Abstract
Background:
In the United States (U.S.), annual influenza vaccination has been recommended for all persons aged ≥6 months with the Healthy People 2020 coverage target of 70%. However, vaccination coverage has remained around 42%–49% during the past eight influenza seasons. We sought to quantify influenza vaccination coverage and factors associated with vaccination in persons seeking outpatient medical care for an acute respiratory infection (ARI).
Methods:
We enrolled outpatients aged ≥6 months with ARI from >50 U.S. clinics from 2011–2012 through 2018–2019 influenza seasons and tested for influenza with molecular assays. Vaccination status was based on documented receipt of the current season’s influenza vaccine. We estimated vaccination coverage among influenza-negative study participants by study site, age, and season, and compared to state-level influenza coverage estimates in the general population based on annual immunization surveys. We used multivariable logistic regression to examine factors independently associated with receipt of influenza vaccines.
Results:
We enrolled 45,424 study participants with ARI who tested negative for influenza during the study period. Annual vaccination coverage among influenza-negative ARI patients and the general population in the participating states averaged 55% (range: 47%– 62%), and 52% (range: 46%–54%), respectively. Among enrollees, coverage was highest among adults aged ≥65 years (82%; range, 80%– 85%) and lowest among adolescents aged 13–17 years (38%; range, 35%- 41%). Factors significantly associated with non-vaccination included non-White race, no college degree, exposure to cigarette smoke, absence of high-risk conditions, and not receiving prior season influenza vaccine.
Conclusions:
Influenza vaccination coverage over eight seasons among outpatients with non-influenza respiratory illness was slightly higher than coverage in the general population but 15% lower than national targets. Increased efforts to promote vaccination especially in groups with lower coverage are warranted to attain optimal health benefits of influenza vaccine.
Keywords: influenza, vaccination, coverage, risk factors, child, adult
INTRODUCTION
Influenza is a major cause of morbidity and mortality among people of all ages in the United States (U.S.) [1]. In the 2018–2019 season alone, influenza infection caused an estimated 31–44 million illnesses, 380,000–760,000 hospitalizations, and 26,000–52,000 influenza-associated deaths [2]. Annual influenza vaccination is the most effective method for preventing influenza and its complications, with varied vaccine effectiveness by season, virus type, and patient characteristics [3, 4]. In 2010, the U.S. Advisory Committee on Immunization Practices (ACIP) recommended that all persons aged ≥6 months without contraindications should receive annual influenza vaccine [5]. Since then, a variety of different formulations of influenza vaccines have become available, with six different vaccine types (egg-based standard dose, egg-based high-dose, cell-culture, recombinant, adjuvanted, and live-attenuated vaccines) licensed and recommended for use in the U.S.
Influenza vaccination coverage in the U.S. remains below the Healthy People 2020 target of 70% for children and adults [6]. National surveys have reported sub-optimal influenza vaccination coverage of 42%–49% among persons of all ages during the past eight influenza seasons [7]. Incremental improvements in influenza vaccination coverage and effectiveness could result in appreciable reductions in influenza disease burden in the U.S. [8, 9]. Previous research has indicated that common reasons for not being vaccinated against influenza include perceived low risk of infection, concerns about vaccine safety, and a perceived lack of benefit, possibly stemming from concerns about low vaccine effectiveness [10–13] Increased contact with the health system (outpatient visits, health care provider recommendations, reminder-recall systems), and having health insurance are strongly associated with a patient’s receipt of vaccines. However, these factors do not fully account for the low vaccination coverage [14, 15].
In this paper, we sought to quantify influenza vaccination coverage and factors associated with vaccination in persons seeking outpatient medical care for an ARI. We analyzed data from a prospective study of outpatients aged ≥6 months in five U.S. communities. Our objectives were to: (1) examine trends in influenza vaccine uptake among a medically attended population and (2) assess factors associated with influenza vaccination over eight influenza seasons.
METHODS
We analyzed data from U.S. Influenza Vaccine Effectiveness (U.S. Flu VE) Network, a prospective study of outpatients seeking care for ARI, from the 2011–2012 through 2018–2019 influenza seasons. We compared overall and age-specific influenza vaccination prevalence from the U.S. Flu VE Network with published state and national estimates from two national data sources: National Immunization Survey-Flu (NIS-Flu) [16] and Behavioral Risk Factor Surveillance System (BRFSS) [17, 18].
Data sources
Details of the U.S. Flu VE Network have been published previously [19–21]. Briefly, the U.S. Flu VE Network sites enrolled approximately 8,000 to 15,000 participants annually from 50–66 ambulatory care facilities (comprising primary care clinics, urgent care clinics, and emergency departments) associated with healthcare institutions in five U.S. states: Michigan, Pennsylvania, Texas, Washington, and Wisconsin. Patients aged ≥6 months seeking outpatient medical care for an ARI with cough within 7 days of illness onset were eligible for enrollment. After obtaining informed consent from patients or their guardians, participants or their proxies were interviewed to collect demographic data, information on general and current health status and symptoms, and influenza vaccination status for the current influenza season. In each season and in each site, study enrollment began after local surveillance identified increasing weekly influenza activity or one or more laboratory-confirmed cases of influenza per week for two consecutive weeks. Enrollment stopped typically in March or April after influenza incidence decreased. All participants were tested for influenza virus infection for research purposes by molecular assays, and participants were classified as influenza-positive or influenza-negative. Study staff collected epidemiological, clinical, vaccination history and vaccine type data from structured interviews, medical chart or health system record reviews, and state immunization information systems. For national and state-level estimates of coverage in the general population, we accessed the National Immunization Survey–Flu (NIS-Flu) database for children aged 6 months to17 years and the Behavioral Risk Factor Surveillance System (BRFSS) database for adults aged ≥18 years [7, 17, 22]
Vaccination status
Vaccination records were extracted from electronic medical records for all participants. Children aged 6 months to 8 years, who are recommended to receive two doses during their first vaccination season, were considered vaccinated for this analysis if they received at least one dose of any seasonal influenza vaccine prior to enrollment according to medical records or immunization registries [23]. In addition, at four sites (excluding Wisconsin), those with parental or self-reported plausible location of vaccination were classified as vaccinated. For participants who were enrolled multiple times within an influenza season, we retained the data of the first enrollment. Prior season vaccination was obtained from electronic medical records, health insurance claims, employee health records, and state/local immunization information systems.
