Abstract
Federal and state enforcement authorities have increasingly intervened on the criminal overprescribing of opioids. However, little is known about the health effects these enforcement actions have on patients experiencing disrupted access to prescription opioids or medication-assisted treatment/medication for opioid use disorder. Simultaneously, opioid death rates have increased. In response, the Maryland Department of Health (MDH) has worked to coordinate mitigation strategies with enforcement partners (defined as any federal, state, or local enforcement authority or other governmental investigative authority). One strategy is a standardized protocol to implement emergency response functions, including rapidly identifying health hazards with real-time data access, deploying resources locally, and providing credible messages to partners and the public. From January 2018 through October 2019, MDH used the protocol in response to 12 enforcement actions targeting 34 medical professionals. A total of 9624 patients received Schedule II-V controlled substance prescriptions from affected prescribers under investigation in the 6 months before the respective enforcement action; 9270 (96%) patients were residents of Maryland. Preliminary data indicate fatal overdose events and potential loss of follow-up care among the patient population experiencing disrupted health care as a result of an enforcement action. The success of the strategy hinged on endorsement by leadership; the establishment of federal, state, and local roles and responsibilities; and data sharing. MDH’s approach, data sources, and lessons learned may support health departments across the country that are interested in conducting similar activities on the front lines of the opioid crisis.
Keywords: opioid, law enforcement, surveillance, preparedness, emergency response
In the United States, the overdose epidemic is partially linked to nonmedical use of opioids and other prescription drugs originally intended for therapeutic use. 1 -5 Both federal and state enforcement authorities have responded by prosecuting medical professionals and others who prescribe opioids and other controlled substances inappropriately. 6 -9 Health professionals are subject to disciplinary action (eg, loss of license to practice and/or prescribe medicine), and their care facilities may close without advance notice to the community. 10 One result of these enforcement actions is the abrupt loss of access to prescription opioids and other controlled substances for patients. In Maryland, state and local public health officials consider such an event a public health emergency, because studies have found that patients who abruptly lose access to their prescriber are at increased risk of withdrawal and may seek nonmedical sources of controlled substances, which come with an increased risk of overdose. 4,11 -15
Research indicates that serious health outcomes occur when patients who are physically dependent on opioid pain medicines suddenly have these medicines discontinued or the dose rapidly decreased. These outcomes include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide. 16 -19 People who stop receiving treatment for opioid use disorder are at increased risk of overdose. 4 A 2019 study found a greater probability of adverse events, including overdose, among patients who discontinued opioid medicines suddenly compared with patients who tapered opioid medicines over time. 20 This sequence of events is particularly concerning in Maryland, which has one of the highest rates of opioid-involved overdose deaths in the United States (38.2 deaths per 100 000 residents). 21,22 Furthermore, reports from 2015-2016 noted that 34.8% of opioid overdose decedents investigated by the Maryland Office of the Chief Medical Examiner received a prescription for controlled substances in the same year as their death. 23
In 2018, the Maryland Department of Health (MDH) established a Controlled Substances Interagency Response Workgroup (hereinafter, Workgroup) when an enforcement action abruptly closed a pain management facility in Baltimore County, resulting in more than 4000 patients losing access to care and their medical records. At the time, only Washington State had publicly available resources to guide public health practice and care for patients associated with a facility affected by an enforcement action. 24 Today, most states still lack established guidelines for coordination between enforcement and public health authorities when an action leads to abrupt loss of care for patients. 25,26 The Centers for Disease Control and Prevention (CDC) has since implemented the Opioid Rapid Response Program, a coordinated interagency effort to mitigate drug overdose risk among patients experiencing disrupted access to prescription opioids or medication-assisted treatment for opioid use disorder. 27
In this case study, we present Maryland’s coordination of preparedness and response activities that link state and local public health, health care providers, and enforcement authorities (defined as any federal, state, and local enforcement body or other governmental investigative authority, including but not limited to the Office of the Inspector General of the US Department of Health and Human Services [HHS OIG], the US Department of Justice, and the MDH Health Professional Licensing Boards) with the aim to mitigate potential adverse health consequences of enforcement actions. We describe a standardized approach, strengthened partnership between enforcement bodies and public health, relevant data sources for response, and lessons learned through the establishment of the Workgroup.
