Abstract
A growing appreciation of NADPH oxidases (NOXs) as mediators of fundamental physiological processes and as important players in myriad diseases has led many laboratories on a search for specific inhibitors to help dissect the role in a given pathway or pathological condition. To date, there are only a few available inhibitors with a demonstrated specificity for a given isozyme. Among those, peptidic inhibitors have the advantage of being designed to target very specific protein-protein interactions that are essential for NOX activity. Herein, we provide the techniques to deliver these inhibitors both in cell culture as well as in vivo.
Keywords: NADPH oxidase, Isoform-specific inhibitors, Peptidic inhibitors, Nox2ds-tat, NoxA1ds
1. Introduction
NADPH oxidases (NOXs), major sources of reactive oxygen species (ROS) in the cell, have emerged as key players both in physiological signal transduction and in disease. Dysregulation of this family of enzymes causing an excess production of reactive oxygen species has been demonstrated to promote the development of pathophysiological conditions including inflammation, cardiovascular diseases, neurodegenerative diseases, and cancer.
To ameliorate the negative impact that dysregulation of NOXs have in disease, and to understand the role that specific isozymes of the enzyme play in a given pathway, intense efforts have been made to develop NOX-specific inhibitors [1]. The main goal is targeting the source of ROS rather than the ROS themselves as had been attempted for a long time with a variety of chemical and enzyme antioxidants. Given the complexity of subunit interactions involved in the assembly of active enzyme and similarities among isozymes in this family, the task of obtaining isoform-specific inhibitors has proven to be extremely difficult. Although the limitation of reduced oral bioavailability is a common refrain, evolving technologies and routes of delivery methods are being employed to avoid this fate (see below for one example) as is the case with other biologics. That notwithstanding, one major advantage that makes this class of inhibitors uniquely valuable is that unlike small molecule inhibitors, they can be designed to competitively block primary protein-protein interactions by precisely replicating a unique peptide-binding domain. This rational approach is intended to minimize off-target effects.
Our laboratory has developed two isoform-specific NOX inhibitors that have proven useful to dissect the role of these isozymes in a variety of processes. One of these inhibitors was directed toward the canonical NOX2 isozyme, Nox2ds-tat (aka gp91ds-tat) [2, 3], and the other was directed against the canonical NOX1 isozyme, NoxA1ds [4]. Below we discuss both of these peptides to exemplify the different strategies used in their design.
Nox2ds-tat (see Fig. 1) is the most widely used NOX2-specific inhibitor. It contains a sequence that mimics a portion of the second intracellular loop of NOX2 (loop B) known to be a site of interaction between NOX2 and p47phox subunits [5, 6], and it is linked to a short 9-amino acid peptide span of HIV-tat viral coat protein (HIV-tat), which has been shown to deliver “cargo” proteins into cells [7]. Rodent and human versions of the peptide differ only in one amino acid; the isoleucine in the rodent version is replaced by a valine in the human version. Using human heterologous systems expressing only one of the NOX isozyme systems at a time, we demonstrated that human Nox2ds-tat is specific for the canonical NOX2 [3]. In in vivo experiments, rodent Nox2ds-tat attenuated angiotensin II-induced increases in blood pressure [2] and suppressed angioplasty-induced superoxide production and neointimal hyperplasia of the carotid artery [8]. Moreover, Quesada et al. demonstrated that treatment with rodent Nox2ds-tat is able to reverse atherosclerotic plaque formation in aorta from ApoE−/− mice fed a high fat diet [9]. The studies described above are a small sample of a wide array of studies that have employed both human and rodent versions of Nox2ds-tat as a tool to define the physiological role of NOX2 and to study the therapeutic potential of NOX2 inhibition.
Fig. 1.

Sequence of Nox2ds-tat containing the tat sequence followed by a sequence of 9 amino acids corresponding to loop B of NOX2 (highlighted in gray). Sequence from rodent and human differ in the isoleucine to valine substitution shown in bold. In scramble control peptides, the loop B sequence is scrambled, but the tat sequence is kept intact
NoxA1ds (see Fig. 2) was designed to target the interaction between NOXA1 and NOX1 of the canonical NOX1 oxidase isozyme by replicating the amino acid region on NOXA1 that is equivalent to the “activation domain” of p67phox [10]. The amino acid sequence of this peptide includes residues between NOXA1 and p67phox that are homologous (residues 200–205) and nonhomologous (residues 195–198) to confer effective blockade and specificity toward the NOX1 system, per se. It also comprises a substitution of Phe-199 for Ala, a modification previously shown to reduce NOX1 activity [4, 11].
