Abstract
Objective: This network meta-analysis will provide a complete toxicity profile, toxicity profile, and safety ranking of immune checkpoint inhibitors (ICIs) for treatment of advanced non-small cell lung cancer (NSCLC).
Methods: We found 12 phase II or III randomized clinical trials (RCTs) including 8,453 patients with NSCLC by searching Pubmed, Embase, and ClinicalTrials.gov. Risk ratios (RRs) and 95% confidence interval (CI) were used to compare the rate of immune-related adverse events (irAEs) for different ICIs-based treatments using pairwise and network meta-analysis with random effects.
Results: For dermatologic irAEs, the corresponding ranking of incidences of the seven groups from high to low was: nivolumab + ipilimumab (97.4%), pembrolizumab (80.1%), nivolumab (67.1%), pembrolizumab + platinum (43.3%), atezolizumab + platinum (39.9%), durvalumab (17.5%), platinum-based chemotherapy (4.7%). For colitis, the corresponding ranking of incidences of the six groups from high to low was: atezolizumab + platinum (77.1%), nivolumab (67.3%), pembrolizumab (60.5%), durvalumab (45.2%), pembrolizumab + platinum (41.4%), platinum-based chemotherapy (8.5%). For endocrine irAEs, the corresponding ranking of incidences of the seven groups from high to low was: nivolumab + ipilimumab (79.1%), durvalumab (69.1%), pembrolizumab (61.9%), atezolizumab + platinum (60.4%),nivolumab (45.7%), pembrolizumab + platinum (33.5%), platinum-based chemotherapy (0.3%). For pneumonitis, the corresponding ranking of incidences of the seven groups from high to low was: pembrolizumab (99.3%), pembrolizumab + platinum (65.1%), durvalumab (62.2%), atezolizumab + platinum (56%), nivolumab (35.9%), platinum-based chemotherapy (18.1%),atezolizumab (13.3%). For hepatitis, the corresponding ranking of incidences of the six groups from high to low was: pembrolizumab (71.2%), pembrolizumab + platinum (64.3%), durvalumab (56.4%), atezolizumab + platinum (53.8%), nivolumab (44.5%), platinum-based chemotherapy (9.8%).
Conlusion: In addition to platinum-based chemotherapy, durvalumab for dermatologic and liver irAEs, pembrolizumab for gastrointestinal irAEs, pembrolizumab + platinum for endocrine irAEs, and atezolizumab for pneumonitis may be associated with lower rates of irAEs than other immune-based regimens. Nivolumab + ipilimumab for dermatologic and endocrine irAEs, atezolizumab + platinum for colitis, and pembrolizumab for pneumonitis and hepatitis may be associated with higher rates of irAEs.
Keywords: immune-related adverse events, non-small cell lung cancer, network meta-analysis, immune checkpoint inhibitor, randomized clinical trial
Introduction
According to the latest statistics, lung cancer is still the leading cause of cancer deaths, although the incidence of cancer has declined in recent years (Goldstraw et al., 2016; Siegel et al., 2020). Nearly 70% of patients with lung cancer are diagnosed with locally advanced or metastatic disease (Torre et al., 2016), and the 5-years survival rate is only 5% (Molina et al., 2008; Bironzo and Di Maio, 2018). However around 80–85% of lung cancer cases are classified as NSCLC (Torre et al., 2015). Platinum-based chemotherapy remains the standard first-line treatment for NSCLC without target gene mutations, with a response rate of only 15% (Sandler et al., 2006; Scagliotti et al., 2008).
In recent years ICIs, which constitute an inhibitory pathway detected in a variety of malignant tumors, have opened a new era of cancer treatment (Ettinger et al., 2017; Hanna et al., 2017). Tumor cells form immune escape through immune checkpoints, however ICIs are to prevent the immune escape caused by the tumor cells by combining with the immune checkpoint, thus enabling the immune cells to resume their killing effect on the tumor cells. Currently, programmed cell death receptor 1(PD-1) and cytotoxic T lymphocyte-associated antigen-4(CTLA4) are widely studied immune checkpoints. PD-1 is a type I transmembrane glycoprotein (He et al., 2015), which is an inhibitory receptor of T cells. It can bind and interact with the specific ligands programmed cell death ligand-1(PD-L1) and programmed cell death ligand-2 (PD-L2). Tumor cells can activate PD-1, thereby promoting it to bind to PD-L1 and PD-L2 on the surface of antigen presenting cells, inhibiting the proliferation of effector T cells and preventing T cells from recognizing dangerous molecules in a timely and effective manner, thus enabling tumor cells to evade the pursuit of immune cells. PD-1/PD-L1 inhibitors promote the activation and proliferation of T cells by inhibiting the expression of PD-1/PD-L1, thus stimulating the killing of tumor cells (Pedoeem et al., 2014). CTLA-4 is a leukocyte differentiation antigen and a transmembrane receptor on T cells. It shares the B7 molecular ligand with CD28, while CTLA-4 induces T cells to be inreactive after binding to B7 molecule, and participates in the negative regulation of immune response. However CTLA-4 inhibitors can inhibit the molecule CTLA-4, allowing T cells to proliferate and attack tumor cells. Data from a series of randomized clinical trials suggest that ICIs alone or in combination as first-line treatment for advanced NSCLC patients provides better clinical benefits and fewer side effects than conventional platinum-based chemotherapy (Corey et al., 2016; Reck et al., 2016; Carbone et al., 2017; Barlesi et al., 2018; Gandhi et al., 2018; Paz-Ares et al., 2018; Hellmann et al., 2019; Tony et al., 2019; West et al., 2019; Jotte et al., 2020; Naiyer et al., 2020; Roy et al., 2020). However, ICIs enhance self immune functions against cancer cells through a unique mechanism that blocks negative regulators expressed on immune or tumor cells, while yielding higher rates of irAEs than platinum-based chemotherapy (Remon et al., 2020). According to statistics, the occurrence of grade 3 or higher irAEs varied from 8 to 10% among patients with advanced NSCLC receiving ICIs (Remon et al., 2020). Common target organs of irAEs included dermatologic irAEs (pruritus and rash), endocrine irAEs (hypothyroidism and hyperthyroidism), colitis, pneumonitis and hepatitis (Wang et al., 2018). Any of these irAEs, if not properly treated and managed in clinical practice, irAEs will lead to treatment termination, failure, and may even be life-threatening. Therefore, it was very necessary for clinicians to better grasp the irAEs most likely to be caused by each treatment regime, and to prevent, detect and treat them as early as possible. However, which treatment regime was more likely to induce irAEs was controversial.
Here, to help clinicians improve early prediction, early detection and early treatment of irAEs, a network meta-analysis was conducted to compare the incidences of irAEs and rank the safety of ICI + chemotherapy, ICI alone, and dual ICIs combination.
