Abstract
Alzheimer disease (AD) is biologically defined by the presence of β-amyloid-containing plagues and tau-containing neurofibrillary tangles. AD is a genetic and sporadic neurodegenerative disease that causes an amnestic cognitive impairment in its prototypical presentation and non-amnestic cognitive impairment in its less common variants. AD is a common cause of cognitive impairment acquired in midlife and late-life but its clinical impact is modified by other neurodegenerative and cerebrovascular conditions. This Primer conceives of AD biology as the brain disorder that results from a complex interplay of loss of synaptic homeostasis and dysfunction in the highly interrelated endosomal/lysosomal clearance pathways in which the precursors, aggregated species and post-translationally modified products of Aβ and tau play important roles. Therapeutic endeavours are still struggling to find targets within this framework that substantially change the clinical course in persons with AD.
Alzheimer disease (AD) is a neurodegenerative disorder characterized by β-amyloid (Aβ)-containing extracellular plaques and tau-containing intracellular neurofibrillary tangles. AD typically presents with prominent amnestic cognitive impairment but can also less commonly manifest as non-amnestic cognitive impairment. The presentation of AD with short-term memory difficulty is most common but impairment in expressive speech, visuospatial processing and executive (mental agility) functions also occurs. Most cases of AD are not dominantly inherited and there is a complex relationship to genetics in many persons with AD.
The severity of cognitive impairment in patients with AD varies. The earliest manifestations can be a subjective decline in mental abilities in the absence of impaired performance on objective cognitive testing1. Mild cognitive impairment (MCI) refers to the earliest symptomatic stage of cognitive impairment in which a single cognitive domain or, possibly, multiple cognitive domains are impaired to at least a mild extent whilst functional capacities are relatively preserved2. By contrast, dementia is defined as cognitive impairment of sufficient magnitude to impair independence and affect daily life. Dementia of gradual onset and ongoing progression with prominent amnestic symptoms and signs is the prototypical clinical phenotype of AD3.
AD was originally considered a clinicopathological entity, meaning that, if the patient fulfilled the clinical syndrome of an amnestic dementia and other conditions were ruled out, one could assume that AD pathology was the cause4. However, increased clinical sophistication together with biomarkers of AD, namely cerebrospinal fluid (CSF) and PET markers for Aβ and tau, has transformed the concept of AD to a neurobiological condition that affects different aspects of cognition. Of note, there is a greater appreciation of the relationship between AD and other aetiologies of cognitive impairment. Although multi-aetiology dementia (which is the preferred term over ‘mixed dementia’) is not the focus of this Primer, it is important to remember that AD pathology rarely occurs in isolation in patients >65 years of age.
This Primer reviews the epidemiology of the cognitive manifestations of AD and highlights the main risk factors of this disorder. In addition, this Primer summarizes the pathophysiology of AD from the perspective of a synaptic disorder and reviews advances in its diagnosis, including clinical diagnosis and advances in biomarkers, as well as the management and quality of life of persons living with cognitive impairment due to AD and that of their caregivers.
Epidemiology
Incidence and prevalence
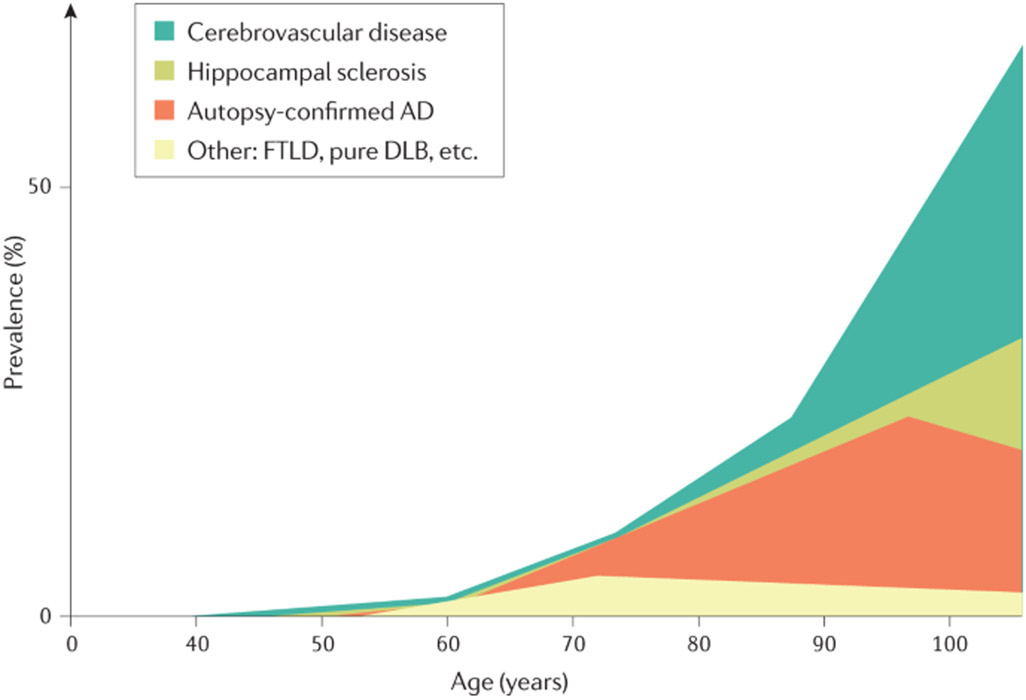
The epidemiology of AD is intertwined with that of all-cause dementia5,6 (FIG. 1). Although AD is the most common cause of dementia7, dementia can be caused by multiple neurodegenerative or cerebrovascular pathologies, particularly in older patients8,9. Indeed, in one study of 184 individuals who met research neuropathological criteria for AD9, 31% had only AD pathology, 22% had AD pathology plus α-synuclein pathology (Lewy bodies outside of the brainstem), 29.5% had AD pathology plus TDP43 pathology (TDP43 inclusions in hippocampi), and 17.5% had AD pathology plus α-synuclein and TDP43 pathology. Within each of these pathologically defined groups, between 29% and 52% of individuals had at least one infarct (microinfarct, lacunar infarct or large infarct)9.
Fig. 1 ∣. Neuropathological diagnoses that cause cognitive impairment across the age spectrum.
Alzheimer disease (AD) may occur without other co-pathologies in those <70 years of age but other pathologies are common in older individuals with AD pathology. DLB, dementia with Lewy bodies; FTLD, frontotemporal lobar degeneration. Adapted from REF.5, Springer Nature Limited.
The prevalence of all-cause dementia is expected to increase from 50 million people in 2010 to 113 million by 2050 worldwide10. Although the prevalence of dementia has increased in both high-income and middle/low-income countries over the past 50 years owing to an extended life expectancy11, the incidence of dementia has slightly decreased in some high-income countries such as the USA, UK and France11. For example, in the Framingham Heart Study, the age-adjusted and sex-adjusted hazard rate for incident dementia in persons aged >60 years was 3.6 per 100 persons in the late 1970s and early 1980s but 2.2 per 100 persons by the late 2000s and early 2010s12. The lower incidence of dementia in people born more recently could be due to the educational, socio-economic, health-care and lifestyle changes that have occurred in the past several decades. In particular, greater educational attainment is a protective factor against dementia presumably because it conveys a greater capacity to withstand the consequences of neurodegenerative and cerebrovascular disease (referred to as ‘cognitive resilience’13). However, attempts at proving cause-and-effect relationships between the various mitigating factors and dementia incidence have proved difficult12,14. Of note, the reduction in dementia incidence cannot be specifically attributed to AD, although one neuropathological study found a trend for a 24% decline in brain Aβ burden from 1972 to 2006 in a sample of 1,599 individuals with a mean age of 82 ± 8 years15.
The prevalence of overt cognitive impairment increases exponentially with advancing age. Indeed, the incidence of dementia increases steeply after 65 years and continues to increase thereafter. The incidence of all-cause dementia in individuals aged 65–70 years is approximately 1 per 100 per year and increases to 4 per 100 per year in those aged 80–90 years16. In a meta-analysis of 20 studies from Europe and North America, the prevalence of clinically diagnosed amnestic dementia (dementia attributed without biomarkers of AD) increased from <1% in persons aged 65–69 years to 7–8% in those aged 80–84 years to 27% in those aged 90–94 years17. In general, the incidence of MCI is about twice that of dementia at any given age18. Studies using MRI and PET to estimate the burden of AD have estimated that MCI with AD pathology contributes to ~50% of all cases of MCI19 and dementia due to AD contributes to ~60–90% of all dementia cases20. Other aetiologies, such as cerebrovascular disease and Lewy body disease, may be contributory in up to half of persons with AD21. The fastest-growing demographic segment in high-income countries is the oldest-old group (people aged >90 years), which is the group with the highest risk of cognitive disorders, particularly multi-aetiology dementia. Improvement in survival into older adulthood in low-income and middle-income countries11 is driving an increased overall societal burden of dementia.
The prevalence and incidence of dementia have been well studied in high-income countries but are less well understood in low-income and middle-income countries. Provisional estimates support the view that dementia is a worldwide illness with little variation in age-specific prevalence between regions22. The prevalence of subjective cognitive complaints was 25% worldwide in one meta-analysis of 16 studies, although individual estimates varied from 6% to 53%23, implying diversity in how subjective cognitive decline is defined both within and across different regions and cultures. Similar to dementia, the prevalence of MCI varies by age — the reported prevalence was 6.7% in individuals aged 60–64 years and 25.2% in those aged 80–84 years in a meta-analysis of 34 studies from high-income countries24.
Risk factors
Age is the most important risk factor for both dementia and AD. In the aged population worldwide, for example, in those aged >65 years, more women than men have dementia owing to an excess all-cause mortality in men aged >45 years11. In addition, the prevalence of mild cognitive impairment may be higher in men; one study found an OR of 1.54 (95% CI 1.2–2.0) for MCI in men versus women in a community comprised of European-Americans25. However, in many studies, the prevalence of dementia is either higher in women or finds no difference between sexes26.
Genetic risk factors for AD include rare dominantly inherited mutations in APP, PSEN1 and PSEN2 and in more common but incompletely penetrant genetic variations such as APOE. Taken together, genetic contributions represent only a modest part of the attributable risk as manifested in the age of onset27.
Dominantly inherited AD has an age of onset that is ~40 years earlier than sporadic late-onset AD but otherwise shares many clinical, biomarker and pathological similarities. Mutations in APP (encoding amyloid precursor protein (APP)), PSEN1 (encoding presenilin 1) and PSEN2 (encoding presenilin 2) account for almost all cases of dominantly inherited AD28. Persons with mutations in these genes are almost always younger than 65 years when they develop symptoms and represent only a minority of persons with AD dementia in this age group, in which AD and frontotemporal degenerations are about equally common. A rare variant of APP (A673T) that is protective against Aβ production and clinical symptomatology has been identified29.
More than 600 genes have been investigated as susceptibility factors for AD. APOE is a susceptibility polymorphism and is the most important genetic risk factor for AD occurring after 65 years. Carriage of the APOE ε4 allele increases the risk for dementia by 3–4 times in heterozygotes and by 12–15 times in homozygotes compared with APOE ε3 carriers30. Some susceptibility genes, such as TREM2, SORL1 and ABCA7 (REF.31), if combined, may be useful in polygenic risk scores, even accounting for the presence of the APOE ε4 genotype, for predicting incident dementia due to AD32. The rare genetic variants convey a lower risk of AD than the APOE ε4 allele; indeed, the rare R47H variant of TREM2 conveys a tenth of the risk of heterozygosity of APOE ε4 and all of the other identified risk genes are 100-fold less potent than APOE ε4 (REF.33). Mutations in MAPT (encoding tau) are not associated with AD, although variants of tau-binding proteins such as BIN1, CD2AP, FERMT2, CASS4 and PTK2B are risk genes in large-scale analyses34. The genetics of rare variants has played and will continue to play a critical role in suggesting important disease mechanisms in AD.
Several potentially modifiable risk factors occurring in midlife, in particular metabolic factors (diabetes mellitus, hypertension, obesity and low HDL cholesterol), hearing loss, traumatic brain injury and alcohol abuse, are associated with an increased risk of later-life dementia, with relative risks of between 1.5 and 1.8 (REF.35). In later life, smoking, depression, low physical activity, social isolation, diabetes mellitus and air pollution are risk factors of similar magnitude for dementia, although several of these, such as low physical activity, social isolation and depression, may have a bi-directional link and may be part of the prodromal phase of dementia36. Diabetes mellitus and hypertension are probably the most common and important risk factors for late-life dementia, especially when those risk factors are present in midlife37 but in late life as well38. Although there is some evidence for an influence of midlife vascular risk factors on later-life brain Aβ39, both diabetes mellitus and hypertension induce cerebrovascular disease and are thought to affect the clinical expression of AD pathology through the modifying effects of atherosclerotic and arteriolosclerotic cerebrovascular disease rather than through a direct effect on Aβ or tau biology40.
Mechanisms/pathophysiology
AD is a synaptic dysfunction disorder encompassing molecular, cellular and macro-scale cortical circuitry system failures that has a predilection for a cognitively eloquent cortex. Synaptic pathophysiology is an attractive theme in unifying observations about genetics, cell biology, neuropathology and the clinical manifestations of AD. The pathology of AD can be viewed as positive (‘overt’) lesions that can be observed using microscopy, that is, tau-containing neurofibrillary tangles, Aβ-containing plaques, activated glia or enlarged endosomes. Alternatively, AD can be viewed as representing negative (‘covert’) phenomena, that is, the loss of synaptic homeostasis, neurons or neuronal network integrity. While the biology of amyloid precursor protein and tau protein is featured prominently throughout this Primer, the apparent primacy of these proteins as embodied in the amyloid cascade hypothesis41 fails to acknowledge the many other covert mechanisms, not all of which can be covered here42,43, that have been hypothesized as relevant. Understanding how the mechanisms that underlie AD lead to synaptic and neuronal loss, which are the likely causes of cognitive impairment, has been a matter of substantial investigation yet much remains to be learned.
The critical molecules
The pathology of canonical AD dementia involves Aβ-containing extracellular neuritic plaques that are found in a widespread distribution throughout the cerebral cortex and tau-containing neurofibrillary tangles that occur initially in the medial temporal lobe, followed by the isocortical regions of the temporal, parietal and frontal lobes44,45.