Methods
Executive leadership from MDH leads the Workgroup by applying CDC’s public health emergency preparedness capabilities for coordinating state and local response activities as an emergency response framework, with expertise from the MDH Office of Preparedness and Response (OP&R). 28 -30 The purpose of using an emergency response framework is to prepare and plan for an enforcement action as MDH would for any emergency, resulting in enhanced ability to assess incidents quickly, deploy resources, and communicate credible information among partners and the public. The Workgroup does not replace normal care coordination or patient referral processes or remove responsibility from health care providers, health insurers, or local health departments (LHDs) after an enforcement action. Partners include representatives from enforcement authorities, policy experts, and data stewards from across MDH (Box). The MDH Institutional Review Board (IRB) determined this case study did not meet the definition of research and therefore did not require IRB approval. Workgroup activities fall into 3 categories: incident preparedness, emergency response, and data sharing.
Box. Representatives and responsibilities of the Controlled Substances Interagency Response Workgroup, Maryland, 2020.
| Internal: Maryland Department of Health (MDH) |
|---|
| Public Health Services: act as MDH trusted agent and initiate convening of representatives. a |
| Behavioral Health Administration: assess situation report, provide subject matter expertise on opioid use disorder, support care coordination, and engage opioid treatment program partners, as appropriate. a |
| Medicaid Administration: assess situation report and extract Medicaid data for the prescriber to identify the number of affected Medicaid recipients and managed care organizations. a |
| Office of Preparedness and Response: coordinate overall response according to protocol, including task delegation. a |
| Maryland Prescription Drug Monitoring Program: extract PDMP practice profile data if requested by a convened medical review committee. Extract PDMP identifiable data if requested for local care coordination. a |
| Principal Counsel: provide counsel on legal issues related to response and planning efforts. a |
| Local health officer(s): assume role of incident commander to manage the response and coordinate with state and local health department staff members in the affected area(s). a |
| Office of Controlled Substances Administration: provide details on the investigative/disciplinary record for prescriber(s). |
| Office of Inspector General: provide information on current Medicaid fraud investigations related to prescriber. |
| Health Professional Licensure Boards: provide details on the investigative/disciplinary record of prescriber(s). Identify who can speak authoritatively about the status of prescriber’s license and timeline for next steps in the disciplinary process. |
| Opioid-involved program directors: participation designated by deputy secretary leadership to provide subject matter expertise. |
| Data analysts (eg, PDMP, ESSENCE, Medicaid): participation designated by deputy secretary leadership to provide subject matter expertise. |
| Office of Communications (optional): monitor situation and issue communications for public release, as appropriate. |
| External |
| Opioid Operational Command Center: monitor situation and issue communications for public release. |
| Law enforcement authority partners: provide details on the investigative/disciplinary record of prescriber(s). b |
| Health system partners (eg, emergency medical services, hospital emergency departments, pharmacists): notify and coordinate local health system partners of potential surge. |
Abbreviations: ESSENCE, Electronic Surveillance System for the Early Notification of Community-based Epidemics; PDMP, prescription drug monitoring program.
aDenotes representatives that are part of the medical review committee. The medical review committee is a subgroup that is formed to review confidential data when an incident occurs.
bLaw enforcement authority partners include the US Drug Enforcement Agency, the Federal Bureau of Investigation, US Department of Justice, and the US Department of Health and Human Services Office of the Inspector General.
