Natsuko Onishi
Natsuko Onishi, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Wen Li
Wen Li, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
David C Newitt
David C Newitt, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Roy J Harnish
Roy J Harnish, MS
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Fredrik Strand
Fredrik Strand, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Alex Anh-Tu Nguyen
Alex Anh-Tu Nguyen, MS
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Vignesh Amal Arasu
Vignesh Amal Arasu, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Jessica Gibbs
Jessica Gibbs, BS
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Ella F Jones
Ella F Jones, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Lisa J Wilmes
Lisa J Wilmes, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
John Kornak
John Kornak, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Bonnie N Joe
Bonnie N Joe, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Elissa R Price
Elissa R Price, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Haydee Ojeda-Fournier
Haydee Ojeda-Fournier, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Mohammad Eghtedari
Mohammad Eghtedari, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Kathryn W Zamora
Kathryn W Zamora, MD, MPH
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Stefanie Woodard
Stefanie Woodard, DO
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Heidi R Umphrey
Heidi R Umphrey, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Michael T Nelson
Michael T Nelson, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
An L Church
An L Church, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Patrick J Bolan
Patrick J Bolan, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Theresa Kuritza
Theresa Kuritza, DO, FAOCR, MBA
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Kathleen Ward
Kathleen Ward, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Kevin Morley
Kevin Morley, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Dulcy Wolverton
Dulcy Wolverton, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Kelly Fountain
Kelly Fountain, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Dan Lopez Paniagua
Dan Lopez Paniagua, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Lara Hardesty
Lara Hardesty, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Kathleen R Brandt
Kathleen R Brandt, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Elizabeth S McDonald
Elizabeth S McDonald, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Mark Rosen
Mark Rosen, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Despina Kontos
Despina Kontos, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Hiroyuki Abe
Hiroyuki Abe, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Deepa Sheth
Deepa Sheth, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Erin Crane
Erin Crane, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Charlotte Dillis
Charlotte Dillis, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Pulin Sheth
Pulin Sheth, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Linda Hovanessian-Larsen
Linda Hovanessian-Larsen, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Dae Hee Bang
Dae Hee Bang, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Bruce Porter
Bruce Porter, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Karen Y Oh
Karen Y Oh, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Neda Jafarian
Neda Jafarian, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Luminita A Tudorica
Luminita A Tudorica, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Bethany Niell
Bethany Niell, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Jennifer Drukteinis
Jennifer Drukteinis, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Mary S Newell
Mary S Newell, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Marina E Giurescu
Marina E Giurescu, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Elise Berman
Elise Berman, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Constance D Lehman
Constance D Lehman, MD, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Savannah C Partridge
Savannah C Partridge, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Kimberly A Fitzpatrick
Kimberly A Fitzpatrick, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Marisa H Borders
Marisa H Borders, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Wei Tse Yang
Wei Tse Yang, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Basak Dogan
Basak Dogan, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Sally Hayward Goudreau
Sally Hayward Goudreau, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Thomas Chenevert
Thomas Chenevert, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Christina Yau
Christina Yau, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Angela DeMichele
Angela DeMichele, MD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Donald A Berry
Donald A Berry, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Laura J Esserman
Laura J Esserman, MD, MBA
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,
Nola M Hylton
Nola M Hylton, PhD
1Author affiliations: From the Department of Radiology
& Biomedical Imaging, University of California San Francisco, 1600
Divisadero St, Room C255, San Francisco, CA 94115 (N.O., W.L., D.C.N., R.J.H.,
F.S., A.A.T.N., V.A.A., J.G., E.F.J., L.J.W., B.N.J., E.R.P., N.M.H.);
Department of Breast Radiology, Karolinska University Hospital, Solna,
Stockholm, Sweden (F.S.); Department of Radiology, Kaiser Permanente Vallejo
Medical Center, Vallejo, Calif (V.A.A.); Department of Epidemiology and
Biostatistics, University of California San Francisco, San Francisco, Calif
(J.K.); Department of Radiology, University of California San Diego, La Jolla,
Calif (H.O.F., M.E.); Department of Radiology, The University of Alabama at
Birmingham, Birmingham, Ala (K.W.Z., S.W., H.R.U.); Department of Radiology,
University of Minnesota, Minneapolis, Minn (M.T.N., A.L.C., P.J.B.); Department
of Radiology, Loyola Medicine, Maywood, Ill (T.K., K.W., K.M.); Department of
Radiology, University of Colorado Denver, Denver, Colo (D.W., K.F., D.L.P.,
L.H.); Department of Diagnostic Radiology, Mayo Clinic Rochester, Rochester,
Minn (K.R.B.); Department of Radiology, University of Pennsylvania,
Philadelphia, Pa (E.S.M., M.R., D.K.); Department of Radiology, University of
Chicago Medical Center, Chicago, Ill (H.A., D.S.); Department of Radiology,
Georgetown University, Washington, DC (E.C., C.D.); Department of Radiology,
University of Southern California, Los Angeles, Calif (P.S., L.H.L.); Department
of Radiology, Swedish Cancer Institute, Seattle, Wash (D.H.B., B.P.); Department
of Radiology, Oregon Health & Science University, Portland, Ore (K.Y.O.,
N.J., L.A.T.); Department of Radiology, Moffitt Cancer Center, Tampa, Fla (B.N.,
J.D.); Department of Women’s Imaging, St. Joseph’s Women’s
Hospital, Tampa, Fla (J.D.); Department of Radiology, Emory University, Atlanta,
Ga (M.S.N.); Department of Radiology, Mayo Clinic Arizona, Phoenix, Ariz
(M.E.G.); Department of Radiology, Inova Health System, Fairfax, Va (E.B.);
Department of Radiology, Harvard Medical School, Massachusetts General Hospital,
Boston, Mass (C.D.L.); Department of Radiology, University of Washington,
Seattle, Wash (S.C.P.); Department of Medical Imaging, Banner University Medical
Center Tucson, Tucson, Ariz (K.A.F., M.H.B.); Department of Radiology,
University of Texas MD Anderson Cancer Center, Houston, Tex (W.T.Y., B.D.);
Department of Radiology, University of Texas Southwestern Medical Center at
Dallas, Dallas, Tex (B.D., S.H.G.); Department of Radiology, University of
Michigan, Ann Arbor, Mich (T.C.); Department of Surgery, University of
California San Francisco, San Francisco, Calif (C.Y., L.J.E.); Department of
Oncology and Hematology, University of Pennsylvania, Philadelphia, Pa (A.D.);
and Berry Consultants, LLC, Austin, Tex (D.A.B.).
1,✉
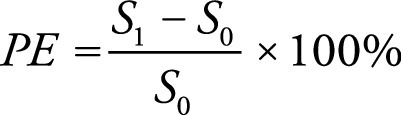 , where
PE is percent enhancement, S0
and S1 are signal intensities at contrast-unenhanced
and early contrast-enhanced phase, respectively. Finally, quantitative BPE was
calculated by averaging the percent enhancement values for all voxels in the
masked volume (Fig 2).
, where
PE is percent enhancement, S0
and S1 are signal intensities at contrast-unenhanced
and early contrast-enhanced phase, respectively. Finally, quantitative BPE was
calculated by averaging the percent enhancement values for all voxels in the
masked volume (Fig 2).