Abstract
Antimicrobial resistance in pathogenic bacteria is increasing worldwide. One solution to this crisis is bacteriophage therapy, a treatment that harnesses naturally occurring bacterial viruses to invade and lyse antimicrobial resistant bacterial hosts. In Gram-negative hosts, a by-product of bacteriophage production is bacterial endotoxin, which can cause serious immune reactions in vivo. Purification methods using organic solvent extraction can remove endotoxin in bacteriophage lysates. In this study, we investigate a method for removal of endotoxin from 16 high-titer Klebsiella pneumoniae lysates by extraction with 1-dodecanol, 1-octanol, dodecane, or decane. In these experiments, treatment with either 1-dodecanol or 1-octanol resulted in removal of 104–105 endotoxin units/mL. Recovery of bacteriophage in lysates treated with dodecanol without dialysis was >90%, and residual dodecanol was low (10–1500 ppm). Overall these results suggest that organic solvent extraction using 1-dodecanol is effective at removing bacterial endotoxin, maintaining bacteriophage titer, and reducing solvent contamination in 16 K. pneumoniae bacteriophage lysates.
Keywords: bacteriophage therapy, bacteriophage purification, antimicrobial resistance, Klebsiella pneumoniae, endotoxin removal
Introduction
In the United States, and around the world, ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are increasing the prevalence of nosocomial infections that are additionally associated with high mortality rates among both healthy and immunocompromised patients.1–4 Treatment options for these multipledrug resistant (MDR) pathogens are limited by an increasing lack of effective antibiotics.5–8 Bacteriophage therapy has been suggested as an alternative treatment method to antibiotics and has been used for treatment of infections resulting from MDR bacterial pathogens such as A. baumannii.9–14 Bacteriophage therapy is effective at reducing bacterial colonization, is pathogen specific, and relatively easy and cost-effective to produce.15,16 Phage therapy products are also becoming more available to a wider consumer and clinical market as a more standard regulatory framework is established.17
The safety of bacteriophage products is well established.18–21 Production of bacteriophage, however, requires specialized postproduction purification to remove bacterial-derived toxins and ensure only bacteriophage particles are in the final product. There are currently multiple methods available for purification of phage lysates including differential centrifugation, density gradient centrifugation, ion exchange chromatography, affinity chromatography, and ultrafiltration; the effectiveness, cost, and production scale of these methods are variable.22–26 As bacteriophages are propagated on live bacterial hosts, unpurified cultures of phage contain considerable quantities of bacterial cell debris as a result of bacteriophage-induced cell lysis. In the production of phages that infect Gram-negative hosts, such as K. pneumoniae, A. baumannii, or Escherichia coli, a major safety concern is residual bacterial lipopolysaccharide also known as endotoxin. Endotoxin induces a strong immune response when introduced to areas outside the gastrointestinal tract in humans and is a major cause of septic shock in patients with bacteremia.27
Previous studies have demonstrated the use of organic solvents for removing endotoxin.25,28 The principle of this approach uses two-phase extraction to partition the hydrophobic endotoxin into an organic solvent, while the charged and more hydrophilic bacteriophage particles partition into the aqueous layer. Organic solvent extraction of bacteriophage solutions has been successful at removing endotoxin while maintaining a minimum bacteriophage titer, and compared with other methods, it is generally rapid, inexpensive, and scalable. A complication of this method, however, is that the residual solvent in the preparation can affect the usability of bacteriophage preparations in humans.
Previously, 1-octanol has been used to remove endotoxin from bacteriophage lysates; with a solubility in water of 540 mg/L at 25°C, multiple downstream dialysis steps are required to remove the residual solvent from the phage before administration.28,29 To reduce the risk of organic solvent contamination, we have explored the use of three new organic solvents. These solvents were selected based on their low solubility in water: 1-dodecanol (solubility 4 mg/L at 25°C), decane (solubility 52 μg/L at 25°C), and dodecane (solubility 3.7 μg/L at 25°C).30–32 1-Dodecanol, also commonly referred to as lauryl alcohol, is a nontoxic generally regarded as safe (GRAS)-registered additive often found in foods and cosmetics.32 Additional removal of solvent can be achieved with dialysis against ethanol or sodium chloride (NaCl) solutions.