Incident Preparedness
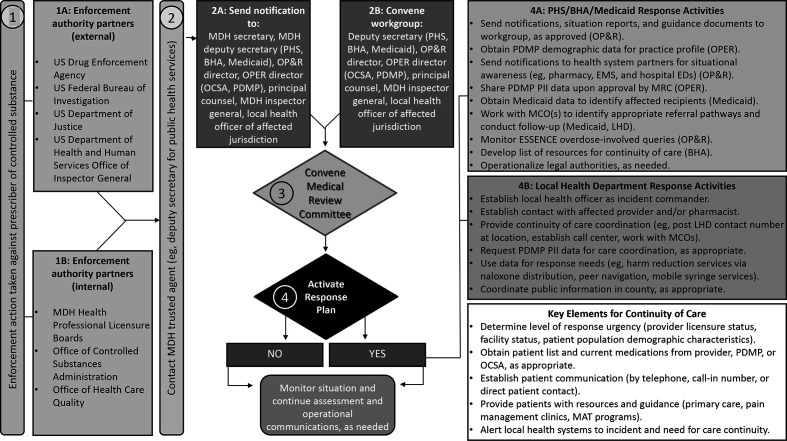
To prepare for enforcement actions, Workgroup members meet with Maryland’s 24 local jurisdictional health officers at a monthly roundtable meeting to collect feedback on policy and procedures for ensuring incident preparedness and response capabilities, local buy-in, and availability of resources. These meetings further inform Workgroup members about the state’s monitoring strategies for controlled substances prescriptions, engage key partners in preparedness and response activities, and identify relevant data to monitor response needs. Moreover, Workgroup members develop resources with input from these key partners (ie, local health officers) to standardize the approach for rapid response to enforcement actions, including a protocol with a built-in checklist, decision flowchart, and surveillance templates (Figure). Lastly, Workgroup members also review after-action reports to identify opportunities to improve future responses (Table).
Figure.
Maryland Department of Health (MDH) Controlled Substances Interagency Response Workgroup enforcement action decision flow chart. In step 1, action is taken against a prescriber from an external or internal enforcement body (ie, any federal, state, or local enforcement authority or other governmental investigative authority; 1A, 1B). In step 2, the MDH trusted agent (the deputy secretary of public health services and/or the OP&R director) is contacted and notified of imminent sanction. The MDH trusted agent alerts executive leadership (2A) and convenes Workgroup members (2B). Workgroup members are directed to gather information and data on where patients reside and receive treatment in preparation for the meeting. In step 3, the Workgroup is convened (2B) to discuss the facts as stated in the enforcement action notification and decide next steps for convening a medical review committee (MRC). The prescriber cannot be identified until the order is public. In step 4, the MRC reviews MDH data to decide whether to activate a response plan. If yes, any of the actions in boxes 4A and 4B may be taken. For example, the state may customize template notifications for local partners about the situation (eg, local hospitals and emergency departments [EDs], pharmacies, local law enforcement, EMS). If not, the MRC will monitor the situation and provide situational updates to leadership. Abbreviations: BHA, Behavioral Health Administration; CS, controlled substance; EMS, emergency medical services; ESSENCE, Early Notification of Community-based Epidemics; LHD, local health department; MAT, medication assisted treatment; MCO, managed care organization; OCSA, Office of Controlled Substances Administration; OP&R, Office of Preparedness and Response; OPER, Office of Provider Engagement and Regulation; PDMP, prescription drug monitoring program; PHS, Public Health Services; PII, personally identifiable information.
Table.
Summary of the emergency preparedness response to opioid prescribing enforcement action by PDMP aggregate demographic characteristics, a syndromic surveillance, b naloxone administration, c and after-action reports, Maryland, January 2018–October 2019
| Incident no. | Government enforcement body | Provider type | No. of prescribers/pharmacists (n = 34) | Facility type | No. of Maryland patients (n = 9270) | No. of patients with prescriptions (n = 9624) | No. of CS prescriptions (n = 86 955) | All drug overdose–related ED alerts | Opioid overdose–related ED alerts | Heroin overdose–related ED alerts | Naloxone-related alerts | After-action items |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Federal | MD | 9 | Pain management | 4019 | 4136 | 44 799 | No | Yes | No | — d | Health officer to contact practice provider |
| 2 | Federal | MD | 1 | Psychiatry | 104 | 156 | 1004 | No | No | No | — d | Establish trusted agent and law enforcement point of contact |
| 3 | Federal | MD | 1 | Endocrinology | 467 | 470 | 5348 | Yes e | Yes | Yes | No | Include health officer and law enforcement representatives |
| 4 | Local | MD | 1 | Family medicine/general practice | 266 | 268 | 1366 | Yes | Yes | No | No | Implement Medical Review Committee infrastructure |
| 5 | Federal | MD, NP, PA | 5 | Pain management | 436 | 442 | 2839 | No | No | No | No | Separately convene medical review committee upon consensus |
| 6 | Joint | MD | 1 | Family medicine/general practice | 105 | 105 | 712 | No | No | Yes e | No | Add average day supply by therapeutic class to profile |
| 7 e | Joint | MD | 1 | Family medicine/general practice | 331 | 338 | 1825 | No | No | No | No | Notify neighboring states of enforcement action |
| 8 | State | MD | 1 | Psychiatry | 33 | 70 | 533 | No | No | No | No | Share information on buprenorphine waivered physicians |
| 9 f | State | MD | 1 | Family medicine/general practice | 302 | 308 | 2016 | No | No | No | No | — d |
| 10 | Joint | MD | 1 | Geriatric | 229 | 230 | 2131 | No | Yes e | No | No | — d |
| 11 | State | MD | 1 | Internal medicine | 459 | 483 | 4992 | No | No | No | No | — d |
| 12 g | Federal | MD, NP, PA, PHR | 11 | Varied | 2519 | 2618 | 19 390 | Yes e | Yes e | Yes e | No | — d |
Abbreviations: CS, controlled substances; ED, emergency department; MD, physician; NP, nurse practitioner; PA, physician assistant; PDMP, prescription drug monitoring program; PHR, pharmacist.
