Abstract
Air pollution levels are uneven within cities, contributing to persistent health disparities between neighborhoods and population sub‐groups. Highly spatially resolved information on pollution levels and disease rates is necessary to characterize inequities in air pollution exposure and related health risks. We leverage recent advances in deriving surface pollution levels from satellite remote sensing and granular data in disease rates for one city, Washington, DC, to assess intra‐urban heterogeneity in fine particulate matter (PM2.5)‐ attributable mortality and morbidity. We estimate PM2.5‐attributable cases of all‐cause mortality, chronic obstructive pulmonary disease, ischemic heart disease, lung cancer, stroke, and asthma emergency department (ED) visits using epidemiologically derived health impact functions. Data inputs include satellite‐derived annual mean surface PM2.5 concentrations; age‐resolved population estimates; and statistical neighborhood‐, zip code‐ and ward‐scale disease counts. We find that PM2.5 concentrations and associated health burdens have decreased in DC between 2000 and 2018, from approximately 240 to 120 cause‐specific deaths and from 40 to 30 asthma ED visits per year (between 2014 and 2018). However, remaining PM2.5‐attributable health risks are unevenly and inequitably distributed across the District. Higher PM2.5‐attributable disease burdens were found in neighborhoods with larger proportions of people of color, lower household income, and lower educational attainment. Our study adds to the growing body of literature documenting the inequity in air pollution exposure levels and pollution health risks between population sub‐groups, and highlights the need for both high‐resolution disease rates and concentration estimates for understanding intra‐urban disparities in air pollution‐related health risks.
Keywords: fine particulate matter, PM2.5‐attributable health impacts, health inequities, environmental justice, intra‐urban baseline disease rates, intra‐urban health risks
Key Points
Fine particulate matter‐attributable health risks are unevenly and inequitably distributed across Washington, DC
Higher PM2.5‐attributable disease burdens are found in neighborhoods with larger proportions of people of color in Washington, DC
High‐resolution disease and concentration estimates are needed to understand intra‐urban disparities in air pollution‐related health risks
1. Introduction
Ambient air pollution in cities is of growing concern due to expected population growth, rapid urbanization, and rising pollution levels in many cities. Extensive epidemiological literature reveals strong associations between ambient fine particulate matter of aerodynamic diameter less than 2.5 μm (PM2.5) and mortality and morbidity outcomes, including cardiovascular and respiratory diseases and lung cancer (Brauer et al., 2012; Burnett et al., 2018; Cohen et al., 2017), and asthma incidence and exacerbation (Khreis et al., 2017; Orellano et al., 2017). An emerging body of literature also suggests associations with additional health outcomes, including diabetes (Bowe et al., 2018; Eze et al., 2015; Yang et al., 2020); neural, behavioral and cognitive changes (de Prado Bert et al., 2018); happiness and well‐being (Zheng et al., 2019); and low birth weight (Bell et al., 2010; Ebisu & Bell, 2012; Malley et al., 2017). Air pollution is considered the leading environmental risk factor and among the leading overall risk factors for global mortality (Aravkin et al., 2020; Cohen et al., 2017; Landrigan et al., 2018). In the US, PM2.5 is estimated to be responsible for 100,000–200,000 premature deaths each year, with the range dependent largely on whether all or only anthropogenic PM2.5 is included, the risk functions used, the mortality causes included, and the year of analysis (Bowe et al., 2019; Fann et al., 2018; Thakrar et al., 2020; Vodonos & Schwartz, 2021).
Overall, air quality in the US has improved dramatically since the 1970 Clean Air Act and its 1990 Amendments (U.S. EPA, 2020). However, it has not improved equitably. Literature reveals that throughout the US, lower income, minority, and marginalized populations experience higher air pollution exposure levels and associated health impacts (Hajat et al., 2015; Tessum et al., 2019). These communities often live near major air pollution sources, such as major roadways, shipping ports, airports, and industrial facilities, resulting from decades of race‐biased policies (both implicit and explicit) in housing, zoning, facility siting, and transportation (Mohai & Saha, 2015). Today, the same communities that bore the greatest burden of harm decades ago continue to face the greatest public health threats associated with long‐term exposure to air pollution (Colmer et al., 2020). The National Ambient Air Quality Standards (NAAQS) in its current form is, essentially, a one‐size‐fits‐all universal approach that lacks specificity and treats all communities and subsects the same. This approach produces unequal impacts and reinforces inequitable outcomes even when implemented with the best of intentions.
Over the last few years, several US states have implemented ground‐breaking laws and policies to address air pollution inequity in their air quality management programs, including California's Assembly Bill (AB) 617 and its resulting Community Air Protection Program (California Legislative Information, 2017), and the New Jersey Law NJ S232 (20R) (STATE OF NEW JERSEY, 219th LEGISLATURE, 2020), which establish community emissions reductions programs and protect communities from projects that pose local health and environmental risks, respectively. Similarly, multi‐state programs have emerged that aim to collaboratively and equitably reduce greenhouse gases and air pollutants, such as the Medium‐and Heavy‐Duty Zero Emission Vehicle (MHD‐ZEV) Initiative (Multi‐State Medium‐ and Heavy‐Duty Zero Emission Vehicle Memorandum of Understanding, 2020), signed onto by 13 states and the District of Columbia, and the Transportation Climate Initiative (The Transportation and Climate Initiative An Agenda for Progress, 2010) supported by 12 Northeast and Mid‐Atlantic states and the District of Columbia. More recently, in January 2021, the Biden Administration issued an Executive Order that elevated the federal government's actions to address environmental injustice (Tackling the Climate Crisis at Home and Abroad, 2021).
Addressing environmental injustice requires information about air pollution exposure levels between and within neighborhoods, which is beyond the intent and capability of the existing network of federal reference monitors throughout North America and the spatial resolution of regional chemical transport models. In the District of Columbia, for example, researchers found that fine‐scale emissions source attribution can reveal environmental injustices that may be obscured when using more coarsely resolved regional data inputs (Northcross et al., 2020). New techniques, both emerging and maturing, are being deployed to conduct air quality characterization and surveillance at high spatial resolutions. Techniques include distributed low‐cost sensor networks (Ahangar et al., 2019; Castillo et al., 2019; Matte et al., 2013), mobile monitoring on vehicles driving through cities (Apte et al., 2017; Messier et al., 2018; Miller et al., 2020; Southerland et al., 2021), and satellite remote sensing (Demetillo et al., 2020; Kerr et al., 2021; Southerland et al., 2021). With relatively high spatial resolution (∼1 × 1 km) and full geographical coverage, satellite remote sensing could be of particular value for targeted assessment of neighborhood‐scale air pollution exposures and health impacts in cities where low‐cost sensor networks and mobile monitoring data are not available.
Beyond information about air quality levels, neighborhood‐scale information on disease rates is important to understand not just inequities in air pollution exposure, but also inequities in air pollution‐related health risks (Southerland et al., 2021). Geographic, economic and racial health inequities are a known issue in the District (Chandra et al., 2013; Health Equity Report: District of Columbia, 2018, 2019). The recently published Health Equity Report for the District of Columbia (DC HER) 2018 analyzed health data in the District by Proximal Neighborhood Groups (PNGs), also referred to as statistical neighborhoods. For simplicity, we refer henceforth to the 51 PNGs as “neighborhoods.” The DC HER reported a high degree of environmental and health inequity within the District, with asthma emergency department (ED) visit rates being one order of magnitude higher in most affected neighborhoods compared to least affected neighborhoods. Furthermore, life expectancy differs by 21 years between neighborhoods at the two ends of the spectrum.
Given the District's disparities in air pollution exposure and disease rates, and the potential it has to become a role model in creating collaborative actions for change, we use the District as a case study to assess intra‐urban heterogeneity in PM2.5‐attributable health impacts. We explore the degree of disparity in estimated PM2.5‐attributable cases of mortality and disease exacerbation between neighborhoods throughout the District using a high‐resolution satellite‐derived PM2.5 concentration data set and two high‐resolution data sets for disease rates ‐ one based on local administrative data and one using a small‐scale estimation technique by the US Centers for Disease Control and Prevention (CDC). By comparing the application of these two data sets, our study shows whether using estimated rather than more cumbersome administrative data for disease rates can identify similar spatial patterns of air pollution‐attributable health risks. We anticipate that our study can both inform mitigation approaches aimed at reducing environmental health disparities in the District and advance the development of technical approaches for estimating air pollution‐related health inequities within cities.
