Abstract
Introduction:
Vascular endothelial growth factor (VEGF)-A is a sought therapeutic target for the treatment of PAD because of its potent role in angiogenesis. However, no therapeutic benefit was achieved in VEGF-A clinical trials, suggesting that our understanding of VEGF-A biology and ischemic angiogenic processes needs development. Alternate splicing in VEGF-A produces pro- and anti-angiogenic VEGF-A isoforms; the only difference being a 6-amino acid switch in the C-terminus of the final 8th exon of the gene. This finding has changed our understanding of VEGF-A biology and may explain the lack of benefit in VEGF-A clinical trials. It presents new therapeutic opportunities for peripheral arterial disease (PAD) treatment.
Areas Covered:
This review examines a newly recognized “anti-angiogenic” VEGF-A isoform and its role in ischemic angiogenesis regulation. A literature search was conducted to include: 1) reports on the predicted mechanism by which the anti-angiogenic VEGF-A isoform would inhibit angiogenesis, 2) findings of the unexpected mechanism of action, and 3) how this mechanism revealed novel signaling pathways that may enhance future therapeutics in PAD.
Expert opinion:
Inhibiting a specific anti-angiogenic VEGF-A isoform in ischemic muscle promotes perfusion recovery in preclinical PAD. Additional efforts focused on the production of these isoforms, and the pathways altered by the presence and removal of different VEGF receptor-ligand interactions, and how this new data may allow bench to bedside progress offers new approaches to PAD.
Keywords: Endothelium, Macrophage polarization, Alternate splicing, Angiogenesis, Ischemia, peripheral artery disease, chronic limb-threatening ischemia, atherosclerotic occlusion, chronic limb ischemia
1. Introduction
Angiogenesis, the formation of blood vessels from the existing vasculature is essential for the development and survival of an organism[1]. Angiogenesis regulates several physiological and pathological processes. While angiogenesis can be an adaptive response to injury, insufficient angiogenesis results in ischemic disorders[2], whereas uncontrolled angiogenesis promotes tumor progression and retinopathies[3,4]. Targeting angiogenesis has been of pivotal interest in several ischemic cardiovascular diseases[5,6] and cancer[7]. Vascular Endothelial Growth Factor-A (VEGF-A) is one of the most extensively studied growth factors in the field of angiogenesis[8,9]. VEGF-A in humans is located on chromosome 6p12 spanning 16,272 bp with eight exons[10,11]. Members of the VEGF-A family are characterized by the presence of eight conserved cysteine residues[11]. VEGFs are highly conserved among species and are found in all vertebrates that have been examined to date[12]. Apart from VEGF-A, other prominent members of the VEGF super-family include VEGF-B, PLGF, VEGF-C, and VEGF-D, all of which are encoded on other chromosomes[13]. These VEGF ligands serve as extracellular signaling molecules for receptor tyrosine kinases including VEGFR1, VEGFR2, and VEGFR3[14]. VEGF-A serves as a ligand for both VEGFR1 and VEGFR2; VEGF-B and PLGF are specific ligands for VEGFR1, and VEGF-C and VEGF-D serve as ligands for VEGFR2 and VEGFR3. While VEGFR2 plays an important role in physiological and pathological angiogenesis[15], VEGFR3 plays an important role in regulating lymphangiogenesis[16]. Though VEGFR2 is regarded as the dominant receptor in post-natal angiogenesis, VEGFR1 also regulates a broad range of physiological and pathological functions[17,18]. Due to our interest in therapeutic angiogenesis for peripheral artery disease (PAD)[19], this review focuses on the recent advances in our understanding of the “anti-angiogenic” VEGF-A isoforms and how differential regulation of the isoforms may effect VEGFR signaling in PAD. To state this more clearly, promoting angiogenesis as a therapeutic in PAD has largely been accomplished by enhancing ligand mediated receptor activation, certainly for VEGF. Recent studies have clearly demonstrated that removal of this anti-angiogenic VEGF165b isoform was not equivalent to the delivery of additional ligand. Indeed, removal of the anti-angiogenic isoform was pro-angiogenic through activating a novel VEGFR1-STAT3 pathway, one that would not have been recognized without the appreciation and systemic interrogation of modulating this specific anti-angiogenic ligand.
1.1. Search methodology
A literature search was conducted to include: 1) reports that covered the predicted mechanism by which the anti-angiogenic VEGF-A isoform would inhibit angiogenesis, 2) findings of the unexpected mechanism of action, and 3) how this mechanism revealed novel signaling pathways that may set the stage for future therapeutics in PAD. The following search terms were used to obtain studies/findings relevant to VEGFs and PAD in Pubmed that were discussed in this review. VEGF165b & angiogenesis; VEGF165b & PAD; preclinical & PAD & models; VEGF-A & PAD & clinical & trials; Cilastozol & PAD; sVEGFRs & pre-eclampsia; sVEGFR & PAD; sVEGFR & Immune & responses; VEGF-A & PAD; VEGF-A & hind & limb & ischemia; VEGF-A & signaling; VEGF165b & signaling; VEGFR1 & signaling; VEGFR2 & signaling; Macrophage & polarization; Macrophages & PAD; Monocyte & phenotypes; Platelets & PAD; Monocyte & phenotypes & Cardiovascular & disease; Monocyte & Phenotypes & PAD.
2. Targeting alternatively spliced VEGF-A isoform as a therapeutic for PAD
2.1. Peripheral Artery Disease
Peripheral artery disease is a disease outcome resulting from atherosclerotic occlusion(s) in the leg(s)[20]. In a large number of symptomatic patients, complete occlusions of blood vessels can result in an inadequate blood flow to meet the demands of everyday walking or profound enough to place the limb at risk for amputation. Hence, the quantity of blood that can be delivered to the distal ischemic leg becomes dependent on the extent of the large vessel (collateral) and microvascular remodeling. Severe PAD (chronic limb-threatening ischemia (CLI) often results in limb amputation[21]. ~200,000 amputations occur in the US/year with PAD as the largest contributing factor for amputations in adults. While surgical and catheter-based revascularization therapies, despite carrying risk, are the preferred first line of treatment for severe PAD, many patients have low or no chance of success from revascularization. Currently, Cilostazol is the only FDA approved drug to treat PAD[22,23], however significant drug interactions with patients that take Cytochrome-P450 inhibitors (CYP34A (erythromycin, diltiazem) or CYP2C19 (Omeprazole)) limits its use[24,25]. Based on its ability to activate VEGFR2 induced angiogenesis, VEGF-A has been extensively sought out as a therapeutic for PAD[26–32]. However, none of the therapies that induce VEGF-A in the ischemic leg were able to provide clinical benefit to PAD patients. Moreover, in some instances, consistent with the known side-effect of VEGF-A in PAD patients, two clinical trials showed the induction of edema[29,32]. These failures of VEGF-A clinical trials and the recent discovery of a novel alternatively spliced isoform family that occurs due to alternate splicing in exon-8 of VEGF-A[33] indicated that our knowledge of VEGF-A isoforms signaling/function in regulating ischemic endothelial angiogenic function is inadequate and more in-depth studies are necessary to allow for successful clinical trials.
2.2. Challenges in translating VEGF-A therapy from bench to bedside in PAD.
VEGFR2 signaling is well known to be the dominant pro-angiogenic signaling in post-natal and pathological angiogenesis[14]. The ability of VEGF-A to induce potent VEGFR2 dependent angiogenesis made it an attractive target for treating ischemic cardiovascular diseases including PAD and Cancer. While blocking VEGF-A has been therapeutically effective for treating cancer[34] and age-related macular degeneration[35], none of the clinical studies conducted in PAD were successful[26,28,30–32]. This clearly indicates that making new vasculature in an ischemic environment is a more formidable challenge than blocking angiogenesis in a tumor environment.
A significant number of preclinical studies to induce hind limb ischemia by femoral artery ligation and resection were conducted to determine the translational potential of VEGF-A treatment[36–42]. In this model, the femoral artery is isolated and ligated proximally just above the inguinal ligament and distally at the start of the popliteal arteries to induce hind limb ischemia (HLI)[43,44]. To a large extent, the pathological features presented in this model faithfully reflect human PAD. In fact, femoral artery ligation and resection in C57BL/6 mice is considered a close match to PAD patients with intermittent claudication, due to their greater resistance to ischemic muscle damage and excellent perfusion recovery to HLI[45–47]. On the other hand, BALB/C mice show a dramatic tissue loss with higher necrosis incidence and poor perfusion recovery to HLI reflecting patients with chronic limb-threatening ischemia[45–47]. Few variations of this model exist, wherein depending on the rationale of the study, either single ligation (proximal) or double ligation (proximal and distal) without resecting the femoral artery are performed[43]. Despite the ability of these models to recapitulate most of the common features of PAD, all these models induce acute ischemia in the hind limb, whereas PAD is a secondary manifestation of atherosclerotic plaque build-up in the in-flow blood vessels that induces chronic ischemia[19].
