Abstract
Vgf (non-acronymic), a neurotrophin stimulated protein which plays a crucial role in learning, synaptic activity, and neurogenesis, is markedly downregulated in the brain of Alzheimer’s disease (AD) patients. However, since vgf is a large polar protein, a safe and efficient gene delivery vector is critical for its delivery across the blood brain barrier (BBB). This research work demonstrates brain-targeted liposomal nanoparticles optimized for delivering plasmid encoding vgf across BBB and transfecting brain cells. Brain targeting was achieved by surface functionalization using glucose transporter-1 targeting ligand (mannose) and brain targeted cell-penetrating peptides (chimeric rabies virus glycoprotein fragment, rabies virus derived peptide, penetratin peptide, or CGNHPHLAKYNGT peptide). The ligands were conjugated to lipid via nucleophilic substitution reaction resulting in more than 75% binding efficiency. The liposomes were formed by film hydration technique demonstrating size less than 200 nm, positive zeta potential (15–20 mV), and polydispersity index less than 0.3. The bifunctionalized liposomes demonstrated ~3 pg/µg protein vgf transfection across in vitro BBB, and ~80 pg/mg protein in mice brain which was 1.5–2 fold (p<0.05) higher compared to untreated control. The nanoparticles were also biocompatible in vitro and in vivo, suggesting a safe and efficient gene delivery system to treat AD.
Keywords: Vgf, Brain-targeting, Alzheimer’s disease, Blood brain barrier, Gene therapy
Graphical Abstract
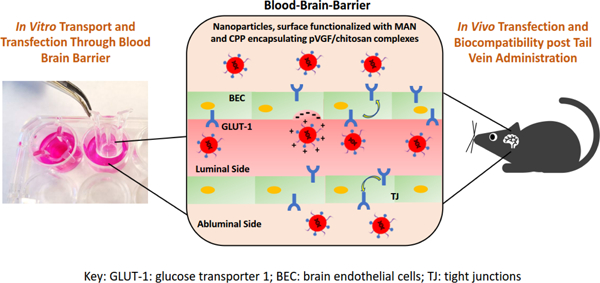
Introduction
Late onset Alzheimer’s disease (AD) is one of the most prevalent dementing illness among the elderly. It is a chronic degenerative disorder of the central nervous system (CNS) which results in the loss of memory, cognition along with the ability to function physically and socially. Although, heritability of late onset AD is as high as 79%, amalgamation of various environmental and genetic factors are the key etiological drivers of this disorder (“2018 Alzheimer’s disease facts and figures,” 2018). Additionally, over the past two decades, limited pathophysiological understanding and modest symptomatic relief by current approved therapeutics have led to a 145% increase in AD related mortalities. Consequently, there is a pressing priority to find new disease modifying therapies with minimal side effects for treatment of AD. Although the pathophysiology of AD is complex, the formation and accumulation of hyperphosphorylated tau protein and β-amyloid plaques are believed to play as the key features of this disorder. Both these phenotypic features are associated with loss of memory and cognition owing to neuronal cell death in the CNS (“2018 Alzheimer’s disease facts and figures,” 2018).
It is widely reported that vgf (non-acronymic), a neurotrophin stimulated protein playing a crucial role in memory formation, learning, enhancement of synaptic activity, and neurogenesis, is markedly downregulated in AD (Alder et al., 2003; Bozdagi et al., 2008; Carrette et al., 2003; Cocco et al., 2010; Rüetschi et al., 2005; Selle et al., 2005; Snyder et al., 1997; Thakker-Varia et al., 2014). Vgf can be stimulated by neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (Bonni et al., 1995; Levi et al., 1985). Following its synthesis, vgf polypeptide is intracellularly processed with tissue and cell specificity to form various secretory bioactive peptides. These peptides are involved in various functions such as memory formation, cognition, learning, neurogenesis and neuroplasticity (Jiang et al., 2019, 2018; Lin et al., 2015). Increase in the expression of vgf protein has shown to attenuate AD related phenotypes including reduction in β-amyloid toxicity and neuroinflammation (El Gaamouch et al., 2020; Lin et al., 2015). Therefore, transport of vgf to the brain can result in beneficial effects for attenuating AD pathophysiology. However, vgf being a large polar protein (~67 kDa) is unable to transport across the blood brain barrier (BBB) and cellular membranes. Intracerebroventricular delivery of vgf derived 21 amino acid peptide over a period of 28 days has shown promising results in attenuating AD related pathophysiology (El Gaamouch et al., 2020). However, in order to deliver high levels of this therapeutic protein to the brain while eliminating highly invasive and complicated procedures, a safe and efficient gene delivery vector is critical. Therefore, our goal is to develop and optimize lipid-based nanoparticles called liposomes for efficient transport and transfection of pVGF (plasmid encoding for vgf protein) inside the brain to restore vgf protein levels in AD.
In our present study, we investigated the transport and transfection efficiency of liposomal nanoparticles as non-viral gene delivery vector for specific delivery of pVGF to the brain. Liposomes were utilized due to their several advantages such as good biocompatibility, lack of immunogenicity, large packaging ability, surface functionalization, and easy scaleup (Nayerossadat et al., 2012; Sharma et al., 2021b). Chitosan polymer was also used to form electrostatic complexes with pVGF gene prior to their encapsulation inside the liposomes. Complex formation with chitosan helps in pDNA condensation, protection from endolysosomes and improvement in transfection efficiency (Banerjee et al., 2020; dos Santos Rodrigues et al., 2020a; Sharma et al., 2019a, 2021a; Sharma and Singh, 2017). In order to overcome the primary hurdle in the path of efficient transport of gene delivery vector inside the brain, i.e. the BBB, surface of liposomal nanaoparticles was functionalized using specific ligands. There are various specific ligands which can target brain via solute carriers (glucose transporters, amino acid transporters and organic anion transporting polypeptide) or receptors (insulin receptors, transferrin receptors, low-density lipoprotein receptors and neurotropic virulence factor receptors) present on BBB (Razzak et al., 2019). Similar to targeting transporters or receptors, adsorption mediated transcytosis is another technique to initiate electrostatic interactions between cell membrane and the carrier vector to enable translocation across the BBB. This is generally achieved via peptides which have the potential to target brain (Niu et al., 2019). To further enhance the transportation potential across the BBB, combination of different brain specific targeting ligands have been used and compared with the mono-functionalized or non-targeted carriers (Dong, 2018; Luo et al., 2020). In our present study, mannose (MAN), a specific substrate for glucose transporter-1 (GLUT-1) was utilized as a brain-targeting ligand to allow for transporter-mediated endocytocis across the BBB. GLUT-1 transporters are present in high density on BBB, normally required for efficient transport of glucose inside the brain (Tsuji, 2005; Umezawa and Eto, 1988). Moreover, expression of GLUT-1 was demonstrated to be either lacking or extremely low in various tissues in humans such as skeletal muscles, stomach, thyroid, uterus, pancreas and ovaries (Godoy et al., 2006). Hence, GLUT-1 targeting has the potential for minimal off-target transport thereby resulting in selective uptake by brain tissue. Lipid-conjugated brain targeting cell penetrating peptides (CPPs) were also used in conjugation with MAN, to improve transport to the brain. Chimeric rabies virus glycoprotein fragment (RVG9R), rabies virus derived peptide (RDP), penetratin (Pen) peptide, or CGN (CGNHPHLAKYNGT) peptide, were selected after careful literature search for brain-specific delivery. RVG9R and RDP both are derived from rabies virus glycoprotein, whereas CGN peptide is obtained from in vivo phage display screening (Fu et al., 2012; Xiang et al., 2011; Zheng et al., 2017). Pen is obtained from Antennapedia protein homeodomain. Dual modification using CPPs and the targeting ligand (MAN) had shown to enhance cellular uptake ability chiefly by diminishing the competition between the ligand and its physiological substrate (glucose and mannose) (Arora et al., 2020b). In our present study, we have developed, optimized and evaluated liposomal nanoparticles with varied surface modifications for their hydrodynamic diameter, zeta potential, entrapment efficiency and cytocompatibility. In vitro BBB model was prepared using murine bEnd.3 cells and primary astrocytes, or hCMEC/D3, human astrocytes (HA) and SHSY5Y cells in a culture insert to further select liposomes with high transport and transfection efficiency across the BBB model. The optimized nanoparticle formulations were then further evaluated in C57BL/6 mice for their transport to the brain, transfection efficacy, and biocompatibility post intravenous (tail-vein) administration.
MATERIALS AND METHODS
Materials
DSPE−PEG2000−NHS and DSPE−PEG2000−Mannose were purchased from Biochempeg Scientific Inc. (MA, USA). Lissamine rhodamine, DOPE (dioleoyl-sn-glycero-3-phosphoethanolamine) and DOTAP (Dioleoyl-3-trimethylammonium-propane chloride) were obtained from Avanti Polar Lipids (AL, USA). Cholesterol was gotten from Sigma-Aldrich (MO, USA). Chimeric rabies virus glycoprotein fragment (YTIWMPENPRPGTPCDIFTNSRGKRASNGGGGRRRRRRRRR), rabies virus derived peptide (KSVRTWNEIIPSKGCLRVGGRCHPHVNGGGRRRRRRRRR), CGN (CGNHPHLAKYNGT) peptide and penetratin (RQIKIWFQNRRMKWKKGG) were purchased from Zhejiang Ontores Biotechnologies Co., Ltd. (Zhejiang, China). Chitosan (molecular weight 30 kDa) was acquired from Glentham Life Sciences (Corsham, UK).