aData source: Information and summary statistics derived from PDMP data for CS prescriptions dispensed in Maryland and reported to the Maryland PDMP. Numbers represent all prescriptions dispensed within 6 months before the incident date. 31
bData source: Electronic Surveillance System for the Early Notification of Community-based Epidemics. 32,33
cData source: These data are based on emergency medical services (EMS) prehospital care reports in which the EMS provider has documented that they administered naloxone. The administration of naloxone is based on the patient’s signs and symptoms and not on any diagnostic tests. These data are reported for trending purposes only. 34
dData not received.
eJurisdiction received >1 alert during the enhanced surveillance period.
fJurisdiction received an additional action investigating the same health care provider at a later date.
gAction occurring across multiple jurisdictions and practices.
Emergency Response
The roles and responsibilities of Workgroup members are defined in the response protocol (Box). For example, the local health officer, who is the incident commander, is responsible for managing the response and coordinating with state health department and LHD staff members in the affected jurisdiction(s). In addition, a designated trusted agent (the deputy secretary of public health services and/or the OP&R director) is responsible for receiving information from enforcement partners to assess event scope and severity, including type of prosecution order, medical record seizure (yes or no), timing, location, duration of the enforcement action, and follow-up point of contact. The receipt of this information triggers the trusted agent to virtually convene the Workgroup and decide whether to establish a medical review committee (MRC) based on an initial workgroup assessment of the enforcement action scope and severity. In accordance with the Maryland Health Occupations Article, MRCs may review sensitive patient information, allowing data sharing from various parts of MDH to better understand the prescriber or practice under investigation. 35 Only Workgroup members representing MDH’s clinical oversight divisions participate in the MRC (Box). The MRC decides whether to activate the response plan, which includes assigning response activities at the state and local level (Figure) following review of the data (eg, size of patient population, number of counties with affected patients, number and type of prescriptions for controlled substances, number of buprenorphine prescriptions, number of overdose-related emergency department (ED) visits, number of naloxone administrations), and assessment of provider status (eg, loss of license to practice and/or prescribe medicine).
Surveillance Systems for Data Sharing
Data sources to characterize response needs include dispensing patterns from the prescription drug monitoring program (PDMP), trends in the number of overdose-related ED visits, and trends in the number of units of naloxone administered by emergency medical services (Table).
The Maryland PDMP provides data on all Schedule II-V controlled substance prescriptions dispensed in the state, which is essential information for scoping an enforcement action. 31 When the MRC convenes, the PDMP generates a practice profile describing prescribing patterns at the site under investigation during the preceding 6 months. This practice profile consists of de-identified aggregate data on several practice characteristics: number of prescriptions for controlled substances written, number of patients (defined as the number of patients who received a prescription by the health care provider at the primary location under investigation), counties of patient residence, prescription payment methods, and number of prescriptions of buprenorphine, a treatment for opioid use disorder. During a response, LHDs can request identified patient-level data from the PDMP to coordinate care in accordance with state laws and regulations. The MRC authorizes this request when a health care provider is unable or unwilling to coordinate patient care (ie, medical license revoked, medical records seized).