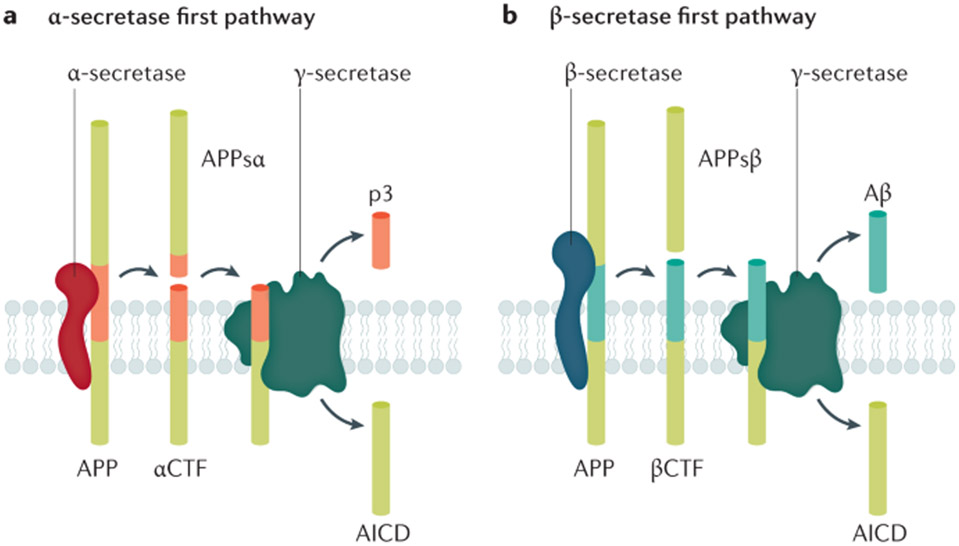
Aβ peptides are derived from APP (a single transmembrane protein that is enriched in neuronal synapses46) following the cleavage of APP by β-secretases and γ-secretases (known as the amyloidogenic pathway)47,48 (FIG. 2). The most abundant species of Aβ are 27–43 amino acids in length. After production, Aβ is secreted into the extracellular space as a monomer47. Owing to its sequence, Aβ (particularly Aβ42) has a high propensity to aggregate, which occurs in a concentration-dependent manner. In addition to Aβ, other molecules — APPsβ, βCTF and AICD — are formed in the amyloidogenic pathway. APP cleavage by α-secretase generates APPsα and αCTF and prevents Aβ formation; αCTF is subsequently cleaved by γ-secretase into p3 and AICD49. Although all cells produce Aβ, it is generated in high levels by synaptic activity and its production and release are regulated by synaptic activity50. Aβ levels are also modulated by the sleep–wake cycle51. Aβ production and release into the extracellular space is higher during wakefulness and Aβ clearance through the glymphatic system is higher during sleep52.
Fig. 2 ∣. APP cleavage pathways.
a ∣ In the non-β-amyloid (Aβ) pathway, successive cleavage by α-secretase leads to the formation of APPsα and αCTF, which in turn is cleaved by γ-secretase to yield the extracellular peptide p3 and the intracellular fragment AICD. APPsα may modulate synaptic transmission through a GABA receptor. b ∣ Aβ is formed in the ‘amyloidogenic’ pathway by the successive cleavage of amyloid precursor protein (APP) by β-secretase into APPsβ and βCTF, the latter being then subjected to γ-secretase, producing Aβ and AICD. βCTF plays a key role in early endosomal abnormalities in AD. Note that the production of Aβ is necessarily in equal amounts to AICD and APPsβ and in inverse amounts to p3 and APPsα. Adapted with permission from REF.48, Elsevier.
In keeping with the hypothesis that Aβ, particularly in its oligomeric form, is toxic, oligomeric Aβ interacts with metabotropic glutamate receptor 5 and NMDA receptors53,54 and seems to interact with several other receptors such as α7 nicotinic acetylcholine receptor and insulin receptors. In addition, Aβ also seems to cause pathological changes in dendritic spines and synaptic efficiency. Although aggregated Aβ is the overt lesion that could cause neurotoxicity, dysregulated APP processing may also have covert’ effects on synaptic function. APP cleavage products have normal physiological functions. For example, APPsα is a ligand for a subtype of GABA receptor that modulates synaptic transmission55. In addition, other cleavage products, such as APPsβ and APPsη, have cellular receptors that suggest they might also modulate synaptic activity49,56 and non-Aβ fragments of APP, such as C-terminal fragments57, seem to play a critical role in endosomal and lysosomal function.
Tau is a microtubule-associated protein that is normally present in the cytoplasm of axons58 but is also present in both presynaptic and postsynaptic compartments and associated with the nuclear membrane59,60. Tau occurs in six isoforms due to alternate splicing of exons 2, 3 and 10; the isoforms are named according to the presence of either three or four microtubule-binding domains as the 3R or 4R isoforms, respectively61. The tau species that occurs in AD is a mixture of 3R and 4R tau. The main function of tau is microtubule stabilization59,60. Tau may become prone to post-translational modifications and to aggregate. When this happens, it accumulates in a hyperphosphorylated form in cell bodies and dendrites. Tau is released into the extracellular space by synaptic activity62,63 and is taken up in postsynaptic neurons and glia64.
Different post-translational modifications of tau might allow it to be differentially taken up in postsynaptic neurons or glia, differentially processed or truncated, or to cause seeded aggregation. In addition, tau post-translational modifications affect the rate of AD progression65. There is emerging evidence that ApoE66, TREM2 (REF.67) and microglia68 play an important role in tau-mediated neurodegeneration although the mechanisms that relate presumed microglial activity to tau toxicity are unknown.
Aggregated 3R and 4R tau appear histologically as neurofibrillary tangles (intracellular tau aggregates), neuropil threads (tau fragments in the neuropil) and dystrophic neurites (tau-containing degenerated axons and dendrites surrounding Aβ plaques). Although these structures can be seen in some brainstem nuclei in young adults69, tauopathy in the medial temporal lobe is the initial site relevant to cognition70,71. Medial temporal tauopathy may occur independently of Aβ pathology72,73. In some patients in whom elevations in Aβ never occur, medial temporal tauopathy (referred to in these patients as primary age-related tauopathy)74 may persist without the involvement of other regions, however, tauopathy of the AD type progresses outside the medial temporal lobe only in those with elevated brain Aβ75-77. Region-specific cognitive deficits occurring in MCI and dementia reflect the sequential expansion of tau accumulation from the medial temporal lobe to interconnected temporal, parietal and frontal association cortices77,78 (see Clinical manifestations).
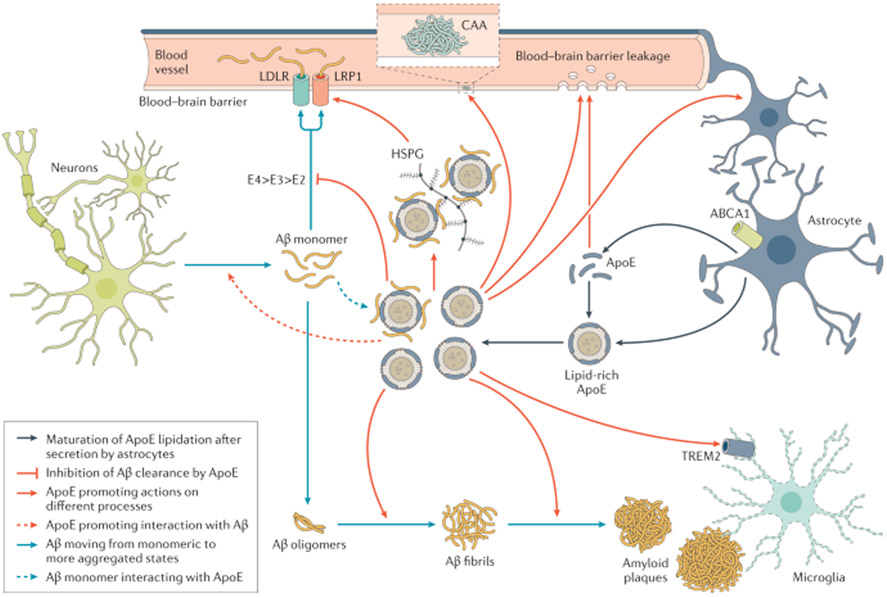
Apolipoprotein E (ApoE) is at the crossroads of clinical, genetic and cellular mechanistic aspects of AD79. The APOE gene encodes the ApoE protein, which is produced in the brain predominantly by astrocytes and activated microglia79. In humans, there are three common isoforms, ApoE2, ApoE3 and ApoE4, which only differ by a single amino acid at position 112 or 158 (REF.79). Carriers of the APOE ε4 allele have a dose-dependent increased risk for AD dementia80. There are several mechanisms by which ApoE may influence AD risk, one of which is by affecting the onset of Aβ aggregation in the brain by altering the clearance and seeding of Aβ80 (FIG. 3; also see Dysfunctional proteostasis, below). The three ApoE isoforms modulate Aβ clearance and seeding to varying extents, with ApoE4 having the strongest effect on the slowing of clearance, followed by ApoE3 then ApoE2 (REF.80). Accordingly, APOE ε4 carriage can cause early Aβ accumulation in a dose-dependent manner before the onset of clinical symptoms81. Aβproximately 10% of APOE ε4 carriers have increased Aβ by age 57 years (as assessed using Aβ-PET), while APOE e4 non-carriers have increased Aβ about 7 years later82.
Fig. 3 ∣. ApoE and Aβ interaction.
In one pathway, monomeric β-amyloid (Aβ) aggregates into oligomers, then into fibrils and finally becomes part of amyloid plagues. ABCA1 participates in the lipidation of ApoE, which has a strong effect on Aβ fibrillization. ApoE complexes with Aβ monomers and, by interacting with the low-density lipoprotein receptor (LDLR) and LDL receptor-related protein 1 (LRP1), transports Aβ into the perivascular spaces around arterioles and venules for removal by the glymphatic pathway. Heparan sulfate proteoglycan (HSPG) also interacts with LRP1 and Aβand influences its oligomerization. Within the perivascular space, Aβ can aggregate, which leads to cerebral amyloid angiopathy (CAA), which in turn can damage the vascular wall, leading to micro haemorrhages. Through the triggering receptor expressed on myeloid cells 2 (TREM2), lipoproteins, including ApoE, influence the phagocytic properties of microglia and might influence plague removal. Adapted with permission from REF.80, Elsevier.
The precise mechanisms by which Aβ/APP and tau interact are not well understood83-85. Transgenic mice with Aβ-overexpression with wild-type tau develop either no tauopathy or one that is dissimilar to AD86,87. Transgenic animals that contain both APP and tau mutants have interactions between Aβ and tau85 but it is not clear whether this model system replicates human AD. Conceptually, there are several cellular systems in which Aβ/APP, tau and ApoE might interact: the synapse53, in microglia88 and in the endosomal/lysosomal/proteasomal system89,90. Alternatively, neural systems that accumulate Aβ and those that accumulate tau might be distinct but interact primarily through the connectome.
Loss of synaptic homeostasis
Synaptic alterations are a central unifying theme53,91 to understand the relationship between Aβ and tau neurotoxicity, the morphological lesions of AD, and cognitive impairment. Synaptic loss is strongly correlated with cognition in patients with AD92,93. Aβ plaques are surrounded by a ring of soluble oligomeric Aβ and decreased synaptic content that extends for ~50 μm, marked by both a loss of presynaptic and postsynaptic markers53,94; given the large number of cortical plaques in patients with AD, this amounts to a substantial number of lost synapses. Moreover, in animal models of plaque deposition in which there is little neuronal loss, an additional 25% loss of synaptic content in the neuropil between plaques can be observed53,94; it is likely that a similar phenomenon occurs in humans. These data suggest a strong interaction between the oligomeric Aβ species thought to surround plaques and the neuropil and synaptic toxicity95. Tau-overexpressing transgenic animals have a similar loss of synapses, with tau accumulation in presynaptic and postsynaptic sites96. These synaptic alterations seem to be synergistically amplified in mice when tau and Aβ mutant transgenes are included in the model85. Patients with AD also have a marked loss of synapses closely associated in cross-sectional studies with the presence of neuronal loss, dendritic loss, loss of dendritic arborization and tangle formation93,97. Importantly, synaptic loss is not simply a part of healthy ageing in the absence of AD pathology in most people98; indeed, cross-sectional studies in human autopsy samples have suggested that synaptic loss can precede neurodegeneration in persons with symptomatic AD99,100 and, in animal models, it is evident that synapse loss can precede tangle formation101.
In addition to a loss of synapses in AD, several studies in animal and laboratory models have suggested the involvement of synaptic dysfunction. In rodent transgenic Aβ models, there is hyperactivity of calcium flux in and around plaques, which can be normalized by inhibiting the generation of Aβ with BACE inhibitors102. How this hyperactivity affects cognitively eloquent neural circuitry is unknown but it is assumed that altered synaptic homeostasis plays a role103,104. In mice with mutant tau transgenes, intracellular and extracellular recordings of neocortical pyramidal neurons show alterations in firing rates and firing patterns, implying alterations in basic electrophysiological homeostasis due to the tauopathy105. When mice expressing human tau and Aβ were examined, there was a tau-dependent quieting of neural system activity in the parietal cortex with or without neuronal loss106, which was normalized by the suppression of tau expression. In this study, tau suppression decreased the amount of soluble tau without affecting aggregated tau, implicating soluble tau in the pathophysiology of AD. Collectively, these data imply that the soluble forms of oligomeric Aβ or of tau, rather than the aggregated forms, have the strongest effect on synaptic function.
Synaptic alterations have consequences on downstream neural circuit integrity. In animal models, a reactive synaptogenesis occurs in the hippocampus after loss of the entorhinal–hippocampal perforant pathway107 and analogous alterations occur in the human hippocampus in AD108,109. Whether this synaptogenesis has functional benefit remains unclear. Synaptic alterations can have remote consequences elsewhere in the brain and are therefore a prime explanation for the unique topographical character of AD, which is discussed further below.
