To the Editor,
Portal vein thrombosis (PVT), defined as thrombosis of the main portal vein (PV), or its branches or the splenic vein (SV) or superior mesenteric vein (SMV) of the spleno-portal axis, is a well-known complication of the hypercoagulable state of cirrhosis.1 However, thrombosis of deep veins, pulmonary microcirculation, and arterial thrombosis has also been reported as a consequence of COVID-19 infection or vaccination.2 The post-COVID-19 thrombophilia is of great relevance in hepatology practice as it predisposes to PVT related increased portal pressures and variceal bleeding, hepatic venous outflow tract obstruction, or post-transplant vascular complications.3
Herein we report six cases of new-onset PVT, who were on hepatocellular carcinoma (HCC) surveillance imaging and were diagnosed with new main PVT or branch PVT following COVID 19 infection in three cases and following vaccination with ChAdOx1 nCoV-19 Coronavirus vaccine (recombinant)in three cases. The surveillance protocol at our institute is based on Ultrasound Doppler imaging every 3 months and a semi-annual triple computed tomography (CT) or magnetic resonance imaging (MRI). All 6 patients had confirmed PVT on either CT or MRI (Table 1). The mean time since the last screening imaging was 95 ± 22.5 days.
Table 1.
Clinical Characteristics of the 6 Patients Who Presented with New Onset PVT.
| Patient Details | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Age (Years) | 38 | 45 | 53 | 55 | 56 | 48 |
| Sex | Male | Male | Male | Female | Female | Male |
| Etiology of Liver Disease | Ethanol | Ethanol | Ethanol | HCV | NAFLD | NAFLD |
| Co morbidity | None | Hypertension | None | None | Diabetes mellitus | Diabetes mellitus |
| COVID19 Infection | Yes | Yes | No | Yes | Yes | No |
| Severity of COVID-infection | Mild | Moderate (Oxygen requiring) | Moderate (Oxygen requiring) | Mild | ||
| Vaccination | No | No | Yes | Yes | No | Yes |
| Number of doses received | 1 | 1 | 2 | |||
| Use of anticoagulation in the last 6 months | No | No | No | Yes. 5 days of LMW heparin. | No | No |
| Use of Steroids in the last 6 months | No | No | No | No | Yes | No |
| Time period between COVID19 to detection of PVT (Days) | 70 | 90 | NA | 90 | 60 | NA |
| Time period between COVID19 vaccination to detection of PVT (Days) | NA | NA | 110 | 60 | NA | 90 |
| Time period between date of last surveillance ultrasound Doppler examination till detection of new PVT (Days) | 120 | 90 | 60 | 90 | 90 | 120 |
| PVT | Main | Main | Eccentric PVT | Branch | Main | Main |
| SMVT | No | No | Yes | No | No | No |
| ST | No | No | Yes | No | No | No |
| Presentation | Pain | Variceal Bleeding | Pain | Increasing ascites | Variceal Bleeding | New onset ascites |
| Other Decompensation | Ascites | Hepatic Encephalopathy | Ascites | |||
| CTP | 9 | 9 | 9 | 12 | 13 | 12 |
| MELDNa | 11 | 13 | 14 | 15 | 18 | 18 |
| COVID antibody titre (CLIA) | 14.9 | 12.5 | Not done | 8.5 | Not done | 7.45 |
| JAK2 mutation | Negative | Negative | Negative | Negative | Negative | Negative |
| Factor V Leiden mutation | Negative | Negative | Negative | Negative | Negative | Negative |
| Treatment | On Variceal Eradication | On Variceal Eradication | On Dabigatran | Expired due to secondary sepsis, bacterial pneumonia | Expired due to systemic sepsis, difficult to treat SBP. | On variceal eradication |
| Hill’s Criteria | ||||||
| Temporality | ++ | ++ | ++ | ++ | ++ | ++ |
| Biological Plausibility | + | + | ++ | + | + | ++ |
| Likelihood of causal relationship with Vaccine | Probable association | Possible association | Probable association | |||
Abbreviations: CTP, Child Turcotte Pugh score; CLIA, chemiluminescent immunoassay; JAK2, Janus kinase 2 gene mutation; PVT, portal vein thrombosis; LMWH, low molecular weight heparin; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; SBP, spontaneous bacterial peritonitis.