2. Methods
2.1. Health Impact Function
We apply widely used epidemiologically derived health impacts functions to estimate mortality‐ and morbidity attributable to PM2.5 (e.g., Anenberg et al., 2010; Fann et al., 2017). We estimate annual PM2.5‐attributable cases of all‐cause mortality, ischemic heart disease (IHD), chronic obstructive pulmonary disease (COPD), stroke, lung cancer, and asthma ED visits. These health outcomes have been determined to be causally associated with PM2.5 by either the U.S. EPA (U.S. EPA, 2019) or the Global Burden of Disease (GBD) study (Aravkin et al., 2020). All analyses are conducted using Geospatial Data Abstraction Library (GDAL) (GDAL Contributors, 2021), Quantum Geographic Information System (QGIS 3.6.2) (QGIS Development Team, 2021) and R version 3.6.3 (R Core Team., 2020).
For each grid cell (∼1 × 1 km) in the District, we estimate the annual excess cases of mortality and asthma ED visits that are attributable to PM2.5 (Mort in Equation 1) for each health outcome separately, applying cause‐specific concentration‐response factors () from the relative risks (RR) shown in Table 1, the baseline disease rates (BDR) described in Table 2, and gridded PM2.5 concentrations (x) and population estimates (Pop). We use log‐linear relationships between concentration and RR, consistent with previous studies (Anenberg et al., 2010; Fann et al., 2012, 2017). We then aggregate the resulting estimated PM2.5‐attributable cases of mortality and morbidity to the neighborhood‐, zip code‐, ward‐, and city‐levels to report results in a way that is meaningful to inform policy.
| (1) |
Table 1.
Relative Risks for All‐Cause and Cause‐Specific Mortality, and Asthma Emergency Department Visits Used in the PM2.5‐Attributable Health Impacts Functions
| Health outcome | Relative risk (95% confidence interval) | Age group (years) | Study | Population studied |
|---|---|---|---|---|
| Asthma ED visits | 1.04 (1.01, 1.07) | 0–99 | Mar et al. (2010) | Greater Tacoma, WA |
| All‐cause mortality | 1.06 (1.04, 1.08) | 0–99 | Turner et al. (2016) | CPS‐II (American Cancer Society) |
| Chronic obstructive pulmonary disease | 1.10 (1.01, 1.19) | 30–99 | Turner et al. (2016) | CPS‐II (American Cancer Society) |
| Ischemic heart disease | 1.14 (1.02, 1.22) | 30–99 | Turner et al. (2016) | CPS‐II (American Cancer Society) |
| Lung cancer | 1.09 (1.03, 1.16) | 30–99 | Turner et al. (2016) | CPS‐II (American Cancer Society) |
| Stroke | 1.11 (1.05, 1.17) | 30–99 | Turner et al. (2016) | CPS‐II (American Cancer Society) |
Table 2.
Characteristics of the Health Data Obtained From the District of Columbia Department of Health for the Years 2000 and 2015, and 2014–2018 for Asthma; Annual Average Health Outcome Cases in the District; Mean (and Range) of the Annual Average Cases Across Neighborhoods, Zip Codes, or Wards; 2010 Population From SEDAC; and Annual Average Health Outcome Rates Computed Using the District's Total Cases and SEDAC Population Data per 100,000 People (and per 10,000 People for Asthma)
| Health outcome of interest | Spatial resolution | Ages included | Mean annual cases (DC‐wide) | Mean (range) cases across neighborhoods, zip codes, or wards | Population (DC‐wide) | Age‐standardized rates (DC‐wide) |
|---|---|---|---|---|---|---|
| Asthma emergency department (ED) visits | Zip code (n = 26) | All ages | 7,103 | 263 (3–1311) | 627,656 | 113 |
| All‐cause mortality | Neighborhood (n = 47) | All ages | 4,702 | 98 (13–177) | 627,656 | 749 |
| Chronic obstructive pulmonary disease | Ward (n = 8) | Ages 30–99 years | 124 | 14 (10–21) | 358,884 | 35 |
| Ischemic heart disease | Neighborhood (n = 47) | Ages 25–99 years | 840 | 17 (8–28) | 358,884 | 234 |
| Lung cancer | Ward (n = 8) | Ages 30–99 years | 258 | 30 (15–45) | 358,884 | 72 |
| Stroke | Ward (n = 8) | Ages 25–99 years | 89 | 10 (6–16) | 358,884 | 25 |
We estimate: (a) annual mean PM2.5‐attributable excess mortality and morbidity from 2000 to 2018 using annual BDR and PM2.5 data and (b) 5‐year mean PM2.5‐attributable excess mortality and morbidity (2014–2018 for asthma ED visits and 2011–2015 for all other health endpoints, consistent with the time periods for which we were able to obtain administrative BDR data) using 5‐year averages of both BDR and PM2.5 concentrations to remove the influence of interannual variability in both of these variables. To disentangle the influence of temporal changes in PM2.5 and disease rates separately, we also estimate PM2.5‐attributable health impacts using PM2.5 concentrations from 2018 (8.7 μg/m3) with year‐specific BDR between 2000 and 2018. We use 2010 population for all calculations, as described in Section 2.4, to isolate the influence of PM2.5 and BDR on spatiotemporal trends in estimated air pollution health risks. PM2.5 concentration and population are gridded and the spatial resolution of BDR varies for different health outcomes as described in Section 2.4.
2.2. Relative Risks
We use epidemiologically derived, cause‐specific RR estimates representing the association between annual average PM2.5 concentration estimates and incidence of the disease outcomes of interest (Table 1), consistent with the US Environmental Protection Agency's (EPA) most recent Regulatory Impact Analysis for PM2.5 (U.S. EPA, 2012). City‐specific RR estimates for the District are not available. For all mortality outcomes, we derive the RRs from the American Cancer Society's (ACS) Cancer Prevention Study II (CPS‐II) which included 1.2 million participants of at least 30 years of age in the US from all states, the District, and Puerto Rico (Turner et al., 2016). For asthma ED visits, we use the RR from a study conducted in the greater Tacoma, Washington area (Mar et al., 2010), which was applied nationally in the most recent US EPA Regulatory Impact Analysis for PM2.5 (U.S. EPA, 2012). While the RR for asthma ED visits that we derive from Mar et al. (2010) is based on daily PM2.5 concentrations, we use annual average PM2.5 and assume that the annual attributable asthma ED visits are approximately equivalent to the sum of daily attributable asthma ED visits.
These RRs are used widely throughout the literature and by the U.S. EPA for regulatory analysis. In the case of mortality outcomes, the studies have the advantage of a nation‐wide cohort with high statistical power. However, extrapolating these RRs to specific populations in the District may obscure differences in concentration‐response relationships between cities. In addition, the population groups in these studies are not reflective of the racial composition of the population in the District, and applying these RRs to multiple population subgroups within an individual city, as we are doing here, ignores differential quality and access to healthcare, as well as other social determinants of health. Without within‐city studies of PM2.5 health effects in the District, extrapolating from these larger studies is necessary.
2.3. PM2.5 Concentrations
We use annual mean PM2.5 concentration estimates from a North American satellite‐derived data set (V4.NA.03) with a spatial resolution of 0.01° × 0.01° (∼1 km2). This data set relates the combined aerosol optical depth (AOD) from multiple satellite retrievals to surface PM2.5 concentrations using the spatiotemporally varying geophysical relationship between AOD and PM2.5 simulated by the GEOS‐Chem chemical transport model. These geophysical values are calibrated to ground‐based monitors using a geographically weighted regression. V4.NA.03 combines the geophysical output of V4.GL.03 (Hammer et al., 2020) with the regional methodology of V4.NA.02 (van Donkelaar et al., 2019). Gridded annual mean PM2.5 concentrations vary within the District by up to ∼2 μg/m3 (Figure S1 in Supporting Information S1). The city‐wide 5‐year average annual PM2.5 concentration decreased from 17.1 μg/m3 in 2000–2004 to 10.0 μg/m3 in 2014–2018 (Table S1 in Supporting Information S1).
While a full evaluation of the satellite‐derived PM2.5 concentrations against ground measurements is not possible with only three Federal Reference Monitors in our study location and period, the satellite‐derived annual average PM2.5 concentrations were generally consistent with observations (Figure S2 in Supporting Information S1). There was a slight overestimation in the satellite‐derived concentrations of ∼0.5 μg/m3 but the spatial distribution agrees well with observations.
