Abstract
Background:
Ivabradine is recommended in heart failure (HF) patients to reduce cardiovascular death and hospitalization due to worsening of HF symptoms.
Aims and Objectives:
To study the effect of Ivabradine in addition to guideline-directed medical therapy (GDMT) in a group of HF patients with HR more than 70 bpm, HF with reduced ejection fraction (HFrEF) left ventricular ejection fraction (LVEF ≤ 40%), and New York Heart Association class II-IV.
Methods:
The study was conducted at Heart Hospital, Hamad Medical Corporation, Qatar. HF patients with age > 18 years, LVEF ≤40%, on GDMT, and HR of ≥70 bpm were included. The study population was divided into two groups: ivabradine group and non-ivabradine group. The primary outcomes were risk, number and length of hospitalizations due to worsening HF, and cardiovascular mortality. The secondary outcome was all-cause mortality. Baseline characteristics were collected at enrollment. Study outcomes were compared in the two groups by applying Chi-square and Fisher's exact tests. Logistic regression model was applied to assess both hospitalizations and cardiovascular mortality.
Results:
A total of 111 patients were studied, 37 (33.94%) ivabradine group and 74 (66.67%) non-ivabradine group. Risk of hospitalization was lower in Ivabradine group compared to non-Ivabradine group (odds ratio: 0.43, 95% confidence interval [CI]: 0.16–1.015, P = 0.094). Average length of hospitalization in ivabradine and non-ivabradine groups was 12.54 and 8.91 days, respectively (incidence rate ratio [IRR]: 1.63, 95% CI: 0.79–3.38, P = 0.187). Compared to non-ivabradine, ivabradine patients had lower number of hospitalizations (IRR: 1.13, 95% CI: 0.61–2.11, P = 0.694). Death rate in both ivabradine and non-ivabradine groups was 3.
Conclusions:
Ivabradine along with GDMT reduces the risk of hospitalization due to worsening HF symptoms. Ivabradine had no significant effect on cardiovascular mortality and all-cause mortality. HFrEF non-Arabs patients have lower risk, number and length of hospitalization, and mortality compared to Arabs.
Keywords: Cardiovascular disease, heart failure, hospitalization, ivabradine
INTRODUCTION
Heart failure (HF) is considered to be a common cardiovascular disorder.[1,2] Approximately 1 in 5 persons will develop HF in their lifetime.[3] Its prevalence has been estimated at 2% in the Western countries and its incidence approaches 5–10 per 1000 persons each year.[4,5] In addition, its prevalence is 7% in 75–84 years old and over 10% in people older than 85 years of age.[6]
In men, 5-year age-adjusted mortality rates after onset are estimated to be 50%, and in women, it is 46%.[7] The treatment for HF often brings very heavy economic burden for patients and their families.[8]
Elevated resting heart rate appears to be a pathophysiological contributor in left ventricular systolic dysfunction.[9] In patients with acute decompensated HF, a heart rate ≥70 bpm and normal sinus rhythm is predictive of in-hospital mortality. The resting heart rate upon discharge influences 12-month hospital readmission and mortality rates.[10]
In patients with left ventricular dysfunction secondary to ischemic cardiomyopathy, heart rates >70 beats/min are associated with a 34% increase in cardiovascular mortality and 53% increase in hospitalization when compared to heart rates below 70 beats/min.[11]
Ivabradine is a novel drug that inhibits the pacemaker current I (f), thereby slowing heart rates without exhibiting negative inotropic effect on the myocardium or altering ventricular action potential.[12] In the SHIFT study,[13] ivabradine improved the composite endpoint of hospitalization and cardiovascular death in patients with HF with reduced ejection fraction (HFrEF) in sinus rhythm with heart rates ≥70.[13] During 23 months of monitoring, HF hospitalizations were decreased by 26% in patients treated with ivabradine.[13] The 2016 American College of Cardiology/American Heart Association (ACC/AHA)/Heart Failure Society of America Focused Update on the Management of HF[14] and the European Society of Cardiology guidelines[5] have given a Class IIa (level of evidence B) recommendation for ivabradine use for patients with chronic HFrEF who are on guideline-directed medical therapy. This includes a maximum tolerated dose of beta-blocker, ACE inhibitors, and mineralocorticoid receptor antagonist and who are in sinus rhythm with a resting heart rate above 70 beats/min. (The European Society of Cardiology considers >75 beats/min)
A meta-nalysis on ivabradine therapy in HF reported that, though the combined endpoint of HF hospitalization or cardiac mortality was reduced, along with improvement in ejection fraction and six-minute walking distance, there was no reduction in all-cause mortality, cardiovascular mortality, or HF hospitalization alone.[15]
A recent study (HEARTS-chronic) reported that chronic HF patients presented young and commonly suffered from severe LV dysfunction.[16] In addition, at 1 year, the all-cause mortality rate was 9% (cardiac related 93.7%), hospitalization rate was 39%, and emergency room visit was 50%. This could be attributed to the relatively high rate of CHF patients with severe LV dysfunction.[16]
The number and length of hospitalizations were not addressed in earlier studies.