High-risk conditions
Participants were defined as high risk if they had ≥1 medical encounter including outpatient visits and inpatient stays during the 12 months before enrollment corresponding to a high-risk condition identified by ACIP [24]. High-risk categories were not mutually exclusive such that individual participants could contribute to more than one category. Participants with no high-risk ICD-9 or ICD-10 codes in medical records during the 12-month period were classified as having no high-risk conditions.
Analysis
For the U.S. Flu VE participants, we estimated overall and age-specific influenza vaccination coverage, defined as proportion of influenza-negative participants that had received at least one dose of current season influenza vaccine. We restricted analysis to participants who tested negative for influenza because influenza positive patients were less likely to be vaccinated given partial vaccine effectiveness. No adjustments were made to national survey data, assuming a small proportion of respondents with influenza. We calculated unweighted overall vaccination coverage estimates by demographic factors, clinical characteristics, and factors associated with vaccination. To examine timing of annual vaccination, we combined data from all seasons and plotted cumulative percent vaccinated by month. Lastly, we examined distributions of influenza vaccine type, stratified by age.
We compared the influenza vaccination coverage estimates in the U.S. Flu VE influenza-negative cohort with coverage in the general population nationally and across the five states with U.S. Flu VE study sites. Among U.S. Flu VE enrollees, we compared vaccinated and unvaccinated participants using Wald chi-squared tests for categorical variables and student’s t-test for continuous variables. We examined associations with receipt of current-season influenza vaccine using multivariable logistic regression models [16], including variables significantly associated with influenza vaccination from bivariate models (p<0.05). A priori, all models contained factors that have been previously associated with influenza vaccination, including participant’s sex, age, race/ethnicity, education level, number of high-risk medical conditions, exposure to cigarette smoke and prior vaccination history [10–12, 23, 25–29]. We reported adjusted odds ratios (aORs) with 95% confidence intervals(95% CIs). Because education levels were not collected until the 2013–2014 season, we evaluated risk factors for non-vaccination from 2013–2014 through 2018–2019. Analyses were conducted using SAS version 9.4 statistical software.
RESULTS
A total of 61,356 participants with ARI were enrolled in the U.S. Flu VE Network during eight influenza seasons. Of these, 15,857 (26%) tested positive for influenza, 75 were inconclusive (0.1%) and 45,424 (74%) tested negative. Among influenza-negative participants, 59% were female, 73% were White, non-Hispanic, and 38% had completed college (Table 1). Distributions of participants’ race/ethnicity and age groups varied among study sites, however, over 60% of participants at each site was white, non-Hispanic, (62% in Texas to 91% in Wisconsin), average age ranged from 29 to 38 years, median ranged from 21 to 39 years (Supplemental Table 1). A majority (68%) of participants self-reported “very good” or “excellent” general health, and 59% did not have any high-risk medical conditions (Table 1). Seventy-four percent had higher than normal BMI (overweight, obese, morbidly obese), 17% were current smokers or were exposed to cigarette smoke in the household, and 47% were vaccinated in the prior season. The distribution of sex, comorbidities, self-reported general health status, and BMI categories were similar among the five sites (Supplemental Table 1).
Table 1.
Demographic and Clinical Characteristics of Influenza-Negative Participants Enrolled in the United States Influenza Vaccine Effectiveness Network, 2011–2012 through 2018–2019 Influenza Seasons
| Vaccination Statusa |
||||
|---|---|---|---|---|
| Total | Vaccinated | |||
| Characteristics | No. | (%)b | No. | (%)c |
| Overall vaccination coverage | 45424 | 100 | 25063 | 55 |
| States | ||||
| Michigan | 7713 | 17 | 4396 | 57 |
| Pennsylvania | 7925 | 17 | 4184 | 53 |
| Texas | 9472 | 21 | 4477 | 47 |
| Washington | 11631 | 26 | 7214 | 62 |
| Wisconsin | 8683 | 19 | 4792 | 55 |
| Sex | ||||
| Female | 27032 | 59 | 15303 | 57 |
| Male | 18391 | 41 | 9759 | 53 |
| Race/ethnicity d | ||||
| White, non-Hispanic | 32889 | 73 | 18825 | 57 |
| Black, non-Hispanic | 3882 | 9 | 1692 | 44 |
| Hispanic, any race | 4275 | 9 | 2081 | 49 |
| Other, non-Hispanic | 4234 | 9 | 2389 | 56 |
| Age Group | ||||
| 6 months-4 yearse | 8047 | 18 | 4967 | 62 |
| 5–12 years | 6252 | 14 | 2849 | 46 |
| 13–17 years | 2831 | 6 | 1066 | 38 |
| 18–49 years | 15190 | 33 | 6824 | 45 |
| 50–64 years | 7569 | 17 | 4800 | 63 |
| ≥65 years | 5535 | 12 | 4557 | 82 |
| Highest education level in household e | ||||
| Some college or less | 22686 | 62 | 11331 | 50 |
| College graduate and above | 13886 | 38 | 8847 | 64 |
| Comorbidities f | ||||
| None | 26623 | 59 | 12817 | 48 |
| 1 High-risk medical condition | 9696 | 21 | 5606 | 58 |
| 2 High-risk medical conditions | 3972 | 9 | 2675 | 67 |
| ≥3 High-risk medical conditions | 5113 | 11 | 3965 | 77 |
| Exposure to cigarette smoke g | ||||
| Yes | 7744 | 83 | 3409 | 44 |
| No | 37510 | 17 | 21575 | 57 |
| Vaccinated in the prior season | ||||
| Yes | 21183 | 47 | 16809 | 79 |
| No | 24241 | 53 | 8254 | 34 |
| Self-reported general health status h | ||||
| Excellent | 14591 | 32 | 7699 | 53 |
| Very good | 16570 | 36 | 9041 | 55 |
| Good | 10993 | 24 | 6303 | 57 |
| Fair/Poorj | 3234 | 7 | 2004 | 62 |
| Body Mass Index (BMI) i | ||||
| Underweight (BMI <18.5) | 241 | 1 | 123 | 51 |
| Normal (18.5 ≤ BMI < 25) | 5724 | 20 | 3098 | 54 |
| Overweight (25 ≤ BMI < 30) | 7769 | 27 | 4630 | 60 |
| Obese (30 ≤ BMI < 40) | 9824 | 34 | 5820 | 59 |
| Morbidly obese (BMI ≥40) | 3272 | 12 | 1835 | 56 |
| Unknown | 1463 | 5 | 675 | 46 |
Defined as having received ≥1 dose of any current seasonal influenza vaccine prior to clinic visit.