In this article, we evaluate four organic solvents octanol, dodecanol, decane, and dodecane as a method to reduce the amount of endotoxin present in bacteriophage preparations targeting Gram-negative MDR bacterial pathogens. We evaluated 16 lysates of K. pneumoniae bacteriophages Soft (distant chi-like), Pharr (T3/T7 like), and JR (T5-like) either alone or in a combination, and quantified success by bacteriophage survival, endotoxin removal, and the level of residual organic solvent. We have used a clinical MDR isolate of K. pneumoniae for this study to investigate the applicability of this method toward the production of phages against ESKAPE pathogens.33,34
Materials and Methods
Media and bacterial strains
Phage lysates were prepared in tryptic soy broth containing 17 g tryptone, 3 g soytone, 2.5 g dextrose, 5 g NaCl, and 3 g K2HPO4 per liter. Tangential flow filtration (TFF) concentrates were buffer exchanged into lactated Ringer's solution purchased from FisherSci Catalog No. NC0767739. K. pneumoniae 39427 (KPN39427, Genbank NZ_CP054268) was obtained from the laboratory of Dr. Tom Walsh, Weill Cornell Medicine. K. pneumoniae 39827 Pharr(r) was generated by in vitro subculturing with bacteriophage Pharr.
Bacteriophage lysate production
Phages Pharr, Soft, and JR were isolated from College Station, TX wastewater samples in 2012, 2016, and 2016, respectively. All phages were isolated from wastewater samples by the culture-enrichment method. In brief, 5–50 mL of a filter-sterilized (0.22 μM polyethersulfone membrane) environmental sample was mixed with an appropriate amount of concentrated growth medium (5 × Tryptone Soy Broth [TSB]), inoculated with 100 μL of an overnight culture of the targeted bacterial strain, then incubated with aeration overnight at 37°C. The resulting enrichment culture was cleared by centrifugation and the supernatant was filter sterilized then spotted on the bacterial host strain using the soft agar overlay method to screen for plaque formation.35
Each phage has additionally been manually annotated and visualized with transmission electron microscopy (Genbank accessions: Spivey/JR MK630230.1, Soft MN106244.1, Pharr MK618658.1). All titering in these experiments was performed in duplicate or triplicate using the soft agar overlay method. Lysates were produced in 1 L batches as follows. One liter TSB was inoculated in a 2.4 L Fernbach flask with 10 mL of the appropriate propagation bacterial host cells, and allowed to grow to optical density (OD) 0.3 at OD550, growth was carried out at 37°C. Bacteriophages were added at multiplicity of infection 0.1 once target OD was reached. After whole culture clearing from cell lysis, cells were removed by centrifugation at 10,000 g for 15 min and filter sterilized using a 0.2 μm vacuum filter. DNase and RNase were added to a final concentration of 1 μg/mL. High titer lysates were concentrated by TFF using Sartorius Model 1084 with Sartorius Slice 200 membranes.
Organic solvent extraction
Organic solvents were purchased from Fisher Chemical (1-octanol [A-402]) or Sigma Aldrich (decane [457116], dodecane [297879], and 1-dodecanol [443816]). The organic solvent extraction method was derived from the method presented in Olearnik (2015). Phage lysates (IDs 1–8 and 11–16) volumes of 0.6 mL were added to 0.4 mL solvent in 1.5 mL polypropylene microcentrifuge tubes. Phage lysates (IDs 9–10) volumes of 6 mL were added to 4 mL solvent in 50 mL polypropylene centrifuge tubes. Dodecanol was prewarmed to 30°C to facilitate a liquid state before the introduction of phage. Samples were mixed for 2 h at 30°C using an inverter for small volumes or floor shaker for larger volumes. Control samples were subjected to these temperature changes and mixing conditions.
After incubation, samples chilled for at least 3 h at 4°C and were centrifuged at 4000 g for 10 min at 4°C. The aqueous layer was then aspirated out and placed into a new tube. At this point, the samples that underwent a second dodecanol extraction were mixed again with fresh dodecanol and subjected to the same steps already described. Samples were then filter sterilized with a 0.22μm filter and the undialyzed samples were ready for quantification. The dialyzed samples were placed in a 10 mL Float-A-Lyzer G2 dialysis system (Spectrum Laboratories, Inc.) and were dialyzed 5 × 4 h in 25% ethanol and then 4 × 4 h in 0.15 M NaCl. After extraction or dialysis was achieved, all samples were stored at 4°C.
