., Heneka M.T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol. Neurobiol. 2014;50(2):534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuan H., Xia Q., Ge P., Wu S. Genetic polymorphism of interleukin 1β -511C/T and susceptibility to sporadic Alzheimer’s disease: a meta-analysis. Mol. Biol. Rep. 2013;40(2):1827–1834. doi: 10.1007/s11033-012-2237-0. [DOI] [PubMed] [Google Scholar]
- 220.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T-C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D.T. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang P., Guan P-P., Wang T., Yu X., Guo J-J., Wang Z-Y. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1β and Aβ between glial and neuron cells. Aging Cell. 2014;13(4):605–615. doi: 10.1111/acel.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lucin K.M., Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64(1):110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Heneka M.T. Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol. 2017;27(2):220–222. doi: 10.1111/bpa.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lévy E., El Banna N., Baïlle D., Heneman-Masurel A., Truchet S., Rezaei H., Huang M-E., Béringue V., Martin D., Vernis L. Causative Links between Protein Aggregation and Oxidative Stress: A Review. Int. J. Mol. Sci. 2019;20(16):E3896. doi: 10.3390/ijms20163896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Walker J.M., Harrison F.E. Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer’s Disease: Impacts on Cognitive Decline. Nutrients. 2015;7(9):7332–7357. doi: 10.3390/nu7095341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 227.Akamine E.H., Kawamoto E.M., Scavone C., Nigro D., Carvalho M.H.C., de Cássia A Tostes R., Britto L.R., Fortes Z.B. Correction of endothelial dysfunction in diabetic female rats by tetrahydrobiopterin and chronic insulin. J. Vasc. Res. 2006;43(4):309–320. doi: 10.1159/000093196. [DOI] [PubMed] [Google Scholar]
- 228.Abudukadier A., Fujita Y., Obara A., Ohashi A., Fukushima T., Sato Y., Ogura M., Nakamura Y., Fujimoto S., Hosokawa M., Hasegawa H., Inagaki N. Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice. Diabetes. 2013;62(9):3033–3043. doi: 10.2337/db12-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nyström T., Nygren A., Sjöholm A. Tetrahydrobiopterin increases insulin sensitivity in patients with type 2 diabetes and coronary heart disease. Am. J. Physiol. Endocrinol. Metab. 2004;287(5):E919–E925. doi: 10.1152/ajpendo.00046.2004. [DOI] [PubMed] [Google Scholar]
- 230.Vandal M., White P.J., Tremblay C., St-Amour I., Chevrier G., Emond V., Lefrançois D., Virgili J., Planel E., Giguere Y., Marette A., Calon F. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes. 2014;63(12):4291–4301. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- 231.Antoniades C., Cunnington C., Antonopoulos A., Neville M., Margaritis M., Demosthenous M., Bendall J., Hale A., Cerrato R., Tousoulis D., Bakogiannis C., Marinou K., Toutouza M., Vlachopoulos C., Leeson P., Stefanadis C., Karpe F., Channon K.M. Induction of vascular GTP-cyclohydrolase I and endogenous tetrahydrobiopterin synthesis protect against inflammation-induced endothelial dysfunction in human atherosclerosis. Circulation. 2011;124(17):1860–1870. doi: 10.1161/CIRCULATIONAHA.111.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chalupsky K., Kračun D., Kanchev I., Bertram K., Görlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid. Redox Signal. 2015;23(14):1076–1091. doi: 10.1089/ars.2015.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hyndman M.E., Verma S., Rosenfeld R.J., Anderson T.J., Parsons H.G. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am. J. Physiol. Heart Circ. Physiol. 2002;282(6):H2167–H2172. doi: 10.1152/ajpheart.00935.2001. [DOI] [PubMed] [Google Scholar]
- 234.Miller A.L. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern. Med. Rev. 2008;13(3):216–226. [PubMed] [Google Scholar]
- 235.Papakostas G.I., Shelton R.C., Zajecka J.M., Etemad B., Rickels K., Clain A., Baer L., Dalton E.D., Sacco G.R., Schoenfeld D., Pencina M., Meisner A., Bottiglieri T., Nelson E., Mischoulon D., Alpert J.E., Barbee J.G., Zisook S., Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry. 2012;169(12):1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]