Synaptic activity is also a likely basis for the propagation of proteopathic seeds trans-synaptically. In AD, the secretion of tau from neurons has been suggested62 and, as tau release is activity dependent, a synaptic mechanism is implied. Moreover, in animal models, tau propagates across synapses to create cytoplasmic deposits in the downstream neurons64,110, although it remains to be tested if these downstream neurons lose function. Synaptic breakdown may facilitate this trans-synaptic deposition of misfolded proteins as observed in an animal model of tau propagation64. In addition, as proteopathic seeds can be taken up at the synapse via LRP1 (REF.111), which interacts with APP and ApoE112,113, competing interactions with, for example, different ApoE isoforms or ApoE protein levels might affect tau propagation.
Synaptic remodelling is a ubiquitous process in the normal brain. Synapses can be tagged for removal, primarily by microglia, which are able to strip synapses from intact dendrites. Dysfunction in the activation of synaptic pruning mechanisms by microglia, mediated in part by complement proteins and ApoE, among other molecules, have been implicated in early AD114. The precise mechanism of this ‘tagging’ is unclear but altered clearance processes plausibly link synaptic dysfunction, innate immune mechanisms and the lysosomal, endosomal and autophagy mechanisms discussed below.
Dysfunctional proteostasis
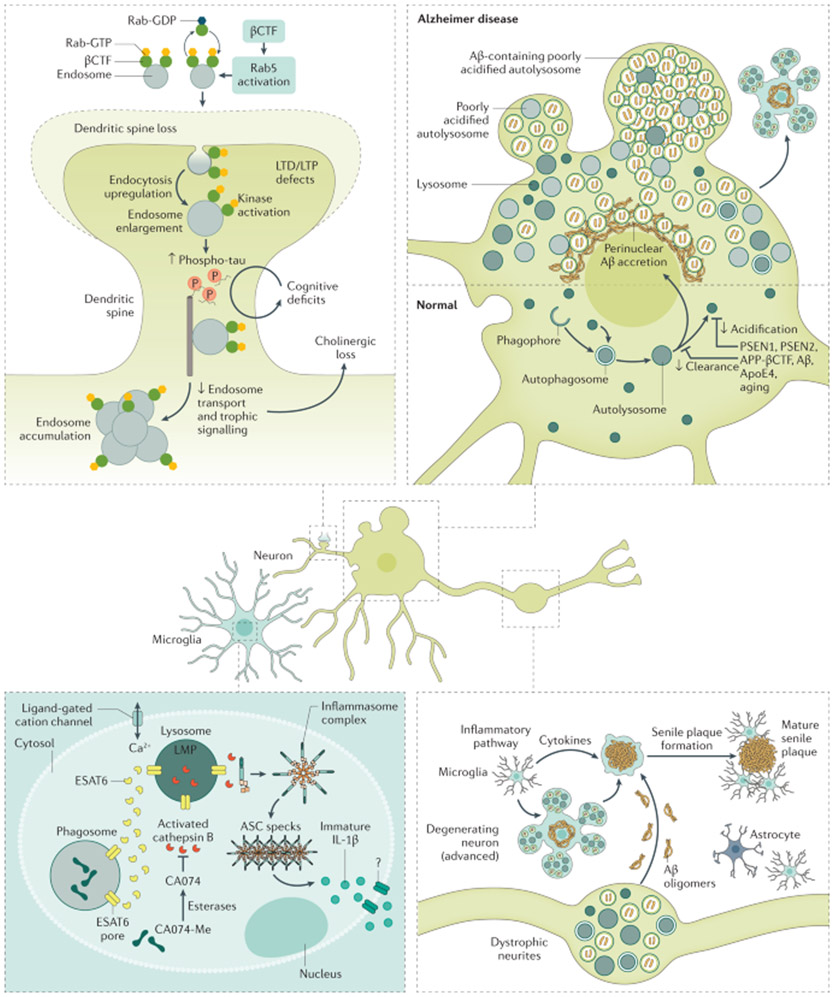
The clearance of damaged proteins is integral to maintaining synaptic homeostasis and the neuronal lysosome (FIG. 4) plays an important role in the aetiology of AD115-117. In the context of normal brain health, the endosomal–lysosomal network (ELN) and autophagy are involved in memory and cognition, with local autophagy activity at synapses modulating memory formation118,119 and autophagy induction attenuating age-related memory decline119.
Fig. 4 ∣. Consequences of the endosomal–lysosomal network and autophagy dysfunction in AD.
The endosomal–lysosomal pathway, consisting of early and late endosomes/multivesicular bodies and lysosomes, serves diverse functions in neurons relevant to Alzheimer disease (AD). In AD, the earliest changes seen in the brain are swelling of neuronal Rab5-endosomes, reflecting the hyper-activation of Rab5 (Rab5-GTP) by amyloid precursor protein (APP)-βCTF or neuronal overexpression of Rab5. Increased Rab5 activation leads to endosome enlargement and increased endocytosis, which have several cellular consequences relevant for AD. For example, increased endocytosis of AMPA receptors (AMPAR) leads to defects in synaptic plasticity and dendritic spine shrinkage and loss. In addition, abnormal growth factor/receptor-mediated signalling results in the downregulation of AKT-mediated prosurvival signalling and increased GSK-3β-mediated tau hyperphosphorylation (p-tau). Corresponding endosomal–lysosomal activities in astrocytes and microglia coordinate bi-directional trafficking of cargo into and out of cells to maintain them and support the clearance of extracellular material in partnership with autophagy. Autophagy encompasses several mechanisms of constituent delivery to lysosomes, including initial entry of the substrate into late endosomes/multivesicular bodies (microautophagy), chaperone-mediated delivery directly to lysosomes, or macroautophagy, the major route depicted here. The increased induction of autophagy, a cellular stress response, becomes counter-productive as the functioning of autolysosomes and lysosomes is progressively corrupted due to multiple genetic and environmental factors. The result is a substantial build-up of autophagic vacuoles, mainly poorly acidified autolysosomes incompetent in clearing Aβ and βCTF, causing a unique pattern of perikaryal membrane blebbing, trafficking deficits producing autophagic vacuole-filled swellings along axons (dystrophic neurites), and accelerated peri-nuclear Aβ aggregation preceding advanced neuronal degeneration and disintegration, initiating senile plaque formation. An ensuing inflammatory response involving the recruitment of phagocytic microglia to compromised neurons/neurites and a release of damaging cytokines as the extracellular debris is being cleared increases bystander neurotoxicity, affecting neighbouring neurons and senile plaque expansion. LMP, lysosomal membrane permeabilization; LTD, long-term depression; LTP, long-term potentiation; ASC, Aβoptosis-associated speck-like protein.
Aβ and the βCTF peptide arising from the β-secretase cleavage of APP are generated mainly within endosomes, which are the first neuronal organelles known to exhibit AD-specific neuropathology120,121. Lysosomes are the principal sites for the clearance of intracellular Aβ and βCTF117,122. In AD, Aβ and βCTF accumulate abnormally in ELN compartments117. In induced pluripotent stem cell lines with APP and PSEN1 mutations, endosomal abnormalities occur that are correlated with βCTF but not with Aβ123. βCTF induces the overactivation of Rab5, a GTPase, which causes several endosome morphological anomalies that occur in early AD117,124 such as accelerated endocytosis, impaired transport of enlarged endosomes and other neurodegenerative processes117. In mouse AD models, elevated levels of Rab5 signalling due to βCTF elevations induce synaptic plasticity deficits, tau hyperphosphorylation and neurodegeneration117. The build-up of βCTF and oxidized substrates, including Aβ, progressively impairs lysosome function and causes autophagy failure125.
Presenilins 1 and 2 are also preferentially localized within the ELN and presenilin 1 is essential for lysosome acidification. Loss-of-function mutations or deletion of PSEN1, independently of γ-secretase, prevent the normal assembly of the lysosomal v-ATPase complex, the proton pump responsible for acidification126, and impede the lysosomal delivery of ClC7, a chloride ion channel also essential for lysosomal acidification in mice and in human fibroblast cultures127. Lysosomal pH dysregulation can instigate broad functional derangements beyond proteostasis, including the effects on synaptic plasticity124, neurotransmitter exocytic release128,129, and synaptic vesicle fusion and recycling130. APOE ε4 carriage has allele-specific effects at every ELN level, from accelerating and accentuating endosome dysfunction120 and impeding exosome release131 to causing lysosomal expansion and lysosomal membrane permeabilization132.
Autophagy has several functions that are relevant for AD, primarily proteostasis but also homeostatic cell signalling, phagocytosis, innate immunity and synaptic function133,134. Macroautophagy is induced by nutrient or metabolic stress and recycles non-essential substrates for energy or selectively targets protein aggregates and damaged organelles134. The early induction of macroautophagy in AD135 is neuroprotective against ageing-related and disease-related sources of oxidative stress136,137; however, continued high rates of delivery of oxidized or damaged cytoplasmic constituents, such as mitochondria, Aβ and other APP products, to lysosomes later in disease overburdens an already compromised lysosomal system135, resulting in a substantial accumulation of waste in neurons138. Accordingly, dystrophic neurites become engorged with waste-filled autophagic vacuoles that contain high levels of APP, β-secretases and γ-secretases, and APP metabolites139. Although neurons can survive for years with neuritic dystrophy140, eventual autophagic vacuole accumulation in the perikarya leads to more rapid autophagy failure and neurodegeneration (FIG. 4). Lysosomal membrane-damaging events, induced by Aβ and other oxidized substrates, trigger the death of neurons and microglia by lysosomal membrane permeabilization, which releases cathepsins that can activate cell death cascades141.
Microglia-expressed genes and genes involved in the complement pathway are risk genes for AD, implying the relevance of microglia and neuroinflammatory mechanisms in AD89. Activated microglia play a key role in AD pathogenesis and link synaptic life cycles and innate immunity to the ELN functions. Microglial lysosomal membrane permeabilization events underlie the brain’s principal inflammatory response and the release of cytokines, particularly IL-1β, which is thought to exacerbate neurodegeneration in AD142,143. Cathepsin B released by lysosomal permeabilization events into the cytosol facilitates the assembly of the microglial NLRP3 inflammasome and activates caspase 1, which is responsible for the maturation and extracellular discharge of cytotoxic IL-1β that subsequently fuels brain inflammation144. The concept of an “autophagy-brake regulation” of inflammation reflects evidence that autophagy suppresses inflammasome activation and IL-1β release145, both of which are elevated when autophagy is impaired in AD and ageing146. The role of the microglial protein TREM2 in AD pathogenesis is complex and may differ at the various disease stages147,148. It might be expected that altered autophagy and ELN function would affect synaptic pruning149 and therefore the integrity of normal synaptic connectivity but there is not a clear demonstration of the interrelationship of these two systems in human AD.
Aβ, tau or other potentially toxic molecules released by dying cells or by aberrant exocytic mechanisms into the extracellular space are degraded by various proteases150, complementing clearance by microglia and astrocytes through endocytic uptake and lysosomal degradation. Molecular chaperones, such as the AD risk factor clusterin, facilitate this glial uptake. These clearance routes, in addition to the brain glymphatic system for the clearance of extracellular pathogenetic proteins through the lymphatic system151, help protect against the transneuronal propagation of tau species.
Aβ, tau and cortical networks
Network connections have a defining role in the initial location of Aβ deposits and may be responsible for the early location and progression of tauopathy through the brain. A central element of AD as a synaptic disorder is the remarkable regional specificity of Aβ and tau accumulation and the extent to which clinical symptoms map on to the latter.
Traditional neuroanatomical investigations established the strong interconnectivity between the entorhinal cortex, the locus of initial cerebral neurofibrillary tangle deposition in AD, and association areas of the temporal, parietal and frontal isocortex70,71. Functional MRI replicated this connectivity in healthy humans and also demonstrated the dysfunction of these pathways in symptomatic AD. Aβ deposition occurs in a network-specific pattern in regions comprising the default mode network (DMN)152,153, a group of areas that are active when the individual is not focused on the external environment and which includes the posterior cingulate gyrus and two subsystems (a medial frontal one and a medial temporal one)152. The association between Aβ deposition and the DMN shows that Aβ accumulates in brain regions with the highest synaptic activity (and, therefore, also regions with the greatest stress on the resident autophagy and ELN). The number of functional connections or the degree of ‘hubness’, which is another measure of synaptic load, is predictive of the amount of measured Aβ in a brain region154.
Tauopathy occurs in networks other than the DMN that support specific cognitive domains and are impaired in symptomatic AD155, with clinical phenotype-specific changes in functional networks as measured with functional MRI156 or FDG-PET157. Regions that are connected to loci with a high tau abundance are more likely to experience an increase in tau accumulation158,159. The major patterns of tau deposition observed with tau-PET show a spatial correspondence to connectivity patterns with variation by clinical phenotypes (see Clinical diagnosis)160. Of note, in contrast to tau, there is no evidence of significant phenotypic variance in this macroscopic Aβ pattern161.
The functional activity of the connectome could selectively alter synaptic homeostasis in a way that offers a plausible account for the co-occurrence of APP/Aβ and tau pathology in AD. In persons without elevated Aβ assessed using PET, those who eventually develop elevated Aβ had DMN hyperconnectivity and increased glucose uptake compared with those who did not later develop increased Aβ162. The loss of homeostasis in areas of high synaptic activity in the DMN could induce dysfunction as well as the loss of homeostasis in remote but connected brain regions that depend on the DMN for proper functioning (such as the medial temporal lobe), a so-called cascading network failure160. One such region of synaptic dysfunction and early Aβ accumulation, the posterior cingulate cortex, is strongly connected to the medial temporal lobe163. The downstream synaptic stress, which is indexed by Aβ accumulation in the posterior cingulate154, is a plausible account of the propensity of the medial temporal lobes to accumulate tau when it loses functional support from the DMN (Supplementary Figure 1). Over a timeframe exceeding a decade or longer, synaptic dysfunction that leads to Aβ accumulation in the DMN could accelerate tauopathy in strongly linked functional modules159,164. Subsequently, the transition from no symptoms to dementia in AD more closely fit with pathological tau expansion from the medial temporal lobe to functionally connected isocortical regions.
Diagnosis, screening and prevention
Clinical manifestations
The severity of cognitive impairment caused by AD ranges from no cognitive impairment to dementia, which includes subjective cognitive impairment and MCI (FIG. 5). The principal cognitive domains that are affected in AD are memory, language, visuospatial function, and executive function and one or more cognitive domains may be affected at any severity of cognitive impairment. The clinical presentations of persons with AD pathology are further modified by the co-presence of non-AD pathologies.