Synthesis of CPP modified lipids
Activated NHS conjugated PEGylated DSPE lipid (NHS-PEG-DSPE) was used for synthesizing CPP conjugated lipid as described previously (Arora et al., 2020b, 2020a; dos Santos Rodrigues et al., 2020a). Briefly, lipid and CPP (1:3 molar ratio) were mixed in dimethylformamide (DMF) alkalified using triethylamine to pH 8.5. The reactants were incubated under constant stirring for 72 h to allow nucleophilic substitution reaction. Post incubation, the resulting mixture was dialyzed for 48 h against distilled water. The resulting solution after dialysis was dried using a lyophilizer and extent of CPP substitution was evaluated using micro-bicinchoninic acid (BCA). The lyophilized product was stored at −20 °C until used for preparation of liposomes.
Synthesis and characterization of liposomal nanoparticles
The lipids, CPP-PEG-DSPE, MAN-PEG-DSPE, DOTAP, DOPE, and cholesterol (4:4:45:45:2) were dissolved in a round bottom flask in a mixture of methanol and chloroform (0.5:1 v/v). A thin lipid film was prepared using Buchi Rotary Evaporator (Rotavapor® R-100, Flawil, Switzerland). The lipid film hydration buffer was prepared by mixing pVGF complexed chitosan (N/P ratio 5:1) and HEPES buffer (pH 7.4, 10 mM). The hydration buffer was added to the vacuum dried lipid film and the resulting dispersion was agitated for 30 min in a bath sonicator. The resulting liposomal nanoparticles were characterized for their hydrodynamic radius and charge by dynamic light scattering technique (DLS) at room temperature. The association of the pVGF/chitosan complex was evaluated using a DNA-binding fluorescent dye (Hoechst 33342) (dos Santos Rodrigues et al., 2018).
Culturing brain cells for in vitro analysis
In vitro assessment of liposomal nanoparticles loaded with pVGF/chitosan complexes were characterized for their cytocompatibility, cellular targeting and transfection efficacy in primary neuronal cells, brain endothelial cells (bEnd.3 cells) and primary astrocytes. Complete DMEM media (base media with 10% fetal bovine serum) was utilized for culturing bEnd.3 cells obtained from ATCC. Primary neurons and astrocytes were cultured from brains of newborn rat pups (less than 3 days old). Previously optimized protocol was followed to extract brain cells’ suspension in complete DMEM media (dos Santos Rodrigues et al., 2019b). Similar procedure was used to culture primary neurons with the aid of neurobasal media supplemented with B-27 and 10 % equine serum. Neuronal cells were culture for 72 h followed by supplementing the culture media with AraC. The culture media was replaced with fresh media on day 5. Anti MAP2 and anti-glial fibrillary acidic protein (GFAP) antibodies were utilized to confirm the identity of the primary neurons and astrocytes, respectively.
In vitro cytocompatibility
Cytotoxicity of the liposomal nanoparticles was evaluated in primary neurons, bEnd.3 cells, and primary astrocytes (dos Santos Rodrigues et al., 2019a; Lipp et al., 2019; Sharma et al., 2019b). Liposomes incorporating pVGF/chitosan complexes were added to the cell culture media in a 96 well plate containing 5,000 cells per well. Post 4 h incubation with increasing concentrations of liposomes (0.05 – 0.6 µM total lipids in DMEM basal medium) the cells were washed with DPBS and further incubated for 48 h in complete media. Finally, MTT (3-(4,5-Dimethylthiazol2-yl)-2,5-Diphenyltetrazolium Bromide) reagent was used to assess the viability of the treated cells against untreated cells (negative control) for comparison.
Cellular uptake
Cellular internalizing ability of the liposomal nanoparticles (100 nM phospholipid concentration) was assessed in primary neurons, bEnd.3 cells, and primary astrocytes. Lissamine rhodamine dye (0.5 mole % of total lipid) loaded liposomes were added to the cells cultured at a density of 105 cells per well of a 24 well plate followed by incubation (up to 4 h). Post incubation, the liposomes were removed by washing the cells with DPBS and the fluorescent dye was extracted in methanol and 0.5 % v/v triton-x mixture. Finally, spectrophotometer was used to analyze the percent cellular uptake of the liposomes inside the cells at λex 553 nm and λem 570 nm.
Cellular transfection
Cellular transfection efficiency of the liposomal nanoparticles (100 nM phospholipid concentration) was performed in primary neurons, bEnd.3 cells, and primary astrocytes seeded at a density of 105 cells per well of a 24 well plate. Cells were treated with liposomes incorporating pVGF/chitosan complexes (1 µg pVGF per well) for 4 h in base media. Post incubation, the formulation was removed by washing the cells followed by further incubation for 48 h in complete media. ELISA was performed to assess vgf protein expression post normalization with respect to total protein content of the cells. Lipofectamine®3000, a marketed transfection reagent, was used as a positive control for this study (Santos Rodrigues et al., 2019).
Blood brain barrier model development
The formation of the BBB model was performed in accordance with the protocol described in literature (dos Santos Rodrigues et al., 2020a). bEnd3 cells and primary astrocytes were used for preparing the BBB model at a density of 15×104 /cm2 and 15×103 /cm2, respectively. Barrier insert (0.4 µm membrane) was used to seed the astrocytes on its bottom side and bEnd3 cells on its top side. Similarly, another barrier was made using hCMEC/D3, human astrocytes (HA) and SHSY5Y cells. HA and SHSY5Y cells (1.5 × 104/cm2) were seeded on the bottom side and hCMEC/D3 (1.5 × 105/cm2) were seeded on top side of the culture inserts. Integrity of the resulting in vitro BBB was assessed using Epithelial Volt/Ohm Meter 2 (EVOM) by measuring the transendothelial electrical resistance (TEER). TEER of the control barrier, made with either b.End3 cells only or hCMEC/D3 cells only was also determined for reference (Santos Rodrigues et al., 2019).
Transport and transfection efficacy across blood brain barrier model
Transcytosis efficacy of the liposomal nanoparticles through the BBB model was determined according to the previously described procedure (Arora et al., 2020b; dos Santos Rodrigues et al., 2020a). Liposomes (100 nM phospholipid concentration) loaded with Lissamine rhodamine dye (λex: 553 nm, λem: 570 nm) were added to the BBB model containing 500 µL of 10% FBS solution in PBS. The BBB model was then incubated in a well containing PBS solution for different time points (up to 24 h). Post each incubation time, the fluorescent intensity in the lower chamber was analyzed using spectrophotometer.
For evaluation transfection efficiency post transcytosis across BBB, the BBB model cell culture insert was placed in a 24-well plate containing primary neurons (Arora et al., 2020b, 2020a). Liposomal nanoparticles (100 nM phospholipid concentration) incorporating pVGF/chitosan complexes (1µg pVGF) were incubated in the BBB model for 16 h. Post incubation the BBB model was removed. Primary neurons were further incubated for 2 days followed by analyzing the transfected vgf protein content using ELISA. Normalization of the expressed protein was performed with respect to total protein content of the cells.
Animal procedure
Institutional Animal Care and Use Committee (IACUC) approved protocol A20091 was used for all live animal procedures. Equal number of male and female C57BL/6 mice (6 mice per group) were subjected to liposomal treatment. All animals were placed in proper housing conditions with food and water access.
In vivo transfection efficiency and biocompatibility
Liposomal nanoparticles incorporating pVGF/chitosan complexes (40 µg pVGF/ 100 g animal) were administered via tail vein injection at a dose of ~15.2 µmoles phospholipids/kg body weight (dos Santos Rodrigues et al., 2019a, 2018). Transfection efficacy was evaluated on 6th day post administration in all tissue samples (dos Santos Rodrigues et al., 2019a, 2018). Tissues were homogenized using RIPA lysis buffer supplemented with phosphatase and protease inhibitor. The cell debris was removed from the resulting lysate by centrifuging at 4 °C and vgf expression was determined using ELISA. In vivo biocompatibility post liposomal treatment was assessed in formalin fixed organs post 5 days of injection. Hematoxylin & Eosin (H&E) stain was used for visualization of inflammatory cells, changes in cell morphology, and any indication of toxicity.
Statistical analysis
All data represents mean ± standard deviation (SD). Statistical analysis was conducted using one-way ANOVA. A p-value of 0.05 or below was considered to be significant.
RESULTS
Synthesis and characterization of liposomes nanoparticles
Prior to the preparation of the liposomes, CPP conjugated lipid was synthesized using RVG9R, RDP, Pen or CGN and activated lipid NHS-PEG-DSPE. Conjugation of different CPPs using identical chemistry has been extensively performed in our lab and characterized using chemical based assay as well as using reverse phase chromatography (Lakkadwala and Singh, 2019). In our current study, the conjugation efficiency of CPPs to the lipid was found to be in excess of 75%. Liposomal nanoparticles were prepared in accordance to our previously reported method (Lakkadwala et al., 2019). Pegylated mannose conjugated lipid (MAN-PEG-DSPE) was used in combination with the synthesized CPP conjugated lipid to produce mannose and CPP (RVG9R, RDP, Pen or CGN) surface functionalized liposomal nanoparticles. Characterization data showed that all the liposomal nanoparticles prepared were less than 200 nm in size. Addition of the ligands on the surface of the nanoparticles resulted in slight increase in their hydrodynamic diameters. The zeta potential of nanoparticles was slightly positive (Table 1). Encapsulation efficiency pVGF/chitosan complexes inside the liposomes was found to be in excess of 80% with no negative effects on size or surface charge of the nanoparticles. Our previous studies also indicated that these lipid nanoparticles are spherical in shape with uniform distribution as seen using transmission electron microscopy (TEM) as well as atomic force microscopy (Arora et al., 2020a; dos Santos Rodrigues et al., 2019b). Both of these microscopic techniques can provide visual morphological characteristics of the nanoparticles. Also, there is no prior requirement for specific preparation or fixation in case of AFM, however, a negative stain is required, usually 0.1% phosphotungstic acid aqueous solution, for imaging using TEM.