Studies of ivabradine included 8% from Asia and 3% from other races, whereas white patients were the majority 89%.[17] No studies targeted effect of ivabradine on cardiovascular outcomes among Arabs and non-Arabs from Asia and Africa or Middle East countries in general.
The aim of this study was to assess the effect of ivabradine on cardiovascular outcomes of HF patients with reduced left ventricular ejection fraction (LVEF).
METHODS
Study design and patients
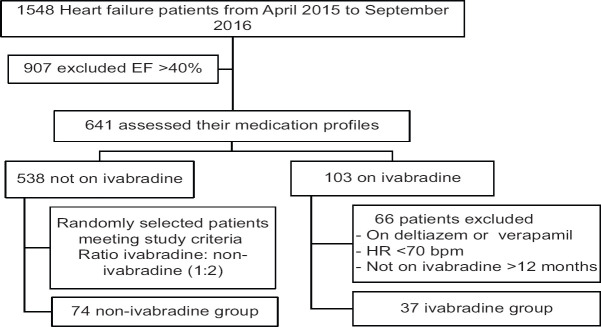
The study was a retrospective cohort study conducted at Heart Hospital, Hamad Medical Corporation (HH-HMC) in Qatar. All patients registered at HF clinic from January 2, 2015, to of June 30, 2016, were eligible to be enrolled in the study [Figure 1]. Their medical profiles were checked to assess the cardiovascular outcomes they developed within 18 months after the exposure to Ivabradine. To ensure that all patients on ivabradine are included in the study, we obtained a list of all patients who have been dispensed ivabradine from all heart hospital pharmacies within that period and merged them with the list provided by HF clinic. The diagnosis of patients was extracted from Cerner which was validated by manual checking. Patients who were older than 18 years, LVEF of ≤40%, on guideline-directed medical therapy (GDMT), and heart rate of ≥70 bpm were enrolled in the study sample.
Figure 1.
Heart failure patient's recruitment based on study inclusion criteria
Data collection was conducted by a pharmacist having access to patient's medication profile. Ivabradine and non-ivabradine groups were not masked during statistical analysis. The inclusion criteria were age ≥18 years, sinus rhythm with LVEF ≤40%, heart rate ≥70 bpm, and using either ACEi or BB. The exclusion criteria were atrial fibrillation or atrial flutter, MI within the last 2 months of recruiting, refilled ivabradine for <12 months, and patients on diltiazem or verapamil.
Data were entered in secured files with a username and a password. A hard copy of the data was kept in a locked cabinet in the hospital to preserve patient confidentiality. The baseline characteristics of participants were collected from the Cerner on the date of patient enrollment, and the patient was followed for 18 months to assess the study outcomes. For each ivabradine patient, two non-ivabradine patients were identified. Data were entered into Excel and transferred to STATA software for further statistical analyses.
The primary outcome of the study was hospitalizations due to worsening HF. It includes both first admissions and readmissions due to worsening HF. The secondary outcomes were cardiovascular and all-cause mortality.
A retrospective cohort study restricts the control for confounders. Compliance to GDMT and diet is an important factor that is associated with the need for hospitalization. Uncontrolled heart rate and fluid accumulation are the indicators of poor compliance. However, based on the data available and nature of the study, compliance as a confounder is not possible to be adjusted at selection phase. Although it is not sufficient, the number of medication refills is considered as a compliance indicator. Other nonmodifiable factors such as race, gender, and age are expected to have some effect on the prediction of hospitalization. Controlling for confounders was not completely achieved in the design phase.
Statistical analysis
The primary outcome of the study is the hospitalization due to worsening HF. The secondary outcomes are cardiovascular and all-cause mortality. Baseline characteristics of the participants are tabulated using proportions (percentage) for categorical variables and mean for continues variables.
Chi-square and Fisher's exact tests were applied to test the difference in the risk of hospitalization between ivabradine and non-ivabradine. The outcome, hospitalization risk, was also evaluated by testing it with other confounding variables such as ethnicity, gender, and GDMT intake.
Chi-square and Fisher's exact tests were also applied to test the association between number of Arabs admitted compared to non-Arabs admitted. The difference between number of Arabs admitted compared to non-Arabs was further investigated as the result was significant.
Fisher's exact test was applied once to test the difference in hospitalization risk between ivabradine and non-ivabradine groups among Arabs. Fisher's exact test was also repeated to test the difference between hospitalization risk for ivabradine and non-ivabradine groups among non-Arabs. To measure the magnitude of difference between Arabs and non-Arabs in outcomes of ivabradine group, Combined Mantel-Haenszel OR was calculated.
The second confounding factor to be tested was gender. Chi-square test was conducted to test the difference between hospitalization risk among female and male patients. GDMT intake among admitted patients was tested using Chi-square test. Risk of hospitalization was assessed using multivariate logistic regression to adjust for the independent variables. Some variables were underreported by the physicians, hence not added to the final model. These variables included smoking status, etiology, and estimated glamorous filtration rate (EGFR). All other variables were included in the final multivariate regression model. Due to the clinical importance of the variables, the forward method of building the model was not applied. The model was validated using receiver operating characteristic curve and specification table.