Methods
Data Sources and Searches
This network meta-analysis (NMA) was based entirely on the preferred reporting items for systematic reviews and meta-analysis (PRISMA) (Liberati et al., 2009; Hutton et al., 2015) and PRISMA extended guidelines for an NMA. RCTs on ICIs versus platinum-based chemotherapy as the first-line treatment of advanced NSCLC from 2015 to 2020 were searched in PubMed, Embase. To search for data not explicitly given in the RCTs, the National Institutes of Health ongoing Trial Registry (Clinicaltrials.gov) was also searched. A combination of MeSH and free-text words was searched according to the PICOS principle.Search terms and their combinations used in the search strategy included (PD-1 OR PD-L1 OR CTLA-4 inhibitor OR nivolumab OR pembrolizumab OR durvalumab OR atezolizumab OR ipilimumab) AND (Non-Small-Cell Lung Carcinoma OR Non-Small Cell Lung Cancer) AND chemotherapy AND (randomized controlled trial). Two independent reviewers (Jingjing Gu and Weidong Zhang) conducted a preliminary screening of the searched topics and abstracts, and if they did not meet the criteria, further read the full text, and all references were evaluated as potentially relevant articles.
Inclusion and Exclusion Criteria
Inclusion criteria for RCTs of this NMA were as follows: 1) study type: only Phase II or III double blind RCTs for advanced NSCLC; 2) participants: included patients that were pathologically diagnosed as advanced NSCLC; 3) experimental group: patients were treated with immunotherapy alone or an immune-based combination as first-line treatment, control group: patients were treated with only platinum-based chemotherapy as first-line treatment; 4) outcome indicators: there was at least one irAE in the RCTs or searched on Clinicaltrials.gov.
Exclusion criteria: non-English articles, studies without valid data, reviews, meta-analyses, editorials, commentary letters, repeat studies.
Data Extraction
By reading the titles, abstracts and full texts, the two authors screened out the articles that met the predetermined inclusion criteria. 1) Trial information, including first author, study year, trial id; 2) Stage information, study endpoint, and sample size of treatment; 3) Patient characteristics at baseline included median age, sex, and the numbers of patients with dermatologic irAEs, colitis, endocrine irAEs, pneumonitis and hepatitis (grade 1–5). And the two authors checked the extracted data before data analysis.
Risk-Of-Bias Assessment
The Cochrane risk bias assessment tool (Higgins and Green, 2011) (Figure1)were used to evaluate the quality of the literature by two reviewers (Jingjing Gu and Weidong Zhang). The seven major sources of biases (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) were classified into three grades including “yellow represents unclear risk”, “green represents low risk” and “red represents high risk” which were assessed using the Cochrane risk bias assessment tool.
FIGURE 1.
The Cochrane risk bias assessment tool was used to evaluate bias from seven key sources: 1. Random sequence generation; 2. Allocation concealment; 3. Blindness of subjects and researchers; 4. Blindness of outcome evaluation; 5. Incomplete data; 6. Selective reporting of results; 7. Other biases. Green represents low risk, yellow represents unclear risk, and red represents high risk. (A) Risk of bias graph; (B) Risk of bias summary.
Outcome Measures
The study endpoints were any grade of dermatologic irAEs including pruritus and rash, colitis, endocrine irAEs including hypothyroidism and hyperthyroidism, pneumonitis and hepatitis. Review criteria were based on the National Cancer Institute Common Terminology Criteria for Adverse Events (Basch et al., 2016). The probability of associated adverse events for each treatment regimen was assessed by the combined RRs and 95% CI.
Data Synthesis and Statistical Analysis
The loops to illustrate the network geometry were generated using Stata13.0. In this NMA, summary RRs and 95% confidence intervals were used as the results of the effect size of ICI-based drugs on the risk of irAEs in NSCLC patients. RR greater than 1 represented a low probability of irAEs in the control group. Markov chain Monte Carlo (MCMC) methods were used to establish random-effects and consistency models calculating the RR and 95% confidence intervals within the Bayesian framework by using R (version 4.0.1) (CoreTeam 2019, Vienna, Austria) and JAGS (version 4.3.0) with the package “getmtc” (version 0.8.2) (Salanti et al., 2008; Sutton et al., 2008). Heterogeneity of the included studies was assessed by I2 statistics. I2 values below 25%, between 25 and 50%, and above 50% represented low, medium, and high heterogeneity, respectively (Mills et al., 2013). If the heterogeneity is low, the fixed effects model would be selected, otherwise the random effects model would be selected. In order to explore the inter-study heterogeneity of each outcome comparison, the values of different parameters of a log-normal distribution were fitted as a prior distribution (Higgins et al., 2003). To obtain the posterior distribution, 10,000 burn-ins and 50,000 iterations of 4 each chain and a thinning interval of 10 were generated for each outcome by using MCMC methods. We judged whether each MCMC chain reached a stable and good iteration during the calculation process through the Brookse-Gelmane-Rubin diagnostic plot with a cut-off value of 1, so as to determine whether the degree of convergence of the model was satisfactory. The surface under the cumulative ranking curve (SUCRA) metric was a tool to rank the probabilities of irAEs of each treatment and identify the best treatment. The SUCRA value was ranged from 0 to 1, the closer to 1 this value approached, the higher its incidence of an immune-related adverse event was in this network meta-analysis (Salanti et al., 2011).A funnel plot was used to assess the publication bias and symmetrical distribution in funnel plot suggests no publication bias (Higgins et al., 2012). Sensitivity analysis was performed to assess the stability of the results which were considered stable if there was significant consistency between direct and indirect results (Dias et al., 2010). We conducted a inconsistency test (that is, the comparison of the differences between direct and indirect comparisons) using the nodal analysis and p > 0.05 indicated that there is no inconsistency (Sterne and Egger, 2001; Furukawa et al., 2016).
