Abstract
We report a detailed clinical examination in a patient with primary coenzyme Q10 deficiency caused by biallelic mutations in the PDSS1 gene who presented clinical features of mitochondrial encephalopathy associated with pulmonary hypertension, livedo reticularis and particularly, chronic distal phalangeal erythema. Laboratory testing showed elevated plasma lactate and 3‐methyl‐glutaconic and tricarboxylic aciduria. Supplementation with high dose of coenzyme Q10 was not effective to control disease progression and the patient died at the age of 3 years old because of a progressive multisystem disorder. Cutaneous involvement in mitochondrial disease is heterogenous, including proliferative, inflammatory, and dystrophic changes among others. The coexistence in our case of phalangeal erythema, livedo reticularis, and pulmonary hypertension suggests microvascular dysfunction as a possible underlying mechanism. This is the first reported patient with PDSS1 mutations presenting with 3‐methyl‐glutaconic aciduria and distal phalangeal erythema, expanding the phenotype of primary coenzyme Q10 deficiency.
Keywords: coenzyme Q10 , cutaneous, phalangeal, erythema, mitochondria, PDSS1
We report the detailed clinical examination of an infant with primary coenzyme Q10 (CoQ10) deficiency, previously published in a case series, 1 presenting unusual finger findings.
He was born prematurely after in vitro fertilization pregnancy, complicated by twin‐twin transfusion.
At the age of 5 months, he presented failure to thrive, hypotonia, feeding difficulties, bilateral hearing impairment, pulmonary hypertension, livedo reticularis, and distal phalangeal erythema (Figure 1). Laboratory tests showed anemia, lactic acidosis, 3‐methyl‐glutaconic, and tricarboxylic aciduria and decreased CoQ10 levels in lymphocytes. At this time, oral ubidecarenone was started at 15 mg/kg/day. Three months later, when biallelic PDSS1 mutations were identified (NM_014317.3: c.716 T > G (p.Val239Gly) and NM_014317.3: c.1183C > T (p.Arg395*)), ubidecarenone was increased to 30 mg/kg/day.
FIGURE 1.
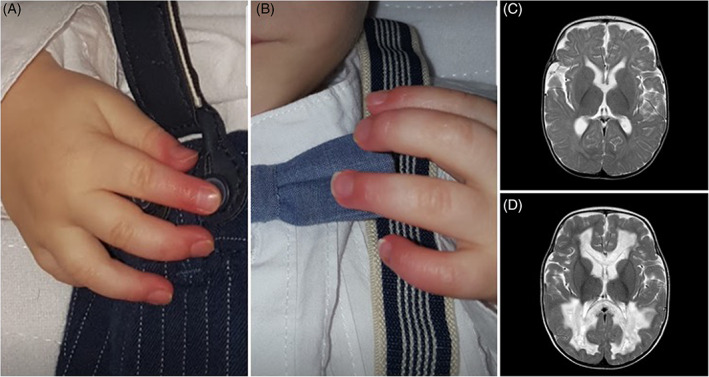
Finger findings and cerebral MRI in the reported patient. A and B, Chronic distal phalangeal erythema, an accompanying sign since the age of 5 months old. C, Axial T2W at the age of 12 months old showing mild cerebral atrophy. D, Axial T2W at the age of 24 months old with appearance of cystic change in white matter. White matter tracts surrounding the lateral ventricles and commissural fibres had signal abnormalities, showing small necrotic or porencephalic cysts. “U” fibres, pyramidal tracts and gray matter were spared
However, he developed a progressive multisystem disorder with severe developmental delay, pyramidal dysfunction, seizures, tremor, optic atrophy and nystagmus, severe visual and hearing impairment, white matter cystic change and chronic heart failure. He died at the age of 3 years old.
Since the age of 5 months, distal phalangeal erythema was an accompanying sign. Cutaneous involvement in mitochondrial disease is heterogenous, including proliferative, inflammatory, and dystrophic changes among others. 2 , 3 , 4 The coexistence in our case of phalangeal erythema, livedo reticularis, and pulmonary hypertension suggests microvascular dysfunction as a possible underlying physiopathogenic mechanism. Symptoms related to microvascular dysfunction and cutaneous involvement have been previously reported in primary CoQ10 deficiencies, 5 (eg, livedo reticularis and pulmonary hypertension in PDSS1, 6 acrocyanosis in COQ4, 7 and lupus‐like symptoms in CoQ8A 8 ). In conclusion, our data expand the phenotypic spectrum associated with PDSS1 variants and primary coenzyme Q10 deficiency.
CONFLICT OF INTEREST
Marcello Bellusci, Maria Teresa García‐Silva, Ana Martínez de Aragón, and Miguel Angel Martín declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
Marcello Bellusci and Maria Teresa García‐Silva are the clinician who attended the reported patient, planned, wrote and revisited critically the article before the submission. Ana Martínez de Aragón is the pediatric radiologist that realized the reported brain MRI studies, wrote the figure legend and revisited critically the article before the submission. Miguel Angel Martín performed and analyzed laboratory tests, revisited critically the article before the submission.
INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study, including for the use of pictures.
ETHICS APPROVAL
No ethics approval is required for case report in our center.
Bellusci M, García‐Silva MT, Martínez de Aragón A, Martín MA. Distal phalangeal erythema in an infant with biallelic PDSS1 mutations: Expanding the phenotype of primary Coenzyme Q10 deficiency. JIMD Reports. 2021;62:3–5. 10.1002/jmd2.12216
REFERENCES
- 1. Bravo‐Alonso I, Navarrete R, Vega AI, et al. Genes and variants underlying human congenital lactic acidosis‐from genetics to personalized treatment. J Clin Med. 2019;8(11):1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sreedhar A, Aguilera‐Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020;11(6):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feichtinger RG, Sperl W, Bauer JW, Kofler B. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23(9):607‐614. [DOI] [PubMed] [Google Scholar]
- 4. Bodemer C, Rötig A, Rustin P, et al. Hair and skin disorders as signs of mitochondrial disease. Pediatrics. 1999;103(2):428‐433. [DOI] [PubMed] [Google Scholar]
- 5. Alcázar‐Fabra M, Trevisson E, Brea‐Calvo G. Clinical syndromes associated with coenzyme Q10 deficiency. Essays Biochem. 2018;62(3):377‐398. [DOI] [PubMed] [Google Scholar]
- 6. Mollet J, Giurgea I, Schlemmer D, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH‐benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117(3):765‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brea‐Calvo G, Haack TB, Karall D, et al. COQ4 mutations cause a broad spectrum of mitochondrial disorders associated with CoQ10 deficiency. Am J Hum Genet. 2015;96(2):309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korkmaz E, Lipska‐Ziętkiewicz BS, Boyer O, et al. ADCK4‐associated glomerulopathy causes adolescence‐onset FSGS. J Am Soc Nephrol JASN. 2016;27(1):63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]


