Abstract
Serology tests for SARS-CoV-2 have proven to be important tools to fight against the COVID-19 pandemic. These serological tests can be used in low-income and remote areas for patient contact tracing, epidemiologic studies and vaccine efficacy evaluations. In this study, we used a semi-stable mammalian episomal expression system to produce high quantities of the receptor-binding domain-RBD of SARS-CoV-2 in a simple and very economical way. The recombinant antigen was tested in an in-house IgG ELISA for COVID-19 with a panel of human sera. A performance comparison of this serology test with a commercial test based on the full-length spike protein showed 100% of concordance between tests. Thus, this serological test can be an attractive and inexpensive option in scenarios of limited resources to face the COVID-19 pandemic.
Keywords: SARS-CoV-2, COVID-19, Serological test, RBD, ELISA
1. Introduction
In December 2019, the coronavirus SARS-CoV-2 produced an outbreak in the Wuhan region of the People's Republic of China. Only 3 months later, the World Health Organization (WHO) declared COVID-19 as a pandemic disease (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline). By May 2021, SARS-CoV-2 had infected more than 153 million people and had caused more than 3.2 million deaths worldwide (https://coronavirus.jhu.edu/map.html).
Rapidly, the State-funded consortium of universities together with research institutes and pharmaceutical companies responded to the COVID-19 pandemic, by working around the clock on the development of vaccines, treatments and diagnostic tools. However, middle and low-income countries have had limited access to these biotechnological products. The distribution of vaccines and diagnostic reagents to face this pandemic worldwide has been unequal, with priority on economic, rather than humanitarian, criteria. Thus, it is essential that countries or regions aim for the autonomous development of crucial biotechnological tools.
As many other infectious diseases, diagnosis of COVID-19 can be done by detection of components of the pathogen (such as nucleic acids or proteins) or of the host's immune response against the pathogen (such as specific antibodies against pathogen's proteins). RT-qPCR assays are the gold standard tests for COVID-19 for the detection of the SARS-CoV-2 genome, even during the incubation period. Other less sensitive tests can also detect SARS-CoV-2 proteins at the very early onset of the disease (Berger et al., 2021). Since low level of antibodies is produced at early stage of SARS-CoV-2 infection, antibody testing is not the best diagnostic strategy (Ojeda et al., 2021). However, antibody tests can be used for the detection of past SARS-CoV-2 infection or vaccination.
Researchers have developed and validated several serological tests for COVID-19 (/open.fda.gov/apis/device/covid19serology/, https://www.centerforhealthsecurity.org/covid-19TestingToolkit/serology/Serology-based-tests-for-COVID-19.html) (Mohit et al., 2021). The main antigenic targets in these tests are the nucleocapsid (N) protein and spike (S) protein, including subunits S1 and S2, as well as the receptor-binding domain (RBD) of SARS-CoV-2.
Although the trimer stabilized version of S proteins allowed the improvement of the recombinant protein yield in a mammalian cell expression system (Hsieh et al., 2020), the tests based on the full-length S proteins demand large volumes of cell cultures for protein production. On the other hand, the RBD of S protein showed good levels of expression in mammalian cells (Stadlbauer et al., 2020).
A recent analysis of the performance of four serology assays for COVID-19 based on the RBD domain resulted in sensitivity and specificity values of 98–87% and 100%, respectively (Ikegami et al., 2021). The study of Ma and collaborators has shown a better diagnostic accuracy of an RBD-based test than that of an N-based test (Ma et al., 2020), with a sensitivity of 100% 16 days after disease onset for this RBD-based test. Regarding ELISA tests based on the full-length S protein, Ojeda and collaborators have reported that, between 2 and 3 weeks after the disease onset of RT-qPCR positive patients, the IgG detection was 74%, and increased up to 90.4% after 3 weeks (Ojeda et al., 2021).
Validated serological tests can be applicable as an indicator of the stage of progression of COVID-19 and therefore can help guide the response to the pandemic. These tests are also useful for patient contact tracing and epidemiologic studies as well as for the evaluation of vaccine efficacy and development of therapeutic antibodies.