It is unclear whether one of the possible reasons for an excellent therapeutic effect of VEGF-A in preclinical murine models but not in human PAD is due to the chronic ischemic environment which may induce pathological features distinct from acute HLI in murine models. A recent report by Krishna et al[48]., developed a new 2-stage variation of femoral artery ligation and resection model to produce a more clinically relevant PAD[48] model. In this model, the authors used an ameroid constrictor to induce a gradual narrowing of the femoral artery for 14 days followed by the resection of the femoral artery. The authors observe a significant decrease in the blood flow in this 2-stage hind limb ischemia model vs. the femoral artery ligation and resection without using ameroid constrictor[48]. The development of murine models that can produce chronic ischemic conditions to match human PAD will enable a more accurate assessment of a gene/molecules’ therapeutic efficacy for PAD treatment. Another possible explanation for the reason behind the failure of VEGF-A in PAD clinical trials can be explained based on the expression of anti-angiogenic VEGF165b isoforms in ischemic muscle[49,50], whose levels and/or function was not accounted for during the VEGF-A clinical trials. Until the discovery of these anti-angiogenic VEGF-A isoforms[33], total VEGF-A in the PAD muscle was considered pro-angiogenic and the focus has been to increase the inadequate VEGF-A levels in the ischemic muscle to activate VEGFR2 signaling and downstream angiogenesis.
2.3. Alternatively spliced anti-Angiogenic VEGF-A isoforms
Alternate splicing in the VEGF-A family is well understood[51]. Alternate stop codons in exons 6 and 7 result in multiple VEGF-A splice variants with prescribed varying lengths and degrees of extracellular matrix binding ability[52]. VEGF-A isoforms that retain heparin-binding sites exhibit strong binding to the extracellular matrix, whereas VEGF-A isoforms that lack the heparin-binding sites show reduced ability to bind to the extracellular matrix resulting in a predominant increase in circulation as soluble isoforms[53]. E.g. VEGF-A189 that retains both exons 6 and 7 is sequestered almost entirely to the extracellular matrix, whereas VEGF-A121 that lacks both exons 6 and 7 is predominantly secreted isoform[53]. Nevertheless, whether membrane-bound or soluble these “exon 6, 7 alternatively spliced isoforms” exhibit comparable angiogenic activity upon binding to VEGFR2.
The discovery of the novel VEGF-A isoform family occurring due to alternative splicing in exon-8 with “anti-angiogenic” properties questioned the inherent pro-angiogenic nature of VEGF-A isoforms[54]. Distal and proximal 3’ splicing regulates the formation of 2 isoform families, with the only known difference so far, being a 6 amino acid switch from CDKPRR in distal splice variants (from hereon called as VEGFxxxa, xxx for the number of amino acids) to SLTRKD in proximal splice variants (called as VEGFxxxb (VEGF165b, most abundantly occurring isoform). However, unlike the isoforms generated by the alternate splicing in exons 6 and 7, isoforms that occur due to splicing in exon-8 display appear to largely display anti-angiogenic properties in-vivo [55]. The recognition of the anti-angiogenic isoforms within the VEGF-A family pushes the boundaries of our understanding of VEGF-A induced angiogenesis. Needless to say that before the discovery of anti-angiogenic VEGFxxxb isoforms, the total amount of VEGF-A identified by either PCR, western blot, ELISA, or immunohistochemical analysis was considered pro-angiogenic, since any reagent that was developed against common sequences/regions in VEGF-A will have in fact detected both the pro- and the anti-angiogenic VEGF-A family members[49,54]. Hence, in physiology or pathology, the actual or relative amounts of pro- vs. anti-angiogenic VEGFxxxa or VEGFxxxb isoforms were not known until the advent of primer sequences and antibodies that are raised/developed specifically against the 6-aminoacid or base-pair sequences[49,54]. Moreover, even though reports demonstrating the expression, as well as the biological activity of VEGFxxxb isoforms, began to increase the mechanistic role of VEGFxxxb isoforms in regulating pathophysiology is still in its infancy; specifically, the precise mechanism(s) by which VEGFxxxb isoforms exert their inhibitory effect on angiogenesis. Additional detail will then be needed to apply these findings to conditions that occur in PAD.
2.4. VEGF165b signaling in endothelium
VEGF165b isoform was first discovered by Bates et al. in renal carcinoma samples[33]. The authors showed that VEGF165b blocked VEGF165a induced human umbilical vein endothelial cells (HUVEC) migration. In a subsequent paper by Woolard et al.[55] the authors report that VEGF165b competitively blocked VEGF165a induced VEGFR2 activation in human microvascular endothelial cells. These reports laid the foundation for the concept that VEGF165b functions as a competitive inhibitor of VEGF165a induced VEGFR2 activation and angiogenesis. The data presented in Woolard et al. also showed that VEGF165b was not able to induce VEGFR2 activation by itself but only blocked VEGF165a induced VEGFR2 activation suggesting that VEGF165b might not have a biological activity by itself. Interestingly, the data in the manuscript also showed that, despite an inability to induce VEGFR2 activation, VEGF165b treated HMVECs showed a significant increase in ERK1/2 activation, one of the other important signaling mediators downstream of VEGFR2 activation[55]. This data suggested the possibility that VEGF165b can induce receptor kinase signaling that is different and/or independent of VEGFR2 activation.
Subsequently, Kawamura et al.[56], using pulmonary arterial endothelial (PAE) cells that express VEGFR2-NRP1 showed that VEGF165b decreases VEGFR2 binding with NRP1 and suggested that decreased VEGFR2 activation by VEGF165b is due to its inhibitory effect on VEGFR2-NRP1 interactions. However, the extent of VEGFR2-NRP1 complex inhibition achieved by VEGF165b did not reflect the relative change in VEGFR2 activation questioning whether VEGFR2-NRP1 complex inhibition was, in fact, responsible for VEGFR2 inhibition by VEGF165b. Later, another report by Catena et al[57]., showed that VEGF165b and its sister isoform VEGF121b isoform are weakly angiogenic isoforms of VEGF-A. In this report, Catena et al[57]., showed that VEGF165b and VEGF121b induced VEGFR2 and Erk1/2 activation albeit to varying degrees compared to VEGF165a. This data contrasts with Woolard et al[55]., who showed that VEGF165b was not able to VEGFR2 activation but suggested the possibility that VEGF165b might not be an inhibitory isoform of VEGFR2. Clearly, data was emerging that VEGF ligand-receptor interactions and down-stream receptor signaling was going to be more complex than a single interaction.
Until recently mechanistic studies on VEGF165b were focused on examining the ability of VEGF165b to block VEGF165a induced VEGFR2 activation[58]. However, data from Catena et al[57]., and Kawamura et al[56]., indicated that VEGF165b not only induces VEGFR2 activation but also downstream ERK1/2 activation suggesting that indeed VEGF165b is not an inhibitory isoform of VEGFR2. Consistently, our recent data showed that VEGF165b induced VEGFR2 activation to the same extent as VEGF165a in physiologically relevant HUVECs, as well as in HEK293 cells that express only VEGFR2[49]. This data suggested that the anti-angiogenic property of VEGF165b is not due to its inhibitory effect on VEGFR2. Furthermore, the ability of VEGF165b to activate VEGFR2 showed that it is not an inactive ligand[49,56,57]. Taken together these findings presented evidence that VEGF165b exerts its anti-angiogenic effects via a receptor other than VEGFR2.