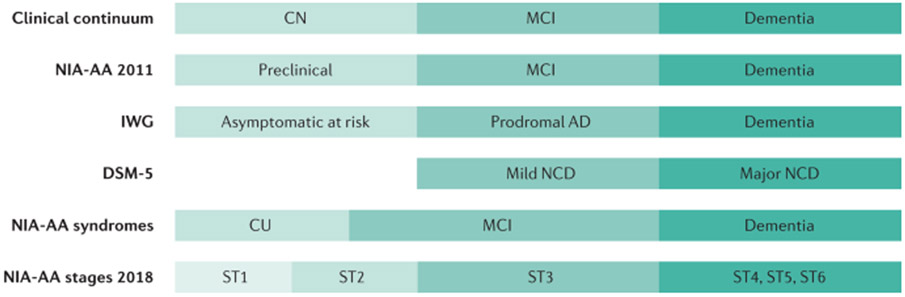
Fig. 5 ∣. Terminologies for characterizing cognitive impairment.
Several different terminology schemes can be used to classify the severity of cognitive impairment from cognitively normal (CN) to dementia. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5)281 uses the terms Mild Neurocognitive Disorder (NCD) and Major NCD to describe symptomatic states. The National Institute of Ageing – Alzheimer’s Association (NIA-AA) Alzheimer disease (AD) framework uses clinical syndromes and defines a six-stage scheme for individuals who have abnormal β-amyloid biomarkers (ST1–ST6)180. In the NIA-AA scheme, mild cognitive impairment (MCI) encompasses both stages 2 and 3. The NIA-AA continuum3,183,282 has evolved slightly at the boundary between cognitively unimpaired (CU) and MCI. The International Work Group (IWG) labels were retrieved from REF.283. The terms CN and CU are equivalent, but the latter is preferred because CU better reflects its definition as the absence of cognitive impairment.
The prototypical patient with AD is one with amnestic MCI progressing to variable degrees of impairment in language, spatial cognition, executive function or working memory that interfere with daily functioning (that is, multidomain dementia). Amnestic presentations are most common with later age of onset (>70 years) while non-amnestic presentations are common in younger persons. The initial appearance and progression of cognitive deficits in typical AD MCI and dementia follow the spread of tauopathy from the medial temporal lobe to the lateral temporal, parietal and frontal isocortex44,70,71. Neuropsychiatric symptoms often co-occur with cognitive deficits, of which depression, anxiety and social withdrawal may be most evident in mild dementia whereas delusions, hallucinations, emotional dyscontrol or physically aggressive behaviours may be observed in more advanced stages165.
AD can also manifest in its earliest symptomatic form with non-amnestic deficits77,166. A common non-amnestic AD clinical presentation — known as posterior cortical atrophy or the visual variant of AD — encompasses prominent visuospatial difficulties, including challenges in reading, face recognition or difficulties processing complex visual scenes167. The logopenic form of primary progressive aphasia is another non-amnestic form of AD168, which typically manifests as a non-fluent aphasia with prominent word-finding pauses, naming and repetition difficulties; approximately 60% of these persons will have underlying AD169. In addition, a dysexecutive presentation of AD is being recognized more frequently particularly in younger patients170. These patients have challenges in executive function, multi-tasking, decision-making and behavioural changes with preserved memory function157. The non-amnestic syndromes of AD are more common in persons aged <70 years although the underlying reasons for this observation are unknown. Both the amnestic and non-amnestic presentations of AD have Aβ deposition throughout the brain but have syndrome-specific distributions of tauopathy78,171.
Clinical diagnosis
Diagnosis in individuals with cognitive complaints.
Clinical diagnosis in the setting where a patient, family member or health professional raises concerns about cognitive decline or dysfunction in the patient is of paramount importance in the overall care of persons with cognitive impairment. Although the level of awareness of dementia has greatly improved in the primary care and specialist communities, heightened awareness of the early signs of cognitive impairment in the clinical setting is still needed.
The diagnostic process begins with a determination of the presence and severity of cognitive impairment. Information from an individual who is familiar with the patient’s daily life and the completion of a cognitive evaluation of the patient (a mental status examination) by a skilled clinician are the cornerstones of diagnosis. Neuropsychological testing can be beneficial to determine the severity of cognitive impairment in mild or high-functioning patients and can identify the involved cognitive domains, which can help the clinician with prognostication and with considerations about the underlying aetiology. This tentative diagnosis can then be confirmed with biomarkers. Although technology can assist in formulating a diagnosis, diagnosis rests on the skill of the clinician in integrating information from the informant, the mental status examination, the neurological examination and the technology. If cognitive impairment is diagnosed, a provisional determination of aetiology is made based on information from the history, the informant, the mental status examination (supplemented by neuropsychological testing in some circumstances) and the rest of the neurological examination.
AD is not the only cause of MCI (FIG. 1). Any neurodegenerative or cerebrovascular aetiology could initially lead to MCI and mild overt symptomatic cognitive impairment could be due to depression, medication misuse or obstructive sleep apnoea. In persons with MCI or dementia, screening for hypothyroidism, B12 deficiency and structural brain lesions, such as neoplasms or subdural hematomas, should be performed, even though the yield of clinically relevant abnormalities is low172. Once persons reach the dementia stage, reversible conditions are very unlikely but a number of non-AD conditions173 have clinical phenotypes that overlap with AD such as Lewy body disease174, frontotemporal degenerations175,176 and hippocampal sclerosis177. Thus, the consideration of alternative aetiologies should occur during the diagnostic evaluation of persons with MCI or dementia in whom AD is suspected. Features of the clinical presentation are the initial clue that a non-AD process may be present.
Screening in those with no cognitive complaints.
Screening for objective evidence of cognitive impairment in the absence of cognitive complaints is not recommended. This topic has been quite controversial178. The United States Preventive Services Task Force has repeatedly indicated that there are insufficient data on improved patient outcomes to recommend the utility of cognitive screening179. One problem with screening is that the cognitive assessments used for screening lack precision, especially for the detection of milder cognitive impairment179. However, in support of screening, the recognition of cognitive limitations in patients by health-care practitioners, even in the absence of cognitive complaints, may be informative as unacknowledged cognitive impairment may have dramatic effects on the adherence to recommended medical interventions or other healthy living activities. Should disease-modifying therapies for AD become available, screening will take on increasing importance despite the challenges in designing studies to address screening178.
Biomarkers
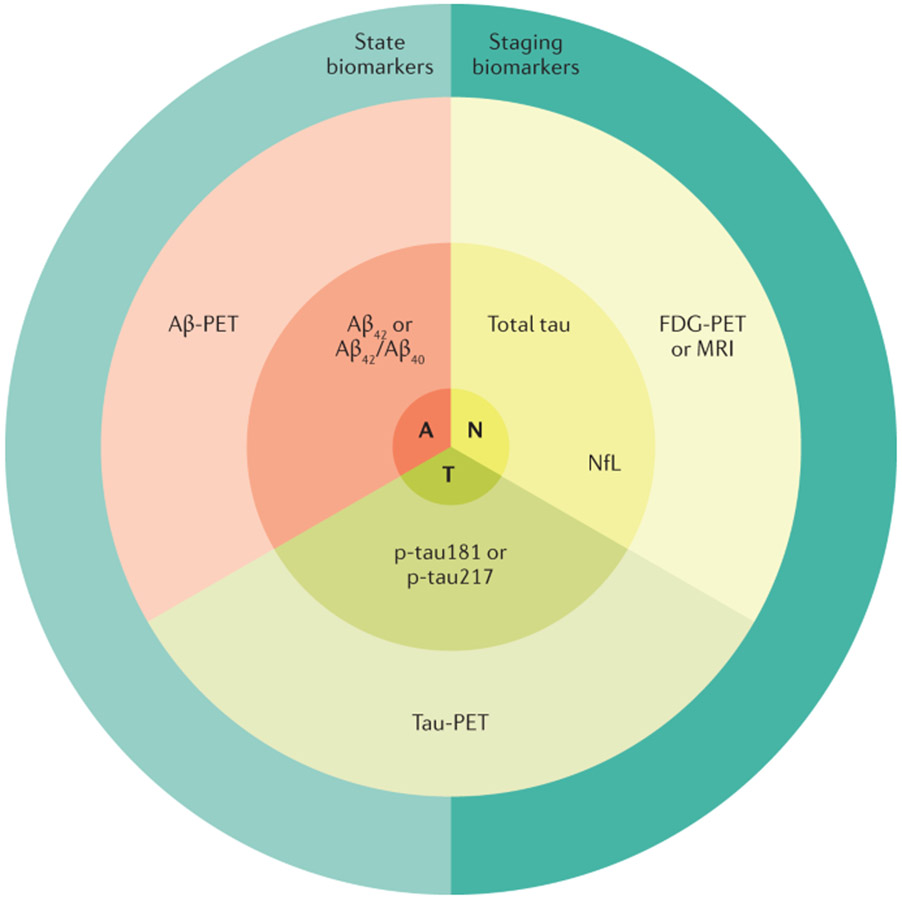
AD-specific antemortem biomarkers used in the context of careful clinical characterization has helped to establish levels of certainty for an AD pathology that was impossible when the only means of verification of the underlying pathology was at autopsy. In addition, the use of these biomarkers has enhanced our knowledge of AD. Combining amyloid and tau biomarkers with non-specific biomarkers of neurodegeneration was at the core of the research framework for AD proposed in 2018 (REF.180). The A-T-N approach is intended to classify individuals in the AD spectrum (FIG. 6). Diagnostic algorithms are being developed to provide recommendations on the most meaningful combination and to order these biomarkers according to the specific clinical situation181.
Fig. 6 ∣. Conceptualizing the A-T-N scheme.
Cerebrospinal fluid and PET imaging biomarkers can be grouped into those that proxy amyloid-β (Aβ; “A”), abnormal tau protein (“T”) or neurodegeneration (“N”). The A biomarkers derived from PET and cerebrospinal fluid (CSF) have an inverse relationship such that higher Aβ-PET corresponds to lower levels of CSF Aβ. The “T” biomarkers derived from PET and CSF are both abnormal at higher values. Quantitatively, tau-PET provides both a measure of regional tau abundance and distribution, whereas CSF p-tau181 or p-tau217 offer only a metric of normal or abnormal tau levels. The “N” markers, by virtue of their lack of specificity to Alzheimer disease (AD) and their heterogeneous underlying biologies, are generally not well correlated with one another. Biomarkers for AD can indicate whether a person is in the AD spectrum (state biomarkers) that include Aβ abnormalities and elevated tau biomarkers. Biomarkers can also be used to indicate the severity of the AD process (stage biomarkers) that include tau-PET and the neurodegeneration biomarkers. NfL, neurofilament light chain.
Imaging biomarkers.
CT, FDG-PET and MRI were the first imaging modalities used to evaluate patients with cognitive impairment but their lack of specificity or sensitivity for AD was difficult to integrate into a conceptual model of AD. The introduction of Aβ-PET imaging in 2004 (REF.182) clarified the roles of FDG-PET and MRI as markers of neurodegeneration, whereas CT was superseded by MRI for research purposes. The 2011 National Institute of Ageing – Alzheimer’s Association criteria183 introduced a diagnostic model using amyloid and neurodegeneration biomarker profiles to characterize the relationship with AD in individuals across the cognitive spectrum.
Structural MRI or CT are necessary in the initial evaluation of a person with suspected cognitive impairment. MRI is often used as a first step to exclude other causes of cognitive impairment and it also allows the assessment of macroscopic brain atrophy as a reflection of tissue loss. Of note, individuals with cognitive impairment and increased Aβ by PET experience accelerated regional atrophy in the temporal and parietal isocortex compared with those without elevated Aβ, with larger volume losses correlating with advancing cognitive impairment184. Hippocampal atrophy observed with MRI is associated with AD but it can also occur in individuals with cognitive impairment caused by hippocampal sclerosis, frontotemporal lobar degeneration or cerebrovascular disease as well as in persons without cognitive impairement185. In younger persons with AD dementia, hippocampal sparing is common171,186. Structural imaging may be used to support a diagnosis of posterior cortical atrophy variant of AD, the most common of the younger-onset, hippocampal-sparing AD variants. Although there is a global reduction in brain volume in AD owing to the loss of synapses, dendrites and neuronal cell bodies187, global brain volume loss is not diagnostically useful because it is not specific for AD.
Structural MRI is also useful to assess patients for cerebral microbleeds that occur as a consequence of cerebral amyloid angiopathy188. Cerebral microbleeds are common in the elderly; in a population-based study, 39% of persons without dementia over the age of 80 years had at least one cerebral microbleed and the burden of cerebral microbleeds was correlated with Aβ-PET levels189. The impact of cerebral microbleeds on cognition is modest190 but difficult to disentangle from the role of the underlying AD pathology.
FDG-PET has revealed a pattern of temporal-parietal and hippocampal hypometabolism that precedes volume loss in the same regions and is highly characteristic of AD191. FDG-PET could be particularly useful after Aβ-PET imaging in those with elevated Aβ for staging, short-term prediction, to differentiate between AD variants and in persons without elevated Aβ on PET scans for the diagnosis of non-AD disorders192,193. The degree and regional extent of hypometabolism measured by FDG-PET roughly correlates with the overall severity of cognitive impairment in AD, supporting the use of clinical severity as a proxy of AD pathology.
The development of Aβ-PET markers that allow the direct visualization of Aβ plaque accumulation has increased the precision of AD diagnosis and has enabled a real-time view of the evolution of β-amyloidosis over time. Many different Aβ-PET tracers have been developed194 and they have been validated against neuropathology195,196 (FIG. 7). Three tracers — florbetapir, florbetaben or flutemetamol — have received FDA and EMA approval and are commercially available. In addition, the 11C tracer Pittsburgh compound B has been widely used in research settings. All these tracers measure fibrillar Aβ deposits and give very similar results in clinical practice191,194. International efforts led to the development of a standardized procedure and a common ‘Centiloid’ scale for Aβ-PET tracers197. Aβ-PET and CSF Aβ42 are closely but inversely correlated198,199. Changes in CSF Aβ42 may precede elevations in Aβ-PET, perhaps because PET cannot detect Aβ species such as oligomers or diffuse plaque Aβ.
