Abstract
The persistence of HIV in the spleen, despite combination antiretroviral therapy, is not well understood. Sustained immune dysregulation and delayed immune recovery, in addition to immune cell exhaustion, may contribute to persistence of infection in the spleen. Eliminating HIV from this secondary lymphoid organ will require a thorough understanding of antiretroviral (ARV) pharmacology in the spleen, which has been minimally investigated. Low ARV exposure within the spleen may hinder the achievement of a functional or sterilizing cure if cells are not protected from HIV infection. In this study, we provide an overview of the anatomy and physiology of the spleen, review the evidence of the spleen as a site for persistence of HIV, discuss the consequences of persistence of HIV in the spleen, address challenges to eradicating HIV in the spleen, and examine opportunities for future curative efforts.
Keywords: antiretrovirals, pharmacology, persistence, reservoirs, spleen, HIV
Introduction
Since the dawn of combination antiretroviral therapy (cART) in 1996, the life expectancy for those living with HIV has increased dramatically to approach near that of non-HIV-infected individuals.1 Following interruption of viral-suppressive therapy, however, rebound viremia occurs after ∼2–3 weeks.2–4 It has been estimated that the half-life of HIV from reservoirs is 44 months, indicating that it would take nearly 73 years of continuous suppressive therapy for full eradication from the body.5,6 The persistence of HIV in the presence of effective cART remains a significant barrier to a cure.
The latent reservoir is established early in infection and comprises memory and follicular helper T cells.7 While the hypothesis of active replication occurring in lymphoid tissue remains controversial,8–10 the persistence of HIV in the lymphoid tissue reservoir has been associated with suboptimal antiretroviral (ARV) concentrations.11,12 The spleen, a lymphoid organ, is one of the first locations to mount the innate immune response against viral infections, as well as to perform foreign antigen presentation. Recently, the detection of cell-associated viral RNA in spleen tissue may indicate either residual replication or HIV release from stable reservoirs.13 ARV penetration has been relatively understudied in the spleen.
Novel clinical pharmacology tools may provide valuable insight into the persistence of HIV in this lymphoid reservoir with current cART and future eradication interventions. Our group has reviewed the pharmacological challenges and opportunities related to the persistence of HIV in the gut-associated lymphoid tissues,14 yet data regarding the persistence of HIV in the spleen have yet to be reviewed. In this study, we review the anatomy and physiology of the spleen, the evidence of HIV persistence and its consequences, challenges to eradicating HIV in the spleen, and the role of the spleen in future HIV cure therapy.
Anatomy and Physiology of the Spleen
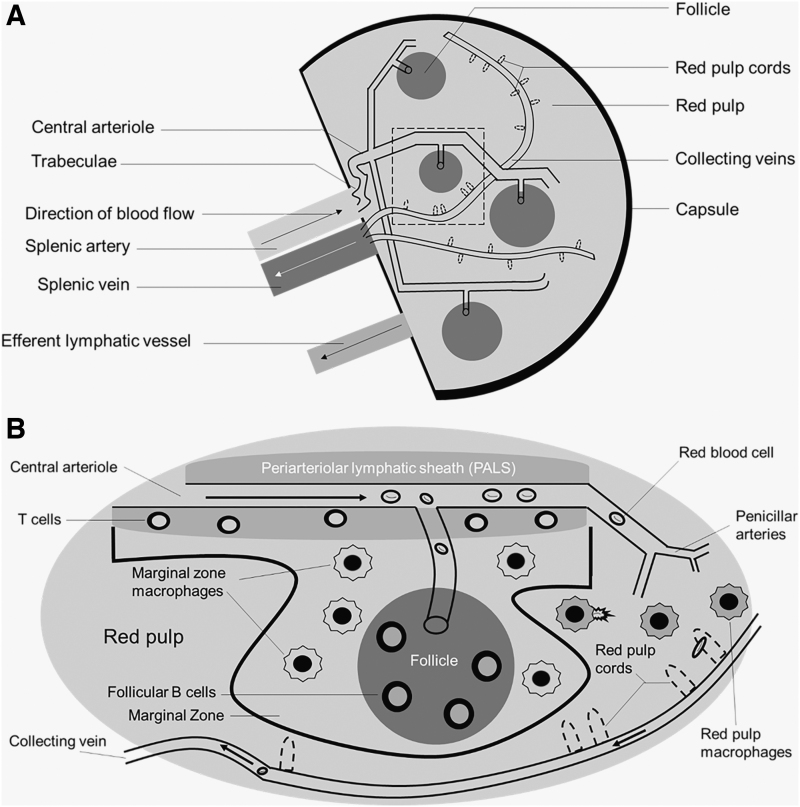
There are two main compartments in the spleen: the red pulp and the white pulp. These two functional units have distinct vascular architecture and cellular compositions. While we briefly review the anatomy and physiology of the spleen, more in-depth discussions of splenic structure have been previously published.15–18 To assist readers with the following section, Figure 1 illustrates the microcirculation of the spleen.
FIG. 1.
Physiologic overview of spleen anatomy and physiology. The splenic artery comes from the celiac trunk of the descending aorta to provide oxygenated blood to the spleen. (A) Provides a high-level view of the microanatomy of the spleen. (B) Represents an enlarged view of the area within the dotted lines. As blood flows through the central arteriole, smaller arterioles branch off to supply blood into the follicles. The white pulp consists of the periarteriolar lymphatic sheaths, marginal zone, and follicles. Blood exits the collecting vein located within the red pulp, drains into the splenic vein, and ultimately enters the portal vein. Arrows represent blood flow.
The spleen comprises red and white pulp. The red pulp has three main functions: filtering blood, recycling iron (following erythrophagocytosis), and storage of platelets.16 Splenic macrophages are responsible for the recycling of iron and compete with bacteria for the utilization of iron for survival. Therefore, the red pulp also serves as a defense against pathogens.
Comprising nearly 25% of the splenic tissue, the white pulp contains numerous immunological cells, including—but not limited to—macrophages, dendritic cells, and plasma B cells.16 T cells are mainly located in the periarterial lymphatic sheaths (PALS, described below). B cells are primarily located in the follicles and are responsible for the production of antibodies following opsonization of foreign antigens (most notably encapsulated bacteria). The marginal zone of the white pulp contains dendritic cells and macrophages responsible for antigen presentation.