Here, we expressed RBD domain in a semi-stable mammalian episomal expression system and developed an inexpensive in house IgG ELISA test for COVID-19, whose performance was compared with that of the commercial IgG ELISA COVIDAR.
2. Material and methods
2.1. Construction of the recombinant plasmid
RBD sequence was amplified with a High-Fidelity DNA Polymerase (Q5® NEB) from a plasmid kindly provided by Florian Krammer (Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, NY, USA) by using primers 5´AAGCTTGCCACCATGTTCGTGTTTCT3´ and 5´AAGCTTTCAATGGTGATGGTGATGGTG3´. The amplified DNA product was cloned in the HindIII site of the plasmid pEAK 8 CMV I Promoter (Magistrelli et al., 2010, Magistrelli et al., 2012). Sequencing of the cloned gene showed no introduction of mutations during PCR amplifications or cloning steps.
2.2. Cell transfection and protein production
PEAK rapid ATCC ® CRL-2828™ cells were seeded in 6-well cell culture plates at a density of 2 × 105 cells per well and incubated in Roswell Park Memorial Institute medium (Sigma-Aldrich) supplemented with 10% of foetal bovine serum (Internegocios) (RPMI-FBS) at 37 °C in 5% CO2. The medium was removed on the following day and 80–90% confluent cell monolayers were transfected with 2 μg of the recombinant plasmid plus 3.5 μl of Lipofectamine 3000 Reagent (ThermoFisher) and 5 μl of P3000 Reagent (ThermoFisher) in RMPI following the manufacturer's recommendations. At day 1 post-transfection the transfection medium was removed and replaced with RPMI-FBS containing 2 μg /ml of puromycin (Sigma-Aldrich) for selection. The transfected cells were detached from the wells and reseeded in successive passages to T25 or T75 flasks with RPMI-FBS or Ex-Cell 293 medium (Sigma-Aldrich) supplemented with glutamine (Sigma-Aldrich) and 1% FBS. After 5–7 days of culturing, culture supernatants were collected, filtered through a 0.22-nm pore membrane (Millipore, Billerica, MA) and kept at 4 °C.
2.3. Protein purification by immobilized ion affinity chromatography
Ni-NTA resin (4 ml, Qiagen) was washed with PBS. The resin was then incubated with 200 ml of filtered culture supernatant in four clean 50-ml conical tubes for 2 h with constantly mixing by inversion in an orbital shaker. The resin was spin at 2000 g for 5 min in a centrifuge and washed with 500 ml of PBS 20 mM imidazole. A polypropylene column was loaded with the washed resin and washed again with 20 ml of PBS 20 mM imidazole. Then, the column was eluted using increasing concentrations of imidazole (250 mM to 1 M imidazole- Biopack). Eight-nine fractions of 500 μl were collected in total. The protein fractions were stored at −20 °C.
2.4. SDS-PAGE and Western blot
Protein samples were resuspended in cracking buffer (60 mM Tris-Cl pH 6.8, 2% SDS, 10% glycerol, 2% β-mercaptoethanol, 0.01% bromophenol blue), heated at 100 °C for 3 min and subsequently subjected to electrophoresis in 12% SDS-PAGE gels. The gels were stained with colloidal Coomasie brilliant blue.
A western blot analysis was performed by first transferring gels to nitrocellulose membranes that were then blocked with TBS (10 mM tris-HCl pH 7.5, 150 mM NaCl) supplemented with 5% skim milk for 30 min before incubating with anti –his antibody (GE) for 2 h. The nitrocellulose membranes were washed with TBS thrice and incubated with a secondary anti-IgG-mouse alkaline phosphatase-conjugated antibody at a 1:10000 dilution for 2 h. Western blots were revealed by incubation with BCIP/NTP solution (Promega).