Fig. 2.
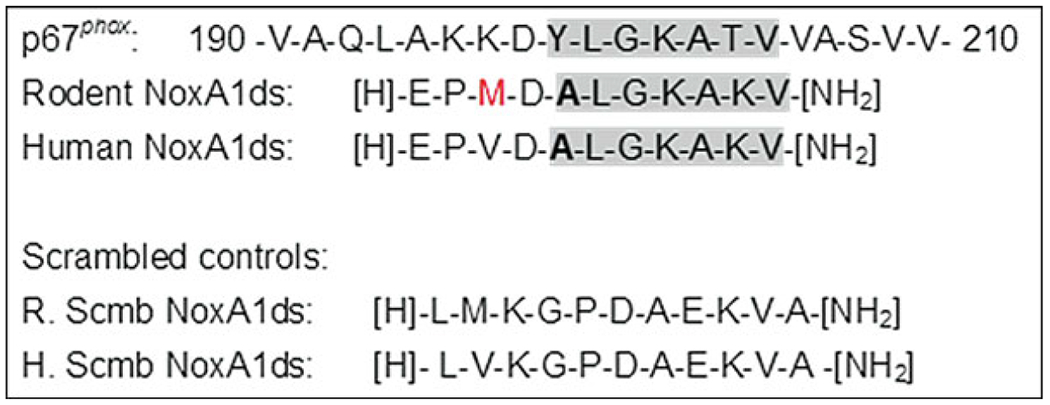
Sequence of NoxA1ds corresponding to amino acids from NoxA1 containing the activation domain (highlighted in gray) and 5 amino acids upstream of it which do not share homology to the equivalent region on p67phox. Shown in bold is the Ala used to substitute Phe-199. Shown in red is the methionine from the mouse NoxA1 that replaces the valine in the human version of the protein. Scrambled control peptides for each version are also shown
This peptide was originally used to delineate the role of NOX1in hypoxia-induced human endothelial cell ROS production and VEGF-stimulated migration. Subsequently, it has been used to test the role of NOX1 in uniaxial stretch-induced phenotypic transitioning of rat vascular smooth muscle cells from a contractile to synthetic phenotype [12], and more recently human NoxA1ds was used as a modality to target senescence-associated endothelial dysfunction [13].
In this chapter, protocols for the delivery ofpeptidic inhibitors both in cell culture and in vivo are detailed.
2. Materials
Peptidic inhibitors: Peptides for use in our studies have generally been synthesized at the Tufts University Core (http://www.tucf.org/peptidesynthesis-f.html) with acetylation of the N-terminus and amidation of the C-terminus. Purity of the peptide stock is usually near 90% (see Note 1). Any peptide synthesis core that meets the same quality standards may be contracted. Please refer to and reference Rey et al. 2001 [2], Csanyi et al. 2011 [3], and Ranayhossaini et al. 2013 [4] for more detail.
Acetic acid-saline solution: 0.01 N acetic acid in 0.9% NaCl (28 μL glacial acetic acid into 50 mL 0.15 M NaCl).
Culture media (recommended formulation is dependent on cell type). For example, human pulmonary artery endothelial cells (HPAEC) purchased from Lonza (Basel, Switzerland) are maintained in the growth medium (EBM-2) suggested by Lonza, while rat aortic smooth muscle cells (RASMC) (Lonza, Walkersville, MD, USA) were grown in DMEM (Cellgro) with 4.5 g/L glucose, l-glutamine, and sodium pyruvate containing 10% heat-inactivated fetal bovine serum (FBS) and 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen).
Opti-MEM™ (Gibco).
Lipofectamine™ LTX with PLUS™ reagent (Invitrogen).
Phorbol 12-myristate 13-acetate (PMA): 20 mM stock dissolved in DMSO (store at −20 °C).