Table 1.
Characterization of liposomes entrapping pVGF/chitosan complexes.
| Liposomal Formulation | Size (nm) | Zeta Potential (mv) | Polydispersity Index (PDI) | pDNA Encapsulation Efficiency (%) |
|---|---|---|---|---|
| Plain | 138.9 ± 11.53 | 14.6 ± 1.1 | 0.239 ± 0.00 | 85.08 ± 3.40 |
| MAN | 144.9 ± 8.12 | 13.1 ± 2.3 | 0.234 ± 0.01 | 83.47 ± 3.58 |
| Pen | 175.6 ± 2.19 | 17.0 ± 1.5 | 0.273 ± 0.06 | 80.31 ± 5.12 |
| PenMAN | 180.0 ± 33.52 | 16.1 ± 1.4 | 0.275 ± 0.04 | 85.37 ± 6.55 |
| CGN | 141.3 ± 17.25 | 15.4 ± 1.0 | 0.155 ± 0.10 | 84.60 ± 2.84 |
| CGNMAN | 174.5 ± 7.63 | 14.9 ± 0.4 | 0.264 ± 0.06 | 84.77 ± 8.29 |
| RVGR9 | 183.7 ± 41.58 | 22.6 ± 0.3 | 0.255 ± 0.18 | 86.7 ± 3.33 |
| RVGR9MAN | 178.1 ± 38.82 | 22.8 ± 2.8 | 0.159 ± 0.12 | 83.56 ± 5.92 |
| RDP | 175.1 ± 20.44 | 20.5 ± 3.0 | 0.219 ± 0.20 | 84.00 ± 3.34 |
| RDPMAN | 190.8 ± 42.21 | 19.0 ± 3.1 | 0.063 ± 0.03 | 82.25 ± 6.34 |
In vitro cytocompatibility
The cytocompatibility of the liposomal nanoparticles was evaluated in primary neurons, bEnd.3 cells, and primary astrocytes (Figure 1) using MTT assay. It was found that the relative viability of the cells compared to untreated cells decreased with increasing phospholipid concentrations in all the brain cell types in vitro. However, up to phospholipid concentration of 100 nM more than 80% of cells were viable in all the cell lines with no significant difference (p>0.05) among different liposomal nanoparticles. Therefore, 100 nM phospholipid concentration was found to be cytocompatible with all brain cell types tested and was selected for all further studies.
Figure 1.
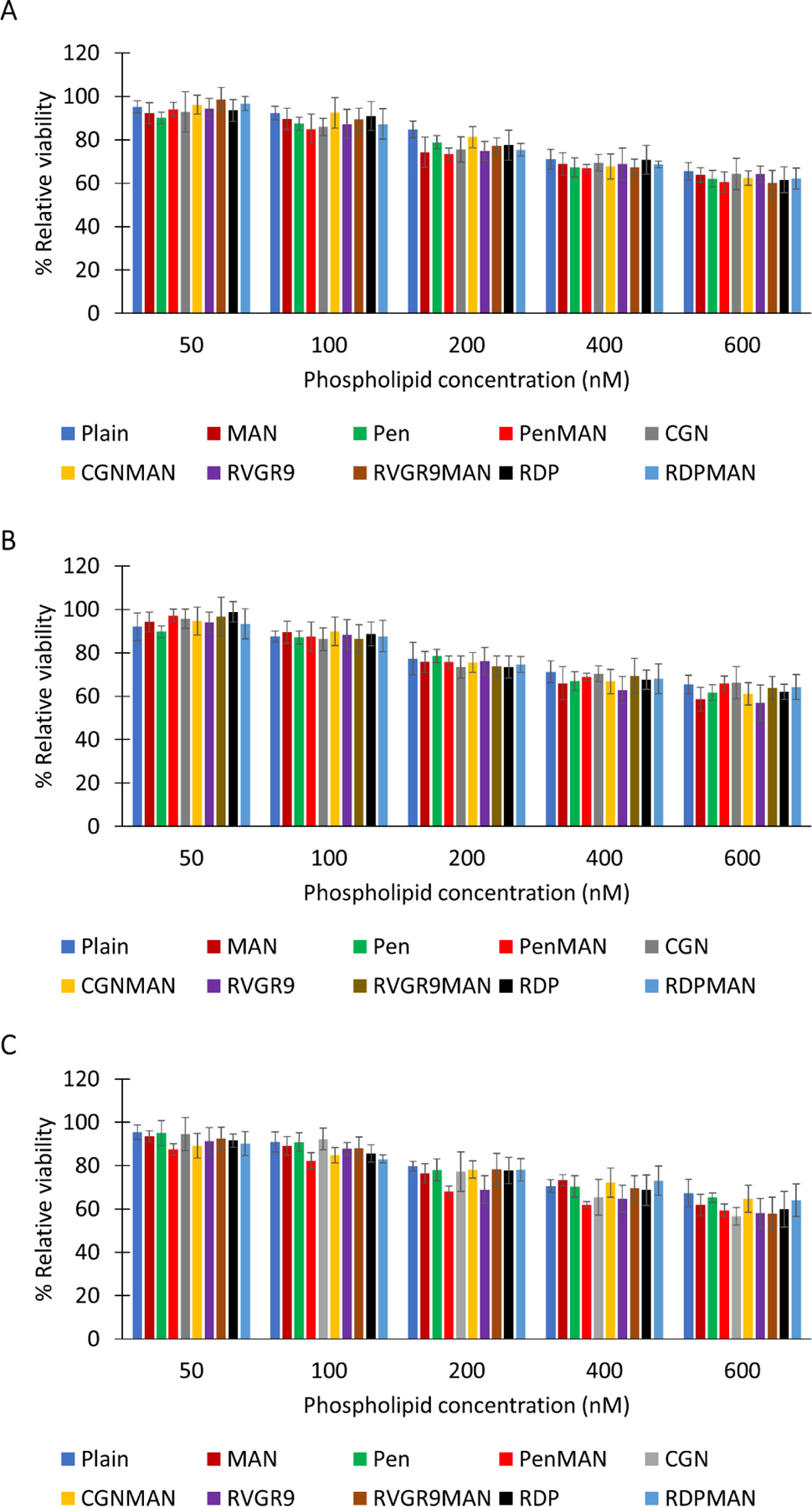
In vitro cytocompatibility. A) bEnd.3, B) primary astrocytes, and C) primary neurons. Data shown as mean (SD) with 4 repeats.
Cellular uptake
The physicochemical characteristics of lipid-based nanoparticles majorly affect their cellular internalization capacity. Therefore, cellular uptake studies were performed in the primary neurons, bEnd.3 cells, and primary astrocytes using lissamine rhodamine dye loaded liposomal nanoparticles as shown in Figure 2. It was observed that as the incubation time of the liposomes with the cells increased their internalization efficiency also increased with no significant difference (p>0.05) between the different formulations. Post 4 h of incubation, the bifunctionalized liposomes (RVG9RMAN, RDPMAN and CGNMAN) were found to be internalized in excess of 80% in primary neurons, bEnd.3 cells, and primary astrocytes. Liposomes surface modified with Pen and PenMAN demonstrated 75–80% cellular uptake within 4 hrs in all the cell lines tested (Arora et al., 2020b). Therefore, 4 h of incubation of liposomal nanoparticles with the cells was selected as the time point for conducting transfection studies.
Figure 2.
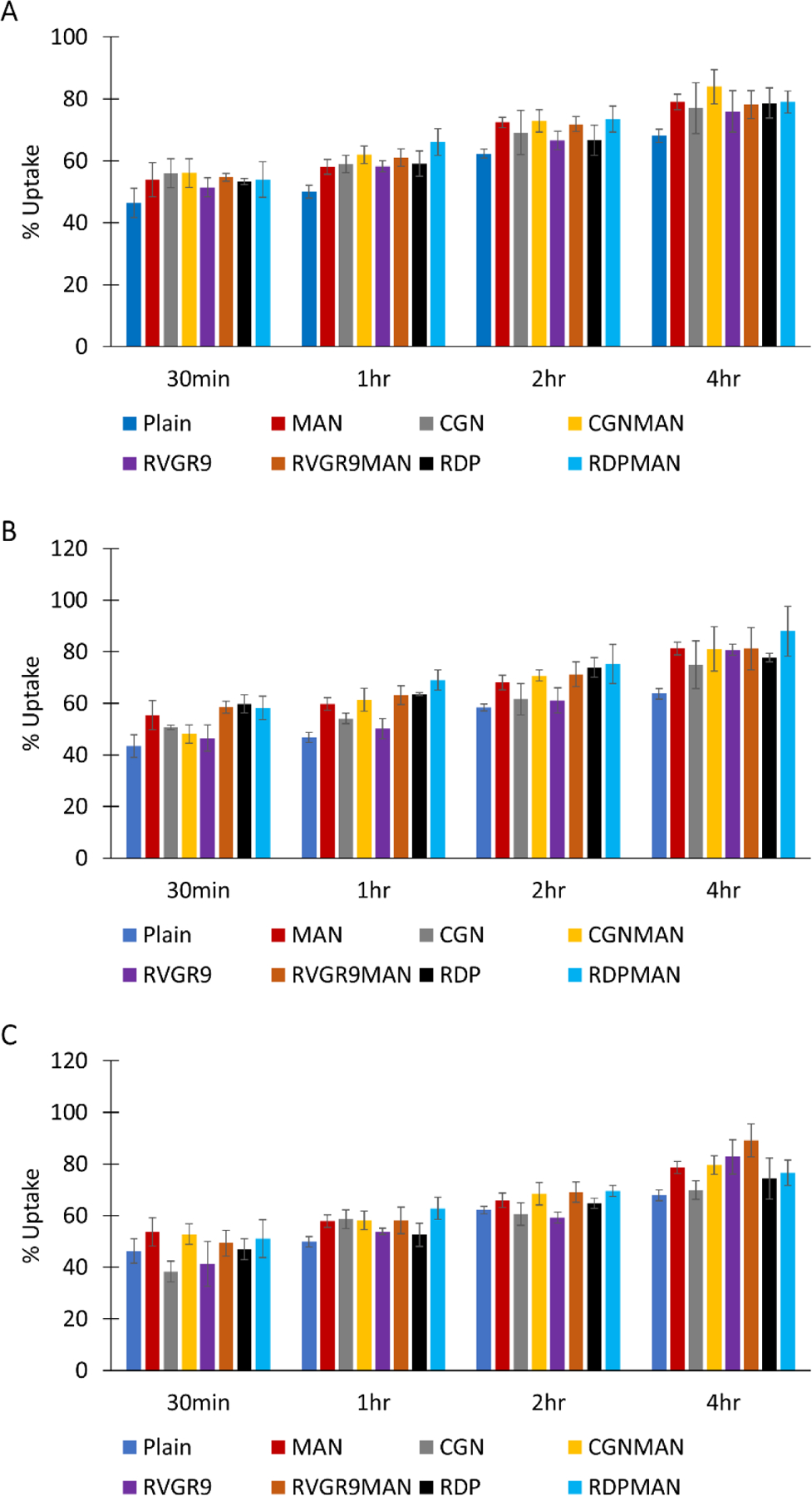
In vitro cellular uptake. A) bEnd.3, B) primary astrocytes, and C) primary neurons. Data shown as mean (SD) with 4 repeats.