2.4. Baseline Disease Rates, Population, and Demographic Data
We use annual baseline mortality counts by neighborhood (n = 51) for the years 2000–2015, and annual baseline asthma ED visits by zip code (n = 26) for the years 2014–2018 from the District's Department of Health (DOH). Baseline counts smaller than five (n < 5) are suppressed to protect privacy, resulting in 50%–95% missing data for COPD, lung cancer and stroke. For these health endpoints, we apply counts aggregated by ward (n = 8) to achieve more spatially complete data (95%). IHD and all‐cause mortality counts are available for 47 out of 51 neighborhoods (Table 2). Remaining neighborhoods and wards with suppressed values are assigned Count = 2.5 (the midpoint of 1–4, the values suppressed by DOH) as the spatiotemporal variability in health outcomes does not allow us to estimate a number to replace missing values. Neighborhoods and wards overlays are presented in Figure S3 in Supporting Information S1.
We use population estimates from the Socioeconomic Data and Applications Center (SEDAC) 2010 population data set. Population counts from SEDAC consist of estimates from the Gridded Population of the World (GPW), Version 4, by the Center for International Earth Science Information Network (CIESIN) at 30 Arc‐Second (∼1 × 1 km) resolution (Center For International Earth Science Information Network‐CIESIN‐Columbia University, 2018). Using this data set, we create two population sub‐categories (shown in Table 2) based on the same age groups that match the RRs in Table 1 and use these in our health impact function (Equation 1). We also use the SEDAC population data set to compute disease rates from the DOH disease count data, as rates are needed to estimate PM2.5‐attributable health impacts at the gridcell level.
To evaluate whether estimated disease rates can be used in lieu of more cumbersome (and sometimes unavailable) city‐specific administrative data to capture intra‐city heterogeneity in air pollution health risks, we compare small‐area disease rate estimates from the CDC 500 Cities with DOH data for four health outcomes: asthma ED visits, COPD, lung cancer, and stroke. While the CDC 500 Cities data have the advantage of high spatial resolution (census tract level) and full spatial coverage across the District, the specific health endpoints and age groups represented in the CDC 500 Cities data do not exactly match those used in the epidemiological studies from which we draw RR estimates nor the DOH data. For example, the CDC 500 Cities data set includes cancer, but not lung cancer specifically; therefore, we assume that the spatial distribution of cancer data also reflects the spatial distribution of lung cancer across the District. The CDC 500 Cities data also represent disease prevalence among adults aged 18 and older, while we need incidence rates to estimate PM2.5‐attributable mortality and morbidity. We therefore develop new estimated tract‐level baseline incidence rates for our diseases and age groups of interest by retaining the District's average disease incidence rate from DOH and the spatial distribution of disease prevalence from CDC 500 Cities. This is an approximation approach, recognizing that the spatial pattern of disease incidence and prevalence may not be fully aligned.
Specifically, for each health outcome included in our study, we use the CDC 500 Cities DC average (CDC 500 Prevalence(city)) and tract‐level prevalence rate (CDC 500 Prevalence(tract)) to calculate the tract‐to‐city prevalence ratio (Equation 2). We then multiply this ratio by our DOH city‐wide baseline disease incidence rate (DOH BDR(city)) to obtain a combined CDC‐DOH tract‐level baseline disease incidence rate (CDC‐DOH BDR(tract)) that retains the total city‐wide incidence rate from DOH and the census tract‐level spatial distribution of prevalence from CDC 500 Cities.
| (2) |
We apply these new integrated CDC‐DOH BDR estimates to calculate PM2.5‐attributable health impacts across DC and compare results with those obtained from applying the DOH rates directly.
We explore differences in estimated PM2.5‐attributable mortality and morbidity outcomes between population sub‐groups using five social, economic, demographic and health outcome factors at the neighborhood level: education (percent residents 25 years or older with high school diploma or higher; the District mean = 92%, range = 79%–99%), unemployment (percent residents 16 years or older unemployed; mean = 8%, range = 2%–30%), income (median household income and percent residents living in poverty; mean = $94,537, range = $25,311–$200,031, and mean = 15%, range = 2%–40%, respectively), race and ethnicity (% Black alone, % White alone, % Latino/Hispanic, % Asian alone; means = 36%, 46%, 11%, and 4%, respectively), and life expectancy at birth (years; mean = 79, range = 68–89). Data were extracted from the DC HER, which uses socio‐demographic data from the US Census Bureau 2011–2015 American Community Survey (ACS) 5‐year estimates, and life expectancy data from the DOH Center for Policy, Planning, and Evaluation.
3. Results
We first report the total number of estimated PM2.5‐attributable deaths and asthma ED visits across the District using 5‐year average PM2.5 concentrations and administrative disease rates (2014–2018 average for asthma ED visits and 2011–2015 for all other health endpoints). Using central estimates of the RRs, we estimate that approximately 220, 10, 90, 20, 10 excess all‐cause, COPD, IHD, lung cancer, and stroke deaths, respectively, and 35 asthma ED visits could be attributed to PM2.5 pollution in the District annually. For all‐cause mortality, we estimated a neighborhood‐level range of 17–90 attributable deaths per 100,000 people [95% confidence interval (CI): 12–61, 22–118). This range was 2–5 per 100,000 people (95% CI: 0–1, 3–9) for COPD mortality, 7–58 per 100,000 (95% CI: 1–9, 10–85) for IHD mortality, 3–9 per 100,000 (95% CI: 1–3, 5–15) for lung cancer mortality, 1–4 per 100,000 (95% CI: 0–2, 1–5) for stroke mortality, and 4–144 per 100,000 (95% CI: 1–37, 8–244) for asthma ED visits. Henceforth we do not report uncertainty bounds as uncertainty from the RR is treated as constant in time and space and thus does not affect results or conclusions for temporal trends or spatial patterns.
We next estimate temporal trends using year‐specific concentrations and administrative disease rates. Declining PM2.5 concentrations and BDR together contribute to an overall decreasing trend in PM2.5‐attributable excess cases in the District, with PM2.5‐attributable all‐cause mortality dropping from 520 excess cases in 2000 to 260 in 2015 (Figure 1). To disentangle the influence of PM2.5 versus BDR changes on the temporal trend in PM2.5‐attributable mortality, we compare PM2.5‐attributable deaths calculated using annually varying PM2.5 concentration and BDR versus those calculated using constant 2018 PM2.5 concentrations (8.7 μg/m3) and annually varying BDR (Figure 1b). Between the years 2000 and 2015, approximately 30% of the cumulative PM2.5‐attributable deaths across this time period (60, 540, 110, 50, or 1,620 deaths from COPD, IHD, LC, stroke, and all‐causes, respectively) could have been avoided if historical PM2.5 concentrations were as low as the 2018 mean (Table S2 in Supporting Information S1).
Figure 1.
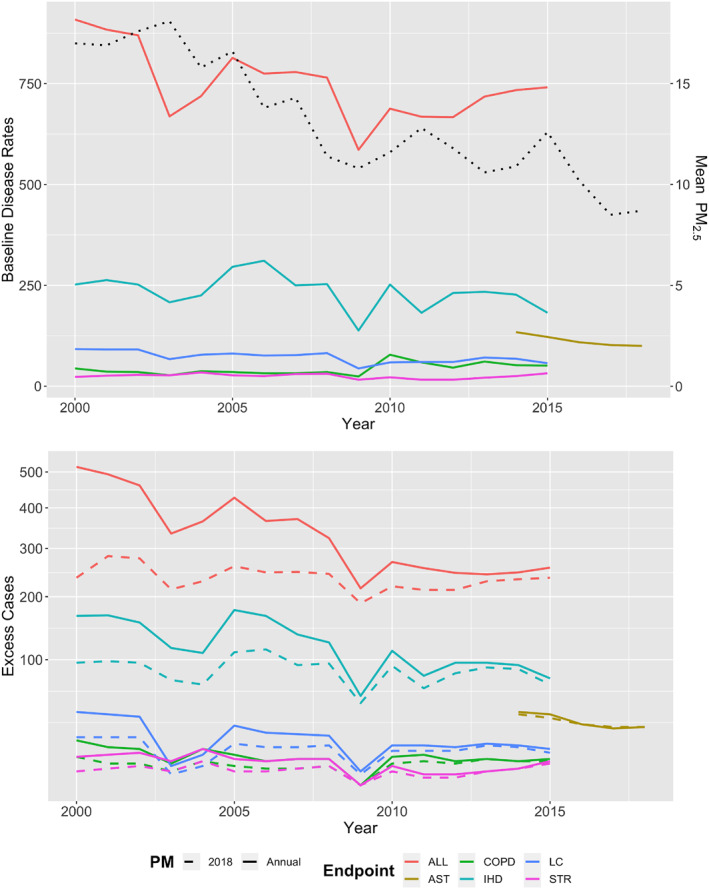
Temporal trends in annual baseline disease rates from District of Columbia Department of Health and annual mean PM2.5 concentrations (μg/m3, black dotted line) between 2000 and 2018 (top panel) and annual PM2.5‐attributable excess cases for premature mortality between 2000 and 2015 and asthma ED visits between 2014 and 2018 (bottom panel). In the bottom panel, solid line represents the application of annual baseline disease rates (BDR) and PM2.5, and dashed lines represent the application of annual BDR with 2018 PM2.5 concentrations. Health endpoints: ALL, All‐cause mortality; AST, Asthma ED visits; COPD, Chronic Obstructive Pulmonary Disease; IHD, Ischemic Heart Disease; LC, Lung Cancer; STR, Stroke.