Previous studies by Waltenberger et al[59]., and Sawano et al[60]., showed that the binding affinity (Kd) of VEGF165a to VEGFR1 is Kd ~1–16pmol/L, whereas for VEGFR2 it is ~410–760pmol/L. However, the extent of VEGFR1 autophosphorylation that follows VEGF165a binding is several magnitude lower compared to VEGFR2[60]. Since the binding sites for VEGFR1 (in exon3) and VEGFR2 (in exon4) are the same in VEGF165a and VEGF165b isoforms, VEGF165b binding affinity to VEGFR1 and VEGFR2 was predicted to be similar to VEGF165a. The intensity of phosphorylation (e.g. measured on western blot) is considered a hallmark for the ability of the receptor to activate the downstream signaling. VEGF165a has a high binding affinity to VEGFR1 (vs. VEGFR2) but cannot induce potent VEGFR1 phosphorylation. This has resulted in the existing paradigm that endothelial VEGFR1 is an anti-angiogenic receptor that functions as a VEGF-A trap to limit angiogenesis. This paradigm was further supported by the developmental studies where VEGFR1 deficient mice die embryonically due to excessive malformed angiogenesis[61,62]. Even though the abnormal angiogenesis was later shown to be due to defective hematopoietic progenitor recruitment, excessive VEGFR2/Akt activation observed in VEGFR1 deficient tissues indicated that lack of VEGFR1 increases the bioavailability of VEGF165a to bind and sustain VEGFR2 activation resulting in excessive angiogenesis. Further experiments using mice that have N-terminal binding regions for VEGFR1, but lack the C-terminal tyrosine kinase region, showed that these mice develop normally indicating that VEGFR1-tyrosine kinase is dispensable for developmental angiogenesis and also suggested a lack of activity for VEGFR1 tyrosine kinase[63]. Even though several reports have presented convincing evidence that VEGFR1 plays critical roles in several pathologies[64–70], only fewer reports have shown a specific and direct pathological role of the VEGFR1 tyrosine kinase[71–73].
In our studies to understand the role of VEGF165b in regulating ischemic angiogenesis in PAD, we anticipated that VEGF165b inhibition (achieved via delivery of an isoform-specific monoclonal antibody) would activate the classical pro-angiogenic VEGFR2-AKT signaling pathway[49]. However, our data showed that VEGF165b inhibition actually decreased VEGFR2 activation in ischemic endothelial cells within the preclinical PAD model. This is consistent with our in vitro data that showed that VEGF165b actually can function as an activating ligand for VEGFR2[49]. What we discovered was that VEGF165b is a potent silencer of VEGFR1 activation. In our studies using HEK-293 cell models (cells that lack VEGFRs but were transfected to be HEK293-VEGFR1 or HEK293-VEGFR2), to determine the competitive inhibitory effect of VEGF165b on VEGFR2 and VEGFR1, we observed that VEGF165b blocked VEGFR1 activation even at 10X lower concentration than VEGF165a, but showed a synergistic effect with VEGF165a in activating VEGFR2[49]. This data was further supported by the evidence that VEGFR1+/− mice have significantly lower perfusion recovery and angiogenesis in ischemic muscle post-hind-limb ischemia[74]. These data confirmed that VEGFR1 deficiency inhibits angiogenesis and VEGF165b blocks VEGFR1 induced angiogenesis to exert its anti-angiogenic effect. A role of VEGFR1 tyrosine kinase in regulating ischemic angiogenesis was also demonstrated in the experiment where VEGFR1 pull-down demonstrated an increased STAT3 binding upon VEGF165b inhibition and overexpressing VEGFR1 in HEK293 cells resulted in STAT3 activation[49]. This data pointed out that VEGFR1 tyrosine kinase has activity and can interact with STAT3 to induce its activation. What remains to be determined is whether the downstream signaling and effects due to VEGFR1+/− may not be the same as VEGFR1-tyrosine kinase deficiency and these differences in disease outcomes and signaling may be more pronounced in cardiovascular pathologies including PAD and cancers.
While we have shown that VEGF165b blocks VEGFR1 to decrease angiogenesis, whether this is due to a direct inhibitory effect of VEGF165b on VEGFR1 or due to competition with VEGF165a for binding sites on VEGFR1 is not completely understood. Furthermore, the molecular mechanism by which VEGF165b inhibits VEGFR1 but activates VEGFR2 is not well understood. The arginine residues in the 8th exon 6 amino acid sequence (CDKPRR) of VEGF165a are positively charged. These positively charged arginine residues can induce a strong conformational change in VEGFR2 resulting in strong receptor dimerization and autophosphorylation and downstream signaling activation. However, in VEGF165b isoforms, the arginine residues are replaced with lysine and aspartic acid (SLTRKD) resulting in a net neutral charge. This net neutral charge was thought to be a “factor” in inadequate internal rotation and weak VEGFR2 autophosphorylation upon VEGF165b binding[58]. However, our experimental data showing the ability of VEGF165b to activate VEGFR2 to the same extent as VEGF165a[49] strongly suggests that this is not the case. We hypothesize that a net positive charge on VEGF-A isoforms is essential to induce strong autophosphorylation that is required for VEGFR1 activation but may not be essential for VEGFR2 activation. Consistent with our hypothesis, a comparison of the C-terminal 6 amino acid sequence in VEGFR1 activating ligands including PLGF and VEGF-B with VEGF165a and VEGF165b showed that all PLGF isoforms and VEGF-B-167 have positive “RR” residues at the C-terminus (Table 1). Further experiments including the structural alterations and receptor dimerization upon specific ligand binding are necessary to decode the mechanism by which VEGF165b activates VEGFR2 but silences VEGFR1.
Table-1:
Last 2 amino acid sequences in VEGFR1 activating ligands. The last 2 amino acids in the c-Terminus of R1 activating ligands are ‘arginine residues’ indicating a positive charge on R1 activating ligands. These arginine residues are replaced with lysine-aspartic acid resides in VEGF165b indicating a neutral charge. This suggests the requirement of a net positive charge on VEGF ligands to activate R1.
| Ligands | last 6 amino acids | Charge |
|---|---|---|
| PIGF-1 (identifier-P49763–2) | DAVPRR | Positive |
| PIGF-2 (identifier: P49763–3) | DAVPRR | Positive |
| PIGF-3 (identifier: P49763–1) | DAVPRR | Positive |
| PIGF-4 (identifier: P49763–4) | DAVPRR | Positive |
| VEGF-B-167 (identifier: P49765–2) | CRKLRR | Positive |
| VEGF165a (identifier: P15692–4) | CDKPRR | Positive |
| VEGF165b (identifier: P15692–8) | SLTRKD | Neutral |
2.5. VEGF165b signaling in macrophages
While VEGFR2 expression is largely confined to endothelial cells, VEGFR1 expression is quite pleiotropic[75–77]. For example, neurons[75], glia[78], adipose[70], and macrophages[77] express VEGFR1 with varying functions. Macrophages are extremely plastic cells that play important roles in maintaining tissue homeostasis[79]. In PAD, studies were focused mainly on the role of macrophages in arterial remodeling[80–83] with very few studies presenting evidence on their role in microvascular remodeling[84]. The ability of macrophages to modulate tissue repair is dependent on their polarization state which in turn is dependent on the tissue microenvironment[85–88]. For example, classically activated (by LPS-IFN-g) cytotoxic M1 macrophages and alternatively activated (IL4) reparative M2 macrophages are at the 2 ends of the macrophage polarization spectrum[89]. New macrophage subsets based on unique marker/cytokine combination are constantly identified making the nomenclature for macrophages more fluidic[90–92]. For example, we have identified that macrophages in the ischemic environment present an M1-like phenotype based on differential arginase and iNos expression[49,84]. Macrophages are also grouped on the basis of the pathological state of the tissue, for example, TAMs (tumor-associated macrophages) in cancer tissues[93], ATMs (Adipose tissue macrophages) in adipose tissue[94], and the tissue they reside in e.g. Kupffer cells in the liver[95], Langerhans cells in the skin[96], and microglia in the brain[97]. Nevertheless, most of the pathologies that study macrophage function focus broadly on M1 and M2 macrophage populations.
