Abstract
Background: Assessing the public perspectives regarding donation of biospecimens to biobanks would be helpful with the establishment of biobanks in the Arab region.
Objective: To develop a biobanking questionnaire in Arabic and assess its psychometric properties.
Design: Multicenter cross-sectional study.
Methods: We used a two-step process for questionnaire development. First, we decided on the important constructs for a questionnaire followed by development of an item pool through review of the scientific literature and published questionnaires. The questionnaire was refined through cognitive interviews and translation. An expert panel assessed content validity. The final questionnaire included five domains: perceptions; aspects important to participation in biobank research; preferences for type of biobank; attitudes toward biobanking; and willingness to participate in biobank research. Second, we distributed the questionnaire to 250 members of the public from Egypt, Jordan, Sudan, and Morocco to assess the questionnaire's psychometric properties, including reliability (internal consistency and Cronbach's alpha) and construct validity (convergent and divergent validity and exploratory factor analysis [EFA]).
Results: Internal consistency yielded a range of Cronbach's alpha for the five domains from 0.62 to 0.80. EFA showed a 12-factorial solution. Kaiser-Meyer-Olkin measure of sampling adequacy was 0.907 and Bartlett's test of sphericity was significant (p < 0.005). Attitudes were positively correlated with willingness to donate (r = 0.30; p < 0.001).
Conclusions: The final biobank Arabic language questionnaire showed excellent reliability and acceptable validity parameters. The newly developed Arabic questionnaire is the first psychometrically tested tool that can be used in the Arab region to assess the public perspectives on participation in biobanking research.
Keywords: biobanking, public, questionnaire, reliability, validity, psychometric properties
Introduction
Genomic research has great promise to advance the health of society. Such research represents the primary means of understanding the genetic basis of illnesses and advancing personalized medicine.1 Population-based genetic research requires biospecimens, from which to extract DNA, as well as associated phenotypic data. Biobanks are entities that are charged with the collection, storage, and distribution of these resources for secondary research. As such, many institutions and countries have established biobanks to collect human biological samples and associated data for genomic research. To maximize the utilization of biobanking resources, regional and transnational biobank networks, such as the Biobanking and Biomolecular Resources Research Infrastructure, the International HapMap Project, and the International Cancer Genome Consortium, have been established.2
Biobanks are well established in high-income countries (HICs) and are only beginning to emerge in low- and middle-income countries (LMICs). In particular, genomic data from Arab countries remain overwhelmingly underrepresented, which prevents understanding genetic variants that are unique to the Arab population.3 Accordingly, genetic research that relies predominantly on resources from HICs leads to discoveries that are mainly focused on the health needs of HICs, which can lead to increased global health inequities.4 It has been suggested that this potential disadvantage could be addressed by the establishment of biobanks and large cohort studies in LMICs.5
Several reasons exist for governments and institutions in the Arab region to establish biobanks and conduct genomic research. First, populations in Arab countries are genetically diverse, and hence, the existence of biobanks can facilitate genetic studies that could unravel the etiology of numerous diseases. Indeed, a significant force behind the national introduction of genomic medicine has been an increasing awareness of the acute need to tackle the high prevalence of genetic diseases in the Arab region. Second, identification of novel disease biomarkers can “eventually lead to lowering disease burden in a nation and consequently reduce costs of diagnosis and treatment.”6,7
However, challenges exist for the establishment and sustainability of biobanks in many Arab countries. These challenges include financial, social, legal, and ethical issues. Ethical issues include proper informed consent, protection of privacy and maintaining confidentiality, returning results to participants, commercialization, data sharing, sample ownership, benefit sharing, and community engagement.8 In addition, there are issues involving proper governance, adequate infrastructure, effective standardized record-keeping system, training, and quality management system under which biobanks will operate.
Finally, conducting pioneering genomic research involving biological samples requires understanding the religious-cultural-social fabric of the Arab community. Indeed, cultural-social norms of the community play an important role in the daily affairs of people, including medical issues, and hence may be influential in the extent to which the public are willing to donate and share their biological samples.9,10
Survey research would be important to assess the willingness of the public to donate biological samples and their health information toward biobanking research. Currently, a limited number of biobanking surveys involving the public exist in the Arab region. These studies, however, have used survey instruments that lack proper validation or are focused on a single domain of measure, such as knowledge or attitude.11–17 Ensuring the reliability and validation of a questionnaire is critical to building cumulative knowledge regarding public behavior; the process of scale development and evaluation continues to be a challenging activity.
Our aim was to develop a questionnaire that would contain the constructs important in understanding the Arabic public behavior toward participating in banking research. Accordingly, this article is divided into two main sections. The first section describes the development of the item pool of the questionnaire, whereas the second section illustrates the study procedures to collect participants' data necessary to evaluate the psychometric properties of questionnaire that include its reliability and construct validity. This second section will review the related statistical concepts and methods used for scale evaluation.
Section 1: Developing the Questionnaire
The Middle East Research Ethics Training Initiative (MERETI) research team18 consisting of individuals from Egypt, Jordan, Sudan, and Morocco held two 2-day meetings in August and October of 2019 to develop a questionnaire to survey the public from the aforementioned countries regarding aspects determinant of the public's behavior toward participation in biobanking research. For a complete discussion of the steps involved with “Questionnaire development,” see Supplementary Data.
Identification of constructs
The first step involved determining the domains of interest that the questionnaire will measure, known as constructs. A construct is the abstract idea, underlying theme, or subject matter that one desires to measure using survey questions. We conceptualized relevant constructs from the existing literature and previously published questionnaires from which to develop an item pool.
