Abstract
Coronavirus disease 2019 (COVID-19) has emerged as a serious threat to global health. The disregulation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) cell signaling pathway observed in patients with COVID-19 has attracted attention for the possible use of specific inhibitors of this pathway for the treatment of the disease. Here, we review emerging data on the involvement of the PI3K/Akt/mTOR pathway in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the clinical studies investigating its tailored inhibition in COVID-19. Current in silico, in vitro, and in vivo data convergently support a role for the PI3K/Akt/mTOR pathway in COVID-19 and suggest the use of specific inhibitors of this pathway that, by a combined mechanism entailing downregulation of excessive inflammatory reactions, cell protection, and antiviral effects, could ameliorate the course of COVID-19.
Keywords: COVID-19, SARS-CoV-2, PI3K/Akt/mTOR pathway, mTOR inhibitors, Akt inhibitors, PI3K inhibitors
Introduction
SARS-CoV-2 is the etiological agent of COVID-19, and was first isolated at the end of 2019 in Wuhan City, Hubei Province, China.1 At the time of writing (October 27, 2021), a total of 244 385 444 confirmed cases of COVID-19 and 4 961 489 deaths had been recorded globally (https://covid19.who.int). These recorded cases and deaths probably represent a gross underestimation. Other strains of coronaviruses, such as SARS-CoV and Middle-East respiratory syndrome coronavirus (MERS-CoV), had previously been shown to provoke acute lung injuries in humans. SARS-CoV-2 shares genetic similarity with SARS-CoV, with modifications of the spike protein leading to enhanced binding affinity toward the angiotensin-converting enzyme 2 (ACE2) receptors of human lung cells.
The spike protein that characterizes SARS-CoV-2 is a transmembrane trimetric glycoprotein that protrudes from the viral surface and comprises two functional subunits: S1 and S2. The S1 subunit is crucial for binding to the host cell receptor, whereas S2 is crucial for the fusion of the viral membrane with that of the host cell.2
When SARS-CoV-2 binds to ACE2, the spike protein undergoes protease cleavage3 at the S1/S2 cleavage site, with consequent generation of S1 and S2 subunits that maintain a connection via a noncovalent bond; the distal S1 subunit stabilizes the S2 subunit, which is anchored to the host cell membrane.4 Subsequently, cleavage occurring at the S2 site triggers membrane fusion. The existence of the furin cleavage site (‘RPPA’ sequence) at the S1/S2 site distinguishes SARS-CoV-2 from other coronaviruses4 and confers strong pathogenicity.
ACE2 is expressed at high levels in the cells of the lungs, heart, ileus, kidneys, and bladder, explaining the variable clinical symptomatology induced by SARS-CoV-2 infection.5 Symptoms of the disease range from asymptomatic, to mild, to severe respiratory failure, and can also lead to multiorgan dysfunction. Patients with severe COVID-19 exhibit serological and clinical signs of excessive immunoinflammatory responses characterized by augmented levels of proinflammatory cytokines, such as TNF-alpha and IL-6, as well as of the anti-inflammatory cytokine IL-10, which might be produced in an attempt to downregulate excessive inflammatory responses. In particular, the increased expression of IL-8 in the lung can exacerbate lung inflammation and tissue damage by in situ recruitment of neutrophils and T cells to the lungs.6., 7. Hence, inhibitors of the synthesis or the action of proinflammatory and/or chemoattractant cytokines could represent a therapeutic target for the prevention and treatment of COVID-19.8., 9.
The PI3K/Akt/mTOR pathway is a crucial cell signaling pathway that regulates various cell functions, such as anabolic processes, nutrient uptake, cell growth, differentiation, survival, proliferation, and motility.10., 11. Upregulated expression of this pathway has been observed in several diseases of neoplastic, autoimmune, and viral origin and is thought to be implicated in the pathogenesis of the disease.10., 12., 13., 14. This has led to the hypothesis that single or dual inhibitors of this pathway could ameliorate the course of SARS-CoV-2 infection. Single inhibitors of PI3K, Akt, and mTOR have been approved for the treatment of different conditions, primarily, but not exclusively, of a neoplastic nature. Only a few dual inhibitors have been approved for clinical use,15 whereas others are under clinical development.10
In this review, we analyze the emerging role of the PI3K/Akt/mTOR axis in COVID-19 infection and the potential therapeutic efficacy of its single and combined inhibitors on the course and possibly prevention of SARS-COV-2 infection.
The PI3K/Akt/mTOR pathway
The PI3K/Akt/mTOR pathway constitutes a signaling cascade regulated through phosphorylation and dephosphorylation by specific kinases, phosphatases, GTP/GDP proteins, adaptor proteins, and scaffolding proteins (Fig. 1 ).16 The different components of the pathway and the mechanisms of their activation are described in the legend to Fig. 1.
Figure 1.
Schematic of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. mTOR forms two complexes (mTORC1 and mTORC2), which regulate each other, are sensitive to distinct stimuli, and regulate cytoskeletal remodeling, cell activation and motility, angiogenesis, metabolism, and autophagy. mTORC1 is sensitive to nutrients, whereas mTORC2 is regulated via PI3K and growth factor signaling. For definitions of abbreviations, please see the main text.