Cellular transfection
Transfection potential of the nanoparticle formulations as a non-viral vector is essential for evaluating their therapeutic effect. Therefore, transfection ability of pVGF/chitosan incorporating liposomes was evaluated in primary neurons, bEnd.3 cells, and primary astrocytes. It was observed that in all the cell lines bifunctionalized liposomes (RVG9RMAN, RDPMAN, PenMAN and CGNMAN) showed significantly higher (p<0.05) vgf transfection efficiency compared to naked pDNA, plain liposomes, and singly modified (MAN, RVG9R, RDP, Pen and CGN) liposomes (Figure 3). RDPMAN functionalized liposomes demonstrated highest transfection efficiency in all the cell lines, which was ~2 fold greater than the single ligand modified liposomes. Similar increase in transfection potential was observed with bifunctionalized PenMAN, RVG9RMAN and CGNMAN liposomes as compared to their single ligand (MAN or CPP only) modified counterparts. However, no significant difference was found between the different bifunctionalized liposomes.
Figure 3.
In vitro vgf transfection. A) bEnd.3, B) primary astrocytes, and C) primary neurons. Data shown as mean (SD) with 4 repeats. ~, |, @, #, *, --, +, and “ show statistically significant difference (p<0.05) from untreated, naked DNA, plain, Pen, MAN, CGN, RVG9R and RDP liposomes, respectively.
Blood brain barrier model development
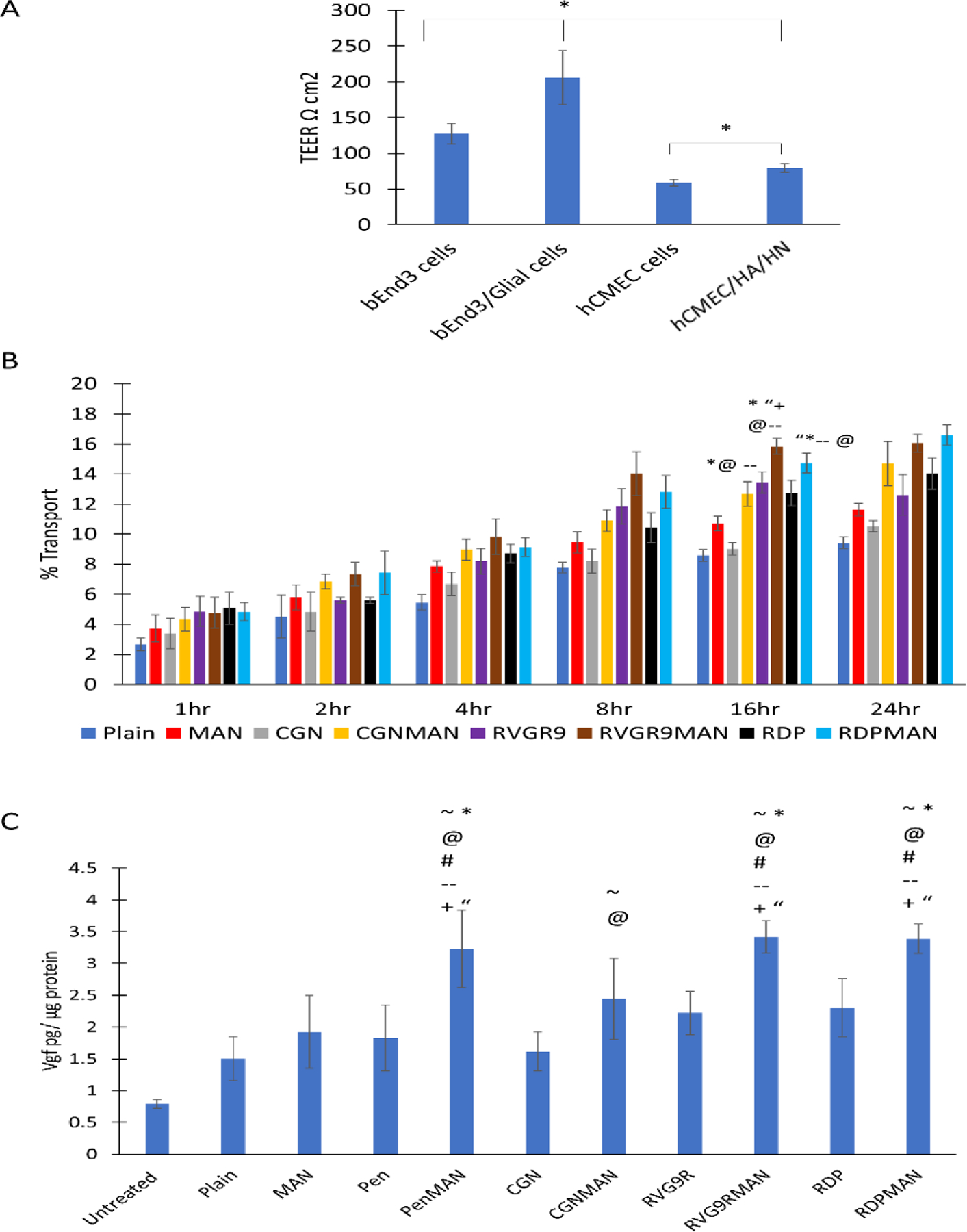
BBB model was prepared using a transwell culture insert. The tightness of the barrier was evaluated utilizing transepithelial electrical resistance (TEER) across the barrier layer. Co-cultured BBB model developed using murine cell lines demonstrated TEER values of 206 ± 37 Ω cm2, which was found to be significantly higher (~1.6 times) than the barrier developed using only b.End3 cells (Figure 4A). Moreover, the barrier cultured using hCMEC/D3, HA and SHSY5Y cells demonstrated TEER of only 79 ± 6 Ω cm2, which was significantly lower (p<0.05) than the barriers developed using the murine cell lines. Therefore, the barrier developed using the murine cells was selected for further evaluation of different liposomal nanoparticles.
Figure 4.