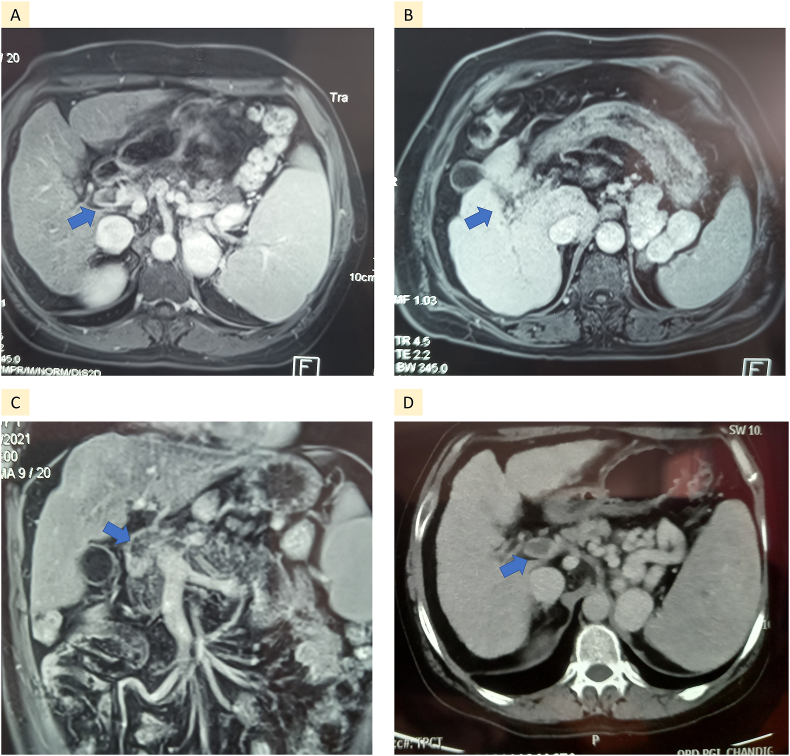
These patients were aged 49.1 ± 7.7 years, 3 (50%) were ethanol related, 66.6% were male, with 2 patients with nonalcoholic fatty liver disease (NAFLD) having diabetes mellitus and one having hypertension as comorbidities. None of the patients had associated HCC, and all tested negative for JAK2 and Factor V Leiden mutation. One patient presented with acute variceal bleeding requiring endotherapy, while the others had mild epigastric pain. Table 1 summarizes the clinical presentations and timeline to thrombosis of each patient. Using Hill’s criteria to establish causality, a probable association was found for two of the patients who had strong temporal association of developing PVT after vaccination. A detailed drug history did not reveal the use of any medicine that could have caused thrombosis. Although cirrhosis per se is a procoagulant condition, we were unable to find any other confounders or underlying hematological conditions that could have caused the PVT. Figure 1 shows the imaging of patients 3, 4, and 6, which shows the acute PVT and early formation of collaterals.
Figure 1.
Imaging of 4 patients with acute main portal vein thrombosis (Blue arrow). Formation of splenorenal shunt is seen in patient B and D.
This case series illustrates the thrombophilia that is associated with the COVID-19 infection per se and reported with the ChAdOx1 nCoV-19 Coronavirus vaccine. It is not possible to establish causality, although all patients tested negative for COVID-19 on RT PCR test at the time of the PVT diagnosis, four of them had prior COVID-19 illness, some of the others who were subsequently vaccinated may have had undiagnosed subclinical COVID-19 infection in the past.4 Nonetheless, this case series shows data that suggests clinicians should be cognizant of vascular complications following COVID-19, and/or vaccination and should assess for venous thrombosis specifically in all patients with cirrhosis, as early diagnosis and treatment with suitable anticoagulation can improve outcomes.5 The incidence of thrombotic disease in patients with COVID-19 is as high as 31% and affects overall outcomes.2 In our series on patients with COVID-19, we used global coagulation tests to identify and treat patients with a hypercoagulable profile with systemic anticoagulation. Of 215 patients with COVID -19, 74 patients requiring intensive care (53 ± 16 years; 64%male) were recruited. The patients were divided into three groups with 11 (14.9%), 34 (45.9% and 29 (39.2%) on low-flow O2 therapy, high-flow O2 therapy, and invasive ventilation, respectively. A procoagulant profile was seen in 45.5%, 32.4%, and 20.7% in low-flow, high-flow, and invasive ventilation.6 We were able to perform COVID antibody testing for 4 (66%) patients, all of whom were reactive for the same.
In conclusion, the key message that needs to be propagated is that COVID-19 vaccines are safe and prevent deaths due to SARS-CoV-2. All eligible patients with chronic liver disease should be offered vaccination to protect them from COVID-19-related mortality. However, the thrombotic perturbations uncovered in the COVID-19 era have critical relevance for patients with cirrhosis and surveillance for venous and arterial thromboembolism needs to be incorporated in clinical practice in the post-COVID era.7
Credit authorship contribution statement
MP: concept, writing, and critical revision, HB, VS, AD: writing and critical revision; TK, HB, SBC: Radiological tests, writing, and critical revision, HK: Data collection and hematological tests.
Conflicts of interest
The authors have none to declare.
Financial support
The authors received no financial support to produce this manuscript.
Ethical clearance
Informed Consent was taken from the patients before writing the manuscript, and all images have been suitably anonymized.
References
- 1.Northup P.G., Garcia-Pagan J.C., Garcia-Tsao G., et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;73:366–413. doi: 10.1002/hep.31646. [DOI] [PubMed] [Google Scholar]
- 2.Rico-Mesa J.S., Rosas D., Ahmadian-Tehrani A., White A., Anderson A.S., Chilton R. The role of anticoagulation in COVID-19-induced hypercoagulability. Curr Cardiol Rep. 2020;22:53. doi: 10.1007/s11886-020-01328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Premkumar M., Sarin S.K. Current concepts in coagulation profile in cirrhosis and acute-on-chronic liver failure. Clin Liver Dis (Hoboken). 2020;16:158–167. doi: 10.1002/cld.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni A.V., Tevethia H.V., Premkumar M., et al. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh K., Na Y., Kim Y.E., Radnaabaatar M., Peck K.R., Jung J. Predicted and observed incidence of thromboembolic events among Koreans vaccinated with ChAdOx1 nCoV-19 vaccine. J Korean Med Sci. 2021;36:e197. doi: 10.3346/jkms.2021.36.e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Premkumar M, Loganathan S, Hazarika A, et al. Hypocoagulable Coagulation Profile and Endogenous Heparinoids Are Associated with Invasive Ventilation and Mortality in COVID-19. Available at SSRN: https://ssrn.com/abstract=3802514 or 10.2139/ssrn.3802514. [DOI]
- 7.Kantarcioglu B., Iqbal O., Walenga J.M., et al. An update on the pathogenesis of COVID-19 and the reportedly rare thrombotic events following vaccination. Clin Appl Thromb Hemost. 2021;27 doi: 10.1177/10760296211021498. 10760296211021498. [DOI] [PMC free article] [PubMed] [Google Scholar]