2.5. Human serum samples
Human serum samples were obtained from the Biobank at the Hospital de Clínicas “José de San Martín” Universidad de Buenos Aires. Informed consent for research use of blood samples were obtained from all donors in order to be part of the Biobank (University Hospital's Institutional Review Board (IRB), Comité de Ética Hospital de Clínicas “José de San Martin” Universidad de Buenos Aires).
A total of 129 human serum samples were included in this study. All serum samples were analysed with a commercial ELISA test to detect IgG antibodies against S protein of SARS-CoV-2 (COVIDAR test) according to the manufacturer's instructions (Ojeda et al., 2021). A total of 53 positive serum samples were obtained from patients with confirmed SARS-CoV-2 infection diagnosed by the CDC 2019 Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)–PCR Diagnostic Panel (available at https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html). Negative serum samples obtained during a community seroprevalence study of SARS-CoV-2 from asymptomatic adults were subsequently analysed for IgG antibodies against S protein (SARS-CoV-2 IgG II Quant assay, Abbott) and N protein (SARS-CoV-2 IgG assay, Abbott). Regarding the negative serum samples, 59 were regarded as “negative” in all three assays aforementioned. A few samples (17) were only tested with COVIDAR (negative = 10, positive = 7).
All these serum samples were obtained from adult patients from the metropolitan area of Buenos Aires city and Buenos Aires Province during 2020. Most SARS-CoV-2 sequences obtained in this area in 2020 belonged to B linage, which was responsible for most infections in Europe and North America during the first phase of the pandemic. Up to December 2020, the only variant of concern (VOC) of SARS-CoV-2 circulating in Argentina was B.1.1.28, whose origin was in Rio de Janeiro, Brasil. UK variant (B.1.1.7) was detected for the first time at the end of December from an imported case (http://pais.qb.fcen.uba.ar/reports.php).
Additionally, 33 serum samples from people vaccinated against COVID-19 obtained in the metropolitan area of Buenos Aires city during 2021 were also included in this study. These sera were taken 2–3 weeks after the administration of the first or second dose of Sputnik V (Gamaleya) (27), Sinopharm (3) or (Oxford –AstraZeneca) (3).
2.6. ELISA
The in-house ELISA protocol was adapted from one previously reported (Stadlbauer et al., 2020). Briefly, 96-well microtiter ELISA plates (nuncMaxisorp) were coated with 50 μl of RBD (2 μg/ml) in PBS overnight (ON) at 4 °C. The plates were blocked for 1 h with 200 μl of PBS-T plus 3% milk powder at room temperature (RT) before incubating 100–200 μl of diluted serum samples (1:50) in PBS-T plus 1% milk powder at RT for 2 h. The wells were washed 12 times with 200 μl of PBS-T before adding 100 μl of 1/3000 Goat anti-Human IgG HRP (Promega) in PBS-T + 1% milk power. The plates were further incubated for 1 h at RT and then washed 12 times with 200 μl of PBS-T. Once the wells were completely dried, 100 μl of 1% 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)-ABTS inphosphate citrate buffer plus 0.1 μl of 30% hydrogen peroxide were added to each well and the colour developing was then stopped with 100 μl of stop solution (50% v/v dimethylformamide (Sigma) 20%w/v SDS (Promega)). The optical density at 405 nm was measured with Multiskanplate reader. This in-house ELISA protocol was used to assay 162 serum samples.
The cut off value of the in-house RBD ELISA was calculated as mean + 3xSD of sera from people that were negative to both RT-qPCR of COVID-19 and commercial IgG ELISA (COVIDAR).
All sera were analysed with COVIDAR ELISA following the manufacturer's recommendations.
3. Results
3.1. Production of RBD from mammalian cells
Transient expression of recombinant proteins in eukaryotic cells demands the production of large quantities of ultrapurified recombinant plasmids as well as expensive reagents and media to optimize the transfection efficiency. Moreover, each round of protein production requires starting all over again beginning at the cellular transfection step. In contrast, semi-stable expression systems use episomal vectors that replicate extra-chromosomally in cells over a long period (Magistrelli et al., 2010, Magistrelli et al., 2012); which allows the expansion of transfected cells at a logarithmic scale.