Blood flow to the spleen ranges from 170 to 330 mL/min in humans, which is ∼5% of the total cardiac output.19 In nonhuman primates (NHPs, a common model for HIV treatment, prevention, and cure), blood flow to the spleen is 21 mL/min, close to 2% of cardiac output, although the spleen weighs significantly less (8 g vs. 180 g in humans).20
Branching off the abdominal aorta and into the celiac artery, the splenic artery provides oxygenated blood to the spleen. As the splenic artery enters the hilus (fissure) of the spleen, the splenic artery begins to branch off and direct into the fibrous trabeculae. These central arteries are surrounded by lymphatic tissue (PALS) that form the characteristic splenic follicles, comprising the white pulp.16,17 The central arteries further branch to form penicillar arteries, which enter the red pulp of the spleen. Greater than 90% of the blood flow to the spleen goes to the white pulp, thereby circumventing the red pulp.21
There are two morphological components of the red pulp that distinguish it from the white pulp: splenic sinuses (sinusoids) and splenic cords (Cords of Billroth). The splenic sinuses are capillaries lined with discontinuous endothelium as well as slits to perform the sifting of erythrocytes in the spleen. These sinuses eventually form veins (venous sinuses). The splenic cords are located within the red pulp and comprise reticular fibers and macrophages. These fibers are composed of collagenous fibers that may be responsible for splenic contraction and are bound by the extracellular matrix and fibroblasts.
From a pharmacologic perspective, the morphology of the splenic sinuses allows the permeation of not only large endogenous molecules, such as albumin, but also large exogenous molecules, such as monoclonal antibodies. For instance, it has been previously demonstrated that rituximab, a monoclonal antibody utilized in the treatment of various cancers and rheumatologically based conditions, distributes into the spleen.22–25
Finally, blood circulating into the red pulp collects in the venous sinuses that enter the trabeculae of the spleen, through the trabecular veins, and ultimately into the splenic vein. The splenic vein then drains into the hepatic portal vein and into the liver. Therefore, there is both anatomical and physiological communication between the spleen and the liver. Similar to lymph nodes, the spleen contains an efferent lymph vessel that drains into the lymphatic system; unlike the lymph nodes, the spleen does not contain any afferent lymph vessels.15,16
HIV Persistence in the Spleen
Although cART can decrease HIV in the plasma to undetectable concentrations, it cannot completely clear HIV infection from the body. Since <2% of total body lymphocytes are in the blood, it is imperative to examine HIV in anatomical sites.26 The spleen is the largest secondary lymphoid organ and contains approximately one-quarter of the body's lymphocytes, which combines the functionality of the innate and adaptive immune responses. Yet, the spleen is understudied. In 1997, Reinhart et al. noted in eight rhesus macaques infected with simian immunodeficiency virus (SIV) deltaB670 that the spleen contains 2 × 108–10 SIV virions [measured by in situ hybridization (ISH) silver staining] and that the viral RNA was observed in the PALS and cells in the red pulp.27
Technological advances in quantifying HIV reservoirs have aided in understanding virology and the spatial distribution of virus. These advances include RNAScope and DNAScope, an ISH approach that utilizes “double-Z” target probes to bind in a contiguous manner to complementary RNA or DNA sequences, respectively.28 In these scenarios, the probability of two probes adjacently nonspecifically binding is low, thereby providing minimal background noise. Deleage et al. observed that the B cell follicles of lymph nodes were anatomical compartments for viral persistence, even after 26 weeks of viral suppressive cART to rhesus macaques.29 The flexibility of this approach allowed for the same authors to demonstrate persistence of SIV RNA on follicular dendritic cells and within the B cell follicles in the spleens of chronically infected rhesus macaques, similar to what had been seen in the lymph nodes.28
To further provide evidence of viral persistence in the spleen, Estes et al. examined 27 rhesus macaques with distinct simian AIDS viruses (nine total spleens) using the RNAScope and DNAScope procedures and demonstrated that even in chronically treated macaques, SHIV and SIV viral RNA persist in the spleen as >99% of the SIV RNA+ cells were located in lymphoid tissues.13 Certainly, the presence of virus in the tissue does not necessarily indicate ongoing viral replication, but the numbers of virions seen in these studies indicate the sources of virus that may reemerge following treatment interruption.
There is evidence from postmortem human spleens that may further indicate persistence of virus. For example, Nolan et al. examined the spleens from five participants on suppressive cART utilizing droplet digital PCR and single-genome sequencing.30 In this analysis, maximum likelihood phylogenies indicated that both the env and nef viral genes showed viral diversity in the spleen and that because both DNA and RNA sequences were detected in two out of five patients, this suggested active virus replication.30 However, this is not without controversy.
Bozzi et al. conducted phylogenetic analyses to investigate HIV populations in infected tissues, including the spleen.31 The authors did not find evidence of ongoing replication or compartmentalization of HIV within the spleen. but did detect clonal expansion of infected cells that predated cART, concluding that the extent of clonal expansion of cells that carry infectious proviruses should be the focus of curative efforts.31 Therefore, while there may potentially be evidence of actively replicating virus, this is still controversial.
Taken together, there is evidence that suggests HIV persists in the spleen. While much of the conclusions are extrapolated from lymph nodes because of their ability to be extracted for further study, morphological similarities between the two organs might indicate similar processes. Furthermore, splenic data are derived from NHP preclinical models, which can recapitulate the infection process. These data can help inform immune dynamics and viral persistence in the human spleen.
Consequences of Persistence of HIV in the Spleen
Immune dysregulation
Like other pathogenic disease states, the spleen plays an important role in initial antigen presentation and subsequent antibody development against HIV. A key component of the immune cascade is the follicular T helper (Tfh) cell, which is responsible for the development of the germinal center, the site of somatic hypermutation and plasma B cells. There are conflicting reports related to the frequency of Tfh in the blood. Earlier reports indicated that during the acute and chronic phase of HIV infection, frequencies are increased.32 Conversely, Boswell et al. indicated decreased frequencies of Tfh cells.33 Within lymphoid tissues, Cubas et al. noted that the triggering of programmed cell death ligand 1 on Tfh cells within the lymph nodes diminishes the B cell responses during HIV infection and potentially lessens control of HIV infection, owing, in part, to the reduction in interleukin (IL)-21 secretion.34
Rodrigues et al. noted in a rhesus macaque model infected with Leishmania infantum, the abortive differentiation of splenic Tfh cells, relating to the failure of these cells to express PD-1.35 Ultimately, this led to altered B cell differentiation and reduced production of antigen-specific immunoglobulins. In fact, immunohistochemical (IHC) analysis has indicated the atrophy and disruption of the germinal center during visceral leishmaniasis. In the context of HIV or SIV, Moukambi et al. utilized rhesus macaques of Indian origin infected with SIVmac251 and showed that—in the acute phase of infection—splenic Tfh cells decrease early after infection and remodeling of normal splenic architecture occurs.32 Accompanying the loss of Tfh cells were the loss of memory B cell subsets and lower titers of immunoglobulins against SIV.32
Collagen deposition
Mechanisms underlying collagen deposition and subsequent depletion of CD4+ T cells are related to the inflammation and chronic immune activation of HIV infection. Fibrosis in preclinical animal models has indicated the mediator role of transforming growth factor (TGF)-β1 on regulatory T cells in the lymphatic tissues of sooty mangabeys and rhesus macaques.36 These regulatory T cells expressing TGF-β1 were directly linked with collagen type 1 expression in fibroblasts and may lend credence to the notion that suppressive cART does not completely recover T cells or reverse collagen deposition in the lymphatic tissues.36–38
Due to infeasibility of serial sampling, this has not been holistically demonstrated in the spleen, although red pulp enlargement and lymph follicle atrophy were noted in the spleens from 34 splenectomized patients secondary to hepatosplenic schistosomiasis.39 Given the morphological and architectural similarities between lymph nodes and the spleen, similar processes with HIV would be expected, although this has not been confirmed.