The effect of ivabradine on patient's survival over time was assessed by plotting Kaplan–Meier curve. Mann–Whitney test was applied to examine the difference between the mean length of hospitalization in ivabradine patients and non-ivabradine patients. To explore the distribution of data around the mean and find the outliers, box plots were plotted between ivabradine group and non-ivabradine group. The effect of comorbidities on length of hospital days was presented by box plots. Negative binomial regression model was fitted for length of hospitalization outcome.
An interaction term was added to the model to analyze the interaction between angiotensin-converting enzyme inhibitor and B-Blockers. The multivariate analysis was repeated without three outliers' observations (days of hospitalization more than 60 days) by observing any change in effect size for more than 20%. As the outcome count of hospitalization was not normally distributed, Wilcoxon Mann–Whitney test was applied to examine the difference between the means of both groups. Poisson regression model was fitted for number of hospitalizations.
Ethical approval
The Medical Research Centre (MRC) of Hamad Medical Corporation classified our research under Category 3 (research involving the study or collection of existing data, documents, and recodes). Our research is approved by The medical research Centre (MRC) of Hamad Medical Corporation (MRC 1443/2018) and Qatar University Research Department (QU-IRB 915-E/18).
RESULTS
Baseline characteristics of the patients showed an even distribution between ivabradine and non-ivabradine groups [Tables 1 and 2]. The analysis included 111 patients. The mean age in ivabradine group was 54 ± 11.47 years and 57 ± 13.47 years for non-ivabradine group. Arabs in ivabradine group were 25 (67.57%), whereas in non-ivabradine groups, they were 45 (60.57%). However, myocardial infarction was 25 (67.57%) and 34 (45.95%) in ivabradine and non-ivabradine patients, respectively.
Table 1.
Baseline characteristics of recruited patients in the study
| Demographic characteristics | Ivabradine group (n=37; 33.33%), n (%) | Nonivabradine group (n=74; 66.67%), n (%) |
|---|---|---|
| Age (years) | ||
| <50 | 15 (40.54) | 36 (48.65) |
| >50 | 22 (59.46) | 38 (51.35) |
| Gender | ||
| Male | 29 (78.38) | 60 (80.18) |
| Female | 8 (21.62) | 14 (18.92) |
| Ethnicity | ||
| Arab | 25 (67.57) | 45 (60.57) |
| Non-Arab | 12 (32.43) | 29 (39.19) |
| Current smoking | ||
| Yes | 7 (18.92) | 8 (10.81) |
| No | 16 (43.24) | 31 (41.89) |
| Unknown | 14 (37.84) | 35 (47.30) |
| Hypertension | 27 (72.97) | 52 (70.27) |
| DM | 27 (72.9) | 44 (59.46) |
| Previous stroke | 1 (2.70) | 6 (8.11) |
| Primary cause of HF | ||
| Ischemic | 25 (67.57) | 39 (52.70) |
| Not-specified | 12 (32.43) | 35 (47.30) |
| MI | 25 (67.57) | 34 (45.95) |
| History of AF/flutter | 0 | 2 (2.70) |
| CRT | 6 (16.22) | 12 (16.22) |
| BMI (kg/m2) | 28.51 (6.53)* | 29.80 (6.50)* |
| Cardiac parameters | ||
| HR (bpm) | 80.86 (8.80)* | 80.59 (9.02)* |
| SBP (mm Hg) | 116.62 (16.98)* | 127.31 (16.98)* |
| DBP (mm Hg) | 68.84 (8.57)* | 76.9 (10.92)* |
| LVEF (%) | ||
| 35-40 | 8 (21.62) | 10 (13.51) |
| 30-35 | 6 (16.22) | 17 (22.97) |
| 25-30 | 8 (21.62) | 18 (24.32) |
| 20-25 | 15 (40.54) | 29 (39.19) |
| eGFR (mL/min per1.73 m2) | ||
| <60 | 1 (2.70) | 18 (24.32) |
| >60 | 25 (67.57) | 27 (36.49) |
| Unknown | 11 (29.73) | 40 (36.04) |
| NYHA class | ||
| Class I | 7 (18.92) | 17 (22.97) |
| Class II | 10 (27.03) | 31 (41.89) |
| Class III-IV | 12 (32.43) | 14 (18.92) |
| Unknown | 8 (21.62) | 12 (16.22) |
*Indicates mean and SD. Categorical variables represented by number of patients and percentage. DM: Diabetes mellitus, HF: Heart failure, AF: Atrial fibrillation, CRT: Cathode-ray tube, BMI: Body mass index, SBP: Systolic blood pressure, DSP: Diastolic blood pressure, LVEF: Left ventricular ejection fraction, EGFR: Estimated glamorous filtration rate, NYHA: New York Heart Association, MI: Myocardial infarction, HR: Heart rate, SD: Standard deviation
Table 2.