Decoding VEGFR1 signaling in endothelial cells is challenging. Several factors including VEGFR1 and VEGFR2 crosstalk, and receptor heterodimerization, contribute to this complexity. However, the lack of VEGFR2 expression on macrophages has enabled us and others to dissect VEGFR1 specific signaling. VEGF165b secreted by macrophages has been suggested to result in increased circulating serum levels in PAD patients[50]. However, in our experiments including in vitro or ex vivo macrophage conditioned medium or human plasma samples we did not detect VEGF165b presence in the circulation[98]. What we found was a significant increase in the macrophage intracellular VEGF165b levels correlating with lower VEGFR1 activation and an M1-like polarization state[98]. This data indicated that the heparin motifs in VEGF165b isoforms[58] enable the cell surface presenting of VEGF165b to VEGFR1 inhibits VEGFR1 activation to induce an M1-like phenotype. Macrophage polarization states are dynamic and reversible with changing tissue environment and cytokine milieu[99]. Hence, inducing and maintaining an M2-like reparative macrophage phenotype in an M1-inducing ischemic tissue environment is extremely challenging. However, VEGF165b inhibition induced and maintained M2-like phenotype in both infiltrating and resident macrophages until day 3 post HLI in a chronic limb-threatening ischemia model[98]. While increased M2-like macrophages in ischemic muscle decreased necrosis and enhanced perfusion, further experiments are necessary to determine how long VEGF165b inhibition can induce and sustain the M2-like phenotype in preclinical PAD models to better understand its therapeutic efficacy.
While VEGF165b inhibition via a VEGF165b antibody allowed VEGFR1 activation to induce STAT3 activation in endothelial cells, in macrophages VEGF165b inhibition modulated VEGFR1 function to induce signaling that is distinct from endothelial cells[49,98]. In VEGFR1+/− macrophages, we have observed a significant increase in S100A8/A9 expression[98]. This increased S100A8/A9 expression played a causal role in driving an M1-like phenotype in VEGFR1+/− macrophages. Interestingly, while we did not see a direct effect of S100A8/A9 on endothelial cells[98], conditioned medium from macrophages that overexpress S100A8/A9 impaired endothelial angiogenesis by a paracrine mechanism in vitro suggesting that not only the signaling but the mechanism of the genes downstream of VEGF165b-VEGFR1 signaling is unique between endothelium and macrophages. Similar to macrophages, monocytes in the circulation also display heterogeneity in the phenotypes[100–102]. We are just beginning to understand monocyte heterogeneity. Differential CD14, CD16 expression (in human monocytes) was used to cluster the monocytes into 3 different subsets[103]. Classical CD14+CD16-, CD14+CD16+ intermediate and CD14-CD16+ non-classical monocyte subsets[100–102]. However, an elegant report by Hamers et al[101]., using Mass Cytof and RNA-Sequencing of human monocyte populations clearly showed the inadequacy of using only CD14 and CD16 markers to distinguish monocyte subsets indicating that more studies are necessary to distinguish specific monocyte subsets using comprehensive marker panels in cardiovascular diseases[103]. Current studies on monocyte heterogeneity in cardiovascular diseases are confined to identifying the 3 major macrophage subsets based on CD14 and CD16 expression. Interestingly even with CD14 and CD16 markers, several papers showed an important correlation with specific monocyte subsets and disease outcomes in coronary artery disease[104], PAD[105], and cardiovascular events[106,107]. Studies using single-cell transcriptomics are underway to decode the molecular machinery that regulates this monocyte subset as well as the possibility of using this monocyte subset as a cell marker to predict adverse coronary outcomes in PAD patients and/or PAD progression.
3. Conclusions
Despite an increasing number of studies demonstrating a potential role of VEGF165b isoforms in several pathologies including stroke[108], PAD[49,50,98], systemic sclerosis[109], tumors[33,55–57], and retinal diseases[110,111], a complete understanding of the mechanism by which these isoforms regulate pathological processes and whether the mechanisms are the same across different processes are still unclear. Our recent studies have expanded the role of VEGF165b function from endothelial cells[49] to macrophages[98] and other studies have demonstrated the presence of VEGF165b in platelets[112] indicating that the functions of VEGF165b are not confined to vasculature. More importantly, the signaling regulated by VEGF165b is distinct between cell types. For example, while VEGF165b regulates VEGFR1-STAT3 signaling in ischemic endothelial cells[49], it regulates VEGFR1-S100A8/S100A9 signaling in ischemic macrophages[98]. These studies indicate that we have just begun to understand the role of VEGF165b isoforms function; and significant gaps remain in our understanding of its signaling, mechanism, and production in ischemic pathologies[58]
4. Expert opinion
What are the key developments and challenges in the area?
Vascular endothelial growth factor receptor (VEGFR)-2-Akt-endothelial nitric oxide synthase (eNOS) mediated nitric oxide generation is widely considered the dominant pathway promoting hypoxia-dependent angiogenesis[15]. Although preclinical studies have focused on VEGF165a induced VEGFR2 activation to achieve therapeutic angiogenesis, numerous human studies targeting this pathway have failed to achieve any meaningful clinical improvement in patients with PAD[21,26–32]. Cloaked within the vascular endothelial growth factor (VEGF) system alternative splicing of VEGF-A results in a 6 amino acid switch that changes the “pro-angiogenic VEGF165a” to the “anti-angiogenic VEGF165b” isoform[54]. Two aspects of this splice variant are of critical importance. First, detailed attention to the presence of this isoform is required for its recognition, and unless specifically sought studies to date on “VEGF” were unable to distinguish VEGF165a vs. VEGF165b, for the 165 and likely other amino acid versions[54]. In PAD our murine and human studies unexpectedly demonstrated that the major effects of the VEGF165b are directly linked to VEGFR1 signaling[49,98]. On ischemic endothelial cells in PAD muscle, greater VEGF165b produced by ischemic/hypoxic conditions reduce the ability of VEGFR1 to promote angiogenesis[49]. On macrophages, greater VEGF165b polarizes macrophages toward an inflammatory phenotype and in a paracrine manner, these inflammatory macrophages inhibit angiogenesis[98] (Figure 1). In both situations, the negative effects of greater VEGF165b are not readily countered by VEGF165a supplementation; the approach of choice in human intervention.
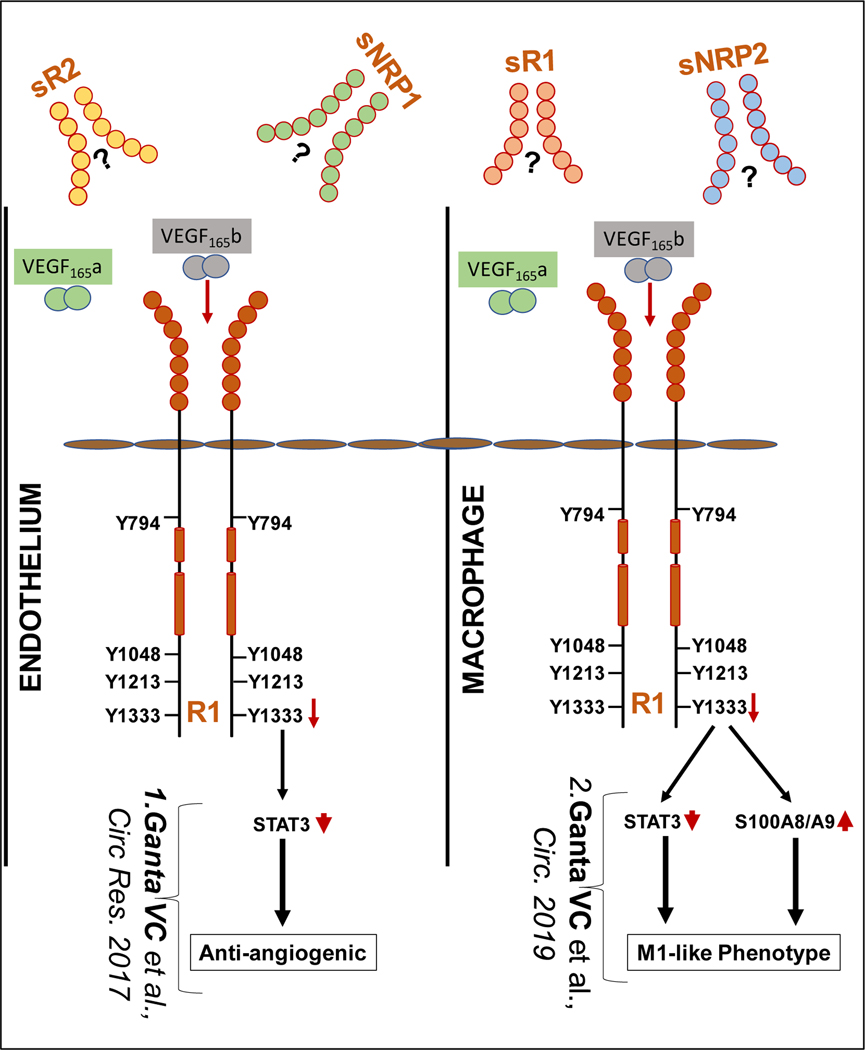
Figure-1:
Recent advances in Anti-angiogenic VEGF165b isoform signaling in PAD. Schematic of our recent findings in VEGF165b signaling. In ischemic ECs, VEGF165b (V165b) blocks VEGFR1 (R1) activation to inhibit angiogenesis (left). In ischemic macrophages, VEGF165b blocks R1 activation to induced M1-like polarization (right). Distinct signaling regulates ischemic angiogenesis and macrophage polarization downstream of R1 activation post VEGF165b inhibition. Limited information on the levels and function of Soluble R1 (sR1), sR2, sNRP1 and sNRP1 significantly increases the complexity of VEGF-VEGFR system in regulating the pathology of PAD.
