Abstract
Heart failure with a preserved ejection fraction (HFpEF) affects half of all patients with HF worldwide, has an increasing prevalence, substantial morbidity and mortality, and very few treatments have proven to be effective. It arguably represents the greatest unmet medical need in cardiovascular disease and is certainly very prominent across all medicine. While initially a disorder characterized by hypertension, hypertrophy and diastolic dysfunction, the syndrome has become greatly impacted by the pandemic of obesity and diabetes and is currently recognized as a multisystem disorder involving heart, pulmonary, renal, skeletal muscle, adipose tissue, immune/inflammatory signalling and vascular systems. This has made it hard to mimic in experimental animals, as it is not simply hypertrophy and hypertension with abnormal relaxation in a mammal. However, new models involving both hemodynamic and metabolic disease, and increasing efforts to examine human pathophysiology, are revealing new signalling and potential therapeutic targets. This Review tackles the basic pathobiology of HFpEF broadly, though a major focus is on mechanisms pertinent to the heart as most of the existing research has focused on this organ. That said, there is also examination of peripheral organ systems, including skeletal muscle, lung, and kidney, as well as systemic biomarkers, and ongoing therapeutic efforts. The goal is to provide a mechanistic road-map of signalling and mechanisms that are being revealed and may finally lead to more patient-specific therapies with clinical impact.
Introduction
Heart failure with a preserved ejection fraction (HFpEF) is a leading cause of morbidity and mortality throughout the industrialized world, and its prevalence is increasing at an alarming rate. HFpEF currently represents 50% of all HF1. Patients with this syndrome develop classic HF symptomatology including exertional intolerance, breathlessness, extravascular fluid accumulation in the lungs, subcutaneous tissues and abdominal cavity, and intermittent cardiovascular decompensation that often leads to hospitalization for urgent diuresis. In using the term HFpEF, we are excluding diseases such as cardiac amyloidosis, genetic hypertrophic cardiomyopathy, valvular disease, and other disorders for which there is a defined etiology. HFpEF refers to the much larger population of patients for which the pathophysiology involves a multi-organ syndrome where cardiac, pulmonary, renal, skeletal, immune/inflammatory, metabolic, and other components collude to cause symptoms and outcomes. Importantly, it is a highly morbid and mortal syndrome, with recent 2-year all-cause mortality or HF hospitalization rates at 35% (compared to 43% for HF with a reduced EF; HFrEF) and a mortality of 14%2. There are also very few effective pharmacological or device treatments for HFpEF1,3,4, making it a major unmet medical need.
Recognition of patients with HF symptoms but a normal-range EF began appearing in the 1970s as case reports of ischemic (but not infarcted) heart disease patients5. The proposed cause was a left ventricle that was stiff in diastole requiring high filling pressures, and became viewed as diastolic HF. The first prospective report of “HF with normal systolic function” appeared in 19846, finding nearly one-third of HF patients had this condition (mean EF of 58%). They were often hypertensive but had otherwise similar demographics, physical and radiographic findings, and measures of diastolic dysfunction as HFrEF. Even these early studies found HFpEF was heterogeneous, with many patients having normal diastolic behaviour7. A common presentation was an elderly woman with systolic hypertension and a small volume hypertrophied and hyperdynamic heart often obliterating the distal cavity during systole, who presented with episodic pulmonary oedema8. Community data confirmed HFpEF was common, finding mortality rates similar or somewhat below HFrEF9,10, and associated with major comorbidities, including advanced age, systolic hypertension and female sex.
Based on these findings, treatments aimed to improve diastole with β-receptor and calcium-channel blockers as used for genetic hypertrophic cardiomyopathy, lower filling volumes with diuretics, and reduce blood pressure with antihypertensives — mostly angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers. While palliative, these measures did not improve overall outcome or blunt the rise in HFpEF cases, and by the early 2000s, the diastolic HF paradigm started to unravel. First off, diastolic dysfunction was common in the elderly without HF and was mild or absent in many with HFpEF11. Other abnormalities were revealed, including arterial stiffening and adverse ventricular–vascular interaction12,13, chronotropic incompetence14-16 and pulmonary hypertension with right HF17-22, spawning the name change to HFpEF. This was a descriptive but still misleading term with respect to pathophysiology, since having a ‘preserved’ EF mostly meant the heart was not dilated, not that systolic function was necessarily normal23-27, or the heart the principal cause of symptoms.
The most dramatic evolution in HFpEF, however, developed over the past 1–2 decades, as the syndrome became closely associated with obesity and metabolic syndrome epidemics28-31. In addition to diffuse hemodynamic abnormalities, HFpEF now also exhibited inflammation and circulating inflammatory biomarkers32-35, vascular insufficiency36-41, pulmonary and renal dysfunction, and abnormal skeletal blood flow and metabolism42-46. Today, obesity and type 2 diabetes occur in most HFpEF patients, with average BMIs in the mid-30s and rising, and are major drivers of the pathophysiology. Hypertensive hypertrophic heart disease in lean patients has become rare in the USA, although it still exists notably in Asia47, though diabetes and lower-BMI visceral adiposity are present in that population as well.
HFpEF is now widely recognized as an integrative systems disorder, with multiple organ pathologies contributing to the syndrome. While, H for heart remains the first letter in HFpEF, its role versus obesity, renal, pulmonary, inflammatory and skeletal muscle disease has become somewhat ambiguous. The heart within a morbidly obese patient works harder to perfuse the extra tissue and propel a higher mass (per Newton’s Law). Most mechanistic understanding of human HFpEF stems from organ and systems physiology studies many described in excellent recent reviews1,3,48-50. Far less understood are the cellular and molecular changes characterizing the syndrome. HFpEF patients rarely receive a heart transplant, and in situ heart biopsies are performed by only a handful of centres worldwide, so tissue data, particularly from live myocytes, are extremely limited. Despite this, hypothetical schemes have been proposed highlighting roles of fibrosis, inflammation, vascular insufficiency, dysfunctional NO and cGMP signalling, abnormal metabolism and other factors29,51.
This Review focuses on cellular and molecular mechanisms as currently understood from the available experimental and clinical data. The figures in various sections are comprehensive and include pathways reported in HFpEF models as well as those likely engaged at least in subsets of these patients. However, many are pertinent to HFrEF as well. Those that are unique to HFpEF are certainly being sought, but proof of specificity in humans remains lacking. That said, the areas discussed are all on current lists of potential therapeutic targets. We put the major focus on the heart, not because this is necessarily the root cause of HFpEF, but that most reported pertains to it. Multi-organ contributors to the syndrome are also discussed, and we conclude with a perspective of current and future therapeutic directions.
Animal models - where we’ve been and where we need to go
Animal models of HFpEF have largely mirrored clinical paradigms at the time they were developed, so not surprisingly, most have emphasized hypertension, left ventricular hypertrophy (LVH) and diastolic dysfunction. Pressure-load-induced LVH in the Dahl salt-sensitive rat (DSSR)52,53 or spontaneously hypertensive rat (SHR)54, renovascular hypertension55, and mice with aortic-constriction or hormone-induced hypertension are prime examples. These rodent models often involve substantial systolic pressure rise, with a hyper-compensated early stage when EF is ‘preserved’, diastolic relaxation delayed, and compliance reduced, that is eventually followed by a dilated phase with depressed function. Recent studies have revealed metabolic abnormalities in DSSRs, such as increased glycolysis with uncoupling of glycolysis from glucose oxidation resulting in proton production56. During the initial months of age EF is still preserved, and calcium homeostasis and Ca2+ transients and myocyte shortening are normal if not increased57. Beyond this time, however, the LV dilates, EF declines and calcium handing looks more like HFrEF53,58. This is a limitation of the model, as this transition is rare in humans59. Another limitation of hypertensive-hypertrophy models is their hearts are substantially benefitted by angiotensin II or other hormone blockade60-63, which is also not true of human HFpEF. Despite the limitations, models of pressure-volume load continue to be used to test new therapies, including most recently histone deacetylase (HDAC) inhibition64,65 and stem cells66.
A parallel universe testing the myocardial impact of diabetes also began in the early 1980s67. While long associated with vascular and ischaemic heart disease, such studies revealed myocardial abnormalities as well. Flash forward 4 decades to Jia and colleagues68, who in their recent review on diabetic cardiomyopathy, list as causative factors: mitochondrial dysfunction, oxidative stress, reduced nitric oxide bioavailability, cardiomyocyte and extracellular matrix-based stiffening, impaired cation channel homeostasis, inflammation, renin–angiotensin–aldosterone system (RAAS) activation, endoplasmic reticulum stress, microvascular dysfunction and multiple metabolic defects. Virtually the same list is found in most contemporary reviews of proposed HFpEF pathophysiology51. In humans, diabetes does not generally exist in a vacuum, but is often accompanied by hypertension, obesity, renal, hepatic, pulmonary and other organ diseases. HF evolving from this constellation is multifactorial. Not quite so in pure diabetic models, wherein hearts demonstrate abnormalities but less often HF. However, HFpEF is not monothematic either, and the growing role of obesity and metabolic defects in this syndrome has sparked animal models combining haemodynamic load and metabolic stress.