Once activated, PI3Ks phosphorylate phosphatidylinositol-4,5-bisphosphate (PIP2) and convert it to phosphatidylinositol-3,4,5-trisphosphate (PIP3). Depending on their substrate specificity and sequence homology, PI3Ks can be categorized into three classes (I–III), each of which can include different isoforms.17
PIP3 recruits to the plasma membrane both phosphoinositide-dependent kinase 1 (PDK1) and Akt, which is activated by PDK1. The Akt kinase family comprises three highly homologous isoforms: Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ).18 Activated Akt phosphorylates tuberous sclerosis 2 (TSC2), which, along with TSC1, constitutes the TSC1–TSC2 complex. This is a GTPase-activating protein (GAP) for the GTP-binding protein Ras homolog enriched in brain (Rheb).19 The Akt-mediated TSC2 phosphorylation inhibits the TSC1–TSC2 complex and enables Rheb to accumulate in a GTP-bound state.20 Consequently, the GTP-loaded Rheb binds and activates mTORC1, which comprises the serine/threonine kinase mTOR, the regulatory protein associated with mTOR (Raptor), and the proline-rich AKT substrate of 40 kDa (PRAS40).20
mTORC1 enhances various anabolic pathways by modulating mRNA translation.20 By phosphorylating the ribosomal protein p70 S6 kinase 1 (p70S6K1) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), mTORC1 enhances cap-dependent and cap-independent translation,20 which leads to pleiotropic effects, including upregulation of lipid metabolism, glycolysis, ribosome biogenesis, and nucleotide synthesis, and inhibition of autophagy.
mTOR is also part of the mTORC2 complex, along with the rapamycin-insensitive companion of mTOR (RICTOR) and the regulatory subunits MAPK-interacting protein 1 (mSin1) and protein associated with RICTOR 1 or 2 (Protor1/2).11., 20., 21. The signaling pathway of mTORC2 is less well characterized.20 The PH domain of mSin1 interacts with the mTOR kinase domain, inhibiting mTORC2 activity. PIP3 binds to mSin1-PH and disactivates its inhibition of mTOR, with consequent mTORC2 activation.20
mTORC2 exerts a central role in regulating cell survival by activating various Protein kinase A/Protein kinase G/Protein kinase C (AGC) family protein kinases, such as Akt and serum and glucocorticoid-induced kinase 1 (SGK1).20 One of the better characterized functions of mTORC2 is the phosphorylation of Akt at Ser, which enhances the kinase activity of Akt for specific substrates.20 Moreover, mTORC2 can regulate cytoskeletal remodeling and cell motility by phosphorylating members of the protein kinase C (PKC) family.20 and also regulates angiogenesis, mitochondrial activity, lipid synthesis, and glucose, amino acid, and nucleotide metabolism.20
Table 1 details specific inhibitors of the PI3K/Akt/mTOR pathway that are used in the clinical setting.20., 21.
Table 1.
FDA-approved PI3K/Akt/mTOR inhibitors in clinical settings.
| PI3K/Akt/mTOR inhibitor | Inhibitor (trade name) | Company | Inhibitor target | Approved indication |
|---|---|---|---|---|
| PI3K inhibitors | Idelalisib (Zydelig) | Gilead Sciences | PI3Kδ isoform | Chronic lymphocytic leukemia, relapsed follicular B cell non-Hodgkin lymphoma, lymphocytic lymphoma |
| Copanlisib (Aliqopa) | Bayer | PI3K-α and PI3K-δ isoforms | Relapsed follicular lymphoma | |
| Duvelisib (Copiktra) | Verastem Oncology | PI3Kδ and PI3Kγ isoforms | Relapsed or refractory chronic lymphocytic leukemia, small lymphocytic lymphoma | |
| Alpesilib (Piqray) | Novartis | PI3Kδ isoform | Breast cancer | |
| Umbralisib (Ukoniq) | TG Therapeutics | PI3Kδ isoform | Relapsed or refractory marginal zone lymphoma, follicular lymphoma | |
| Akt inhibitors | Miltefosine (Impavido) | Profounda | Inhibits cytochrome c-oxidase | Visceral and cutaneous leishmaniasis |
| mTOR inhibitors | Everolimus | Novartis | Selectively inhibits mTOR | Advanced neuroendocrine tumors, advanced breast cancer, advanced nonfunctional gastrointestinal, lung, and neuroendocrine tumors |
| Sirolimus | Pfizer | Selectively inhibits mTOR | Immunosuppressant, lymphangioleiomyomatosis | |
| Temsirolimus | Wyeth | Selectively inhibits mTOR | Advanced-stage renal cell carcinoma |
Activation of the PI3K/Akt/mTOR cascade in SARS-CoV-2 infection
In a proteomic study, ∼12.5% of the ∼12 000 screened phosphorylation sites showed significant changes in response to SARS-CoV-2 infection, suggesting the involvement of a variety of kinases known to control many key cellular processes, such as RPS6Ks (involved in cell activation and survival), Akt (which regulates cell growth, survival, and motility), p38, JNK, and ERK (associated with the stress response).22
In another proteotranscriptomics study on the Huh7 liver cancer cell line, the ErbB, HIF-1, mTOR, and TNF signaling pathways were significantly upregulated during SARS-CoV-2 infection.23 These pathways share several proteins, such as Akt, mTOR, 4E-BP1, and S6K1, which showed increased levels of phosphorylation, with prominent activation at 24 h post-infection, suggesting that SARS-CoV-2 activates the Akt-mTOR signaling during the initial phases of infection.23 Of note, this signaling cascade is also activated by several other viruses, including influenza viruses, HIV, and MERS.19., 24., 25., 26.
By using an anti-signature analysis approach, novel options for the treatment of SARS-CoV-2 infection have been previously predicted. Among the predicted drugs, glucocorticoids, as well as Mitogen-activated protein kinase kinase (MAP2K, MEK, and MAPKK), serine-threonine kinase (Akt), mTOR ,and I kappa B Kinase (IKK) inhibitors have been predicted.27 The predicted beneficial action of glucocorticoids in COVID-19 was confirmed by clinical studies demonstrating the positive effects of steroid treatment in COVID-19, in particular for its pulmonary complications.