In the context of immune system homeostasis, the secondary lymphoid tissues—specifically the spleen—play a vital role. The architecture of these lymphoid organs is ideal to mount an immune response following the introduction of an antigen. For instance, the T cell zone (TZ) is the primary site of HIV activation as the majority of CD4+ T cells reside here.40 It has been previously demonstrated that the TZ decreases in size during the progression of HIV infection.41 Schacker et al.41 prospectively examined histological sections of inguinal lymph nodes from HIV-positive patients on suppressive cART and noted depletion of the CD4+ T cell population in all stages of HIV infection secondary to damage from collagen. The area of tissue occupied with collagen was significantly negatively correlated (r = −0.55, p = .0008) with tissue-naive CD4+ T cells.41
This was further supported by a separate, follow-up study by the same group, indicating the deposition of collagen was associated with the depletion and impaired reconstitution of CD4+ T cells.42 In 2008, Estes et al. expanded these findings to the gut-associated lymphoid tissue (GALT), demonstrating a significant, inverse relationship (r = −0.60; p = .004) between the percent area occupied by collagen in the TZ of the Peyer patches and the CD4+ cell population.43 Furthermore, treatment in early infection can increase a specific subtype of T cell—CD4+ central memory T cell—in the Peyer patches, even after 6 months of suppressive cART.43
In the inguinal lymph nodes, Zeng et al. noted that collagen damages the fibroblastic reticular cell (FRC) network, contributing to the loss of naive T cells, mediated by decreased IL-7 production and presentation to the FRC, even following suppressive cART administration.44 Tien et al. found that plasma fibrinogen and C-reactive protein were independent predictors of 5-year mortality risk in HIV-infected patients.45 This increased inflammatory state secondary to HIV infection causes damage to the lymphoid tissue. Given these results, adjunct antifibrotic and/or anti-inflammatory therapy have been speculated and trialed with mixed results.46,47
Inflammatory sequelae
Although cART has allowed for the reconstitution of peripheral CD4+ T cells, HIV-infected adults are at greater risk of non-AIDS-related morbidity, including cardiovascular disease, osteoporosis, and liver disease.48 One reason for the increased risk of these morbidities is inflammation. A higher frequency of inflammatory monocytes, cytokines, and hypercoagulability biomarkers has been noted, even with consistent, viral suppressive cART.45,49–52
Another key sequelae of the chronic inflammatory state is the activation of macrophages and monocytes. Table 1 displays the four main macrophage subsets in the spleen. The activation is accompanied by increased turnover and frequency of circulating monocytes, which may be regulated by the marginal zone macrophages.53–56
Table 1.
Macrophage Subsets in the Spleen
| Macrophage type | Cellular marker(s) | Function | Effect of HIV infection59,114 |
|---|---|---|---|
| Red pulp macrophages | Spi-C,115 F4/80+,116,117 VCAM-1,118 CD163+,119 HO-1,116 ferroportin116 | • Clearance of effete red blood cells15 | • Increase in acute infection |
| • Iron metabolism15,115,120 | • Increase in chronic infection | ||
| • Scavenging blood-borne debris120 | |||
| • Production of type 1 interferons121 | |||
| Marginal zone metallophilic macrophages | CD68+,117,120 SIGN-R1,120 CD169,117 sialoadhesin122 | • Immune surveillance117 | • Decrease in acute infection |
| • Degradation and clearance of viruses123 | • Decrease in chronic infection | ||
| Marginal zone macrophages | CD68+,117,120 CD169+, MARCO,124 LXRα,125 Tim4125 | • Clearance of blood-borne encapsulated bacteria125 | • Decrease in acute infection |
| • Decrease in chronic infection | |||
| White pulp macrophages (tingible body macrophages) | CD68+120 | • Phagocytosis of apoptotic B cells within germinal centers117,125 | • Decrease in acute infection |
| • Regulation of germinal center reactions125 | • Decrease in chronic infection (replaced by CD163+ and CD163+/CD68+ macrophages) |
F4/80, epidermal growth factor-like module-containing mucin-like hormone receptor-like 1; VCAM-1, vascular cell adhesion molecule 1; HO-1, heme oxygenase 1; SIGN-R1, specific intracellular adhesion molecule-grabbing nonintegrin receptor 1; MARCO, macrophage receptor with collagenous structure; LXRα, liver X receptor-alpha; Tim4, T cell membrane protein 4.
This local, splenic activation may explain the findings seen by IHC. In 72 spleens from postmortem AIDS patients, histological evidence suggested drastic changes ranging from follicular hyperplasia to follicular atrophy, which had been directly attributable to HIV infection rather than concomitant opportunistic infections.57 Furthermore, the inflammation resulted in splenomegaly (median splenic weight was 280 g in those without secondary pathologic lesions).57 It had been noted from the spleens of these patients that progressive HIV-impaired activation of macrophages in the spleen secondary to follicular morphological changes.57,58
This was further explored by Williams et al. using an accelerated SIV-infected NHP model.59 In this study, uninfected, acutely infected, infected, but asymptomatic, and chronically infected untreated NHP spleens were immunologically stained for CD163+CD68+ macrophages. Two important findings were demonstrated. First, the immunology of marginal zone macrophages changed during the acute and chronic phases of infection. In the acute phase, universal upregulation of CD163 was noted, with accompanying loss of CD68 throughout the organ. In the chronic phase, CD163+CD68+ macrophages entered the germinal center to compensate for the acute phase loss of CD68.59 Second, the authors noted lymphoid depletion, illustrated by hyposplenism, white pulp depletion, and germinal center burnout related to T cell depletion from infection.59
Taken together, the pathogenesis of HIV infection directly affects the spleen early in infection and the physical presence of virus in the lymphoid organ causes morphological changes that impair responses to other immunological insults. Furthermore, these morphological changes continue to occur in the chronic phase of infection, although the modulatory effect of cART—if present—has yet to be determined.