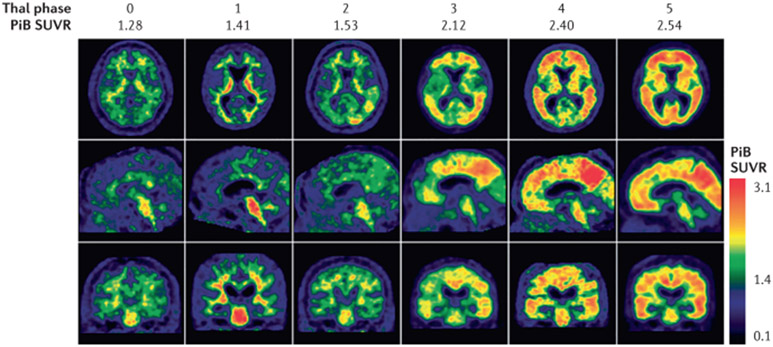
Fig. 7 ∣. Aβ-PET scans closely approximate neuropathology.
Findings from antemortem 11C Pittsburgh Compound B (PiB) scans showing different standardized uptake value ratios (SUVR) of a global region of interest on the last scan prior to death are similar to findings from post-mortem-derived amyloid-β (Aβ) burden as rated on the “Thal phase”. The Thal Aβ staging system284 is a sequence of five levels of Aβ accumulation that reflect the expanding territory occupied by Aβ plaques. The relevant PiB signal is on the cortical surface, whereas binding in the subcortical white matter and brainstem represents the non-specific binding of the tracer. Adapted with permission from REF.196, Oxford University Press.
Quantitative Aβ-PET imaging provides evidence on the extent and location of amyloid deposition, which is its main advantage over CSF-Aβ biomarkers. Although the pattern observed on Aβ-PET does not differ across AD subtypes or clinical phenotypes161, it allows the staging and monitoring of Aβ accumulation and the detection of Aβ in the earliest stages of AD200. Longitudinal Aβ-PET studies demonstrate a very low rate of accumulation of Aβ, with a lag time of 10–20 years between initiation of accumulation and symptomatic cognitive impairment and a deceleration of Aβ accumulation coinciding with the onset of symptomatic disease201. Approximately 20% of persons at 65 years of age and nearly 60% at 85 years have elevated brain Aβ but are cognitively normal202. This demonstrates both the strong effect of age on brain Aβ accumulation and the lack of clinical consequences of elevated Aβ until something else happens, namely the expansion of tauopathy outside of the medial temporal lobe.
Appropriate use criteria have been developed to identify patients most likely to benefit from Aβ-PET for diagnosis203. Aβ-PET imaging is not necessary for many patients with clinically diagnosed dementia due to AD; however, there is evidence for clinical utility in some symptomatic individuals not fulfilling these criteria204,205. With the introduction of tau-PET into clinical research, the Aβ-PET appropriate use criteria need to be revised along with the development of similar criteria for tau-PET.
Several tau-PET ligands have been developed, of which 18F-flortaucipir has been approved by the FDA. Second-generation tracers with an improved signal-to-noise ratio as well as less off-target and lower non-specific binding are available for research purposes206-208. Tau-PET imaging allows the detection of tauopathy that is largely, though not entirely, specific for AD209. The tau-PET binding topography strongly correlates with cognitive performance210 and its regional patterns map onto the different AD clinical phenotypes77,78 (FIG. 8). There is a close correspondence between the regional accumulation of a tau-PET tracer and FDG hypometabolism. Tau-PET abnormalities are highly predictive of subsequent cognitive decline in both asymptomatic and symptomatic individuals211-213 and sensitive to the regional extension of tauopathy over time158. Whereas Aβ begins to accumulate 10–20 years prior to cognitive symptoms and then shows a reduced rate of accumulation around the time of symptom onset201, tau accumulates in the temporal and parietal isocortex at a time much more proximate to cognitive impairment and continues to accumulate in parallel with disease progression77,214,215. It is very uncommon to encounter a patient with substantial tau accumulation outside of the medial temporal lobe who does not have cognitive impairment209.
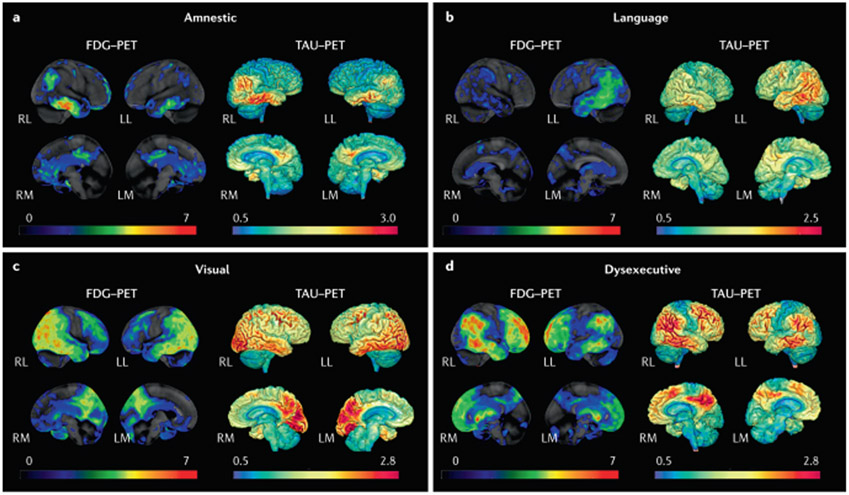
Fig. 8 ∣. Tau-PET and FDG-PET patterns in different clinical syndromes in persons with high β-amyloid-PET.
a ∣ Typical amnestic-predominant Alzheimer disease with temporoparietal hypometabolism and tauopathy. b ∣ Language syndrome (also known as logopenic variant primary progressive aphasia) with a highly asymmetric pattern in which hypometabolism and tauopathy are highly left hemisphere predominant. c ∣ Visual syndrome (also known as posterior cortical atrophy) with a pattern of hypometabolism and tauopathy that is posterior temporal, parietal and occipital lobar in distribution. d ∣ Dysexecutive syndrome with temporal, parietal and prominent frontal hypometabolism and tauopathy. Red colour on FDG-PET indicates greater hypometabolism, whereas red colour on tau-PET indicates a higher intensity of tracer retention. LL, left lateral; LM, left medial; RL, right lateral; RM, right medial.
CSF biomarkers.
The most validated and widely accepted CSF biomarkers for AD are decreases in Aβ42 (or Aβ42 normalized to Aβ40 or total tau (t-tau)) and increases in phosphorylated tau (p-tau181). These biomarkers are recognized by research guidelines216 for their diagnostic utility217 and are used clinically in many European countries and the USA. Collaborative efforts over the last few years have sought to advance standardization and to improve the technology for CSF analysis218.
Aβ42 is reduced in CSF in individuals with symptomatic AD of any severity and in asymptomatic persons who later develop symptoms. However, there is individual variability in Aβ42 levels in CSF and normalization using CSF Aβ40 or p-tau levels has shown better diagnostic performance compared with Aβ42 alone219,220.
In the A-T-N scheme, p-tau181 is considered a specific biomarker for tau pathology, whereas t-tau is considered a general marker of neurodegeneration. Both p-tau181 and t-tau are typically increased in MCI and dementia due to AD221. Both CSF p-tau181 and t-tau are of little value in the staging of disease severity222. Neuropathological studies have shown that p-tau181 correlates only moderately with neurofibrillary tangles223,224. p-Tau181 strongly correlates with t-tau in CSF and is markedly increased in AD but not in most other neurodegenerative diseases222, supporting the use of p-tau181 as an AD-specific biomarker. p-Tau217 has recently been proposed as a more sensitive alternative to p-tau181 (REFS225,226); p-tau217 is not yet available for clinical practice.
New CSF biomarkers need to be incorporated into a diagnostic or prognostic framework for AD. CSF neurofilament light chain227 will likely emerge as an accepted biomarker for neurodegeneration of diverse aetiologies, and other CSF biomarkers228 on the verge of acceptance include neurogranin229, synaptosome-associated protein 25 (SNAP25) and synaptotagmin 1 (SYT1), although their specificities for AD are yet to be established. Biomarkers for non-AD processes, such as cerebrovascular pathology, α-synuclein and TDP43, will also be of value in establishing diagnostic frameworks for other neurodegenerative disorders and in recognizing co-occurrence in persons with abnormal AD biomarkers.
Blood-based biomarkers.
Blood-based biomarkers for AD are rapidly expanding, although effect sizes and other statistics to distinguish AD or MCI from controls are lower than those for CSF biomarkers (https://www.alzforum.org/alzbiomarker) and more studies are needed to fully understand the links with more validated CSF and PET biomarkers. The development of blood-based biomarkers for Aβ42 (REF.230), p-tau181 (REF.231), p-tau217 (REF.232) and neurofilament light chain233 will greatly expand the capability with which AD as an aetiology can be included or excluded. One blood-based assay assessing the Aβ42/Aβ40 ratio was approved in the USA and in the European Union in 2020 based on a mass spectroscopy assay. This assay closely correlates with Aβ-PET status, with an area under the curve value of 0.88 (95% CI 0.82–0.93)230. Blood-based biomarkers should still be considered as candidate screening tools and not as diagnostic biomarkers234.
Prognosis and rate of progression
Both routine clinical practice and clinical trial experience has shown that the rate of cognitive progression in persons with AD is highly variable235. Variability in the rate of progression may be due to the biology of AD65, the involvement of non-AD pathologies8 or the presence of comorbidities. Age may not be particularly relevant after accounting for comorbidities and life expectancy235. In general, patients with milder degrees of cognitive impairment experience a more gradual decline than those with more advanced impairment236 but, once the severe stages are reached, an apparent plateau may occur.
Although persons with subjective cognitive complaints without objective evidence of cognitive decline have a highly variable prognosis, having subjective cognitive complaints approximately doubles the risk of developing MCI237,238. In one study with long-term follow-up, the transition from subjective memory complaint to MCI took an average of 9.4 years238. In addition, subjective cognitive complaints are associated with higher burdens of imaging changes such as elevated Aβ on PET or hippocampal atrophy associated with AD239,240. Similar to subjective cognitive impairment, clinically diagnosed MCI is associated with a substantially higher risk for progression to dementia than no cognitive impairment within 2 years24. Of note, the risk of progression of MCI is highly variable, even in the more rigorous clinical trial setting241. Some of the risk is predicted by the presence or absence of abnormal AD biomarkers.
Individuals with abnormal Aβ biomarkers who are either cognitively unimpaired or who have MCI242,243 are at increased risk for cognitive decline compared with persons with normal Aβ biomarker levels, especially if they also have an abnormal neurodegeneration biomarker, an abnormal tau-PET biomarker244,245 or elevated CSF tau levels243,246. For example, 6 years after a CSF profile with both abnormal Aβ and tau, ~80% of cognitively normal individuals had developed MCI or dementia (HR 33.8; 95% CI 6.1–187)246. In individuals with MCI, those with elevated Aβ by PET have an increased risk of progression to dementia (HR 2.6; 95% CI 1.3–5.3)242. APOE genotypes are also associated with the risk of progression, either slowing in those with the APOE ε2 allele247 or accelerating in those with the APOE ε4 allele248. In individuals with dominantly inherited AD, CSF tau species at different phosphorylation states are associated with different stages of disease225.
Prevention
Although there are no proved pharmacological nor non-pharmacological249-252 approaches for the prevention of cognitive impairment due to AD, there are grounds for optimism that multidimensional interventions that involve exercise, lifestyle changes and cognitive stimulation253, combined with focused attention on other modifiable behaviours or conditions, might delay the onset of overt cognitive impairment. Lifestyle and medical strategies, if instituted at least by midlife, may reduce the burden of cerebrovascular disease-related brain injury254. A trial of aggressive blood pressure reduction in persons who were thought to be cognitively unimpaired at enrolment across the age range of 50 years and older (including 28% who were over the age of 75 years) resulted in a lower rate of incident MCI and dementia, although the safety of targeting a systolic blood pressure of 120 mmHg in older persons is debated255. A reduction in the total burden of brain disease of any aetiology in cognitively eloquent brain regions would increase the amount of AD-related pathology needed to produce symptoms and, through that indirect mechanism, delay symptomatic disease due to AD.
Management
The majority of care and management of patients with MCI and dementia due to AD (as well as most all-cause MCI and dementia) occurs in the outpatient setting whilst patients are still living in the community. Management begins from the first moment of a clinician’s interaction with patients and their families. Much of the burden of the disease falls on family caregivers. For most families, having a family member with cognitive impairment is a novel experience. Compassion, patience and a lack of condescension are critical to establishing rapport, trust and realistic expectations. For many, explicit training in this new role may be helpful. Supporting caregivers is a major mission of the Alzheimer’s Association in the USA and its sister organizations in other countries. As there are many uncertainties on how best to deliver caregiver support, there are ongoing pragmatic trials of the best way to deliver formal care for patients with dementia256.
Acknowledgements of the diagnosis on the part of the family is a critical step before more sophisticated treatment interventions can be introduced. Many patients with MCI or dementia due to AD have a profound loss of insight into their own deficits and limitations. The brunt of the diagnostic disclosure may fall on the family caregiver, usually a spouse, adult child or a sibling. As each patient and their family caregivers differ in levels of sophistication and in emotional preparedness, the disclosure of the diagnosis must be tailored to the situation257. The introduction of biomarkers into the diagnostic process has made the delivery of the diagnosis of MCI or dementia more challenging258 because distinctions between syndromes and aetiologies become more complex and may be unfamiliar to laypersons.
Patients with MCI to moderate dementia due to AD should be encouraged to be as socially, mentally and physically active and engaged as possible. As diet has not been shown to affect the course of symptomatic cognitive impairment in AD, eating a ‘heart-healthy’ diet can be encouraged without specific recommendations for foods to eat or to avoid. In addition, rather than systematic group interventions like cognitive stimulation, individualized interventions should be preferred as they may delay institutionalization259.
Treatments for comorbidities
Many patients with cognitive impairment due to AD have comorbidities that can exacerbate cognitive dysfunction and worsen the performance of daily activities; of note, many comorbidities may not be clinically recognized. Patients with concomitant depression or anxiety may benefit from the use of pharmacological interventions. Treating depression and anxiety in persons with MCI or dementia due to AD differs from the treatment of the general population as drugs with an anti-cholinergic pharmacology should be avoided and lower doses of psychoactive drugs should be used. Optimizing medication regimens should include minimizing other psychoactive medications unless clearly indicated. For example, avoiding medications that induce alterations in gait and balance is a paramount consideration. Antidepressants such as citalopram or sertraline may be effective for both anxiety and depression and can be used safely in patients with cognitive impairment.