Notably, by using a different bioinformatics approach, based on the construction of a drug–target network for the human coronavirus (HCoV)–host interactome, Zhou and colleagues also identified the mTOR inhibitor rapamycin, among other drugs, as a potential suitable medication for the treatment of patients with COVID-19.28
In vivo evidence for the possible beneficial effects of mTOR inhibition in COVID-19 also stems from a retrospective study on patients with diabetes hospitalized with COVID-19 and treated with the antidiabetic agent metformin, which is also a mTOR inhibitor,29 showing that the in-hospital mortality of these patients was significantly reduced compared with the the no-metformin-treated group (2.9% versus 12.3%).30 Another retrospective cohort study reported a reduction in 30-day mortality after SARS-CoV-2 infection in nursing-home residents taking metformin-containing diabetes regimens.31 These clinical findings are in line with data from Duarte and collaborators,32 who found, via computational analysis, that metformin can reverse the transcriptional signature induced by SARS-CoV-2 in vitro.32 A case of complete recovery from COVID-19 in a recipient of a kidney-pancreas transplant who continued baseline immunosuppression with everolimus has also been reported.33 Considering the positive outcome despite the presence of risk factors for severe COVID-19, the potential beneficial role of immunosuppression in this condition was highlighted.33
Unfortunately, no data are yet available on whether mutations of the spike protein characterizing the variants of concern of SARS-CoV-2, in particular the Delta and Deltaplus variants, impact the activation of the PI3K/Akt/mTOR pathway. Given that these mutations, such as Leu452Arg, Thr478Lys, and Glu484Gln, of the spike protein increase the affinity for the hACE2 receptor, explaining the greater infectivity of SARS-CoV-2B.1.617.2 and B.1.617.2.1,34 beneficial effects of using PI3K/Akt/mTOR inhibitors can be envisaged.
Antiviral effects of PI3K/Akt/mTOR inhibitors against SARS-COV-2 infection
Viruses can activate the PI3K/Akt/mTOR signaling pathway and its downstream targets, including S6K1 and 4EBP1, leading to rapid activation of the translation machinery involved in the synthesis of viral protein.26., 35. In particular, it has been shown that SARS-CoV-2 infection induces several intracellular phosphorylation events related to the mTOR and JAK1 pathways.22., 23., 24., 25., 26. In particular, the activation of CD147 and furin (involved in the entry of SARS-CoV-2 into the cell) can induce the activation of the PI3K/Akt signaling pathway.36 Moreover, SARS-CoV-2 endocytosis, occurring via a clathrin-mediated pathway, is regulated by the PI3K/Akt pathway, the suppression of which inhibits the entry of certain viruses.36
Inhibitors of the PI3K/Akt/mTOR pathway can exert antiviral effects, including tailored anti-SARS-CoV-2 effects. mTOR inhibitors have previously bee shown to suppress HIV infection in vitro and in vivo, in preclinical settings.13 In agreement with these data, everolimus significantly reduced HIV RNA levels in ART-suppressed individuals who received solid organ transplantation.37 In addition, Kindrachuk et al. showed that rapamycin inhibited MERS infection in vitro by 61% at a 10 μM concentration.26
Although neither rapamycin nor the mTORC1/mTORC2 inhibitor Torin-1 blocked SARS-CoV-2 infection in vitro,23 the Akt inhibitor MK-2206 exerted significant antiviral effects on Sars-CoV-2 in vitro,23 likely via induction of enhanced autophagy, secondary to the stabilization of Beclin-1 (BECN-1).38 Autophagy is involved in the clearance and recycling of intracellular materials and elimination of intracellular pathogens, such as viruses.39 Miller et al. reported that coronaviruses might inhibit the autophagic mechanism by increasing viral replication.40., 41. MERS-CoV interferes with host cell autophagy by triggering the degradation of BECN1, which is promoted by the phosphorylation of the cellular E3 ubiquitin ligase SKP2, following the activation AKT1.40., 41. Accordingly, when infected cells are treated with an SKP2 inhibitor, the consequent increase in BECN1 levels is associated with decreased MERS-CoV replication.41 Baggen et al. found that PI3K type 3, which controls the initiation of canonical and non-canonical autophagy, is a common host factor for SARS-CoV-2 and other coronaviruses.42 In fact, inhibition of the class III PI3K Vps34, by using the class III PI3K inhibitor VPS34-IN1, significantly inhibited SARS-CoV-2 replication in vitro. 39 Moreover, VPS34-IN1 and its bioavailable analog VVPS34-IN1 strongly inhibited SARS-CoV-2 infection in human lung tissue cultures.39 Hence, SARS-CoV-2 could hijack the autophagy machinery by using specific components, particularly class III PI3K, highlighting the possibility of evaluating the use of class III PI3K inhibitors for the treatment of COVID-19.39 Moreover, growth factor receptor signaling could have a crucial role in SARS-CoV-2 infection and can be activated upon infection, inducing PI3K signaling.43 In fact, the PI3K inhibitor pictilisib and the dual PI3K and mTOR inhibitor omipalisib efficiently inhibited SARS-CoV-2 replication in vitro.43 However, in contrast to these data, neither the PI3K inhibitor wortmannin nor the S6K1 inhibitor BI-D1870 exerted any significant viral inhibition.23
In a preprint article, Acharya and colleagues showed that the dual mTOR and PI3K-α/(BRD2/BRD4) inhibitor SF2523 blocked the replication of SARS-CoV-2 in lung bronchial epithelial cells in a manner comparable to that of remdesivir, the only US Food and Drug Administration (FDA)-approved antiviral agent used to treat COVID-19 to date.44They also reported a synergistic effect of SF2523 with remdesivir.44
The PI3K/Akt/mTOR pathway and the immune system: Implications for COVID-19
Severe cases of COVID-19 are characterized by hyperinflammatory reactions, cytokine storm, followed by acute respiratory distress syndrome (ARDS), lung damage, and multiorgan failure. The presence of CD25 + hyperactivated T cells, displaying Th1 and Th2 effector characteristics and reduced expression of the marker of regulatory T cells FOXP3, has been observed in the lungs of patients with severe COVID-19, along with that of CD25-expressing hyperactivated T cells, which produce the protease Furin, which promotes the entry of SARS-CoV-2.45