Challenges to Eradicating HIV in the Spleen
Clonal expansion
Clonal expansion describes the process of T and B lymphocytes interacting with an antigen or antigen-presenting cell and subsequently generating clones through mitosis against a specific antigen. It has been shown that clonal expansion of HIV-infected cells can persist for over 11 years, even on suppressive cART.60 Indeed, the identical HIV DNA sequences of infected cells in peripheral blood and tissues lend credence to cellular proliferation being the contributor to the persistence of HIV.61–63
Lee et al.64 performed cross-sectional genetic analyses on RNA sequences from T cell subsets in blood, lymph nodes, and gut tissues from patients on suppressive cART for 3–17.8 years and demonstrated two important findings. First, the number of infected memory T cells decreases with cART initiation. Second, effector memory T cells from the lymph nodes can produce infectious virus through clonal expansion. This maintains HIV persistence and offsets the decay of HIV-infected cells.64 The effector memory T cells from the lymph node contained provirus, which was genetically identical to those in plasma.64
Similarly, von Stockenstrom et al. isolated intracellular HIV-1 genomes from CD4+ T cell subsets of peripheral blood, GALT, and lymph node tissues, from patients receiving suppressive cART, separated by 7–9 months.65 The authors noted that DNA integrant frequencies were stable over time and that clonally expanded populations were found in effector memory T cells isolated from lymph nodes.65 While these data are derived from lymphoid tissues, data for the spleen remain sparse, and although morphological similarities between lymph nodes and spleen may permit extrapolation, it is important to be able to examine the spleen directly.
Bozzi et al., utilizing phylogenetic analyses of infected postmortem tissues of treated HIV-infected individuals, did not find evidence of ongoing replication in tissues.31 In the two spleen samples examined (cART duration: 8.0 and 22.7 years, respectively), there were identical HIV sequences determined by average pair wise differences (0.26% and 1.7%, respectively), compared to the variants sequenced from peripheral blood mononuclear cells (PBMCs) after years of cART.31 Although this is a sample size of 2, these data do point to the spleen potentially containing clones of virus present before starting cART, representing another challenge to fully eradicate HIV.
ARV penetration and modulation
Although the spleen plays a crucial role in immunoregulation, the study of the spleen as a reservoir for HIV is challenging. The spleen is the first place of antigen presentation, but the in-depth study of the organ is limited to postmortem individuals or situations in which the spleen must be removed (e.g., splenectomy secondary to ruptured spleen following motor vehicle accident). However, there have been a few studies examining the distribution of ARVs into the spleen of animal models.
Di Mascio et al. administered 5 mg/kg intravenous bolus doses of a radiolabeled derivative of tenofovir to Sprague-Dawley rats and detailed the biodistribution of the compound into the spleens after 120 min.66 The authors noted that compared to plasma, splenic concentrations were ∼2-fold lower.66 Furthermore, positron emission tomography imaging indicated a slower accumulation and clearance of the drug from the spleen compared to plasma.66 Similarly, Lee et al. described the biodistribution of a one-time administration of radiolabeled tenofovir disoproxil fumarate to two beagle dogs, which were sacrificed at 24 h.67 The percent injected dose per gram of tissue in the spleen observed by Lee et al. (<0.01%) was similar to Di Mascio et al. (<0.05%).66,67
Devanathan et al. demonstrated that NHPs achieved higher splenic penetration than humanized mouse models or humans alongside higher molar percentages of the active intracellular metabolites of nucleoside reverse transcriptase inhibitors (NRTIs), indicating a higher rate of active metabolite conversion.68 However, for the NRTIs and metabolites in humans, inhibitory quotient values did not exceed 1, which may result in lack of virologic control.68
In a separate analysis, it was noted that between plasma and tissue concentrations, the NRTIs emtricitabine and tenofovir had positive linear relationships and raltegravir had inconsistent relationships in the secondary lymphoid organs, highlighting the heterogeneity of ARV penetration into tissues.69 Fletcher et al. examined ARV concentrations in PBMCs and mononuclear cells from lymph nodes, ileum, and rectum, and noted that lower drug concentrations correlated with slower HIV decay rate in the tissues.12
All taken together, this would lead one to believe that it is the lack of ARV tissue penetration that contributes to the persistence of HIV. However, this conclusion remains controversial.31 Should this be the case, drug resistance would likely occur during the setting of subtherapeutic ARV concentrations, as seen in the plasma of patients with poor drug adherence, but this is not observed.70 Instead, it has been previously noted that the concentrations in tissue reservoirs may be low enough as to not permit the development of resistance, but allows wild-type virus to continue to replicate, thereby outcompeting resistant variants.71
Optimizing cART in the spleen is difficult for many reasons. First, as mentioned previously, there are limited data of ARV exposure in the spleen. In addition, drug transporters and metabolizing enzymes may modulate the disposition of ARVs in the spleen. While a comprehensive discussion of drug transporters is beyond the scope of this review, it is well known that ARVs interact with the ATP-binding cassette membrane-associated efflux transporters and solute carrier uptake transporters.72 The expression of these transporters can be altered endogenously or exogenously, thereby affecting ARV concentrations. In preclinical species, quantifiable Mrp4, Bcrp, and Ent1 concentrations have been demonstrated, although these did not significantly correlate with ARV concentrations .68
Drug-metabolizing enzymes (DMEs) are highly responsible for the disposition of ARVs. Macrophages derived from bone marrow and blood express cytochrome P450 (CYP) 1A1, 2A6/7, 2D6, 2E1 and 3A4 mRNA, which are responsible for the biotransformation of numerous ARV classes, such as protease inhibitors and non-NRTIs.73–75 Furthermore, gene expression of DMEs has been shown in human PBMCs.76 While the DMEs in the spleen have yet to be fully characterized, it is possible that due to the wealth of lymphocytes in the spleen, DMEs may play a role in the intracellular disposition and metabolism of small molecules, including ARVs.
The role of inflammation on potential modulation of ARV disposition into reservoir tissues has yet to be elucidated. In the context of antimicrobials, pathophysiological changes in critical illness (alterations in protein binding, pH, etc.) dictated by increases in acute phase proteins affect distribution into the active site of infection, resulting in concentrations below the minimum inhibitory concentration.77 Although it has been previously demonstrated that the protein binding potential was lower than that in plasma, these values did not differ between NHPs infected with recombinant SHIV and uninfected controls.68 Taken together, there may potentially be a role of inflammation or inflammatory sequelae (e.g., collagen deposition as above) associated with HIV infection that modulates ARV penetration and leads to inadequate penetration in lymphoid tissues, but this needs further study.