Results
Literature Search Results and Study Characteristics
Through the literature search, 539 studies were initially retrieved. After removing duplicates, 352 studies were used to filter through screening titles and abstracts, then 39 studies were assessed by screening full text. Finally, 12 RCTs (Reck et al., 2016; Corey et al., 2016; Carbone et al., 2017; Barlesi et al., 2018; Paz-Ares et al., 2018; Gandhi et al., 2018; Hellmann et al., 2019; Tony et al., 2019; West et al., 2019; Roy et al., 2020; Jotte et al., 2020; Naiyer et al., 2020) including 8,453 patients with advanced NSCLC were considered eligible for inclusion in this network meta-analysis. The literature retrieval strategy is shown in Figure 2. The included RCTs involved 1 Phase Ⅰ/Ⅱ trial and 11 Phase III trials and described eight treatment regimes (pembrolizumab, nivolumab, durvalumab, atezolizumab, pembrolizumab + platinum, atezolizumab + platinum, nivolumab + ipilimumab and platinum-based chemotherapy). The Network diagrams of comparisons on all outcomes in this network meta-analysis are presented in Figure 3. The dermatologic irAEs (pruritus and rash) and endocrine irAEs (hypothyroidism and hyperthyroidism) involved seven different treatment regimens (pembrolizumab, nivolumab, durvalumab, pembrolizumab + platinum, atezolizumab + platinum, nivolumab + ipilimumab and platinum-based chemotherapy) in 11 studies (Reck et al., 2016; Corey et al., 2016; Carbone et al., 2017; Paz-Ares et al., 2018; Gandhi et al., 2018; Barlesi et al., 2018; Tony et al., 2019; West et al., 2019; Hellmann et al., 2019; Naiyer et al., 2020; Jotte et al., 2020) (Figure 3A).The pneumonitis involved seven different treatment regimens (pembrolizumab, nivolumab, durvalumab, atezolizumab, pembrolizumab + platinum, atezolizumab + platinum, nivolumab + ipilimumab and platinum-based chemotherapy) in 11 studies (Reck et al., 2016; Corey et al., 2016; Carbone et al., 2017; Paz-Ares et al., 2018; Gandhi et al., 2018; Barlesi et al., 2018; West et al., 2019; Tony et al., 2019; Naiyer et al., 2020; Jotte et al., 2020; Roy et al., 2020) (Figure3B). Colitis and hepatitis involved six different treatment regimens (pembrolizumab, nivolumab, durvalumab, atezolizumab, pembrolizumab + platinum, atezolizumab + platinum and platinum-based chemotherapy) in 10 studies (Corey et al., 2016; Reck et al., 2016; Carbone et al., 2017; Barlesi et al., 2018; Gandhi et al., 2018; Paz-Ares et al., 2018; Tony et al., 2019; West et al., 2019; Jotte et al., 2020; Naiyer et al., 2020) (Figure3C). Key features of all the studies are shown in Table 1 and Table 2.
FIGURE 2.
Flowchart of the systematic search PRISMA flow diagram.
FIGURE 3.
Network diagrams of comparisons on all outcomes in this network meta-analysis. (A) Comparisons on dermatologic and endocrine irAEs in patients with advanced NSCLC. (B) Comparisons on pneumonitis in patients with advanced NSCLC. (C) Comparisons on colitis and hepatitis in patients with advanced NSCLC.(The circles represent treatment regimens and the size of each circle represents the number of participants, while the yellow line represents double-blind RCTs and the green is not blind.)
TABLE 1.
General characteristics of the included randomised control trials for this network meta-analyses (Abbreviations: MN,multinational; NA, not applicable.)
| First author, year | Study ID | Region | Trial phase | Trail number | Experimental group | Control group |
|---|---|---|---|---|---|---|
| Carbone 2017 | Checkmate 026 | MN | II | 423 | Nivolumab 3 mg/kg | Platinum-based chemotherapy |
| Naiyer 2020 | Mystic | MN | III | 721 | Durvalumab 20 mg/kg | Platinum-based chemotherapy |
| Paz-Ares 2018 | Keynote-407 | United States | III | 559 | Pembrolizumab 200 mg + platinum-based chemotherapy | Platinum-based chemotherapy |
| Gandhi 2018 | Keynote-189 | MN | III | 616 | Pembrolizumab 200 mg + platinum-based chemotherapy | Platinum-based chemotherapy |
| Mok 2019 | Keynote-042 | MN | III | 1,274 | Pembrolizumab 200 mg | Platinum-based chemotherapy |
| Reck 2019 | Keynote-024 | MN | III | 305 | Pembrolizumab 200 mg | Platinum-based chemotherapy |
| Borghaei 2016 | Keynote-021 | United States, Taiwan | I/II | 123 | Pembrolizumab 200 mg + platinum-based Chemotherapy | Platinum-based chemotherapy |
| Rivero 2018 | Impower 132 | MN | Ⅲ | 578 | Atezolizumab 1200 mg + platinum-based Chemotherapy | Platinum-based chemotherapy |
| Robert 2018 | Impower 131 | MN | Ⅲ | 1,021 | Atezolizumab 1200 mg + platinum-based Chemotherapy | Platinum-based chemotherapy |
| West 2019 | Impower 130 | MN | Ⅲ | 724 | Atezolizumab 1200 mg + platinum-based Chemotherapy | Platinum-based chemotherapy |
| Spigel 2019 | Impower 110 | MN | III | 572 | Atezolizumab 1200 mg | Platinum-based chemotherapy |
| Hellmann 2018 | Checkmate 227 | MN | III | 1,537 | Nivolumab 3 mg/kg Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg | Platinum-based chemotherapy |
TABLE 2.
Patient characteristics and extracted data for study end-points in the included randomised controlled trials.
| Study ID | Treatment | Trail number | Dermatologic irAEs (pruritus and rash | Colitis | Endocrine irAEs (hypothyroidism and hyperthyroidism | Pneumonitis | Hepatitis |
|---|---|---|---|---|---|---|---|
| Checkmate 026 | Nivolumab 3 mg/kg | 267 | 92 | 3 | 20 | 23 | 60 |
| Platinum-based chemotherapy | 263 | 48 | 0 | 7 | 17 | 36 | |
| Mystic | Durvalumab 20 mg/kg | 369 | 107 | 1 | 40 | 26 | 33 |
| Platinum-based chemotherapy | 352 | 88 | 0 | 4 | 10 | 37 | |
| Keynote-407 | Pembrolizumab 200 mg + platinum-based chemotherapy | 278 | 92 | 7 | 82 | 18 | 5 |
| Platinum-based chemotherapy | 280 | 58 | 4 | 14 | 6 | 0 | |
| Keynote-189 | Pembrolizumab 200 mg + platinum-basedchemotherapy | 405 | 145 | 9 | 43 | 18 | 5 |
| Platinum-based chemotherapy | 202 | 50 | 0 | 11 | 5 | 0 | |
| Keynote-042 | Pembrolizumab 200 mg | 636 | 107 | 7 | 116 | 53 | 9 |
| Platinum-based chemotherapy | 615 | 44 | 2 | 13 | 3 | 0 | |
| Keynote-024 | Pembrolizumab 200 mg | 154 | 42 | 6 | 27 | 12 | 1 |
| Platinum-based chemotherapy | 150 | 6 | 0 | 5 | 1 | 0 | |
| Keynote-021 | Pembrolizumab 200 mg + platinum-based chemotherapy | 59 | 22 | 2 | 14 | 3 | 1 |
| Platinum-based chemotherapy | 62 | 11 | 0 | 4 | 0 | 0 | |
| Impower 132 | Atezolizumab 1200 mg + platinum-based chemotherapy | 291 | 75 | 5 | 23 | 16 | 3 |
| Platinum-based chemotherapy | 274 | 60 | 0 | 6 | 6 | 1 | |
| Impower 131 | Atezolizumab 1200 mg + platinum-based chemotherapy | 334 | 74 | 6 | 34 | 23 | 4 |
| Platinum-based chemotherapy | 334 | 39 | 0 | 4 | 5 | 0 | |
| Impower 130 | Atezolizumab 1200 mg + platinum-based chemotherapy | 473 | 119 | 4 | 53 | 28 | 3 |
| Platinum-based chemotherapy | 232 | 28 | 0 | 1 | 14 | 0 | |
| Impower 110 | Atezolizumab 1200 mg | 286 | NA | NA | NA | 14 | NA |
| Platinum-based chemotherapy | 263 | NA | NA | NA | 17 | NA | |
| Checkmate 227 | Nivolumab 3 mg/kg | 576 | 73 | NA | NA | NA | NA |
| Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg | 391 | 117 | NA | NA | NA | NA | |
| Platinum-based chemotherapy | 570 | 34 | NA | NA | NA | NA |
(Abbreviations: irAE, immune-related adverse event; MN,multinational; NA, not applicable.).