Development of the item pool of the questionnaire
After the initial two meetings, the MERETI research team continued with discussions through virtual web meetings and emails to develop the item pool of the questionnaire. The initial questionnaire consisted of 85 items divided into the following six domains: Privacy and Trust; Concerns about Participation in a Biobank; Perceptions about Biobanks; Attitudes toward participating in biobank research; Preferences for biobanks defined by types of informed consent and methods of storage of health information; and Opinions regarding biobank practices (Supplementary File S1; Version December 4, 2019).
Instrument refinement
Cognitive interviews
To ensure that the scientific intent of the questions and, at the same time, makes sense to respondents, we performed cognitive interviews among 12 members of the public. Cognitive interviewing is an evidence-based tool that captures the thought processes of participants, while they are engaged with answering survey questions.19 Trained members of the research team conducted the cognitive interviews using an interview guide (Supplementary File S2) that consisted of probes to assess comprehension and difficulty of the items, for example, participants were asked to interpret the items and paraphrase in their own words each item. Investigators also observed whether participants had difficulty understanding the instructions of the questions and whether their responses were answered in a similar manner to like-kind questions. Examples of information obtained from the cognitive interviews are presented in Supplementary File S3.
Content validity
Based on the results of the cognitive interviews, we revised the questionnaire and proceeded to assess for content validity. Assessing content validity represents a nonstatistical systematic examination of the survey content to determine the extent to which the items in a questionnaire are representative of the entire theoretical construct the questionnaire is designed to assess and whether the items are sufficient to measure the domain of interest. The process of content validation involves a panel of experts who are familiar with the construct.20 Accordingly, we recruited five experts who had knowledge and expertise on biobanking and served on regional biobanking networks.
Translating the questionnaire into Arabic
The modified questionnaire developed after the process of performing the cognitive interviews and establishing content validity was translated into Arabic followed by a back-translation into English performed by two additional translators. The back translators compared their translations with the previous English version. Minor discrepancies were identified and resolved by discussions between the researchers and the translators. During the development of the questionnaire, previous versions of the questionnaire are shown in Supplementary Files S4–S6. The final version of the questionnaire comprised of 47 items (Supplementary File S7; Version July 13, 2020). The Arabic version of the questionnaire is available in Supplementary File S8.
Section 2: Psychometric Analyses—Obtaining a Data Set for Testing of Reliability and Validity
Once the process of item development, cognitive interviewing, and evaluation of content validity was completed, the next step involved administering the final version of the questionnaire to a large representative sample of respondents for whom the questionnaire is intended to assess for reliability and validity. Before explaining our methods of data collection, we will review the statistical concepts of testing for reliability and validity.
Testing of reliability
Assessing for reliability involves statistical measures to determine how reproducible the survey instrument's data are. Reliability considers the extent to which the questions used in a questionnaire consistently elicit the same results each time it is asked in the same situation on repeated occasions. A questionnaire is said to have high reliability if it produces similar results under consistent conditions and any change would be due to a true change in the attitude, as opposed to changing interpretation (i.e., a measurement error). The reliability of a questionnaire represents the consistency of the survey results, which can be tested by calculating its internal consistency and test-retest reliability.
Internal consistency
Internal consistency is commonly estimated using the coefficient alpha also known as Cronbach's alpha.21 Items in a domain found to have poor internal consistency are deleted if its removal causes an increase in Cronbach's alpha. Cronbach's alpha = 0 indicates no internal consistency (i.e., none of the items correlated with one another), whereas alpha = 1 reflects perfect internal consistency (i.e., all the items are perfectly correlated with one another). In practice, a criterion of adequate internal consistency reliability is a Cronbach's alpha of 0.70 or higher.22
Test-retest reliability
We determined the “test-retest” reliability by obtaining data from a subsample of 100 randomly selected participants who completed the survey again at some time between 7 and 10 days after their first completion. From these data, we calculated the intra-class correlation coefficient (ICC). The higher the correlation, the higher the test-retest reliability, with values close to zero indicating low reliability. A value of the ICC <0.4 is considered a weak agreement; 0.4 to 0.75 represents good agreement; and value ≥0.75 is excellent agreement.
Validity
The validity of an instrument can be examined in numerous ways. The most common tests of validity include the following: (1) content validity (described earlier and is done before the instrument is administered to the target population) is used to assess whether the items on a questionnaire adequately represent the construct of specific interest and (2) construct validity and exploratory factor analysis (EFA), both of which occur after survey administration.
Construct validity answers the question, “Does this instrument really measures the construct it is intended to measure?” Construct validity is the most important concept in evaluating a questionnaire that is designed to measure a construct that is not directly observable. If a questionnaire lacks construct validity, it will be difficult to interpret results from the questionnaire, and inferences cannot be drawn from questionnaire responses to a behavior domain.
Convergent and divergent (discriminant) validity are considered to be subcategories or subtypes of construct validity. In other words, measures of constructs that theoretically should be related to each other are, in fact, observed to be related to each other (i.e., one should be able to show a correspondence or convergence between similar constructs) and measures of constructs that theoretically should not be related to each other are, in fact, observed to not be related to each other (i.e., one should be able to discriminate between dissimilar constructs).
We assessed convergent validity by analyzing inter-item and item-to-total correlations, which are used to examine relationships that exist between individual items in a questionnaire pool. Inter-item correlation examines the extent to which scores on one item are related to scores on all other items in a scale. It also examines the extent to which items on a scale are assessing the same content. Items with very low correlations (<0.30) are less desirable and could be a cue for potential deletion from the tentative scale. Item-total correlations aim at examining the relationship between each item versus the total score of scale items. Items with very low adjusted item-to-total correlations (<0.30) are also subject to deletion from the scale.23
We used several methods to assess for divergent validity. First, we compared the total mean scores of the scales between subgroups of the participants based on their demographic characteristics: age, gender, residence, education, religion, marital status, country medical condition, and medical research participation.24 Second, we calculated the inter-total correlations for each of the different domains. Finally, evidence of divergent validity was demonstrated if the inter-factor correlations generated from the factor correlation matrix (see Exploratory Factor Analysis section below) were less than 0.7.25
Exploratory factor analysis
Factor analysis is a generic term that describes several methods to analyze inter-relationships within a set of variables, resulting in the construction of a few hypothetical “factors.” EFA can be an aid in evaluating construct validity using two functions. It identifies the factor structure or the number of factors (also referred to as components or domains) that underlie a set of variables (e.g., all of the questionnaire items) and determines as to whether the factors are correlated or uncorrelated.23 EFA is generally considered to be more of a theory-generating than a theory-testing procedure.