Amplex Red reagent (Life Technologies/Invitrogen): 20 mM dissolved in DMSO (store at −20° C in 100 μL aliquots).
AR assay buffer (1× stock): 25 mM HEPES, 0.12 M NaCl, 3 mM KCl, 1 mM MgCl2. Adjust the pH to 7.4 with HCl.
2× Amplex Red/ HRP solution: 55 μL of 20 mM Amplex Red, 3.5 μL of 1 U/μL HRP stock, 5.5 mL AR assay buffer (prepared fresh at the time of assay).
NADPH (12.5× stock): 0.45 M NADPH in 1× AR assay buffer (prepared fresh at the time of assay).
Alzet™ osmotic minipumps (volumes, rate of infusion, and length of treatment should be chosen according to animal model and outcome of interest). Insertion of pumps can be for delivery of peptides intravenously (i.v.), subcutaneously (s.c.), or intraperitoneally (i.p.). Delivery s.c. or i.p. will be described here in detail. For all three routes of administration and particularly i.v. delivery, see Alzet’s protocols (http://www.alzet.com/products/guide_to_use/implantation_and_explantation.html).
Surgical supplies.
CompAir XLT nebulizer (Omron) for aerosolized delivery to lungs and airways.
Fiberglass nebulization chambers for aerosolized delivery.
3. Methods
3.1. Design of Peptidic Inhibitors
The design of specific and efficient peptidic inhibitors is based on the knowledge of protein-protein interactions essential for the catalytic activity of the enzyme (see Fig. 3). Some of the strategies employed for identification of regions of interaction among the various subunits of NOX (mainly NOX2 isozyme) include the use of peptide phage display libraries [6] and “peptide walking” [14]. Many of these interactions have been published and described in various reviews [15–17]. Dahan and Pick [18] have proposed a host of potential strategies for inhibition. Nox2ds-tat and NoxA1ds are good examples of inhibitors based on available information and rational design; in the case of Nox2ds-tat, the strategy involved the use of the tat sequence to overcome the difficulty of penetration [2, 3], while in the case of NoxA1ds, the strategy involved the inclusion of amino acids outside the activation domain to afford specificity [4].
Once the sequence of the region of interest is determined, use searching engines like BLAST™ (basic local alignment search tool, https://blast.ncbi.nlm.nih.gov) as described in Rey et al. 2001 [2] to assess conservation or lack thereof of this sequence in other proteins. The objective is to choose a peptide with no or very minimal homology to other proteins.
Based on the selected peptide sequence, generate its scrambled peptide control by randomly changing the order of the amino acid sequence and checking for the minimum number of hits in the search engine. In the case of a chimeric peptide containing the tat sequence, only the selected peptide sequence is scrambled; the tat is kept intact.
Analysis of the hydrophobicity of the selected peptide will determine if the inclusion of a tat sequence is necessary (see Note 2).
Testing of the designed peptide should include effect on NOX activity, scavenging properties, specificity, cell penetration, toxicity, etc.
Although rational design is expected to yield very selective inhibitors, success is dependent not just on the sequence chosen but also on how tight the interactions being targeted are, as was implicated in the failure of multiple attempts to target NOX4 intramolecular or NOX4-p22phox interactions [19].
Fig. 3.