In this study, the sequence encoding the polypeptide RBD of S protein was cloned in pEAK 8 CMV I Promoter. This vector is an episomal replicative plasmid that confers puromycin resistance to the transfected cells. In the presence of puromycin only transfected cells replicate. The sequence encoding a six histidine tag was added at the end of the gene for purification purposes. The recombinant plasmid was used to transfect 2 × 106 of PEAKrapid ATCC ® CRL-2828™ cells. The transfected culture was expanded by successive passages in standard complete medium or serum-free medium containing puromycin until reaching 8.5x107cells. Recombinant RBD was purified from culture supernatant by a single step of metal ion affinity chromatography.
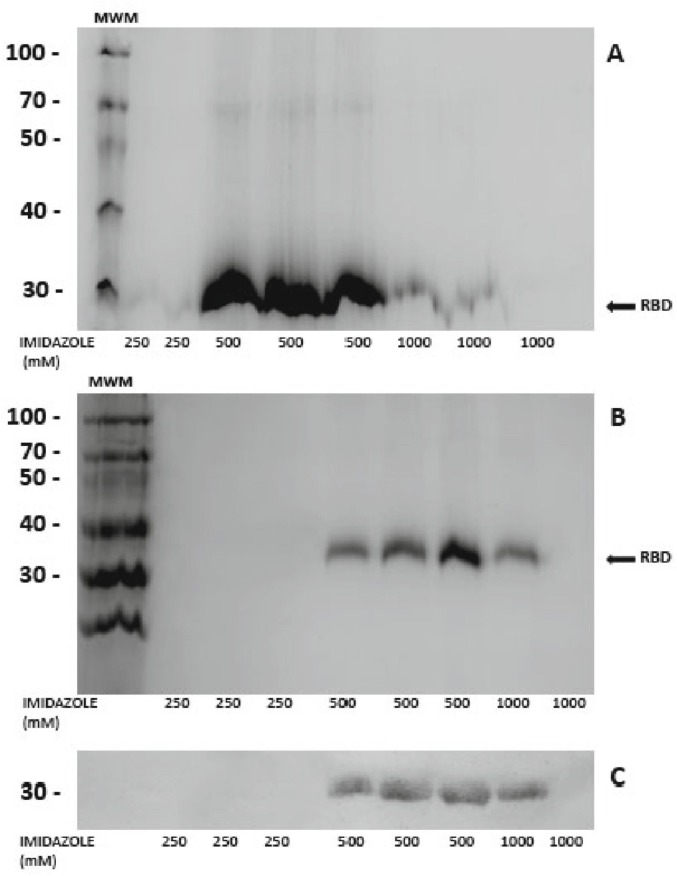
SDS-polyacrylamide gel electrophoresis of elution fractions showed the presence of RBD migrating at the expected molecular weight of around 30 kDa (Fig. 1 ). The recombinant protein yield was 10 mg/l.
Fig. 1.
RBD protein production.
SDS-PAGE of elution fractions containing RBD. Recombinant RBD was eluted from the immobilized metal affinity chromatography (IMAC) with increasing concentrations of imidazole. (A) Coomasie brilliant blue stain of protein fractions obtained from cells grown in complete medium (A) or reduced-serum medium (B), (C) Western blot of protein fractions in B using anti-his as primary antibody (GE) 1/1000 and anti mouse as secondary antibody (Sigma) 1/30,000. Imidazole concentrations used to elute the proteins are indicated in x axis. MWM: 14–100 kDa Blue Plus Protein Marker (TransGen Biotech).
Transfected cells were viable after freezing and thawing, with production of equivalent amounts of secreted recombinant protein to that of the non-frozen cells (10 mg/l).
3.2. Evaluation of RBD antigenicity against human sera
In order to assess the antigenicity power of RBD, we developed an in-house ELISA that was subsequently evaluated with a panel of human sera. The serum panel included 53 samples positive for a commercial IgG ELISA based on the entire S protein of SARS-CoV-2 (COVIDAR) and 59 negative sera according to this ELISA test.