Potential role for PI3K in COVID-19
The activation of the PI3K pathway in T cells favors survival and cell cycle progression, controlling differentiation12., 46., 47. and regulating the acquisition of effector and memory phenotypes.48. It is of interest, in the context of this review, that the PI3K pathway, in particular the PI3Kδ isoform, represents an emerging target for treating airway disorders, such as asthma, chronic obstructive pulmonary disease, and allergic rhinitis.49 It has been suggested that the PI3Kδ inhibitor idelalisib, alone or in combination with the antihistaminic ebastine, might be a promising therapeutic strategy for COVID-19.49 Accordingly, ebastine in association with an antiviral is being investigated for the treatment of COVID-19.49
Targeting Akt in SARS-CoV-2 infection
In addition, Akt inhibition might dampen excessive inflammation, cytokine storm, fibroproliferation, and platelet activation in patients with COVID-19 and might also prevent scarring and favor resolution of damaged lungs.50 Moreover, Akt inhibition downregulates the expression of ACE2.50 In support of these observations, the Akt inhibitors triciribine and MK-2206 increased the number of regulatory T cells (Tregs), favoring injury resolution and recovery in a murine model of endotoxin-induced experimental lung injury. Hence, Akt suppression might increase Tregs in the lungs of patients with COVID-19, thus reducing inflammation and promoting injury resolution.51
Role of mTOR in immune responses to SARS-CoV-2
mTOR has a pleiotropic role in immune responses. The activation of the mTOR cascade is usually associated with high metabolic activity, whereas low mTOR activation is characteristic of a quiescent state. Hence, the priming of naive T helper cells induces an increase in mTOR activity, which promotes the differentiation of Th1, Th2, and Th17 cells. By contrast, in the absence of mTOR signaling, Treg and T follicular helper cell (Tfh) polarization is predominant. In addition, mTOR activation promotes the generation of cytotoxic effector cells, whereas low mTOR activity is associated with a switching toward a memory CD8 + T cell phenotype (reviewed in52. Therefore, mTOR inhibitors might exert beneficial effects against COVID-19 through a reduction in the proliferation of conventional T lymphocytes, which could mitigate the cytokine storm, and preserve Treg growth and activity, which, in turn, might reduce the hyper-reactivity during the critical phase of the disease.
In a study on 76 patients with COVID-19 and 69 healthy individuals from Hong Kong and Atlanta, GA, USA,53 Arunachalam and colleagues observed in the myeloid cells from patients with COVID-19 reduced expression of human leukocyte antigen class DR (HLA-DR) and of proinflammatory cytokines, as well as impaired mTOR signaling and interferon-alpha (IFN-α) production by plasmacytoid dendritic cells (DCs), which correlated with disease severity.53 These results suggest that SARS-CoV-2 infection is characterized by spatial discrepancies in both innate and adaptive immune responses, with suppression of peripheral innate immunity and increased proinflammatory responses in the lung tissues, which could be restored by targeting the mTOR cascade.
Given that the mTOR pathway regulates B cell development in the germinal center,54 mTOR inhibitors might also improve the course of COVID-19 by downregulating excessive B cell activation, which can occur during the course of COVID-19 infection.55 The upregulated humoral immune response in COVID-19 infection is of particular relevance in the pathogenesis of COVID-19 because uncontrolled B cell responses might worsen the course of the disease. Indeed, low-titer antibodies can enhance infection through antibody-dependent enhancement (ADE). ADE has been reported following vaccination or secondary infections with other coronaviruses, as well as with Ebola and Dengue virus. Detailed analysis has shown that antibodies to any viral epitope can induce ADE when present in suboptimal titers or when of low affinity.56 Accordingly, two studies analyzing 222 and 173 patients with COVID-19, respectively, reported that patients with severe disease frequently had an increased IgG response and a higher titer of total antibodies, which were associated with worse outcome.57., 58. In addition, compared with uninfected individuals, patients with COVID-19 display augmented autoantibodies directed against immunomodulatory proteins (such as cytokines, chemokines, complement components, and cell surface proteins) that perturb immune function and impair virological control by inhibiting immunoreceptor signaling and by altering peripheral immune cell composition.59
The occurrence of an excessive humoral immune response in COVID-19 is particularly important given the multiple organ damage that is often observed in severe COVID-19. Indeed, patients can have proteinuria, hematuria,60 and neurological symptoms,61 and can develop thromboembolic events,62 which could be the consequence of a secondary antiphospholipid syndrome (APS) (reviewed in63or other autoimmune diseases.64 Accumulating data demonstrate that anti-CL and anti-b2-GPI autoantibodies65., 66., 67., 68. and lupus anticoagulant66., 69., 70. are found in patients with COVID-19. Interestingly, activation of the mTOR pathway was observed in the vascular endothelium of proliferating intrarenal vessels from patients with APS nephropathy.71 In addition, patients with APS nephropathy who underwent transplantation and were receiving rapamycin had no recurrence of vascular lesions and showed lower vascular proliferation on biopsy, compared with patients not treated with rapamycin.71 Hence, it is reasonable to believe that the use of specific inhibitors targeting the mTOR pathway would benefit patients, protecting them from the thrombotic events that can complicate COVID-19.