Data sources to identify overdose events and optimize resource allocation include Maryland’s syndromic surveillance platform, known as the Electronic Surveillance System for the Early Notification of Community-based Epidemics (ESSENCE), and patient care reports from emergency medical services partners. 32 -34 ESSENCE collects real-time, de-identified data from all ED visits statewide. The ESSENCE team uses keyword queries developed for all drug, opioid, and heroin overdoses to monitor overdose-related ED visits in affected jurisdictions before, during, and after enforcement actions. Increased activity is detected by computational algorithms built into ESSENCE that trigger an alert. 36,37 Emergency medical services provide weekly reports to OP&R epidemiologists on the use of naloxone in local jurisdictions, which provides situational awareness.
Outcomes
From January 2018 through October 2019, Maryland used the protocol to respond to 12 enforcement actions targeting 34 medical professionals (Table).
Incident Preparedness
Preparedness activities resulted in a productive Workgroup (Box), development of resources, and a standard approach in response to enforcement actions (Figure). Convening the Workgroup upon notification of an enforcement action prompted assessment of response activities from the LHD and the Public Health Services, Behavioral Health, and Medicaid administrations of MDH (Figure). The incident preparedness phase helped ensure feasibility of response resources, enhanced familiarity with protocols, and strengthened relationships across Workgroup members to facilitate a coordinated response to future enforcement actions.
The Workgroup incorporated corrective actions into the protocol following after-action reports of each incident (Box). For example, in Incident 1, enforcement authorities arrived at the scene without prior notification and seized all medical records, limiting legal avenues for patient support. For future incidents, a designated trusted agent was established for law enforcement partners to confidentially notify MDH before or immediately after an enforcement action to mobilize the Workgroup without jeopardizing the investigation. By Incident 12, law enforcement partners provided MDH’s trusted agent with 1 week’s advance notice of impending actions and remained in close contact as the enforcement action occurred. The advance notification allowed the Workgroup to prepopulate surveillance templates and compile resources for the affected areas upon completion of the enforcement action.
Emergency Response
Each of the 12 enforcement actions was initiated by various federal, state, and/or local government bodies in Maryland. The affected medical professionals included 12 physicians, 2 nurse practitioners, 2 physician assistants, and 1 pharmacist (Table). The most frequently affected specialty was pain management, followed by family medicine/general practice and psychiatry.
The Workgroup provided resources and technical assistance to local Workgroup members virtually via conference call and email. State-level staff members did not deploy locally upon notification of an enforcement action. Local response activities followed protocol but depended on capacity and response needs. For example, all LHDs posted their contact information on the door of the affected facility for patient follow-up, whereas one county established a designated help line for resources and referrals. During Incident 1, approximately 5% of affected patients used the designated help line. Reports indicated calls were mostly for reasons other than referral (eg, complaints about the abrupt closure and pharmacy issues). Although one primary location was typically the focus of an enforcement action, ESSENCE and PDMP data indicated that patients in surrounding jurisdictions and states were also affected. Local health officers and state health officials (eg, deputy secretary equivalent or public health emergency preparedness director) in neighboring counties and states were contacted for awareness—another example of an identified after-action item later built into the protocol.
Surveillance Systems for Data Sharing
A total of 9624 patients received prescriptions for Schedule II-V controlled substances from affected prescribers in the 6 months before the respective enforcement action, 9270 (96%) of whom were residents of Maryland (Table). Within the 6-month window before the actions, targeted health care providers wrote 86 955 prescriptions for Schedule II-V controlled substances (dispensed in or into Maryland and reported to the PDMP) or about 1% of all prescriptions for Schedule II-V controlled substances dispensed during the same period in or into Maryland and reported to the PDMP. The practice profile was used for Workgroup decision making during all 12 enforcement actions and was critical when the prescriber under investigation was cooperative and able to coordinate care pathways. In these scenarios, the local health officer worked directly with the health care provider and office staff members. In addition, the MRC used the practice profile to identify when a substantial proportion of patients were Medicaid enrollees to engage Medicaid to support care transition. The practice profile template was updated iteratively for public health intervention needs based on Workgroup feedback, including adding opioid use disorder treatment data and average days’ supply by therapeutic class (Table).