What are knowledge gaps and how should they be tackled?
Biomarkers are generally invaluable for guiding human therapeutics. One key question that remains to be answered about these elusive VEGF isoforms is our inability to detect VEGF165b in circulation. In contrast to other studies that used the human serum to detect VEGF165b, we have used human plasma samples. Since plasma is devoid of platelets, platelets may contribute to the circulating VEGF165b levels. Consistent with this hypothesis, Hirigoyen et al[112]., showed that platelets from systemic sclerosis secrete significantly higher VEGF165b/VEGF-A levels. Single antiplatelet therapy with aspirin or clopidogrel is recommended as a treatment for symptomatic patients to decrease cardiovascular risk[113–115]. However, more studies are necessary to understand whether platelets serve to deliver VEGF165b or VEGF165b expression modulates platelet function in PAD.
Furthermore, increased binding of plasma VEGF165b to soluble VEGFR1 in the circulation can mask its detection. In addition to sVEGFR1[116], other soluble VEGFRs and NRPs including sVEGFR2[117], sVEGFR3[118], sNRP1[119], and sNRP2[120] have been reported in various physiological and pathological conditions. However, a systematic analysis of the expression or function of these soluble forms beyond their assumed role as a growth factor sink in PAD is not clear[121,122]. For e.g., sVEGFR1 has been shown to interact with α5β1 integrin to inhibit tumor angiogenesis[123]. The function of soluble VEGFR1 has been extensively studied in pre-eclampsia[124]. Increased sVEGFR1 levels have been shown to contribute to the pathogenesis of pre-eclampsia by sequestering VEGF-A and PLGF leading to decreased angiogenesis[124,125]. Very limited information exists on soluble VEGFRs in PAD[121,122]. The ability of sVEGFR1 to sequester VEGF-A strongly indicates the possibility of sequestering VEGF165b as well[126]. However, if there is a preferential binding between VEGF isoforms to sVEGFR1 (and other sVEGFRs) and how these interactions regulate membrane VEGFR1 (vs. VEGFR2) signaling needs to be further studied. More importantly, whether and how sequestering of VEGF165b to sVEGFR1 regulates circulating monocyte phenotype needs to be further examined. It is appealing to speculate that targeting VEGF165b may have the potential to decrease cardiovascular events (via effecting VEGF165b+CD14+CD16+ monocyte subsets and/or VEGF165b expressing platelets in the circulation) in PAD patients.
What should be unfolding in the next 5 years?
Structural studies will be one essential aspect for advancing our understanding of this problem Data to date has shown that, unlike VEGF165a that has 8 cysteine disulfide bonds, VEGF165b has only 7 suggesting that the dimer formed by VEGF165b is weaker compared to VEGF165a. This is evident from our experimental observations (data not shown) that it is relatively easier to observe ~20kD VEGF165b monomer than VEGF165a monomer in western blot analysis. Further experiments are needed to understand whether and how the VEGF165b monomer regulates VEGFR1 vs. VEGFR2 receptor dimerization and signaling.
While progress has been made in understanding the pathological consequences of VEGF165b expression in ischemic muscle, the upstream processes that regulate VEGF165b production is still in their infancy. For example, the splicing machinery that regulates VEGF165b seems to be cell/tissue-specific[50,127–130] and it is yet to be seen what regulates the preferential production of VEGF165b in endothelial cells in non-ischemic and ischemic tissue. In general, splice factors control target and process multiple genes, rendering it difficult to target a splicing factor to achieve therapeutic benefit. However, identifying a 3’ specific slice factor regulated by ischemia might provide a way to target VEGF165b upstream. This is an important aspect to consider due to the loss of VEGFR2 signaling upon VEGF165b inhibition. Even though VEGF165b inhibition enhanced perfusion despite decreased VEGFR2 activation in preclinical models[49], human pathology is more complex to simply overlook the possibility of VEGFR2 signaling inhibition. Hence, more studies are needed to understand the upstream mechanism of preferential VEGF165b production in ischemic tissues.
Conventional therapies were focused on increasing growth factor levels e.g., VEGF-A in PAD muscle to achieve a therapeutic effect[28–32]. However, a 3-fold increased expression of VEGF165b over VEGF165a in PAD muscle and the ability of VEGF165b to inhibit VEGFR1 even at 10 times lower concentration than VEGF165a indicates a 30Molar excess of VEGF165b activity in PAD muscle[49]. This suggests that simply increasing VEGF-A levels to obtain a therapeutic effect in PAD muscle may not be clinically feasible and can also be partly attributed to the failure of VEGF-A clinical trials. Several VEGF-A modulators are in clinical use for cancer[34,131] and macular degeneration[132,133]. Hence, it is not very far to move the beachside findings to the clinic in using VEGF165b monoclonal antibodies to achieve perfusion benefit for PAD patients.
What potential do the latest approaches hold? Are there niche questions?
Given the prevalence and consequences of PAD, likely a number of areas will be the focus of activity for years ahead regarding VEGF165b, its relationship to VEGF receptor activity, and its’ targeting as a therapeutic. Though we have not approached this in this review PAD studies targeting VEGF165b have used monoclonal antibodies and beyond the fact that monoclonal antibodies are emerging as excellent human therapeutics, RNA levels of the VEGF165b are far below that what would be expected when compared to its protein. At least with our current knowledge, RNA-directed inhibition can be anticipated to be much more challenging than antibody-guided inhibition. Recognition of the amino acid composition differences between the VEGF165b and VEGF165a splice variants and its amino acid composition difference may well provide important insight into the isoform interactions with VEGFR1. Does VEGF165b inhibition lead to the same angiogenic signaling pathways that occur with VEGF165a activation or are these distinct and exploitable for investigation? Can promoting angiogenesis independent of VEGFR2 offer therapeutic advantages? The ability for VEGF165b and its particular relationship to the only VEGF receptor on monocytes/macrophages may well provide a way in which processes within PAD muscle in humans may explain the enigmatic role that PAD plays in the increased risks these patients have for heart attack and stroke[134–136]. Finally, the extent to which the finding of this particular isoform will be valuable across other disciplines given the widespread role that the VEGF ligand and receptor play across a host of physiologic and disease processes.
Article Highlights.
Alternative splicing in VEGF-A 8th exon, near its C-terminus, results in the formation of pro- or anti-angiogenic VEGF-A isoforms of the same amino acid length.
The pre-existing paradigm would have suggested that the anti-angiogenic VEGF-A isoforms simply exert their effect by blocking pro-angiogenic VEGF-A induced VEGFR2 activation to inhibit angiogenesis
Our recent studies have shown that anti-angiogenic VEGF-A isoforms are in fact agonists of VEGFR2 but antagonists of VEGFR1 and targeted ligand removal have unveiled novel VEGFR1 signaling pathways in PAD relevant conditions.
In the ischemic endothelial cells, the anti-angiogenic VEGF-A isoform inhibits VEGFR1 signaling to directly decrease angiogenesis.
In the ischemic macrophages, the anti-Angiogenic VEGF-A isoform inhibits VEGFR1 signaling to induce an M1-like phenotype which inhibits angiogenesis in a paracrine manner.
Acknowledgments
Funding
BH Annex is supported by R01HL141325, R01HL148590, RO1HL150003, R01HL101200 (Popel, Johns Hopkins, PI), R01GM129074 (Mac Gabhann, Johns Hopkins, PI). VC Ganta is supported by R01HL146673.