Endotoxin quantification
Endotoxin quantification was performed using Biomerieux EndoZyme II Recombinant Factor C Assay Kit according to product instructions. Endotoxin-free glassware was used for appropriate steps. Fluorescence of diagnostic 96-well plates was quantified by a Tecan Infinite 200 Pro multimode plate reader.
Dodecanol quantification by gas chromatography-mass spectrometry
Residual dodecanol in samples was quantified by Selected Reaction Monitoring (SRM) Gas Chromatography-Mass Spectrometry (GC-MS) at the Texas A&M University Protein Chemistry Laboratory (PCL). A standard curve was generated using eight concentrations of dodecanol in hexane between 2500 and 20 ppm, peak values were set for each of the standard concentrations. Sample values were then compared with the standard curve by comparing the peak area of the sample. As the only definite quantification came from the standard curve, we defined the quantification for the samples by noting where they fell in a range between two of the standard samples. As an example, if a sample fell between 78 and 39 ppm on the standard curve, the greater number was used in Table 2 to define it as being lower than the known value 78 ppm.
Table 2.
Dodecanol Quantification by Selected Reaction Monitoring Gas Chromatography-Mass Spectrometry
| Lysate ID | Dodecanol (less than no. PPM) | Dodecanol w/dialysis (less than no. PPM) | Secondary dodecanol (less than no. PPM) |
|---|---|---|---|
| 1 | 78 | LOD | ND |
| 2 | 39 | LOD | ND |
| 3 | 78 | LOD | ND |
| 4 | 78 | LOD | ND |
| 5 | LOD | LOD | ND |
| 6 | LOD | LOD | ND |
| 7 | LOD | LOD | ND |
| 8 | 78 | LOD | ND |
| 9 | 39 | ND | 625 |
| 10 | 39 | ND | 78 |
| 11 | LOD | ND | LOD |
| 12 | 312 | ND | 312 |
| 13 | 312 | ND | 78 |
| 14 | LOD | ND | LOD |
| 15 | 78 | ND | 78 |
| 16 | 156 | ND | 1250 |
Dodecanol residual amounts were quantified using gas chromatography-mass spectrometry and a reference curve of pure dodecanol. The LOD was 10 ppm dodecanol. ND no sample exists for this cell.
LOD, limit of detection; ND, no data; PPM, parts per million.
Results
Octanol and dodecanol reduce endotoxin in single-phage lysates and dialysis decreases phage survival
Bacteriophage Pharr lysates with an initial titer of 108 PFU/mL were treated with octanol, dodecanol, decane, or dodecane (40%v/v) with or without dialysis against 25% ethanol (5 × 4 h) and 0.15 M NaCl (4 × 4h). The treated lysates were then assessed by bacteriophage survival, and endotoxin content was measured in endotoxin units (EU)/mL (IDs 1–2; Table 1). A reduction in EU/mL for the samples extracted with octanol, octanol w/dialysis, dodecanol, and dodecanol w/dialysis was observed with the amount of endotoxin reduced by at least 95% in these four samples (Fig. 1a). Statistical analysis is not appropriate for these samples, thus p-values were not generated. Bacteriophage recovery was negatively affected by dialysis with dialyzed samples maintaining <50% bacteriophage recovery (Fig. 1b). The low recovery may be due to the levels of ethanol exposure. Undialyzed samples in this experiment maintained an average of at least 60% bacteriophage recovery. Decane and dodecane treatments did not reduce endotoxin without dialysis in these samples and were subsequently removed as extraction solvents for further experiments due to their lack of efficacy.
Table 1.