In 4 of the 12 responses to enforcement actions, PDMP patient-identifiable data were requested for local use when a heightened need to deploy resources existed (eg, if a health care provider did not cooperate with sanctions, in case of facility closure or loss of access to medical records, and/or if prescribers surrendered their license to both prescribe controlled substances and practice medicine). In these scenarios, the public health response was limited because it was difficult to ascertain whether patients were being directed appropriately for care coordination and resources were limited. To assist in this effort, notifications were sent directly to patients asking that they call the LHD or a special hotline.
The ESSENCE team monitored the surveillance system time series for overdose-related alerts using queries developed for all drug, opioid, and heroin overdose. Six of 12 jurisdictions received overdose-related ED alerts that indicated heightened activity for the primarily affected area (Table). No jurisdictions experienced an increase in naloxone administrations by emergency medical services. When an alert occurred, health care facilities and first responders in the affected jurisdiction were contacted for situational awareness and resource allocation using customized template notifications. Using the checklist embedded in the response protocol as a guide, the local health officer, acting as incident commander, considered further actions in respective communities (Figure).
Preliminary data from Incident 1 indicate that fatal overdose events occurred among the patient population experiencing disrupted access to their health care provider/prescriber within weeks of the practice abruptly closing. In addition, MDH analyzed PDMP data to better understand engagement with prescribers of controlled substances among patients affected by Incident 11 and Incident 12. Of 2978 patients, 2051 (69%) received a prescription for a controlled substance in the 6 months after the date of Incident 12, indicating that they were able to continue to access care. Of the 2051 patients, 1158 (56%) had seen those same health care providers before the incident, which may imply that not all patients who had seen a prescriber involved in a practice closure used that health care provider as their only source of prescriptions. The remaining 893 (44%) patients who did not appear to have reengaged in care are cause for concern, especially patients who may have been on high-dose treatment or opioid use disorder treatment. In addition, half of the incidents resulted in an increase in overdose-related ED visits seen in ESSENCE data, suggesting that these actions may have additional health consequences.
Lessons Learned
The experience in Maryland demonstrates key lessons that may be of value to other states and localities navigating enforcement actions related to controlled substances prescriptions, including engaged leadership, multidisciplinary contribution to protocol development, functional communication channels between public health and law enforcement, and a legal framework for sharing data across programs.
The Workgroup was limited early on by inconsistent participation. This challenge was partially mitigated when Maryland’s governor declared the opioid crisis a state of emergency, which secured engagement from subject matter experts across MDH and law enforcement. 38 Inconsistent participation was also addressed by narrowing the scope of the Workgroup to preparedness and response activities outlined in the protocol. Data sharing was a substantial barrier to overcome but proved a keystone to public health decision making during the Workgroup assessment phase. Principal counsel advised on a framework conforming to the legality of sharing data among Workgroup members that resulted in formation of the MRC. The framework also helped to emphasize that shared data would be used for the public health goals of reducing the number of overdose deaths and the risk associated with disruption of care, and not for enforcement or punitive purposes. In addition, the Workgroup found that de-identified data were essential for coordination efforts to assess the scale and scope of a given response, including insight into the number of patients and the type of resources required. During response mode, establishment of a protocol addressed challenges of ambiguity about roles and responsibilities for coordination. For example, although the timing of law enforcement notification varied (eg, on-scene, 24 hours, 1 week), the existence of established points of contact created trust and enhanced communication among Workgroup members with various responsibilities.
The outcomes presented in this case study are descriptive and process oriented; therefore, assessment of public health outcomes is limited. However, preliminary analyses at the ecological level indicate that increases in both fatal and nonfatal overdose occurred after some of the enforcement actions and loss of care engagement. Incorporation of a plan for patients who did not appear to have reengaged in care after an enforcement action will be important for subsequent iterations of the protocol. These results highlight the need for future research to characterize the efficacy of MDH’s efforts for improved public health intervention at the state level, including evaluation of temporal changes in the rate of overdose events after an enforcement action, the relationship between time to opioid discontinuation and risk of an opioid-related ED visit or hospitalization, and the contributions of Medicaid to facilitate local care coordination.
Conclusion
Although this case study represents a snapshot in time, the response protocol and Workgroup continue to be used for enforcement actions to provide cross-agency coordination for situational awareness and communication to LHDs that remain on the front lines. The public health emergency preparedness approach may be applied as a framework for other state or local public health programs as they enhance their ability to prepare for, respond to, and recover from an emergency.