mTOR inhibition might also be useful to tackle the process of ‘inflammaging’ that occurs in older individuals and that appears to be associated with more severe forms of COVID-19. Indeed, aging is associated with immune hyperfunction diseases, which might explain, in part, the higher rate of mortality observed in older patients. This is consistent with preclinical studies showing that the excessive inflammatory response rather than high virus titer leads to death in SARS-CoV-infected old nonhuman primates.72 Given that rapamycin is a known antiaging and senolytic agent, it could inhibit immune hyperfunctions and cellular senescence and decrease cytokine secretion.73
Considering the worse clinical outcomes of COVID-19 infection in older patients and the ability of mTOR inhibitors to counteract age-related decline of the immune responses, Bischof and colleagues highlighted the need to carry out large-scale clinical trials with mTOR inhibitors in older patients to evaluate the impact of this class of drugs on the prevention and clinical course of COVID-19 in these high-risk individuals.74
Despite the above-mentioned data, Shi et al. reported that rapamycin and rapalogs impaired intrinsic immunity and promoted viral cell-entry driven by SARS-CoV-2 Spike in nasal cells and primary small airway cells. Rapalogs appear to act by the degradation of interferon-inducible transmembrane (IFITM) proteins, which inhibit virus infection. Moreover, reduced levels of phosphorylated ribosomal protein S6 (pS6), a canonical target of mTOR activation, have been observed in plasmacytoid DCs from patients with COVID-19, and correlated with impaired production of IFN-alpha.75 By contrast, the authors found increased levels of circulating cytokines despite inhibition of plasmacytoid DCs.75 This suggests that SARS-CoV-2 infection is characterized by spatial discrepancies in both innate and adaptive immune responses, with suppression of peripheral innate immunity and increased proinflammatory responses in the lung tissues, which could ultimately be restored by specific inhibitors of the PI3K/Akt/mTOR pathway.75
The PI3K/Akt/mTOR pathway in SARS-CoV-2 vaccination: A feasible target?
Although no studies or case reports are yet available on the effects of PI3K/Akt/mTOR inhibition following SARS-CoV-2 vaccination, scientific evidence supports the hypothesis that specific inhibitors improve the immune responses elicited by vaccines. In particular, frequent administration of low-dose rapamycin induces the polarization of T cells toward central memory phenotypes (TCMs), which exhibit greater plasticity and proliferative capacity compared with effector memory T cells.76 In addition, local delivery of low doses of rapamycin to lymph nodes during vaccination in mice enhanced T cell function in response to melanoma antigens, by increasing antigen-specific TCMs.76 Finally, rapamycin at low doses modulates DC function, promoting the secretion of proinflammatory cytokines.76
Moreover, it was observed that specific PI3K–Akt inhibitors selectively inhibit Tregs, while exerting low effects on conventional T cells. Accordingly, in tumor-bearing mice, the effect of an anticancer vaccine was more pronounced when the animals were treated concomitantly with the PI3K inhibitor, wortmannin, and this effect was reversed when mice were reconstituted with Tregs.77
On a final note, Keating and collaborators found that rapamycin can promote protection against various subtypes of influenza virus when administered to mice upon immunization against a single viral strain. Inhibition of mTOR resulted in reduced formation of germinal centers and inhibited B cell class-switching, with the generation of a unique repertoire of antibodies that mediated cross-strain protection. Hence, rapamycin appears able to skew the antibody response away from high-affinity variant epitopes, leading to the generation of antibodies targeting more conserved elements of the virus, which might be useful to improve the efficacy of the vaccine against viral variants.78
Clinical trials
Eight clinical trials with drugs targeting the PI3K/AKT/mTOR pathway in COVID-19 are listed with ClinicalTrial.gov (https://clinicaltrials.gov/) (Table 2 ). However, NCT04409327 was terminated because of insufficient accrual of patients, and NCT04482712 and NCT04371640 have been withdrawn. In addition, it is unlikely that NCT04374903 will advance further, because the study aimed to combine sirolimus with hydroxychloroquine (HCQ), which has been shown to be ineffective in COVID-19. So far, NCT04341675, NCT04461340, and NCT04372602 are active and recruiting, whereas NCT04374903 and NCT04584710 appear active but not yet recruiting.
Table 2.
Clinical trials investigating the effects of drugs targeting the PI3K/Akt/mTOR pathway in COVID-19.