Head-To-Head Comparisons for the Endpoints
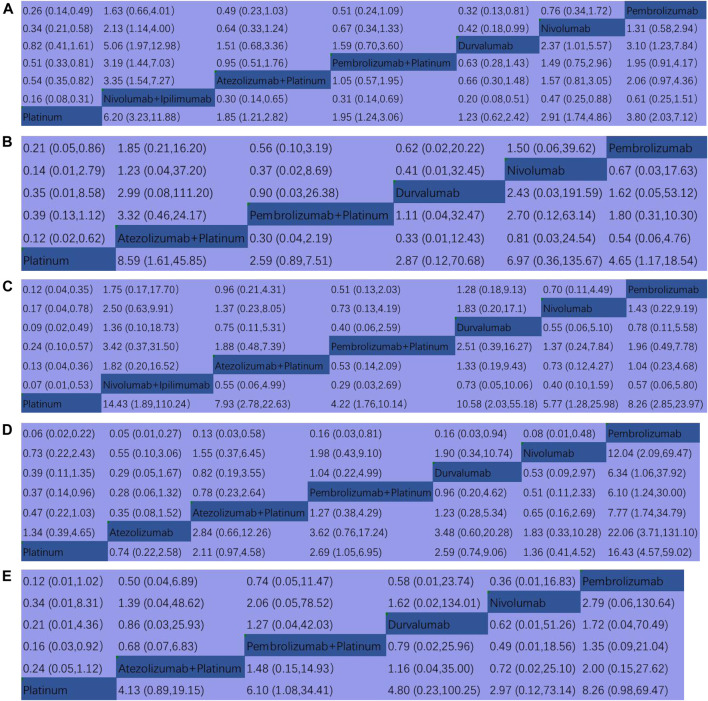
In terms of dermatologic irAEs, platinum-based chemotherapy had the lowest rate compared to durvalumab (RR, 1.23, 95% CI, 0.62–2.42), atezolizumab + platinum (RR, 1.85, 95% CI, 1.21–2.82), pembrolizumab + platinum (RR, 1.95,95% CI, 1.24–3.06), nivolumab (RR, 2.91, 95%CI, 1.74–4.86), pembrolizumab (RR, 3.80, 95% CI, 2.03–7.12), nivolumab + ipilimumab (RR, 6.20, 95% CI, 3.23–11.88) (Figure 4A). In terms of colitis, platinum-based chemotherapy had the lowest rate compared to pembrolizumab + platinum (RR, 2.59, 95% CI, 0.89–7.51), durvalumab (RR, 2.87, 95% CI, 0.12–70.68), pembrolizumab (RR, 4.65, 95% CI, 1.17–18.54), nivolumab (RR, 6.97, 95% CI, 0.36–135.67), atezolizumab + platinum (RR, 8.59, 95% CI, 1.61–45.85), (Figure 4B). For endocrine irAEs, platinum-based chemotherapy had the lowest rate compared to pembrolizumab + platinum (RR, 4.22, 95% CI, 1.76–10.14), nivolumab (RR, 5.77, 95% CI, 1.28–25.98), Atezolizumab + platinum (RR, 7.93, 95% CI, 2.78–22.63), pembrolizumab (RR, 8.26, 95% CI, 2.85–23.97), durvalumab (RR, 10.58, 95% CI, 2.03–55.18), and nivolumab + ipilimumab (RR, 14.43, 95% CI, 1.89–110.2) (Figure 4C). For pneumonitis, atezolizumab had the lowest rate compared to platinum-based chemotherapy (RR,1.34, 95% CI, 0.39–4.65), nivolumab (RR, 1.83, 95% CI, 0.33–10.28), atezolizumab + platinum (RR, 2.84, 95% CI, 0.66–12.26), durvalumab (RR, 3.48, 95% CI, 0.60–20.28), pembrolizumab + platinum (RR, 3.62, 95% CI, 0.76–17.24), and pembrolizumab (RR, 22.06, 95% CI, 3.71–131.10) (Figure 4D). For hepatitis, pembrolizumab + platinum had the lowest rate compared to nivolumab (RR, 2.97, 95% CI, 0.12–73.14), atezolizumab + platinum (RR, 4.13, 95% CI, 0.89–19.15), durvalumab (RR, 4.80, 95% CI, 0.23–100.25), pembrolizumab + platinum (RR, 6.10, 95% CI, 1.08–34.41), pembrolizumab (RR, 8.26, 95% CI, 0.98,69.47) (Figure 4E).
FIGURE 4.
Pooled estimates of the network meta-analysis. (A) Multiple treatment comparison for dermatologic irAEs based on network consistency. (B) Multiple treatment comparison for colitis based on network consistency model. (C) Multiple treatment comparison for endocrine irAEs based on network consistency model. (D) Multiple treatment comparison for pneumonitis based on network consistency model. (E) Multiple treatment comparison for hepatitis based on network consistency model. (OR>1 means the treatment in top left is worse; Platinum = Platinum based chemotherapy).