We next explore the spatial distribution of these PM2.5‐attributable health impacts across the District. Estimated PM2.5‐attributable mortality and morbidity rates are higher along the east to south city border for all health endpoints, and are also relatively high in the northeast (Figure 2). While neighborhoods located closer to downtown are more densely populated (Figure S1 in Supporting Information S1), PM2.5‐attributable mortality rates (per 100,000 people) generally increase with increasing distance from the city center. The highest PM2.5‐attributable all‐cause mortality rates are more than four times higher in the most (Fort Dupont and Marshall Heights, both located in Ward 7) versus least (Woodley Park and Georgetown East, located in Wards 3 and 2, respectively) impacted neighborhoods, as shown in Figure 3 and Table S3 in Supporting Information S1. The neighborhoods with the 10 highest (mostly in wards 5, 7, and 8) and 10 lowest (mostly in wards 1–3) PM2.5‐attributable all‐cause mortality rates are geographically segregated. The neighborhoods with the highest PM2.5‐attributable mortality rates have 10% lower education and employment rates, 10% more residents living in poverty, $61,000 lower median household income, and about 10 fewer years of life expectancy (Figure S4 in Supporting Information S1). The top 10 neighborhoods also have 54% higher proportions of Blacks and 44% lower proportions of Whites (Figure 4 and Table S3 in Supporting Information S1).
Figure 2.
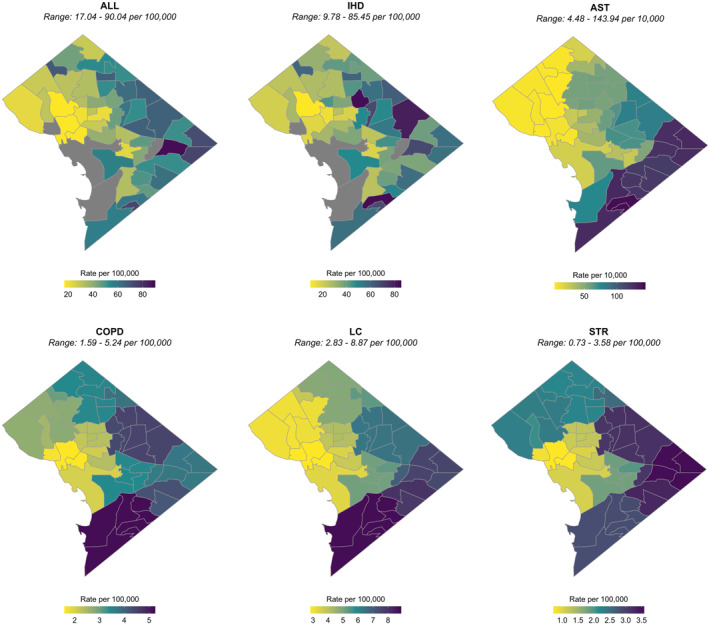
PM2.5‐attributable excess mortality and asthma ED visit rates at the neighborhood scale (2011–2015 average). Baseline disease rates underlying these estimates from the DC DOH are at the neighborhood‐level for all‐cause mortality (ALL) and ischemic heart disease (IHD); zip code‐level for asthma ED visits (AST); and ward‐level for chronic obstructive pulmonary disease (COPD), lung cancer (LC), and stroke (STR).
Figure 3.
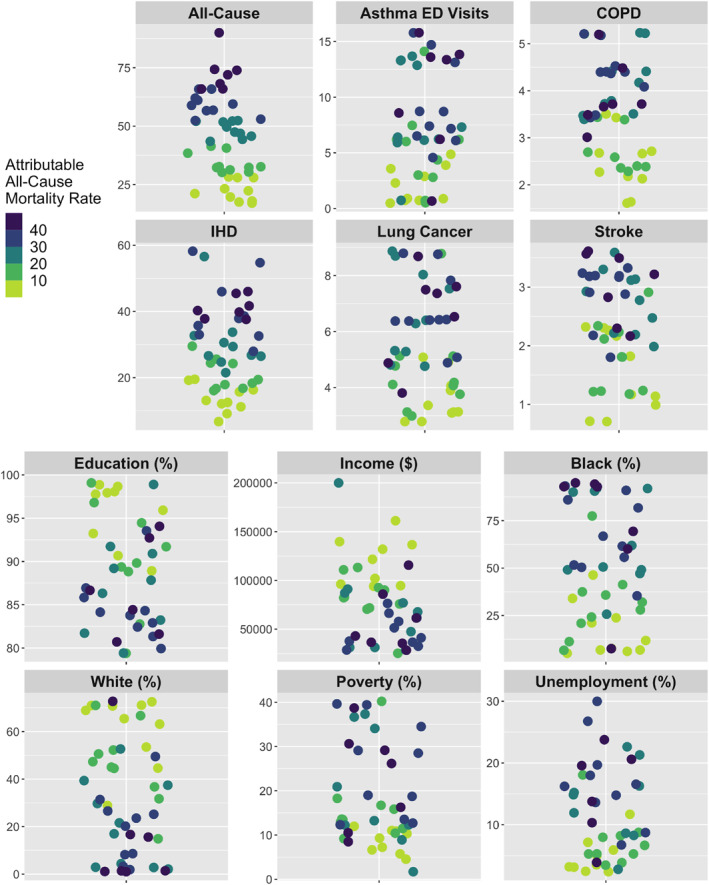
PM2.5‐attributable health impacts (2011–2015 average) at the neighborhood scale. Top: The distribution of PM2.5‐attributable mortality rates (per 100,000 people for all mortality outcomes and per 10,000 people for asthma ED visits) for each health endpoint and each of the 47 DC neighborhoods with available health data. Bottom: The distribution of sociodemographic variables across DC neighborhoods (see Section 2 for variable definitions). The color gradient used in all panels represents that of the PM2.5‐attributable all‐cause mortality rates (inset legend). Data points are randomly scattered across the x‐axis for plotting purposes.
Figure 4.
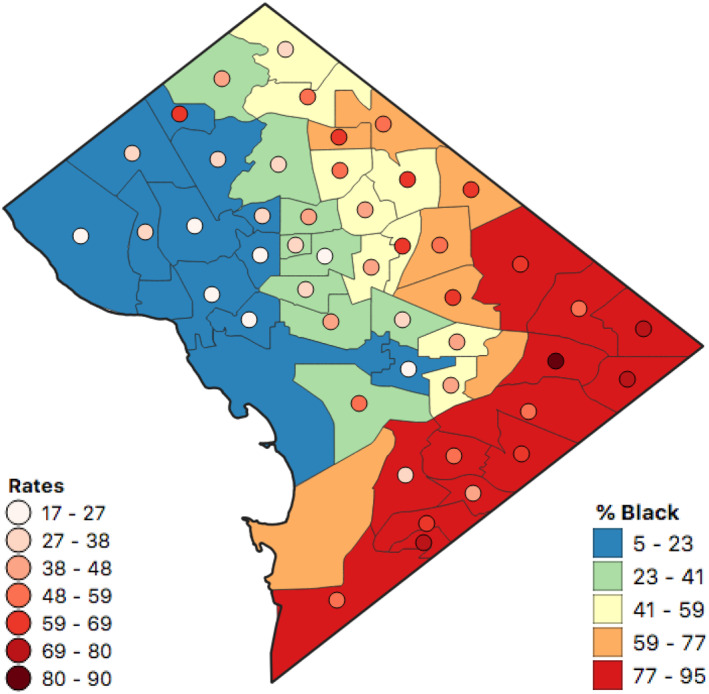
PM2.5‐attributable mortality rates (per 100,000 people) for all‐cause mortality and percent (%) Black distribution by neighborhood across Washington, DC. Data represent equal intervals and 2011–2015 means.