Declaration of interest
BH Annex is the founder of Merand Pharmaceuticals which is seeking to develop microRNAs for PAD. VC Ganta received a grant from Merand Pharm. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–84. [DOI] [PubMed] [Google Scholar]
- 2.Dragneva G, Korpisalo P, Yla-Herttuala S. Promoting blood vessel growth in ischemic diseases: challenges in translating preclinical potential into clinical success. Disease Models & Mechanisms. 2013. March;6(2):312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nature Reviews Cancer. 2017. August;17(8):457–474. [DOI] [PubMed] [Google Scholar]
- 4.Ribatti D. Judah Folkman, a pioneer in the study of angiogenesis. Angiogenesis. 2008;11(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent KA, Jiang C, Boltje I, et al. Gene therapy progress and prospects: therapeutic angiogenesis for ischemic cardiovascular disease. Gene Therapy. 2007. May;14(10):781–789. [DOI] [PubMed] [Google Scholar]
- 6.Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. Journal of Molecular and Cellular Cardiology. 2001. March;33(3):379–393. [DOI] [PubMed] [Google Scholar]
- 7.Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nature Reviews Clinical Oncology. 2018. May;15(5):310–324. [DOI] [PubMed] [Google Scholar]
- 8.Harry LE, Paleolog EM. From the cradle to the clinic: VEGF in developmental, physiological, and pathological angiogenesis. Birth Defects Res C Embryo Today. 2003. Nov;69(4):363–74. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005. (94):209–31. [DOI] [PubMed] [Google Scholar]
- 10.Arcondeguy T, Lacazette E, Millevoi S, et al. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Research. 2013. September;41(17):7997–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitt UA, Hsu SY, Hsueh AJW. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Molecular Endocrinology. 2001. May;15(5):681–694. [DOI] [PubMed] [Google Scholar]
- 12.Gong BW, Liang D, Chew TG, et al. Characterization of the zebrafish vascular endothelial growth factor A gene: comparison with vegf-A genes in mammals and Fugu. Biochimica Et Biophysica Acta-Gene Structure and Expression. 2004. January 5;1676(1):33–40. [DOI] [PubMed] [Google Scholar]
- 13.Tammela T, Enholm B, Alitalo K, et al. The biology of vascular endothelial growth factors. Cardiovascular Research. 2005. February 15;65(3):550–563. [DOI] [PubMed] [Google Scholar]
- 14.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011. December;2(12):1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abhinand CS, Raju R, Soumya SJ, et al. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. Journal of Cell Communication and Signaling. 2016. December;10(4):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the Fms-Like Tyrosine Kinase-4 Gene Becomes Restricted to Lymphatic Endothelium during Development. P Natl Acad Sci USA. 1995. April 11;92(8):3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceci C, Atzori MG, Lacal PM, et al. Role of VEGFs/VEGFR-1 Signaling and its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int J Mol Sci. 2020. February 18;21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts DM, Kearney JB, Johnson JH, et al. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004. May;164(5):1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nature Reviews Cardiology. 2013. July;10(7):387–396. [DOI] [PubMed] [Google Scholar]
- 20.Zemaitis MR, Boll JM, Dreyer MA. Peripheral Arterial Disease. StatPearls. Treasure Island (FL) 2020. [PubMed] [Google Scholar]
- 21.Levin SR, Arinze N, Siracuse JJ. Lower extremity critical limb ischemia: A review of clinical features and management. Trends in Cardiovascular Medicine. 2020. Apr;30(3):125–130. [DOI] [PubMed] [Google Scholar]
- 22.Pratt CM. Analysis of the cilostazol safety database. Am J Cardiol. 2001. Jun 28;87(12a):28d–33d. [DOI] [PubMed] [Google Scholar]
- 23.Regensteiner JG, Ware JE Jr., McCarthy WJ, et al. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002. Dec;50(12):1939–46. [DOI] [PubMed] [Google Scholar]
- 24.Abbas R, Chow CP, Browder NJ, et al. In vitro metabolism and interaction of cilostazol with human hepatic cytochrome P450 isoforms. Hum Exp Toxicol. 2000. March;19(3):178–184. [DOI] [PubMed] [Google Scholar]
- 25.Suri A, Bramer SL. Effect of omeprazole on the metabolism of cilostazol. Clin Pharmacokinet. 1999;37:53–59. [DOI] [PubMed] [Google Scholar]
- 26.Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Therapy. 2012. June;19(6):622–629. [DOI] [PubMed] [Google Scholar]
- 27.Makinen K, Manninen H, Hedman M, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: A randomized, placebo-controlled, double-blinded phase II study. Molecular Therapy. 2002. July;6(1):127–133. [DOI] [PubMed] [Google Scholar]
- 28.Kusumanto YH, van Weel V, Mulder NH, et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum Gene Ther. 2006. June;17(6):683–91. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998. March 31;97(12):1114–23. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen HS, Rasmussen CS, Macko J. VEGF gene therapy for coronary artery disease and peripheral vascular disease. Cardiovasc Radiat Med. 2002. Apr-Jun;3(2):114–7. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Mohler E 3rd, Lederman RJ, et al. Regional Angiogenesis with Vascular Endothelial Growth Factor (VEGF) in peripheral arterial disease: Design of the RAVE trial. Am Heart J. 2003. June;145(6):1114–8. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Mohler ER 3rd, Lederman RJ, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003. October 21;108(16):1933–8. [DOI] [PubMed] [Google Scholar]
- 33.Bates DO, Cui TG, Doughty JM, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002. July 15;62(14):4123–31. [PubMed] [Google Scholar]
- 34.Zirlik K, Duyster J. Anti-Angiogenics: Current Situation and Future Perspectives. Oncol Res Treat. 2018;41(4):166–171. [DOI] [PubMed] [Google Scholar]
- 35.Campa C, Harding SP. Anti-VEGF Compounds in the Treatment of Neovascular Age Related Macular Degeneration. Curr Drug Targets. 2011. Feb;12(2):173–181. [DOI] [PubMed] [Google Scholar]
- 36.Jazwa A, Florczyk U, Grochot-Przeczek A, et al. Limb ischemia and vessel regeneration: Is there a role for VEGF? Vascul Pharmacol. 2016. Nov;86:18–30. [DOI] [PubMed] [Google Scholar]
- 37.Rivard A, Silver M, Chen DF, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999. February;154(2):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couffinhal T, Silver M, Kearney M, et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE(−/−) mice. Circulation. 1999. June 22;99(24):3188–3198. [DOI] [PubMed] [Google Scholar]
- 39.Dai QS, Thompson MA, Pippen AM, et al. Alterations in endothelial cell proliferation and apoptosis contribute to vascular remodeling following hind-limb ischemia in rabbits. Vasc Med. 2002. May;7(2):87–91. [DOI] [PubMed] [Google Scholar]
- 40.Dai QS, Huang JH, Klitzman B, et al. Engineered zinc finger-activating vascular endothelial growth factor transcription factor plasmid DNA induces therapeutic angiogenesis in rabbits with hindlimb ischemia. Circulation. 2004. October 19;110(16):2467–2475. [DOI] [PubMed] [Google Scholar]
- 41.Xie DH, Li YJ, Reed EA, et al. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg. 2006. July;44(1):166–175. [DOI] [PubMed] [Google Scholar]
- 42.Li YJ, Hazarika S, Xie DH, et al. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes. 2007. March;56(3):656–665. [DOI] [PubMed] [Google Scholar]
- 43.Aref Z, de Vries MR, Quax PHA. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. Int J Mol Sci. 2019. August;20(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu HY, Li YL, Tran P. A Murine Model of Acute Hindlimb Ischemia/Reperfusion Injury. Faseb J. 2009. April;23. [Google Scholar]
- 45.Sealock R, Zhang H, Lucitti JL, et al. Congenic Fine-Mapping Identifies a Major Causal Locus for Variation in the Native Collateral Circulation and Ischemic Injury in Brain and Lower Extremity. Circ Res. 2014. February 14;114(4):660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okeke E, Dokun AO. Role of genetics in peripheral arterial disease outcomes; significance of limb-salvage quantitative locus-1 genes. Exp Biol Med. 2018. January;243(2):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Md AOD, Keum S, Hazarika S, et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical Hindlimb ischemia. Circulation. 2008. March 4;117(9):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishna SM, Omer SM, Li JZ, et al. Development of a two-stage limb ischemia model to better simulate human peripheral artery disease. Sci Rep-Uk. 2020. February 26;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganta VC, Choi M, Kutateladze A, et al. VEGF165b Modulates Endothelial VEGFR1-STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circ Res. 2017. January 20;120(2):282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kikuchi R, Nakamura K, MacLauchlan S, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nature Medicine. 2014. December;20(12):1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poltorak Z, Cohen T, Neufeld G. The VEGF splice variants: properties, receptors, and usage for the treatment of ischemic diseases. Herz. 2000. Mar;25(2):126–9. [DOI] [PubMed] [Google Scholar]