Future Approaches to Understanding ARV Tissue Distribution
Mass spectrometric imaging
Evaluations of ARVs in tissues have conventionally utilized liquid chromatography-mass spectrometry (LC-MS) of tissue homogenates, which has been regarded as the gold standard of tissue quantification.78 However, a limitation to this analysis is that tissue homogenates lose spatial and distributional configurations within the tissue due to total consumption of sample. This would lead to significant overestimation of concentrations at the site of action, and misleading conclusions regarding exposure-response relationships.79 Our group has demonstrated that distribution of ARVs is not uniform across the tissue reservoirs.80,81
These data were generated through a novel mass spectrometry imaging method known as infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI).82,83 The process and workflow of IR-MALDESI have been described previously.83 IR-MALDESI combines the technical specifications of traditional LC-MS/MS methods, with heat-map style imaging of variable drug concentrations across a snap-frozen, unaltered tissue section. In addition, IR-MALDESI uses ice as a matrix, which avoids organic matrix peak interference, allows collection of multiple masses within the tissue, reduces voxel-to-voxel variability, and increases sensitivity.83,84
The heat-map style imaging data generated from MALDESI require tissue slices for analyses. Adjacent spleen tissue slices can be sectioned for IHC and ISH analyses and registered with IR-MALDESI images using numerical programming language (e.g., MATLAB) algorithms. Correlation analyses can be assessed qualitatively by visual comparisons and quantitatively through image analysis of the total percentage of the image occupied by a specific ARV (IR-MALDESI), CD4+ cells (IHC), and vRNA/vDNA using RNAScope/DNAScope (ISH). This combinatorial approach allows for the visualization of ARVs, HIV RNA/DNA, and immunological cells, possibly demonstrating that merely achieving quantifiable splenic ARV concentrations may not be sufficient for effective viral control, as suggested previously.11,12
Furthermore, the quantitative data abstracted from IR-MALDESI can lend credence to the ARV concentrations needed within the tissue compartment to suppress virus. Thompson et al. assessed the distribution of ARVs in gut tissues across three species and measured the distribution relative to CD3+ T cells and the expression of HIV and SHIV RNA.81 Across the three species, it was demonstrated that 40%–60% of the CD3+ T cells remained unexposed to ARVs, indicating that ARV exposure in some areas where HIV target cells reside may be limited.81 These analyses should be similarly performed in the spleen and other tissue reservoirs (lymph nodes, genital tract, etc.) to elucidate the relationship between potential suboptimal ARV exposure and HIV persistence, and represent ongoing work by our group.
Opportunities for HIV Eradication
Small molecules
The concept of “kick and kill” strategies describes latency-reversing agents (LRAs) combined with immunotherapeutic intervention, as described below.85 Numerous LRA classes have been investigated, but the most widely explored are the histone deacetylase inhibitors (HDACi). Deacetylation of histones leads to compacted chromatin, hindering gene expression. Inhibition allows for the unpacking of the chromatin and subsequent immune recognition of the latent cells.86 Examples of HDACi are vorinostat, panobinostat, and romidepsin.
Tissue concentrations of HDACi are scarce. Recently, Perrin et al. dosed three rats intravenously with 10 mg/kg panobinostat and concentrations in tissues were quantified, and the mean concentration in the spleen was 2.9 μM.87 Compared to EC50 values of HIV-1 wild-type and mutant strains determined by Norton et al. (1.6–12.86 nM),88 the concentrations are over 200-fold higher.
Regarding romidepsin, it was noted that when cutaneous burn-injured and control mice were dosed with 5 mg/kg intraperitoneal injections twice daily, there was an increase in histone acetylation.89 Within the spleen tissue, there was a 17% increase in histone acetylation (a biomarker of romidepsin's effect). This suggests splenic bioavailability of romidepsin, although drug concentrations are unknown. Since LRAs are used in combination with immunotherapeutic agents, a discussion of these follows.
Monoclonal antibody therapy
Monoclonal antibodies represent a novel approach for the treatment and potential eradication of HIV. Most recently, the sole monoclonal antibody approved in the United States for the treatment of HIV infection is the postattachment inhibitor ibalizumab, which targets the extracellular CD4 domain.90–94 Due to their designed specificity for a particular antigen, monoclonal antibodies have been utilized for decades in other immune-related diseases, such as cancer, rheumatoid arthritis, and psoriasis. Monoclonal antibodies greatly differ from conventional small molecules as they are highly polar and have molecular weights of ∼150 kDa.95
As a result of these different physicochemical properties, distribution of antibodies into tissues occurs primarily through convection.96–98 Similar to other proteins, monoclonal antibodies experience a sieving process, which is mathematically represented by reflective coefficients. Most tissues have capillary beds comprising reflective coefficients of 0.95–0.98, indicating virtual impermeability to plasma proteins. In contrast, spleen sinusoidal capillaries contain reflective coefficients of 0.85, lending credence to the notion that plasma proteins circulate into the spleen.97,99 Indeed, the spleen has been shown to be a preferential site of accumulation of therapeutic antibodies.
FCγ receptors in neutrophils, monocytes, and macrophages are responsible for the internalization and destruction of antibodies.100 However, binding to FcRn (also known as the Brambell receptor) allows for the recycling of the antibody into the plasma upon acidification.100 It has been demonstrated that the spleen expresses FcRn receptors.101 Indeed, in FcRn knockout mice, immunoglobulin accumulation occurred in the liver and spleen, both organs that contain the aforementioned sinusoidal capillary system.102 A well-documented example is the monoclonal antibody rituximab, which targets splenic antigens (i.e., CD20 antigens on B cell splenocytes), and we direct readers to the numerous references detailing the pathway of rituximab as it relates to the spleen.22–25
While therapeutic antibodies were proposed initially as a curative strategy for HIV, suboptimal efficacy in preclinical animal models and early unsuccessful clinical trials in humans has tempered enthusiasm. Currently, broadly neutralizing antibodies may potentially regenerate the interest in large protein therapeutics to treat and prevent HIV infection. While a review of antibody therapeutics is beyond the scope of this article, interested readers are directed to a review by Gruell and Klein.103
Adoptive T cell therapy
Another novel approach to HIV therapy is adoptive cell therapy, particularly the engineering of T cells with chimeric antigen receptors (CARs) that are directed toward the HIV envelope.104 Currently, CAR T cell therapy is explored and approved for cancer immunotherapies, including B cell leukemia. In the 1980s, early attempts of CARs utilized soluble CD4 molecules to prevent HIV infection by blocking the interaction between CD4 and the envelope; however, resistance and a short half-life of the soluble CD4 hampered their efficacy.105,106
Advances have enhanced technical features of T cell therapies in the context of hematological malignancies. One improved design has been to introduce antibodies against viral antigens versus the extracellular regions of CD4 cells, allowing for specific binding to the viral antigen and limited off-target effects, although differences in affinities of the antibody component are being evaluated.107,108
Despite the advancements in T cell therapies, the pharmacokinetics remain relatively unknown. Biodistribution studies of T cells in mice prepared from splenocytes or tumors concentrated primarily in the spleen and lung after 20–24 h.109,110 Early development of mathematical models relied on incomplete biodistribution data or a lack of experimental data.111 Khot et al. utilized chromium-51-labeled T cells from splenocytes that were expanded using anti-CD3, anti-CD28, and IL-2, and administered to a melanoma mouse model.112 After noting that the T cells are rapidly cleared from the blood following administration, >90% of the T cells distributed in the spleen, liver, lungs, kidneys, bone, and lymph nodes.112
Other studies utilized chromium-labeled lymphocytes and noted high body surface counts of lymphocytes in the spleen and liver, both of which have an intricate reticuloendothelial system as aforementioned.113 More sophisticated mathematical modeling approaches will be warranted to characterize the biodistribution of CAR T cell therapies that utilize antibodies in the future.