Bacteriophage Lysates Used in This Study
| Lysate ID | Bacteriophagea,b preparation | Relative endotoxinc content (EU/108 PFU) | Titerd (PFU/mL) | EU/mLe |
|---|---|---|---|---|
| 1 | Pharr lysate | 3870 | 2.42E+08 | 9366 |
| 2 | Pharr lysate | 17,732 | 3.27E+08 | 57,894 |
| 3 | Soft concentrated lysate | 411 | 7.80E+10 | 320,377 |
| 4 | Soft concentrated lysate | 65 | 6.17E+11 | 400,302 |
| 5 | Pharr concentrated lysate | 9678 | 3.95E+09 | 382,272 |
| 6 | Pharr concentrated lysate | 486 | 3.90E+10 | 189,532 |
| 7 | JR concentrated lysate | 4537 | 8.30E+09 | 376,539 |
| 8 | JR concentrated lysate | 4638 | 7.00E+09 | 324,690 |
| 9 | Combined pharr, soft, JR concentrated lysate | 30,920 | 1.27E+08 | 39,113 |
| 10 | Combined pharr, soft, JR concentrated lysate | 466,148 | 4.80E+07 | 223,751 |
| 11 | Soft concentrated lysate | 1163 | 3.55E+10 | 412,792 |
| 12 | Soft concentrated lysate | 468 | 2.22E+11 | 1,037,371 |
| 13 | Pharr concentrated lysate | 9686 | 2.20E+09 | 213,096 |
| 14 | Pharr concentrated lysate | 3316 | 2.45E+09 | 81,231 |
| 15 | JR concentrated lysate | 5975 | 1.50E+09 | 89,628 |
| 16 | JR concentrated lysate | 14,133 | 1.47E+09 | 207,753 |
Bacteriophage lysates used in this study included individual bacteriophage lysates of phages Soft, Pharr, or JR as well as a triphage mixture of all three phages. For each lysate, titer and endotoxin content have been investigated.
Bacteriophages Soft and Pharr host—Klebsiella pneumoniae 39427 WT.
Bacteriophage JR host—K. pneumoniae 39427 Pharr resistant.
Relative endotoxin content is a comparative measure of how much endotoxin is present in each lysate relative to the titer, all samples are normalized to endotoxin units per 108 PFU/mL.
Titer determined by two independent replicates of traditional plaque assays.
EU is defined by EndoZyme as 100 pg endotoxin.
EU, endotoxin unit.
FIG. 1.
Quantification of endotoxins and bacteriophage titer in Pharr lysates treated with octanol, dodecanol, decane, and dodecane. Two independently prepared bacteriophage Pharr lysates were treated with octanol, dodecanol, decane, or dodecane to remove endotoxin through organic solvent extraction. A second set of treated samples were additionally dialyzed against 25% ethanol and 0.15 M NaCl to remove residual solvent. Bars represent the averages of two lysates, which were quantified twice. (a) Percentage endotoxin remaining in the samples after treatment as compared with the untreated control. Endotoxin levels are measured in EU per mL lysate. (b) Bacteriophage recovery was measured as the percentage of the phage recovered compared with the titer of the untreated control lysate. EU, endotoxin units.
Octanol and dodecanol extraction reduces endotoxin in concentrated phage lysates and is bacteriophage dependent
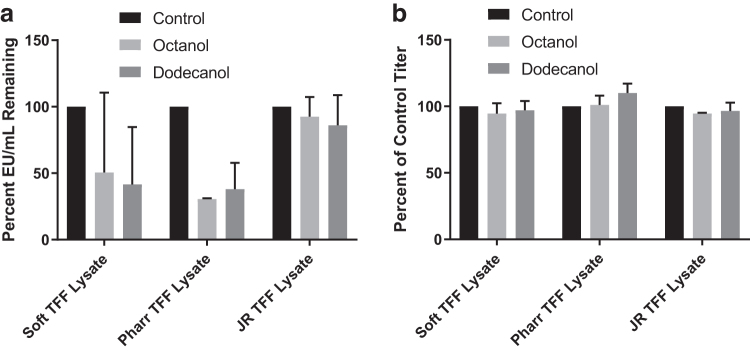
Bacteriophage lysates from three phages Soft (distant chi-like), Pharr (T3/T7 like), and JR (T5-like) were concentrated by TFF to achieve higher titer lysates. These high titer concentrates represent a potential phage therapy product that can be given in low volumes to human patients or used in in vivo models (IDs 3–8, Table 1, which denotes starting endotoxin content and titer of the lysates). Two independent concentrated lysates of each phage were treated with either octanol or dodecanol, and endotoxin removal and phage survival were quantified. A reduction in endotoxin was observed in all cases, and the effectiveness of the organic extraction was phage dependent (Fig. 2a).