Acknowledgments
The authors thank members of the Maryland Department of Health (MDH) Controlled Substances Interagency Response Workgroup for their time and expertise in developing these resources, MDH leadership for their support of this Workgroup, Michael Baier for his historical perspective, and local health departments for sharing their best practices and lessons learned to develop and implement these resources. We also thank our Centers for Disease Control and Prevention partners from the Center for Preparedness (Victor M. Caceres, MD, MPH, and Edward C. Weiss, MD, MPH) and the National Center for Injury Prevention and Control (Douglas Roehler, PhD, MPH) for technical assistance in developing this case study for public health practice.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Jessica C. Acharya, MPH https://orcid.org/0000-0001-5957-8729
References
- 1. Bohnert ASB., Valenstein M., Bair MJ. et al. Association between opioid prescribing patterns and opioid overdose–related deaths. JAMA. 2011;305(13):1315-1321. 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 2. Liu Y., Logan JE., Paulozzi LJ., Zhang K., Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648-658. [PubMed] [Google Scholar]
- 3. Mack KA., Zhang K., Paulozzi L., Jones C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J Health Care Poor Underserved. 2015;26(1):182-198. 10.1353/hpu.2015.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strickler GK., Kreiner PW., Halpin JF., Doyle E., Paulozzi LJ. Opioid prescribing behaviors—prescription behavior surveillance system, 11 states, 2010-2016. MMWR Surveill Summ. 2020;69(1):1-14. 10.15585/mmwr.ss6901a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Substance Abuse and Mental Health Services Administration . Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. US Department of Health and Human Services; 2010. [Google Scholar]
- 6. Justice Department’s Criminal Division creates Appalachian regional prescription opioid strike force to focus on illegal opioid prescriptions [news release] . US Department of Justice, Office of Public Affairs; October 25, 2018. Accessed July 10, 2020. https://www.justice.gov/opa/pr/justice-department-s-criminal-division-creates-appalachian-regional-prescription-opioid
- 7. Kim DD., Sibai N. The current state of opioid prescribing and Drug Enforcement Agency (DEA) action against physicians: an analysis of DEA database 2004-2017. Pain Physician. 2020;23(3):E297-E304. [PubMed] [Google Scholar]
- 8. Goldenbaum DM., Christopher M., Gallagher RM. et al. Physicians charged with opioid analgesic—prescribing offenses. Pain Med. 2008;9(6):737-747. 10.1111/j.1526-4637.2008.00482.x [DOI] [PubMed] [Google Scholar]
- 9. Attorney General William P. Barr delivers remarks at the Department of Justice’s 2020 National Opioid Summit [news release] . US Department of Justice, Office of Public Affairs; March 6, 2020. Accessed July 10, 2020. https://www.justice.gov/opa/speech/attorney-general-william-p-barr-delivers-remarks-department-justices-2020-national-opioid
- 10.Md. Code, Criminal Law § 5-101 (2014).