In 2000, Tofovic and colleagues69 crossed the Zucker Diabetic Fatty Rat (missense mutation in leptin receptor) with an inbred SHHF rat (a cross between SHR and SHROB, the latter a SHR rat with spontaneous leptin receptor mutation) to generate the ZSF-1 rat. The ZSF-1 rat develops LVH, hypertension, diastolic dysfunction, fibrosis, obesity, hyperlipidaemia, renal dysfunction, reduced NO signalling and aortic stiffening70. While not developing the severe fluid dys-homeostasis found in human HFpEF, it has still become a popular model in academia and industry. It was recently used to show therapeutic benefits from novel guanylyl cyclase 1 stimulators that augment cGMP71, nitrite to improve GLUT4 signalling via a SIRT3–AMP kinase pathway72, and neuregulin 1 to improve diastolic distensibility by enhancing ERK1/2 activity to phosphorylate titin73. However, the ZSF-1 rat also benefits from ACE inhibition74, and it does not display substantial diastolic pressure elevation or natriuretic peptide (NP) increases until old age. Also, as a genetic mix, the potency of each of its components is diluted.
Another recent model developed in C57BL/6 mice combines chronic NOS1 and NOS3 inhibition by l-NAME (N-nitroarginine methyl ester) with metabolic stress from a high-fat diet75. This rapidly gained popularity given its ease of use, short development duration, and phenotype consistency. It is also among the best-characterized models for HFpEF, including rest and exercise data, extensive histopathological, functional, and molecular assays, and does display multiple relevant features75. However, it also has limitations. l-NAME does not mimic the pathophysiology of hypertension, volume/salt load, and related pathophysiologies in human HFpEF, and may itself bias towards unique oxidant/nitrosative imbalances. The model is less impactful in female mice with or without their sex hormones which differs from humans where post-menopausal females comprise a majority of HFpEF patients76. Also, skeletal muscle changes that are prominent in human HFpEF were not observed in the model.
Lastly, in an era dominated by mouse models, it is recognized that behaviour in rodents does not guarantee translation to humans, so investigators have returned to larger mammals, including dogs, pigs and non-human primates. Studies in aged dogs subjected to peri-nephritis-induced hypertension produced hearts with characteristics and haemodynamic load responses similar to those in humans with HFpEF12,77. More recent pig models combine deoxycorticosterone acetate (DOCA) and salt loading to stimulate volume expansion and hypertension with a Western diet (high fat, sugar and salt) have been generated78-80. They develop some characteristic HFpEF cardiac morphology including left atrial dilatation with reduced systolic function, myocardial fibrosis, oxidative and nitrosative stress, and titin hypophosphorylation78,80. However, unlike HFpEF patients, hearts in these models have minimally elevated diastolic pressures and relaxation delay, and LV chambers are smaller, with compensatory hypertrophy79,80. Alas, it is proving hard to replicate the syndrome in large mammals at anywhere near the severity and associated morbidity common to humans.
Table 1 summarizes the various models discussed, highlighting what they do and do not capture with respect to HFpEF. A persistent problem with pre-clinical models of HFpEF is that while each reflect some features, none really model the human syndrome. The continued use of pure hypertension - hypertrophy models, which is no longer a common HFpEF phenotype should be discouraged. Combination models that engage multiple factors such as obesity and hemodynamic stress are better, but there also needs to be more consideration of the quantitative extent of the various components. Many HFpEF patients have Class II or higher obesity, and this is not often generated in the animal models studied. In humans, blood pressure elevation is treated and so generally below 130 mmHg systolic, so pre-clinical models with substantial untreated hypertension are less relevant. Alternatively, some studies employ very mild stressors, or induce diastolic dysfunction that while technically present is very mild and would not compromise cardiovascular function. Evidence that the model captures exertional impairment and ideally fluid retention and redistribution is also important. Another feature that maybe decisive in the human disease yet rarely appears in preclinical models is defects in skeletal muscle blood flow and metabolism. This needs more focused efforts. Therapeutic studies should ideally test several different models including perhaps one in a larger mammal, though the latter remains difficult as none have yet come close to mirroring the human syndrome. Lastly, to truly move us closer to translationally relevant models, they need to be easily implemented in multiple laboratories so that replication/validation can occur.
Table 1 ∣.
Animal models of HFpEF
| Animal Model | LVH | HTN | Obesity T2DM |
Diastolic Dysfunction |
Skeletal Muscle Abnl and/or Exercise Intolerance |
Fluid overload >Lung wt |
Improved by ACE/ARB |
|---|---|---|---|---|---|---|---|
| Dahl-salt sensitive rats | ++ | ++ | − | ++ | + | Not pre-HF | +/+ |
| Spontaneously hypertensive rats | ++ | ++ | − | + | + | Not pre-HF | +/+ |
| ZSF-1 rat | + | + | + | + | + | + | |
| Aortic constriction (mouse, rat) | + | − | − | +/− | ND | mild | +/+ |
| Aldosterone Infusion (mouse, rat) | + | + | − | ++ | ND | mild | + |
| Ageing models (e.g. SAM-WD) mouse | Mild | − | +/− | + | + | − | + |
| L-Name + HFD, mouse | + | ++ | + | + | + | + | Not tested |
| db/db or ob/ob mice | + | + | + | + | + | ND | + |
| Aged dogs subjected to peri-nephritis-induced hypertension | + | + | − | + | ND | − | Not tested |
| Aortic Banded Cat | + | ++ | − | ++ | ND | + | + |
| DOCA and salt loaded pigs on HFD | + | + | + | + | + | ND | Not tested |
Animal models that focus solely on LV pressure/volume overload such as the Dahl-salt sensitive or spontaneously hypertensive rat, aortic banded cat, or aldosterone infusion – generate principally cardiac disease such as hypertrophy, diastolic dysfunction, and fibrosis. Pure obesity models, such as the leptin or leptin receptor deficient models (db/db or ob/ob) generate marked obseity and some cardiac disease. Other models such as L-NAME+high fat diet (HFD) in mice, or the DOCA-HFD pig model, or the senescence accelerated mouse on western diet (SAM-WD) attempt to integrate both components. The latter two do not develop significant heart failure however, reflected by less increase in diastolic pressure and fluid accumulation in the lungs.
Cardiac molecular/cellular pathways and mechanisms
The bulk of the research into HFpEF basic mechanisms has focused on the heart, likely due to its historical dominance in the syndrome, the fact that patient symptoms are similar to those of humans in whom the heart has clearly failed, and our clinical approach. Most animal models have imposed haemodynamic and more recently combined haemodynamic and metabolic stresses, with their primary criterion being to induce diastolic dysfunction with an EF >50%. Based on these and available human data, cardiac HFpEF mechanisms can be grouped as: cardiac hypertrophy, fibrosis, excitation–contraction coupling, sarcomere dysfunction, cGMP–PKG signalling deficiency, nitrosative–oxidative stress, microvascular insufficiency, inflammation, and mitochondrial and metabolic defects. There are undoubtedly others, but this covers the major ones.
Cardiac hypertrophy: Role of neurohormones and signaling pathway
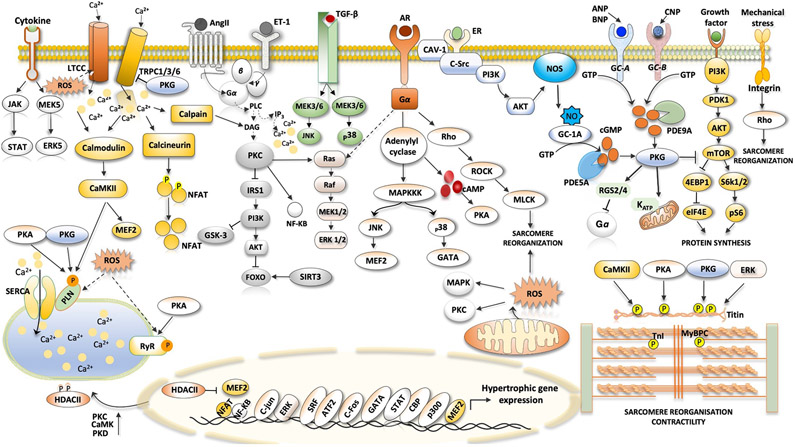
LVH has long been considered a cardinal feature of HFpEF and major cause for diastolic dysfunction and elevated diastolic filling pressures despite a normal-range EF. That said, recent clinical trials find LVH in 30–60% of HFpEF patients81-85 so this is certainly not a requirement for the syndrome. However, it is one of the best well studied aspects. In their excellent 2018 review of physiological and pathophysiological cardiac hypertrophy, Nakamura and Sadoshima86 listed the components of pathological hypertrophy as: impaired calcium handling, fibrosis, oxidant stress, cell death, insufficient angiogenesis, mitochondrial dysfunction, metabolic reprogramming, cell growth and protein synthesis, and induction of fetal gene programmes. All of these are also on the hit list for HFpEF. Key molecular players in this signalling are G-protein-coupled receptors (GPCRs) and their hormone ligands (angiotensin II, endothelin 1, α-adrenergic receptors and β- adrenergic receptors); signalling kinases (p38, ERK1/2, JNK, CAMKII, PKC, PKG, PKA, mTORC1 and AMPK), epigenetic modulators (NFAT, MEF2, GATA4, class II HDACs and Hippo) and mechanosensitive plasma-membrane cation channels (TRPC and TRPV) (Figure 1). These have been well described in HFrEF, and most are also observed in models of non-dilated hypertrophy induced by pathological haemodynamic or neurohumoral stress, such as aortic banding in mice.