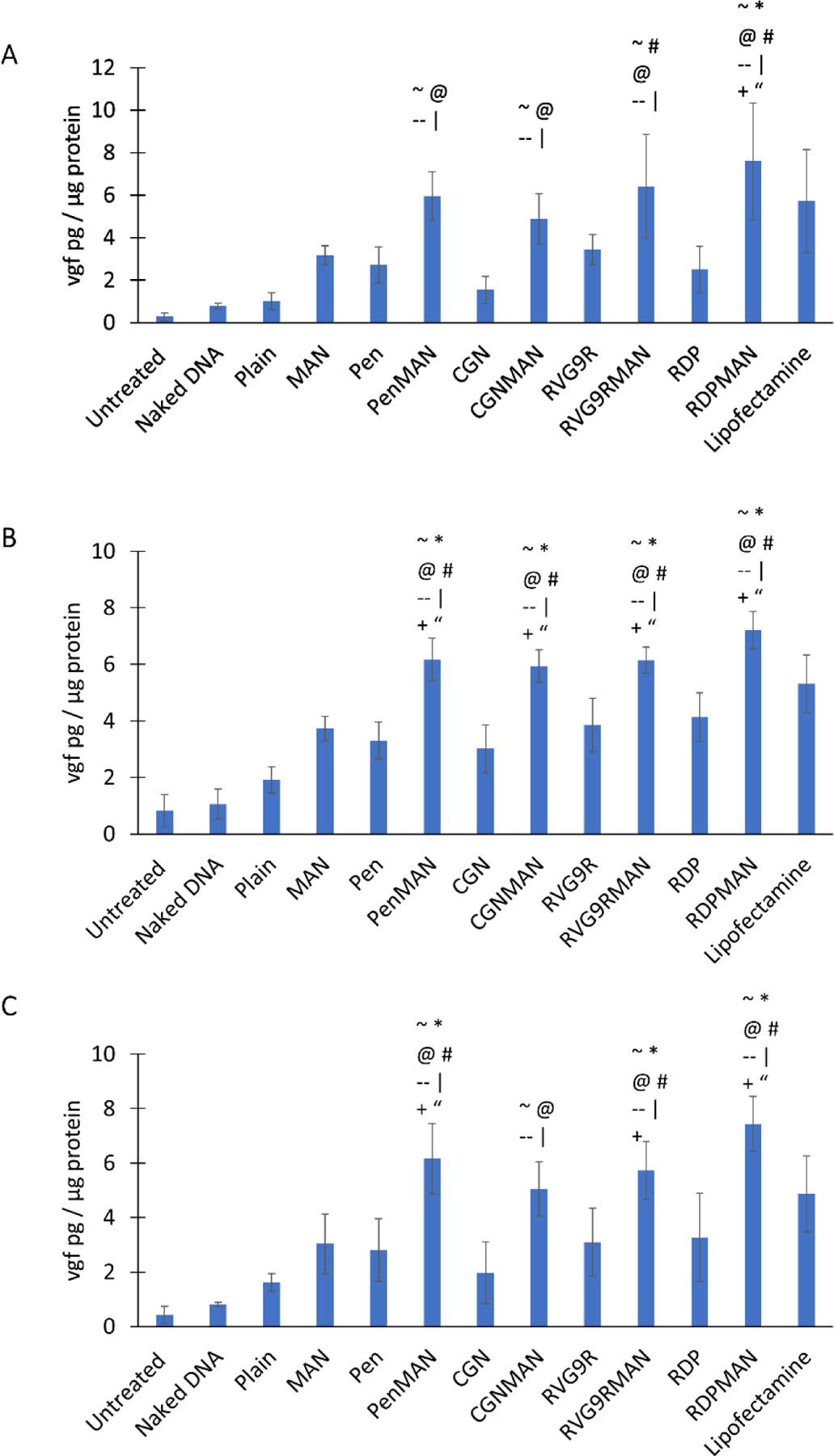
A) In vitro BBB transendothelial electrical resistance, B) Transport across BBB model, and C) pVGF transfection across BBB model. Data shown as mean (SD) with 4 repeats. ~, @, #, *, --, +, and “ shows statistically significant difference (p<0.05) from untreated, plain, Pen, MAN, CGN, RVG9R and RDP liposomes, respectively.
Transport and transfection across blood brain barrier model
Transportation of nanoparticles across the BBB is the major challenge for current non-viral vectors for treatment of brain specific disorders. Therefore, effect of surface functionalization of liposomal nanoparticles was at first evaluated in in vitro BBB model. Transcytosis of liposomes loaded with lissamine rhodamine dye was assessed using co-cultured BBB model for up to 24 h. It was found that as the incubation time increased the liposomal transport across the barrier layer also increased (Figure 4B). Transportation saturation was observed in about 16 h and no significant enhancement in transport was observed post 16 h of incubation. The bifunctionalized liposomes RVG9RMAN and RDPMAN were found to be transported ~15% by 16 h, which was significantly greater than plain, and monofuctionalized (MAN, RVG9R, RDP and CGN) liposomes. The findings are consistent with our earlier reported transport for PenMAN and Pen modified liposomes, respectively (Arora et al., 2020b).
Following translocation across the BBB, the delivered gene must express the desired protein in the brain cells to elicit the intended therapeutic effect. Transfection efficacy of liposomal nanoparticles prepared in this study was evaluated in primary neurons seeded across the BBB model. As depicted in Figure 4C, RVG9RMAN, PenMAN and RDPMAN liposomal nanoparticles demonstrated ~2 times higher vgf expression compared to plain liposomes. Transfection efficacy was notably improved with ligand conjugation as evident with significantly higher (p<0.05) transfection of vgf protein using bifunctionalized liposomes RVG9RMAN, PenMAN and RDPMAN when compared to monofuctionalized liposomes (MAN, RVG9R, RDP, Pen and CGN). Although CGNMAN functionalized liposomes demonstrated higher transfection, no significant difference (p>0.05) was observed as compared to the liposomes surface modified with single targeting ligand. Therefore, RVG9RMAN, PenMAN and RDPMAN liposomes were selected for in vivo evaluation in our further studies.
In vivo transfection efficiency and biocompatibility
Transportation of therapeutics across the BBB is one of the major challenges for CNS diseases. Liposomal nanoparticles incorporating pVGF/chitosan complexes were administrated via tail vein and transfection efficiency was evaluated in all major tissues on 6th day following injection. Bifunctionalized liposomes RVG9RMAN, PenMAN, and RDPMAN demonstrated significantly higher (p<0.05) vgf protein transfection in the brain of wild type mice compared to mice treated with saline and naked DNA (Figure 5A). RVG9RMAN, PenMAN and RDPMAN functionalized liposomes demonstrated ~70 pg vgf protein per mg total protein in the brain, which was ~1.5 times higher than the expression demonstrated by monofunctionalized liposomes. All three bifunctionalized liposomes showed similar transfection potential in the brain with no statistically significant difference (p>0.05). Monofunctionalized (MAN, RVG9R, Pen and RDP) and plain liposomes showed comparable transfection efficiency in brain although significantly lower than bifunctionalized liposomal nanaoparticles (RVG9RMAN, PenMAN and RDPMAN). Vgf protein was also found to be increased in plasma and other organs (liver, spleen, kidney, heart, and lungs), however the levels were found to be comparable to their baseline levels.
Figure 5.
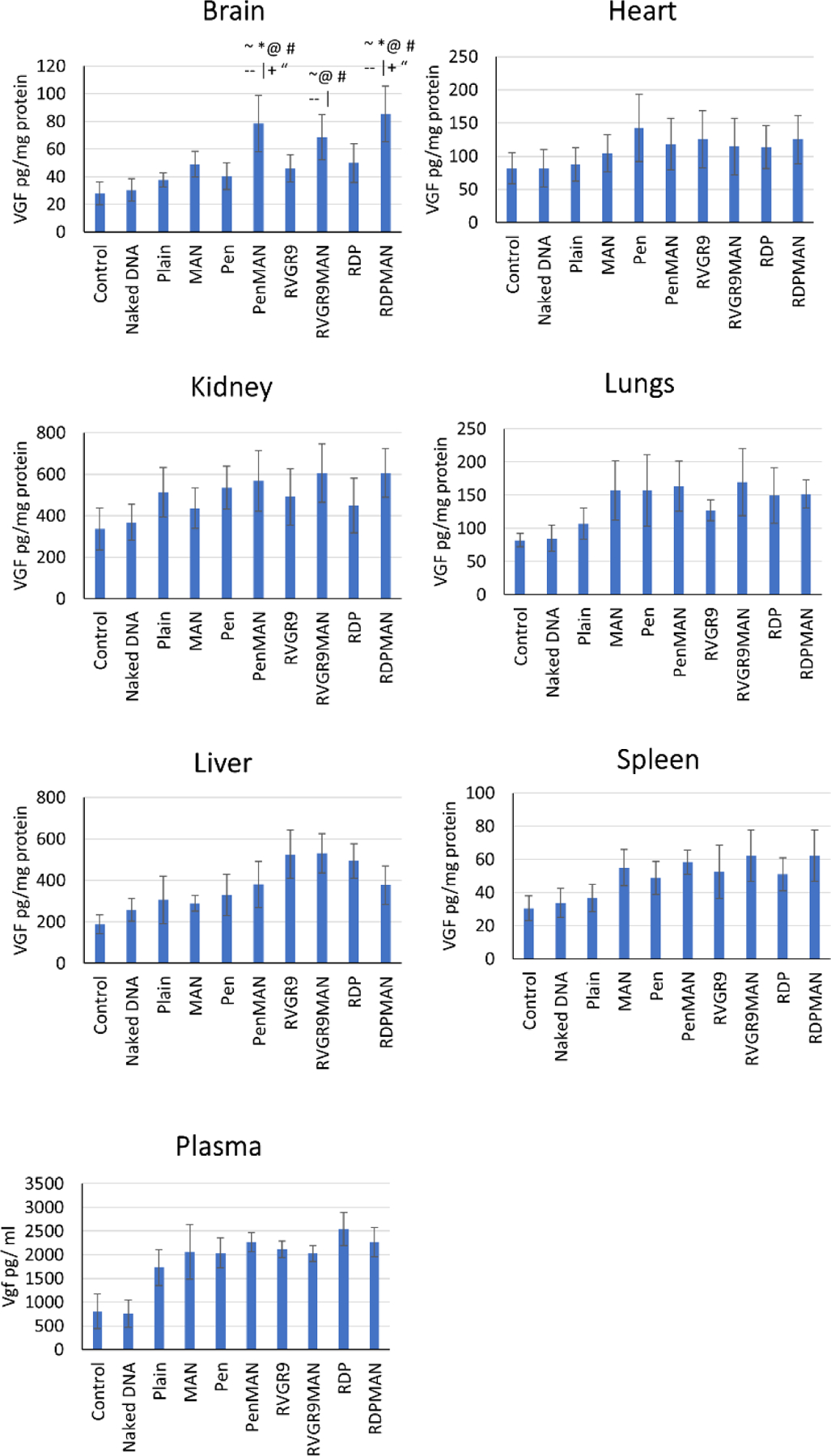
In vivo vgf transfection. Different organs are indicated as follows: A) Brain, B) Heart, C) Kidney, D) Lungs, E) Liver, F) Spleen, and G) Plasma. Data shown as mean (SD) of 6 animals per group. ~, |, @, #, *, --, +, and “ show statistically significant difference (p<0.05) from control, naked DNA, plain, Pen, MAN, CGN, RVG9R and RDP liposomes, respectively.