Determining the Ranking
We ranked the probabilities of immune-related adverse events for all treatments by estimating the SUCRA value. A higher SUCRA value indicated a higher probability of irAEs and a poorer treatment regimen. For dermatologic irAEs, the corresponding ranking of incidences of the seven groups from high to low was: nivolumab + ipilimumab (97.4%), pembrolizumab (80.1%), nivolumab (67.1%), pembrolizumab + platinum (43.3%), atezolizumab + platinum (39.9%), durvalumab (17.5%), platinum-based chemotherapy (4.7%) (Figure 5A, Supplementary figure S1A). For colitis, the corresponding ranking of incidences of the six groups from high to low was: atezolizumab + platinum (77.1%), nivolumab (67.3%), pembrolizumab (60.5%), durvalumab (45.2%), pembrolizumab + platinum (41.4%), platinum-based chemotherapy (8.5%) (Figure 5B, Supplementary figure S1B). For endocrine irAEs, the corresponding ranking of incidences of the seven groups from high to low was: nivolumab + ipilimumab (79.1%), durvalumab (69.1%), pembrolizumab (61.9%), atezolizumab + platinum (60.4%),nivolumab (45.7%), pembrolizumab + platinum (33.5%), platinum-based chemotherapy (0.3%) (Figure 5C, Supplementary figure S1C). For pneumonitis, the corresponding ranking of incidences of the seven groups from high to low was: pembrolizumab (99.3%), pembrolizumab + platinum (65.1%), durvalumab (62.2%), atezolizumab + platinum (56%), nivolumab (35.9%), platinum-based chemotherapy (18.1%),atezolizumab (13.3%) (Figure 5D, Supplementary figure S1D). For hepatitis, the corresponding ranking of incidences of the six groups from high to low was: pembrolizumab (71.2%), pembrolizumab + platinum (64.3%), durvalumab (56.4%), atezolizumab + platinum (53.8%), nivolumab (44.5%), platinum-based chemotherapy (9.8%) (Figure 5E, Supplementary figure S1E).
FIGURE 5.
Sequence diagram of the network meta-analysis. (A) Multiple treatment comparison for dermatologic irAEs based on SUCRA. (B) Multiple treatment comparison for colitis based on SUCRA. (C) Multiple treatment comparison for endocrine irAEs based on SUCRA. (D) Multiple treatment comparison for pneumonitis based on SUCRA. (E) Multiple treatment comparison for hepatitis based on SUCRA. (Platinum = Platinum based chemotherapy).
Convergence, Heterogeneity and Publication Bias
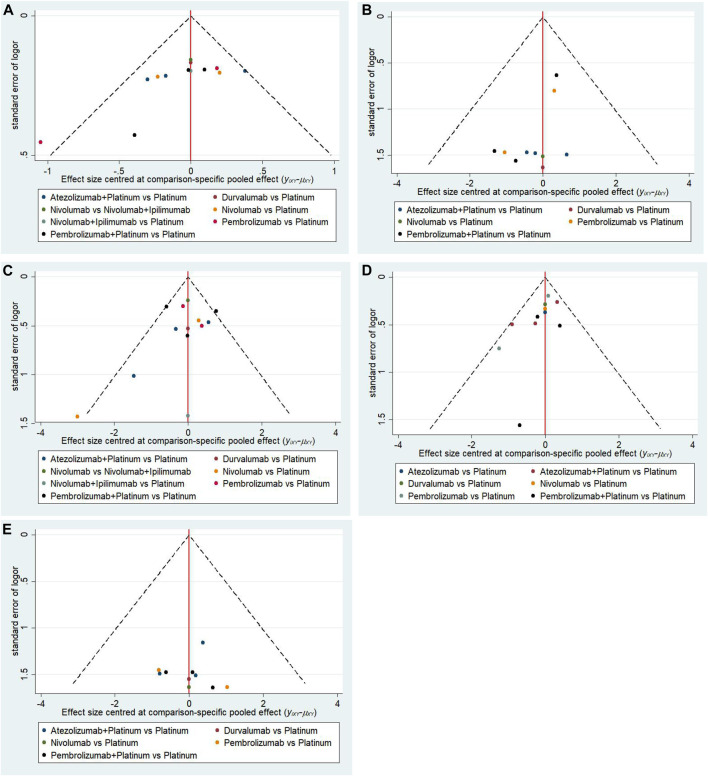
As shown in Supplementary figure S2, all comparisons of different irAEs all suggested that the contraction factor in Brookse-Gelmane-Rubin diagnostic plots were equal to the predefined cut-off value 1, suggesting that the study model had good convergence. In this network meta-analysis the comparisons showed low, medium or high heterogeneity (Supplementary figure S3), then the random effects model was selected. The funnel plots were all symmetrically distributed, suggesting no publication bias (Figure 6).
FIGURE 6.
Funnel plot of (A) dermatologic irAEs, (B) colitis, (C) endocrine irAEs, (D) pneumonitis and (E)hepatitis in the network meta-analysis.(Platinum = Platinum based chemotherapy).
Risk-Of-Bias Assessment and Sensitivity Analysis
As shown in Figure 1, 9 included RCTs (Corey et al., 2016; Reck et al., 2016; Carbone et al., 2017; Barlesi et al., 2018; Hellmann et al., 2019; West et al., 2019; Jotte et al., 2020; Naiyer et al., 2020; Roy et al., 2020) were regarded as high risk on blinding of participants and personnel and 5 included RCTs (Gandhi et al., 2018; Paz-Ares et al., 2018; Tony et al., 2019; Jotte et al., 2020; Naiyer et al., 2020) were considered high risk on incomplete outcome data. Other domains indicated unclear risk or low risk. The inconsistency test conducted that p values>0.05 indicated there was significant consistency between direct and indirect results of all comparisons, and then the sensitivity analysis indicated stable results (Supplementary figure S4A-E).
Discussion
In recent years, a number of RCTs had successively evaluated ICI + chemotherapy, ICI alone, dual ICIs combination versus chemotherapy in terms of efficacy and safety in the first-line treatment of advanced NSCLC (Corey et al., 2016; Reck et al., 2016; Carbone et al., 2017; Barlesi et al., 2018; Gandhi et al., 2018; Paz-Ares et al., 2018; Hellmann et al., 2019; Tony et al., 2019; West et al., 2019; Jotte et al., 2020; Naiyer et al., 2020; Roy et al., 2020). These RCTs suggested that the efficacy and treatment-related side effects of ICI + chemotherapy, ICI alone or dual ICIs were better than chemotherapy alone. Because of this, ICIs including nivolumab (Kazandjian et al., 2016), pembrolizumab (Pai-Scherf et al., 2017) and atezolizumab (Weinstock et al., 2017) were approved for NSCLC by FDA and had became an important part of treatment options in advanced NSCLC (Antonia et al., 2017). However with the widespread use of ICIs, the irAEs showed a tendency to be more prevalent in ICIs than in chemotherapy. IrAEs represented an entirely new toxicity spectrum of ICIs, and it could affect any tissue or organ, if mishandled, could reduce the survival benefit of antitumor efficacy, which might be related to the mechanism by which ICI alters the balance of immune cells in the body, inducing damage to some organ systems. The incidence and severity of irAEs were usually related to the drug type, tumor type, irAEs history and other immune-related medical history (Chen et al., 2015), and different ICIs had different immune mechanisms, and even belonging to the same mechanism had different tolerability to different irAEs. However which treatment regime could be better tolerated was worth studying.