FIG. 2.
Quantification of endotoxins and bacteriophage titer in TFF concentrated Soft, Pharr, and JR lysates. (a) Two independently grown TFF concentrates of each phage (Soft, Pharr, and JR) were prepared and treated with either octanol or dodecanol and the residual endotoxin was quantified as EU/mL for each phage type. (b) Bacteriophage recovery was measured as stated previously with all lysates losing <10% of the original titer. TFF, tangential flow filtration.
The phage Soft lysates had endotoxin concentrations reduced by 49.4% and 58.5% for octanol and dodecanol, respectively. The endotoxin in the Pharr lysates was reduced further with a 69.5% reduction for octanol extraction and a 61% reduction for dodecanol extraction. Endotoxin in the phage JR concentrate was only marginally reduced, with a 7.5% decrease with octanol extraction and a 14.0% decrease with dodecanol extraction. These reductions correspond to a removal of ∼105 EU/mL in the Pharr and Soft concentrates and a removal of ∼104 EU/mL in the JR concentrates. Titers in all treatment groups remained >90%, indicating good phage recovery with both octanol and dodecanol (Fig. 2b).
Endotoxin removal is variable in combined bacteriophage lysates
Two mixed lysates containing equimolar concentrations of bacteriophages Soft, Pharr, and JR were subjected to endotoxin removal by octanol and dodecanol extraction. In these two lysates (ID 9–10; Table 1), 32.5% of endotoxin was removed by octanol and 41.5% of endotoxin was removed by dodecanol on average (Fig. 3a). In all treatments, at least 104 EU/mL was removed. Loss of titer was again observed to be <10% in all treatment groups.
FIG. 3.
Quantification of endotoxins and bacteriophage recovery in triphage preparation of Soft, Pharr, and JR. (a) Endotoxin quantification by EU/mL of the average of two individually produced mixed lysates containing equimolar concentrations of Soft, Pharr, and JR phage particles when treated with octanol or dodecanol. EU concentration was observed to be reduced by at least 104 EU/mL for all three treatment groups. (b) Bacteriophage recovery was measured as stated previously. Titer was not significantly affected by solvent extraction with all titers remaining >95% recovered compared with the control group.
Repeated extraction with dodecanol has no effect on endotoxin removal
Endotoxin removal using either a single or double extraction with dodecanol was performed on both TFF-concentrated single-bacteriophage lysates and tri-phage mixed lysates (IDs 9–16; Table 1). The endotoxin was reduced in the mixed lysates by 41.5% and 52.0% for primary and secondary dodecanol extractions, respectively (Fig. 4a). The endotoxin removal in the TFF-concentrated lysates appeared to be bacteriophage dependent with removals varying, >95% (Soft), >60% (Pharr), and >25% (JR). In both the concentrated lysates and mixed lysates, only small disparities of removal between the two treatment groups were observed, indicating that a second dodecanol extraction did not improve endotoxin content. The titers from these treatment groups were >90% recovery except for one JR lysate that was treated with dodecanol twice and only had 36% of the bacteriophage recovered (Fig. 4b).
FIG. 4.

Quantification of endotoxins and bacteriophage recovery in TFF-concentrated preparations of Soft, Pharr, and JR and combined preparations after single or double extraction with dodecanol. (a) Endotoxin removal was quantified by EU/mL as previously described. There were only very moderate differences between a single- or double-dodecanol extraction in any of the lysate groups. (b) Bacteriophage recovery was measured as stated previously. Titer remained stable after solvent extraction with a single lysate suffering a >60% loss in titer. All remaining lysates lost <15% titer.
Dodecanol quantification
After the extractions, the amount of dodecanol left in the samples was quantified by SRM GC-MS. Dodecanol amounts were compared with a pure dodecanol standard curve. The greatest amount of dodecanol observed in single-dodecanol extractions was <312 ppm, and five samples were below the limit of detection at 10 ppm (Table 2). All dialyzed dodecanol samples were below the limit of detection. A double-extracted dodecanol sample recorded the highest measured reading of 1250 ppm.