Fig. 1 ∣. Signalling pathways in cardiac hypertrophy.
Ventricular hypertrophy in heart failure with preserved ejection fraction, particularly in association with hypertension and neurohormonal stress, can involve many pathways identified in other hypertrophic syndromes. The figure shows the major pathways in cardiomyocytes that are thought to stimulate pathological muscle growth of the heart. The hormones angiotensin II (AngII), endothelin 1 (ET-1) and catecholamines bind to their cognate receptors, which are coupled to heterotrimeric G proteins to activate downstream signalling, such as the phospholipase C (PLC)–protein kinase C (PKC) axis. Activated PKC inhibits the insulin receptor substrate 1 (IRS1)–RACα serine/threonine-protein kinase (AKT)–forkhead box protein (FOXO) signalling pathway. β1-Adrenergic receptor (AR) stimulated protein kinase A (PKA) raises cytosolic Ca2+ levels by phosphorylation of Ca2+-handling proteins. Transient receptor potential channel 1 (TRPC1), TRPC3 and TRPC6 have been linked to pathological hypertrophy through elevated NFAT signalling. TRPC1 and TRPC6 are also mechanosensitive. Integrin transmembrane receptors also transduce intracellular hypertrophic signalling by activating downstream effectors such as Rho. Transforming growth factor-β (TGFβ) signalling and receptors transmitting signals through Gq-protein-coupled receptors promote activation of Rho-associated protein kinase (ROCK), extracellular-signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK), which contributes to pathological cardiac hypertrophy and fibrosis. Growth factor-mediated stimulation of mechanistic target of rapamycin (mTOR) signalling is linked to induction of protein synthesis and inhibition of autophagy. Hypertrophy is also associated with depressed cGMP–protein kinase G (PKG) signalling (both nitric oxide (NO)-mediated and natriuretic-peptide-mediated) and increased phosphodiesterase type 5A (PDE5A) and PDE9A expression. Many of these kinases can affect sarcomeric proteins, altering myofilament Ca2+ sensitivity and passive stiffness. Cytokines augment cardiac hypertrophy through their receptors (such as the IL-6 receptor). 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; AC, adenylyl cyclase; ANP, atrial natriuretic peptide; AT1R, angiotensin II receptor type 1; ATF2, cAMP-dependent transcription factor ATF2; BNP, B-type natriuretic peptide; CAMKII, Ca2+/calmodulin-dependent protein kinase II; CAV1, caveolin 1; CBP, CREB-binding protein; CNP, C-type natriuretic peptide; cTnI, cardiac troponin I; DAG, diacylglycerol; eIF4E, eukaryotic translation initiation factor 4E; ER, oestrogen receptor; FOS, proto-oncogene c-Fos; GATA4, transcription factor GATA4; HDAC, histone deacetylase; IP3, inositol trisphosphate; JAK, Janus kinase; JNK, JUN N-terminal kinase; JUN, proto-oncogene c-Jun; KATP, ATP-dependent K+ channel; LTCC, L-type Ca2+ channel; MAPKKK, mitogen-activated protein kinase kinase kinase; MEF2, myocyte enhancer factor 2; MEK, MAPK/ERK kinase; MLCK, myosin light chain kinase; MYBPC, cardiac myosin-binding protein C; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; NOS3, endothelial nitric oxide synthase; NPR-A, natriuretic peptide receptor A; NPR-B, natriuretic peptide receptor B; P, phosphate; p300, histone acetyltransferase p300; PDK1, 3-phosphoinositide-dependent protein kinase 1; PI3K, phosphoinositide 3-kinase; PKD, protein kinase D; PLN, cardiac phospholamban; pS6, ribosomal protein S6 kinase; RAF, RAF proto-oncogene serine/threonine-protein kinase; RGS2, regulator of G-protein signalling 2; RGS4, regulator of G-protein signalling 4; ROS, reactive oxygen species; RYR2, ryanodine receptor 2; S6K1, ribosomal protein S6 kinase-β1; S6K2, ribosomal protein S6 kinase-β2; SERCA2A, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; sGC, soluble guanylyl cyclase; SIRT3, mitochondrial NAD-dependent protein deacetylase sirtuin 3; SR, sarcoplasmic reticulum; SRC, proto-oncogene tyrosine-protein kinase SRC; SRF, serum response factor; STAT, signal transducer and activator of transcription; TGFBR, transforming growth factor-β receptor.
Activation of the RAAS is a hallmark of HFrEF and is linked to many of the above-cited defects. Genetically modified mice long ago established a critical role of Gαq/α11 signalling triggered by angiotensin II and pathological cardiac hypertrophy87-89. There are few human data on RAAS hormone levels in ambulatory HFpEF or patients during exertion, although one study found them similarly elevated in patients with acute decompensated HFrEF or HFpEF90. However, the lack of benefit from multiple studies using RAAS blockade in HFpEF50,91 makes a primary role unlikely. The exception may be aldosterone, which signals via mineralocorticoid receptors expressed in the distal convoluted tubule and cortical and medullary regions of the collecting duct in the nephron, the brain, vascular smooth muscle, cardiomyocytes, fibroblasts and inflammatory cells92. While classically a transcription-factor-regulating pathway, non-genomic signalling engaging MAP kinases, PKC, sSrc kinase and NADPH oxidases are reported92. Cardiomyocyte-targeted ablation of the mineralocorticoid receptor (MR) is protective against pressure-load stress, reduces fibrosis, and blocks the DOCA-salt induced inflammatory and fibrotic response93,94. Vascular MR signalling is coupled with MMP, TGFβ1, CTGF and galectin-3 expression with extracellular matrix remodelling. HFpEF patients who are obese, diabetic, have chronic kidney disease, concentric LVH and high renin levels seem particularly responsive to MR antagonism with spironolactone95. While the international randomized trial of spironolactone in HFpEF was neutral96, subgroup analysis from North and South America where baseline disease was better documented and metabolite analysis supported the drug was actually taken97, report benefits98. Many have focused on the drug’s antifibrotic effects99, but this role remains unclear100, as its capacity to reduce sodium retention to lower intravascular volume and cardiac wall stress is also likely to be important101.
Sustained catecholamine hyperstimulation contributes to hypertrophy and myocardial dysfunction, and is a central component of HFrEF for whichh β-AR blockade is a proven therapy. No so in HFpEF. While β-AR was hypothesized to prolong diastolic filling time and lower oxygen demand, trial data is scant. The recent J-DHF trial102 of 245 HFpEF patients treated with carvedolol is the largest, and found no overall benefit in prognosis except perhaps in patients who tolerated the highest doses but also may have been less sick. HFpEF patients also exhibit chronotropic incompetence14,16 which limits cardiac output reserve, and this can be worsened by β-AR blockade. The funny channel blocker ivabradine slows sinus rate without negative inotropy and worsened HFpEF symptoms103, conferring no long-term benefits104.
Kinase and phosphatase signalling cascades in pathological hypertrophy are well recognized and reviewed elsewhere86. Despite their prominent roles, their relevance to human HFpEF remains unproven, though several are current therapeutic targets. Hyperactivated CAMKII contributes to the pathophysiology of atrial fibrillation105, hypertrophy106, mitochondrial energetics107, inflammation108,109, and diabetes110, so some role in HFpEF seems likely. Pro-fibrotic signalling coupled to non-voltage gated cation channels such as transient receptor potential canonical channel type 6 (TRPC6) which are activated by CAMKII111 are another potential contributor. The channels are normally expressed at low levels in multiple tissues, but are upregulated in a feed-forward manner coupled to stimulation of calcineurin–NFAT activation that reduces TRPC6 phosphorylation, increasing its Ca2+/Na+ conductance, and raises expression of TRPC6112. While first revealed as a mechanism of pathological hypertrophy112, TRPC6 now appears to play a prominent role in pathological fibrosis113 via both NFAT and SRF–p38 signalling pathways114. Another recent discovery is role of HDACs that remove N-acetyl lysine from histone or non-histone proteins. Early studies focused on hypertrophic and fibrotic influences and role in redox modulation of both, and HDAC inhibitors have been shown to suppress both in experimental pressure-overload and neurohumoral activation models115-117. Results with HDAC inhibitors have varied. In one report, ITF-2357 improved diastolic dysfunction but without reducing hypertrophy or fibrosis in both DSSR and aged mouse models64. However, another study using a cat pressure-overload model found suberoylanilide hydroxamic acid, a pan-HDAC inhibitor approved for treatment of cutaneous T-cell lymphoma, reduced LVH, improving diastolic function65. Both studies showed enhanced myofilament relaxation rates, and the cat model revealed improved mitochondrial function associated with differential acetylation of components of the electron transport chain and metabolic pathways. These and other pathways are actively being explored (Figure 1).