The process of EFA results in the smallest and most compatible number of underlying factors from a larger set of initial variables on a questionnaire item. The identification of a group of questionnaire items that belongs to a “factor” is achieved through a process of “factor loading,” which shows the degree to which a question item loads or correlates with the factor or component.26 There are rules to determine whether an item “loads” in a meaningful way on a factor.23 To define the number of factors that can be extracted, one can determine the number of Eigenvalues above 1, construct a scree plot,27 or perform a parallel analysis.28
The EFA process can be summarized as follows: (1) the researcher collects raw data on an instrument without having a preconceived notion as to the number of underlying factors, (2) presents this information in data matrices, (3) correlates the variables, and (4) identifies the factors underlying the variables.29
It is important to point out that factor analysis does not tell the researcher what labels or meanings are attached to each of the factors. Instead “[t]he substantive meaning given to a factor is typically based on the researcher's careful examination of what the high loading variables of a factor measure. [In other words], the researcher must ask what the variables of a factor have in common.”30
The extraction of factors can also be used to reduce the number of questionnaire items. With factor analysis, items with factor loadings below 0.30 are considered inadequate as they contribute <10% variation of the latent construct measured. Hence, it is often recommended to retain items that have factor loadings of 0.40 and above. Also, items with cross-loadings or that appear not to load uniquely on individual factors can be deleted.
One final point, to obtain a “more easily interpretable solution regarding the factors…[that is,] determine the simplest solution among a potentially infinite number of solutions that are equally compatible with the observed correlations,” one performs a statistical process known as “rotation.”30
We used EFA on the 47 items of the survey to evaluate the factor structure of the Arabic version of the survey.31 Factorability was initially assessed with both the Kaiser-Meyer-Olkin (KMO) Index test and Bartlett's test of sphericity. The KMO statistics range from 0 to 1, with values closer to 1 denoting greater adequacy of the factor analysis (KMO ≥0.6 low adequacy, KMO ≥0.7 medium adequacy, KMO ≥0.8 high adequacy, and KMO ≥0.9 very high adequacy). We used the KMO test to check for sampling adequacy where a minimum value of 0.5 indicates that factor analysis is appropriate. We used the Bartlett's test of sphericity to check whether the variables were correlated in an identity matrix. A significant p-value associated with the Bartlett's test also indicates the appropriateness of factor analysis.32
To determine the type of rotation to use, we first ran the EFA using the principal component analysis (PCA) with an oblique direct Oblimin rotation to calculate the inter-factor correlations. If the absolute values of the component correlation matrix are less than 0.32, then we shifted the EFA by using the PCA with Varimax rotation.31 We then used a scree plot to determine the optimal number of factors that exist from all the questionnaire items. Based on the number of factors shown by the scree plot, we are then able to calculate the factor loadings for each question item. For each domain in the questionnaire, and using a cutoff value of 0.30, question items with high factor loadings are associated with a distinct factor within the domain.33 Items were not allowed to cross-load on more than one single factor. The last step in the EFA was to calculate the correlations between each of the factors (inter-factor correlation matrix). Correlation coefficients between any two factors that demonstrate statistically significant differences and are less than 0.70 confirm that each factor represents a distinct entity from the other factors. In other words, the correlation matrix substantiates discriminant (divergent) validity between any two factors.
Methods of Data Collection
We distributed the final draft of the questionnaire to 250 members of the public proportionally distributed between Egypt, Jordan, Sudan, and Morocco. After obtaining written consent, research coordinators requested each participant to complete the Arabic version of the survey, which included several definitions, for example, biospecimen, DNA, and biobanks, and instructions for completing the survey. Participants were asked to complete questions involving sociodemographic data (i.e., gender, age, residence, marital status, education, and religion), history of cancer, and previous medical research participation. Research staff assisted participants who had difficulty reading or completing surveys. Participants were provided a $2.00 reimbursement for time and effort for completing the pilot test survey.
Data management
Quantitative data were presented either as mean ± SD or with percent and frequency. For each domain, we assigned a score to each item, for example, values of “4” to “0” were assigned to “definitely yes” to “definitely no,” respectively. We calculated a “total score” for each domain by adding the scores of all items within each domain. Negatively worded questions (in the sections consisting of “perception,” “aspects of biobanking,” and “attitude” items) were reverse scored to be consistent with positively worded statements.
We used t-test and an ANOVA test to compare the demographic characteristics and the total scores. We used Pearson's correlation analysis to calculate inter-item, item-to-total, and inter-total correlations. We used the Statistical Package for the Social Sciences (SPSS), version 22.0, for Windows and STATA version 11 for the analyses. The tests were two tailed and p < 0.05 was considered statistical significance.
Results
Characteristics of the study participants
Table 1 shows the baseline characteristics of 250 participants who were recruited for the psychometric study. The mean age of the study sample was 42 ± 14 years and males constituted 43.8% of the total sample. The majority (86%) lived in urban regions. Of the study participants, 11.2% gave a history of cancer. More than one-third (36.4%) were university graduates, 64.4% were married, and 97.2% were Muslims. Almost one half (49.4%) were from Egypt, 13.6% from Sudan, 12.8% from Jordan, and 24% from Morocco. Of these participants, 11.6% reported previous participation in medical research and 8.4% mentioned that they heard about the term “biobank.”