Discussion
The data presented here suggest that 1-dodecanol is comparable with the previously investigated solvent 1-octanol as a solvent for removal of bacterial endotoxin from bacteriophage lysates. The experiments conducted in this study corroborated previous findings that concluded that 1-octanol is capable of removing ∼104 to ∼105 EU/mL, and that removal of endotoxin appears to be bacteriophage dependent.22,24,36 These two observations were also true for 1-dodecanol that performed comparably with octanol at endotoxin removal in each experimental set. Bacteriophage recovery for samples treated with octanol has previously been reported as ranging from 15% to 60%, but typically included dialysis steps that the data presented here show can greatly lower bacteriophage recovery. In the experiments performed here, samples dialyzed after octanol or dodecanol treatment were within this published range.22,24,36 Samples that were undialyzed, however, generally maintained bacteriophage recovery of 90% or better. These data suggest that dialysis may introduce significant losses in yield during bacteriophage production.
The quantification of 1-dodecanol in the samples tested has shown that lysates treated with dodecanol retain <1250 ppm of residual dodecanol, and more commonly contain no detectable dodecanol residue (<10 ppm). Dodecanol is a GRAS food additive and is found in many products such as chewing gum that has been found to have 16–27 ppm dodecanol. Although there is limited toxicity data available, one study concludes that the LD50 of dodecanol by intravenous injection in rats is 390 mg/kg and that by ingestion is 4150 mg/kg. Extrapolating from these numbers, an estimate of toxicity in a 62 kg human would translate to an LD50 of 24 g administered intravenously or 257 g ingested.32 As the experimental data indicated that residual dodecanol would be in the parts per million range in bacteriophage lysates, toxicity should not be an issue. In addition, with a melting point of 24°C, dodecanol is solid at room temperature and refrigerated conditions, making the separation of aqueous and organic layers uncomplicated in these experiments.
A further hypothesis stating that a second treatment with dodecanol would improve endotoxin removal was generated based on the possibility that the dodecanol or solvent–water interface in the previous experiments was becoming saturated with bacterial cell debris. This hypothesis ultimately was determined to be false, as there was no difference in the endotoxin content between the single- and double-dodecanol extracted lysates. The double-extracted lysates did have higher residual dodecanol with the maximum single-extracted lysate having <156 ppm, and the maximum double-extracted lysate having <1250 ppm.
The versatility of 1-dodecanol was challenged by using several different bacteriophage preparation methods, including single-phage and mixed-phage lysates. In the single-bacteriophage lysates, endotoxin was reduced to 0.5–38% (Pharr), 3.5–41% (Soft), and 67–86% (JR) of the original endotoxin content. These variations between individual lysates using the same phage and between individual bacteriophages is common to endotoxin removal using solvent extraction. As others have stated, it appears that endotoxin removal is bacteriophage dependent, an issue that could become important when choosing bacteriophage for large-scale production.36,37
A major point of contention regarding bacteriophage therapy is the emergence of phage-resistant bacteria during bacteriophage treatment. In a simplified description, a bacterial pathogen exposed to a predatory phage is able to undergo mutation to lose the phage binding receptor and is thus protected from any subsequent infection by that phage.38 To overcome this challenge, multiple phages using different receptors can be used in concert as a mixed lysate, a solution that may make phages more effective in vivo. In the experiments described here, investigated mixed lysates included three bacteriophages that were also tested individually, and after a single treatment with dodecanol, an average of 58% of the EU/mL remained. As endotoxin removal was higher in Soft and Pharr lysates but lower in JR lysates, it is consistent that the mixed lysate removal rate is somewhere in between, though that number appears to be variable in this small sample size. In addition, it should be reiterated that phages Pharr and Soft use a different bacterial host strain (KPN39427 WT) than bacteriophage JR (KPN39427 Pharr resistant). It is possible that this host strain produces more endotoxin or is generally more endotoxic than the WT strain.39
Bacteriophage therapy that is administered intraperitoneally (i.p.) rather than orally is subject to a 5 EU/kg/h standard for i.p. administration in humans.40 The amount of endotoxin allowed in bacteriophage preparations for human administration thus depends on both the mass of the patient and the amount of bacteriophage administered. Assuming a 62 kg patient, this would indicate a 7400 EU/day or 310 EU/h limit. If the effective dose of these phages was 1 × 108 PFU/day, then 13 of the 16 samples treated with dodecanol (lysate ID 1–8, 11–15) would already be within the safe limit for daily administration. If the required effective titer were higher, however, most of these samples would require subsequent purification. As the method currently stands, the remaining endotoxin would need to be removed by more conventional methods such as an Endo-Trap column. In addition, it is possible that the preremoval of endotoxin by dodecanol could make conventional methods such as Endo-Trap more successful than it would be on its own, or allow other filtration methods to become more feasible for large volumes as they would start with a more pure sample. This is currently unknown and could be explored in the future.