Myocardial fibrosis
Hypertension and LVH stimulate interstitial fibrosis which has long been viewed as a cause for passive muscle stiffening and reduced chamber compliance in HFpEF118,119. Fibrosis also results from diabetic heart disease via multiple signalling cascades and alterations in the matrix proteins themselves, such as insoluble advanced glycation end-products29,68,120-122. Obesity is similarly linked to increased myocardial123,124 and hepatic fibrosis95,125, and combined haemodynamic and metabolic stress can synergize to stimulate fibrosis75. Pro-inflammatory and oxidant stress conditions also stimulate fibrosis. Thus, many HFpEF co-morbidities can be coupled to a profibrotic process. The still somewhat existential question is when is it pathophysiologically important? Despite a lot of research and interest, we still do not know. The presence of connective tissue per se is only a part of the story, as fibroblasts are active participants in myocyte and vascular cell crosstalk, and their molecular phenotypes and secreted signals are likely as if not more important than the collagen that is synthesized. The distribution and cross-linking of matrix proteins also potently impacts their mechanical properties so it is not simply a matter of how much fibrosis but what type and where it is. The presence of a primarily fibrotic HFpEF phenotype seems fairly rare given the mild-moderate levels observed in most patients126.
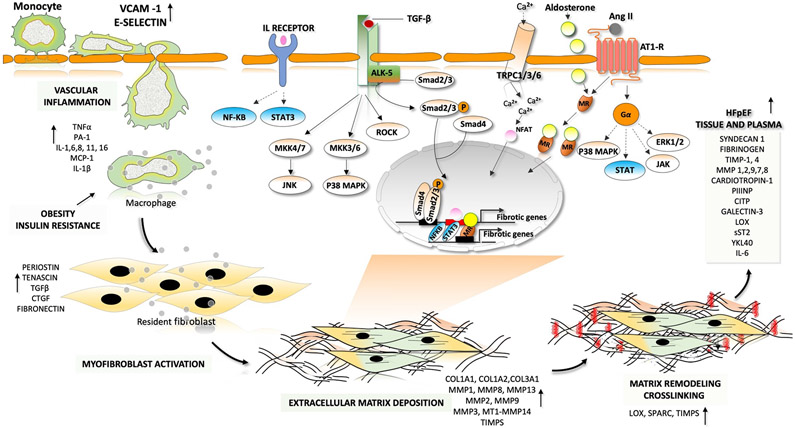
With these caveats, it remains useful to examine recent advances in understanding the fibrotic process and potential therapeutic interventions (Figure 2). Among its signalling mechanisms are RAAS activation, insulin resistance, oxidative stress, advanced glycation end-product signalling, TGFβ, endothelin 1, Rho-kinase and leptin-mediated signalling, and upregulation of matricellular proteins (such as thrombospondin 1)123. Fibroblast cell types are complex and multiple, with different cell lineages differentially transforming into synthetic versus stable scar-structure-related subtypes after injury127. Fibroblast activation and transition to myofibroblasts has been linked to a Yap-induced Hippo suppression, transcriptionally regulating ER stress and unfolded protein responses to enhance collagen synthesis128. Matrix is composed of fibrillar collagen, glycoproteins (e.g. thrombospondins and tenascins), proteoglycans (e.g. versican and syndecans) and glycosaminoglycans (e.g. hyaluronan and heparan sulfate). Collagen content reflects a balance between synthesis, post-synthetic processing, post-translational modification, and degradation. Synthesis involves secretion of procollagen into the interstitium where it undergoes end-terminal cleavage by procollagen N-proteinase and C-proteinase to form mature collagen. Zinc-dependent matrix metalloproteinases (MMPs) degrade ECM to remodel the matrix (see review129).
Fig. 2 ∣. Fibrotic–inflammatory remodelling in heart failure with preserved ejection fraction.
In patients with heart failure with preserved ejection fraction, endothelial cells produce factors that induce inflammation and recruit monocytes for transendothelial migration. These factors include interleukins (IL-1, IL-6 and IL-8), colony-stimulating factors (granulocyte-colony stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) and chemotactic factors (C-C motif chemokine 2 (CCL2)). Pro-inflammatory cytokines such as tumour necrosis factor (TNF), IL-1 and IL-6 are involved in the initiation and propagation of inflammatory signals. This inflammatory signalling stimulates monocytes and endothelial cells to initiate weak interactions and the subsequent rolling of the monocytes over the endothelial cells, mediated by adhesion molecules (such as vascular cell adhesion protein 1 (VCAM1) and E-selectin). Finally, monocytes migrate through the intercellular clefts between the endothelial cells to the underlying tissue. Macrophage-derived mediators and pro-inflammatory cytokines drive the transformation of quiescent fibroblasts into proliferative and matrix-synthesizing active myofibroblasts. The communication between inflammatory cells and resident fibroblasts occurs through direct cell–cell interactions and through paracrine signalling. Sustained activation of myofibroblasts produces structural extracellular matrix (ECM) proteins and matricellular proteins. Crosslinking of collagen molecules by matrix-crosslinking enzymes, such as lysyl oxidase (LOX), prevents the enzymatic degradation of collagen, leading to an increase in collagen content and stiffness. The dynamic alterations in the composition of the ECM and the prolonged deposition of ECM modulates cardiac function. Both canonical transforming growth factor-β (TGFβ)– mothers against decapentaplegic homologue 2 (SMAD2)–SMAD3 signalling and non-canonical TGFβ signalling through Rho-associated protein kinase (ROCK), extracellular-signal-regulated kinase (ERK) and p38 contribute to the development of pathological hypertrophy and fibrosis. Phosphorylation and activation of ERK, p38, Janus kinase (JAK) and signal transducer and activator of transcription (STAT) by activation of G-protein-coupled receptors also leads to the development of fibrosis. Activation of the calcineurin–nuclear factor of activated T cells (NFAT) pathway by cardiac transient receptor potential channels (TRPCs) also has a major role in fibrotic remodelling. Mineralocorticoid receptors (MRs) are ligand-activated transcription factors that induce transcription of profibrotic genes upon aldosterone binding. AngII, angiotensin II; AT1R, angiotensin II receptor type 1; CITP, carboxy-terminal propeptide of procollagen type I; COL, collagen; CTGF, connective tissue growth factor; JNK, JUN N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; MEK, MAPK/ERK kinase; NF-κB, nuclear factor-κB; P, phosphate; PIIINP, amino-terminal propeptide of procollagen type III; sST2, soluble protein ST2; TGFBR1, transforming growth factor-β receptor type 1; TIMP, metalloproteinase inhibitor; YKL40, chitinase-3-like protein 1.
Direct support for myocardial fibrosis in human HFpEF comes mostly from studies of hypertensive–hypertrophic heart disease patients showing correlates with diastolic dysfunction130-132, from autopsy studies37, image-based analysis of myocardial extracellular volume and correlation to diastolic dysfunction133, epicardial biopsy analysis in hypertensive patients presenting for coronary artery bypass graft surgery134. In 2020, Hahn and colleagues126 reported results from >100 endocardial biopsies from well-phenotyped HFpEF patients126. The population was obese (median BMI of 37.6 kg/m2) with nearly 60% having T2DM; most also hypertensive (median systolic blood pressure: 141 mmHg), with a median sex-adjusted LV mass index of 105 mg/m2 (90% in normal population). Interstitial fibrosis was found in most all the biopsies, but it was moderate or severe in only 26%.