- 52.Grunstein J, Masbad JJ, Hickey R, et al. Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Molecular and Cellular Biology. 2000. October;20(19):7282–7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JE, Keller GA, Ferrara N. Vascular Endothelial Growth-Factor (Vegf) Isoforms - Differential Deposition into the Subepithelial Extracellular-Matrix and Bioactivity of Extracellular Matrix-Bound Vegf. Molecular Biology of the Cell. 1993. December;4(12):1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates DO, Mavrou A, Qiu Y, et al. Detection of VEGF-A(xxx)b isoforms in human tissues. PLoS One. 2013;8(7):e68399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004. November 1;64(21):7822–35. [DOI] [PubMed] [Google Scholar]
- 56.Kawamura H, Li XJ, Harper SJ, et al. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Research. 2008. June 15;68(12):4683–4692. [DOI] [PubMed] [Google Scholar]
- 57.Catena R, Larzabal L, Larrayoz M, et al. VEGF(1)(2)(1)b and VEGF(1)(6)(5)b are weakly angiogenic isoforms of VEGF-A. Mol Cancer. 2010. December 31;9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008. November;8(11):880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waltenberger J, Claesson-Welsh L, Siegbahn A, et al. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994. October 28;269(43):26988–95. [PubMed] [Google Scholar]
- 60.Sawano A, Takahashi T, Yamaguchi S, et al. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth & Differentiation. 1996. February;7(2):213–221. [PubMed] [Google Scholar]
- 61.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of Blood-Island Formation and Vasculogenesis in Flk-1-Deficient Mice. Nature. 1995. July 6;376(6535):62–66. [DOI] [PubMed] [Google Scholar]
- 62.Fong GH, Rossant J, Gertsenstein M, et al. Role of the Flt-1 Receptor Tyrosine Kinase in Regulating the Assembly of Vascular Endothelium. Nature. 1995. July 6;376(6535):66–70. [DOI] [PubMed] [Google Scholar]
- 63.Hiratsuka S, Minowa O, Kuno J, et al. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. P Natl Acad Sci USA. 1998. August 4;95(16):9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albonici L, Giganti MG, Modesti A, et al. Multifaceted Role of the Placental Growth Factor (PlGF) in the Antitumor Immune Response and Cancer Progression. Int J Mol Sci. 2019. June 2;20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002. December;979:80–93. [DOI] [PubMed] [Google Scholar]
- 66.Lal N, Puri K, Rodrigues B. Vascular Endothelial Growth Factor B and Its Signaling. Frontiers in Cardiovascular Medicine. 2018. April 20;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao RH, Xue Y, Hedlund EM, et al. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. P Natl Acad Sci USA. 2010. Jan 12;107(2):856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pipp F, Heil M, Issbrucker K, et al. VEGFR-1-selective VEGF homologue PlGF is arteriogenic - Evidence for a monocyte-mediated mechanism. Circ Res. 2003. March 7;92(4):378–385. [DOI] [PubMed] [Google Scholar]
- 69.Boscolo E, Mulliken JB, Bischoff J. VEGFR-1 Mediates Endothelial Differentiation and Formation of Blood Vessels in a Murine Model of Infantile Hemangioma. Am J Pathol. 2011. November;179(5):2266–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robciuc MR, Kivela R, Williams IM, et al. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metabolism. 2016. April 12;23(4):712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park K, Amano H, Ito Y, et al. Vascular endothelial growth factor receptor 1 (VEGFR1) tyrosine kinase signaling facilitates granulation tissue formation with recruitment of VEGFR1(+) cells from bone marrow. Journal of Pharmacological Sciences. 2016. March;130(3):S115–S115. [DOI] [PubMed] [Google Scholar]
- 72.Amano H, Mastui Y, Ito Y, et al. The role of vascular endothelial growth factor receptor 1 tyrosine kinase signaling in bleomycin-induced pulmonary fibrosis. Biomedicine & Pharmacotherapy. 2019. September;117. [DOI] [PubMed] [Google Scholar]
- 73.Murakami M, Zheng YJ, Hirashima M, et al. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arteriosclerosis Thrombosis and Vascular Biology. 2008. April;28(4):658–664. [DOI] [PubMed] [Google Scholar]
- 74.Amano H, Kato S, Ito Y, et al. The Role of Vascular Endothelial Growth Factor Receptor-1 Signaling in the Recovery from Ischemia. Plos One. 2015. July 2;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sselvaraj D, Gangadharan V, Michalski CW, et al. A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain (vol 27, pg 780, 2015). Cancer Cell. 2015. August 10;28(2):270–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao J, Wu XM, Zhuang GL, et al. Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. P Natl Acad Sci USA. 2011. Jul 12;108(28):11590–11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekiguchi K, Ito Y, Hattori K, et al. VEGF Receptor 1-Expressing Macrophages Recruited from Bone Marrow Enhances Angiogenesis in Endometrial Tissues. Sci Rep-Uk. 2019. May 7;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lisi L, Ciotti GMP, Chiavari M, et al. Vascular endothelial growth factor receptor 1 in glioblastoma-associated microglia/macrophages. Oncology Reports. 2020. June;43(6):2083–2092. [DOI] [PubMed] [Google Scholar]
- 79.Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cellular & Molecular Immunology. 2020. September 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Frontiers in Physiology. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jetten N, Donners MMPC, Wagenaar A, et al. Local Delivery of Polarized Macrophages Improves Reperfusion Recovery in a Mouse Hind Limb Ischemia Model. Plos One. 2013. July 24;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong HL, Tian XY. The Role of Macrophages in Vascular Repair and Regeneration after Ischemic Injury. Int J Mol Sci. 2020. September;21(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gallagher KA. Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia INVITED COMMENTARY. J Vasc Surg. 2018. June;67(6):1920–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganta VC, Choi MH, Kutateladze A, et al. A MicroRNA93-Interferon Regulatory Factor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Ischemic Muscle. Circulation. 2017. June 13;135(24):2403-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murray PJ. Macrophage Polarization. Annual Review of Physiology, Vol 79. 2017;79:541–566. [DOI] [PubMed] [Google Scholar]
- 86.Hoeksema MA, Glass CK. Nature and nurture of tissue-specific macrophage phenotypes. Atherosclerosis. 2019. February;281:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das A, Sinha M, Datta S, et al. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am J Pathol. 2015. October;185(10):2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daemen S, Schilling JD. The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity. Frontiers in Immunology. 2020. January 22;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goerdt S, Politz O, Schledzewski K, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67(5–6):222–226. [DOI] [PubMed] [Google Scholar]
- 90.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014. July 17;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guilliams M, van de Laar L. A Hitchhiker’s Guide to Myeloid Cell Subsets: Practical Implementation of a Novel Mononuclear Phagocyte Classification System. Front Immunol. 2015;6:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou JW, Tang ZW, Gao SY, et al. Tumor-Associated Macrophages: Recent Insights and Therapies. Frontiers in Oncology. 2020. February 25;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016. May;59(5):879–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takei Y, Arteel GE, Bergheim I, et al. Roles of Kupffer cells in alcoholic liver disease. Alcoholism-Clinical and Experimental Research. 2005. June;29(6):1116–1120. [Google Scholar]