Table 1.
Baseline Characteristics of the Study Population
| No. (%) | |
|---|---|
| Gender | |
| Male | 112 (43.8) |
| Female | 138 (55.2) |
| Residence | |
| Urban | 215 (86) |
| Rural | 35 (14) |
| History of cancer | |
| Yes | 28 (11.2) |
| No | 222 (88.8) |
| Education | |
| No schooling | 18 (7.2) |
| Less than primary school | 14 (5.6) |
| Primary school | 30 (12) |
| Vocational/technical training diploma | 23 (9.2) |
| Secondary school | 28 (11.2) |
| University degree | 91 (36.4) |
| Postgraduate degree | 28 (11.2) |
| Other | 5 (2) |
| Religion | |
| Muslim | 243 (97.2) |
| Christian | 4 (1.6) |
| Marital status | |
| Never married | 70 (28) |
| Married | 161 (64.4) |
| Divorced | 3 (1.2) |
| Widowed | 10 (4) |
| Have children | |
| Yes | 161 (64.4) |
| No | 77 (33.8) |
| Degree of religion | |
| Not at all religious | 2 (0.8) |
| Not very religious | 24 (9.6) |
| Somewhat religious | 176 (70.4) |
| Very religious | 42 (16.8) |
| Country | |
| Egypt | 124 (49.6) |
| Sudan | 34 (13.6) |
| Jordan | 32 (12.8) |
| Morocco | 60 (24) |
| Prior medical research participation | |
| Yes | 29 (11.6) |
| No | 212 (84.8) |
| Type of the medical researcha (n = 29) | |
| Drug clinical trial | 0.0 |
| Blood sample research | 8 (27.5) |
| Genetic research | 9 (31.0) |
| Questionnaire/interview study | 27 (93.0) |
| Type of the research you would volunteer for in futurea | |
| Drug clinical trial | 40 (16.0) |
| Blood sample research | 128 (51.2) |
| Genetic research | 96 (38.4) |
| Questionnaire/interview study | 185 (74.0) |
| Familiar with the term “biobank” previously | |
| Yes | 21 (8.4) |
| No | 196 (78.4) |
| Not sure | 23 (9.2) |
Multiple response question. The total is more than 250.
Table 2 shows the aggregate scale scores for each domain. Older people (>40 years of age) had a significantly higher score for the “perception” scale (p < 0.05). Men compared with women had significantly (p < 0.05) higher scores for the “perception” and “willingness” domains. Those from Egypt had significantly higher scores for the “perception” scale and those from Morocco had a significantly higher score for “preferences.” Individuals with cancer had significantly higher scores (p < 0.05) for “aspects,” “attitudes,” and “willingness” domains. Those who expressed being “very religious” had a lower score for “perception” scale (p = 0.04) and a higher score for the “preferences” scale (p = 0.005). Those who gave previous history of participation in medical research had a higher score for the “attitudes” scale (p = 0.01).
Table 2.
Comparison of Domain Scores for Baseline Characteristics of the Public (n = 250)
| Perception | Aspects important | Preferences to type of informed consent | Attitude | Willingness | |
|---|---|---|---|---|---|
| Age groups | |||||
| ≤40 years | 22.05 ± 3.58 | 51.33 ± 5.78 | 14.33 ± 3.48 | 46.54 ± 4.43 | 19.82 ± 3.57 |
| >40 years | 23.60 ± 3.35 | 52.18 ± 5.53 | 14.81 ± 2.74 | 47.25 ± 5.47 | 19.41 ± 5.65 |
| p | 0.001 | 0.22 | 0.24 | 0.27 | 0.52 |
| Gender | |||||
| Male | 23.50 ± 2.98 | 51.74 ± 5.21 | 14.66 ± 2.55 | 46.42 ± 5.40 | 20.94 ± 3.85 |
| Female | 22.52 ± 3.72 | 51.94 ± 5.34 | 14.69 ± 3.38 | 47.35 ± 4.61 | 18.85 ± 5.21 |
| p | 0.03 | 0.76 | 0.95 | 0.15 | 0.001 |
| Residence | |||||
| Urban | 22.74 ± 3.37 | 51.66 ± 5.49 | 14.63 ± 3.21 | 47.07 ± 5.16 | 19.81 ± 4.53 |
| Rural | 23.54 ± 3.94 | 52.54 ± 3.59 | 14.62 ± 2.00 | 46.34 ± 2.80 | 19.60 ± 5.82 |
| p | 0.21 | 0.36 | 0.99 | 0.41 | 0.81 |
| Medical condition | |||||
| Cancer | 22.93 ± 4.31 | 53.75 ± 5.16 | 13.82 ± 2.40 | 49.04 ± 4.28 | 21.43 ± 2.59 |
| Others | 22.84 ± 3.34 | 51.53 ± 5.24 | 14.73 ± 3.13 | 46.72 ± 4.93 | 19.57 ± 4.89 |
| p | 0.90 | 0.03 | 0.14 | 0.02 | 0.003 |
| Education | |||||
| No education | 24.03 ± 3.98 | 51.69 ± 4.60 | 15.53 ± 1.87 | 47.28 ± 7.48 | 20.78 ± 4.34 |
| Received some education | 22.69 ± 3.36 | 51.79 ± 5.37 | 14.50 ± 3.19 | 46.93 ± 4.42 | 19.63 ± 4.78 |
| p | 0.09 | 0.92 | 0.08 | 0.71 | 0.20 |
| Religion | |||||
| Muslim | 22.81 ± 3.49 | 51.79 ± 5.24 | 14.64 ± 3.10 | 46.95 ± 4.95 | 19.72 ± 4.75 |
| Christian | 24.75 ± 3.10 | 52.50 ± 8.34 | 13.25 ± 0.50 | 49.50 ± 2.38 | 20.25 ± 2.87 |
| p | 0.27 | 0.79 | 0.002 | 0.31 | 0.83 |
| Marital status | |||||
| Never married | 22.68 ± 2.99 | 50.84 ± 4.98 | 14.23 ± 2.79 | 47.57 ± 4.42 | 20.22 ± 3.24 |
| Ever married | 22.92 ± 3.63 | 52.14 ± 5.35 | 14.78 ± 3.16 | 46.74 ± 5.07 | 19.61 ± 5.17 |
| p | 0.62 | 0.08 | 0.20 | 0.23 | 0.26 |
| Having children | |||||
| Yes | 23.06 ± 3.59 | 51.94 ± 5.52 | 14.80 ± 3.23 | 46.66 ± 5.22 | 19.96 ± 4.90 |