Many fibrosis-related plasma biomarkers correlate with features of diastolic dysfunction in HFpEF patients including: syndecan 1, TIMP1 and MMP1/TIMP1 ratio130, cardiotropin 1, carboxy-terminal propeptide of procollagen type I (CITP), amino-terminal propeptide of procollagen type III (PIIINP),135, galectin 3136,137, lysyl-oxidase, CITP/MMP1 ratio, an inverse index of myocardial collagen cross-linking99, interleukin-11 (IL-11), a key downstream effector of TGFβ in fibroblasts that induces myocardial fibrosis and contractile dysfunction138, and sST2 (soluble ST2) a member of the IL-1 receptor family secreted by fibroblasts and cardiomyocytes in response to mechanical strain. Membrane bound ST2 normally binds to IL-33 ligand eliciting antihypertrophic and antifibrotic responses, but this is negated by sST2 acting as an IL-33 decoy. Other markers include osteopontin and FGF23. In a recent study examining nearly 50 biomarkers from patients in the TOPCAT trial, a machine-learning algorithm identified several profibrotic markers: FGF23, YKL40 (a hepatic fibrosis marker), IL-6, ST2 and MMP7, as being among the eight that best predicted prognosis35. Importantly, the tissue source for these biomarkers remains uncertain, given the multisystem disease in HFpEF, as renal, pulmonary, and vascular dysfunction could be as much if not more involved as the myocardium139. At least one study used one of the biomarkers, CITP/MMP1 ratio, to stratify patients with more or less collagen crosslinking, and found those with the lowest ratio (most crosslinking) were least responsive to aldosterone antagonism99. Demonstration of HFpEF therapy efficacy to diminish markers of fibrosis or, better yet, myocardial fibrosis itself in those impacted by it, remains lacking.
Excitation–contraction coupling
In HFrEF, the process linking myocyte depolarization to calcium–myofilament interaction and contraction, excitation–contraction coupling, is beset by multiple abnormalities140. The expression of proteins involved with calcium uptake into the sarcoplasmic reticulum — phospholamban and SERCA2a — is often reduced and/or the proteins are hypophosphorylated, depressing calcium transients and contraction while delaying relaxation. To compensate and lower diastolic Ca2+ despite reduced SR uptake, reverse mode Na+/Ca2+ exchange is enhanced. The L-type Ca2+ channel (Cav1.2) current–voltage response is depressed, limiting inotropy and lusitropy. In addition, the Ca2+ concentration needed for 50% maximal myofilament force is lower, related to reduced phosphorylation of troponin I, and reduced myosin binding protein C phosphorylation causes depressed adrenergic reserve141. These changes have not yet been documented in HFpEF. When studied in hypertension–LVH models, and recently the ZSF-1 rat, the data usually show peak Ca2+ transients and SR calcium load as normal or even enhanced in the ‘preserved EF’ state, although Ca2+ decay transients can be prolonged54,57,142,143. Abnormal excitation–contraction coupling is described in diabetes models68 and associated with pathological CAMKII signaling110. However, without obtaining live, intact myocytes from HFpEF patients, which only happens for HFrEF due to heart transplantation, the status of excitation–contraction coupling is likely to remain uncertain.
Cardiomyocyte sarcomere function
In contrast to live, beating myocytes or muscle, frozen tissue (even small pieces such as endocardial biopsy samples) can be used to study sarcomere function. While few in number, such studies have been performed in human HFpEF, with heart tissue obtained mostly from elderly patients with hypertension–hypertrophy with or without diabetes. The work reports passive force–length dependence to be stiffer but systolic force–Ca2+ essentially unchanged120,144,145. Stiffening was attributed to hypophosphorylation of the sarcomere protein and molecular spring titin, that was in turn linked to depressed protein kinase G activity146. Stiffer titin correlated with reduced diastolic chamber compliance134. Both in vitro and in vivo, activating PKG or PKA reversed these changes and improved muscle and chamber compliance134,147. Analogous force–Ca2+ relation and titin hypophosphorylation findings have been reported in the ZSF-1 rat148. This was not documented in compensated (preserved EF) hypertension–LVH models149, and remains to be determined in other contemporary HFpEF models. Myofilament acetylation also regulates sarcomere function in systole and diastole150-152, and two recent studies reported HDAC inhibitors in experimental HFpEF models quickened myofilament relaxation kinetics associated with improved chamber relaxation64,65. The precise protein targets and mechanisms for this remain unknown.
PKG signalling
The most widely known cardiovascular roles for cGMP and its cognate kinase PKG are their regulation of vascular tone and endothelial function. PKG activates myosin light chain phosphatase to reduce MLC kinase activity and relax vascular smooth muscle. It also phosphorylates RhoA, suppressing Rho kinase and associated smooth muscle proliferation. Endothelial cells play a critical paracrine role, responding to ligands and mechanical stress to activate NO synthase, with NO diffusing to smooth muscle cells, activating sGC and generating cGMP. In skeletal muscle, endothelial NOS plays an important role in myocyte cGMP signalling as well. A similar cascade is often shown for the heart, arguing that depressed endothelial NO generation is a primary cause of reduced myocyte cGMP–PKG signalling. This paracrine scheme surprisingly still awaits definitive proof, as myocytes have the autonomous capacity to generate and stimulate cGMP–PKG. Nonetheless, stimulation of the pathway generally confers antihypertrophic, antifibrotic and proangiogenic effects in the myocardium153-155 (Figure 3).
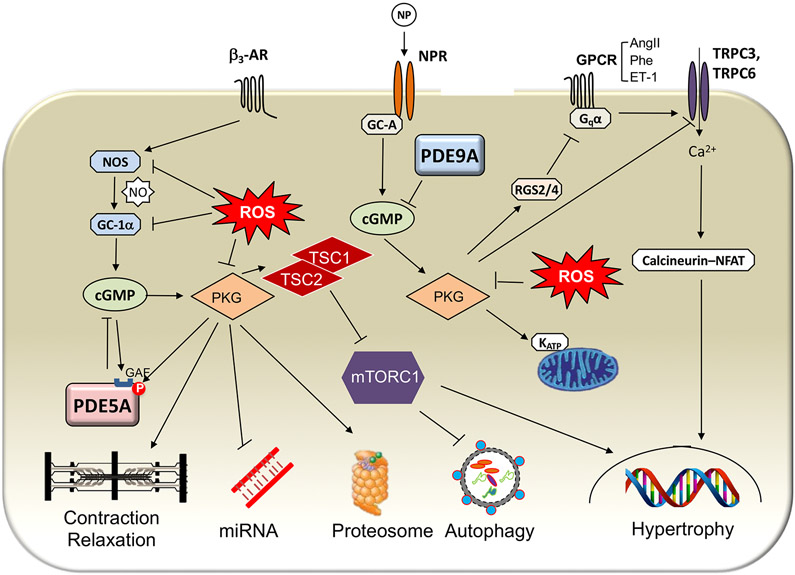
Fig. 3 ∣. Components of the cGMP–PKG signalling systems and their cellular effectors.
cGMP is generated by one of two different cyclases: guanylyl cyclase A (GC-A), which is coupled to the natriuretic peptide (NP) receptor (NPR), and soluble guanylyl cyclase (sGC), which is the target of nitric oxide (NO). Each relative pool of cGMP has a different primary targeting phosphodiesterase (PDE): PDE5A for GC-1α-derived cGMP and PDE9A for GC-A-derived cGMP. Increased cGMP levels in turn activate protein kinase G (PKG). PKG is the primary effector protein for many cGMP-mediated effects. Phosphorylation of sarcomeric proteins enhances relaxation and diastolic compliance and blunts β3-adrenergic receptor (β3-AR)-stimulated contractility. PKG stimulation, controlled by PDE5A but not PDE9A, reverses the abnormally altered expression of many microRNAs that is associated with hypertrophic remodelling induced by pressure stress. PKG activation also increases proteasome activity and stimulates autophagy, the latter coupled to the suppression of mechanistic target of rapamycin complex 1 (mTORC1) signalling. NP-stimulated PKG coupled to NPR-A (for which both atrial NP and B-type NP are ligands) has less of an effect on sarcomeres or microRNA levels, but augments autophagy. PKG stimulated by either NO or NPs activates regulator of G-protein signalling 2 (RGS2) and RGS4 to suppress Gq-coupled receptor signalling and phosphorylates transient receptor potential channel 3 (TRPC3) and TRPC6 to suppress hypertrophy and fibrosis. AngII, angiotensin II; ET-1, endothelin 1; GAF, GAF domain; GPCR, G-protein-coupled receptor; KATP, ATP-dependent K+ channel; NFAT, nuclear factor of activated T cells; NOS, nitric oxide synthase; P, phosphate; Phe, phenylephrine; ROS, reactive oxygen species; TSC1, hamartin; TSC2, tuberin.
There are multiple mechanisms for PKG amelioration of heart disease, and this list continues to grow. One is its phosphorylation and inhibition of TRPC6 conductance to suppress calcineurin–NFAT signalling and thus prohypertrophic and fibrotic programmes156,157. Another is PKG activation of RGS2 and RGS4, inhibiting Gαq-coupled signaling158,159. A particularly potent effect is from PKG activation of TSC2 to suppress hyperactivity of the mechanistic target of rapamycin complex 1160. In mice expressing a homozygous knock-in mutation of Tsc2 that prevents this PKG modification (S1365A mutation), TAC-induced hypertrophy, LV dysfunction, and cardiovascular mortality is markedly increased and cannot be rescued by use of a PDE5 inhibitor to stimulate PKG. By contrast, mice with a heterozygous mutation, providing one wild-type allele for PKG phosphorylation, are rescued by the same treatment. PKG phosphorylates and enhances proteasome clearance of misfolded proteins161 and stimulates autophagy160, confers anti-inflammatory effects29,162, improves mitochondrial energetics163, can supress miRNA changes otherwise induced by pressure overload164 and counters obesity165,166. Other than TSC2, the identification of specific PKG-modified protein residues underlying these important effects remains lacking. Sarcomere protein modifications in troponin I and titin are known and have are potent regulators of diastolic function147,167.