| Trial | Status | Last update | Estimated enrolment | Study start date | Intervention/treatment | Placebo comparator |
|---|---|---|---|---|---|---|
| NCT04371640: Safety and tolerability of Sirolimus adjuvant therapy in patients with COVID-19 | Withdrawn (study population not regularly admitted to hospital; approaches have shifted away from repurposing old drugs) | December 7, 2020 | 0 | May 1, 2020 | Sirolimus + standard medical care Day 1: 10 mg; Days 2–7: 5 mg | Placebo + standard medical care Day 1: 10 ml Days 2–7: 5 ml |
| NCT04341675: Effect of sirolimus in improving clinical outcomes in hospitalized patients with COVID-19 | Recruiting | May 20, 2020 | 30 participants | April 24, 2020 | Sirolimus 6 mg daily on Day 1 followed by 2 mg daily for next 13 days for total treatment duration of 14 days or hospital discharge, whatever happens sooner | Matching placebo + standard medical care |
| NCT04461340: The efficacy and safety of sirolimus as an adjuvant agent to the standard treatment protocol in patients with COVID-19 infection | Recruiting | September 9, 2020 | 40 participants | August 15, 2020 | 20 patients will receive sirolimus (oral dose of 6 mg on Day 1 followed by 2 mg daily for 9 days) plus national standard-of-care therapy against COVID-19; daily administration of sirolimus (1 mg) for up to 4 weeks while hospitalized | Matching placebo + standard medical care |
| NCT04482712: The clinical effectiveness of mTOR inhibition with rapamycin in minimizing or decreasing the ALI/ARDS in participants infected with mild to moderate COVID-19 infection | Withdrawn (study was never submitted to IRB or approved because of feasibility issues) | June 1, 2021 | 20 participants | April 2021 | Administration of rapamycin (sirolimus) 1 mg daily during hospitalization | Matching placebo + standard medical care |
| NCT04374903: The clinical improvement in COVID-19 Patients treated with Hydroxychloroquine in Combination with Azithromycin or Sirolimus.: | Not yet recruiting | May 5, 2020 | 58 participants | May 1, 2020 | Subjects will receive either HCQ with azithromycin (AZ) or HCQ with sirolimus (SIR). Study A: subjects will receive HCQ 600 mg orally for 10 days and AZ 250 mg orally daily for 10 days; study B: subjects will receive HCQ 600 mg orally for 10 days and SIR 4 mg orally on Day 1, then 2 mg orally daily for 9 days | Matching placebo + standard medical care |
| NCT04409327: Phase 2 Study to Determine if RTB101 Reduces the Severity of COVID-19 in Older Adults (≥65 years) Residing in Nursing Homes | Terminated (insufficient accrual rate) | February 10, 2021 | 36 participants | July 11, 2020 | Oral RTB101 (TORC1 inhibitor) 10 mg hard gelatin capsule once daily for 4 weeks | Oral-matching placebo once daily for 4 weeks |
| NCT04584710: A Phase 2 Study of RTB101 as COVID-19 Post-Exposure Prophylaxis in Older Adults (≥65 years) | Active, not recruiting | February 9, 2021 | 60 participants | October 13, 2020 | Oral RTB101 (TORC1 inhibitor) 10 mg hard gelatin capsule once daily for 2 weeks | Oral matching placebo once daily for 2 weeks |
| NCT04372602: The efficacy of the PI3K inhibitor Duvelisib in improving the overall survival and advanced disease manifestations of SARS-CoV-2 infection | Recruiting | May 25, 2021 | 28 participants | October 12, 2020 | Duvelisib 25 mg twice daily for up to 10 days | Placebo 25 mg twice daily for up to 10 days |
Topline results generated from these ongoing studies will provide key important insights into the feasibility of using single or dual inhibitors of the PI3K-Akt-mTOR pathway in the prevention and treatment of COVID-19.
The observations of patients will also be instrumental to understand whether the known side effects of this class of drugs, including possible generalized immunosuppression, are outweighed by their clinical efficiency.
Concluding remarks
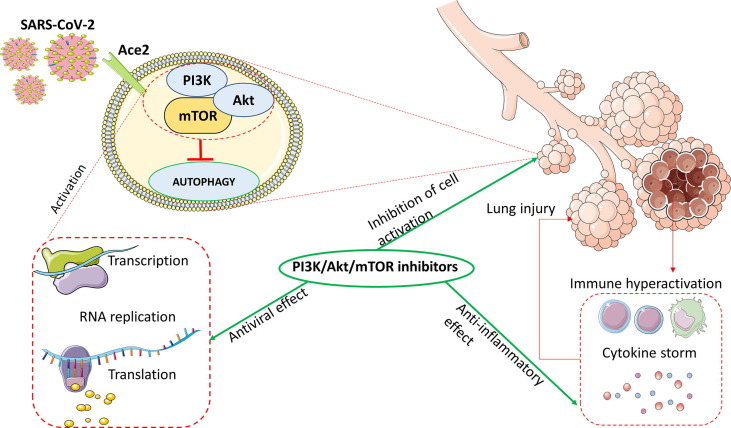
This review summarizes the potential role of the PI3K/Akt/mTOR pathway in the cellular response to SARS‐CoV‐2 and current clinical trials evaluating specific inhibitors in the prevention and treatment of COVID-19. Although the current vaccination campaign is slowing the diffusion of COVID-19, the poor distribution of the vaccines in developing countries, along with the emergence of virus variants, which might evade the immune protection conferred from current vaccines, warrant the parallel development of alternative pharmacological strategies for the treatment of SARS-CoV-2. The increasing and converging literature data that we have presented herein suggest that inhibitors of the PI3K/Akt/mTOR pathway represent a valuable asset in the management of COVID-19 infection (Fig. 2 ).
Figure 2.