Percentage refers to column percentage
Percentage refers to row percentage
Enrollees were categorized into one of four mutually exclusive racial/ethnic populations: white, black, other race, and Hispanic. Persons identifying as Hispanic might have been of any race. Persons identifying as white, black, or other race were non-Hispanic. Race/ethnicity data were missing for 144 enrollees (<1%).
Data on education level were only collected from 2013–2014 through 2018–2019 influenza seasons, data missing/refused for 155 enrollees (<1%)
Comorbidities are defined as ≥1 medical record-documented high-risk code in prior year, as defined by Advisory Committee on Immunization Practices guidance for conditions that increase risk for complications from influenza. High-risk conditions were categorized into groups: asthma, cerebrovascular diseases, chronic obstructive pulmonary diseases (COPD) and other lung diseases, diabetes, cardiovascular diseases, hematologic conditions/blood disorders, liver diseases, neurologic conditions, renal diseases, and immunosuppressive conditions (including rheumatoid arthritis, immune system disorders or immunodeficiency, antineoplastic chemotherapy, HIV, organ transplants).
Data on exposure to cigarette smoke in household were missing/refused for 170 enrollees.
“Fair” and “Poor” were grouped together for self-reported health status. Data missing for 36 enrollees (<1%)
Body mass index was calculated as kg/m2 from height and weight recorded in the electronic medical record. Calculated for adults aged ≥18 years only
Vaccination coverage in influenza-negative participants
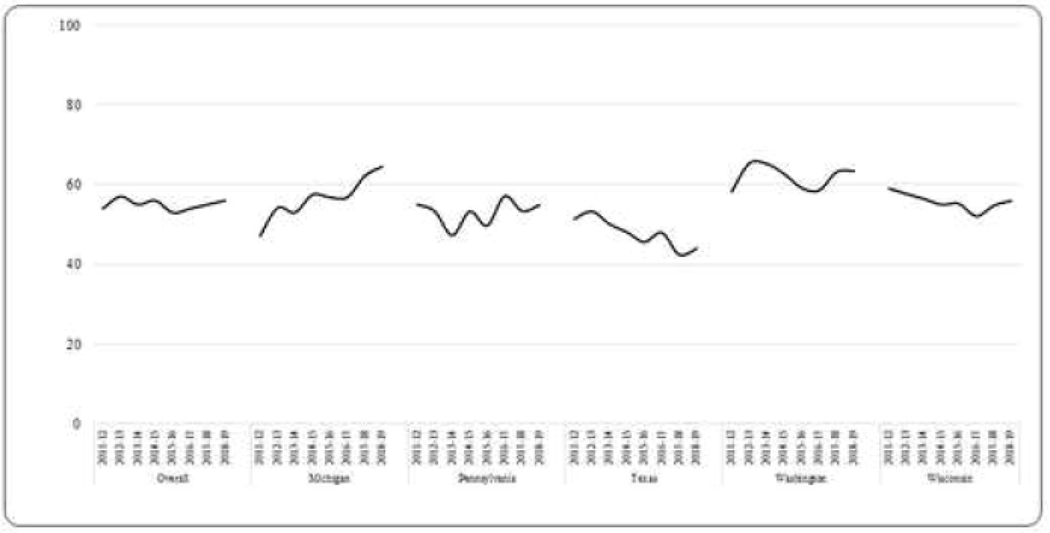
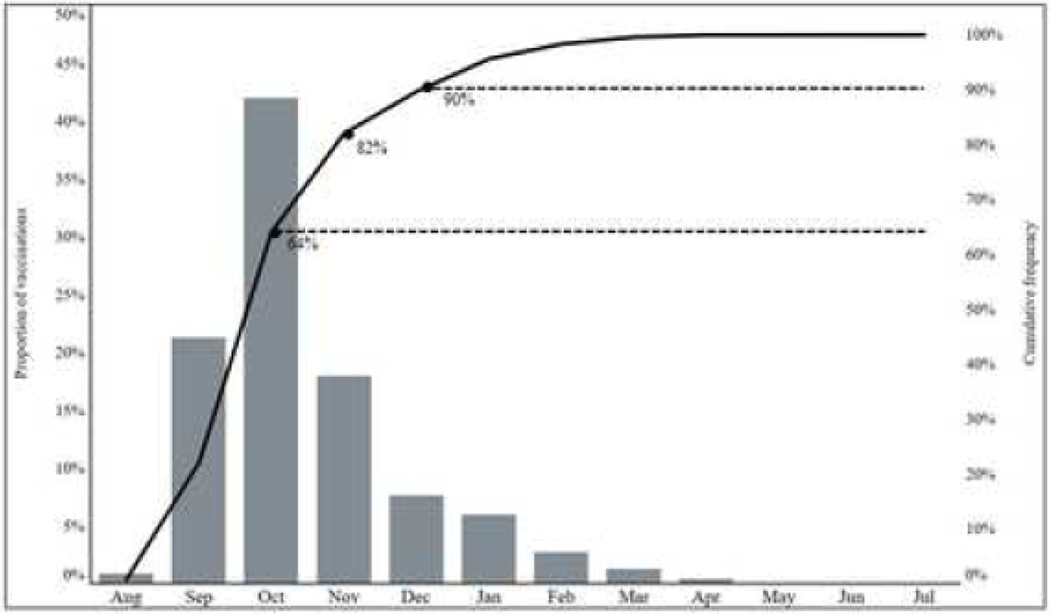
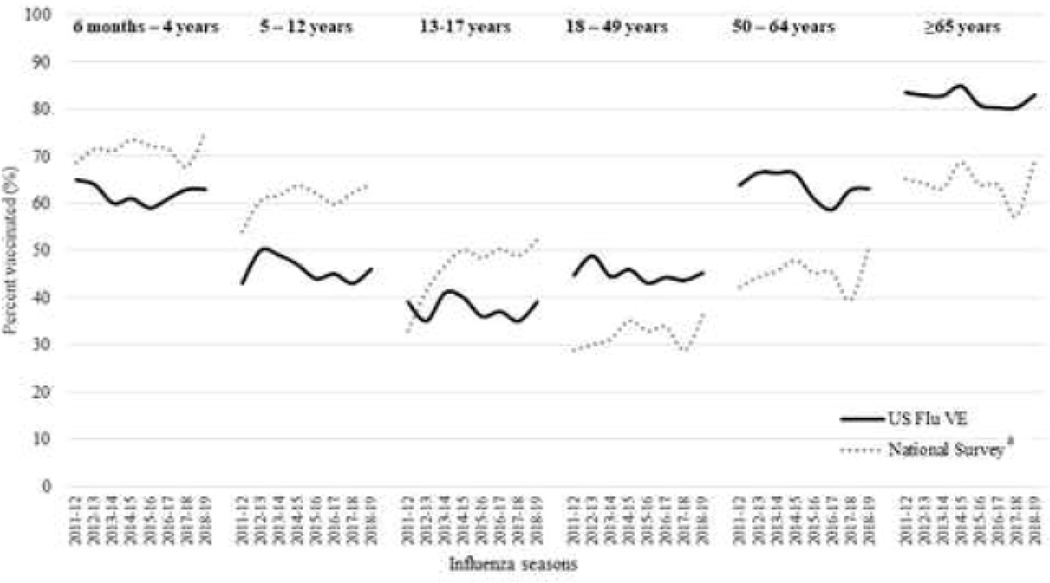
Over the eight influenza seasons studied, the average prevalence of receipt of ≥1 dose of influenza vaccine among all influenza-negative participants aged ≥6 months was 55% (range: 53%−57%) (Figure 1). Among those vaccinated, 90% were vaccinated by end of December of the respective influenza season (Figure 2). Vaccination coverage varied widely by age groups in a “U” shaped curve; coverage was higher among adults aged ≥50 years (63%, range: 59%−67% in 50–64 years; 82%, range: 80%−85% in adults ≥65 years) and young children aged 6 months-4 years (62%, range: 59%−65%) compared to older children aged 5–12 years (46%, range: 43%−50%). Coverage was lower among adolescents aged 