PM2.5‐attributable mortality rates appear to follow the spatial patterns of the BDR inputs (Figure S5 in Supporting Information S1) more so than that of the PM2.5 inputs (Figure S1 in Supporting Information S1). For all‐cause mortality and IHD, health outcomes for which BDR were available at the neighborhood‐level, PM2.5‐attributable excess mortality rates range by a factor of five and eight (from 17 to 90, and 7 to 58 cases per 100,000 people) respectively, across the District's neighborhoods. Contrastingly, for the health outcomes with ward‐level BDR (COPD, LC and STR), PM2.5‐attributable mortality rates show less variation, with ranges differing by a factor of ∼3–5. PM2.5‐attributable asthma ED visit rates (with BDR at the zip code‐level, n = 26), also show spatial homogeneity between neighborhoods within zip codes (i.e., more heterogeneity within wards but not within zip codes).
We next compare the application of administrative versus estimated BDR on PM2.5‐attributable mortality rates. The overall spatial distributions of BDR across the District differ between the DOH data set and the integrated CDC‐DOH data set, though are more similar for asthma, COPD, and stroke than for lung cancer (Figure 5). Differences in PM2.5‐attributable mortality and morbidity rates estimated using the two BDR data sets were more widespread for asthma ED visits compared with COPD, lung cancer and stroke (mean percent differences of 12, −7, and −9, respectively, compared with 187 for asthma, although with relatively large standard deviations of 32, 45, and 25, respectively). Over‐ and under‐estimation by the application of the integrated CDC‐DOH estimated BDR are more unevenly distributed for COPD and stroke. The differences seen in Figure 5 are driven solely by the difference in BDR data set, as all other health impact assessment inputs (PM2.5 concentration, population, and relative risk) are identical in the two calculations.
Figure 5.
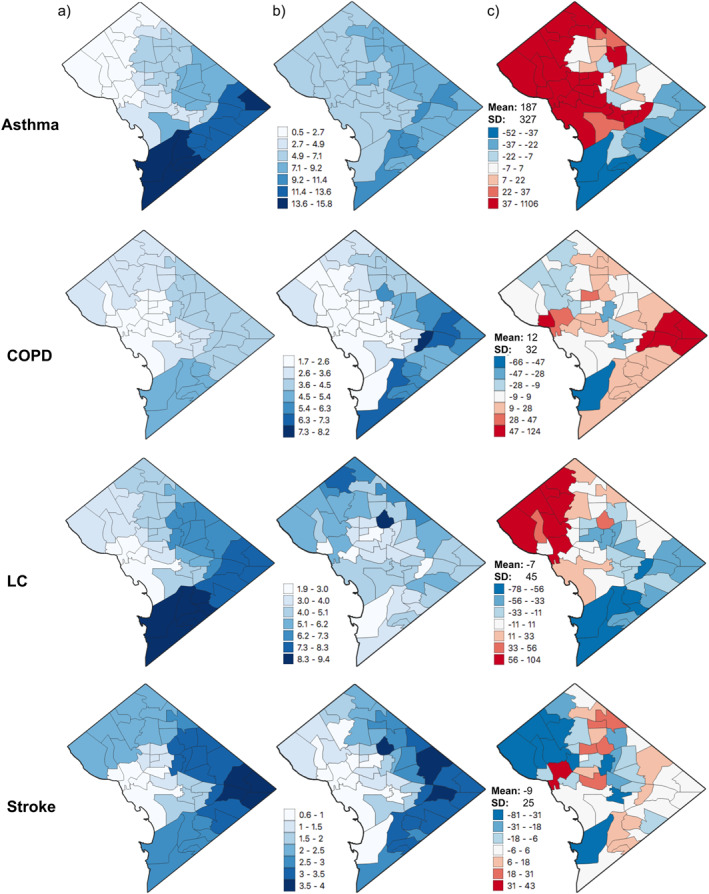
Neighborhood‐level PM2.5‐attributable rates for asthma ED visits (Asthma) per 10,000 people and PM2.5‐attributable mortality rates for COPD, lung cancer (LC) and stroke per 100,000 people using (a) DOH disease rates and (b) the integrated CDC‐DOH disease rates, and (c) percent difference between (a and b) [(CDC‐DOH ‐ DOH)/DOH]. Results are aggregated to neighborhood scale for all health outcomes, though DOH disease rate inputs for panel (a) are at zip code level for asthma ED visits and ward level for COPD, lung cancer, and stroke.
4. Discussion
Following national trends, PM2.5 concentrations and PM2.5‐attributable deaths have halved locally in the District during our study period. The District‐wide mean annual average PM2.5 concentrations decreased from 17 to 8.7 μg/m3 between 2000 and 2018. Consequently, total estimated PM2.5‐attributable excess deaths for four cause‐specific mortality outcomes combined (COPD, IHD, lung cancer and stroke) dropped from approximately 240 in 2000 to 120 in 2015. PM2.5‐attributable asthma ED visits also declined, from approximately 40 cases in 2014 to 30 cases in 2018. Estimated PM2.5‐attributable mortality and morbidity rates differed by up to five times between wards and up to eight times between neighborhoods. For example, PM2.5‐attributable all‐cause deaths ranged from 17 to 90 per 100,000 people across neighborhoods, and PM2.5‐attributable IHD deaths ranged from 7 to 58 per 100,000 people.
This spatial heterogeneity reveals both racial and sociodemographic inequities in the District's PM2.5‐attributable health burden. Specifically, we find that PM2.5 health risks are largest for neighborhoods with a high proportion of people of color, located in Wards 7 and 8 in the District's southeast. These neighborhoods also have lower income levels and lower educational attainment compared with the District average. Our analysis suggests that the same neighborhoods have substantially larger PM2.5‐attributable mortality and morbidity rates compared with neighborhoods that have a higher percentage of White populations and higher levels of household income and educational attainment. These results are consistent with the prior literature demonstrating that PM2.5 exposure and associated health impacts are unevenly and inequitably distributed across race/ethnicity, age and socioeconomic categories (Ebisu & Bell, 2012; Southerland et al., 2021; Tessum et al., 2019; Yitshak‐Sade et al., 2019), and adds to the literature documenting inequity in air pollution exposure levels and pollution health risks between population sub‐groups in the District (Chandra et al., 2013). By considering the influence of intra‐city heterogeneity in disease rates, we extend the literature to incorporate not just inequitable exposure, but also population vulnerability to pollution, similar to the analyses for New York City by Kheirbek et al. (2013) and the San Francisco Bay Area, California by Southerland et al. (2021).
The intra‐city variation in our estimated PM2.5‐attributable mortality and morbidity cases is driven by both disease rates and PM2.5 concentrations. While gridded PM2.5 varies spatially across the District by ∼2 μg/m3 (with higher concentrations in the northeast), BDR are five times higher in the southeast wards for COPD, lung cancer and stroke, up to nine times higher in southeast neighborhoods for all‐cause mortality and IHD, and over 30 times higher in southeast zip codes for asthma ED visits, compared with wards, neighborhoods and zip codes in the District's northwest, respectively. We found that variation in fine‐scale BDR drives the spatial heterogeneity in estimated PM2.5‐attributable mortality and morbidity, consistent with Southerland et al. (2021), though the coarse resolution of the data inputs to the PM2.5 concentration model preclude our ability to draw a strong conclusion from this result. Our results may suggest that the satellite‐derived PM2.5 concentrations are not showing the extent of heterogeneity of PM2.5 concentrations at the street and block level.
Our study has several limitations and uncertainties, similar to other air pollution health impact assessments (Fuentes, 2009). Each variable input to the health impact function is uncertain to different degrees. Previous health impact assessments have found that the choice of relative risk has the greatest impact on estimated air pollution‐attributable health risks (Anenberg et al., 2012); therefore, we have focused on incorporating the error from the relative risk estimate in our results. For PM2.5, the variability of resolutions associated with data sets used to produce the satellite‐derived PM2.5 concentrations may limit their ability to fully represent intra‐city variation. While these input data sets have resolutions as high as 1 × 1 km, the combined effect of coarser inputs may reduce the spatial heterogeneity indicated between neighborhoods. Exposure misclassification also results from our inability to account for time activity patterns, home ventilation, and other factors that lead to differences between concentration and exposure. Exposure misclassification may be exacerbated for vulnerable populations and can in turn create uncertainties in risk estimates and potentially disadvantage already vulnerable populations (Northcross et al., 2020).