Potential protective effects of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) inhibitors in coronavirus 2019 (COVID-19). Abbreviations: Ace2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carvalho T., Krammer F., Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ou X., Liu Y., Lei X., Li P., Mi D., Ren, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Yan H., Xu Z., Yang B., Luo P., He Q. Molecular basis for class side effects associated with PI3K/AKT/mTOR pathway inhibitors. Expert Opin Drug Metab Toxicol. 2019;15:767–774. doi: 10.1080/17425255.2019.1663169. [DOI] [PubMed] [Google Scholar]
- 11.Liu G.Y., Sabatini D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mammana S., Bramanti P., Mazzon E., Cavalli E., Basile M.S., Fagone P., et al. Preclinical evaluation of the PI3K/Akt/mTOR pathway in animal models of multiple sclerosis. Oncotarget. 2018;9:8263–8277. doi: 10.18632/oncotarget.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donia M., McCubrey J.A., Bendtzen K., Nicoletti F. Potential use of rapamycin in HIV infection. Br J Clin Pharmacol. 2010;70:784–793. doi: 10.1111/j.1365-2125.2010.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabbah D.A., Hajjo R., Bardaweel S.K., Zhong H.A. Phosphatidylinositol 3-kinase (PI3K) inhibitors: a recent update on inhibitor design and clinical trials (2016–2020) Expert Opin Ther Pat. 2021;31:877–892. doi: 10.1080/13543776.2021.1924150. [DOI] [PubMed] [Google Scholar]
- 15.Janku F., Yap T.A., Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15:273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 16.Martelli A.M., Evangelisti C., Chappell W., Abrams S.L., Bäsecke J., Stivala F., et al. Targeting the translational apparatus to improve leukemia therapy: Roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia. 2011;25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- 17.Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez E., McGraw T.E. The Akt kinases: Isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicoletti F., Fagone P., Meroni P., McCubrey J., Bendtzen K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov Today. 2011;16:715–721. doi: 10.1016/j.drudis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Martelli A.M., Buontempo F., McCubrey J.A. Drug discovery targeting the mTOR pathway. Clin Sci. 2018;132:543–568. doi: 10.1042/CS20171158. [DOI] [PubMed] [Google Scholar]
- 21.Alzahrani A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Stukalov A., Girault V., Grass V., Karayel O., Bergant V., Urban C., et al. Multi-level proteomics reveals host-perturbation strategies of SARS-CoV-2 and SARS-CoV. Nature. 2021;594:246–252. doi: 10.1038/s41586-021-03493-4. [DOI] [PubMed] [Google Scholar]
- 23.Appelberg S., Gupta S., Akusjärvi S.S., Ambikan A.T., Mikaeloff F., Saccon E., et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg Microbes Infect. 2020;9:1–36. doi: 10.1080/22221751.2020.1799723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranadheera C., Coombs K.M., Kobasa D. Comprehending a Killer: the Akt/mTOR signaling pathways are temporally high-jacked by the highly pathogenic 1918 influenza virus. EBioMedicine. 2018;32:142–163. doi: 10.1016/j.ebiom.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochim Biophys Acta - Mol Basis Dis. 2005;1741:4–10. doi: 10.1016/j.bbadis.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagone P., Ciurleo R., Lombardo S.D., Iacobello C., Palermo C.I., Shoenfeld Y., et al. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clements A., Gao B., Yeap S.H.O., Wong M.K.Y., Ali S.S., Gurney H. Metformin in prostate cancer: two for the price of one. Ann Oncol. 2011;22:2556–2560. doi: 10.1093/annonc/mdr037. [DOI] [PubMed] [Google Scholar]
- 30.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lally M.A., Tsoukas P., Halladay C.W., O’Neill E., Gravenstein S., Rudolph J.L. Metformin is associated with decreased 30-day mortality among nursing home residents infected with SARS-CoV2. J Am Med Dir Assoc. 2021;22:193–198. doi: 10.1016/j.jamda.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte R.R.R., Copertino D.C., Jr, Iñiguez L.P., Marston J.L., Bram Y., Han Y., et al. Identifying FDA-approved drugs with multimodal properties against COVID-19 using a data-driven approach and a lung organoid model of SARS-CoV-2 entry. Mol Med. 2021;27:105. doi: 10.1186/s10020-021-00356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heron V.C., Bach C.A.T., Holmes N.E., Whitlam J.B. Complete recovery from COVID-19 of a kidney-pancreas transplant recipient: potential benefit from everolimus? BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-238413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajj-Hassan H., Hamze K., Abdel Sater F., Kizilbash N., Khachfe H.M. Probing the increased virulence of severe acute respiratory syndrome coronavirus 2 B.1.617 (Indian variant) from predicted spike protein structure. Cureus. 2021;13 doi: 10.7759/cureus.16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuss-Duerkop S.K., Wang J., Mena I., White K., Metreveli G., Sakthivel R., et al. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khezri M.R. PI3K/AKT signaling pathway: a possible target for adjuvant therapy in COVID–19. Hum Cell. 2021;1:1. doi: 10.1007/s13577-021-00484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henrich T.J., Schreiner C., Cameron C., Hogan L.E., Richardson B., Rutishauser R.L., et al. Everolimus, an mTORC1/2 inhibitor, in ART-suppressed individuals who received solid organ transplantation: a prospective study. Am J Transplant. 2021;21:1765–1779. doi: 10.1111/ajt.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gassen N., Papies J., Bajaj T., Emanuel J., Dethloff F., Chua R.L., et al. SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat Commun. 2021;12:3818. doi: 10.1038/s41467-021-24007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuen C.K., Wong W.M., Mak L.F., Wang X., Chu H., Yuen K.Y., et al. Suppression of SARS-CoV-2 infection in ex-vivo human lung tissues by targeting class III phosphoinositide 3-kinase. J Med Virol. 2020;93:2076–2083. doi: 10.1002/jmv.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller K., McGrath M.E., Hu Z., Ariannejad S., Weston S., Frieman M., et al. Coronavirus interactions with the cellular autophagy machinery. Autophagy. 2020;16:2131–2139. doi: 10.1080/15548627.2020.1817280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gassen N.C., Niemeyer D., Muth D., Corman V.M., Martinelli S., Gassen A., et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat Commun. 2019;10:1–16. doi: 10.1038/s41467-019-13659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baggen J., Persoons L., Vanstreels E., Jansen S., Van Looveren D., Boeckx B., et al. Genome-wide CRISPR screening identifies TMEM106B as a proviral host factor for SARS-CoV-2. Nat Genet. 2021;53:435–444. doi: 10.1038/s41588-021-00805-2. [DOI] [PubMed] [Google Scholar]
- 43.Klann K., Bojkova D., Tascher G., Ciesek S., Münch C., Cinatl J. Growth factor receptor signaling inhibition prevents SARS-CoV-2 replication. Mol Cell. 2020;80:164–174. doi: 10.1016/j.molcel.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acharya A, Pandey K, Thurman M, Challagundala KB, Vann KR, Kutateladze TG, et al. Blockade of SARS-CoV-2 infection in vitro by highly potent PI3K-α/mTOR/BRD4 inhibitor. bioRxiv 2021; 2021: 2021.03.02.433604.