13–17 years (38%, range: 35%−41%) and adults aged 18–49 years (45%, range: 42%−50%) and (Figure 3). Among children recommended to receive 2 doses, most of those vaccinated received both doses (range among seasons, 74%−84%). Vaccination coverage varied across study sites; coverage was highest in Washington (62%) and lowest in Texas (47%). During the study period, overall coverage increased in Michigan (44% to 63%) and Washington (58% to 63%).
Figure 1:
Prevalence of Influenza Vaccination Among Influenza-Negative Participants Enrolled in the United States Influenza Vaccine Effectiveness Network, 2011–2012 through 2018–2019 (n = 45,424)
Figure 2:
Proportion of Influenza-Negative Participants Vaccinated by Calendar Month in the United States Influenza Vaccine Effectiveness Network Sites, 2011–2012 through 2018–2019 Influenza Seasonsa
Note: gray bars are represented on left axis and solid line on right axis
a: The proportion of vaccinations occurring during these months did not vary by influenza season
Figure 3.
Seasonal Influenza Vaccination Coverage Among Children aged ≥6 months, by age group — United States Influenza Vaccine Effectiveness Network and National Survey Estimatesa, 2011–2012 through 2018–2019. Panel A: Children aged 6 months – 17 years. Panel B: Adults aged ≥18 years
US Flu VE denotes United States Influenza Vaccine Effectiveness Network
a: National Survey estimates (dotted line) represent state specific estimates from National Immunization Survey-Flu (ages 6 months-17 years) and Behavioral Risk Factor Surveillance System (age≥18 years)
Coverage was lower among those who were non-white (44% vs. 57%), had not completed college (50% vs. 64%), had no high-risk conditions (48% vs. 69%), were exposed to cigarette smoke (44% vs. 57%) and had not received prior season’s influenza vaccine (34% vs. 79%) (Table 1). Across all influenza seasons, 64% and 90% were vaccinated by the end of October and December, respectively (Figure 2). Within age groups, coverage did not change substantially during the eight seasons included (Figure 3). When contrasted with state surveys, average coverage among children aged 6 months through 17 years was 8% lower (range: −11% to 1%) in the U.S. Flu VE population compared to NIS-Flu (Figure 3). In contrast, coverage among adults aged ≥18 years was 10% higher (range: 6% to 14%) in the U.S. Flu VE Network population compared to BRFSS state estimates.