Conclusion
In summary, the spleen is an understudied organ in the context of HIV, and the gaps in knowledge range from ARV concentration analyses to distributional data examining ARVs in relationship to immunological cells and virus. New pharmacologic methods can help bridge those knowledge gaps. These data will help inform HIV treatment and cure clinical trials that would potentially harness immunologically related therapies with small molecules.
Authors' Contributions
A.S.D. and A.D.M.K. wrote the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The authors are grateful for the National Institutes of Health Grant R01AI111891 (to A.D.M.K.). A.S.D. is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM086330.
References
- 1. van Sighem AI, Gras LAJ, Reiss P, Brinkman K, de Wolf F, ATHENA National Observational Cohort Study: Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010;24:1527–1535. [DOI] [PubMed] [Google Scholar]
- 2. Wong JK, Hezareh M, Günthard HF, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997;278:1291–1295. [DOI] [PubMed] [Google Scholar]
- 3. Chun T-W, Justement JS, Murray D, et al. : Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: Implications for eradication. AIDS 2010;24:2803–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong JK, Yukl SA: Tissue reservoirs of HIV. Curr Opin HIV AIDS 2016;11:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perelson AS, Essunger P, Cao Y, et al. : Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997;387:188–191. [DOI] [PubMed] [Google Scholar]
- 6. Siliciano JD, Kajdas J, Finzi D, et al. : Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003;9:727–728. [DOI] [PubMed] [Google Scholar]
- 7. Banga R, Procopio FA, Noto A, et al. : PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016;22:754–761. [DOI] [PubMed] [Google Scholar]
- 8. García M, Górgolas M, Cabello A, et al. : Peripheral T follicular helper cells make a difference in HIV reservoir size between elite controllers and patients on successful cART. Sci Rep 2017;7:16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aid M, Dupuy FP, Moysi E, et al. : Follicular CD4 T helper cells as a major HIV reservoir compartment: A molecular perspective. Front Immunol 2018;9:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siliciano JD, Siliciano RF: A long-term latent reservoir for HIV-1: Discovery and clinical implications. J Antimicrob Chemother 2004;54:6–9. [DOI] [PubMed] [Google Scholar]
- 11. Cory TJ, Schacker TW, Stevenson M, Fletcher CV: Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS 2013;8:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fletcher CV, Staskus K, Wietgrefe SW, et al. : Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA 2014;111:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estes JD, Kityo C, Ssali F, et al. : Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 2017;23:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson CG, Gay CL, Kashuba ADM: HIV persistence in gut-associated lymphoid tissues: Pharmacological challenges and opportunities. AIDS Res Hum Retroviruses 2017;33:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mebius RE, Kraal G: Structure and function of the spleen. Nat Rev Immunol 2005;5:606–616. [DOI] [PubMed] [Google Scholar]
- 16. Cesta MF: Normal structure, function, and histology of the spleen. Toxicol Pathol 2006;34:455–465. [DOI] [PubMed] [Google Scholar]
- 17. Steiniger BS: Human spleen microanatomy: Why mice do not suffice. Immunology 2015;145:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cataldi M, Vigliotti C, Mosca T, Cammarota M, Capone D: Emerging role of the spleen in the pharmacokinetics of monoclonal antibodies, nanoparticles and exosomes. Int J Mol Sci 2017;18:1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crean PA, Pratt T, Davies GJ, Myers M, Lavender P, Maseri A: The fractional distribution of the cardiac output in man using microspheres labelled with technetium 99m. Br J Radiol 1986;59:209–215. [DOI] [PubMed] [Google Scholar]
- 20. Davies B, Morris T: Physiological parameters in laboratory animals and humans. Pharm Res 1993;10:1093–1095. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt EE, MacDonald IC, Groom AC: Comparative aspects of splenic microcirculatory pathways in mammals: The region bordering the white pulp. Scanning Microsc 1993;7:613–628. [PubMed] [Google Scholar]
- 22. Cioc AM, Vanderwerf SM, Peterson BA, Robu VG, Forster CL, Pambuccian SE: Rituximab-induced changes in hematolymphoid tissues found at autopsy. Am J Clin Pathol 2008;130:604–612. [DOI] [PubMed] [Google Scholar]
- 23. Bennett M, Schechter GP: Treatment of splenic marginal zone lymphoma: Splenectomy versus rituximab. Semin Hematol 2010;47:143–147. [DOI] [PubMed] [Google Scholar]
- 24. Kalpadakis C, Pangalis GA, Dimopoulou MN, et al. : Rituximab monotherapy is highly effective in splenic marginal zone lymphoma. Hematol Oncol 2007;25:127–131. [DOI] [PubMed] [Google Scholar]
- 25. Kalpadakis C, Pangalis GA, Angelopoulou MK, et al. : Treatment of splenic marginal zone lymphoma with rituximab monotherapy: Progress report and comparison with splenectomy. Oncologist 2013;18:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Svicher V, Ceccherini-Silberstein F, Antinori A, Aquaro S, Perno CF: Understanding HIV compartments and reservoirs. Curr HIV/AIDS Rep 2014;11:186–194. [DOI] [PubMed] [Google Scholar]
- 27. Reinhart TA, Rogan MJ, Huddleston D, Rausch DM, Eiden LE, Haase AT: Simian immunodeficiency virus burden in tissues and cellular compartments during clinical latency and AIDS. J Infect Dis 1997;176:1198–1208. [DOI] [PubMed] [Google Scholar]
- 28. Deleage C, Chan CN, Busman-Sahay K, Estes JD: Next-generation in situ hybridization approaches to define and quantify HIV and SIV reservoirs in tissue microenvironments. Retrovirology 2018;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deleage C, Wietgrefe SW, Del Prete G, et al. : Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun 2016;1:68–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nolan DJ, Rose R, Rodriguez PH, et al. : The spleen is an HIV-1 sanctuary during combined antiretroviral therapy. AIDS Res Hum Retroviruses 2018;34:123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bozzi G, Simonetti FR, Watters SA, et al. : No evidence of ongoing HIV replication or compartmentalization in tissues during combination antiretroviral therapy: Implications for HIV eradication. Sci Adv 2019;5:eaav2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moukambi F, Rabezanahary H, Rodrigues V, et al. : Early loss of splenic Tfh cells in SIV-infected rhesus macaques. PLoS Pathog 2015;11:e1005287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boswell KL, Paris R, Boritz E, et al. : Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog 2014;10:e1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cubas RA, Mudd JC, Savoye A-L, et al. : Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 2013;19:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodrigues V, Laforge M, Campillo-Gimenez L, et al. : Abortive T follicular helper development is associated with a defective humoral response in Leishmania infantum-infected macaques. PLoS Pathog 2014;10:e1004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Estes JD, Wietgrefe S, Schacker T, et al. : Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis 2007;195:551–561. [DOI] [PubMed] [Google Scholar]
- 37. Estes JD: Role of collagen deposition in lymphatic tissues and immune reconstruction during HIV-1 and SIV infections. Curr HIV/AIDS Rep 2009;6:29–35. [DOI] [PubMed] [Google Scholar]
- 38. Estes JD, Li Q, Reynolds MR, et al. : Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis 2006;193:703–712. [DOI] [PubMed] [Google Scholar]