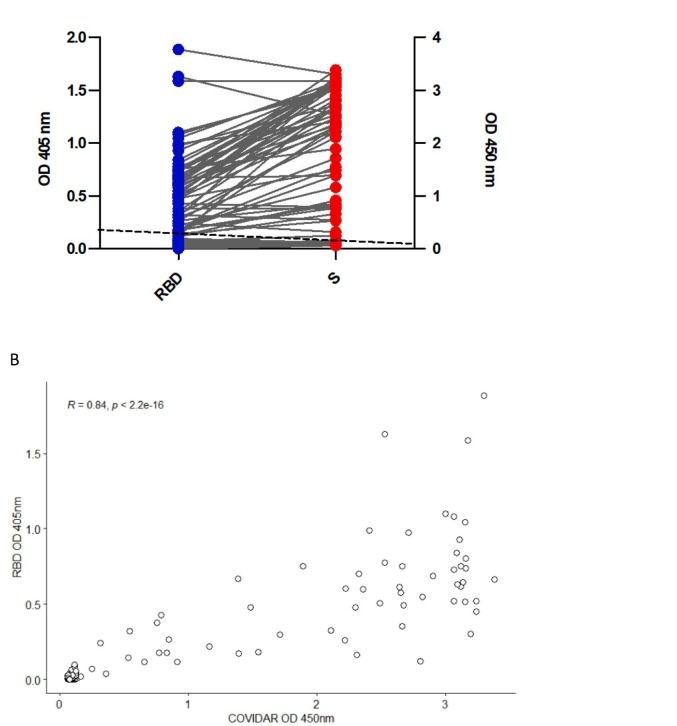
The concordance between the commercially available S IgG ELISA test and in-house IgG RBD ELISA was 100% (Fig. 2A). Linear regression analysis showed a very good correlation (R = 0.84) between the OD values obtained by the in-house RBD ELISA and COVIDAR test used as a reference (Fig. 2B). However, in general, the ratio of positive serum to the average value of the negative sera was higher for the S-based test than for the RBD-based test (Table 1 ).
Fig. 2.
Validation of in-house IgG ELISA using RBD.
ELISA for detection of SARS-CoV-2–specific IgG antibodies. (A) Blue dots indicate serum reactivity to RBD and red dots indicate serum reactivity to S. The dotted line indicates ELISA cut-off value = 0.1 (mean of negative sera +3xSD) and 0.25 for IgG RBD ELISA and COVIDAR, respectively. (B) Spearman's rank correlation analysis between the OD values obtained by the in-house RBD ELISA (y) and COVIDAR (x). R = 0.84, p-value <2.2 e-16.
Table 1.
Intensity of antibody recognition against RBD and S protein related to average value of negative sera.
| Serum sample # | RBD⁎ | S⁎ |
|---|---|---|
| 1 | 10.8 | 41.3 |
| 2 | 4.3 | 25.6 |
| 3 | 11 | 35 |
| 4 | 7.9 | 29.5 |
| 5 | 9.3 | 36.3 |
| 6 | 6.5 | 36.2 |
| 7 | 7.2 | 36.2 |
| 8 | 3 | 14.2 |
| 9 | 4.3 | 17.2 |
| 10 | 4.1 | 5.6 |
Ratio of serum sample#/average negative sera.