Uptake of vaccine types by age group
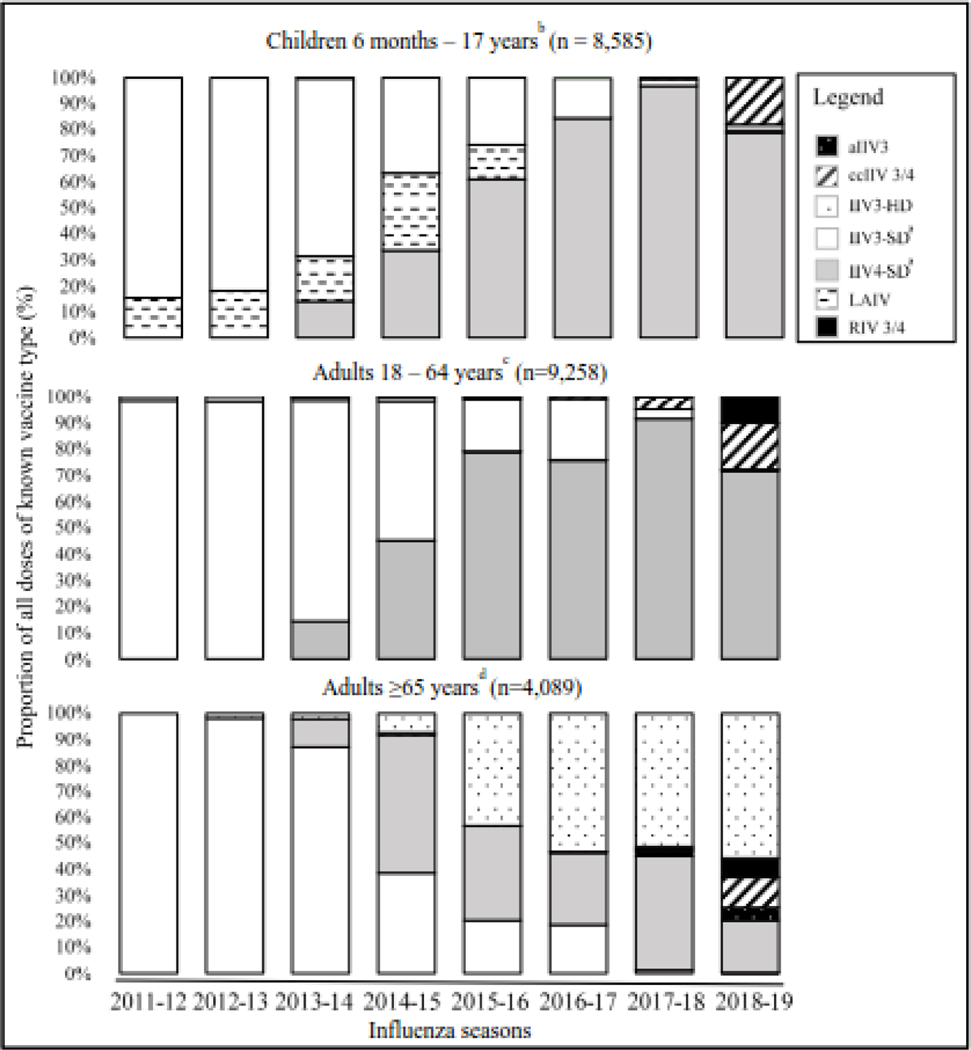
Several different vaccine formulations were used in the U.S. Flu VE network population during the study period. Trivalent standard dose inactivated influenza vaccine (IIV3-SD) was the predominant vaccine type (66%−100%) from 2011–2012 through 2013–2014 influenza seasons for all age groups (Figure 4). Among children aged ≤18 years, live attenuated influenza vaccine (LAIV) accounted for 11% to 25% of influenza vaccine during 2011–2012 through 2015–2016, although LAIV uptake in the following three seasons was limited. Uptake of quadrivalent standard-dose (IIV4-SD) dramatically increased from 14% in 2013–2014 to a peak of 95% in 2017–2018 among persons aged ≤64 years. Uptake of high-dose vaccine (IIV3-HD) among adults aged ≥65 years increased from 1% in 2012–2013 to 57% in 2018–2019. Distribution of vaccine type changed further in 2018–2019, with the increased use of cell-based (ccIIV4) or recombinant (RIV4) vaccines, principally at two study sites (data not shown). Uptake of adjuvanted influenza vaccine was limited (<1%) in the U.S. Flu VE cohort during the study years.
Figure 4.
Trends in Influenza Vaccine Type, by Age Group, US Influenza Vaccine Effectiveness Network, 2011–2012 through 2018–2019 Influenza Seasons (n = 21,932)
IIV3/4 denotes trivalent/quadrivalent inactivated influenza vaccine; a denotes adjuvanted; HD denotes high dose; SD denotes standard dose; cc denotes cell-culture; LAIV denotes live attenuated influenza vaccine; RIV denotes recombinant influenza vaccine
a: Fluzone Intradermal Trivalent included into IIV3-SD (5), IIV4, (8)
b: Data missing for 297 enrollees (2%)
c: Data missing for 2366 enrollees (20%)
d: Data missing for 468 enrollees (10%)
Risk factors for non-vaccination in the U.S. Flu VE Network
Factors significantly associated with non-vaccination were age, comorbidities, education level, and race/ethnicity (Table 2). Not being vaccinated in the prior season was found to be the strongest predictor of non-vaccination (OR = 7.78; 95% CI = 7.42–8.16) but was removed in the final model in order to assess other risk factors independently associated with non-vaccination. Odds of non-vaccination among children aged 5 – 17 years and young adults aged 18 – 49 years were 3 to 4 times higher (aOR 3.14 – 4.40) than persons aged ≥65 years. Other significant factors associated with non-vaccination included zero vs. ≥3 high risk conditions (aOR= 2.66; 95% CI = 2.44–2.90), less than a college degree vs college degree or higher education (aOR = 1.61, 95% CI = 1.58–1.74), black race compared with non-Hispanic whites (aOR =1.62; 95% CI = 1.49–1.76) and exposure to cigarette smoke (aOR=1.39, 95% CI = 1.31–1.48).
Table 2.
Risk factors associated with Non-vaccination Among Influenza-Negative Participants in the United States Influenza Vaccine Effectiveness Network 2013–2019 Influenza Seasons (n=36707)
| OR | 95% CI | AOR | 95% CI | |
|---|---|---|---|---|
| States | ||||
| Michigan | 1.23 | (1.16–1.32) | 1.06 | (0.99–1.14) |
| Pennsylvania | 1.47 | (1.37–1.54) | 1.22 | (1.14–1.31) |
| Texas | 1.82 | (1.72–1.92) | 1.52 | (1.42–1.63) |
| Washington | REF | REF | REF | REF |
| Wisconsin | 1.33 | (1.25–1.41) | 1.20 | (1.12–1.29) |
| Sex | ||||
| Female | REF | REF | REF | REF |
| Male | 1.15 | (1.11–1.20) | 1.24 | (1.18–1.30) |
| Race/Ethnicity a | ||||
| White, non-Hispanic | REF | REF | REF | REF |
| Black, non-Hispanic | 1.72 | (1.61–1.85) | 1.62 | (1.49–1.76) |
| Hispanic, any race | 1.41 | (1.33–1.52) | 1.06 | (0.98–1.15) |
| Other, non-Hispanic | 1.03 | (0.97–1.10) | 1.05 | (0.97–1.14) |
| Age Group | ||||
| 6 months-4 months | 2.94 | (2.70–3.23) | 1.63 | (1.46–1.82) |
| 5–12 years | 5.56 | (5.00–6.25) | 3.14 | (2.81–3.51) |
| 13–17 years | 7.69 | (6.67–8.33) | 4.40 | 3.86–6.00) |
| 18–49 years | 5.88 | (5.26–6.25) | 3.74 | (3.41–4.10) |
| 50–64 years | 2.70 | (2.50–2.94) | 3.74 | (3.41–4.10) |
| ≥65 years | REF | REF | REF | REF |
| Highest education level in household b | ||||
| College graduate or less | 1.76 | (1.68–1.84) | 1.61 | (1.58–1.74) |
| Advanced | REF | REF | REF | REF |
| Comorbidities c | ||||
| None | 3.70 | (3.45–4.00) | 2.66 | (2.44–2.90) |
| 1 High risk condition | 2.50 | (2.27–2.70) | 1.83 | (1.67–2.00) |
| 2 High risk conditions | 1.64 | (1.49–1.82) | 1.46 | (1.31–1.62) |
| ≥3 High risk conditions | REF | REF | REF | REF |
| Smoking status d | ||||
| Yes | 1.72 | (1.64–1.82) | 1.39 | (1.31–1.48) |
| No | REF | REF | REF | REF |
| Self-reported general health status | ||||
| Excellent | 1.44 | (1.32–1.57) | ||
| Very good | 1.34 | (1.23–1.46) | ||
| Good | 1.21 | (1.10–1.32) | ||
| Fair/poore | REF | REF | ||
| Body Mass Index (BMI) f | ||||
| Underweight (BMI <18.5) | 1.37 | (1.04–1.82) | 1.18 | (0.87–1.61) |
| Normal (18.5 ≤ BMI < 25) | REF | REF | REF | REF |
| Overweight (25 ≤ BMI < 30) | 1.20 | (1.12–1.20) | 1.08 | (1.00–1.18) |
| Obese (30 ≤ BMI < 40) | 1.02041 | (0.94–1.09) | 0.99 | (0.92–1.06) |
| Morbidly obese (BMI ≥40) | 1.16279 | (1.06–1.28) | 1.18 | (1.07–1.31) |
Enrollees were categorized into one of four mutually exclusive racial/ethnic populations: white, black, other race, and Hispanic. Persons identifying as Hispanic might have been of any race. Persons identifying as white, black, or other race were non-Hispanic. Race/ethnicity data were missing for 121 enrollees (<1%).