| No | 22.09 ± 3.19 | 51.64 ± 4.41 | 14.04 ± 2.76 | 47.71 ± 4.28 | 19.62 ± 4.01 |
| p | 0.06 | 0.66 | 0.08 | 0.10 | 0.33 |
| Degree of religiosity | |||||
| Very religious | 22.09 ± 2.17 | 52.21 ± 5.27 | 15.83 ± 2.84 | 48.14 ± 6.44 | 20.59 ± 4.87 |
| Not, not very, somewhat religiousa | 23.00 ± 3.66 | 51.69 ± 5.27 | 14.39 ± 3.06 | 46.74 ± 4.51 | 19.61 ± 4.68 |
| p | 0.04 | 0.60 | 0.005 | 0.18 | 0.22 |
| Country | |||||
| Egypt | 23.06 ± 3.73 | 52.21 ± 5.56 | 14.75 ± 3.28 | 47.29 ± 4.23 | 20.60 ± 3.59 |
| Sudan | 20.94 ± 3.26 | 51.18 ± 4.58 | 12.76 ± 2.31 | 46.56 ± 4.32 | 21.62 ± 2.44 |
| Jordan | 24.08 ± 2.89 | 51.52 ± 5.12 | 15.10 ± 2.72 | 46.68 ± 5.64 | 17.07 ± 6.47 |
| Morocco | 21.97 ± 2.41 | 51.25 ± 5.14 | 15.97 ± 2.63 | 46.75 ± 6.39 | 19.72 ± 4.57 |
| P | <0.001 | 0.63 | <0.001 | 0.79 | 0.70 |
| Medical research participation | |||||
| Yes | 23.38 ± 3.48 | 52.48 ± 3.93 | 15.03 ± 3.93 | 47.31 ± 4.96 | 19.21 ± 2.96 |
| No | 22.75 ± 3.53 | 51.86 ± 5.19 | 14.56 ± 2.99 | 44.90 ± 4.61 | 19.75 ± 5.96 |
| P | 0.40 | 0.53 | 0.54 | 0.01 | 0.40 |
Combined categories.
Tests of reliability of the questionnaire
The mean scores for the question items, the total scores, and the Cronbach's alpha for each domain and the ICC for each question are shown in Supplementary File S9.
Cronbach's alpha and ICCs for each of the domains
As shown in Supplementary File S9 for all domains, the Cronbach's alpha and the ICC values were all indicative of adequate reliability. The specific results are as follows:
For the domain “perception”: Cronbach's alpha was 0.71 and the ICC values ranged from 0.60 to 0.92.
For the domain “aspects important regarding participation in biobank research”: Cronbach's alpha was 0.62 and the ICC values ranged from 0.65 to 0.96.
For the domain “preferences for the type of the biobank”: Cronbach's alpha was 0.72 and the ICC values ranged between 0.63 and 0.87.
For the domain “attitude toward taking part in biobank and medical research”; Cronbach's alpha was 0.70 and the ICC values ranged between 0.63 and 0.96.
For the domain “willingness to participate in a research domain”; Cronbach's alpha was 0.80 and the ICC values ranged from 0.68 to 0.94.
Tests of construct validity
Convergent validity
We assessed convergent validity by analyzing the inter-item and item-to-total correlations.
-
1.
Inter-item correlations (data not shown): the result for this statistic demonstrates more than adequate convergent validity. A summary of the results for each domain is as follows:
All items of the “perception” domain showed high significant inter-correlation (p < 0.001), except for the following items: “people will have to spend monies to donate biological samples; “researchers are more interested in making money from donated biological samples than doing good research”; “biobank research can lead to improvement in an individual's health”; and “researchers will always contact people if the analysis of their biospecimens show risk for disease.”
All items of the “aspects important toward participation in biobank research” domain was significantly inter-correlated (p < 0.05), except for the following items: “future research on my data could improve healthcare for people in the future,” “I will be able to obtain my genetic results from the research done on my biological samples,” and “If future research might reveal stigmatizing information about me this will be kept private.”
All items of the “participants' preferences toward informed consent and data security” domain were significantly inter-correlated (p < 0.05).
All items of the “attitudes toward taking part in biobank and medical research” domain were significantly inter-correlated (p < 0.05).
All items for the “willingness to participate in a research” domain were highly significantly inter-correlated (p < 0.001).
-
2.
The item-to-total correlations for the questionnaire items in each domain are shown in Supplementary File S10. As shown, all questionnaire items were significantly correlated with the total scores of each domain (p < 0.05), except Preference 1 in the “participants' preferences toward informed consent and data security” domain (p = 0.08). These results demonstrate adequate convergent validity.
Divergent validity
We used three methods to assess for divergent validity.
-
1.
Table 2 shows comparisons between the total mean scores of scales between subgroups of participants based on their demographic characteristics.24 Subgroups with significant p-values shown in “bold” are emblematic of divergent validity.
-
2.