While we used administrative BDR data which we consider to be less uncertain, our results may be subject to the modifiable areal unit problem, since due to privacy constraints we obtained BDR at different spatial aggregation levels for different diseases. To explore the potential influence of uncertainty in BDR, we explored differences between results using administrative versus estimated disease rates to improve understanding of the influence of this choice on estimated air pollution health impacts. We found that using more easily accessible estimated BDR from the CDC 500 Cities project in lieu of administrative data yielded considerably different spatial patterns of estimated PM2.5‐attributable disease rates. Future studies should seek to advance methods to estimate disease rates for a wider range of diseases and population sub‐groups and with greater accuracy beyond what is currently available from the CDC 500 Cities and other similar efforts. Without estimated disease rates for small administrative units (e.g., census tract) that are both accurate and consistent with the health outcomes and populations used in epidemiological studies, administrative data remains the most appropriate data source for disease rates in intra‐city air pollution health impact assessments.
Our results suggest that evaluating PM2.5 in regards to the health‐based National Ambient Air Quality Standard must consider both intra‐urban variation in concentrations and disease rates to address impacts on certain vulnerable populations, in particular communities of color. Black and Native American people have statistically significantly higher asthma rates than their counterparts in other races (CDC, 2019). Although persistent, these health inequities are neither natural nor inevitable (Health Equity Report: District of Columbia, 2018, 2019). Given the relationship between air pollution and asthma exacerbation, an outcomes‐focused equity lens that is intentional in its protection of historically marginalized communities, especially those that experience worse air quality‐related health effects, is critical to reduce air pollution inequities. The D.C. Law 23–181. Racial Equity Achieves Results (REACH) Amendment Act of 2020, which requires that racial equity impact analysis be conducted by each agency and council for each new piece of legislation, underscores growing interest and need for analysis focused on differential racial impacts, such as premature mortality, on historically disadvantaged and highly impacted communities (DOEE Ozone NAAQS Comment Letter, 2020; DOEE Ozone NAAQS Public Hearing, 2020).
Our results also indicate that quantitatively characterizing neighborhood‐scale differences in PM2.5‐related health risks would continue to benefit from advances in fine resolution information on both PM2.5 concentration data and intra‐city baseline disease rates. In alignment with Kheirbek et al. (2013) and Southerland et al. (2021), we found that fine‐scale baseline disease data better characterize population subgroups' susceptibility and disparities, which is necessary to aid in policy‐making to reduce urban health inequities, even for pollutants that are relatively spatially homogeneous, as is PM2.5. However, the racial and ethnic inequities may be underestimated in this and other recent studies that apply generalizable relative risks from large nation‐wide cohorts and/or that extrapolate relative risks from one population to another, which can obscure differences in concentration‐response relationships between neighborhoods and population sub‐groups. Future air pollution health impact assessments should consider using relative risks specific to individual sub‐populations to explore distributional impacts of air pollution health risks. There is a trade‐off, as large cohorts have more statistical power and population‐specific studies may be limited by lower statistical power to detect effects (e.g., Alexeeff et al., 2018). Future studies may assess the potential for using population‐specific relative risks to characterize inequities in air pollution‐related health risks.
While PM2.5 concentrations have been decreasing across the US since 1990, owing to effective environmental policies, PM2.5 air pollution still contributes 60,000–100,000 premature deaths each year nationally (Aravkin et al., 2020; Fann et al., 2017; Goodkind et al., 2019), and these air pollution‐related health risks continue to be inequitably distributed (e.g., Colmer et al., 2020; Tessum et al., 2019). Furthermore, ground‐based monitoring continues to be sparsely distributed, which is insufficient for assessing the spatial distribution of pollution levels and associated health impacts within cities. Future studies may consider improving intra‐city PM2.5 concentration estimates by integrating multiple exposure assessment approaches, including low‐cost sensors, mobile monitoring, statistical techniques such as land use regression modeling, chemical transport modeling, and satellite observations to capture air pollution exposure inequities more fully (Ahangar et al., 2019; Castillo et al., 2019; Hammer et al., 2020). Estimation techniques to generate high‐resolution baseline disease rates are also needed to consider population vulnerability to air pollution, as inequities exist not just in exposure levels, but in the health outcomes attributable to those exposure levels.
5. Conclusion
We assessed spatiotemporal trends in the health burden of PM2.5 pollution in Washington, DC and its 51‐statistical neighborhoods. While annual average PM2.5 concentrations have decreased between 2000 and 2018, PM2.5 still contributes to disease burdens in the District, and PM2.5‐attributable health impacts are unevenly and inequitably distributed. The highest attributable burdens are estimated to occur in neighborhoods that have larger proportions of people of color, as well as lower household income and lower educational attainment. Our results also indicate that quantitatively characterizing neighborhood‐scale differences in PM2.5‐related health risks within cities, either in the US or globally, would benefit from advances in fine resolution information on both PM2.5 concentration data and intra‐city baseline disease rates.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
This work was supported by NASA grant #80NSSC19K0193. R. V. Martin acknowledges support from NASA grant #80NSSC21K0508 and CIHR grant #CEU‐147101. The authors acknowledge with appreciation the help of Rebecca Winter and Patricia Lloyd from the Washington, DC Department of Health for providing access to health data, and Dan Goldberg and Arash Mohegh for assisting with geospatial data analysis and interpretation.
Castillo, M. D. , Kinney, P. L. , Southerland, V. , Arno, C. A. , Crawford, K. , van Donkelaar, A. , et al. (2021). Estimating intra‐urban inequities in PM2.5‐attributable health impacts: A case study for Washington, DC. GeoHealth, 5, e2021GH000431. 10.1029/2021GH000431
Data Availability Statement
Health data at the neighborhood level for Washington, DC used in this study are not publicly available due to confidentiality of patient information. The baseline disease data for this study were made available to us through an Institutional Review Board request to the Washington, DC Department of Health. Centers for Disease Control and Prevention (CDC) 500 Cities Project data are publicly available at https://www.cdc.gov/places/about/500-cities-2016-2019/index.html. Surface PM2.5 data sets for this study are referenced in Hammer et al. (2020) and van Donkelaar et al. (2019), and are compiled and made publicly available at https://sites.wustl.edu/acag/datasets/surface-pm2-5/. The population data sets from the Socioeconomic Data and Applications Center (SEDAC) are publicly available at https://sedac.ciesin.columbia.edu/data/set/gpw-v4-basic-demographic-characteristics-rev11. Sociodemographic data used in this study are referenced in the Health Equity Report: District of Columbia 2018 (2019), and are publicly available in the US Census Bureau 2011–2015 American Community Survey (ACS) 5‐year estimates website (https://www.census.gov/programs-surveys/acs/technical-documentation/table-and-geography-changes/2015/5-year.html), and life expectancy data is available from the Washington, DC Department of Health Center for Policy, Planning and Evaluation (https://dchealth.dc.gov/page/center-policy-planning-and-evaluation).