Treatment at the time of enrollment
| Treatment at enrollment | Ivabradine group (n=37; 33.33%), n (%) | Nonivabradine group (n=74; 66.67%), n (%) |
|---|---|---|
| Ivabradine dose (mg BID) | ||
| 2.5 | 15 (40.54) | |
| 5 | 16 (43.24) | |
| 7.5 | 6 (16.22) | |
| B-blocker | 33 (89.19) | 73 (98.65) |
| ACE inhibitor | 23 (62.16) | 58 (78.38) |
| ARB | 9 (24.32) | 13 (17.57) |
| Non | 5 (13.51) | 3 (4.05) |
| Diuretics (excluding MRAs) | 33 (89.19) | 69 (93.24) |
| MRAs | 21 (56.76) | 37 (50.00) |
| Glycosides | 4 (10.81) | 8 (10.81) |
Indicates mean and SD. Categorical variables represented by number of patients and percentage. ACE: Angiotensin-converting enzyme, ARB: Angiotensin receptor blocker, MRAs: Mineralocorticoid receptor antagonist, SD: Standard deviation
Hospitalization risk
Chi-square test showed that there is no association (crude odds ratio [OR] =0.608, P = 0.24) between ivabradine and non-ivabradine patients in hospitalization risk [Table 3].
Table 3.
Comparison of study endpoints
| Study endpoint | Ivabradine group (n=37; 33.33%), n (%) | Nonivabradine group (n=74; 66.67%), n (%) | Total (n=111) | P |
|---|---|---|---|---|
| Risk of hospitalization | 23 (62.16) | 54 (72.97) | 77 | 0.24 |
| All cause mortality | 3 (8.11) | 3 (4.05) | 6 | - |
Testing the outcome by the factor of ethnicity instead of the treatment group had different results. The number of Arabs admitted was 55 (78.57%) compared to non-Arabs 22 (53.66%) (Chi-square test, P = 0.006). The combined OR was found to be similar to that of the crude OR of hospitalization in Arabs and non-Arabs (P = 0.005). It was lower (OR = 0.158) in the ivabradine group.
These results exclude any possibility of the Simpson's paradox. The difference between hospitalization risk and gender was also assessed using Chi-square test. Males admitted were 62 (69.66%) compared to females 15 (68.18%) (P = 0.893).
The admitted patients were also assessed for their GDMT intake using Chi-square and Fisher's exact tests. Out of all admitted patients, 40 (51.94%) were on both diuretics and MRAs and 5 (6.49%) were not using neither diuretics nor MRAs (Fisher's exact = 0.022). The admitted ivabradine patients who were on B-Blockers were 20 (86.96%), whereas the admitted non-ivabradine patients on B-Blockers were found to be 54 (100%) (Fisher's exact = 0.024).
After adjusting for the variables in the multivariate logistic model, non-ivabradine patients were found to have lower risk than ivabradine patients (OR = 0.43, P = 0.09, confidence interval [CI]: 0.16–1.15) [Table 4]. The ethnicity showed a significant reduction (OR = 0.36, P = 0.028, 95% CI: 0.14–0.90) in hospitalization of non-Arab patients compared to Arabs.
Table 4.
Multivariate logistic regression model for hospitalization (yes/no)
| OR | P | 95% CI | |
|---|---|---|---|
| Ivabradine patients | 0.43 | 0.094 | 0.16-1.15 |
| Ethnicity (non-Arab) | 0.36 | 0.028 | 0.14-0.90 |
| Age | 1.01 | 0.66 | 0.97-1.05 |
| NYHA | |||
| Unclassified | 3.08 | 0.162 | 0.64-14.91 |
| Class II | 1.30 | 0.642 | 0.43-3.99 |
| Class III-IV | 2.23 | 0.218 | 0.62-8.00 |
| Etiology (ischemic) | 1.51 | 0.373 | 0.61-3.74 |
| DM | 1.45 | 0.438 | 0.57-3.70 |
NYHA: New York Heart Association, DM: Diabetes mellitus, OR: Odds ratio, CI: Confidence interval
Length of hospitalization (days)
The ivabradine patients reported the maximum number of days of hospitalization (79 days) compared to non-ivabradine (57 days). Total days of hospitalization for non-ivabradine were 660, compared to 464 for ivabradine. However, the mean hospital days was higher in the ivabradine (mean = 12.5) group than non-ivabradine group (mean = 8.9). Mann–Whitney test was applied to examine the difference between the mean length of hospitalization in two groups. The difference in mean days of hospitalization between the groups was found to be non-significant (P = 0.45).
A negative binomial regression model was estimated. The model adjusted for most of the predictors in the study [Table 5]. Length of hospitalization days is 63% higher (incidence rate ratio [IRR] =1.63, P = 0.187) in patients with ivabradine, compared to the non-ivabradine, while all other variables held constant. However, this increase in length of hospitalization was not statistically significant.
Table 5.