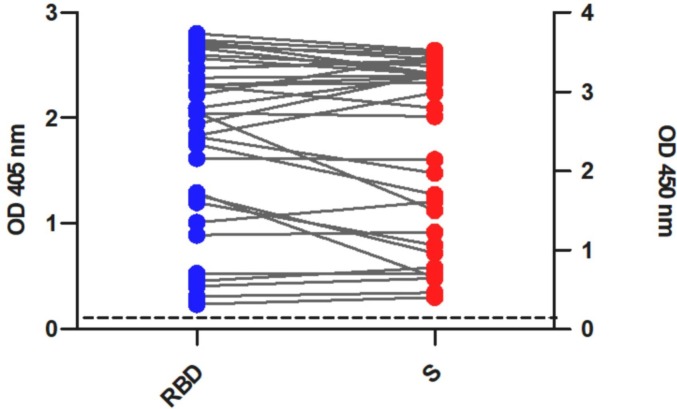
To determine the usefulness of the in-house RBD IgG ELISA test as a tool to estimate the rate of COVID19 vaccination, we subsequently evaluated the reactivity of this test against sera of vaccinated people with different vaccine formulations. All serum samples (33) were positive for RBD IgG (Fig. 3 ) and COVIDAR tests. This result indicates that, at least for the analysed samples, the test identified vaccinated people.
Fig. 3.
Reactivity of human sera to RBD and S proteins.
ELISA for detection of SARS-CoV-2–specific IgG antibodies in serum samples from vaccinated people. Blue dots indicate serum reactivity to RBD and red dots indicate serum reactivity to S. The dotted line indicates ELISA cut-off value = 0.1 (mean of negative sera +3xSD) and 0.25 for IgG RBD ELISA and COVIDAR, respectively. These results represent one of two independent replicates.
4. Discussion
In this study, we used an easy and inexpensive mammalian expression system to produce the RDB domain of S protein of SARS-CoV-2. Starting with the transfection of a cell monolayer in a 9.6 cm2 well with the 2 μg of the episomal recombinant plasmid, at the end of the process we purified 2 mg of the RBD protein. The entire protocol avoids the use of expensive reagents or media and any eventual low cell transfection efficiency can be easily resolved by expanding the transfected cells in medium containing puromycin. According to Thermofisher official web page, 1 l of transfected cells produced with Expi293 Expression System or other FreeStyle 293 Medium costs $1531 and $548, respectively. Although the production levels vary significantly among culture media, which makes it impossible to make an adequate comparison of production costs, for this study we estimated a value of $2.35 for lipofectamine 3000 (ThermoFisher, the most expensive reagent used in our protocol) to produce 200 ml of semi-stable transfected cells expressing RBD. Thus, the system expression and protocol here described is an attractive option for low budget scenarios.
Interestingly, the results (positive/negative) obtained with the in-house RBD IgG ELISA matched exactly to those of a commercially available ELISA test that employs the full-length S protein of SARS-CoV-2. The results of this study were reproducible in two independent determinations made in a time interval of six months and performed by different operators. The commercial test used here for comparison, called COVIDAR, has been tested in RT-qPCR SarsCoV-2 positive patients and in a large panel of human sera obtained before 2020 (Ojeda et al., 2021). The sensitivity of COVIDAR reported for IgG detection was 72% to 74% between 2 and 3 weeks from the onset of symptoms and seroconversion increased up to 90.4% after 3 weeks (Ojeda et al., 2021). Based on the high concordance of our test with COVIDAR, we may estimate similar performance in both assays. However, further analysis with a larger panel of serum samples is necessary to precisely determine the sensitivity and specificity of this RBD IgG ELISA test.
As previously reported (Amanat et al., 2020; Stadlbauer et al., 2020), we have found that the antibody reactivity against COVID-19 was stronger against the S protein than against the RBD domain. This was an expected result since more B epitopes are present in the full-length S than in RBD region. However, despite its lower reactivity, RBD is the target of 90% of the neutralizing activity present in SARS-CoV-2 immune sera (Piccoli et al., 2020). Therefore, the use of RBD in serological studies would allow a more direct estimation of neutralizing antibodies in serum samples. This feature makes the test developed in this study a simple and very useful tool for first screening of sera with neutralizing capacity.
Another important outcome of this study is the good performance of the in-house RBD IgG ELISA to identify vaccinated people. All serum samples from vaccinated people (33) were positive when tested with RBD IgG ELISA; which is in agreement with the general believe that antibodies appear at least 15 days after vaccination (Mohit et al., 2021).