References
- Ahangar, F. , Freedman, F. , & Venkatram, A. (2019). Using low‐cost air quality sensor networks to improve the spatial and temporal resolution of concentration maps. International Journal of Environmental Research and Public Health, 16(7), 1252. 10.3390/ijerph16071252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeff, S. E. , Roy, A. , Shan, J. , Liu, X. , Messier, K. , Apte, J. S. , et al. (2018). High‐resolution mapping of traffic related air pollution with Google street view cars and incidence of cardiovascular events within neighborhoods in Oakland, CA. Environmental Health, 17(1), 38. 10.1186/s12940-018-0382-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anenberg, S. C. , Horowitz, L. W. , Tong, D. Q. , & West, J. J. (2010). An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environmental Health Perspectives, 118(9), 1189–1195. 10.1289/ehp.0901220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anenberg, S. C. , Schwartz, J. , Shindell, D. , Amann, M. , Faluvegi, G. , Klimont, Z. , et al. (2012). Global air quality and health co‐benefits of mitigating near‐term climate change through methane and black carbon emission controls. Environmental Health Perspectives, 120(6), 831–839. 10.1289/ehp.1104301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte, J. S. , Messier, K. P. , Gani, S. , Brauer, M. , Kirchstetter, T. W. , Lunden, M. M. , et al. (2017). High‐resolution air pollution mapping with Google street view cars: Exploiting big data. Environmental Science & Technology, 51(12), 6999–7008. 10.1021/acs.est.7b00891 [DOI] [PubMed] [Google Scholar]
- Aravkin, A. Y. , Zheng, P. , Abbafati, C. , Abbas, K. M. , Abbasi‐Kangevari, M. , Abd‐Allah, F. , et al. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396(10258), 1223–1249. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, M. L. , Belanger, K. , Ebisu, K. , Gent, J. F. , Lee, H. J. , Koutrakis, P. , & Leaderer, B. P. (2010). Prenatal exposure to fine particulate matter and birth weight: Variations by particulate constituents and sources. Epidemiology, 21(6), 884–891. 10.1097/EDE.0b013e3181f2f405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe, B. , Xie, Y. , Li, T. , Yan, Y. , Xian, H. , & Al‐Aly, Z. (2018). The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. The Lancet Planetary Health, 2(7), e301–e312. 10.1016/S2542-5196(18)30140-2 [DOI] [PubMed] [Google Scholar]
- Bowe, B. , Xie, Y. , Yan, Y. , & Al‐Aly, Z. (2019). Burden of cause‐specific mortality associated with PM2.5 air pollution in the United States. JAMA Network Open, 2(11), e1915834. 10.1001/jamanetworkopen.2019.15834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer, M. , Amann, M. , Burnett, R. T. , Cohen, A. , Dentener, F. , Ezzati, M. , et al. (2012). Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environmental Science & Technology, 46(2), 652–660. 10.1021/es2025752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, R. , Chen, H. , Szyszkowicz, M. , Fann, N. , Hubbell, B. , Pope, C. A. , et al. (2018). Global estimates of mortality associated with long‐term exposure to outdoor fine particulate matter. Proceedings of the National Academy of Sciences, 115(38), 9592–9597. 10.1073/pnas.1803222115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Legislative Information . (2017). Bill Text—AB‐617 Nonvehicular air pollution: Criteria air pollutants and toxic air contaminants. Retrieved from https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201720180AB617 [Google Scholar]
- Castillo, M. D. , Wagner, J. , Casuccio, G. S. , West, R. R. , Freedman, F. R. , Eisl, H. M. , et al. (2019). Field testing a low‐cost passive aerosol sampler for long‐term measurement of ambient PM2.5 concentrations and particle composition. Atmospheric Environment, 216, 116905. 10.1016/j.atmosenv.2019.116905 [DOI] [Google Scholar]
- CDC . (2019). Measures to identify and track racial disparities in childhood asthma. Retrieved from https://www.cdc.gov/asthma/asthma_disparities/risk_factors.htm [Google Scholar]
- Center For International Earth Science Information Network‐CIESIN‐Columbia University . (2018). Gridded population of the world, version 4 (GPWv4): Basic demographic characteristics, revision 11 [Data set]. NASA Socioeconomic Data and Applications Center (SEDAC). 10.7927/H46M34XX [DOI] [Google Scholar]
- Chandra, A. , Blanchard, J. C. , & Ruder, T. (2013). District of Columbia Community Health Needs Assessment. [PMC free article] [PubMed] [Google Scholar]
- Cohen, A. J. , Brauer, M. , Burnett, R. , Anderson, H. R. , Frostad, J. , Estep, K. , et al. (2017). Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. The Lancet, 389(10082), 1907–1918. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer, J. , Hardman, I. , Shimshack, J. , & Voorheis, J. (2020). Disparities in PM2.5 air pollution in the United States. Science, 369(6503), 575–578. 10.1126/science.aaz9353 [DOI] [PubMed] [Google Scholar]
- Demetillo, M. A. G. , Navarro, A. , Knowles, K. K. , Fields, K. P. , Geddes, J. A. , Nowlan, C. R. , et al. (2020). Observing nitrogen dioxide air pollution inequality using high‐spatial‐resolution remote sensing measurements in Houston, Texas. Environmental Science & Technology, 54(16), 9882–9895. 10.1021/acs.est.0c01864 [DOI] [PubMed] [Google Scholar]
- de Prado Bert, P. , Mercader, E. M. H. , Pujol, J. , Sunyer, J. , & Mortamais, M. (2018). The effects of air pollution on the brain: A review of studies interfacing environmental epidemiology and neuroimaging. Current Environmental Health Reports, 5(3), 351–364. 10.1007/s40572-018-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOEE Ozone NAAQS Comment Letter . (2020). RE: Review of the Ozone NAtional Ambient Air Quality Standards (Docket Number: EPA‐HQ‐OAR‐2018‐0279), U.S. Environmental Protection Agency, 4 (2020) (testimony of District of Columbia Department of Energy and Environment (DOEE)).
- DOEE Ozone NAAQS Public Hearing . (2020). Transcript O3 NAAQS Public Hearing 08‐31‐2020 PM Session, U.S. Environmental Protection Agency, Public Hearing 08‐31‐2020 PM Session, 40 (2020) (testimony of District of Columbia Department of Energy and Environment (DOEE)). Retrieved from https://www.regulations.gov/document/EPA-HQ-OAR-2018-0279-0372
- Ebisu, K. , & Bell, M. L. (2012). Airborne PM2.5 chemical components and low birth weight in the northeastern and mid‐Atlantic regions of the United States. Environmental Health Perspectives, 120(12), 1746–1752. 10.1289/ehp.1104763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze, I. C. , Hemkens, L. G. , Bucher, H. C. , Hoffmann, B. , Schindler, C. , Künzli, N. , et al. (2015). Association between ambient air pollution and diabetes mellitus in Europe and North America: Systematic review and meta‐analysis. Environmental Health Perspectives, 123(5), 381–389. 10.1289/ehp.1307823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, N. , Coffman, E. , Timin, B. , & Kelly, J. T. (2018). The estimated change in the level and distribution of PM2.5‐attributable health impacts in the United States: 2005–2014. Environmental Research, 167, 506–514. 10.1016/j.envres.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, N. , Kim, S.‐Y. , Olives, C. , & Sheppard, L. (2017). Estimated changes in life expectancy and adult mortality resulting from declining PM2.5 exposures in the contiguous United States: 1980–2010. Environmental Health Perspectives, 125(9), 097003. 10.1289/EHP507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, N. , Lamson, A. D. , Anenberg, S. C. , Wesson, K. , Risley, D. , & Hubbell, B. J. (2012). Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Analysis, 32(1), 81–95. 10.1111/j.1539-6924.2011.01630.x [DOI] [PubMed] [Google Scholar]
- Fuentes, M. (2009). Statistical issues in health impact assessment at the state and local levels. Air Quality, Atmosphere & Health, 2(1), 47–55. 10.1007/s11869-009-0033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GDAL Contributors . (2021). GDAL/OGR geospatial data abstraction software library. Open Source Geospatial Foundation. Retrieved from https://gdal.org/ [Google Scholar]
- Goodkind, A. L. , Tessum, C. W. , Coggins, J. S. , Hill, J. D. , & Marshall, J. D. (2019). Fine‐scale damage estimates of particulate matter air pollution reveal opportunities for location‐specific mitigation of emissions. Proceedings of the National Academy of Sciences, 116(18), 8775–8780. 10.1073/pnas.1816102116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat, A. , Hsia, C. , & O'Neill, M. S. (2015). Socioeconomic disparities and air pollution exposure: A global review. Current Environmental Health Reports, 2(4), 440–450. 10.1007/s40572-015-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, M. S. , van Donkelaar, A. , Li, C. , Lyapustin, A. , Sayer, A. M. , Hsu, N. C. , et al. (2020). Global estimates and long‐term trends of fine particulate matter concentrations (1998–2018). Environmental Science & Technology, 54(13), 7879–7890. 10.1021/acs.est.0c01764 [DOI] [PubMed] [Google Scholar]
- Health Equity Report: District of Columbia 2018 . (2019).