Results from a negative binomial regression model to estimate hospital days
| Variable | IRR | P | 95% CI |
|---|---|---|---|
| Ivabradine | 1.63 | 0.187 | 0.79-3.38 |
| Ethnicity (non-Arab) | 0.45 | 0.015 | 0.23-0.86 |
| BMI | 1.035 | 0.301 | 0.97-1.10 |
| HR | 0.94 | 0.003 | 0.91-0.98 |
| SBP | 1.00 | 0.997 | 0.98-1.03 |
| DBP | 1.02 | 0.273 | 0.99-1.05 |
| LVEF overall | |||
| 25-30 | 0.59 | 0.225 | 0.25-1.38 |
| 30-35 | 0.25 | 0.007 | 0.09-0.68 |
| 35-40 | 0.47 | 0.124 | 0.18-1.23 |
| Etiology (ischemic) | 3.01 | 0.005 | 1.40-6.45 |
| DM | 1.05 | 0.879 | 0.55-2.01 |
| MI | 0.98 | 0.952 | 0.45-2.12 |
| ACE or ARB | |||
| ACE | 0.48 | 0.240 | 0.14-1.65 |
| ARB | 0.46 | 0.269 | 0.11-1.83 |
| ARNI overall (mg BID) | |||
| 100 | 0.19 | 0.040 0.219 |
0.01-2.65 |
| 50 | 0.07 | 0.029 | 0.01-0.76 |
IRR: Incidence rate ratio, CI: Confidence interval, DM: Diabetes mellitus, BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, LVEF: Left ventricular ejection fraction, MI: Myocardial infarction, ACE: Angiotensin-converting enzyme, ARB: Angiotensin receptor blocker, ARNI: Angiotensin receptor neprilysin inhibitor, HR: Heart rate, BID: Twice daily
Compared to Arabs, non-arabs spent more days in the hospital (OR=0.145, P= 0.015). If the heart rate of a patient was to increase by one unit, the rate for hospital days would decrease by a factor 0.94, while holding other predictors in the model constant (P = 0.003).
Patients with LVEF (30%–35%) compared to other groups (while holding other variables constant in the model) had a rate 0.25 times less for days spent in the hospital (P = 0.007). The overall test for P among the strata of LFEV was significant (P = 0.057). Patients on angiotensin receptor neprilysin inhibitor (ARNI) compared to patients who are not taking ARNI (while holding other variables constant in the model) were expected to have a rate 0.07–0.19 times less for days spent in the hospital (P = 0.040). Patients on ACE compared to patients who are not taking ACE or ARB (while holding other variables constant in the model) had a rate 0.48 times less for days spent in the hospital (P = 0.240), while patients on ARB had a rate 0.46 less for the days spent in the hospital (P = 0.269).
Number of hospitalizations
The total count of hospitalizations reported was 207 hospitalizations. The ivabradine patients reported a total of 80 (38.65%) hospitalizations compared to non-ivabradines 127 (61.35%) hospitalizations. However, the mean count of hospitalizations was higher in ivabradine (mean = 2.16) patients than non-ivabradines (mean = 1.72).
The total count of hospitalizations was reported for each patient in ivabradine and non-ivabradine groups. The dependent variable count of hospitalization is not normally distributed (mean = 1.86, SD = 2.40, skewness = 2.65). Wilcoxon Mann–Whitney test was applied to examine the difference between the means of both groups. The difference between count of hospitalizations between the groups was found to be non-significant (P = 0.67).
A Poisson regression was used to estimate the number of hospitalization and adjusted for a number of predictors. Non-ivabradine patients had 13% higher number of hospitalizations compared to ivabradine patients (IRR = 1.13, P = 0.694) [Table 6]. However, these findings were not statistically significant.
Table 6.
Poison regression for count of hospitalization
| Variable | IRR | P | 95% CI |
|---|---|---|---|
| Ivabradine group | 1.13 | 0.694 | 0.61-2.11 |
| Gender (male) | 0.98 | 0.932 | 0.59-1.62 |
| Age | 1.00 | 0.789 | 0.98-1.02 |
| Ethnicity (non-Arab) | 0.44 | 0.004 | 0.25-0.76 |
| LVEF | |||
| Overall | 0.050 | ||
| 25-30 | 1.05 | 0.881 | 0.56-1.96 |
| 30-35 | 0.52 | 0.013 | 0.32-0.87 |
| 35-40 | 0.96 | 0.887 | 0.54-1.70 |
| Etiology (ischemic) | 1.43 | 0.169 | 0.86-2.38 |
| MI | 1.08 | 0.765 | 0.67-1.73 |
| B-blockers | 0.41 | 0.027 | 0.18-0.90 |
| ACE or ARB | |||
| Overall | 0.100 | ||
| ACE | 0.54 | 0.032 | 0.31-1.07 |
| ARB | 0.59 | 0.138 | 0.14-2.68 |
| ARNI | |||
| Overall | 0.168 | ||
| 100 mg BID | 0.44 | 0.070 | 0.18-1.07 |
| 50 mg BID | 0.62 | 0.523 | 0.14-2.68 |
| Diuretics | 1.68 | 0.194 | 0.77-3.68 |
ACE: Angiotensin-converting enzyme, ARB: Angiotensin receptor blocker, ARNI: Angiotensin receptor neprilysin inhibitor, LVEF: Left ventricular ejection fraction, MI: Myocardial infarction, BID: Twice daily
Non-Arab compared to Arab group (while holding other variables constant in the model) had a rate 0.444 times less for count of hospitalizations (P = 0.004).