Data on education level were only collected from 2013–2014 through 2018–2019 influenza seasons, data missing/refused for 153 enrollees (<1%)
Comorbidities are defined as ≥1 medical record-documented high-risk code in prior year, as defined by Advisory Committee on Immunization Practices guidance for conditions that increase risk for complications from influenza. High-risk conditions were categorized into groups: asthma, cerebrovascular diseases, chronic obstructive pulmonary diseases (COPD) and other lung diseases, diabetes, cardiovascular diseases, hematologic conditions/blood disorders, liver diseases, neurologic conditions, renal diseases, and immunosuppressive conditions (including rheumatoid arthritis, immune system disorders or immunodeficiency, antineoplastic chemotherapy, HIV, organ transplants).
Includes current smoker and exposure to cigarette smoke in the household. Data on exposure to cigarette smoke in household were missing/refused for 170 enrollees.
“Fair” and “Poor” were grouped together for self-reported health status.
Body mass index was calculated as kg/m2 from height and weight recorded in the electronic medical record. Calculated for adults aged ≥18 years only.
DISCUSSION
Reducing morbidity and mortality associated with influenza requires vaccination programs to reach a large percentage of the U.S. population annually. The primary purpose of influenza vaccine effectiveness networks is to evaluate protection provided by vaccination, but these networks can also provide information on coverage by age, vaccine type, geography, and factors associated with vaccination. Influenza vaccination coverage among medically attended outpatients in the U.S. Flu VE network was approximately 55%, which is 15% below the Healthy People 2020 targets of 70%, and did not change substantially over eight influenza seasons after the 2009 A(H1N1) influenza pandemic [30]. These trends in influenza vaccination rates mirrored telephone survey data from representative samples of state residents [7, 16, 18]. Increased uptake of quadrivalent vaccine formulations and introduction of new vaccines designed to be more immunogenic, especially among older individuals, did not substantially improve coverage although perception of improved effectiveness may have influenced vaccine choice, purchase or provider recommendations.
Consistent with the literature, our findings suggest that persons with more frequent contact with the health care system are more likely to be vaccinated. Adults aged ≥65 years, children aged 6 months-4 years, and older adults aged 50–64 years, age groups that are known to access medical care more frequently than other age groups, had consistently higher coverage throughout the past decade [23, 31]. Perception of risk of complications and severe disease may also contribute to increased coverage in these age groups, while perception of lower risk may lead to decreased vaccination in younger adults [10, 11, 13]. Vaccination coverage was lowest among adolescents aged 13–17 years and adults aged 18– 49 years. Healthy adults who have low perceived risk of influenza infection, fewer interactions with the health system, or low confidence in vaccine effectiveness are less likely to be vaccinated [10–12, 25]. Differences in coverage by age may reflect gradual expansion of vaccine recommendations between 2000 and 2010 from young children to include older children and adolescents [30, 32]. However, between 2012 and 2019, coverage in this population plateaued [17, 33].
Overall vaccination coverage and trends in this medically attended population were similar to state estimates for the general population. However, we observed considerable differences when stratified by age groups. Coverage among children was lower in the U.S. Flu VE network compared to state estimates, possibly due to parental over-reporting of children’s vaccination in telephone surveys [7, 17, 34]. In contrast, coverage among adults was 13%−18% higher in the U.S. Flu VE Network compared to state estimates, which may reflect greater access to medical care among patients seeking care for mild illness. Differences in demographic groups and other determinants of vaccination could also explain differences in coverage between participants in our network and the estimates from the general population [13, 23, 34].
Despite the lack of preferential recommendations, with the exception of LAIV in the 2013–2014 influenza season, we observed usage patterns of influenza vaccine types changed drastically in this study population after 2014–2015. Among children and adults aged 18–64 years, quadrivalent replaced the trivalent, standard dose vaccine, and high-dose vaccine use increased among those aged ≥65 years. We also reported a sudden decline in LAIV uptake in the 2016–2017 season, following ACIP’s withdrawal of its recommendation for use of LAIV that season [35]. Changing patterns of vaccine type were not associated with an increase in coverage. This indicates a shift in vaccine type usage among those routinely vaccinated rather than previously unvaccinated persons seeking a new influenza vaccine product.