- 96.Thorbecke GJ, Silberberg-Sinakin I, Flotte TJ. Langerhans cells as macrophages in skin and lymphoid organs. J Invest Dermatol. 1980. July;75(1):32–43. [DOI] [PubMed] [Google Scholar]
- 97.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018. April;18(4):225–242. [DOI] [PubMed] [Google Scholar]
- 98.Ganta VC, Choi M, Farber CR, et al. Antiangiogenic VEGF(165)b Regulates Macrophage Polarization via S100A8/S100A9 in Peripheral Artery Disease. Circulation. 2019. January 8;139(2):226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okabe Y, Medzhitov R. Tissue-Specific Signals Control Reversible Program of Localization and Functional Polarization of Macrophages. Cell. 2014. May 8;157(4):832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guilliams M, Mildner A, Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity. 2018. October 16;49(4):595–613. [DOI] [PubMed] [Google Scholar]
- 101.Hamers AAJ, Dinh HQ, Thomas GD, et al. Human Monocyte Heterogeneity as Revealed by High-Dimensional Mass Cytometry. Arteriosclerosis Thrombosis and Vascular Biology. 2019. January;39(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature Reviews Immunology. 2005. December;5(12):953–964. [DOI] [PubMed] [Google Scholar]
- 103.Ong SM, Teng KR, Newell E, et al. A Novel, Five-Marker Alternative to CD16-CD14 Gating to Identify the Three Human Monocyte Subsets. Frontiers in Immunology. 2019. July 26;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shantsila E, Tapp LD, Wrigley BJ, et al. Monocyte subsets in coronary artery disease and their associations with markers of inflammation and fibrinolysis. Atherosclerosis. 2014. May;234(1):4–10. [DOI] [PubMed] [Google Scholar]
- 105.Wildgruber M, Aschenbrenner T, Wendorff H, et al. The “Intermediate” CD14(++)CD16(+) monocyte subset increases in severe peripheral artery disease in humans. Sci Rep-Uk. 2016. December 19;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hopfner F, Jacob M, Ulrich C, et al. Subgroups of monocytes predict cardiovascular events in patients with coronary heart disease. The PHAMOS trial (Prospective Halle Monocytes Study). Hellenic Journal of Cardiology. 2019. Sep-Oct;60(5):311–321. [DOI] [PubMed] [Google Scholar]
- 107.Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14(++)CD16(−) Monocytes Predict Cardiovascular Events. Circulation-Cardiovascular Genetics. 2012. February;5(1):122–131. [DOI] [PubMed] [Google Scholar]
- 108.Chaitanya GV, Cromer WE, Parker CP, et al. A Recombinant Inhibitory Isoform of Vascular Endothelial Growth Factor(164/165) Aggravates Ischemic Brain Damage in a Mouse Model of Focal Cerebral Ischemia. Am J Pathol. 2013. September;183(3):1010–1024. [DOI] [PubMed] [Google Scholar]
- 109.Manetti M, Guiducci S, Ibba-Manneschi L, et al. Impaired Angiogenesis in Systemic Sclerosis: The Emerging Role of the Antiangiogenic VEGF(165)b Splice Variant. Trends in Cardiovascular Medicine. 2011. Oct;21(7):204–210. [DOI] [PubMed] [Google Scholar]
- 110.Magnussen AL, Churchill A, Harper S, et al. VEGF165b is more potent at inhibiting endothelial cell migration than Pegabtanib and is cytoprotective for retinal pigmented epithelial cells. Faseb J. 2008. April;22. [Google Scholar]
- 111.Konopatskaya O, Churchill A, Harper S, et al. VEGF(165)b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Molecular Vision. 2006. May 26;12(67–69):626–632. [PubMed] [Google Scholar]
- 112.Hirigoyen D, Burgos PI, Mezzano V, et al. Inhibition of angiogenesis by platelets in systemic sclerosis patients. Arthritis Research & Therapy. 2015. November 19;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gurbel PA, Fox KAA, Tantry US, et al. Combination Antiplatelet and Oral Anticoagulant Therapy in Patients With Coronary and Peripheral Artery Disease Focus on the COMPASS Trial. Circulation. 2019. April 30;139(18):2170–2185. [DOI] [PubMed] [Google Scholar]
- 114.Lee WH, Chu CY, Hsu PC, et al. Comparison of Antiplatelet and Antithrombotic Therapy for Secondary Prevention of Ischemic Stroke in Patients With Peripheral Artery Disease - Population-Based Follow-up Study in Taiwan. Circulation Journal. 2013. April;77(4):1046–1052. [DOI] [PubMed] [Google Scholar]
- 115.Melfi R, Ricottini E. Antiplatelet therapy for peripheral artery disease. Cardiovascular Diagnosis and Therapy. 2018. October;8(5):663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006. October 26;443(7114):993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ebos JML, Bocci G, Man S, et al. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004. June;2(6):315–326. [PubMed] [Google Scholar]
- 118.Mouawad R, Spano JP, Comperat E, et al. Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: Correlation with clinical parameters and outcome. Eur J Cancer. 2009. May;45(8):1407–1414. [DOI] [PubMed] [Google Scholar]
- 119.Gagnon ML, Bielenberg DR, Gechtman Z, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. P Natl Acad Sci USA. 2000. March 14;97(6):2573–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parker MW, Linkugel AD, Goel HL, et al. Structural Basis for VEGF-C Binding to Neuropilin-2 and Sequestration by a Soluble Splice Form. Structure. 2015. April 7;23(4):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hazarika S, Dokun AO, Li Y, et al. Impaired angiogenesis after Hindlimb ischemia in type 2 diabetes Mellitus - Differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007. October 26;101(9):948–956. [DOI] [PubMed] [Google Scholar]
- 122.Wieczor R, Gadomska G, Ruszkowska-Ciastek B, et al. Impact of type 2 diabetes on the plasma levels of vascular endothelial growth factor and its soluble receptors type 1 and type 2 in patients with peripheral arterial disease. J Zhejiang Univ-Sc B. 2015. November;16(11):948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orecchia A, Lacal PM, Schietroma C, et al. Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the alpha 5 beta 1 integrin. J Cell Sci. 2003. September 1;116(17):3479–3489. [DOI] [PubMed] [Google Scholar]
- 124.Tripathi R, Ralhan R, Saxena S, et al. Soluble VEGFR-1 in pathophysiology of pregnancies complicated by hypertensive disorders: the Indian scenario. J Hum Hypertens. 2013. Feb;27(2):107–114. [DOI] [PubMed] [Google Scholar]
- 125.Hornig C, Barleon B, Ahmad S, et al. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000. April;80(4):443–454. [DOI] [PubMed] [Google Scholar]
- 126.Kendall RL, Thomas KA. Inhibition of Vascular Endothelial-Cell Growth-Factor Activity by an Endogenously Encoded Soluble Receptor. P Natl Acad Sci USA. 1993. November 15;90(22):10705–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008. October 15;121(20):3487–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Batson J, Toop HD, Redondo C, et al. Development of Potent, Selective SRPK1 Inhibitors as Potential Topical Therapeutics for Neovascular Eye Disease. Acs Chemical Biology. 2017. March;12(3):825–832. [DOI] [PubMed] [Google Scholar]
- 129.Amin EM, Oltean S, Hua J, et al. WT1 Mutants Reveal SRPK1 to Be a Downstream Angiogenesis Target by Altering VEGF Splicing. Cancer Cell. 2011. December 13;20(6):768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoon C, Kim D, Kim S, et al. MiR-9 regulates the post-transcriptional level of VEGF165a by targeting SRPK-1 in ARPE-19 cells. Graefes Archive for Clinical and Experimental Ophthalmology. 2014. September;252(9):1369–1376. [DOI] [PubMed] [Google Scholar]
- 131.Meadows KL, Hurwitz HI. Anti-VEGF Therapies in the Clinic. Cold Spring Harbor Perspectives in Medicine. 2012. October;2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Desideri LF, Traverso CE, Nicolo M. Abicipar pegol: an investigational anti-VEGF agent for the treatment of wet age-related macular degeneration. Expert Opinion on Investigational Drugs. 2020. Jul 2;29(7):651–658. [DOI] [PubMed] [Google Scholar]
- 133.Barakat MR, Kaiser PK. VEGF inhibitors for the treatment of neovascular age-related macular degeneration. Expert Opinion on Investigational Drugs. 2009. May;18(5):637–646. [DOI] [PubMed] [Google Scholar]
- 134.Kolls BJ, Sapp S, Rockhold FW, et al. Stroke in Patients With Peripheral Artery Disease. Stroke. 2019. June;50(6):1356–1363. [DOI] [PubMed] [Google Scholar]
- 135.Olivier CB, Mulder H, Hiatt WR, et al. Incidence, Characteristics, and Outcomes of Myocardial Infarction in Patients With Peripheral Artery Disease: Insights From the EUCLID Trial. JAMA Cardiol. 2019. January 1;4(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annual Review of Pathology: Mechanisms of Disease, Vol 15, 2020. 2020;15:123–147. [DOI] [PMC free article] [PubMed] [Google Scholar]