If the systolic blood pressure of a patient increased by one unit, the rate for count of hospitalizations increased by a factor 1.01 while holding other predictors in the model constant (P = 0.018). Patients with LVEF (30%–35%) compared to other groups (while holding other variables constant in the model) had a rate 0.47 times less for count of hospitalizations (P = 0.01). The overall test for P among the strata of LFEV was significant (P = 0.003).
Again, the above results indicate that the number of hospitalizations is approximately 30% higher (IRR = 1.30, P = 0.694) in patients with ivabradine, compared to non-ivabradine patients after adjusting for all other variables, which is not statically significant.
Patients on B-Blockers compared to patients who are not taking B-Blockers (while holding other variables constant in the model) are expected to have a rate 0.41 times less for count of hospitalizations (P = 0.027). Patients on ACEi compared to patients who are not taking ACEi (while holding other variables constant in the model) are expected to have a rate 0.354 times less for count of hospitalizations (P = 0.032).
All-cause mortality
Death status was followed up within an 18-month period from the date of enrollment in the study. The total number of patients who died was six; all of them were Arabs, and five of them were diabetic and hypertensive. Four of the patients who died were <60 years old and two of them were more than 60 years old.Two patients were categorized clinically as NYHA class II and III whereas the remaining four patients' NHYA classification was not documented in their profiles. The ivabradine patients who died had previous MI, whereas the non-ivabradine patients who died did not experience MI before. All the 6 patients who died did not have stroke before. Out of the six dead patients, two (one ivabradine and one non-ivabradine) had an implemented CRT.
The effect of ivabradine had relative risk reduction of 14.81% (95% CI: 1.36–0.90) and absolute risk reduction of 4.1% (95% CI: −0.06 to − 0.14), which are not statistically significant. Based on the absolute risk reduction, 23 patients needed to be treated to prevent one death (P = 0.37). Kaplan–Meier curve [Figure 2] shows that the curves of ivabradine and non-ivabradine are overlapping.
Figure 2.
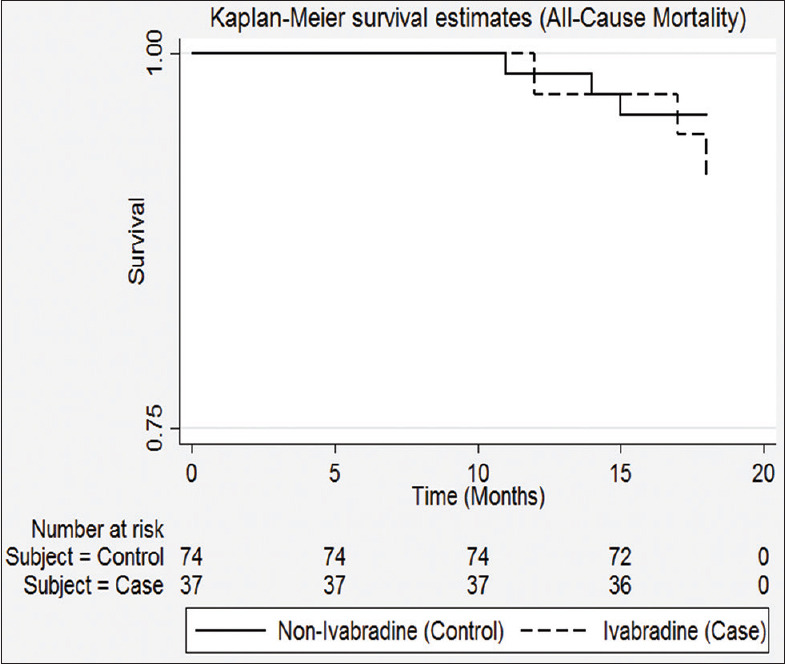
Kaplan-Meier Curve of treatment groups
DISCUSSION
Our results show that ivabradine reduced the risk for HFrEF patients of being hospitalized by 43%. Although it is not statically significant (P = 0.09) which might be due to small sample size, it incorporates a clinical significance in line with available evidence. The number and length of hospitalizations in ivabradine patients were not significantly different than those not taking ivabradine. Our results for risk of hospitalizations were consistent with the SHIFT study trail where the reduction of hospitalization risk fell by 18%.[13]
Pei et al.[18] analyzed the RR of cardiovascular composite endpoint events between the added ivabradine group and the standard anti-HF therapy group. The results showed that the RRs of all-cause mortality and cardiovascular mortality were not significantly different between the two groups. The RRs of cardiovascular death or worsening HF and HF were decreased in the added ivabradine group. However, the analysis of the causes of death in patients with HF showed that the RR of patients who died from HF was significantly decreased by the treatment with added ivabradine compared to that in the standard anti-HF therapy group.
However, a 3-year follow-up study by Tumasyan et al.[19] confirmed that the hospitalization rates at 1, 2, and 3 years were decreased by 31.3%, 27.5%, and 24.5%, respectively, and the 3-year mortality rate decreased by 14%, 10.5%, and 17.1%, respectively, in the 15 mg ivabradine group compared with those in the standard anti-HF therapy group.[19] Lopatin et al.[20] also postulated a similar conclusion.