Here, we indirectly compared the probability of irAEs caused by ICI + platinum, ICI alone, dual ICIs combination for advanced NSCLC through NMA. Data on irAEs from published studies and Clinicaltrials.gov from 2015 to 2020 were collected. Finally, 12 head-to-head phase II and III RCTs (Corey et al., 2016; Reck et al., 2016; Carbone et al., 2017; Barlesi et al., 2018; Gandhi et al., 2018; Paz-Ares et al., 2018; Hellmann et al., 2019; Tony et al., 2019; West et al., 2019; Jotte et al., 2020; Naiyer et al., 2020; Roy et al., 2020)including 8,453 patients with advanced NSCLC were included in this network meta-analysis. Notably, this NMA concluded that for dermatologic irAEs, colitis, endocrine irAEs, pneumonitis and hepatitis, ICI-based therapy showed a higher incidence of irAEs than platinum-based chemotherapy. Qiang Su (Su et al., 2019) concluded that compared with chemotherapy, PD-1/PD-L1 inhibitors showed significant increase in grade 1–5 and grade 3–5 pneumonitis, which was consistent with the results of ours. And for dermatologic irAEs, colitis, endocrine irAEs, pneumonitis and hepatitis, pembrolizumab + platinum showed a higher incidence of irAEs than pembrolizumab, which was consistent with Slater et al.'s (Slater et al., 2002) conclusion that chemotherapy may produce immunosuppression. Interestingly, only dermatologic and endocrine irAEs involved nivolumab + ipilimumab and we concluded that nivolumab + ipilimumab showed a higher incidence of dermatologic and endocrine irAEs than ICI + platinum and ICI alone and the explanation for this might be that the combination of the dual ICIs increased the imbalance of immune cells and led to more irAEs. And we found something new that except for chemotherapy, durvalumab for dermatologic irAEs, pembrolizumab + platinum for colitis, pembrolizumab + platinum for endocrine irAEs, atezolizumab for pneumonitis and nivolumab for hepatitis had the least incidence of irAEs, which were different from the conclusion of Cheng Xu et al. (Xu et al., 2018) and Xinru Chen et al. (Chen et al., 2020). Cheng Xu et al. (Xu et al., 2018) compared the occurrences of irAEs with different immunotherapy regimens for all cancers in 2018, and they compared the efficacy of ICI monotherapy, ICI monotherapy plus chemotherapy, and chemotherapy in lung cancer and concluded that atezolizumab had the best overall safety, while nivolumab had the best overall safety in the treatment of lung cancer with a combined approach. Xinru Chen et al. (Chen et al., 2020) investigated different immunotherapy regimens for immune-related pneumonia (IRP), and they concluded that ICIs increased the risk of IRP compared to chemotherapy and that ICI + chemotherapy was associated with a lower risk of IRP than dual ICIs combination and ICI monotherapy. However the reason why my results were different from theirs might be: firstly, compared to previous studies, we only included patients with advanced NSCLC and excluded other cancer types in this NMA, which was relatively specific, and all data were up to date. Secondly, the studies of us that only platinum-based chemotherapy was included, and those involving docetaxel were excluded. Thirdly, instead of integrating different drugs with similar mechanisms into the same arm, we analyzed each of the treatment regimens separately.
The current analysis has several strengths. Firstly, to our knowledge, this NMA analyzed the probability of irAEs caused by different treatment regimens for advanced NSCLC more specifically. Secondly, some conclusions this study drew were innovative compared to previous studies, which will have novel significance for guiding clinical treatment and subsequent studies. Thirdly, all of the articles included in this NMA were RCTs and the Cochrane risk bias assessment tool were used to examine the quality of included studies and to ensure their inherent authenticity by assessing the potential risk of bias in various aspects of RCT design, implementation, and outcome evaluation. Fourthly, in this NMA, in order to detect the heterogeneity of the included literature and data, we used heterogeneity test, and in order to detect publication bias, we used funnel plots, and the results showed that no significant publication bias was found. Fifthly, we conducted sensitivity analysis on all studies to investigate inconsistencies between direct and indirect comparisons by inconsistency test, and the results all suggested that p>0.05, indicating that our research results are stable. All of these suggested that our study is quite reliable.
Nonetheless, there were a few unavoidable limitations to our article. Firstly, in all of the studies of irAEs included, almost all comparisons showed low heterogeneity except for some comparisons. Secondly, although we found that the rating systems and terminology used in the reports were consistent and compatible, the diagnosis of each irAE was based solely on the experience of each clinician, rather than on a centralized review, which can lead to bias in irAE evaluation. Thirdly, some irAEs were delayed diseases, and clinicians could not have observed the symptoms of patients within the clinical timeframe, leading to the loss of clinical data, which would bring some potential heterogeneity to this study. Fourthly, the median follow-up time for each RCT was different, which would increase the frequency of irAEs associated with immunotherapy and increase the confounding factors for these events. Fifthly, although we included the latest RCT data that could be retrieved, some studies had a small sample size, which might be the reason why some comparisons showed moderate or high heterogeneity.
Despite there are some limitations in our study, we still have some prospects for the prediction and treatment of irAEs. According to the reports (Wang et al., 2019), most irAEs have been reported to be mild, such as cutaneous and endocrine, but death from moderate, severe or life-threatening irAEs have been reported in 1–2% of patients, such as pneumonia and colitis and T. W. Chen et al. (Chen et al., 2015) suggest that seventeen percent of studies over stated the safety of the experimental regimen, and although glucocorticoids and steroids (Weber et al., 2009) can be used for irAES, some patients still die because of late diagnosis, which emphasize the importance of determining the predictors of irAEs and early management and prevention. Studies have suggest that after treatment of ICI peripheral blood eosinophil is associated with irAEs (Schindler, 2014). Circulating IL-17 (Callahan, 2011), expression of CD177 and CEACAM1 (Shahabi et al., 2013), and neutrophil infiltration in the lamina propria of the colon (Berman et al., 2010) is suggested to be associated with gastrointestinal toxicity, however the occurrence mechanism of other irAEs is not clear, and at present, the evaluation system of irAEs is not perfect. In the future, more effective predictors of irAEs will be needed and with the extensive application of ICIs treatment and the development of related trials, people will have a fuller understanding of the mechanism of irAEs, and the management measures will be more standardized.