- Kerr, G. H. , Goldberg, D. L. , & Anenberg, S. (2021). COVID‐19 pandemic reveals persistent disparities in nitrogen dioxide pollution. Atmospheric Sciences. 10.1002/essoar.10504561.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek, I. , Wheeler, K. , Walters, S. , Kass, D. , & Matte, T. (2013). PM2.5 and ozone health impacts and disparities in New York City: Sensitivity to spatial and temporal resolution. Air Quality, Atmosphere & Health, 6(2), 473–486. 10.1007/s11869-012-0185-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khreis, H. , Kelly, C. , Tate, J. , Parslow, R. , Lucas, K. , & Nieuwenhuijsen, M. (2017). Exposure to traffic‐related air pollution and risk of development of childhood asthma: A systematic review and meta‐analysis. Environment International, 100, 1–31. 10.1016/j.envint.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Landrigan, P. J. , Fuller, R. , Acosta, N. J. R. , Adeyi, O. , Arnold, R. , Basu, N. et al. (2018). The Lancet Commission on pollution and health. The Lancet, 391(10119), 462–512. 10.1016/S0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- Malley, C. S. , Kuylenstierna, J. C. I. , Vallack, H. W. , Henze, D. K. , Blencowe, H. , & Ashmore, M. R. (2017). Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environment International, 101, 173–182. 10.1016/j.envint.2017.01.023 [DOI] [PubMed] [Google Scholar]
- Mar, T. F. , Koenig, J. Q. , & Primomo, J. (2010). Associations between asthma emergency visits and particulate matter sources, including diesel emissions from stationary generators in Tacoma, Washington. Inhalation Toxicology, 22(6), 445–448. 10.3109/08958370903575774 [DOI] [PubMed] [Google Scholar]
- Matte, T. D. , Ross, Z. , Kheirbek, I. , Eisl, H. , Johnson, S. , Gorczynski, J. E. , et al. (2013). Monitoring intraurban spatial patterns of multiple combustion air pollutants in New York City: Design and implementation. Journal of Exposure Science & Environmental Epidemiology, 23(3), 223–231. 10.1038/jes.2012.126 [DOI] [PubMed] [Google Scholar]
- Messier, K. P. , Chambliss, S. E. , Gani, S. , Alvarez, R. , Brauer, M. , Choi, J. J. , et al. (2018). Mapping air pollution with Google street view cars: Efficient approaches with mobile monitoring and land use regression. Environmental Science & Technology, 52(21), 12563–12572. 10.1021/acs.est.8b03395 [DOI] [PubMed] [Google Scholar]
- Miller, D. J. , Actkinson, B. , Padilla, L. , Griffin, R. J. , Moore, K. , Lewis, P. G. T. , et al. (2020). Characterizing elevated urban air pollutant spatial patterns with mobile monitoring in Houston, Texas. Environmental Science & Technology, 54(4), 2133–2142. 10.1021/acs.est.9b05523 [DOI] [PubMed] [Google Scholar]
- Mohai, P. , & Saha, R. (2015). Which came first, people or pollution? Assessing the disparate siting and post‐siting demographic change hypotheses of environmental injustice. Environmental Research Letters, 10(11), 115008. 10.1088/1748-9326/10/11/115008 [DOI] [Google Scholar]
- Multi‐State Medium‐ and Heavy‐Duty Zero Emission Vehicle Memorandum of Understanding . (2020).
- Northcross, A. L. , Hsieh, S. , Wilson, S. , Roper, E. , Dickerson, R. R. , Norouzi, P. , & Morris, V. (2020). Monitoring neighborhood concentrations of PM2.5 and black carbon: When using citywide averages underestimates impacts in a community with environmental justice issues. Environmental Justice, 13(2), 27–35. 10.1089/env.2019.0026 [DOI] [Google Scholar]
- Orellano, P. , Quaranta, N. , Reynoso, J. , Balbi, B. , & Vasquez, J. (2017). Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta‐analysis. PLoS One, 12(3), e0174050. 10.1371/journal.pone.0174050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- QGIS Development Team . (2021). QGIS geographic information system. Open Source Geospatial Foundation Project. Retrieved from https://www.qgis.org/ [Google Scholar]
- R Core Team . (2020). R Foundation for Statistical Computing. Retrieved from https://www.r-project.org/ [Google Scholar]
- Southerland, V. A. , Anenberg, S. C. , Harris, M. , Apte, J. , Hystad, P. , van Donkelaar, A. , et al. (2021). Assessing the distribution of air pollution health risks within cities: A neighborhood‐scale analysis leveraging high‐resolution data sets in the Bay Area, California. Environmental Health Perspectives, 129(3), 037006. 10.1289/EHP7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- STATE OF NEW JERSEY, 219th LEGISLATURE . (2020). Retrieved from https://www.njleg.state.nj.us/2020/Bills/S0500/232_I1.HTM
- Tackling the Climate Crisis at Home and Abroad . (2021). Federal Register. Retrieved from https://www.federalregister.gov/documents/2021/02/01/2021-02177/tackling-the-climate-crisis-at-home-and-abroad [Google Scholar]
- Tessum, C. W. , Apte, J. S. , Goodkind, A. L. , Muller, N. Z. , Mullins, K. A. , Paolella, D. A. , et al. (2019). Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. Proceedings of the National Academy of Sciences, 116(13), 6001–6006. 10.1073/pnas.1818859116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakrar, S. K. , Balasubramanian, S. , Adams, P. J. , Azevedo, I. M. L. , Muller, N. Z. , Pandis, S. N. , et al. (2020). Reducing mortality from air pollution in the United States by targeting specific emission sources. Environmental Science & Technology Letters, 7(9), 639–645. 10.1021/acs.estlett.0c00424 [DOI] [Google Scholar]
- The Transportation and Climate Initiative An Agenda for Progress . (2010). Retrieved from https://www.transportationandclimate.org/sites/default/files/TCI-declaration.pdf
- Turner, M. C. , Jerrett, M. , Pope, C. A. , Krewski, D. , Gapstur, S. M. , Diver, W. R. , et al. (2016). Long‐term ozone exposure and mortality in a large prospective study. American Journal of Respiratory and Critical Care Medicine, 193(10), 1134–1142. 10.1164/rccm.201508-1633OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA . (2012). Regulatory impact analysis for the proposed revisions to the National Ambient Air Quality Standards for Particulate Matter. [Google Scholar]
- U.S. EPA . (2019). Integrated Science Assessment (ISA) for Particulate Matter (Final report, December 2019). [Google Scholar]
- U.S. EPA . (2020). Air quality trends show clean air progress. EPA Our Nation's Air. Retrieved from https://gispub.epa.gov/air/trendsreport/2020/ [Google Scholar]
- van Donkelaar, A. , Martin, R. V. , Li, C. , & Burnett, R. T. (2019). Regional estimates of chemical composition of fine particulate matter using a combined geoscience‐statistical method with information from satellites, models, and monitors. Environmental Science & Technology, 53(5), 2595–2611. 10.1021/acs.est.8b06392 [DOI] [PubMed] [Google Scholar]
- Vodonos, A. , & Schwartz, J. (2021). Estimation of excess mortality due to long‐term exposure to PM2.5 in continental United States using a high‐spatiotemporal resolution model. Environmental Research, 196, 110904. 10.1016/j.envres.2021.110904 [DOI] [PubMed] [Google Scholar]
- Yang, B.‐Y. , Fan, S. , Thiering, E. , Seissler, J. , Nowak, D. , Dong, G.‐H. , & Heinrich, J. (2020). Ambient air pollution and diabetes: A systematic review and meta‐analysis. Environmental Research, 180, 108817. 10.1016/j.envres.2019.108817 [DOI] [PubMed] [Google Scholar]
- Yitshak‐Sade, M. , Kloog, I. , Zanobetti, A. , & Schwartz, J. D. (2019). Estimating the causal effect of annual PM2.5 exposure on mortality rates in the Northeastern and mid‐Atlantic states. Environmental Epidemiology, 3(4), e052. 10.1097/EE9.0000000000000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, S. , Wang, J. , Sun, C. , Zhang, X. , & Kahn, M. E. (2019). Air pollution lowers Chinese urbanites’ expressed happiness on social media. Nature Human Behaviour, 3(3), 237–243. 10.1038/s41562-018-0521-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Health data at the neighborhood level for Washington, DC used in this study are not publicly available due to confidentiality of patient information. The baseline disease data for this study were made available to us through an Institutional Review Board request to the Washington, DC Department of Health. Centers for Disease Control and Prevention (CDC) 500 Cities Project data are publicly available at https://www.cdc.gov/places/about/500-cities-2016-2019/index.html. Surface PM2.5 data sets for this study are referenced in Hammer et al. (2020) and van Donkelaar et al. (2019), and are compiled and made publicly available at https://sites.wustl.edu/acag/datasets/surface-pm2-5/. The population data sets from the Socioeconomic Data and Applications Center (SEDAC) are publicly available at https://sedac.ciesin.columbia.edu/data/set/gpw-v4-basic-demographic-characteristics-rev11. Sociodemographic data used in this study are referenced in the Health Equity Report: District of Columbia 2018 (2019), and are publicly available in the US Census Bureau 2011–2015 American Community Survey (ACS) 5‐year estimates website (https://www.census.gov/programs-surveys/acs/technical-documentation/table-and-geography-changes/2015/5-year.html), and life expectancy data is available from the Washington, DC Department of Health Center for Policy, Planning and Evaluation (https://dchealth.dc.gov/page/center-policy-planning-and-evaluation).