The emergence and rapid spread of new VOC of SARS-CoV-2 around the world is complicating the control of the COVID-19 pandemic. Most of these VOCs carry mutations on RBD (Vasireddy et al., 2021) since they give the virus a genetic advantage in terms of transmissibility, morbidity/mortality or vaccine escape, thus resulting in antigenic drifts to escape from immune recognition. Also, it has been reported that some mutations in the RBD of VOCs may increase their binding to the ACE2 cell receptor. These variants have shown increased transmissibility, greater disease severity and higher mortality. Importantly, some VOCs have shown an in vitro partial resistance to neutralizing antibodies from vaccinated people, suggesting a reduced vaccine efficacy against these VOCs (Planas et al., 2021; González-Candelas et al., 2021). Although no significant failure has been reported with the currently available diagnostic tests in areas where VOCs are prevalent (Vasireddy et al., 2021), the potential capacity of VOCs to avoid antibody recognition alerts to the need for diagnostic strategies that anticipate the appearance of new virus variants. In this regard, since RBD is a protein of a lower molecular weight compared to S, modifying its gene sequence according to the new adaptive variants would be quick and easy. The optimal size of RBD gene sequence facilitates its PCR amplification and further cloning steps in the multiple cloning site of pEAK 8 CMV episomal plasmid.
In conclusion, the protein expression system and the assay developed and tested in this study is an inexpensive alternative in serological surveillance and vaccine evaluation studies.
Funding
This work was supported by the Instituto Nacional de Tecnología Agropecuaria (INTA) Grant i102 and by ANPCyT PICT-2018-01113 and 2017-1721.
Competing financial and non-financial interests
All authors declare no competing or financial interests.
Author contributions statement
Luciana Villafañe, Lucía Gallo Vauletand Marcelo Rodríguez Fermepin: serology studies, resources and writing. Laura I. Klepp and Marina A. Forrellad: protein expression and purification; Florencia M. Viere: conceptualization and resources; María M. Bigi: statistical analysis. María I. Romano: conceptualization and writing; Giovanni Magistrelli: analysis, resource, review and editing; Fabiana Bigi: conceptualization, formal analysis, writing the original draft and editing.
Acknowledgements
We thank COVIDAR consortium and F. Krammer very much for providing us with COVIDAR kit and the plasmid expressing RDB domain, respectively. We are grateful to people from the community who kindly provided us with blood samples. We also thank Laura Alcaraz for her assistance in sera selection at HCJSM and Dr. Julia Sabio Garcia for critical reading of this paper. LIK, MAF and F. Bigi are CONICET fellows.
References
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D.S., Lugo L.A., Kojic E.M., Stoever J., Liu S.T.H., Cunningham-Rundles C., Felgner P.L., Moran T., García-Sastre A., Caplivski D., Cheng A.C., Kedzierska K., Vapalahti O., Hepojoki J.M., Simon V., Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26 doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A., Nsoga M.T.N., Perez-Rodriguez F.J., Aad Y.A., Sattonnet-Roche P., Gayet-Ageron A., Jaksic C., Torriani G., Boehm E., Kronig I., Sacks J.A., de Vos M., Jacquerioz Bausch F., Chappuis F., Renzoni A., Kaiser L., Schibler M., Eckerle I. Diagnostic accuracy of two commercial SARSCoV- 2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Candelas F., Shaw M.A., Phan T., Kulkarni-Kale U., Paraskevis D., Luciani F., Kimura H., Sironi M. One year into the pandemic: short-term evolution of SARS-CoV-2 and emergence of new lineages. Infect. Genet. Evol. 2021 doi: 10.1016/j.meegid.2021.