Conclusion
This systematic review and NMA suggests that, in addition to platinum-based chemotherapy, durvalumab for dermatologic and liver irAEs, pembrolizumab for gastrointestinal irAEs, pembrolizumab + platinum for endocrine irAEs, and atezolizumab for pneumonitis may be associated with lower rates of irAEs than other immune-based regimens. Nivolumab + ipilimumab for dermatologic and endocrine irAEs, atezolizumab + platinum for colitis, and pembrolizumab for pneumonitis and hepatitis may be associated with higher rates of irAEs. These conclusions will be helpful for clinical management, early prediction, early detection and early treatment. However a large number of multicenter clinical trials and studies are still needed to balance the therapeutic efficacy and the occurrence of irAEs of ICIs to maximize patient benefit.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Author GH and CB have given substantial contributions to the conception or the design of the manuscript, author JG and author WZ to acquisition, analysis and interpretation of the data. All authors have participated to drafting the manuscript, author GH revised it critically. All authors read and approved the final version of the manuscript. All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.686876/full#supplementary-material
Abbreviations
ICI, immune checkpoint inhibitor; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; NSCLC, non-small cell lung cancer; RCTs, randomized clinical trials; RRs, risk ratios; 95%CI, 95% confidence interval; NMA, network meta-analysis; CTLA4, cytotoxic T lymphocyte-associated antigen-4; PD-1, programmed cell death receptor 1; PD-L1, programmed cell death ligand-1; PD-L2, programmed cell death ligand-2; PRISMA, preferred reporting items for systematic reviews and meta-analysis; MCMC, Markov chain Monte Carlo; SUCRA, surface under the cumulative ranking curve.
References
- Antonia S. J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. (2017). Durvalumab after Chemoradiotherapy in Stage III Non-small-cell Lung Cancer. N. Engl. J. Med. 377, 1919–1929. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- Barlesi F., Nishio M., Cobo M. (2018). Efficacy of Atezolizumab (Atezo) 1 Carboplatin (Carbo)/cisplatin (Cis) 1 Pemetrexed (Pem) as 1L Treatment in Key Subgroups with Stage IV Non-squamous Non-small Cell Lung Cancer (NSCLC). Ann. Oncol. 29, 743–744. 10.1093/annonc/mdy424.066 [DOI] [Google Scholar]
- Basch E., Iasonos A., McDonough T., Barz A., Culkin A., Kris M. G., et al. (2016). Patient versus Clinician Symptom Reporting Using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a Questionnaire-Based Study. Lancet Oncol. 7 (11), 903–909. 10.1016/S1470-2045(06)70910-X [DOI] [PubMed] [Google Scholar]
- Berman D., Parker S. M., Siegel J., Chasalow S. D., Weber J., Galbraith S., et al. (2010). Blockade of Cytotoxic T-Lymphocyte Antigen-4 by Ipilimumab Results in Dysregulation of Gastrointestinal Immunity in Patients with Advanced Melanoma. Cancer Immun. 10, 11. [PMC free article] [PubMed] [Google Scholar]
- Bironzo P., Di Maio M. (2018). A Review of Guidelines for Lung Cancer. J. Thorac. Dis. 10 (Suppl. 13), S1556–S1663. 10.21037/jtd.2018.03.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan M. K. (2011). Evaluation of Serum IL-17 Levels during Ipilimumab Therapy: Correlation with Colitis. ASCO Meet. Abstr. 29. 10.1200/jco.2011.29.15_suppl.2505 [DOI] [Google Scholar]
- Carbone D. P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., et al. (2017). First-Line Nivolumab in Stage IV or Recurrent Non-small-cell Lung Cancer. N. Engl. J. Med. 376, 2415–2426. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. W., Razak A. R., Bedard P. L., Siu L. L., Hansen A. R. (2015). A Systematic Review of Immune-Related Adverse Event Reporting in Clinical Trials of Immune Checkpoint Inhibitors. Ann. Oncol. 26, 1824–1829. 10.1093/annonc/mdv182 [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang Z., Hou X., Zhang Y., Zhou T., Liu J., et al. (2020). Immune-related Pneumonitis Associated with Immune Checkpoint Inhibitors in Lung Cancer: a Network Meta-Analysis. J. Immunother. Cancer 8, e001170. 10.1136/jitc-2020-001170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey J. L., Shirish M. G., Hossein B. (2016). Carboplatin and Pemetrexed with or without Pembrolizumab for Advanced, Non-squamous Non-small-cell Lung Cancer: a Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. The Lancet 17 (11), 30498–30510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias S., Welton N. J., Caldwell D. M., Ades A. E. (2010). Checking Consistency in Mixed Treatment Comparison Meta-Analysis. Stat. Med. 29, 932–944. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- Ettinger D. S., Wood D. E., Aisner D. L., Akerley W., Bauman J., Chirieac L. R., et al. (2017). Non-small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 15, 504–535. 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- Furukawa T. A., Salanti G., Atkinson L. Z., Leucht S., Ruhe H. G., Turner E. H., et al. (2016). Comparative Efficacy and Acceptability of First-Generation and Second-Generation Antidepressants in the Acute Treatment of Major Depression: Protocol for a Network Meta-Analysis. BMJ Open 6, e010919. 10.1136/bmjopen-2015-010919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., et al. (2018). Pembrolizumab Plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N. Engl. J. Med. 378, 2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W. E., et al. (2016). The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 11 (1), 39–51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Hanna N., Johnson D., Temin S., Baker S., Brahmer J., Ellis P. M., et al. (2017). Systemic Therapy for Stage IV Non-small-cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 35, 3484–3515. 10.1200/JCO.2017.74.6065 [DOI] [PubMed] [Google Scholar]
- He J., Hu Y., Hu M., Li B. (2015). Development of PD-1/pd-L1·pathway in Tumor Immune Microenvironment and Treatment for Non-small Cell Lung Cancer. Sci. Rep. 5, 13100. 10.1038/srep13110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann M. D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S. W., Carcereny Costa E., et al. (2019). Nivolumab Plus Ipilimumab in Advanced Non-small-cell Lung Cancer. N. Engl. J. Med. 381, 2020–2031. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- Higgins J., Green S. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0. Chichester, England: The Cochrane Collaboration. Confidence intervals. [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Jackson D., Barrett J. K., Lu G., Ades A. E., White I. R. (2012). Consistency and Inconsistency in Network Meta-Analysis: Concepts and Models for Multi-Arm Studies. Res. Synth. Methods 3, 98–110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton B., Salanti G., Caldwell D. M., Chaimani A., Schmid C. H., Cameron C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162, 777–784. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- Jotte R., Cappuzzo F., Vynnychenko I., Stroyakovskiy D., Rodríguez-Abreu D., Hussein M., et al. (2020). Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results from a Randomized Phase III Trial. J. Thorac. Oncol. 15, 1351–1360. 10.1016/j.jtho.2020.03.028 [DOI] [PubMed] [Google Scholar]