Comorbid sleep disorders, such as obstructive sleep apnoea, should be treated using oral appliances or nasal devices that create expiratory positive airway pressure; patients with dementia may have difficulty adapting to sleeping with a mask. Hearing loss is common in patients with AD and can exacerbate short-term memory problems. Hearing loss represents a management challenge in persons with MCI or dementia because the small and expensive hearing aids require careful adjustments that may be challenging for patients. Misplacing and losing the devices is what usually terminates attempts to employ hearing aids in persons with cognitive impairment. In addition, visual loss in persons with cognitive impairment, except in persons with the posterior cortical atrophy syndrome, represents an unrelated but confounding disability, the management of which is made more difficult by the impaired memory and judgment of persons with dementia.
Comorbid gait and balance disorders in persons with presumed AD should raise questions about alternative diagnoses such as Lewy body disease, normal pressure hydrocephalus or cerebrovascular disease. However, gait and balance disorders in older persons can occur due to orthopaedic or neurological diseases that are unrelated to the cognitive disorder such as peripheral neuropathy or spine disease. As with sensory losses, the compounding effects of a gait and balance disorder and impaired memory and judgment present vexing management challenges for which common-sense interventions offer benefit260.
The treatment of pain in persons with cognitive impairment is challenging owing to the difficulties that persons with dementia have in describing their pain. Further, as many potent analgesic agents may cause sedation or reduced attentional abilities, there are substantial limitations on the use of medications beyond acetaminophen and NSAIDs. In patients with more than moderate dementia, a pain condition may present with agitation and irritability, whilst the cause of the pain remains covert.
Pharmacological approaches for AD
Pharmacological approaches that are specific to AD are limited to three cholinesterase inhibitors (donepezil, rivastigmine and galantamine) and the NMDA receptor antagonist memantine. The cholinesterase inhibitors are approved in the USA and Europe for mild to moderate dementia due to AD and are not approved for persons with MCI; donepezil is also approved for severe dementia only in the USA. In clinical trials, these drugs have consistently shown modest benefits in providing a delay in symptom progression by 6 months or slightly longer250,261. The adverse effects of the cholinesterase inhibitors include nausea, vomiting, loose stools or loss of appetite in a minority of individuals and, less commonly, muscle cramps, headaches and unpleasant dreams. Memantine is approved in the USA only for moderate to severe dementia due to AD; its effects are also rather modest250. The adverse effects of memantine are minor. Neither the cholinesterase inhibitors or memantine have any relevant effects on the underlying biology of AD.
Up until 2020, clinical trials of anti-Aβ agents of a variety of different mechanisms had failed to produce any benefits262 (BOX 1). One drug, aducanumab (a monoclonal antibody that targets Aβ protofibrils), is being considered by the FDA and the EMA for AD based on the results of two phase III clinical trials263. Claims of efficacy for aducanumab were made based on a slowing of decline on the Clinical Dementia Rating scale but are complicated because the trials were terminated prematurely for futility; however, additional data review found evidence supporting an efficacy claim. Donanemab264, which targets plaque Aβ, reported both a lowering of aggregated Aβ and a reduction in the rate of cognitive and functional decline in a phase II study; a larger phase III trial (NCT#04437511) is under way. Another monoclonal antibody, lecanemab265 (which targets Aβ oligomer protofibrils) is also in late-stage trials (NCT#03887455). Several other therapies are in various stages of clinical development such as drugs directed against tau accumulation or spread in later-phase development266. Two secondary prevention studies for AD are ongoing, both with passive Aβ immunization; neither the API study267 nor the A4 study268 will report results until at least 2022.
Box 1 ∣. Anti-Aβ antibody agents: future role still uncertain.
The status of amyloid-β (Aβ)-lowering agents as therapies for AD has been a matter of vigorous debate for several years as a result of repeated failures. In 2019, the drug aducanumab gained attention due to the claims of its sponsor that, in at least one of its two pivotal trials, benefits were seen263. As of this writing, the regulatory status of aducanumab has not been clarified. If one were to focus on the one trial that showed benefits on the Clinical Dementia Rating Scale, it was the degree of Aβ lowering and the target of the drug that led to success. On the other hand, if one were to view the two studies together and draw the conclusion that there were minimal to no clinical benefits from high-dose aducanumab, then one could conclude the opposite. That is, neither targeting oligomeric/protofibrillar Aβ nor the degree of Aβ lowering were sufficient. Into this contentious debate, the report that the drug donanemab demonstrated significant reductions in the rate of decline on a combined cognitive and functional scale264 has added to the complexity of the determination of the clinical benefit of anti-Aβ therapies. In contrast to aducanumab, donanemab targeted aggregated Aβ species found in plaques. In addition, while aducanumab had a dramatic effect on Aβ lowering, donenamab was so effective in lowering Aβ that the drug was discontinued in many participants during the trial because their Aβ levels by PET fell essentially to zero; however, the clinical impact of donanemab was very small. As a replication study of donanemab is under way, a discussion of the clinical meaningfulness of the effect size of donanemab will have to await its outcome. In addition, the outcome of the phase III trial of lecanemab265 will also serve to inform the field on whether Aβ-lowering strategies will be endorsed as clinically meaningful.
Behavioural dyscontrol in persons with dementia due to AD, usually in the moderate to severe stages, is a particular challenge to manage. Frightening hallucinations, delusions that lead to socially disruptive behaviours or physically aggressive behaviours will invariably require pharmacological intervention. Pimavanserin, a selective serotonin inverse agonist269, is currently approved in the USA and Europe for Parkinson disease dementia psychosis but it is being examined in the USA for a broader indication to include dementia-related psychosis in general. Pimavanserin may be the only medication to gain a specific indication for the treatment of behaviours collectively referred to as agitation, namely frightening hallucinations and delusional thinking, physically aggressive behaviours and other socially disruptive behaviours. Atypical antipsychotics, such as quetiapine, are generally the main-stays of treatment for agitation in patients with dementia. However, this class of drugs is associated with a risk of increased all-cause mortality. Other antipsychotics that may be less sedating have a risk for the development of extrapyramidal signs. Drug-induced parkinsonism in persons with dementia is an unacceptable consequence of attempts to control agitation.
Quality of life
The degree of cognitive impairment in AD has a dramatic effect on the patient’s desires and abilities to engage in some activities. The severity of cognitive impairment and the pattern of cognitive domain losses are inversely related to the capability of insight on the part of the patient. Giving up previously enjoyed pastimes and hobbies may be less distressing for patients than it is for family caregivers who observe that change in behaviour. In fact, patient and caregiver goals are often divergent270.
MCI and dementia are a family affair and quality of life is as much of an issue for the primary family caregiver as it is for the patient. Even if a potent therapy were available, the changes in the daily life of a patient with cognitive impairment and their family can take an emotional toll and affect their quality of life. The stress of a diagnosis of MCI or dementia may be especially high in families in which the patient is under the age of 65 years and who had been working or had dependent children still at home271. Care of the caregiver will always be as important as care of the patient. The quality of life of both patient and caregiver is affected by several factors such as other comorbidities, physical limitations, hearing limitations, visual limitations, mood disorders, pain disorders and sleep disorders. Some of these are modifiable or manageable. The quality of life of a patient and caregiver is also affected by unrelated culture and environmental factors such as the composition of the family unit, the physical amenities or challenges of the place of residence, the family financial situation, or the health of key family members on whom the patient may depend. Although health-care providers may not have the time or expertise to provide social care consultations and such services are not available in all communities, volunteer organizations such as the Alzheimer’s Association and the Alzheimer’s Disease International can be a resource for providing consultation to families of persons with dementia. Interventions for patients and their families can be of value in improving a sense of well-being of both parties272.
Patients with AD and their families have several key encounters with medical professionals over the course of the illness. Both the explicit message and the implicit comfort and empathy that those encounters convey can have a major effect on the sense of well-being of the patient and their family. When the diagnosis is rendered for the first time, it can be very stressful257. It is never easy to give a life-changing diagnosis but doing so with empathy, patience and a touch of optimism can make a huge difference for families. An honest discussion of therapeutic options and lifestyle activities that enhance the quality of life of the patient and family is deeply appreciated, although sometimes that discussion needs to take place after the implications of the diagnosis can be digested by the family and patient. Some but not all families and patients perceive that there is a stigma associated with a diagnosis of cognitive impairment, dementia or AD. Discouraging such a belief must originate with the health-care provider and the equanimity about the diagnosis that they project. Another difficult point for patient and family occurs around life-changing events such as the need to leave employment, to stop driving or to move out of one’s home and into some other living arrangement. The decision to place a patient in a residential care facility is a particularly difficult time. Additionally, of course, end-of-life decisions will have to be faced. Health-care professionals can make these passages through the saga of dementia much less painful by offering empathy, knowledge, validation of the caregiver’s decision-making and perhaps sometimes a little nudge to make a painful decision.
Outlook
Managing cognitive impairment due to AD is an issue of colossal scale and breadth, including basic cell biology, systems biology, clinical practice, human therapeutics and population-health implementation science. In clinical care, the timely recognition of symptomatic cognitive impairment remains an obstacle to quality care. In all countries, expertise in cognitive disorders at the primary care level could be improved substantially. New approaches to timely diagnosis using innovative technologies, such as smartphones and virtual medical encounters, might be transformative and create more efficient but informative clinical assessments. Although clinical diagnosis will always rely on direct interactions between clinicians, patients and family caregivers, various technologies may provide objective documentation of changes in a patient’s behaviour that had escaped detection by family caregivers. For example, social withdrawal is a common accompaniment of changes in cognitive function. Devices that measure mobility could be used to identify when decreases in activities outside the home began. Of course, such technology-derived information would need to be validated against human observations.
The last decade has seen much effort into prevention strategies for dementia using low-cost available techniques and technologies such as diet, exercise, cognitive training and attention to vascular risk factor mitigation. The FINGER study253 is unique in its design and its claims of success compared to other multidimensional intervention programmes. This study recruited persons with lower ratings of vascular health, thereby enriching the trial with persons likely to benefit from interventions for lifestyle changes, diet and exercise. Lessons learned from the unsuccessful trials273-275 should enable researchers to choose the right participants and develop the right intervention strategies to improve upon the FINGER study paradigm. The translation of interventions suitable for highly motivated research subjects into strategies for persons who have less health-conscious world-views must also be part of implementing non-pharmacological interventions. The development of strategies that enable longer periods of follow-up for interventions of all types but especially non-pharmacological ones should be a goal for the field. There are practical and ethical limitations on the duration of randomized controlled trials; therefore, alternative trial designs that minimize the loss of scientific rigour are needed.
Acceptance of the multi-aetiologic nature of later-life cognitive impairment must be recognized, although it increases the complexity of defining the relative contributions of individual pathophysiologies6. Blood-based biomarkers for AD, vascular injury, TDP43, α-synuclein and synaptic markers will dramatically improve aetiological characterization and assist in phenotyping patients in ways that might be therapeutically relevant. In addition, identifying effective treatments for cognitive impairment will also help to identify the aetiological relevance and will create the impetus throughout the health-care delivery system to become more proactive in diagnosing and treating cognitive impairment due to AD. Ongoing and future work in genomics276, proteomics277 and lipidomics278 must be pursued to help clarify promising targets for therapy. In addition, discovery at the interface of clinical AD and basic science should be directed to biological variables that substantially affect the rate of disease progression. Basic laboratory science in AD has expanded our knowledge dramatically and perhaps the critical target that will change how we approach AD therapeutics has already been reported. Rodent models of AD have not proved to be good predictors of success in humans279 and therefore new model systems need to be developed such as induced pluripotent stem cells280 or three-dimensional culture systems278.
A major bottleneck in the process of discovering effective therapeutics is the performance of clinical trials. As of 2020, our technology has not substantially improved upon a trial design that requires a large fraction of participants to receive a placebo, a minimum of 18 months per person, hundreds if not a thousand persons per treatment arm and clinical outcome measures that are noisy. Until the field can qualify a surrogate disease biomarker that reduces the number of participants and can find techniques to shorten the time to an efficacy readout, the process of working through a very small number of promising agents per year worldwide is far too slow.
Supplementary Material
Acknowledgements
The authors acknowledge research support from NIH (D.S.K. and R.C.P, P30 AG062677 and U01 AG006786; B.T.H., P30AG062421; R.A.N. P01 AG017617 and R01 AG062376).
Footnotes
Competing interests
D.S.K. served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety Monitoring Board for a tau therapeutic for Biogen but receives no personal compensation. He is a site investigator in a Biogen aducanumab trial. He is an investigator in a clinical trial sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche and Alzeca Biosciences but receives no personal compensation. He receives research support from the NIH. G.C. serves on the Scientific Advisory Board of the Fondation Vaincre Alzheimer but receives no personal compensation. She receives personal fees from Fondation d’entreprise MMA des Entrepreneurs du Futur and from Fondation Alzheimer as she serves in the Operational Committee. She receives research support from European Union Horizon 2020 research and innovation programme (grant agreement number 667696), Inserm, Fondation d’entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer et maladies apparentées and Fondation Vaincre Alzheimer. R.C.P. is a consultant for Biogen, Inc., Roche, Inc., Merck, Inc., Genentech Inc. and Eisai, Inc., has given educational lectures for GE Healthcare, receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), UpToDate, and receives research support from the NIH. B.T.H. has a family member who works at Novartis and owns stock in Novartis; he serves on the SAB of Dewpoint and owns stock. He serves on a scientific advisory board or is a consultant for Biogen, Novartis, Cell Signalling, the US Dept of Justice, Takeda, Vigil, W20 group and Seer. His laboratory is supported by sponsored research agreements with Abbvie, F Prim, and research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, Brightfocus and the JPB Foundation. H.A. serves on the Scientific Advisory Board of the Observatoire des Mémoires but receives no personal compensation. She receives research support from Spoelberch Foundation, Association France Alzheimer et maladies apparentées, the Regional Health Agency of Aquitaine and National Research Agency. D.M.H. reports being a Co-founder for C2N Diagnostics LLC and participating in scientific advisory boards/consulting for Genentech, C2N Diagnostics, Denali, Merck and Idorsia. He is an inventor on patents licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies (this anti-tau antibody programme is licensed to Abbvie) and to Eli Lilly on the therapeutic use of an anti-amyloid-β antibody. His laboratory receives research grants from the National Institutes of Health, Cure Alzheimer’s Fund, Tau Consortium, the JPB Foundation, Good Ventures, Centene, BrightFocus and C2N Diagnostics. All other authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41572-021-00269-y.