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.C., Javanmardi K., Le K.C., Wrapp D., Lee A.G., Liu Y., Chou C.W., Byrne P.O., Hjorth C.K., Johnson N.V., Ludes-Meyers J., Nguyen A.W., Park J., Wang N., Amengor D., Lavinder J.J., Ippolito G.C., Maynard J.A., Finkelstein I.J., McLellan J.S. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369 doi: 10.1126/SCIENCE.ABD0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Benirschke R.C., Fakhrai-Rad H., Motamedi M.H., Hockett R., David S., Lee H.K., Kang J., Gniadek T.J. Target specific serologic analysis of COVID-19 convalescent plasma. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Zeng W., He H., Zhao D., Yang Yunru, Jiang D., Zhou P., Qi Y., He W., Zhao C., Yi R., Wang X., Wang B., Xu Y., Yang Yun, KombeKombe A.J., Ding C., Xie J., Gao Y., Cheng L., Li Y., Ma X., Jin T. COVID-19 diagnosis and study of serum SARS-CoV-2 specific IgA, IgM and IgG by chemiluminescence immunoanalysis. medRxiv. 2020 doi: 10.1101/2020.04.17.20064907. [DOI] [Google Scholar]
- Magistrelli G., Malinge P., Lissilaa R., Fagte S., Guilhot F., Moine V., Buatois V., Delneste Y., Kellenberger S., Gueneau F., Ravn U., Kosco-Vilbois M., Fischer N. Rapid, simple and high yield production of recombinant proteins in mammalian cells using a versatile episomal system. Protein Expr. Purif. 2010;72 doi: 10.1016/j.pep.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Magistrelli G., Malinge P., Elson G., Fischer N. Protein Purification. 2012. Episomal vectors for rapid expression and purification of proteins in mammalian Cells. [DOI] [Google Scholar]
- Mohit E., Rostami Z., Vahidi H. A comparative review of immunoassays for COVID-19 detection. Expert. Rev. Clin. Immunol. 2021 doi: 10.1080/1744666x.2021.1908886. [DOI] [PubMed] [Google Scholar]
- Ojeda D.S., Lopez Ledesma M.M.G., Pallarés H.M., Costa Navarro G.S., Sanchez L., Perazzi B., Villordo S.M., Alvarez D.E., Echavarria M., Oguntuyo K.Y., Stevens C.S., Lee B., Carradori J., Caramelo J.J., Yanovsky M.J., Gamarnik A.v., Long-Ueira Y., Polo M.L., Salvatori M., Azzolina S., Ghiglione Y., Salomon H., Quiroga M.F., Turk G., Laufer N. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E., Acton O.J., Jaconi S., Guarino B., Minola A., Zatta F., Sprugasci N., Bassi J., Peter A., de Marco A., Nix J.C., Mele F., Jovic S., Rodriguez B.F., Gupta S.v., Jin F., Piumatti G., Lo Presti G., Pellanda A.F., Biggiogero M., Tarkowski M., Pizzuto M.S., Cameroni E., Havenar-Daughton C., Smithey M., Hong D., Lepori V., Albanese E., Ceschi A., Bernasconi E., Elzi L., Ferrari P., Garzoni C., Riva A., Snell G., Sallusto F., Fink K., Virgin H.W., Lanzavecchia A., Corti D., Veesler D. Mapping neutralizing and Immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183 doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., Planchais C., Buchrieser J., Rajah M.M., Bishop E., Albert M., Donati F., Prot M., Behillil S., Enouf V., Maquart M., Smati-Lafarge M., Varon E., Schortgen F., Yahyaoui L., Gonzalez M., de Sèze J., Péré H., Veyer D., Sève A., Simon-Lorière E., Fafi-Kremer S., Stefic K., Mouquet H., Hocqueloux L., van der Werf S., Prazuck T., Schwartz O. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021;27 doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E., Meade P., Brito R.N., Teo C., McMahon M., Simon V., Krammer F. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 2020;57 doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasireddy D., Vanaparthy R., Mohan G., Malayala S.V., Atluri P. Review of COVID-19 variants and COVID-19 vaccine efficacy: what the clinician should know? J. Clin. Med. Res. 2021;13 doi: 10.14740/jocmr4518. [DOI] [PMC free article] [PubMed] [Google Scholar]