Conclusions
As compared with the previously described approach using1-octanol, 1-dodecanol is a promising choice for large-scale removal of endotoxin in bacteriophage lysates by organic solvent extraction. Treatment of bacteriophage preparations with either octanol or dodecanol resulted in removal of 104–105 EU/mL depending on the initial EU concentration. Bacteriophage recovery with treatment of dodecanol without dialysis was on average >90%, which is an improvement over other methods. Dodecanol residual concentrations were far below presumed toxic levels with concentrations in the 10–1000 ppm range even for lysates that were extracted with dodecanol twice. The combination of these results indicates that dodecanol, which would not require additional dialysis, could both remove significant amounts of endotoxin and maintain a high bacteriophage titer if used as described. Overall, these findings suggest an opportunity to improve current methods of bacteriophage lysate purification using solvent extraction by replacing 1-octanol with 1-dodecanol, a method that would be efficient, cost-effective, and scalable.
Acknowledgments
We thank Smriti Shankar, research associate at the Texas A&M PCL, for her GC-MS expertise and sample quantification. We also thank Dr. Thomas Walsh and Michael Satlin of Weill Cornell Medical College for their provision of bacterial strains and continued collaboration.
Authors' Contributions
Authors Michalik-Provasek, Parker, and Lessor performed data collection and contributed to article production. Authors Michalik-Provasek and Parker contributed to study design. Author Michalik-Provasek also performed data analysis. Author Gill contributed to study design, data analysis, and article production and review. All coauthors have reviewed and approved of the article before submission. This article has only been submitted to this journal and is not published elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This project was supported by NIH-NIAID project AI121689 and by Texas AgriLife Research.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance-biggest threats. Centers for Disease Control and Prevention Web site. 2018. https://www.cdc.gov/drugresistance/biggest_threats.html (accessed July 15, 2021).
- 3. Potter RF, D'Souza AW, Dantas G. The rapid spread of carbapenem-resistant enterobacteriaceae. Drug Resist Updates. 2016;29:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in US hospitalized patients, 2012–2017. N Engl J Med. 2020;382(14):1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rice LB. Progress and challenges in implementing the research on ESKAPE pathogens. Infect Control Hosp Epidemiol. 2010;31(S1):S7–S10. [DOI] [PubMed] [Google Scholar]
- 6. Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11(3):297–308. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; ISBN: 9789241564748, 2014. [Google Scholar]
- 8. Broncano-Lavado A, Santamaría-Corral G, Esteban J, et al. Advances in bacteriophage therapy against relevant multidrug-resistant pathogens. Antibiotics. 2021;10(6):672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61(10):e00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adhya S, Merril CR, Biswas B. Therapeutic and prophylactic applications of bacteriophage components in modern medicine. Cold Spring Harb Perspect Med. 2014;4(1):a012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cisek AA, Dabrowska I, Gregorczyk KP, et al. Phage therapy in bacterial infections treatment: One hundred years after the discovery of bacteriophages. Curr Microbiol. 2017;74(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulani MS, Kamble EE, Kumkar SN, et al. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front Microbiol. 2019;10:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altamirano FLG, Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev. 2019;32(2):e00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nale JY, Clokie MR. Preclinical data and safety assessment of phage therapy in humans. Curr Opin Biotechnol. 2021;68:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kingwell K. Bacteriophage therapies re-enter clinical trials. Nat Rev Drug Discov. 2015;14(8):515. [DOI] [PubMed] [Google Scholar]