- 45.Kalfaoglu B., Almeida-Santos J., Tye C.A., Satou Y., Ono M. T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.589380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delgoffe G.M., Pollizzi K.N., Waickman A.T., Heikamp E., Meyers D.J., Horton M.R., et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–304. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee K., Gudapati P., Dragovic S., Spencer C., Joyce S., Killeen N., et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araki K., Youngblood B., Ahmed R. The role of mTOR in memory CD8+ T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palma G., Pasqua T., Silvestri G., Rocca C., Gualtieri P., Barbieri A., et al. PI3Kδ inhibition as a potential therapeutic target in COVID-19. Front Immunol. 2020;11:2094. doi: 10.3389/fimmu.2020.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somanath P.R. Is targeting akt a viable option to treat advanced-stage COVID-19 patients? Am J Physiol Lung Cell Mol Physiol. 2020;319:L45–L47. doi: 10.1152/ajplung.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artham S., Verma A., Alwhaibi A., Adil M.S., Manicassamy S., Munn D.H., et al. Delayed Akt suppression in the lipopolysaccharide-induced acute lung injury promotes resolution that is associated with enhanced effector regulatory T cells. Am J Physiol Lung Cell Mol Physiol. 2020;318:L750–L761. doi: 10.1152/ajplung.00251.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keating R., McGargill M.A. mTOR regulation of lymphoid cells in immunity to pathogens. Front Immunol. 2016;7:180. doi: 10.3389/fimmu.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R.A.P.M., Scott M., Hagan T., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:eabc6261. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raybuck A.L., Cho S.H., Li J., Rogers M.C., Lee K., Williams C.L., et al. B cell-intrinsic mTORC1 promotes germinal center-defining transcription factor gene expression, somatic hypermutation, and memory B cell generation in humoral immunity. J Immunol. 2018;200:2627–2639. doi: 10.4049/jimmunol.1701321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavalli E., Petralia M., Basile M., Bramanti A., Bramanti P., Nicoletti F., et al. Transcriptomic analysis of COVID-19 lungs and bronchoalveolar lavage fluid samples reveals predominant B cell activation responses to infection. Int J Mol Med. 2020;46:1266–1273. doi: 10.3892/ijmm.2020.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cloutier M., Nandi M., Ihsan A.U., Chamard H.A., Ilangumaran S., Ramanathan S. ADE and hyperinflammation in SARS-CoV2 infection: comparison with dengue hemorrhagic fever and feline infectious peritonitis. Cytokine. 2020;136 doi: 10.1016/j.cyto.2020.155256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20:339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 60.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV–2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 63.Cavalli E., Bramanti A., Ciurleo R., Tchorbanov A.I., Giordano A., Fagone P., et al. Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: diagnostic and therapeutic perspectives. Int J Mol Med. 2020;46:903–912. doi: 10.3892/ijmm.2020.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harzallah I., Debliquis A., Drenou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zayet S., Klopfenstein T., Kovacs R., Stancescu S., Hagenkötter B. Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg Infect Dis. 2020;26:2258–2260. doi: 10.3201/eid2609.201791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV–2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sieiro Santos C., Nogal Arias C., Moriano Morales C., Ballesteros Pomar M., Diez Alvarez E., Perez S.T. Antiphospholipid antibodies in patient with acute lower member ischemia and pulmonary thromboembolism as a result of infection by SARS-CoV2. Clin Rheumatol. 2020;39:2105–2106. doi: 10.1007/s10067-020-05194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canaud G., Bienaimé F., Tabarin F., Bataillon G., Seilhean D., Noël L.H., et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371:303–312. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]
- 72.Smits S.L., de Lang A., van den Brand J.M.A., et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blagosklonny M.V. From causes of aging to death from COVID-19. Aging. 2020;12:10004–10021. doi: 10.18632/aging.103493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bischof E., Siow R.C., Zhavoronkov A., Kaeberlein M. The potential of rapalogs to enhance resilience against SARS-CoV-2 infection and reduce the severity of COVID-19. Lancet Heal Longev. 2021;2:e105–e111. doi: 10.1016/S2666-7568(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.G S, AI C, S M, Lai KK, Das S, Beare PA, et al. Rapalogs downmodulate intrinsic immunity and promote cell entry of SARS-CoV-2. bioRxiv 2021; 2021: 2021.04.15.440067. [DOI] [PMC free article] [PubMed]
- 76.Gammon J.M., Gosselin E.A., Tostanoski L.H., Chiu Y.C., Zeng X., Zeng Q., et al. Low-dose controlled release of mTOR inhibitors maintains T cell plasticity and promotes central memory T cells. J Control Release. 2017;263:151–161. doi: 10.1016/j.jconrel.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abu-Eid R., Samara R.N., Ozbun L., Abdalla M.Y., Berzofsky J.A., Friedman K.M., et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keating R., Hertz T., Wehenkel M., Arris T.L., Edwards B.A., McClaren J.L., et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013;14:1266–1276. doi: 10.1038/ni.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]