Lastly, we identified that not being vaccinated in the prior season, younger age, lower education levels, non-Hispanic, black ethnicity, and having no high-risk medical conditions were independently associated with non-vaccination in this medically attended population. Prior season vaccination was by far the strongest predictor of vaccination in the current season as past vaccination was highly correlated with current season vaccination. Because of this strong correlation, the association of age, education, ethnicity, and risky medical conditions with vaccination uptake in the current season was not adjusted for vaccination in the prior season was not included in the final multivariable model. These findings are consistent with several studies and systematic reviews which document that older, white (non-Hispanic) adults with preexisting medical conditions are more likely to interact with a health care provider and be vaccinated [10, 12, 14, 23, 27, 28]. Our findings indicate that even in populations seeking medical care, racial disparities existed among those who identified as black, non-Hispanic, when compared to those who identified as white, non-Hispanic.
Limitations
Several limitations of our study should be considered. First, ARI cases who were tested positive for influenza because they are less likely to be vaccinated, which could potentially bias results toward an overestimation of vaccination coverage. Our overall coverage estimates were geographically limited to research sites in five states, thus, our results are not generalizable to all U.S. populations. The U.S. Flu VE network consists of a predominantly white, non-Hispanic population seeking outpatient medical care for ARI, and are not nationally representative populations. In addition, our data do not capture important indicators of vaccination such as health provider recommendation, number of physician contacts, income, or socioeconomic status as shown in other studies [10, 13, 14, 26]. The U.S. Flu VE network was designed to evaluate influenza vaccine effectiveness, and not to investigate individual decisions and beliefs leading to non-vaccination. Nevertheless, we documented that coverage is suboptimal in specific age groups, socio-demographic groups, and sites even in this medically attended population [12, 26].
Conclusions
Overall vaccination coverage was below national targets in all age groups, especially among older children and young adults. It is plausible that vaccination coverage has plateaued due to perceptions of the influenza vaccine offering only modest protection. Advancements in vaccine technologies that result in products conferring increased protection may boost public perception [8, 23, 36]. Further work identifying determinants of non-vaccination could inform efforts to promote vaccination in target populations and reduce the burden of influenza illness in the community.
Supplementary Material
Highlights.
Between 2011–2019, influenza vaccination coverage among US outpatients was ~55%
Influenza vaccination coverage was lower than national targets of 70%
Coverage was highest among adults older than 65 years and children aged<5 years
Coverage was lowest among persons aged 13–49 years
Acknowledgements:
The authors would like to thank the research staff at all study sites and individuals who participated in this study.
Joshua G.Petrie, Lois E. Lamerato, Ryan E. Malosh, E.J. McSpadden, Hannah Segaloff, Caroline K.Cheng, Rachel Truscon, Emileigh Johnson, Armanda Kimberly, Anne Kaniclides, Amy Getz, Kim Beney, Sarah Bauer, Michelle Groesbeck. University of Michigan, Ann Arbor, and Henry Ford Health System, Detroit, Michigan; Rose Azrak, G.K. Balasubramani, Todd M. Bear, Duane Eisaman, Heather Eng, Andrew Fackler, Edward Garofolo, Robert Hickey, Philip Iozzi, Monika Johnson, Stephanie Kirk, Jason A. Lyons, Donald B. Middleton, Krissy K. Moehling, Jonathan M. Raviotta, Evelyn C. Reis, Bret Rosenblum, Sean Saul, Theresa Sax, Michael Susick, Joe Suyama, Leonard F. Urbanski, Alexandra Weissman, John V. Williams, University of Pittsburgh Schools of the Health Sciences and University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
Funding: The US Influenza Vaccine Effectiveness Network was supported by the Centers for Disease Control and Prevention through [cooperative agreements U01IP001034-U01IP001039); At Pittsburgh, the project was also supported by the National Institutes of Health through grant UL1TR001857.
Abbreviations:
- ACIP
Advisory Committee of Immunization Practices
- aOR
Adjusted Odds Ratio
- ARI
Acute Respiratory Illness
- CI
Confidence Interval
- NIS-Flu
National Influenza Survey-Flu
- BRFSS
Behavioral Risk Factor Surveillance System
- aIIV3
Adjuvanted Inactivated Influenza Vaccine (Trivalent)
- ccIIV 3/4
Cell-Based Inactivated Influenza Vaccine (Trivalent & Quadrivalent)
- IIV3-SD
Standard Dose Trivalent Inactivated Influenza Vaccine
- IIV3-HD
High-Dose Trivalent Inactivated Influenza Vaccine
- IIV4
Quadrivalent Inactivated Influenza Vaccine
- LAIV
Live Attenuated Influenza Vaccine
- OR
Odds Ratio
- RIV 3/4
Recombinant Influenza Vaccine (Trivalent & Quadrivalent)
- RT-PCR
Real-Time Reverse Transcription Polymerase Chain Reaction
- U.S. Flu VE
United States Influenza Vaccine Effectiveness
Footnotes
Conflict of interest: The authors have indicated they have no potential conflicts of interest to disclose.