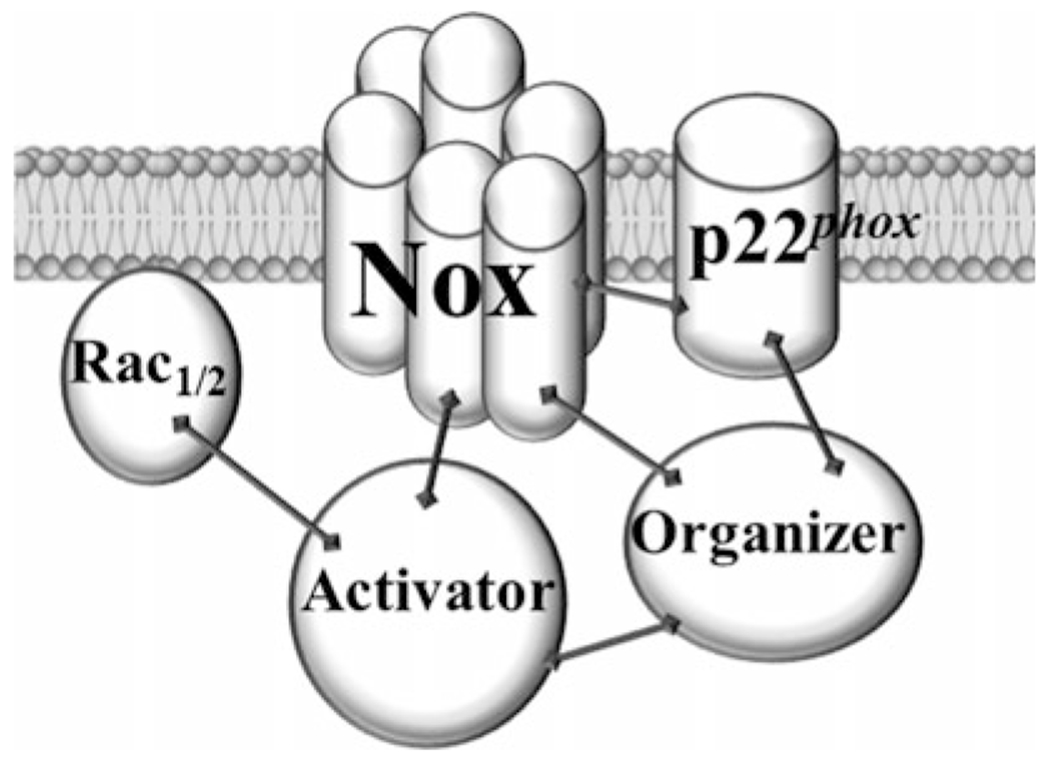
Protein-protein interactions important for the assembly of a fully active prototype NOX
3.2. Test of Peptide Inhibitors in Cells In Vitro
In order to determine specificity and potency of inhibitors, it is ideal to have a heterologous cell system expressing all the subunits needed for the NOX isoform of interest. A stably transfected cell line commonly used for this purpose is COS-phox, developed by Dr. Dinauer [20], which expresses all the components necessary for NOX2 activity. For other NOX isoforms, transiently transfected HEK-293 or COS7 cells can be used.
3.2.1. Cell Transfection
Grow HEK-293 or COS7 cells in 100 mm plates to 70% confluency.
Change media to Opti-MEM (Gibco) (13.5 mL).
In a 15 mL conical tube containing 2.7 mL of Opti-MEM, add 20 μg total plasmid DNA (if plasmids for four subunits are going to be transfected, then add 5 μg of each). Mix.
Add 6.75 μL PLUS reagent, mix, and let stand for 5 min at room temperature.
Add 30 μL Lipofectamine LTX, mix, and let stand for 30 min.
Add DNA-Lipofectamine mix dropwise to cells.
Incubate overnight at 37 °C.
3.2.2. Cell Treatments
Grow cells of choice in appropriate media to 70–80% confluency (see Note 3).
Serum starve the cells to promote cell cycle synchronization by changing the growth medium for a medium containing no more than 10% of the original media’s growth factors/serum concentrations (see Note 3), and incubate overnight.
Prepare peptide solution by weighing the appropriate amount of peptide to have at least 100× working solution and resuspending it in acetic acid in saline solution (see Note 4). Peptide solution is prepared freshly the day of the experiment. The 1:100 dilution or greater ensures that the acetic acid-saline solution has no effect on the pH of the medium.
Pretreat the cells with the peptide, scrambled peptide, or vehicle for 30 min before addition of stimuli.
Stimulate the cells with phorbol 12-myristate 13-acetate, PMA (1–5 μM) for NOX2 activity, or 10 μM ionomycin plus 1 μM PMA for NOX5. For NOX1 and NOX4, activity is compared to that of untransfected control cells.
3.2.3. ROS Assay
Assess ROS production in the presence and absence of the inhibitors using whole cells or homogenates of stimulated cells. In general, the ROS assay of choice in this case is Amplex Red as it is more sensitive than the gold standard assay, cytochrome C, as described by Csanyi 2011 et al. [3]. Assay can be done with whole cells or homogenates of stimulated cells.