- 39. Freitas CR, Barbosa AA, Fernandes AL, Andrade ZA: Pathology of the spleen in hepatosplenic schistosomiasis. Morphometric evaluation and extracellular matrix changes. Mem Inst Oswaldo Cruz 1999;94:815–822. [DOI] [PubMed] [Google Scholar]
- 40. Zhang ZQ, Notermans DW, Sedgewick G, et al. : Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci U S A 1998;95:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schacker TW, Nguyen PL, Beilman GJ, et al. : Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest 2002;110:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schacker TW, Brenchley JM, Beilman GJ, et al. : Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol 2006;13:556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Estes J, Baker JV, Brenchley JM, et al. : Collagen deposition limits immune reconstitution in the gut. J Infect Dis 2008;198:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng M, Southern PJ, Reilly CS, et al. : Lymphoid tissue damage in HIV-1 infection depletes naïve T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog 2012;8:e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tien PC, Choi AI, Zolopa AR, et al. : Inflammation and mortality in HIV-infected adults: Analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010;55:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toribio M, Fitch KV, Sanchez L, et al. : Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 2017;31:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Utay NS, Kitch DW, Yeh E, et al. : Telmisartan therapy does not improve lymph node or adipose tissue fibrosis more than continued antiretroviral therapy alone. J Infect Dis 2018;217:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freiberg MS, Chang C-CH, Kuller LH, et al. : HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hunt PW, Martin JN, Sinclair E, et al. : T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003;187:1534–1543. [DOI] [PubMed] [Google Scholar]
- 50. French MA, King MS, Tschampa JM, da Silva BA, Landay AL: Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009;200:1212–1215. [DOI] [PubMed] [Google Scholar]
- 51. Neuhaus J, Jacobs DR, Baker JV, et al. : Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010;201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deeks SG, Tracy R, Douek DC: Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013;39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burdo TH, Soulas C, Orzechowski K, et al. : Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010;6:e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burdo TH, Lentz MR, Autissier P, et al. : Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011;204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bronte V, Pittet MJ: The spleen in local and systemic regulation of immunity. Immunity 2013;39:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Swirski FK, Nahrendorf M, Etzrodt M, et al. : Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009;325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Falk S, Müller H, Stutte HJ: The spleen in acquired immunodeficiency syndrome (AIDS). Pathol Res Pract 1988;183:425–433. [DOI] [PubMed] [Google Scholar]
- 58. Falk S, Stutte HJ: The spleen in HIV infection—morphological evidence of HIV-associated macrophage dysfunction. Res Virol 1990;141:161–169. [DOI] [PubMed] [Google Scholar]
- 59. Williams DW, Engle EL, Shirk EN, et al. : Splenic damage during SIV infection: Role of T-cell depletion and macrophage polarization and infection. Am J Pathol 2016;186:2068–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wagner TA, McLaughlin S, Garg K, et al. : HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014;345:570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kulpa DA, Chomont N: HIV persistence in the setting of antiretroviral therapy: When, where and how does HIV hide? J Virus Erad 2015;1:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simonetti FR, Sobolewski MD, Fyne E, et al. : Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A 2016;113:1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kearney MF, Wiegand A, Shao W, et al. : Origin of rebound plasma HIV includes cells with identical proviruses that are transcriptionally active before stopping of antiretroviral therapy. J Virol 2016;90:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee E, von Stockenstrom S, Morcilla V, et al. : Impact of antiretroviral therapy duration on HIV-1 infection of T cells within anatomic sites. J Virol 2020;94:e01270-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. von Stockenstrom S, Odevall L, Lee E, et al. : Longitudinal genetic characterization reveals that cell proliferation maintains a persistent HIV type 1 DNA pool during effective HIV therapy. J Infect Dis 2015;212:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Di Mascio M, Srinivasula S, Bhattacharjee A, et al. : Antiretroviral tissue kinetics: In vivo imaging using positron emission tomography. Antimicrob Agents Chemother 2009;53:4086–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee WA, He G-X, Eisenberg E, et al. : Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 2005;49:1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Devanathan AS, Fallon JK, White NR, et al. : Antiretroviral penetration and drug transporter concentrations in the spleens of three preclinical animal models and humans. Antimicrob Agents Chemother 2020;64:e01384-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Devanathan AS, Pirone JR, Akkina R, et al. : Antiretroviral penetration across three preclinical animal models and humans in eight putative HIV viral reservoirs. Antimicrob Agents Chemother 2019;64:e01639-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shen L, Siliciano RF: Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol 2008;122:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. : Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016;530:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kis O, Robillard K, Chan GNY, Bendayan R: The complexities of antiretroviral drug-drug interactions: Role of ABC and SLC transporters. Trends Pharmacol Sci 2010;31:22–35. [DOI] [PubMed] [Google Scholar]
- 73. Hodges VM, Molloy GY, Wickramasinghe SN: Demonstration of mRNA for five species of cytochrome P450 in human bone marrow, bone marrow-derived macrophages and human haemopoietic cell lines. Br J Haematol 2000;108:151–156. [DOI] [PubMed] [Google Scholar]
- 74. Wickramasinghe SN: Evidence of drug metabolism by macrophages: Possible role of macrophages in the pathogenesis of drug-induced tissue damage and in the activation of environmental procarcinogens. Clin Lab Haematol 1987;9:271–280. [DOI] [PubMed] [Google Scholar]
- 75. Wickramasinghe SN, Barden G, Gardner B: Ability of unstimulated and phorbol-ester-stimulated human blood-monocyte-derived macrophages to metabolize drugs and its implications. Clin Lab Haematol 1991;13:41–50. [DOI] [PubMed] [Google Scholar]
- 76. Elliott JI, Raguz S, Higgins CF: Multidrug transporter activity in lymphocytes. Br J Pharmacol 2004;143:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Joukhadar C, Frossard M, Mayer BX, et al. : Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med 2001;29:385–391. [DOI] [PubMed] [Google Scholar]
- 78. Thompson CG, Cohen MS, Kashuba ADM: Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr 2013;63(Suppl 2):S240–S247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O: Tissue concentrations: Do we ever learn? J Antimicrob Chemother 2008;61:235–237. [DOI] [PubMed] [Google Scholar]