Therapeutic stimulation of PKG involves either enhanced NO or NP-related cGMP synthesis, or blocking cGMP hydrolysis by inhibiting PDE1, PDE2, PDE5 or PDE9168. Of the PDEs, PDE5 and PDE9 are cGMP selective and have been most studied to date. Each tactic augments cGMP but does so in different cell types within different intracellular compartments, so they are not interchangeable. For example, while both PDE5 and PDE9 inhibition counter pressure-overload hypertrophy, fibrosis, and dysfunction, PDE5 depends on the presence of NOS signalling, whereas PDE9 regulates cGMP coupled to NP stimuli155. Conditions where NOS activation is compromised include the loss of oestrogen in post-menopausal women and, indeed, PDE5 inhibition is ineffective to counter pressure overload in ovariectomized female mice169,170. Effectiveness is restored by exogenous oestrogen replacement. This is related to non-nuclear dependent oestrogen–ERα-dependent signalling that couples via PI3K to NOS activation. Bypassing NOS to directly stimulate sGC and generate cGMP is effective even in these mice. These findings are potentially relevant to HFpEF, which involves many post-menopausal women. Another strategy to circumvent NOS-deficiency states leverages PDE9 inhibition, and work in progress is testing this. Stimulation of cGMP synthesis has been largely achieved by organic nitrates, and by inorganic nitrates and nitrites, the latter aimed at preventing tachyphylaxis. NP stimulation still requires peptide administration, although alternative NP-receptor agonists are being investigated.
Despite the exciting science suggesting utility of stimulating the cGMP/PKG system for HFpEF, clinical data to date has been disappointing. A major problem is that the impact on blood pressure from many of the strategies used limits their capacity to chronically engage signaling in other tissues (e.g. heart, lung, kidney). This is more a problem with NO-donors or surrogates or enhancers of natriuretic peptides, that potently alter vascular tone. PDE inhibitors are more cell-type specific and function in nano-domains, so their interference can augment cGMP signaling in tissue without altering blood pressure. Inhibitors of PDE9 are prime examples of this. They may also be unknown differences between human and rodent cGMP/PKG effects in tissue.
Oxidative–nitrosative stress
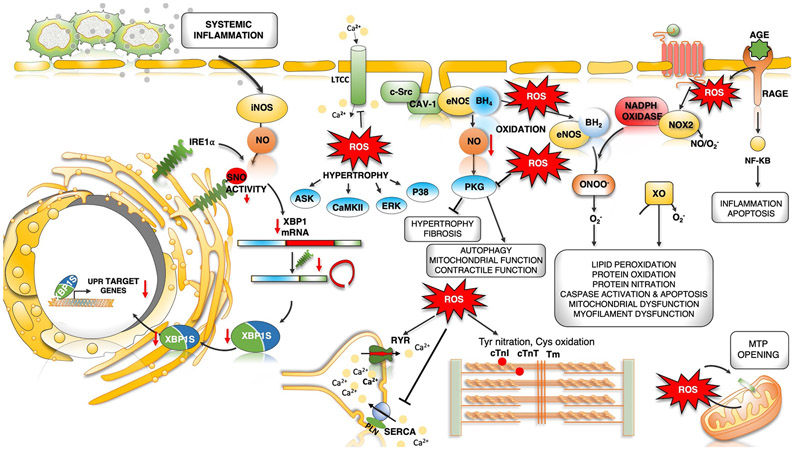
Oxidative stress is common to metabolic diseases such as diabetes and haemodynamic diseases such as pressure overload, and is thought to be a potent contributor to the pathophysiology. Specific sources of nitrosative–oxidative stress are NADPH oxidases NOX2 and NOX4171, ROS from mitochondrial injury or dysfunction172, xanthine oxidase173, monoamine oxidase174, inducible nitric oxide synthase (iNOS or NOS2)75, and the uncoupling of NOS3 (or eNOS)175 (Figure 4). The latest chapter in these studies revealed nitrosative stress is linked to iNOS S-nitrosylation of endonuclease inositol-requiring protein 1α (IRE1α), culminating in defective splicing and downregulation of protein expression of ER-stress protein X-box binding protein 1 (XBP1)75. The result is the HFpEF phenotype in an l-NAME plus high-fat diet mouse model. Blocking iNOS pharmacologically or genetically restored XBP1 expression and reversed the pathophysiology. XBP1 is also downregulated in human myocardium from HFpEF patients75. NOS3 uncoupling involves oxidation of the enzyme or its critical cofactor, tetrahydrobiopterin, reducing generation of NO to favour superoxide production. It occurs in pressure-overload hypertrophy in mice, coupled to heart dysfunction and fibrosis175, and in ZSF-1 rat and HFpEF pig models39,80, but not yet documented in human HFpEF. Broad in vivo antioxidant or anti-nitrosative strategies have thus far been disappointing, although targeting specific sources – such as iNOS - remains conceptually attractive.
Fig. 4 ∣. Dysregulated oxidative and nitrosative stress in HFpEF pathogenesis.
Increased microvascular inflammation and pro-inflammatory cytokine levels result in increased expression of inducible nitric oxide synthase (NOS2) in cardiomyocytes. NOS2-derived nitric oxide (NO) mediates S-nitrosylation (SNO) of the unfolded protein response (UPR) regulator IRE1α, leading to a progressive decline in IRE1α-mediated generation of the spliced form of X-box-binding protein 1 (XBP1), known as XBP1s. Reduced XBP1s levels lead to decreased XBP1s-dependent expression of UPR target genes, compromised UPR and endoplasmic reticulum (ER) function, and prolonged ER stress. IRE1α activity and XBP1s levels are reduced in the hearts of patients with heart failure with preserved ejection fraction (HFpEF). Oxidative stress and increased reactive oxygen species (ROS) formation can directly modulate cardiac redox status by reacting with NO to decrease its bioavailability. Oxidative stress uncouples endothelial nitric oxide synthase (NOS3) by oxidation and depletion of its cofactor tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2). NOS3-derived NO has antihypertrophic and antifibrotic effects primarily by activation of cGMP–protein kinase G (PKG) signalling. Uncoupled NOS3 generates oxidant species promoting protein tyrosine nitration, cysteine oxidation and lipid peroxidation, damaging proteins, lipids and DNA. Receptor-induced activation of NADPH oxidase 2 (NOX2) and mitochondrial redox mismatch are other major sources of ROS and stimulate mitochondrial transition pore (MTP) opening, Ca2+ overload and mitochondrial dysfunction. Advanced glycation end products (AGEs) that bind to cell surface receptors for AGEs (RAGEs) can stimulate NADPH oxidase, thereby increasing the production of ROS and aggravating inflammation by activation of the nuclear factor-κB (NF-κB) pathway. ROS can directly or indirectly activate various kinases, resulting in hypertrophy. Post-translational redox modifications of protein kinases (such as Ca2+/calmodulin-dependent protein kinase II (CAMKII), protein kinase A and PKG), sarcoplasmic reticulum (SR) proteins (ryanodine receptor 2 (RYR2) and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2A (SERCA2A)) and myofilament proteins (cardiac troponin I (cTnI), cardiac troponin T (cTnT), tropomyosin (Tm) and cardiac myosin binding protein C can alter their activity and contribute to hypertrophy and altered excitation–contraction coupling. AngII, angiotensin II; ASK, apoptosis signal-regulating kinase; AT1R, angiotensin II receptor type 1; CAV1, caveolin 1; ERK, extracellular-signal-regulated kinase; LTCC, L-type Ca2+ channel; O2−, superoxide anion; ONOO−, peroxynitrite; PLN, cardiac phospholamban; SRC, proto-oncogene tyrosine-protein kinase SRC; XO, xanthine oxidoreductase.
Mitochondrial and metabolic defects
Cardiac mitochondrial function and metabolism is abnormal in HFrEF and in the diabetic heart (see recent reviews68,176,177) and is likely to play an important role in HFpEF as well (Figure 5). Ultimately, this may reduce high-energy phosphate generation and reserve, with resting myocardial ATP 20–40% of normal178-181 and reduced phosphocreatine (PCr) stores182-184. In human HFpEF, Phan and colleagues185 found PCr/ATP ratio reduced by 27% versus controls accompanied by depressed exercise augmented cardiac output, oxygen uptake, and relaxation. PCr in skeletal muscle during exercise must be restored, and this process is delayed in HFpEF patients185,186.