Table 3 shows the inter-total correlations between the domains of the questionnaire. The domains of “Participants' preferences toward type of biobank” and that of “Willingness to participate in biobank research” showed a significant negative correlation between each other. As one would not expect these domains to be related with each other, this result is demonstrative of divergent validity.
Table 3 also shows further evidence of convergent validity as there were significant correlations between domains expected to be related with each other. For example, “perceptions” with “attitudes toward” biobanks (r = 0.14, p = 0.03); participants' preferences toward informed consent” with “willingness to participate in research” (r = −0.17, p = 0.008); and “attitudes” toward taking part in biobank” with “willingness to participate in research” (r = 0.30, p < 0.001).
-
3.
Supplementary File S11 shows the inter-factor correlation matrix obtained from Explanatory Factor Analysis (see below section “Factorial analysis”). There were both negative and positive correlations among the 12 factors. The largest negative correlation was between the factors “Willingness” and “Attitude 2” (−0.229), and the smallest negative correlation was between the factors “Willingness” and “Aspect 2” (−0.015). The largest positive correlation was between the factors “Aspect 1” and “Perception 2” (0.213), and the lowest positive correlation was between the factors “Attitude 1 and Aspect 3 (0.011). There were no correlation coefficients larger than 0.7; hence, the factors derived from EFA revealed adequate discriminant validity between the factors.
Table 3.
Inter-Total Correlation for Each of the Domains (Assessment of Convergent and Divergent Validity)
| Domain | Aspects important toward participation in biobank research | Participants' preferences toward type of biobank | Attitude toward participation in biobank research | Willingness to participate in biobank research |
|---|---|---|---|---|
| Perceptions | −0.09 (0.15) | 0.09 (0.18) | 0.14 (0.03) | 0.04 (0.54) |
| Aspects important toward participation in biobank research | −0.11 (0.10) | 0.24 (<0.001) | −0.04 (0.55) | |
| Participants' preferences toward type of biobank | −0.01 (0.10) | −0.18 (0.008) | ||
| Attitude toward participating in biobank research | 0.30 (<0.001) |
Factorial analysis
To determine whether to perform the EFA, we assessed the sampling adequacy and sphericity assumptions. The KMO measure of sampling adequacy was 0.64, which is above the recommended value of 0.60, and the Bartlett's test of sphericity was found to be highly significant (p < 0.001).
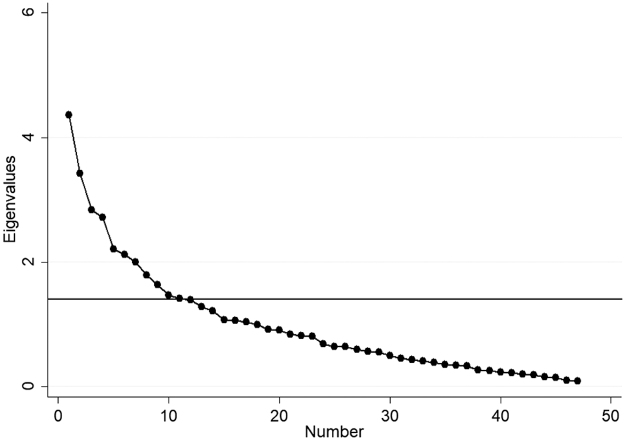
The Scree Plot (Fig. 1) shows that a 12-factor solution is optimal for the EFA. This is indicated by the number of graph points above the horizontal line. Therefore, we ran the EFA with the 12-factor model. The 12 factors explained 62% of the model variance.
FIG. 1.
Scree plot showing that the questionnaire has a 12-factor solution.
Using the result of a 12-factor solution, we first ran the EFA using PCA with Direct Oblimin rotation. All absolute values of correlations between the factors were <0.32 (Supplementary File S11). Hence, we re-ran the final EFA using the PCA with Varimax rotation. This analysis produced the factor loadings for all items of the questionnaire, which are shown in Supplementary File S12.
For the domain “perceptions about biobanks,” items loaded on three factors with factor loadings ranging between 0.321 and 0.822.
For the domain “aspects important regarding participation in biobank research,” items loaded on three factors, with factor loadings ranging between 0.380 and 0.880.
For “preference toward type of biobank,” items loaded on two factors, with factor loadings between 0.467 and 0.882.
For the “attitude toward taking part in biobank and medical research,” items loaded on three factors, with factor loadings between 0.309 and 0.810.
For the “willingness to participate in biobanking research,” all items loaded on one factor, with factor loadings ranging between 0.728 and 0.827.
Discussion
This article reports on the scale development and validation (reliability and construct validity) of an Arabic version of a public survey involving participation in biobanking research. To our knowledge, this is the first validated questionnaire developed for the public in the Arab region related to aspects of biobanking. The evaluation of the psychometrics properties of the instrument involved collecting data from representative samples of populations from Egypt, Sudan, Morocco, and Jordan.
In developing the questionnaire, we considered the religious-cultural-social fabric of the Arab community as well as the nuanced cultural differences between the representative Arab countries participated in the survey development to ensure that the final version of the survey would be applicable for the entire Arab region. Forward and backward translations were performed by bilingual translators and cognitive testing was done among members of the public from the region to assess their understanding of the question items and elicit their opinions and feedback regarding the relevancy of the questions.
The statistical testing of the psychometric properties of the questionnaire showed that its 47 items covering perceptions, aspects important in participating in a biobank, attitudes toward biobanks, and willingness to donate biological samples represent a reliable and valid tool. In particular, the results obtained for the Cronbach's alpha values and the ICCs showed that the questionnaire is a reliable tool. Also, within our sample population, the evidence regarding convergent and divergent validity confirmed the construct validity of our questionnaire.