- 16. Young R, Gill JJ. MICROBIOLOGY. Phage therapy redux—What is to be done? Science. 2015;350(6265):1163–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brives C, Pourraz J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020;6(1):1–11. [Google Scholar]
- 18. Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45(3):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fabijan AP, Lin RC, Ho J, et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5(3):465–472. [DOI] [PubMed] [Google Scholar]
- 20. Ooi ML, Drilling AJ, Morales S, et al. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to staphylococcus aureus. JAMA Otolaryngol Head Neck Surg. 2019;145(8):723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barr JJ. A bacteriophages journey through the human body. Immunol Rev. 2017;279(1):106–122. [DOI] [PubMed] [Google Scholar]
- 22. Bonilla N, Rojas MI, Netto FC, et al. Phage on tap—A quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ. 2016;4:e2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gill JJ. Phage choice, isolation, and preparation for phage therapy. Curr Pharm Biotechnol. 2010;11(1):2–14. [DOI] [PubMed] [Google Scholar]
- 24. Smrekar F, Ciringer M, Peterka M, et al. Purification and concentration of bacteriophage T4 using monolithic chromatographic supports. J Chromatogr B Biochromatogr Nanotechnol Symp. 2008;861(2):177–180. [DOI] [PubMed] [Google Scholar]
- 25. Van Belleghem JD, Merabishvili M, Vergauwen B, et al. A comparative study of different strategies for removal of endotoxins from bacteriophage preparations. J Microbiol Methods. 2017;132:153–159. [DOI] [PubMed] [Google Scholar]
- 26. García R, Latz S, Romero J, et al. Bacteriophage production models: An overview. Front Microbiol. 2019;10:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71(1):635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szermer-Olearnik B, Boratyński J. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS One. 2015;10(3):e0122672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National center for biotechnology information. PubChem compound summary for CID 957, 1-octanol. https://pubchem.ncbi.nlm.nih.gov/compound/1-Octanol (accessed March 26, 2021).
- 30. National center for biotechnology information. PubChem compound summary for CID 15600, decane. https://pubchem.ncbi.nlm.nih.gov/compound/Decane (accessed March 26, 2021).
- 31. National center for biotechnology information. PubChem compound summary for CID 8182, dodecane. https://pubchem.ncbi.nlm.nih.gov/compound/Dodecane (accessed March 26, 2021).
- 32. National center for biotechnology information. PubChem compound summary for CID 8193, 1-dodecanol. https://pubchem.ncbi.nlm.nih.gov/compound/1-Dodecanol (accessed March 26, 2021).
- 33. Dorman MJ, Short FL. Klebsiella pneumoniae: When a colonizer turns bad. Nat Rev Microbiol. 2017;15(7):384. [DOI] [PubMed] [Google Scholar]
- 34. Satlin MJ, Chen L, Patel G, et al. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant enterobacteriaceae (CRE) in the CRE epicenter of the united states. Antimicrob Agents Chemother. 2017;61(4):e02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adams MH. Bacteriophages. New York: Interscience Publishers; 1959. [Google Scholar]
- 36. Hietala V, Horsma-Heikkinen J, Carron A, et al. The removal of endo-and enterotoxins from bacteriophage preparations. Front Microbiol. 2019;10:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adriaenssens EM, Lehman SM, Vandersteegen K, et al. CIM® monolithic anion-exchange chromatography as a useful alternative to CsCl gradient purification of bacteriophage particles. Virology. 2012;434(2):265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nobrega FL, Vlot M, de Jonge PA, et al. Targeting mechanisms of tailed bacteriophages. Nat Rev Microbiol. 2018. 10.1038/s41579-018-0070-8. DOI: 10.1038/s41579-018-0070-8. [DOI] [PubMed]
- 39. Luchi M, Morrison DC. Comparable endotoxic properties of lipopolysaccharides are manifest in diverse clinical isolates of gram-negative bacteria. Infect Immun. 2000;68(4):1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. US Food and Drug Administration. Bacterial endotoxins/pyrogens. Inspections, Compliance, Enforcement, and Criminal Investigations, FDA, Bethesda, MD. 1985. www.fda.gov/ICECI/Inspections/InspectionGuides/InspectionTechnica (accessed July 15, 2021).