Disrupt cells/tissues in ice-cold disruption buffer (HBSS containing protease inhibitor cocktail) by glass-on-glass homogenizer (tissues) or five freeze/thaw cycles (cells), and pass through a 30-gauge needle five times to further lyse the cells. Centrifuge the cell lysate at 1000 × g for 10 min at 4 °C to remove unbroken cells, nuclei, and debris. Throughout all these procedures, extreme care should be taken to maintain the lysate at a temperature close to 0 °C.
Measure protein concentrations.
-
Prepare working solutions (Subheading 2, step 8–10) and cell or cell homogenates for ROS assays.
If whole cells are going to be tested in a 384-well plate, seed ~40,000 cells per well in Opti-MEM. If cell homogenates are going to be tested, amount of protein needs to be optimized for each tissue/cell type. For 384-well plate, test in a range between 0.1 and 1 μg/well. Dilute appropriate amount of cell homogenate in AR assay buffer.
Prepare H2O2 standards by serial dilution of stock H2O2 in AR assay buffer starting at 10 μM concentration. Plate 25 μL/well of each dilution, and then add 25 μL/well of 2× AR/HRP solution.
-
For a 384-well plate, add cell homogenate and then 2× Amplex Red/ HRP solution to wells. Read four points for baseline fluorescence. Add NADPH and read for 1 h.
– Reaction (in microliters)
Cells or cell homogenate: 21
2× AR/HRP: 25
0.45 mM NADPH: 4
Fluorescence measurements are made using a microplate reader with a 530 nm excitation and a 590 nm emission filter.
Data are calculated as the rate of RFU/min.
Although depending on the cell type and endogenous SOD levels, superoxide dismutation happens spontaneously. To measure superoxide production, SOD can be added to the reaction mixture to ensure full conversion of superoxide into H2O2.
Repeat the test using cells expressing each of the other isoforms to determine isoform specificity.
3.3. Use of Peptide Inhibitors in Cells In Vitro
Treat cells as described above (see Subheading 3.2.2, step 1–3).
Pretreat the cells of interest with the peptide, scrambled peptide, or vehicle for 30 min–4 h before addition of stimuli.
Stimulate the cells with the agent to be tested (i.e., angiotensin II, TNFα, etc.) for the length of time necessary for the response of interest (e.g., migration, proliferation, protein expression). If treatment time with stimulant is longer than 6 h, at 4 h intervals after the stimulus administration, add peptide again to the same final concentration as the initial administration (see Note 5).
Assess biochemical and physiological readouts of interest.
3.4. Application of Peptide Inhibitors In Vivo
The application of peptides in animals can be performed by simple injections intraperitoneally or procedures that are less stressful for the animal such as via minipump or aerosolization.
3.4.1. Preparation of Osmotic Minipumps for In Vivo Delivery
Prepare Alzet osmotic minipumps containing either vehicle (0.01 N acetic acid in saline solution), stimulus solution (e.g., angiotensin II delivered at a rate of 0.75 mg/kg/day), or combined stimulus + peptide (10 mg/kg/day) or combined stimulus + scrambled peptide (10 mg/kg/day).
-
Calculate the amounts of peptide needed:
Amount of peptide per day = Dosing rate (in mg/kg/day) × Weight of animal (in gm)/1000.
Volume of infusion per day (μL/day) = 24 × mean pumping rate ascribed by the manufacturer (in μL/h).
Concentration of peptide solution (mg/μL) = Amount of peptide per day/Volume of infusion per day.
Amount (weight) of peptide per pump = Concentration of peptide solution × volume of pump.
This amount of peptide is multiplied per the number of animals per group and resuspended in acetic acid-saline solution in the appropriate volume required by each pump (for an example of calculations, see Note 6), calculating ~10–20% in excess for handling. It is important also to take into account the solubility of the peptide for the dosage chosen in case treatments require more than one pump.
Sterilize the peptide and vehicle solutions by loading them into a syringe and passing the contents through a 0.22 μm sterile disc filter.