- 11. Cicero TJ., Ellis MS. Abuse deterrent formulations of prescription opioids—reply. JAMA Psychiatry. 2015;72(8):850-851. 10.1001/jamapsychiatry.2015.0674 [DOI] [PubMed] [Google Scholar]
- 12. Compton WM., Jones CM., Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. 10.1056/NEJMra1508490 [DOI] [PubMed] [Google Scholar]
- 13. Harocopos A., Allen B., Paone D. Circumstances and contexts of heroin initiation following non-medical opioid analgesic use in New York City. Int J Drug Policy. 2016;28:106-112. 10.1016/j.drugpo.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 14. Mars SG., Bourgois P., Karandinos G., Montero F., Ciccarone D. “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy. 2014;25(2):257-266. 10.1016/j.drugpo.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krawczyk N., Mojtabai R., Stuart EA. et al. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction. 2020;115(9):1683-1694. 10.1111/add.14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Accessed June 1, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
- 17. Tofighi B., Grossman E., Williams AR., Biary R., Rotrosen J., Lee JD. Outcomes among buprenorphine-naloxone primary care patients after Hurricane Sandy. Addict Sci Clin Pract. 2014;9(1):3. 10.1186/1940-0640-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cepeda A., Valdez A., Kaplan C., Hill LE. Patterns of substance use among Hurricane Katrina evacuees in Houston, Texas. Disasters. 2010;34(2):426-446. 10.1111/j.1467-7717.2009.01136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis JR., Wilson S., Brock-Martin A., Glover S., Svendsen ER. The impact of disasters on populations with health and health care disparities. Disaster Med Public Health Prep. 2010;4(1):30-38. 10.1017/S1935789300002391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mark TL., Parish W. Opioid medication discontinuation and risk of adverse opioid-related health care events. J Subst Abuse Treat. 2019;103:58-63. 10.1016/j.jsat.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. National Center for Health Statistics . Drug overdose mortality by state. 2021. Accessed July 9, 2020. https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm [PubMed]
- 22. Hedegaard H., Minino AM., Warner M. Drug overdose deaths in the United States, 1999-2019. NCHS Data Brief. 2020;394:1-8. [PubMed] [Google Scholar]
- 23. Eisenberg MD., Saloner B., Krawczyk N. et al. Use of opioid overdose deaths reported in one state’s criminal justice, hospital, and prescription databases to identify risk of opioid fatalities. JAMA Intern Med. 2019;179(7):980-982. 10.1001/jamainternmed.2018.8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Washington State Nurses Association . Update: closure of Seattle Pain Centers—treating patients and reminder on pain management rules. August 3, 2016. Accessed September 29, 2020. https://www.wsna.org/news/2016/update-closure-of-seattle-pain-centers-treating-patients-and-reminder-on-pain-management-rules
- 25. Association of State and Territorial Health Officials . ASTHO’s Expert Panel on Building State Opioid Preparedness. Accessed July 10, 2020. https://www.astho.org/t/event.aspx?eventid=20446
- 26. Association of State and Territorial Health Officials . Responding to Pain Clinic Closures: A Guide for State Health Departments. Association of State and Territorial Health Officials; 2020. [Google Scholar]
- 27. Centers for Disease Control and Prevention . Opioid Rapid Response Program (ORRP): mitigate drug overdose risks among patients experiencing disrupted access to prescribers. Published September 2, 2021. Accessed April 5, 2021. https://www.cdc.gov/opioids/opioid-rapid-response-teams.html
- 28. Gibson PJ., Theadore F., Jellison JB. The common ground preparedness framework: a comprehensive description of public health emergency preparedness. Am J Public Health. 2012;102(4):633-642. 10.2105/AJPH.2011.300546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention . Public health emergency preparedness (PHEP) cooperative agreement. Accessed May 24, 2021. https://www.cdc.gov/cpr/readiness/phep.htm
- 30. Centers for Disease Control and Prevention . Public health emergency preparedness and response capabilities: national standards for state, local, tribal, and territorial public health. Accessed July 11, 2020. https://www.cdc.gov/cpr/readiness/capabilities.htm
- 31. Maryland Department of Health, Behavioral Health Administration . Maryland Prescription Drug Monitoring Program: updates. Accessed July 10, 2020. https://health.maryland.gov/pdmp/Pages/pdmp-updates.aspx
- 32. Maryland Department of Health . Office of Preparedness and Response. Accessed August 24, 2020. https://preparedness.health.maryland.gov/Pages/Home.aspx
- 33. Governor’s Office of Homeland Security . Core goal #5: biosurveillance. Accessed August 23, 2020. https://gohs.maryland.gov/biosurveillance
- 34. ImageTrend EMS Service Bridge . Maryland eMEDS: electronic patient care reporting system. Accessed June 3, 2021. https://www.mdemeds.com
- 35.Maryland Code, Health Occupations § 1-401 (2018).
- 36. Naus J., Wallenstein S. Temporal surveillance using scan statistics. Stat Med. 2006;25(2):311-324. 10.1002/sim.2209 [DOI] [PubMed] [Google Scholar]
- 37. Wallenstein S., Naus J. Scan statistics for temporal surveillance for biologic terrorism. MMWR Suppl. 2004;53:74-78. [PubMed] [Google Scholar]
- 38. Maryland Department of Health . Maryland Opioid Operational Command Center. Published 2017. Accessed September 29, 2020. https://beforeitstoolate.maryland.gov/about-the-opioid-operational-command-center