H&E staining was performed to assess biocompatibility of the administered liposomal nanoparticles in major tissues. Saline treated group was used as a control. Tissue sections were assessed for signs of aberrations, nucleus enlargement, inflammation, or abnormalities in cellular morphology. The results indicated that the liposomal nanoparticles did not demonstrate any form of toxicity in the brain, liver, spleen, kidney, heart, and lungs (Figure 6). Disruption of muscle fibers were not observed in cardiac tissue. Lung and liver tissues did not show any sign of fibrosis and ballooning, respectively. Also, abnormal alveoli thickening was not observed in the lungs. Examination of spleen and kidney tissue sections depicted no signs of nuclei enlargement, necrosis, or defects in cell morphology. Moreover, mice treated with liposome nanoparticles did not demonstrate any changes in water/food intake, behavioral alterations, or loss of physical activity, indicating good safety profile of this delivery system.
Figure 6.
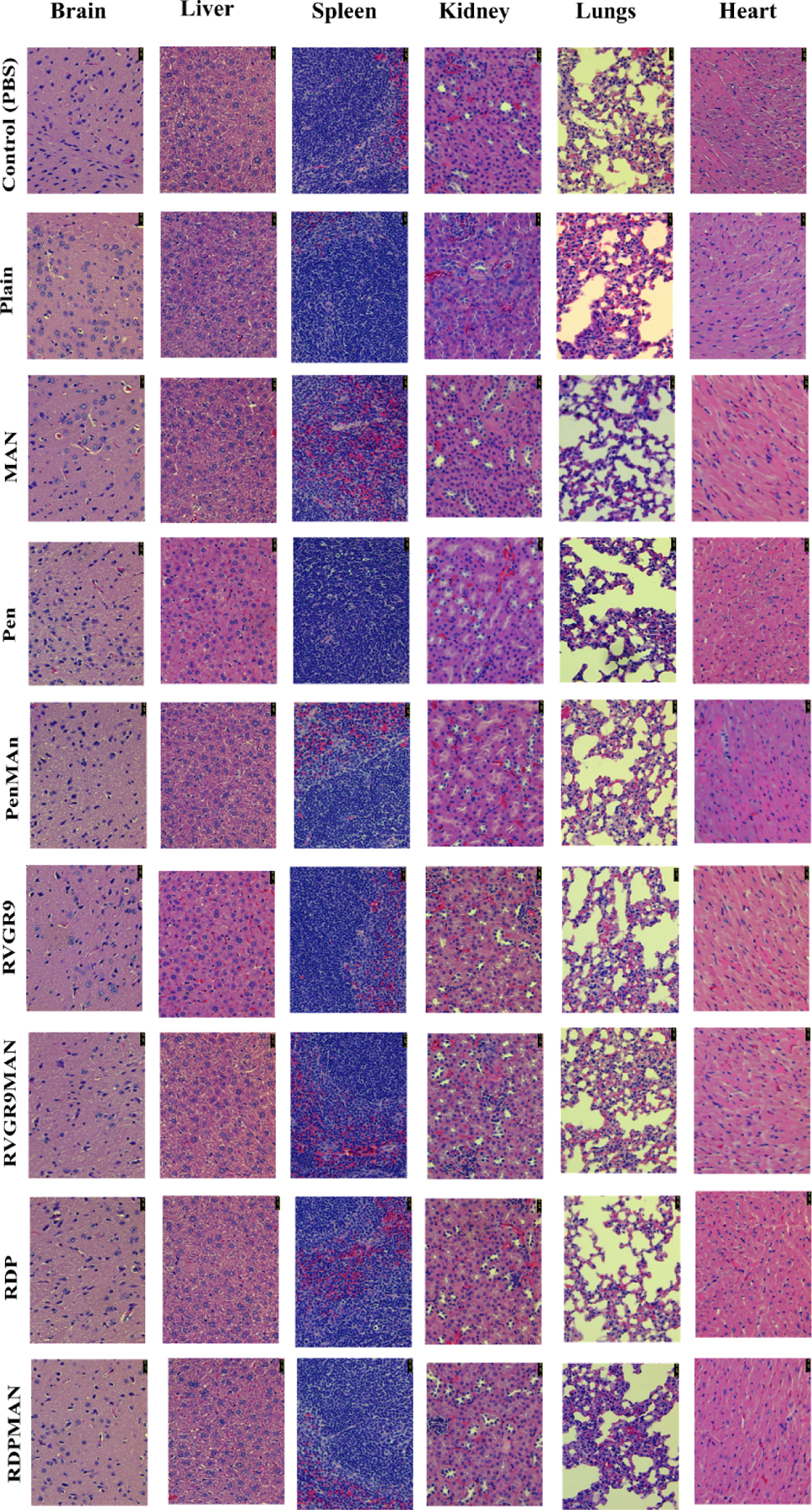
In vivo histological assessment of different liposomal nanoparticle formulations in C57BL/6 mice after 6 days post formulation administration. The figure depicts H&E stained tissue sections of Brain, Liver, Spleen, Kidney, Lungs, and Heart post administration of PBS (negative control) and different liposomal nanoparticles prepared in this study.
DISCUSSION
Despite the astonishing progress in medical treatment over recent years in various fields, there remains a wide gap for brain targeted treatments for CNS disorders (Ventola, 2017). Over the past decade, various delivery systems have been researched for gene delivery to the brain using numerous targeting ligands. However, shortcomings such as safety, efficacy, ease of administration, off target adverse reactions, suitability for clinical translation, and chiefly inefficient translocation across the blood brain barrier (BBB) have been some of the major hurdles to overcome for development of therapeutic strategies to treat brain-related malignancies (Bunker et al., 2016; Zylberberg et al., 2017). The protective and selectively permeable BBB has been rightly termed as one of the greatest challenges for neurological disorders. Adding on to the complexity, Alzheimer’s disease (AD) pathophysiology is poorly understood and difficult to treat with current therapies. Consequently, novel brain specific treatments are an essential need to reduce the socioeconomic burden of AD, one of the world’s most fatal disorders of the elderly. In our present study, we designed and optimized brain-targeted liposomal nanoparticles surface modified with MAN in combination with CPPs to enhance the translocation of genetic cargo across the BBB. In our extensive experience with multiple brain-targeted formulations with various conjugating ligands, due to the complex nature of different transfection mechanisms along with the potential gaps between in vitro and in vivo cellular environment, both in vitro and in vivo cellular uptake as well transfection studies need to be reviewed closely to identify a potential lead formulation (Arora et al., 2020a; dos Santos Rodrigues et al., 2020b, 2020c). To the same effect, due to the differences in biochemical properties, half-life, and molecular weight of the expressed proteins, different genes may show varied transfection and efficacy. In our present study, we have explored the gene transfection efficiency of vgf (non-acronymic) utilizing different liposomal formulations. Vgf is a critical protein for improving learning, memory formation process, synaptic activity and neurogenesis, that is found to be downregulated in AD (Cocco et al., 2010). Plasmid encoding vgf protein was encapsulated inside the liposomal nanoparticles and their safety and efficacy in transfecting brain cells was tested in vitro and in vivo.
From a quality standpoint, nanoparticle formulations must be stable, homogenous and be able to carry required genetic load for therapeutic efficacy (Zylberberg et al., 2017). The liposomal nanoparticles prepared in this study showed size below 200 nm, uniformity in their hydrodynamic diameter as depicted by their low PDI and high entrapment efficiency of the therapeutic gene (pVGF/chitosan complexes). Nanoparticle hydrodynamic diameter below 200 nm aids in crossing the tight junction between endothelial cells of BBB and helps escape the immune system resulting in improved half-life in the body (Masserini, 2013). Low PDI (below 0.3) indicates homogeneity in particle size without any evidence of instability such as aggregation or precipitation (Danaei et al., 2018). Another concern with lipid-based non-viral vectors is cellular toxicity owing to high cationic charge. Nanoparticles with cationic charge can interact with the membranes of various cells and its organelles resulting in undesirable safety profile of the formulation (Fröhlich, 2012). However, net positive surface charge of nanoparticles is beneficial in promoting cellular internalization and transfection. The various functionalized liposomes prepared in this study showed good biocompatibility in vitro as well as in vivo. In our optimized formulation, low cytotoxicity (i.e. >80% cell viability) and high transfection efficiency was promoted by utilizing cationic phospholipid DOTAP and helper phospholipid DOPE in equimolar ratios (Kim et al., 2015). Additionally, PEGylation on liposomal surface in our formulation assisted in shielding from opsonization and accumulation, which is eventually helpful in increasing their half-life in the biological system (Kim et al., 2012; Klibanov et al., 1990; Suk et al., 2016).
Effective transport of liposomes inside the cells is vital for eliciting their desired therapeutic effects. Improved physicochemical characteristics of these nanoparticles owing to surface functionalization primarily increases their cellular internalization capacity. Modification using MAN ligand helped target GLUT-1 transporter mediated endocytosis through the BBB. Moreover, co-functionalizing with brain-specific CPPs aided in enhanced cellular penetration and overall higher uptake of these nanoparticles as compared to plain liposomes (Lakkadwala et al., 2019; Pan et al., 2017). The internalization of the nanoparticles initiates with the activation of the cellular transport pathways upon interaction between cell membrane and liposomes components resulting in either direct translocation or endocytosis across the cell membrane (Behzadi et al., 2017; Kettler et al., 2014). Based on our previous results with various conjugating ligands, the presence of targeting ligands changes the surface characteristics of the nanoparticles such as particle charge density as well as the size, which results in dissimilar nanoparticle-cell interactions, and endosomal escape properties in comparison to the unconjugated nanoparticles. Therefore, higher cellular uptake does not always correlate with the higher cellular transfection as seen in previous publications from our lab (dos Santos Rodrigues et al., 2020b; Sharma et al., 2012). Post internalization of the liposomes inside the cells the next step is efficient transfer of genetic payload to the nucleus for inducing transfection of the desired protein. Previously, our group had optimized the N/P ratio (5:1) of pDNA/chitosan complexes to attain desired pDNA encapsulation, condensation, protection against enzymatic degradation along with efficient endosomal escape and release inside the cells (Lavertu et al., 2006; Rodrigues et al., 2020). The superior endosomal escape ability of the bifunctionalized liposomes encapsulating pVGF/chitosan complexes is one of the important aspects of enhanced plasmid transport inside the nucleus and high transfection efficiency (dos Santos Rodrigues et al., 2019a; Sharma et al., 2016, 2014).