Altogether, the above analyses indicated that treatment with added ivabradine was beneficial for the long-term prognosis of patients with HF and did not induce cardiovascular death.
The study by Zugck et al.[21] also found that the proportion of patients hospitalized within 1 year decreased from 23% before treatment to 5% with ivabradine therapy. Hence, the treatment with 5–7.5 mg ivabradine decreased the occurrence of cardiovascular events, especially HF relapse and hospitalization.
According to the guideline, for symptomatic HF patients (NYHA grade II-III) with a stable, chronic decrease in ejection fraction (LVEF ≤35%) who have received the maximum tolerated dose of β-blockers to treat a sinus rhythm with HR ≥70 bpm, ivabradine treatment could reduce the rate of hospitalization due to HF. Our study also found that ivabradine is beneficial for patients with HF who are still at high HR after full GDMT (ideal heart rate is 55–60 bpm at rest).
Lakobishvili et al.[22] conducted an observational study of 550 patients and found that approximately only a quarter of the patients were apparently suitable for consideration for ivabradine treatment. After a 12-month follow-up, 76.1% of the patients using ivabradine combined with β-blockers received at least half of the target dose of β-blockers compared to the 65.5% who received this dose in the only β-blockers group (P < 0.05).[20]
Another study[23] proved that a proportion of patients with HF and SHIFT-like characteristics may potentially benefit from ivabradine treatment. Thus, a substantial improvement in β-blocker therapy can be achieved by initiating treatment with ivabradine. Furthermore, CHF patients treated with the maximum dose of β-blocker may potentially benefit from the treatment with ivabradine. It is noteworthy that, in most studies, especially in the SHIFT study and the BEAUTIFUL study, ivabradine efficacy was obtained in patients who had received standard anti-HF treatment, and the use rate and dosage of the beta-blocker were far superior to the real world.
In our study, the ethnicity of the population was different than the landmark trails done on ivabradine. In the SHIFT study, the majority of patients were Western white people (89%). Our study population were mainly Arab and non-Arabs from Asia and Africa. We found that patients from non-Arab group had 31.9% less risk to be admitted compared to Arab after controlling for other factors. Non-Arab also showed a significant lower rate of length of hospital stay and number of hospitalizations compared to Arabs. Moreover, all six patients who died during the study period were Arabs. We also found that male gender had a nonsignificantly higher risk of hospitalization than females. These findings were consistent with a study that reported race, gender, and age are factors that are expected to have some effect on prediction of hospitalization.[24]
We have considered cardiovascular mortality as a second primary endpoint unlike in the SHIFT study, where it was a composite of cardiovascular death and HF hospitalization. We had similar results to SHIFT in terms of relative risk reduction. In SHIFT, RRR was 14.8% and we found it to be 14.81.
Comorbidities and other triggering variables were studied among all the outcomes in our study. Diabetes, hypertension, MI, and stroke. Increased heart rate and blood pressure showed a significant higher rate of risk of hospitalizations. Our results on GDMT intake were also in concordance with the recommendations of ACC/AHA 2016 guidelines which recommend GDMT to reduce morbidity. Patients on GDMT were found to have less rate for number and length of hospitalization compared to patients who are not on GDMT. However, patients on diuretics had higher expected rate 1.68 for days spent in the hospital. This could be due to their clinical deteriorating status that needs more care and longer stay in the hospital. Diuretics and mineralocorticoid receptor antagonist (MRAs) are prescribed for symptomatic HF patients to relieve fluid congestion.[14]
Strengths and weaknesses
Our study is the first observational study that assessed ivabradine effect on number and length of hospitalizations. Earlier studies focused on risk of hospitalization due to worsening HF symptoms.[11,17] We tried to fill the gap of lack of studies on ivabradine effect on cardiovascular outcomes in Arabs and non-Arabs from Asia and Africa. Earlier landmark studies included 8% of their sample from Asia and 3% from other races where white were the majority at 89%.[17] We also specifically measured the effect of ivabradine on death due to HF rather than considering all cardiovascular deaths that could be to other reasons.
The limitations of our study were raised due to the underreported three variables (smoking, EGFR, and NYHA classification) that may restrict the generalizability of the results. However, NYHA classification can be subjective according to patient's description of symptoms and physician assessment. Compliance to medication was considered by including patients on ivabradine and GDMT for more than 12 months from pharmacy. These patients were collected from the pharmacy. However, this is not a sufficient tool for measuring patient's compliance to medications.
Future research
Our study emphasizes on the need to direct health practitioners to optimize guidelines on directed medical therapy and encourage patients for pay attention to diet, lifestyle, and therapy rather than adding more medications to their regimen.
We suggest future studies to include patient's compliance and adherence to medications and lifestyle modifications with other predictors for the assessment of the effect of medications on HF patients' outcomes.
We also suggest to further assess the reasons behind the difference between Arabs and non-Arabs HF patients in their cardiovascular outcomes.
CONCLUSIONS
Ivabradine along with GDMT reduces the risk of hospitalizations due to worsening HF symptoms. Ivabradine had no significant effect on cardiovascular mortality and all-cause mortality. HFrEF non-Arabs patients have lower risk, number and length of hospitalization, and mortality compared to Arabs.