While wearing gloves, and in a sterile culture hood, fill pumps using insertion “needle” provided by the pump manufacturer, being careful not to introduce bubbles into the solutions. Then, immerse pumps into a sterile 0.9% NaCl saline solution in 50 mL conical tubes, and cover the tubes to maintain sterility.
Place tubes at 37 °C for 3 to 4 h in an incubator to allow pumping to commence (priming of the pump).
3.4.2. Surgical Implantation of Osmotic Minipumps
Anesthetize the animal as required, shave abdomen or nape of the neck, and place animal on a heating pad.
For i.p. delivery, under aseptic conditions, make a midline incision of 1/3 in. on the skin; separate skin from peritoneal wall using forceps. For s.c. delivery make a similar incision in the scapular region in the nape (backside) of the neck.
Cut into the peritoneal wall or subdermal scapula, with care not to damage internal organs, to make an incision just large enough to insert pump. Using a hemostat spread the tissue to create a pocket for the pump.
Insert mini-osmotic pump holding it from the bottom and sliding it into the pocket, delivery portal side first.
Close the wound opening and skin using silk sutures or wound clips.
Apply betadine solution to the wound, and keep the animal on the heating pad until it recovers from the anesthesia.
When the animal is awake and moving, return it to cage and monitor for postoperative pain/distress (examples include failure to eat/drink, hovering in the corner, shallow breathing, and failure to groom). To alleviate pain and distress, analgesic (buprenex −0.5 m g/kg s.c.) can be administered once during recovery post minipump implantation, and repeat every 6–12 h for 24 h post-surgery. Monitor animals daily.
Depending of the experiment, animals are maintained under normal husbandry conditions, following appropriate IACUC regulations, for the appropriate length of time.
Take appropriate measurements according to outcome of interest.
3.4.3. Aerosolization
In order to deliver a therapeutic dose of the inhibitor in the form of an aerosol ofrespirable particles, a nebulizer is used. The mist to be inhaled is created by a flow of air going through the narrow opening in the nebulizer cup.
Prepare peptide solution. Depending on the number of animals that can be accommodated in an enclosed chamber, calculate the amount of peptide necessary for the desired dose. For example, for a four-animal chamber, to have a dose of 5.1 mg/kg for a rat of 275 g (=1.4 mg/rat), weigh 5.6 mg of peptide.
Dilute appropriate amount of peptide in a minimum of 5 mL (see Note 7).
Place the animals in the chamber.
Set nebulizer compressor to an air flow rate of 6 L/min (equivalent to a nebulizer rate of 0.2 mL/min).
Aerosolize for 30 min per treatment.
Repeat treatment as appropriate (see Note 8).
Acknowledgments
Research in the Pagano Lab is supported by the National Institutes of Health Grants R01HL079207 and P01HL103455-01 and receives support from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
4 Notes
Commonly used Nox2ds-tat has a molecular weight of 2452.98 gr/mol for the rodent form RKKRRQRRRCSTRIRRQL and 2438.96 gr/mol for the human form that has the isoleucine substituted by a valine. For NoxA1ds the molecular weight is 1157.4 gr/mol for the rodent form, EPMDALGKAKV, and 1167.38 gr/mol for the human version that has the methionine substituted by a valine.
Hydrophobicity can be calculated using the calculator in: https://www.peptide2.com/N_peptide_hydrophobicity_hydrophilicity.php. For example, the values obtained for Nox2ds are as follows: hydrophobic, 22.22%; acidic, 0%; basic, 33.33%; and neutral, 44.44%. These values suggest that hydrophobicity of Nox2ds sequence is low so it required a carrier, such as the tat sequence, to allow for membrane translocation. In contrast to the values calculated for NoxA1ds, hydrophobic is 54.55%, acidic is 18.18%, basic is 18.18%, and neutral is 9.09% that suggested its ability to permeate the plasma membrane.
Depending on the cells used, the level of confluency may negatively affect NOX activity; thus preliminary assays are required to determine the optimal confluency to achieve an optimal NOX activity.
The sensitivity of the cells to serum starvation should also be determined beforehand, i.e., some cells such as endothelial cells do not tolerate lower than 10% of the original growth factors/serum concentrations in the growing media.