References
- 1.Jessen F et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10, 844–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC Mild cognitive impairment as a diagnostic entity. J. Intern. Med 256, 183–194 (2004). [DOI] [PubMed] [Google Scholar]
- 3.McKhann GM et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 7, 263–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC How early can we diagnose Alzheimer disease (and is it sufficient)? The 2017 Wartenberg lecture. Neurology 91, 395–402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson PT et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121, 571–587 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle PA et al. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann. Neurol 83, 74–83 (2018). A clinical-neuropathological analysis of >1,000 persons demonstrating how multiple aetiologies relate to late-life cognition.
- 7.Schneider JA, Arvanitakis Z, Leurgans SE & Bennett DA The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol 66, 200–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapasi A, DeCarli C & Schneider JA Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karanth S et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 77, 1299–1307 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodaty H et al. The world of dementia beyond 2020. J. Am. Geriatr. Soc 59, 923–927 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Wu YT et al. The changing prevalence and incidence of dementia over time — current evidence. Nat. Rev. Neurol 13, 327–339 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Satizabal CL et al. Incidence of dementia over three decades in the Framingham Heart Study. N. Engl. J. Med 374, 523–532 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11, 1006–1012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tom SE et al. Trends in incident dementia and early life socieoeconomic status by birth cohort in the adult changes in thought study. JAMA Open 3, e2011094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovari E, Herrmann FR, Bouras C & Gold G Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology 82, 326–331 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Niu H, Álvarez-Álvarez I, Guillén-Grima F & Aguinaga-Ontoso I Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia 32, 523–532 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Hy LX & Keller DM Prevalence of AD among whites: a summary by levels of severity. Neurology 55, 198–204 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Gillis C, Mirzaei F, Potashman M, Ikram MA & Maserejian N The incidence of mild cognitive impairment: a systematic review and data synthesis. Alzheimers Dement. 11, 248–256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC et al. Mild cognitive impairment due to Alzheimer’s disease: criteria in the community. Ann. Neurol 74, 199–208 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degenhardt EK et al. Florbetapir F18 PET amyloid neuroimaging and characteristics in patients with mild and moderate Alzheimer dementia. Psychosomatics 57, 208–216 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Robinson JL et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141, 2181–2193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince M et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther 8, 23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Röhr S et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res. Ther 12, 167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen RC et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90, 126–135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen RC et al. Prevalence of mild cognitive impairment is higher in men than in women. The Mayo Clinic Study of Aging. Neurology 75, 889–897 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mielke MM, Vemuri P & Rocca WA Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol 6, 37–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thambisetty M, An Y & Tanaka T Alzheimer’s disease risk genes and the age-at-onset phenotype. Neurobiol. Aging 34, 2696.e1–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haass C, Kaether C, Thinakaran G & Sisodia S Trafficking and proteolytic processing of APP. Cold Spring Harb. Perspect. Med 2, a006270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonsson T et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012). [DOI] [PubMed] [Google Scholar]
- 30.van der Lee SJ et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 17, 434–444 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Bellenguez C et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol. Aging 59, 220.e1–220.e9 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Leonenko G et al. Polygenic risk and hazard scores for Alzheimer’s disease prediction. Ann. Clin. Transl. Neurol 6, 456–465 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karch CM, Cruchaga C & Goate AM Alzheimer’s disease genetics: from the bench to the clinic. Neuron 83, 11–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunkle BW et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet 51, 414–430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livingston G et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 396, 413–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh-Manoux A et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry 74, 712–718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottesman RF et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 74, 1246–1254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samieri C et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 320, 657–664 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottesman RF et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317, 1443–1450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vemuri P et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 138, 761–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golde TE, DeKosky ST & Galasko D Alzheimer’s disease: the right drug, the right time. Science 362, 1250–1251 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Herrup K The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci 18, 794–799 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Zlokovic BV et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 16, 1714–1733 (2020). [DOI] [PubMed] [Google Scholar]
- 44. Arnold SE, Hyman BT, Flory J, Damasio AR & Van Hoesen GW The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1, 103–116 (1991). Graphic demonstration of the different regional distributions of Aβ and tau at autopsy.
- 45.Montine TJ et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 123, 1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haass C & Selkoe DJ Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Holtzman DM, Morris JC & Goate AM Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med 3, 77sr71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thinakaran G & Koo EH Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem 283, 29615–29619 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haass C & Willem M Secreted APP modulates synaptic activity: a novel target for therapeutic intervention? Neuron 101, 557–559 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Cirrito JR et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48, 913–922 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Kang JE et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boespflug EL & Iliff JJ The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-β, and sleep. Biol. Psychiatry 83, 328–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spires-Jones TL & Hyman BT The intersection of amyloid β and tau at synapses in Alzheimer’s disease. Neuron 82, 756–771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benarroch EE Glutamatergic synaptic plasticity and dysfunction in Alzheimer disease: emerging mechanisms. Neurology 91, 125–132 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Rice HC et al. Secreted amyloid-β precursor protein functions as a GABA(B)R1a ligand to modulate synaptic transmission. Science 363, eaao4827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Müller UC, Deller T & Korte M Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci 18, 281–298 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Small SA & Petsko GA Endosomal recycling reconciles the Alzheimer’s disease paradox. Sci. Transl. Med 12, eabb1717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallardo G & Holtzman DM Amyloid-β and tau at the crossroads of Alzheimer’s disease. Adv. Exp. Med. Biol 1184, 187–203 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Pooler AM, Noble W & Hanger DP A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 76(Pt A), 1–8 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Eftekharzadeh B et al. Tau protein disrupts nucleocytoplasmic transport in Alzheimer’s disease. Neuron 99, 925–940.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kent SA, Spires-Jones TL & Durrant CS The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 140, 417–447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada K et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med 211, 387–393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu JW et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci 19, 1085–1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Calignon A et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dujardin S et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med 26, 1256–1263 (2020). A clinical-neuropathological-molecular demonstration of how post-translational modifications of tau might play a role in the rate of clinical progression.
- 66.Shi Y et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gratuze M et al. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Invest 130, 4954–4968 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med 216, 2546–2561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braak H, Thal DR, Ghebremedhin E & Del Tredici K Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Braak H & Braak E Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- 71.Delacourte A et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52, 1158–1165 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Price JL & Morris JC Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol 45, 358–368 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Delacourte A et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp. Gerontol 37, 1291–1296 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Crary JF et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jack CR et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain 142, 3230–3242 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanseeuw BJ et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 76, 915–924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ossenkoppele R et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graff-Radford J et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20, 222–234 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Basak JM & Holtzman DM The role of apolipoprotein E in Alzheimer’s disease. Neuron 63, 287–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huynh TV, Davis AA, Ulrich JD, Holtzman DM & Apolipoprotein E and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res 58, 824–836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reiman EM et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl Acad. Sci. USA 106, 6820–6825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jack CR Jr. et al. Age, sex, and APOE e4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 72, 511–519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Kant R, Goldstein LSB & Ossenkoppele R Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci 21, 21–35 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Small SA & Duff K Linking Aβ and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron 60, 534–542 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Busche MA & Hyman BT Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci 23, 1183–1193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurt MA, Davies DC, Kidd M, Duff K & Howlett DR Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol. Dis 14, 89–97 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Le R et al. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J. Neuropathol. Exp. Neurol 60, 753–758 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Leyns CEG et al. TREM2 function impedes tau seeding in neuritic plaques. Nat. Neurosci 22, 1217–1222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Small SA, Simoes-Spassov S, Mayeux R & Petsko GA Endosomal traffic jams represent a pathogenic hub and therapeutic target in Alzheimer’s disease. Trends Neurosci. 40, 592–602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nixon RA & Yang DS Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol. Dis 43, 38–45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palop JJ & Mucke L Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci 17, 777–792 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Terry RD et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol 30, 572–580 (1991). [DOI] [PubMed] [Google Scholar]
- 93. DeKosky ST & Scheff SW Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol 27, 457–464 (1990). A real-time demonstration of a close correspondence between synapse loss and degree of cognitive impairment in brain biopsies from patients with AD.
- 94.Spires TL et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci 25, 7278–7287 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arbel-Ornath M et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener 12, 27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou L et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun 8, 15295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masliah E, Hansen L, Albright T, Mallory M & Terry RD Immunoelectron microscopic study of synaptic pathology in Alzheimer’s disease. Acta Neuropathol. 81, 428–433 (1991). [DOI] [PubMed] [Google Scholar]
- 98.Henstridge CM et al. Post-mortem brain analyses of the Lothian Birth Cohort 1936: extending lifetime cognitive and brain phenotyping to the level of the synapse. Acta Neuropathol. Commun 3, 53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lleo A et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s Disease cerebrospinal fluid. Mol. Cell Proteom 18, 546–560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeVos SL et al. Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease brain. Front. Neurosci 12, 267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshiyama Y et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Keskin AD et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl Acad. Sci. USA 114, 8631–8636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Busche MA et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321, 1686–1689 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Kuchibhotla KV et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59, 214–225 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menkes-Caspi N et al. Pathological tau disrupts ongoing network activity. Neuron 85, 959–966 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Busche MA et al. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci 22, 57–64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson KJ, Scheff SW & DeKosky ST Reactive synaptogenesis in hippocampal area CA1 of aged and young adult rats. J. Comp. Neurol 252, 374–384 (1986). [DOI] [PubMed] [Google Scholar]
- 108.Geddes JW & Cotman CW Plasticity in hippocampal excitatory amino acid receptors in Alzheimer’s disease. Neurosci. Res 3, 672–678 (1986). [DOI] [PubMed] [Google Scholar]
- 109.Hyman BT, Kromer LJ & Van Hoesen GW Reinnervation of the hippocampal perforant pathway zone in Alzheimer’s disease. Ann. Neurol 21, 259–267 (1987). [DOI] [PubMed] [Google Scholar]
- 110.Liu L et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7, e31302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rauch JN et al. LRP1 is a master regulator of tau uptake and spread. Nature 580, 381–385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kounnas MZ et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 82, 331–340 (1995). [DOI] [PubMed] [Google Scholar]
- 113.Rebeck GW, Reiter JS, Strickland DK & Hyman BT Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 11, 575–580 (1993). [DOI] [PubMed] [Google Scholar]
- 114.Wilton DK, Dissing-Olesen L & Stevens B Neuron-glia signaling in synapse elimination. Annu. Rev. Neurosci 42, 107–127 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Nixon RA The role of autophagy in neurodegenerative disease. Nat. Med 19, 983–997 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Menzies FM et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93, 1015–1034 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Nixon RA Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J. 31, 2729–2743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shehata M et al. Autophagy enhances memory erasure through synaptic destabilization. J. Neurosci 38, 3809–3822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glatigny M et al. Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol. 29, 435–448.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Cataldo AM et al. Endocytic pathway abnormalities precede amyloid β deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol 157, 277–286 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Acker ZP, Bretou M & Annaert W Endo-lysosomal dysregulations and late-onset Alzheimer’s disease: impact of genetic risk factors. Mol. Neurodegener 14, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suire CN et al. Cathepsin D regulates cerebral Aβ 42/40 ratios via differential degradation of Aβ42 and Aβ40. Alzheimers Res. Ther 12, 80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kwart D et al. A large panel of isogenic APP and PSEN1 mutant human iPSC neurons reveals shared endosomal abnormalities mediated by APP β-CTFs, not Aβ. Neuron 104, 256–270.e5 (2019). Evidence from induced pluripotent stem cells on the critical role of endosomal dysregulation in the pathogenesis of Aβ and tau-induced disease.
- 124.Pensalfini A et al. Endosomal dysfunction induced by directly over-activating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer’s disease. Cell Rep. 33, 108420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lauritzen I et al. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol. 132, 257–276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee JH et al. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12, 1430–1444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee JH et al. β2-adrenergic agonists rescue lysosome acidification and function in PSEN1 deficiency by reversing defective ER-to-lysosome delivery of ClC-7. J. Mol. Biol 432, 2633–2650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morel N & Poea-Guyon S The membrane domain of vacuolar H+ATPase: a crucial player in neurotransmitter exocytotic release. Cell. Mol. Life Sci 72, 2561–2573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Higashida H, Yokoyama S, Tsuji C & Muramatsu SI Neurotransmitter release: vacuolar ATPase V0 sector c-subunits in possible gene or cell therapies for Parkinson’s, Alzheimer’s, and psychiatric diseases. J. Physiol. Sci 67, 11–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Far O & Seagar M A role for V-ATPase subunits in synaptic vesicle fusion? J. Neurochem 117, 603–612 (2011). [DOI] [PubMed] [Google Scholar]
- 131.Peng KY et al. Apolipoprotein E4 genotype compromises brain exosome production. Brain 142, 163–175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu RQ et al. Membrane localization of β-amyloid 1-42 in lysosomes: a possible mechanism for lysosome labilization. J. Biol. Chem 285, 19986–19996 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morishita H & Mizushima N Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol 35, 453–475 (2019). [DOI] [PubMed] [Google Scholar]
- 134.Levine B & Kroemer G Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bordi M et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12, 2467–2483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moreira PI, Carvalho C, Zhu X, Smith MA & Perry G Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 1802, 2–10 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Nixon RA The aging lysosome: an essential catalyst for late-onset neurodegenerative diseases. Biochim. Biophys. Acta Proteins Proteom 1868, 140443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whyte LS, Lau AA, Hemsley KM, Hopwood JJ & Sargeant TJ Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease? J. Neurochem 140, 703–717 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Pensalfini A et al. Intracellular amyloid and the neuronal origin of Alzheimer neuritic plaques. Neurobiol. Dis 71, 53–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Adalbert R et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 132, 402–416 (2009). [DOI] [PubMed] [Google Scholar]
- 141.Nixon RA & Yang DS Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb. Perspect. Biol 4, a008839 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nakanishi H Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regenerate Res. 15, 25–29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lowry JR & Klegeris A Emerging roles of microglial cathepsins in neurodegenerative disease. Brain Res. Bull 139, 144–156 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Sarlus H & Heneka MT Microglia in Alzheimer’s disease. J. Clin. Invest 127, 3240–3249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deretic V, Saitoh T & Akira S Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol 13, 722–737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cho MH et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10, 1761–1775 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jay TR et al. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J. Neurosci 37, 637–647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lewcock JW et al. Emerging microglia biology defines novel therapeutic approaches for Alzheimer’s disease. Neuron 108, 801–821 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Kim HJ et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 22, 1576–1584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saido T & Leissring MA Proteolytic degradation of amyloid β-protein. Cold Spring Harb. Perspect. Med 2, a006379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rasmussen MK, Mestre H & Nedergaard M The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Buckner RL, Andrews-Hanna JR & Schacter DL The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci 1124, 1–38 (2008). Demonstration of the relationship between the default mode network and amyloid accumulation.