REFERENCES
- 1.White SM. Evidence-based strategies for advanced heart failure. Crit Care Nurs Clin North Am. 2019;31:1–13. doi: 10.1016/j.cnc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Isomi M, Sadahiro T, Ieda M. Progress and challenge of cardiac regeneration to treat heart failure. J Cardiol. 2019;73:97–101. doi: 10.1016/j.jjcc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu YC, Pfister O. How to diagnose heart-failure? Ther Umsch. 2018;75:151–4. doi: 10.1024/0040-5930/a000982. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC).Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 6.Jorsal A, Wiggers H, McMurray JJV. Heart failure: Epidemiology, pathophysiology, and management of heart failure in diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47:117–35. doi: 10.1016/j.ecl.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg E, Di Palo KE, Piña IL. Sex differences in heart failure. Clin Cardiol. 2018;41:211–6. doi: 10.1002/clc.22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varughese S. Management of acute decompensated heart failure. Crit Care Nurs Q. 2007;30:94–103. doi: 10.1097/01.CNQ.0000264253.52381.2a. [DOI] [PubMed] [Google Scholar]
- 9.Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101:865–9. doi: 10.1016/j.amjcard.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Laskey WK, Alomari I, Cox M, Schulte PJ, Zhao X, Hernandez AF, et al. Heart rate at hospital discharge in patients with heart failure is associated with mortality and rehospitalization. J Am Heart Assoc. 2015;4:e001626. doi: 10.1161/JAHA.114.001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–21. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 12.Savelieva I, Camm AJ. I f inhibition with ivabradine: Electrophysiological effects and safety. Drug Saf. 2008;31:95–107. doi: 10.2165/00002018-200831020-00001. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomized placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. 2016;68:1476–88. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Anantha Narayanan M, Reddy YN, Baskaran J, Deshmukh A, Benditt DG, Raveendran G. Ivabradine in the treatment of systolic heart failure – A systematic review and meta-analysis. World J Cardiol. 2017;9:182–90. doi: 10.4330/wjc.v9.i2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhabeeb W, Elasfar A, AlBackr H, AlShaer F, Almasood A, Alfaleh H, et al. Clinical characteristics, management and outcomes of patients with chronic heart failure: Results from the heart function assessment registry trial in Saudi Arabia (HEARTS-chronic) Int J Cardiol. 2017;235:94–9. doi: 10.1016/j.ijcard.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 17.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo controlled trial. Lancet. 2010;376:886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 18.Pei H, Miao W, Xie WZ, Wang W, Zhao D, Su GH, et al. Ivabradine improves cardiac function and increases exercise capacity in patients with chronic heart failure. Int Heart J. 2019;60:899–909. doi: 10.1536/ihj.18-559. [DOI] [PubMed] [Google Scholar]
- 19.Tumasyan LR, Adamyan KG. P1704 Comparative efficacy of long-term digoxin and ivabradine therapy on prognosis, left and right heart functional parameters in patients with chronic heart failure and preserved ejection fraction. Eur J Heart Fail. 2017;19:413. [Google Scholar]
- 20.Lopatin Y, Grebennikova A, Sisakian H, Hayrapetyan H, Pagava Z, Glezer M, et al. (2017) Benefits of Early Co-administration of Beta-blockers and Ivabradine in Patients Hospitalized Due to Worsening Heart Failure. Circulation. 136(1):A12310. [Google Scholar]
- 21.Zugck C, Stork S. RELIf-CHF Study Investigators. Long-term treatment with Ivabradine over 12 months in patients with chronic heart failure in clinical practice: Effect on symptoms, quality of life and hospitalizations. Int J Cardiol. 2017;240:258–64. doi: 10.1016/j.ijcard.2017.03.131. [DOI] [PubMed] [Google Scholar]
- 22.Pei H, Miao W, Xie W, Wang W, Zhao D, Su G, et al. (2019). Ivabradine Improves Cardiac Function and Increases Exercise Capacity in Patients with Chronic Heart Failure. International Heart Journal. 60(4):899–909. doi: 10.1536/ihj.18-559. doi: 10.1536/ihj.18-559. [DOI] [PubMed] [Google Scholar]
- 23.Roth S, Fernando C, Azeem S, Moe GW. Is there a role for ivabradine in the contemporary management of patients with chronic heart failure in academic and community heart failure clinics in Canada? Adv Ther. 2017;34:1340–8. doi: 10.1007/s12325-017-0529-4. [DOI] [PubMed] [Google Scholar]
- 24.Colucci WS, Gottlieb SS, Yeon SB, Moe GW. “Prognosis of Heart Failure – UpToDate.”. [Last accessed on 2017 Jan 08]. Available from: https://0-www.u p t o d a t e . c o m . m y l i b r a r y . q u . e d u . q a / c o n t e n t s /prognosis-of-heart-failure?source=machineLearning and search=causesoffailurehearthospitalization and selectedTitle=1 ~ 150 and sectionRank=1 and anchor=H3#H3 .