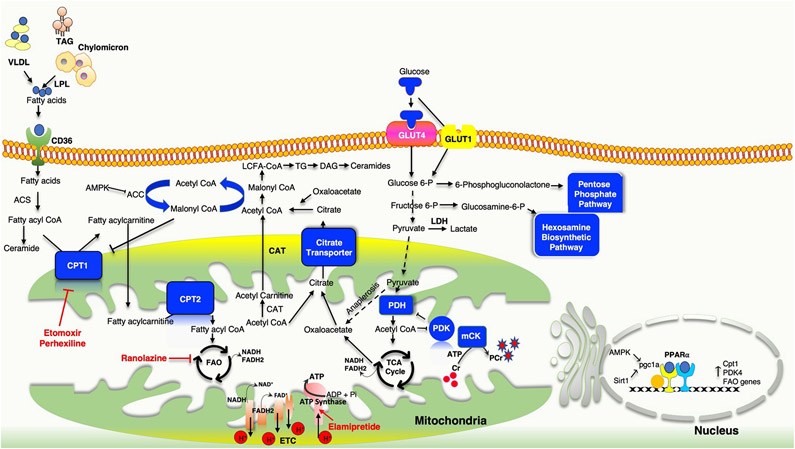
Fig. 5 ∣. Metabolic flexibility and HFpEF.
Heart failure involves alterations in multiple metabolic pathways that can alter mitochondrial ATP production and substrate utilization, and ultimately myocardial energy reserve and efficiency. Obesity, which is very common in patients with heart failure with preserved ejection fraction (HFpEF), induces systemic fatty acid oversupply from adipose tissue that is re-routed to peripheral organs, including the heart. Mismatch between cardiac fatty acid uptake and fatty acid β-oxidation (FAO) leads to intramyocardial accumulation of diglycerides and ceramides, uncoupling their oxidative phosphorylation. Hypoxia facilitates the metabolic shift towards glycolysis with a subsequent increase in lactate and pyruvate accumulation owing to impaired activity of pyruvate dehydrogenase (PDH). A compensatory increase in anaplerosis diverts the products of the glycolysis towards the hexosamine biosynthetic pathway and the pentose phosphate pathway. Several metabolism-modulating pharmaceuticals are being examined in HFpEF, as in other forms of heart failure. Ranolazine is a partial inhibitor of FAO, which reciprocally increases glucose oxidation and PDH activity. Etomoxir inhibits carnitine palmitoyl transferase 1 (CPT1), which controls the access of long-chain fatty acids (LCFAs) to the mitochondria for FAO. Elamipretide selectively targets the electron transport chain to increase energy efficiency. ACC, acetyl-CoA carboxylase; ACS, acetyl-CoA synthetase; AMPK, AMP-activated protein kinase; CAT, carnitine acyltransferase; CD36, fatty acid translocase; Cr, creatine; DAG, diacylglycerol; GLUT, glucose transporter; LDH, lactate dehydrogenase; LPL; lipoprotein lipase, mCK, muscle creatine kinase; PCr, phosphocreatine; PDK, pyruvate dehydrogenase kinase; PGC1α, peroxisome proliferator-activated receptor-γ coactivator 1α; Pi, inorganic phosphate; PPARα, peroxisome proliferator-activated receptor-α; SIRT1, NAD-dependent protein deacetylase sirtuin 1; TAG, triacylglycerol; TCA, tricarboxylic acid; TG, triglyceride; VLDL, very low density lipoprotein.
Both the HFrEF and diabetic hearts display changes mitochondrial biogenesis, with downregulation of PGC1α expression, which is considered a central factor for high-capacity mitochondrial oxidative function187. In addition, the critical feedback balance between redox modulation of metabolism and vice versa via NAD+/NADH balance is disrupted188. Pathological hypertrophy results in a decline in NAD, perhaps related to oxidative stress and increased consumption of NAD by poly ADP ribose polymerases required for DNA repair189. In addition, reduced NAD synthetic pathways coupled to NAMPT may play a role. NAD+ is required for metabolic substrate oxidation and is regenerated by the electron transport chain, and the ratio of NAD+/NADH declines in HFrEF and the diabetic hearts188,190-192. This adversely impacts intermediary metabolism of glucose and fatty acids, increases acetyl-CoA levels which impacts lysine acetylation of proteins to contribute to heart dysfunction, and impacts glucose–fat oxidation balance188. Proper NAD+/NADH ratio is also required for normal SIRT3 function193, a deacetylase fuelling metabolism by activating glycolysis, fatty acid oxidation, the tricarboxylic acid cycle and electron transport chain, and depressed SIRT3 signalling has been described in these syndromes and experimental HFpEF72.
Abnormalities of intermediary metabolism are also well documented HFrEF and are also thought to play an important role in HFpEF. By impairing fuel utilization and inducing conditions of substrate toxicity, they impede energy reserve and adaptability of the heart and skeletal muscle to stress. Reduced mitochondrial oxidative metabolism is accompanied by increased glycolysis178,181,194,195, but this does not translate to higher glucose uptake via pyruvate dehydrogenase complex, uncoupling glycolysis from pyruvate oxidation196. Impaired insulin signalling inhibits glucose oxidation by a negative feedback regulation via the Randle cycle, further enhancing glycolysis197. These changes are observed in multiple models of obesity and diabetes. There is also compensatory anaplerosis, redirecting glycolysis products to ancillary biosynthetic pathways such as hexosamine biosynthetic and pentose pyrophosphate pathways, further diverting pyruvate towards anaerobic glycolysis198.
In diabetes, impaired glucose oxidation is offset by increased fatty acid oxidation178,199, whereas in HFrEF, fatty acid oxidation is also depressed200. Enzymes catalysing fatty acid β-oxidation are downregulated in HFrEF, associated with a downregulated gene regulatory network coordinated by PPARα and its transcriptional cofactor PGC1α. The resulting mismatch between enhanced fatty acid uptake and oxidation results in intramyocardial accumulation of diglycerides and ceramides, further increasing reactive oxygen species, ER stress, mitochondrial dysfunction and lipotoxicity. Elevated myocardial triglyceride levels are reported in human HFpEF, and the extent of myocardial steatosis is positively and independently correlated with impaired diastolic strain rate and reduced exercise capacity in patients with HFpEF201,202. Increased plasma fatty acid levels are associated with greater risk of HFPEF development203. Plasma metabolomic profiling in HFpEF has found altered β-oxidation intermediates204-206, and these metabolic signatures may differentiate between HF types206.
Lastly, both HFrEF and the diabetic heart exhibit an increase in the use of ketone bodies as fuel for oxidation177,207-209. Evidence for human HFpEF is scant, although peripheral ketone levels are reportedly increased210. Ketones can suppress glucose and fatty acid oxidation, yielding more ATP per molecule of oxygen invested, which makes them the energetically efficient fuel. Therefore, maintaining cardiac ketone levels is viewed as beneficial, and methods to augment this source is being tested for HFrEF conditions. β-Hydroxybutyrate, the major ketone body, also inhibits HDACs211, and in light of recent reports on HDAC inhibitory beneficial effects on myofibril relaxation kinetics in experimental HFpEF64,65, ketone body supplementation may provide additional benefits beyond metabolic regulation. The cardioprotective effect of empagliflozin is associated with increased plasma ketone levels212 that may provide a mechanism for improving myocardial metabolism and heart function in HFpEF.
Plasma biomarkers and inflammation
An increasingly popular theory regarding HFpEF is that it reflects a pro-inflammatory state (see Figure 2). Westermann and colleagues32 were the first to report on myocardial tissue and examined 20 right ventricular biopsy samples, finding increased staining for CD3+, CD11a+, and CD45+ cells, (the last two are pan-leukocyte markers) as compared to eight controls. The only other report is from Hahn and colleagues126, who examined >100 right ventricular HFpEF biopsy samples and found increased numbers of CD68+ cells, indicating macrophages and other monocytes. Animal models, even those due purely to pressure-overload stress, also display myocardial inflammation66,213,214. Blocking specific cell types, such as infiltrating CCR2+ monocyte-derived macrophages215 or CD4+ T cells216, or using abatcept (a broad T-cell inhibitor)217, improves cardiac remodelling induced by aortic banding in mice. Inflammation is well described in diabetes and obesity, so the combination of all three in HFpEF only further increases the likelihood of an importance of pro-inflammatory processes. The triggers stem from multiple cell types, as myocytes, fibroblasts and vascular cells all synthesize various cytokines, including IL-1, IL-6, IL-8, colony-stimulating factors such as G-CSF, M-CSF, and GM-CSF; and chemotactic factors like MCP, to enhance migration and extravasation of inflammatory cells into tissue.