- 153.Greicius MD, Srivastava G, Reiss AL & Menon V Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101, 4637–4642 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buckner RL et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimers disease. J. Neurosci 29, 1860–1873 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Agosta F et al. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol. Aging 33, 1564–1578 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Whitwell JL et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. Neurobiol. Aging 36, 1245–1252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Townley RA et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2, fcaa068 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Franzmeier N et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat. Commun 11, 347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sintini I et al. Tau and amyloid relationships with resting-state functional connectivity in atypical Alzheimer’s disease. Cereb. Cortex 10.1093/cercor/bhaa319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jones DT et al. Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex 97, 143–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sepulcre J et al. Hierarchical organization of tau and amyloid deposits in the cerebral cortex. JAMA Neurol. 74, 813–820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jack CR Jr. et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81, 1732–1740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Protas HD et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 70, 320–325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Franzmeier N et al. Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Sci. Adv 6, eabd1327 (2020). Analysis showing how regional tau expansion follows connectivity patterns.
- 165.D’Onofrio G et al. Neuropsychiatric symptoms and functional status in Alzheimer’s disease and vascular dementia patients. Curr. Alzheimer Res 9, 759–771 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Lehmann M et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136, 844–858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Crutch SJ et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 13, 870–884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gorno-Tempini ML et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 71, 1227–1234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bergeron D et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann. Neurol 84, 729–740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ossenkoppele R et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 138, 2732–2749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murray ME et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10, 785–796 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Clarfield AM The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch. Intern. Med 163, 2219–2229 (2003). [DOI] [PubMed] [Google Scholar]
- 173.Beach TG, Monsell SE, Phillips LE & Kukull W Accuracy of the clinical diagnosis of Alzheimer disease at national institute on aging Alzheimer disease centers, 2005-2010. J. Neuropathol. Exp. Neurol 71, 266–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McKeith IG et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89, 88–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rascovsky K et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gorno-Tempini ML et al. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Botha H et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain 141, 1201–1217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Petersen RC & Yaffe K Issues and questions surrounding screening for cognitive impairment in older patients. JAMA 323, 722–724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Patnode CD et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services TaskFforce. JAMA 323, 764–785 (2020). [DOI] [PubMed] [Google Scholar]
- 180.Jack CR Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chetelat G et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer”s disease and other dementias. Lancet Neurol. 19, 951–962 (2020). [DOI] [PubMed] [Google Scholar]
- 182.Klunk WE et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol 55, 306–319 (2004). [DOI] [PubMed] [Google Scholar]
- 183.Jack CRJ et al. Introduction to revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer Association Workgroups. Alzheimer’s Dement. 7, 257–262 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Knopman DS et al. Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol. Aging 46, 32–42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schröder J & Pantel J Neuroimaging of hippocampal atrophy in early recognition of Alzheimer’s disease–a critical appraisal after two decades of research. Psychiatry Res. Neuroimaging 247, 71–78 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Petersen C et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. 138, 597–612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ridha BH et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 5, 828–834 (2006). [DOI] [PubMed] [Google Scholar]
- 188.Greenberg SM et al. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat. Rev. Neurol 16, 30–42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Graff-Radford J et al. Cerebral microbleeds: prevalence and relationship to amyloid burden. Neurology 92, e253–e262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li X et al. The significant effects of cerebral microbleeds on cognitive dysfunction: an updated meta-analysis. PLoS ONE 12, e0185145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Laforce R Jr. et al. Parallel ICA of FDG-PET and PiB-PET in three conditions with underlying Alzheimer’s pathology. Neuroimage Clin. 4, 508–516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Caroli A et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology 84, 508–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Iaccarino L, Sala A & Perani D Predicting long-term clinical stability in amyloid-positive subjects by FDG-PET. Ann. Clin. Transl. Neurol 6, 1113–1120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Villemagne VL, Doré V, Burnham SC, Masters CL & Rowe CC Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol 14, 225–236 (2018). [DOI] [PubMed] [Google Scholar]
- 195.Clark CM et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678 (2012). [DOI] [PubMed] [Google Scholar]
- 196.Murray ME et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138, 1370–1381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klunk WE et al. The centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 11, 1–15.e1-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Landau SM et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol 74, 826–836 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leuzy A et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain 139, 2540–2553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Grothe MJ et al. In vivo staging of regional amyloid deposition. Neurology 89, 2031–2038 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jagust WJ & Landau SM Temporal dynamics of β-amyloid accumulation in aging and Alzheimer’s disease. Neurology 96, e1347–e1357 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jack CR Jr. et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using THE National Institute on Aging-Alzheimer’s association research framework. JAMA Neurol. 76, 1174–1183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Johnson KA et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the Alzheimer’s association. J. Nucl. Med 54, 476–490 (2013). [DOI] [PubMed] [Google Scholar]
- 204. Rabinovici GD et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321, 1286–1294 (2019). A very large pragmatic trial of the value of Aβ imaging in clinical dementia practice.
- 205.de Wilde A et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 75, 1062–1070 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jeong HJ et al. [18F]THK5351 PET imaging in patients with mild cognitive impairment. J. Clin. Neurol 16, 202–214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pascoal TA et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143, 2818–2830 (2020). [DOI] [PubMed] [Google Scholar]
- 208.Leuzy A et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 77, 955–965 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ossenkoppele R et al. Discriminative accuracy of [18F] flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA 320, 1151–1162 (2018). Demonstration of the remarkable specificity of an elevated tau PET signal outside of the medial temporal lobe for persons with elevated Aβ.
- 210.Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC & Hassenstab JJ Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91, e859–e866 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brier MR et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med 8, 338ra366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Harrison TM et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann. Neurol 85, 229–240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lu M et al. Aggregated tau measured by visual interpretation of flortaucipir positron emission tomography and the associated risk of clinical progression of mild cognitive impairment and Alzheimer disease: results from 2 phase III clinical trials. JAMA Neurol. 78, 445–453 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lowe VJ et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141, 271–287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pontecorvo MJ et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140, 748–763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shaw LM et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 14, 1505–1521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Molinuevo JL et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 136, 821–853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hansson O et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 1, 30029–30023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hansson O et al. Prediction of Alzheimer’s disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord 23, 316–320 (2007). [DOI] [PubMed] [Google Scholar]
- 220.Wiltfang J et al. Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low- and high-CSF Aβ40 load. J. Neurochem 101, 1053–1059 (2007). [DOI] [PubMed] [Google Scholar]
- 221.Mattsson N et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393 (2009). [DOI] [PubMed] [Google Scholar]
- 222.Skillbäck T et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138, 2716–2731 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Buerger K et al. No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 130, e82 (2007). [DOI] [PubMed] [Google Scholar]
- 224.Seppälä TT et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78, 1568–1575 (2012). [DOI] [PubMed] [Google Scholar]
- 225.Barthélemy NR et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med 26, 398–407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Janelidze S et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun 11, 1683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kern S et al. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol. 76, 187–193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zetterberg H & Bendlin BB Biomarkers for Alzheimer’s disease-preparing for a new era of disease-modifying therapies. Mol. Psychiatry 26, 296–308 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tarawneh R et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 73, 561–571 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schindler SE et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Karikari TK et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020). [DOI] [PubMed] [Google Scholar]
- 232.Palmqvist S et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.de Wolf F et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 143, 1220–1232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ashton NJ et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol 16, 265–284 (2020). [DOI] [PubMed] [Google Scholar]
- 235.Wattmo C & Wallin A K Early- versus late-onset Alzheimer’s disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res. Ther 9, 70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wilson RS et al. Cognitive decline in incident Alzheimer’s disease in a community population. Neurology 74, 951–955 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.van Harten AC et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology 91, e300–e312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kryscio RJ et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 83, 1359–1365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Stewart R et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br. J. Psychiatry 198, 199–205 (2011). [DOI] [PubMed] [Google Scholar]
- 240.Amariglio RE et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Petersen RC et al. Randomized controlled trials in mild cognitive impairment: sources of variability. Neurology 88, 1751–1758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Roberts RO et al. Prevalence and outcomes of amyloid positivity among persons without dementia in a longitudinal, population-based setting. JAMA Neurol. 75, 970–979 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vos SJ et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138, 1327–1338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jack CR Jr. et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 321, 2316–2325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Knopman DS et al. Entorhinal cortex tau, amyloid-β, cortical thickness and memory performance in non-demented subjects. Brain 142, 1148–1160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vos SJ et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Serrano-Pozo A, Qian J, Monsell SE, Betensky RA & Hyman BT APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann. Neurol 77, 917–929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Craft S et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology 51, 149–153 (1998). [DOI] [PubMed] [Google Scholar]
- 249.Butler M et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann. Intern. Med 168, 52–62 (2018). [DOI] [PubMed] [Google Scholar]
- 250.Fink HA et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann. Intern. Med 168, 39–51 (2018). [DOI] [PubMed] [Google Scholar]
- 251.Brasure M et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann. Intern. Med 168, 30–38 (2018). [DOI] [PubMed] [Google Scholar]
- 252.Kane RL et al. in Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia (Agency for Healthcare Research and Quality, 2017). [PubMed] [Google Scholar]
- 253. Ngandu T et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 (2015). A first demonstration of non-pharmacological means of delaying cognitive decline in elderly persons at risk for dementia.
- 254.Debette S et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Williamson JD et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 321, 553–561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Reuben DB et al. D-CARE: the dementia care study: design of a pragmatic trial of the effectiveness and cost effectiveness of health system-based versus community-based dementia care versus usual dementia care. J. Am. Geriatr. Soc 68, 2492–2499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fisk JD, Beattie BL, Donnelly M, Byszewski A & Molnar FJ Disclosure of the diagnosis of dementia. Alzheimers Dement. 3, 404–410 (2007). [DOI] [PubMed] [Google Scholar]
- 258.Alpinar-Sencan Z & Schicktanz S Addressing ethical challenges of disclosure in dementia prediction: limitations of current guidelines and suggestions to proceed. BMC Med. Ethics 21, 33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Amieva H et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int. Psychogeriatr 28, 707–717 (2016). [DOI] [PubMed] [Google Scholar]
- 260.Tricco AC et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA 318, 1687–1699 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mohs RC et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 57, 481–488 (2001). [DOI] [PubMed] [Google Scholar]
- 262.Panza F, Lozupone M, Logroscino G & Imbimbo BP A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol 15, 73–88 (2019). [DOI] [PubMed] [Google Scholar]
- 263.Rogers MB https://www.alzforum.org/news/research-news/aducanumab-still-needs-prove-itself-researchers-say (2020). [Google Scholar]
- 264.Mintun MA et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med 10.1056/NEJMoa2100708 (2021). [DOI] [PubMed] [Google Scholar]
- 265.Shugart J https://www.alzforum.org/news/conference-coverage/banish-av-ban2401-antibody-makes-its-move-phase-3-program (2020). [Google Scholar]
- 266.VandeVrede L, Boxer AL & Polydoro M Targeting tau: clinical trials and novel therapeutic approaches. Neurosci. Lett 731, 134919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rios-Romenets S et al. Baseline demographic, clinical, and cognitive characteristics of the Alzheimer’s prevention initiative (API) autosomal-dominant Alzheimer’s disease Colombia trial. Alzheimers Dement 16, 1023–1030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Insel PS, Donohue MC, Sperling R, Hansson O & Mattsson-Carlgren N The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann. Clin. Transl. Neurol 7, 776–785 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ballard C et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 17, 213–222 (2018). [DOI] [PubMed] [Google Scholar]
- 270.Jennings LA et al. Patient and caregiver goals for dementia care. Qual. Life Res 26, 685–693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bannon S et al. In it together: a qualitative meta-synthesis of common and unique psychosocial stressors and adaptive coping strategies of persons with young-onset dementia and their caregivers. Gerontologist 10.1093/geront/gnaa169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Moon H & Adams KB The effectiveness of dyadic interventions for people with dementia and their caregivers. Dementia 12, 821–839 (2013). [DOI] [PubMed] [Google Scholar]
- 273.van Charante EP et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 388, 797–805 (2016). [DOI] [PubMed] [Google Scholar]
- 274.Andrieu S et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16, 377–389 (2017). [DOI] [PubMed] [Google Scholar]
- 275.Sink KM et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the life randomized trial. JAMA 314, 781–790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.De Jager PL, Yang HS & Bennett DA Deconstructing and targeting the genomic architecture of human neurodegeneration. Nat. Neurosci 21, 1310–1317 (2018). [DOI] [PubMed] [Google Scholar]
- 277.Rehiman SH et al. Proteomics as a reliable approach for discovery of blood-based Alzheimer’s disease biomarkers: a systematic review and meta-analysis. Ageing Res. Rev 60, 101066 (2020). [DOI] [PubMed] [Google Scholar]
- 278.Mahajan UV et al. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: a targeted metabolomic and transcriptomic study. PLoS Med. 17, e1003012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dawson TM, Golde TE & Lagier-Tourenne C Animal models of neurodegenerative diseases. Nat. Neurosci 21, 1370–1379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.van der Kant R et al. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-β in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell 24, 363–375.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual of Mental Disorders 5th ed. (American Psychiatric Association, 2013). [Google Scholar]
- 282.Albert M et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging– Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 270–279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Dubois B et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014). [DOI] [PubMed] [Google Scholar]
- 284.Thal DR, Rub U, Orantes M & Braak H Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.