Within our questionnaire, we measured perceptions, attitudes, and willingness to participate in biobanking research. Perceptions are important as they represent individuals' interpretation of reality based on their beliefs and experiences. Attribution theory of behavior explains that “fundamental attribution error occurs when the influence of external factors is underestimated and the influence of internal factors (i.e., perceptions) is overestimated in regard to making judgments about behavior.”34 Accordingly, it would be helpful to the biobank community to be aware of any misguided perception that the public hold regarding biobanking research that could be the target of future public educational programs.
Our results demonstrated that “perceptions” was positively correlated with “attitudes.” If one considers “perceptions” to be closely related to knowledge, such a correlation between “perceptions” and “attitudes” holds significant importance, as attitudes serve as precondition “for someone to consider applying their learned knowledge or skills.”35 Furthermore, measurement of the public attitudes toward aspects of biobank research is fundamental as it represents an important downstream influence on behavior.36 This significance of attitudes relies on the work of social scientists who demonstrated a link between attitudes and behavior. Specifically, Ajzen and Fishbein used two components to predict intentions, which in turn predict behaviors.37 One component involves the motivation to comply with normative expectations, whereas the other component is the person's attitude toward the particular act in question. Such a link between “attitudes” and “behavior” was implied by our results showing that “attitudes” and “willingness to participate in biobank research” held a significant positive correlation between these domains.
Assessment of the public's perceptions and attitudes toward biobanking is essential for understanding the likelihood of the public's actual behavior toward donating biospecimens to a biobank. Conversely, surveying the public also allows for understanding the public's specific concerns with biobank participation. Such concerns might include issues involving privacy, type of informed consent (e.g., broad, tiered consent, or specific consent for every instance of secondary research), return of genetic results, benefit sharing, data sharing, and community engagement. Acknowledging and addressing these issues with the public and other stakeholders help toward building trust.38,39 This in turn will ensure suitable decision-making and customized awareness among members of the public. Therefore, our questionnaire was designed to facilitate a better understanding of aspects considered determinant of public behavior, which highlights the importance of developing a reliable and valid tool.
In a previous study that developed and validated the Biobanking Attitudes AND Knowledge Survey (BANKS) in the English language, the questionnaire consisted of three scales: knowledge, attitudes, and self-efficacy in participating in a biobank.40 The questionnaire showed satisfactory reliability and validity. The BANKS questionnaire was also translated into the Spanish language and with the demonstration of acceptable reliability and validity statistics will serve as a valid tool to be used among the Spanish community.41
Strengths and limitations
Although our sample population was from four countries in the Arab region, it still might not have generalizability to the other countries in the Arab region. For example, the Gulf countries might have populations of slightly different cultures and demographics from the populations tested in our study.
There are several strengths of our validated questionnaire. First our methodology of testing for reliability and construct validity relied on several statistical tests. Also, our questionnaire included multi-item scales that assess biobanking-related perceptions, aspects important to donating biospecimens, attitudes, and willingness to donate. These subscales will give investigators and directors of biobanks good information regarding areas to explore with the public who might be hesitant to participate in biobank research. Such information will also be important in developing education to enhance the public's knowledge and address misperceptions that they might hold.
Conclusion
We conclude that our questionnaire proves to be a reliable tool, with adequate construct Future research conducted with this questionnaire among a larger sample of members of the public will help in identifying the public's views toward biobanking and the likelihood of their participation in biobanking research performed in the Arab region.
Ethical Approval
We obtained ethical approval for the institutional review boards in Morocco (Avis Du Comite D'Ethique Hospital-Universitaire Fes No 13/19); Egypt (the University of Cairo, No. 19/10/15 and University of Beni Suef, Approval No. FMBSUREC/01102019); Jordan (Jordan University Hospital, Ref 67/209/5766); Sudan (University of Khartoum, Ref: FM/DO/ED); and USA (University of Maryland, HP-00095620). We obtain informed consent from the research participants.
Supplementary Material
Authors' Contributions
S.A.E. collected data from participants, performed statistical analysis of the data, and contributed to the writing of the article. M.A., A.S.A., M.E.I., N.T.M., A.E., Z.M., F.A., E.E., E.G., M.S., L.A., and K.E.R. collected data from participants, and contributed to the analysis of data and to writing of the article. H.J.S. conceived the idea for the study, interpreted de-identified data, and contributed to writing of the article. All authors read and approved the final article.
Author Disclosure Statement
No conflicting financial interests exist.
Funding Information
Supported by Award No. R25TW007090 of the Fogarty International Center at the National Institutes of Health.