All authors attest they meet the ICMJE criteria for authorship
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1.].Lu PJ, Santibanez TA, Williams WW, Zhang J, Ding H, Bryan L, et al. Surveillance of influenza vaccination coverage--United States, 2007–08 through 2011–12 influenza seasons. MMWR Surveill Summ. 2013;62(4):1–28 [PubMed] [Google Scholar]
- [2.].Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States—2018–2019 influenza season. 2020. June 17, 2020 [Google Scholar]
- [3.].Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. The Lancet Infectious diseases. 2016;16(8):942–51. 10.1016/s1473-3099(16)00129-8 [DOI] [PubMed] [Google Scholar]
- [4.].Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza and other respiratory viruses. 2018;12(1):132–7. 10.1111/irv.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5.].Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2016;65(5):1–54. 10.15585/mmwr.rr6505a1 [DOI] [PubMed] [Google Scholar]
- [6.].Health UDo, Services H, Prevention OoD, Health Promotion. Healthy people 2020. Washington, DC:; 2010. [Google Scholar]
- [7.].Centers for Disease Control and Prevention. 2010–11 through 2018–19 Influenza Seasons Vaccination Coverage Trend Report 2019 [Available from: https://www.cdc.gov/flu/fluvaxview/reportshtml/trends/index.html. September 26, 2019
- [8.].Hughes MM, Reed C, Flannery B, Garg S, Singleton JA, Fry AM, et al. Projected Population Benefit of Increased Effectiveness and Coverage of Influenza Vaccination on Influenza Burden in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020;70(12):2496–502. 10.1093/cid/ciz676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9.].Fry AM, Kim IK, Reed C, Thompson M, Chaves SS, Finelli L, et al. Modeling the effect of different vaccine effectiveness estimates on the number of vaccine-prevented influenza-associated hospitalizations in older adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(3):406–9. 10.1093/cid/ciu328 [DOI] [PubMed] [Google Scholar]
- [10.].Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML. Barriers of Influenza Vaccination Intention and Behavior - A Systematic Review of Influenza Vaccine Hesitancy, 2005 – 2016. PloS one. 2017;12(1):e0170550. 10.1371/journal.pone.0170550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11.].Santibanez TA, Kennedy ED. Reasons given for not receiving an influenza vaccination, 2011–12 influenza season, United States. Vaccine. 2016;34(24):2671–8. 10.1016/j.vaccine.2016.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12.].Lutz CS, Fink RV, Cloud AJ, Stevenson J, Kim D, Fiebelkorn AP. Factors associated with perceptions of influenza vaccine safety and effectiveness among adults, United States, 2017–2018. Vaccine. 2020;38(6):1393–401. 10.1016/j.vaccine.2019.12.004 [DOI] [PubMed] [Google Scholar]
- [13.].Kang GJ, Culp RK, Abbas KM. Facilitators and barriers of parental attitudes and beliefs toward school-located influenza vaccination in the United States: Systematic review. Vaccine. 2017;35(16):1987–95. 10.1016/j.vaccine.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14.].Lu PJ, O’Halloran A, Williams WW. Impact of health insurance status on vaccination coverage among adult populations. American journal of preventive medicine. 2015;48(6):647–61. 10.1016/j.amepre.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15.].Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, et al. Surveillance of Vaccination Coverage Among Adult Populations - United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. 10.15585/mmwr.ss6501a1 [DOI] [PubMed] [Google Scholar]
- [16.].Centers for Disease Control and Prevention. National Immunization Surveys. [Available from: https://www.cdc.gov/vaccines/imz-managers/nis/about.html. March 5, 2020
- [17.].Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2018–19 influenza season. 2019;21:2019
- [18.].Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System [Available from: https://www.cdc.gov/brfss/about/index.htm. March 5, 2020
- [19.].Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, et al. Influenza Vaccine Effectiveness Against 2009 Pandemic Influenza A(H1N1) Virus Differed by Vaccine Type During 2013–2014 in the United States. The Journal of infectious diseases. 2016;213(10):1546–56. 10.1093/infdis/jiv577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20.].Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, et al. Influenza Vaccine Effectiveness in the United States during the 2015–2016 Season. N Engl J Med. 2017;377(6):534–43. 10.1056/NEJMoa1700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21.].Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(3):319–27. 10.1093/cid/cit736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22.].Lu P-j, Hung M-C, O’Halloran AC, Ding H, Srivastav A, Williams WW, et al. Seasonal Influenza Vaccination Coverage Trends Among Adult Populations, US, 2010–2016. 2019;57(4):458–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23.].Abbas KM, Kang GJ, Chen D, Werre SR, Marathe A. Demographics, perceptions, and socioeconomic factors affecting influenza vaccination among adults in the United States. PeerJ. 2018;6:e5171–e. 10.7717/peerj.5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24.].Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, et al. Influenza Vaccine Effectiveness in the United States during the 2015–2016 Season. N Engl J Med. 2017;377(6):534–43. 10.1056/NEJMoa1700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25.].Lu PJ, Hung MC, O’Halloran AC, Ding H, Srivastav A, Williams WW, et al. Seasonal Influenza Vaccination Coverage Trends Among Adult Populations, U.S., 2010–2016. American journal of preventive medicine. 2019;57(4):458–69. 10.1016/j.amepre.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26.].Kahn KE, Santibanez TA, Zhai Y, Bridges CB. Association between provider recommendation and influenza vaccination status among children. Vaccine. 2018;36(24):3486–97. 10.1016/j.vaccine.2018.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27.].Okoli GN, Abou-Setta AM, Neilson CJ, Chit A, Thommes E, Mahmud SM. Determinants of Seasonal Influenza Vaccine Uptake Among the Elderly in the United States: A Systematic Review and Meta-Analysis. Gerontology & geriatric medicine. 2019;5:2333721419870345. 10.1177/2333721419870345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28.].Harrison SM, Wei MY, Lamerato LE, Petrie JG, Toth Martin E. Multimorbidity is associated with uptake of influenza vaccination. Vaccine. 2018;36(25):3635–40. 10.1016/j.vaccine.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29.].Webb NS, Dowd-Arrow B, Taylor MG, Burdette AM. Racial/Ethnic Disparities in Influenza Vaccination Coverage Among US Adolescents, 2010–2016. Public health reports (Washington, DC : 1974). 2018;133(6):667–76. 10.1177/0033354918805720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30.].Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2005;54(Rr-8):1–40 [PubMed] [Google Scholar]
- [31.].Lu PJ, O’Halloran A, Ding H, Srivastav A, Williams WW. Uptake of Influenza Vaccination and Missed Opportunities Among Adults with High-Risk Conditions, United States, 2013. The American journal of medicine. 2016;129(6):636.e1–.e11. 10.1016/j.amjmed.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32.].Santibanez TA, Lu PJ, O’Halloran A, Meghani A, Grabowsky M, Singleton JA. Trends in childhood influenza vaccination coverage--U.S., 2004–2012. Public health reports (Washington, DC : 1974). 2014;129(5):417–27. 10.1177/003335491412900505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33.].Santibanez TA, Srivastav A, Zhai Y, Singleton JA. Trends in Childhood Influenza Vaccination Coverage, United States, 2012–2019. Public health reports (Washington, DC : 1974). 2020;135(5):640–9. 10.1177/0033354920944867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34.].Linn ST, Guralnik JM, Patel KV. Disparities in influenza vaccine coverage in the United States, 2008. J Am Geriatr Soc. 2010;58(7):1333–40. 10.1111/j.1532-5415.2010.02904.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35.].Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines recommendations of the Advisory Committee on Immunization Practices—United States, 2016–17 influenza season. 2016;65(5):1–52 [DOI] [PubMed] [Google Scholar]
- [36.].Ainslie KEC, Haber M, Orenstein WA. Challenges in estimating influenza vaccine effectiveness. Expert review of vaccines. 2019;18(6):615–28. 10.1080/14760584.2019.1622419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.