Dilution of peptides in acetic acid-saline solution is done to minimize peptide binding to the plastic or glass vial.
Until the stability of the peptide inhibitor in the preparation or animal can be determined, repeated doses of peptide may ensure that the desired concentration is maintained for the duration of the experiment.
For a 7-day infusion of peptide at 10 mg/kg/day in a 30-gram mouse using an Alzet 2001 pump (specifications for a 7-day pump: 1 μL/h mean pumping rate, 200 μL reservoir volume):
Amount of peptide per day = 10 (mg/kg/day) × 30 (gr)/1000 = 0.3 mg /mouse/day
Volume of infusion per day = 24 × 1 μL/h = 24 μL/day
Concentration of peptide solution (mg/μL) = 0.3 mg/24 μL = 0.0125 mg/μL (=12.5 mg/mL)
Amount of peptide per pump: 0.0125 mg /μL × 200 μL = 2.5 mg
For four mice in each group (2.5 mg × 4 = 10 mg), 12 mg of peptide should be resuspended in a 960 μL acetic acid-saline solution (a 20% excess allows for small losses during pump loading).
For protracted treatment protocols, for example, for hypoxia regimen lasting 3 weeks, NoxA1ds was aerosolized to rodents, as described, every 3 days for the duration of the treatment. The profound right ventricular hypertrophy and increased Fulton index induced by hypoxia were significantly ameliorated by the aerosolization with NoxA1ds (unpublished data).
References
- 1.Cifuentes-Pagano ME, Meijles DN, Pagano PJ (2015) Nox inhibitors & therapies: rational design of peptidic and small molecule inhibitors. Curr Pharm Des 21(41):6023–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ (2001) Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O 2 - and systolic blood pressure in mice. CircRes 89:408–414 [DOI] [PubMed] [Google Scholar]
- 3.Csanyi G et al. (2011) Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51 (6):1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranayhossaini DJ et al. (2013) Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem 288 (51):36437–36450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLeo FR, Quinn MT (1996) Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol 60(6):677–691 [DOI] [PubMed] [Google Scholar]
- 6.DeLeo FR et al. (1995) Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc Natl Acad Sci U S A 92 (15):7110–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawell S et al. (1994) Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A 91(2):664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson GM et al. (2003) Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res 92(6):637–643 [DOI] [PubMed] [Google Scholar]
- 9.Quesada IM et al. (2015) Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 242 (2):469–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD (1998) Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox). J Biol Chem 273(27):16663–16668 [DOI] [PubMed] [Google Scholar]
- 11.Maehara Y et al. (2010) A conserved region between the TPR and activation domains of p67phox participates in activation of the phagocyte NADPH oxidase. J Biol Chem 285 (41):31435–31445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez AI et al. (2015) MEF2B-Nox1 signaling is critical for stretch-induced phenotypic modulation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 35(2):430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijles DN et al. (2017) The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci Signal 10(501):eaaj1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph G, Pick E (1995) “Peptide walking” is a novel method for mapping functional domains in proteins. Its application to the Rac1-dependent activation of NADPH oxidase. J Biol Chem 270(49):29079–29082 [DOI] [PubMed] [Google Scholar]
- 15.Brandes RP, Kreuzer J (2005) Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65(1):16–27 [DOI] [PubMed] [Google Scholar]
- 16.Brandes RP, Weissmann N, Schroder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76C:208–226 [DOI] [PubMed] [Google Scholar]
- 17.Sumimoto H (2008) Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275 (13):3249–3277 [DOI] [PubMed] [Google Scholar]
- 18.Dahan I, Pick E (2012) Strategies for identifying synthetic peptides to act as inhibitors of NADPH oxidases, or “all that you did and did not want to know about Nox inhibitory peptides”. Cell Mol Life Sci 69 (14):2283–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csanyi G, Pagano PJ (2013) Strategies aimed at Nox4 oxidase inhibition employing peptides from Nox4 B-LOOP and C-terminus and p22 (phox) N-terminus: an elusive target. Int J Hypertens 2013:842827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price MO et al. (2002) Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood 99(8):2653–2661 [DOI] [PubMed] [Google Scholar]