- 80. Thompson CG, Bokhart MT, Sykes C, et al. : Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs. Antimicrob Agents Chemother 2015;59:2944–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Thompson CG, Rosen EP, Prince HMA, et al. : Heterogeneous antiretroviral drug distribution and HIV/SHIV detection in the gut of three species. Sci Transl Med 2019;11:eaap8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bokhart MT, Rosen E, Thompson C, Sykes C, Kashuba ADM, Muddiman DC: Quantitative mass spectrometry imaging of emtricitabine in cervical tissue model using infrared matrix-assisted laser desorption electrospray ionization. Anal Bioanal Chem 2015;407:2073–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bokhart MT, Muddiman DC: Infrared matrix-assisted laser desorption electrospray ionization mass spectrometry imaging analysis of biospecimens. Analyst 2016;141:5236–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Robichaud G, Barry JA, Garrard KP, Muddiman DC: Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) imaging source coupled to a FT-ICR mass spectrometer. J Am Soc Mass Spectrom 2013;24:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Deeks SG: HIV: Shock and kill. Nature 2012;487:439–440. [DOI] [PubMed] [Google Scholar]
- 86. Bose P, Dai Y, Grant S: Histone deacetylase inhibitor (HDACI) mechanisms of action: Emerging insights. Pharmacol Ther 2014;143:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perrin J, Werner T, Kurzawa N, et al. : Identifying drug targets in tissues and whole blood with thermal-shift profiling. Nat Biotechnol 2020;38:303–308. [DOI] [PubMed] [Google Scholar]
- 88. Norton NJ, Mok HP, Sharif F, Hirst JC, Lever AML: HIV silencing and inducibility are heterogeneous and are affected by factors intrinsic to the virus. mBio 2019;10 [Epub ahead of print]; DOI: 10.1128/mBio.00188-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guo Y, Benson C, Hill M, et al. : Therapeutic potential of Pak1 inhibition for pain associated with cutaneous burn injury. Mol Pain 2018;14:1744806918788648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jacobson JM, Kuritzkes DR, Godofsky E, et al. : Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother 2009;53:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Emu B, Fessel J, Schrader S, et al. : Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med 2018;379:645–654. [DOI] [PubMed] [Google Scholar]
- 92. Bettiker RL, Koren DE, Jacobson JM: Ibalizumab. Curr Opin HIV AIDS 2018;13:354–358. [DOI] [PubMed] [Google Scholar]
- 93. Iacob SA, Iacob DG: Ibalizumab targeting CD4 receptors, an emerging molecule in HIV therapy. Front Microbiol 2017;8:2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sheikh V, Murray JS, Sherwat A: Ibalizumab in multidrug-resistant HIV-accepting uncertainty. N Engl J Med 2018;379:605–607. [DOI] [PubMed] [Google Scholar]
- 95. Buss NAPS, Henderson SJ, McFarlane M, Shenton JM, de Haan L: Monoclonal antibody therapeutics: History and future. Curr Opin Pharmacol 2012;12:615–622. [DOI] [PubMed] [Google Scholar]
- 96. Wang W, Wang EQ, Balthasar JP: Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008;84:548–558. [DOI] [PubMed] [Google Scholar]
- 97. Shah DK, Betts AM: Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 2012;39:67–86. [DOI] [PubMed] [Google Scholar]
- 98. Cao Y, Jusko WJ: Survey of monoclonal antibody disposition in man utilizing a minimal physiologically-based pharmacokinetic model. J Pharmacokinet Pharmacodyn 2014;41:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kaufman S, Deng Y: Splenic control of intravascular volume in the rat. J Physiol 1993;468:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tabrizi M, Bornstein GG, Suria H: Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J 2010;12:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Akilesh S, Christianson GJ, Roopenian DC, Shaw AS: Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 2007;179:4580–4588. [DOI] [PubMed] [Google Scholar]
- 102. Yip V, Palma E, Tesar DB, et al. : Quantitative cumulative biodistribution of antibodies in mice: Effect of modulating binding affinity to the neonatal Fc receptor. MAbs 2014;6:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gruell H, Klein F: Antibody-mediated prevention and treatment of HIV-1 infection. Retrovirology 2018;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuhlmann A-S, Peterson CW, Kiem H-P: Chimeric antigen receptor T-cell approaches to HIV cure. Curr Opin HIV AIDS 2018;13:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Moore JP, McKeating JA, Huang YX, Ashkenazi A, Ho DD: Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol 1992;66:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhen A, Carrillo MA, Kitchen SG: Chimeric antigen receptor engineered stem cells: A novel HIV therapy. Immunotherapy 2017;9:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yang G, Holl TM, Liu Y, et al. : Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med 2013;210:241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McCoy LE, Burton DR: Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev 2017;275:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wallace PK, Palmer LD, Perry-Lalley D, et al. : Mechanisms of adoptive immunotherapy: Improved methods for in vivo tracking of tumor-infiltrating lymphocytes and lymphokine-activated killer cells. Cancer Res 1993;53:2358–2367. [PubMed] [Google Scholar]
- 110. Melder RJ, Munn LL, Stoll BR, et al. : Systemic distribution and tumor localization of adoptively transferred lymphocytes in mice: Comparison with physiologically based pharmacokinetic model. Neoplasia 2002;4:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stekel DJ, Parker CE, Nowak MA: A model of lymphocyte recirculation. Immunol Today 1997;18:216–221. [DOI] [PubMed] [Google Scholar]
- 112. Khot A, Matsueda S, Thomas VA, Koya RC, Shah DK: Measurement and quantitative characterization of whole-body pharmacokinetics of exogenously administered T cells in mice. J Pharmacol Exp Ther 2019;368:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hersey P: The separation and 51 chromium labeling of human lymphocytes with in vivo studies of survival and migration. Blood 1971;38:360–371. [PubMed] [Google Scholar]
- 114. Diaz LK, Murphy RL, Phair JP, Variakojis D: The AIDS autopsy spleen: A comparison of the pre-anti-retroviral and highly active anti-retroviral therapy eras. Mod Pathol 2002;15:406–412. [DOI] [PubMed] [Google Scholar]
- 115. Kohyama M, Ise W, Edelson BT, et al. : Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 2009;457:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kurotaki D, Uede T, Tamura T: Functions and development of red pulp macrophages. Microbiol Immunol 2015;59:55–62. [DOI] [PubMed] [Google Scholar]
- 117. Davies LC, Jenkins SJ, Allen JE, Taylor PR: Tissue-resident macrophages. Nat Immunol 2013;14:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dutta P, Hoyer FF, Grigoryeva LS, et al. : Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 2015;212:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kristiansen M, Graversen JH, Jacobsen C, et al. : Identification of the haemoglobin scavenger receptor. Nature 2001;409:198–201. [DOI] [PubMed] [Google Scholar]
- 120. Den Haan JMM, Kraal G: Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun 2012;4:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yadava A, Kumar S, Dvorak JA, Milon G, Miller LH: Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc Natl Acad Sci U S A 1996;93:4595–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Munday J, Floyd H, Crocker PR: Sialic acid binding receptors (siglecs) expressed by macrophages. J Leukoc Biol 1999;66:705–711. [DOI] [PubMed] [Google Scholar]
- 123. Vanderheijden N, Delputte PL, Favoreel HW, et al. : Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J Virol 2003;77:8207–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Elomaa O, Kangas M, Sahlberg C, et al. : Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 1995;80:603–609. [DOI] [PubMed] [Google Scholar]
- 125. A-Gonzalez N, Guillen JA, Gallardo G, et al. : The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat Immunol 2013;14:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]