- Kazandjian D., Suzman D. L., Blumenthal G., Mushti S., He K., Libeg M., et al. (2016). FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-small Cell Lung Cancer with Progression on or after Platinum-Based Chemotherapy. Oncologist 21, 634–642. 10.1634/theoncologist.2015-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 339, b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. J., Thorlund K., Ioannidis J. P. (2013). Demystifying Trial Networks and Network Meta-Analysis. BMJ 346, f2914. 10.1136/bmj.f2914 [DOI] [PubMed] [Google Scholar]
- Molina J. R., Yang P., Cassivi S. D., Schild S. E., Adjei A. A. (2008). Non-small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 83, 584–594. 10.4065/83.5.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiyer A., Byoung C., Niels R. (2020). Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-Line Treatment of Metastatic Non–Small Cell Lung Cancer. JAMA Oncol. 6 (5), 237–251. 10.1016/j.cllc.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai-Scherf L., Blumenthal G. M., Li H., Subramaniam S., Mishra-Kalyani P. S., He K., et al. (2017). FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and beyond. Oncologist 22, 1392–1399. 10.1634/theoncologist.2017-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gümüş M., Mazières J., et al. (2018). Pembrolizumab Plus Chemotherapy for Squamous Non-small-cell Lung Cancer. N. Engl. J. Med. 379, 2040–2051. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- Pedoeem A., Azoulay-Alfaguter I., Strazza M., Silverman G. J., Mor A. (2014). Programmed Death-1 Pathway in Cancer and Autoimmunity. Clin. Immunol. 153 (1), 145–152. 10.1016/j.clim.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A. G., Hui R., Csőszi T., Fülöp A., et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-small-cell Lung Cancer. N. Engl. J. Med. 375, 1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- Remon J., Passiglia F., Ahn M. J., Barlesi F., Forde P. M., Garon E. B., et al. (2020). Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J. Thorac. Oncol. 15, 914–947. 10.1016/j.jtho.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Roy S. H., Giuseppe G., Filippo D. M. (2020). Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 383, 1328–1339. 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- Salanti G., Higgins J. P., Ades A. E., Ioannidis J. P. (2008). Evaluation of Networks of Randomized Trials. Stat. Methods Med. Res. 17, 279–301. 10.1177/0962280207080643 [DOI] [PubMed] [Google Scholar]
- Salanti G., Ades A. E., Ioannidis J. P. (2011). Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J. Clin. Epidemiol. 64, 163–171. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M. C., Brahmer J., Schiller J. H., Dowlati A., et al. (2006). Paclitaxel-carboplatin Alone or with Bevacizumab for Non-small-cell Lung Cancer. N. Engl. J. Med. 355, 2542–2550. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- Scagliotti G. V., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. (2008). Phase III Study Comparing Cisplatin Plus Gemcitabine with Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients with Advanced-Stage Non-small-cell Lung Cancer. J. Clin. Oncol. 26, 3543–3551. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- Schindler K. (2014). Correlation of Absolute and Relative Eosinophil Counts with Immune-Related Adverse Events in Melanoma Patients Treated with Ipilimumab. ASCO Meet. Abstr. 32,9096–9096. 10.1200/jco.2014.32.15_suppl.9096 [DOI] [Google Scholar]
- Shahabi V., Berman D., Chasalow S. D., Wang L., Tsuchihashi Z., Hu B., et al. (2013). Gene Expression Profiling of Whole Blood in Ipilimumab-Treated Patients for Identification of Potential Biomarkers of Immune-Related Gastrointestinal Adverse Events. J. Transl. Med. 11, 75. 10.1186/1479-5876-11-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2020). Cancer Statistics, 2016. CA Cancer J. Clin. 66, 7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- Slater L. M., Stupecky M., Sweet P., Osann K. E. (2002). Enhancement of Leukemia Rejection by Mice Successfully Treated for L1210 Leukemia Due to Low Dose Compared to High Dose VP-16. Leuk. Res. 26, 203–206. 10.1016/s0145-2126(01)00105-9 [DOI] [PubMed] [Google Scholar]
- Sterne J. A., Egger M. (2001). Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of axis. J. Clin. Epidemiol. 54, 1046–1055. 10.1016/s0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- Su Q., Zhu E. C., Wu J. B., Li T., Hou Y. L., Wang D. Y., et al. (2019). Risk of Pneumonitis and Pneumonia Associated with Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 10, 108. 10.3389/fimmu.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Ades A. E., Cooper N., Abrams K. (2008). Use of Indirect and Mixed Treatment Comparisons for Technology Assessment. Pharmacoeconomics 26, 753–767. 10.2165/00019053-200826090-00006 [DOI] [PubMed] [Google Scholar]
- Tony S. K., Wu Y-L., Kudaba I. (2019). Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-small-cell Lung Cancer (KEYNOTE-042): a Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 6736, 32409–32421. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. (2015). Global Cancer Statistics, 2012. CA Cancer J. Clin. 65, 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- Torre L. A., Sauer A. M., Chen M. S., Kagawa-Singer M., Jemal A., Siegel R. L. (2016). Cancer Statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging Incidence in Males and Females. CA Cancer J. Clin. 66, 182–202. 10.3322/caac.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Y., Salem J. E., Cohen J. V., Chandra S., Menzer C., Ye F., et al. (2018). Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 4, 1721–1728. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou S., Yang F., Qi X., Wang X., Guan X., et al. (2019). Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol. 5, 1008–1019. 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Thompson J. A., Hamid O., Minor D., Amin A., Ron I., et al. (2009). A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Comparing the Tolerability and Efficacy of Ipilimumab Administered with or without Prophylactic Budesonide in Patients with Unresectable Stage III or IV Melanoma. Clin. Cancer Res. 15 (17), 5591–5598. 10.1158/1078-0432.CCR-09-1024 [DOI] [PubMed] [Google Scholar]
- Weinstock C., Khozin S., Suzman D., Zhang L., Tang S., Wahby S., et al. (2017). U.S. Food and Drug Administration Approval Summary: Atezolizumab for Metastatic Non-small Cell Lung Cancer. Clin. Cancer Res. 23, 4534–4539. 10.1158/1078-0432.CCR-17-0540 [DOI] [PubMed] [Google Scholar]
- West H., McCleod M., Hussein M., Morabito A., Rittmeyer A., Conter H. J., et al. (2019). Atezolizumab in Combination with Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-squamous Non-small-cell Lung Cancer (IMpower130): a Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 20, 924–937. 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- Xu C., Chen Y. P., Du X. J., Liu J. Q., Huang C. L., Chen L., et al. (2018). Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ 363, k4226. 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.