Most drug/gene delivery candidates for treatment of CNS disorders fail due to their inability to circumvent the BBB. Thus, it is essential to assess the ability of these therapeutics to translocate across BBB in an efficient in vitro model. A good BBB cell culture model can also help in optimization and screening of the potential formulations for treating brain disorders (Hatherell et al., 2011; Helms et al., 2015). In our study, BBB models were developed utilizing either murine cell lines or human cell lines and was assessed using TEER across the barrier. This is one of the most commonly used techniques to evaluate BBB integrity (Crone and Olesen, 1982). TEER values in vivo was found to be ~5900 Ωcm2 in rats, which is greater than currently available BBB models (Butt et al., 1990). Current in vitro BBB models yield TEER values ranging from 100 – 300 Ωcm2 achieved utilizing combination of immortalized and primary cells (Daniels et al., 2013; Helms et al., 2015). The TEER of the co-cultured BBB model was significantly higher than the monolayer model in case of both human and murine BBB models. The BBB model developed using human cell lines showed lower TEER compared to BBB model prepared using murine cells which can be due to the absence (or presence in very low amount) of several tight junction proteins in the human endothelial cells, which are present in the endothelial cells of murine origin (Biemans et al., 2017; Brown et al., 2007; Eigenmann et al., 2013; Rahman et al., 2016). Moreover, the ability to retain phenotype post numerous passages and rapid growth in bEnd.3 cells make them suitable for the development of an efficient BBB model (Brown et al., 2007). Low TEER of BBB model developed using human endothelial cells has also been reported by other researchers resulting in poor integrity of the barrier (Eigenmann et al., 2013; Hatherell et al., 2011; Markoutsa et al., 2011). Therefore, in our study, transport and transfection efficiency of liposomal nanoparticles was investigated in the murine BBB model. Transfection studies using different liposomal nanoparticles showed significantly improved vgf transfection using bifunctionalized liposomes compared to non-functionalized (plain) and monofunctionalized groups, which can be rationalized via their dual transport mechanisms leading to enhanced transport of the therapeutic gene to the nucleus of cells. These observations advocate the effect of targeting ligands (MAN and CPPs) on developing liposomal nanoparticles as suitable candidates for brain-targeted gene therapy for treating AD.
Furthermore, in vivo studies in C57BL/6 mice confirmed the ability of the liposomal nanoparticles to enhance transcytosis across the BBB and transfect the brain cells. The dual targeting effect with CPP and MAN was evident by the enhanced translocation of bifunctionalized liposomal nanoparticles inside the brain cells in comparison to other formulations. Subsequently, the bifunctionalized nanoparticles also showed superior transfection and increased vgf level ~ 2-fold higher than baseline level in the mice brain. Further dose optimization and efficacy studies will need to be performed in AD mice model to evaluate the overall efficacy of the delivery system in treating AD. Increase in vgf protein was also found in other vital organs, which may be due to the accumulation of nanoparticles in these organs owing to the reticuloendothelial system (RES) leading to nonspecific transfection (Haute and Berlin, 2017; Li and Huang, 2009). However, histopathological analysis performed in all vital tissues did not show any signs of pathology such as inflammation, toxicity, changes in cellular morphology or vasculature, which was also supported by our previous studies indicating good safety profile of this delivery system (Arora et al., 2020b, 2020a).
CONCLUSION
Overall, AD is a very complex disorder which surfaces various challenges for an effective treatment strategy. However, delivery of crucial brain development protein vgf via gene therapy using brain-targeted non-viral vector evaluated in this study may be beneficial in restoring lost cognitive function in AD or at minimum halting the progression of the disease. The results indicate that the bifunctionalized liposomal nanoparticles evaluated in this study are efficient in transporting genetic cargo inside the brain cells with no signs of toxicity. These liposomal nanoparticles will be further assessed for their therapeutic efficiency in transgenic mouse model of AD in our future studies.
Acknowledgment
This research was supported by National Institutes of Health Grants R01 AG051574 and RF1 AG068034.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 2018 Alzheimer’s disease facts and figures, 2018. . Alzheimer’s Dement 14, 367–342. [Google Scholar]
- Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB, 2003. Brain-Derived Neurotrophic Factor-Induced Gene Expression Reveals Novel Actions of VGF in Hippocampal Synaptic Plasticity. J. Neurosci 23, 10800–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Layek B, Singh J, 2020a. Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood–Brain Barrier for Effective Treatment of Alzheimer’s Disease. Mol. Pharm [DOI] [PMC free article] [PubMed]
- Arora S, Sharma D, Singh J, 2020b. GLUT-1: An Effective Target To Deliver Brain-Derived Neurotrophic Factor Gene Across the Blood Brain Barrier. ACS Chem. Neurosci 11, 1620–1633. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Sharma D, Trivedi R, Singh J, 2020. Treatment of insulin resistance in obesity-associated type 2 diabetes mellitus through adiponectin gene therapy. Int. J. Pharm 583, 119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M, 2017. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev [DOI] [PMC free article] [PubMed]
- Biemans EALM, Jäkel L, de Waal RMW, Kuiperij HB, Verbeek MM, 2017. Limitations of the hCMEC/D3 cell line as a model for Aβ clearance by the human blood-brain barrier. J. Neurosci. Res 95, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni A, Ginty DD, Dudek H, Greenberg ME, 1995. Serine 133-Phosphorylated CREB Induces Transcription via a Cooperative Mechanism That May Confer Specificity to Neurotrophin Signals. Mol. Cell. Neurosci 6, 168–183. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, Alberini CM, Huntley GW, Salton SRJ, 2008. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J. Neurosci 28, 9857–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Morris AP, O’Neil RG, 2007. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res 1130, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker A, Magarkar A, Viitala T, 2016. Rational design of liposomal drug delivery systems, a review: Combined experimental and computational studies of lipid membranes, liposomes and their PEGylation. Biochim. Biophys. Acta - Biomembr 1858, 2334–2352. [DOI] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ, 1990. Electrical resistance across the blood‐brain barrier in anaesthetized rats: a developmental study. J. Physiol 429, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P, Hochstrasser DF, Sanchez JC, 2003. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease, in: Proteomics pp. 1486–1494. [DOI] [PubMed]
- Cocco C, D’Amato F, Noli B, Ledda A, Brancia C, Bongioanni P, Ferri GL, 2010. Distribution of VGF peptides in the human cortex and their selective changes in Parkinson’s and Alzheimer’s diseases. J. Anat 217, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Olesen SP, 1982. Electrical resistance of brain microvascular endothelium. Brain Res 241, 49–55. [DOI] [PubMed] [Google Scholar]
- Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR, 2018. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BP, Cruz-Orengo L, Pasieka TJ, Couraud PO, Romero IA, Weksler B, Cooper JA, Doering TL, Klein RS, 2013. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods 212, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, 2018. Current strategies for brain drug delivery. Theranostics 8, 1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Rodrigues B, Arora S, Kanekiyo T, Singh J, 2020a. Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res 1734, 146738. [DOI] [PubMed] [Google Scholar]
- dos Santos Rodrigues B, Banerjee A, Kanekiyo T, Singh J, 2019a. Functionalized liposomal nanoparticles for efficient gene delivery system to neuronal cell transfection. Int. J. Pharm 566, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Rodrigues B, Kanekiyo T, Singh J, 2020b. In vitro and in vivo characterization of CPP and transferrin modified liposomes encapsulating pDNA. Nanomedicine Nanotechnology, Biol. Med 28, 102225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Rodrigues B, Kanekiyo T, Singh J, 2019b. ApoE-2 Brain-Targeted Gene Therapy Through Transferrin and Penetratin Tagged Liposomal Nanoparticles. Pharm. Res 36, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Rodrigues B, Lakkadwala S, Kanekiyo T, Singh J, 2020c. Dual-Modified Liposome for Targeted and Enhanced Gene Delivery into Mice Brain. J. Pharmacol. Exp. Ther jpet.119.264127. [DOI] [PMC free article] [PubMed]
- dos Santos Rodrigues B, Oue H, Banerjee A, Kanekiyo T, Singh J, 2018. Dual functionalized liposome-mediated gene delivery across triple co-culture blood brain barrier model and specific in vivo neuronal transfection. J. Control. Release 286, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M, 2013. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gaamouch F, Audrain M, Lin WJ, Beckmann N, Jiang C, Hariharan S, Heeger PS, Schadt EE, Gandy S, Ehrlich ME, Salton SR, 2020. VGF-derived peptide TLQP-21 modulates microglial function through C3aR1 signaling pathways and reduces neuropathology in 5xFAD mice. Mol. Neurodegener 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E, 2012. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine 7, 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A, Wang Y, Zhan L, Zhou R, 2012. Targeted delivery of proteins into the central nervous system mediated by rabies virus glycoprotein-derived peptide. Pharm. Res 29, 1562–1569. [DOI] [PubMed] [Google Scholar]
- Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, De Los Angeles García M, Medina RA, Carrasco M, Barberis S, Castro T, Martínez F, Koch X, Vera JC, Poblete MT, Figueroa CD, Peruzzo B, Pérez F, Nualart F, 2006. Differential subcellular distribution of glucose transporters GLUT1–6 and GLUT9 in human cancer: Ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J. Cell. Physiol 207, 614–627. [DOI] [PubMed] [Google Scholar]
- Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ, 2011. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 199, 223–229. [DOI] [PubMed] [Google Scholar]
- Haute D. Van, Berlin JM, 2017. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: Lessons from gold nanoparticles. Ther. Deliv 8, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud PO, Deli MA, Förster C, Galla HJ, Romero IA, Shusta EV, Stebbins MJ, Vandenhaute E, Weksler B, Brodin B, 2015. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab 36, 862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lin WJ, Labonté B, Tamminga CA, Turecki G, Nestler EJ, Russo SJ, Salton SR, 2019. VGF and its C-terminal peptide TLQP-62 in ventromedial prefrontal cortex regulate depression-related behaviors and the response to ketamine. Neuropsychopharmacology 44, 971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lin WJ, Sadahiro M, Labonté B, Menard C, Pfau ML, Tamminga CA, Turecki G, Nestler EJ, Russo SJ, Salton SR, 2018. VGF function in depression and antidepressant efficacy. Mol. Psychiatry 23, 1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettler K, Veltman K, van de Meent D, van Wezel A, Hendriks AJ, 2014. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem 33, 481–492. [DOI] [PubMed] [Google Scholar]
- Kim BK, Hwang GB, Seu YB, Choi JS, Jin KS, Doh KO, 2015. DOTAP/DOPE ratio and cell type determine transfection efficiency with DOTAP-liposomes. Biochim. Biophys. Acta - Biomembr 1848, 1996–2001. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim PH, Kim SW, Yun CO, 2012. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials 33, 1838–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov AL, Maruyama K, Torchilin VP, Huang L, 1990. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268, 235–237. [DOI] [PubMed] [Google Scholar]
- Lakkadwala S, dos Santos Rodrigues B, Sun C, Singh J, 2019. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 307, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkadwala S, Singh J, 2019. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surfaces B Biointerfaces 173, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavertu M, Méthot S, Tran-Khanh N, Buschmann MD, 2006. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials 27, 4815–4824. [DOI] [PubMed] [Google Scholar]
- Levi A, Eldridge JD, Paterson BM, 1985. Molecular cloning of a gene sequence regulated by nerve growth factor. Science (80-. ) 229, 393–395. [DOI] [PubMed] [Google Scholar]
- Li SD, Huang L, 2009. Nanoparticles evading the reticuloendothelial system: Role of the supported bilayer. Biochim. Biophys. Acta - Biomembr 1788, 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WJ, Jiang C, Sadahiro M, Bozdagi O, Vulchanova L, Alberini CM, Salton SR, 2015. VGF and its C-terminal peptide TLQP-62 regulate memory formation in hippocampus via a BDNF-TrkB-dependent mechanism. J. Neurosci 35, 10343–10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp L, Sharma D, Banerjee A, Singh J, 2019. Controlled Delivery of Salmon Calcitonin Using Thermosensitive Triblock Copolymer Depot for Treatment of Osteoporosis. ACS Omega 4, 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Yang H, Zhou YF, Hu B, 2020. Dual and multi-targeted nanoparticles for sitespecific brain drug delivery. J. Control. Release 317, 195–215. [DOI] [PubMed] [Google Scholar]
- Markoutsa E, Pampalakis G, Niarakis A, Romero IA, Weksler B, Couraud PO, Antimisiaris SG, 2011. Uptake and permeability studies of BBB-targeting immunoliposomes using the hCMEC/D3 cell line. Eur. J. Pharm. Biopharm 77, 265–274. [DOI] [PubMed] [Google Scholar]
- Masserini M, 2013. Nanoparticles for Brain Drug Delivery. ISRN Biochem [DOI] [PMC free article] [PubMed]
- Nayerossadat N, Ali P, Maedeh T, 2012. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Chen J, Gao J, 2019. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci 14, 480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kang X, Zeng Y, Zhang W, Peng H, Wang J, Huang W, Wang H, Shen Y, Huang Y, 2017. A mannosylated PEI-CPP hybrid for TRAIL gene targeting delivery for colorectal cancer therapy. Polym. Chem 8, 5275–5285. [Google Scholar]
- Rahman NA, Rasil A.N. ain H.M., Meyding-Lamade U, Craemer EM, Diah S, Tuah AA, Muharram SH, 2016. Immortalized endothelial cell lines for in vitro blood-brain barrier models: A systematic review. Brain Res 1642, 532–545. [DOI] [PubMed] [Google Scholar]
- Razzak RA, Florence GJ, Gunn-Moore FJ, 2019. Approaches to cns drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues B. dos S., Kanekiyo T, Singh J, 2020. Nerve Growth Factor Gene Delivery across the Blood–Brain Barrier to Reduce Beta Amyloid Accumulation in AD Mice. Mol. Pharm 17, 2054–2063. [DOI] [PubMed] [Google Scholar]
- Rüetschi U, Zetterberg H, Podust VN, Gottfries J, Li S, Hviid Simonsen A, McGuire J, Karlsson M, Rymo L, Davies H, Minthon L, Blennow K, 2005. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp. Neurol 196, 273–281. [DOI] [PubMed] [Google Scholar]
- Santos Rodrigues B. Dos, Lakkadwala S, Kanekiyo T, Singh J, 2019. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int. J. Nanomedicine 14, 6497–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle H, Lamerz J, Buerger K, Dessauer A, Hager K, Hampel H, Karl J, Kellmann M, Lannfelt L, Louhija J, Riepe M, Rollinger W, Tumani H, Schrader M, Zucht H-D, 2005. Identification of Novel Biomarker Candidates by Differential Peptidomics Analysis of Cerebrospinal Fluid in Alzheimers Disease. Comb. Chem. High Throughput Screen 8, 801–806. [DOI] [PubMed] [Google Scholar]
- Sharma D, Arora S, Banerjee A, Singh J, 2021a. Improved insulin sensitivity in obese-diabetic mice viachitosan Nanomicelles mediated silencing of pro-inflammatory Adipocytokines. Nanomedicine Nanotechnology, Biol. Med 33, 102357. [DOI] [PubMed] [Google Scholar]
- Sharma D, Arora S, dos Santos Rodrigues B, Lakkadwala S, Banerjee A, Singh J, 2019a. Chitosan-Based Systems for Gene Delivery - Functional Chitosan: Drug Delivery and Biomedical Applications, in: Jana Sougata, Jana Subrata (Eds.), Functional Chitosan Springer Singapore, Singapore, pp. 229–267. [Google Scholar]
- Sharma D, Arora S, Singh J, 2019b. Smart thermosensitive copolymer incorporating chitosan–zinc–insulin electrostatic complexes for controlled delivery of insulin: effect of chitosan chain length. Int. J. Polym. Mater. Polym. Biomater 1–15. [DOI] [PMC free article] [PubMed]
- Sharma D, Arora S, Singh J, Layek B, 2021b. A review of the tortuous path of nonviral gene delivery and recent progress. Int. J. Biol. Macromol 183, 2055–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Singh J, 2017. Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymer and Their Nanomicelles for Nonviral Gene Delivery Applications. Bioconjug. Chem 28, 2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Lakkadwala S, Modgil A, Singh J, 2016. The role of cell-penetrating peptide and transferrin on enhanced delivery of drug to brain. Int. J. Mol. Sci 17, 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Modgil A, Sun C, Singh J, 2012. Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood-brain barrier model. J. Pharm. Sci 101, 2468–2478. [DOI] [PubMed] [Google Scholar]
- Sharma G, Modgil A, Zhong T, Sun C, Singh J, 2014. Influence of short-chain cell-penetrating peptides on transport of doxorubicin encapsulating receptor-targeted liposomes across brain endothelial barrier. Pharm. Res 31, 1194–1209. [DOI] [PubMed] [Google Scholar]
- Snyder SE, Cheng HW, Murray KD, Isackson PJ, McNeill TH, Salton SRJ, 1997. The messenger RNA encoding VGF, a neuronal peptide precursor, is rapidly regulated in the rat central nervous system by neuronal activity, seizure and lesion. Neuroscience 82, 7–19. [DOI] [PubMed] [Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, 2016. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev 99, 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S, Behnke J, Doobin D, Dalal V, Thakkar K, Khadim F, Wilson E, Palmieri A, Antila H, Rantamaki T, Alder J, 2014. VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling. Stem Cell Res 12, 762–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A, 2005. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx 2, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa F, Eto Y, 1988. Liposome targeting to mouse brain: Mannose as a recognition marker. Biochem. Biophys. Res. Commun 153, 1038–1044. [DOI] [PubMed] [Google Scholar]
- Ventola CL, 2017. Progress in nanomedicine: Approved and investigational nanodrugs. P T 42, 742–755. [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Zhou R, Fu A, Xu X, Huang Y, Hu C, 2011. Targeted delivery of large fusion protein into hippocampal neurons by systemic administration. J. Drug Target 19, 632–636. [DOI] [PubMed] [Google Scholar]
- Zheng X, Pang X, Yang P, Wan X, Wei Y, Guo Q, Zhang Q, Jiang X, 2017. A hybrid siRNA delivery complex for enhanced brain penetration and precise amyloid plaque targeting in Alzheimer’s disease mice. Acta Biomater 49, 388–401. [DOI] [PubMed] [Google Scholar]
- Zylberberg C, Gaskill K, Pasley S, Matosevic S, 2017. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther 24, 441–452. [DOI] [PubMed] [Google Scholar]