Supplementary Material
References
- 1. De Souza YG, Greenspan JS. Biobanking past, present and future: Responsibilities and benefits. AIDS 2013;27:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen H, Pang T. A call for global governance of biobanks. Bull World Health Organ 2014;93:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Ali M, Osman W, Tay GK, AlSafar HS. A 1000 Arab genome project to study the Emirati population. J Hum Genet 2018;63:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drake TM, Knight SR, Harrison EM, Søreide K. Global inequities in precision medicine and molecular cancer research. Front Oncol 2018;8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernando B, King M, Sumathipala A. Advancing good governance in data sharing and biobanking—International aspects. Wellcome Open Res 2019;4:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al Kuwari H, Al Thani A, Al Marri A, et al. The Qatar Biobank: Background and methods. BMC Public Health 2015;15:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alahmad G, Dierickx K. Ethics of research biobanks: Islamic perspectives. Biopreserv Biobank 2018;16:179–185. [DOI] [PubMed] [Google Scholar]
- 8. Budimir D, Polasek O, Marusić A, et al. Ethical aspects of human biobanks: A systematic review. Croat Med J 2011;52:262–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coppola L, Cianflone A, Grimaldi AM, et al. Biobanking in health care: Evolution and future directions. J Transl Med 2019;17:172-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahram M. Ethics of biobanking in the Arab region. In: Silverman H (ed). Research Ethics in the Arab Region. London: Springer; 2017:95–106. [Google Scholar]
- 11. Merdad L, Aldakhil L, Gadi R, et al. Assessment of knowledge about biobanking among healthcare students and their willingness to donate biospecimens. BMC Med Ethics 2017;18:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samir Abdelhafiz A, Sultan E, Ziady H, et al. What Egyptians think. Knowledge, attitude, and opinions of Egyptian patients towards biobanking issues. BMC Med Ethics 2019;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ormond KE, Cirino AL, Helenowski IB, Chisholm RL, Wolf WA. Assessing the understanding of biobank participants. Am J Med Genet A 2009;149a:188–198. [DOI] [PubMed] [Google Scholar]
- 14. Saida L, Boulouiz R, Abda N, Tajir M, Bellaoui M, Ouarzane M. Assessment of knowledge, attitudes and support of health professionals towards biobanks in Eastern Morocco. Open J Epidemiol 2019;09:191–201. [Google Scholar]
- 15. Ziady H, El Zeiny N, Sultan E, El Sharef Y. Assessment of medical students' knowledge and attitude towards biobanks assessment of medical students' knowledge and attitude towards biobanks and biospecimens donationbiospecimens donation. J Med Res Inst 2017;38:1–9. [Google Scholar]
- 16. El-khadry S, Abdallah A, Yousef M, abdeldayem H, Ezzat S, Dorgham L. Effect of educational intervention on knowledge and attitude towards research, research ethics, and biobanks among paramedical and administrative teams in the National Liver Institute, Egypt. Egypt Liver J 2020;10:Article number 1. [Google Scholar]
- 17. Ahram M, Othman A, Shahrouri M. Public perception towards biobanking in Jordan. Biopreserv Biobanking 2012;10:361–365. [DOI] [PubMed] [Google Scholar]
- 18. Middle East Research Ethics Training Initiative (MERETI). MERETI-NETWORK. 2021. Available at www.mereti-network.net (Last accessed May 11, 2021).
- 19. Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications; 2004. [Google Scholar]
- 20. Zamanzadeh V, Ghahramanian A, Rassouli M, Abbaszadeh A, Alavi-Majd H, Nikanfar AR. Design and implementation content validity study: Development of an instrument for measuring patient-centered communication. J Caring Sci 2015;4:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika 1951;16:297–334. [Google Scholar]
- 22. Nunnally JC. Psychometric Theory 3E. New York, NY: Tata McGraw-Hill Education; 1994. [Google Scholar]
- 23. Boateng GO, Neilands TB, Frongillo EA, Melgar-Quinonez HR, Young SL. Best practices for developing and validating scales for health, social, and behavioral research: A primer. Front Public Health 2018;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drost EA. Validity and reliability in social science research. Educ Res Perspect 2011;38:105. [Google Scholar]
- 25. Field A. Discovering Statistics Using IBM SPSS Statistics. London, UK: Sage; 2013. [Google Scholar]
- 26. Quantitative Specialists. Factor Loadings—What Do They Mean? Factor Analysis; PCA; Eigenvalues. Available at: https://youtu.be/XnsHe_c23_g (last accessed February 24, 2021).
- 27. Quantitative Specialists. How to Interpret a Scree Plot in Factor Analysis; EFA; Eigenvalue; PCA. Available at: https://youtu.be/vFUvNICWVz4 (last accessed February 24, 2021).
- 28. Field A. P. Discovering Statistics Using SPSS, 2nd edn. London: Sage; 2005. [Google Scholar]
- 29. Stapleton CD. Basic Concepts in Exploratory Factor Analysis (EFA) as a Tool to Evaluate Score Validity: A Right-Brained Approach. Institute of Education Services.. 1997. Available at: https://eric.ed.gov/?id=ED407419 (Last accessed May 10, 2021).
- 30. Kim J, Mueller C. Introduction to Factor Analysis. Beverly Hills: Sage Publications; 1978. [Google Scholar]
- 31. Field A. Exploratory factor analysis. Discovering statistics using SPSS. London, UK: Sage; 2005:619–680. [Google Scholar]
- 32. Samuels P. Advice on Exploratory Factor Analysis. Technical Report. ResearchGate. 2017. Available at: https://www.researchgate.net/publication/319165677_Advice_on_Exploratory_Factor_Analysis (Last accessed January 4, 2021).
- 33. Field A. Discovering Statistics Using SPSS, 4th edn. London: SAGE Publications Ltd.; 2013. [Google Scholar]
- 34. Kelley HH. Attribution theory in social psychology. In: Levine D (ed). Nebraska Symposium on Motivation, Vol. 15. Lincoln: University of Nebraska Press; 1967. [Google Scholar]
- 35. Kalichman MW, Plemmons DK. Research agenda: The effects of responsible-conduct-of-research training on attitudes. J Empir Res Hum Res Ethics 2015;10:457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ajzen I. The theory of planned behavior. Organizational Behav Hum Decis Processes 1991;50:179–211. [Google Scholar]
- 37. Ajzen I, Fishbein M. The prediction of behavioral intentions in a choice situation. J Exp Social Psychol 1969;5:400–416. [Google Scholar]
- 38. Ahram M, Othman A, Shahrouri M, Mustafa E. Factors influencing public participation in biobanking. Eur J Hum Genet 2014;22:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moodley K, Singh S. “It's all about trust”: Reflections of researchers on the complexity and controversy surrounding biobanking in South Africa. BMC Med Ethics 2016;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wells KJ, Arevalo M, Meade CD, et al. Development and validation of the Biobanking Attitudes and Knowledge Survey (BANKS). Cancer Epidemiol Biomarkers Prev 2014;23:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arevalo M, Jacobsen PB, Gwede CK, et al. Development and validation of the Biobanking Attitudes and Knowledge Survey-Spanish (BANKS-SP). J Community Genet 2016;7:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.