The greatest support for inflammatory conditions in HFpEF comes from peripheral blood biomarker analyses, and the data are overwhelmingly clinical. Among the many biomarkers commonly identified are C-reactive protein, TNF and TNFR, IL-1β, ILT6, IL-6, IL-10 and MPO35. Although common, high-sensitivity CRP was found in the normal range in 40% of HFpEF patients in the RELAX trial36. Increases in circulating inflammatory biomarkers correlate with acute decompensation in HFpEF34. While providing an overall indication of an inflammatory state, it is hard to solve the inverse problem to interpret a panel of biomarkers to discern which organ(s) are inflamed. Therefore, their use has been primarily to segregate HFpEF patients into lower-risk versus higher-risk groups. Importantly, these same markers are commonly observed in HFrEF, and there is little evidence to date that this is a unique or pathognomonic feature of HFpEF. Table 2 lists major plasma biomarkers for inflammation, renal, fibrotic, and other pathways that have been generally confirmed in various studies as being indicative of specific HFpEF phenotypes.
Table 2 ∣.
Plasma biomarkers for HFpEF
| Pathway | Biomarker | References |
|---|---|---|
| Inflammation | PAI-1, uPAR GDF-15 Pentraxin-3 vWF IL-1, IL-6, IL-16, IL-8 Heparin-binding EGF-like growth factor Platelet Growth Factor sTNFR1, sTNFR2, TRAIL-R2, TNF-alpha MCP-1 C-reactive protein PTX-3 CCL20 AGRP |
29, 35, 285, 286, 287, 288
|
| Remodeling/Fibrosis | sST2 Galectin-3 PIIINP ICTP Fibrinogen MMP-1, MMP-2, MMP-7, MMP-8, MMP-9, TIMP-1, TIMP-4 |
35, 286, 289 |
| Renal function | UACR BUN Cystatin-C |
285, 290 |
| Hypertrophy | Natriuretic peptides, BNP, NT-pro BNP Endothelin 1 High Sensitivity Troponin I, C, or T Renin, Aldosterone/angiotensin II FGF-23, FGF-21 NEMO TIE2 |
35, 285, 286, 291 , 292
|
| Vascular | FABP4 YKL40 OPG MPO Fas P-Selectin Tenascin-C Endostatin |
35 |
PAI; Plasminogen activator inhibitor-1, uPAR; urokinase-type plasminogen activator receptor, GDF-15; Growth differentiation factor-15, vWF; von Willebrand factor, IL; Interleukin, EGF; Epidermal growth factor, sTNFR; soluble tumor necrosis factor receptors, TRAIL-R2; TNF-related apoptosis-inducing ligand receptor, TNF; Tumor necrosis factor, MCP; Monocyte chemoattractant protein-1, PTX; pentraxin 3, CCL20; C–C motif chemokine 20, AGRP; Agouti-related protein, sST2; soluble suppression of tumorigenecity 2, PIIINP; procollagen type III N-terminal propeptide, ICTP; collagen type I carboxy-terminal telopeptide, MMP; Matrix metalloproteinases , TIMP; Tissue inhibitors of metalloproteinases, UACR; urine albumin-to- creatinine ratio, BUN; blood urea nitrogen, BNP; Brian natriuretic peptide, ET1; Endothelin1, hs-TnC; high-sensitivity cardiac troponin, FGF; Fibroblast growth factor, NF-κ-B; nuclear factor kappa-light-chain-enhancer of activated B cells, NEMO; NF-κB essential modulator, TIE2; angiopoietin receptor TEK tyrosine kinase, FABP; fatty-acid-binding protein , YKL40; Chitinase 3-like 1, OPG; Osteoprotegerin, MPO; Myeloperoxidase
Vascular disease: large and small vessels
At the systems level, older HFpEF patients generally have large-artery stiffening. This increases arterial blood pressure amplification late in systole due to early arriving wave reflections and reduced aortic compliance. The results is an increased in LV afterload imposed on the heart late in systole, stimulating myocardial remodelling such as hypertrophy, and depressing diastolic function detected as slowed early filling velocity and delaying relaxation218. The resulting suboptimal ventricular–arterial coupling12,219,220 impairs systolic reserve by limiting the capacity of the heart to enhance stroke volume without incurring substantial metabolic costs12,221. Diabetes mellitus also results in aortic stiffening and thus increased pulsatile arterial load, coupled to ventricular hypertrophy, fibrosis and adverse ventricular–vascular coupling222.
Microvascular disease also plays an important role in HFpEF. As this is harder to study in mice and even rats, some of the best preclinical work was performed decades ago in larger mammals. For example, LVH induced by aortic banding of dogs results in endocardial hypoperfusion during rapid pacing or exercise associated with depressed flow reserve223,224. The endocardium is particularly vulnerable to ischaemia, perhaps due to myocardial compression that results in microvascular retrograde flow during systole that must be refilled during diastole, while flow is primarily antegrade during systole in epicardial vessels225. Diffuse microvascular disease is also a common feature of diabetes68. Mechanisms include depressed nitric oxide signalling, oxidative stress, inflammation, impaired angiogenesis and other molecular abnormalities41. One factor attracting recent attention is the NAD-dependent deacetylase sirtuin 3 (SIRT3). SIRT3-deficient mice develop microvascular rarefaction, mitochondrial dysfunction and fibrosis, with depressed angiogenesis226. Application of hypertrophic stimuli results in markedly amplification of the maladaptive response227. Endothelial cell-targeted SIRT3 knock-down impairs glycolysis and angiogenesis and is coupled to diastolic dysfunction228. The protein has also been identified as a prime contributor to metformin–AMPK dependent improvement in a mouse model of HFpEF and pulmonary hypertension72. Proof of a role in human HFpEF remains lacking.
While precise mechanisms and therapeutic targets have yet to be identified, there is substantial support for microvascular defects in human HFpEF. Reactive hyperaemia after 5-min limb ischemia is depressed229,230. In coronary arteries, endothelium-dependent (flow response to acetylcholine) and endothelium-independent (flow response to adenosine) are often depressed. A reduced endothelial-independent response correlated with slower diastolic relaxation velocity and higher estimated diastolic pressures231. Depressed flow reserve also predicted worse outcome, including a fivefold increase in HFpEF hospitalizations232. Moreover, while HFpEF patients have greater resting cardiac external work, myocardial blood flow, and myocardial oxygen consumption compared with healthy controls, myocardial perfusion rises less and oxygen extraction more as work is increased by dobutamine β-adrenergic stimulation233. Microvascular disease also potently impacts skeletal muscle HFpEF, playing a prominent role to inadequate nutrient and oxygen supply and correlating with depressed exercise performance45,234.
MicroRNA signatures
With the discovery that microRNAs are released into the blood stream and may convey information about disease processes, studies began looking at these as potential biomarkers to discriminate between forms of HF as well as provide prognostic insight within HFpEF itself235,236. The largest such study was reported from ~1,700 HF patients from Singapore and New Zealand, divided into model group to derive an optimized eight-element microRNA for differentiating between HF forms, and separate validation groups237. The authors also measured NT-proBNP, and found this provided much of the HF discrimination, with the additive impact of microRNAs being rather modest. KEGG pathways corresponding to the eight-element microRNA set were involved with mRNA and ER processing, ubiquitin proteolysis, Hippo pathway, extracellular matrix interactions and fatty acid biosynthesis. Somewhat worrisome is that among five such miRNA studies to date, there appears to be minimal concordance in the miRNAs identified238.
Right ventricular Disease
Before abandoning the heart for extracardiac HFpEF contributors, it is important to note that though HFpEF traditionally focuses on left ventricular disease and disease models, growing evidence in humans supports a major role for right ventricular dysfunction19,20,239-244. RV dysfunction is a major risk factor for worse outcome in HFpEF patients240,242. It most often evolves in the setting of type II pulmonary hypertension, and longitudinal studies have shown its gradual evolution following LV disease that ultimately becomes a dominant limiting factor239. The dysfunctional RV is not hypercontractile in HFpEF, but exhibits reduced contractile and adverse systolic-vascular coupling during exercise19. Molecular underpinnings of RV disease associated with increased pulmonary afterload include many pathways shared by the LV that emphasize metabolic and energetic pathways245, but this remains a work in progress as RV-specific pathobiology has long been neglected. Experimental studies are problematic here as small rodents do not develop the type of RV failure observed in larger mammals or humans. Ongoing studies obtaining RV endocardial biopsies from humans with HFpEF are now performing broad molecular analyses along with sarcomere studies with the hope of elucidating signatures unique to HFpEF that might be therapeutic targets in the future.
Extracardiac Components
Skeletal muscle perfusion and metabolism
In the early 1980s, as vasodilator therapy was first being tested as a treatment for HF, investigators found that exercise capacity was not only limited by the heart but also by insufficient skeletal muscle vasodilatation246,247. In a classic study, LeJemtel and colleagues248 showed that lower-limb blood flow was the same in HF patients whether they exercised one or both legs, whereas controls had greater flow with one leg exercise. This indicated an inability of HF skeletal muscle to vasodilate appropriately to receive the cardiac output the heart could offer. Many studies followed identifying endothelium-dependent dysfunction and skeletal metabolic defects that limited its capacity to do the work required, including the work of breathing.
Fast forward several decades and much the same pathophysiology is now well recognized to play a critical role in HFpEF249,250. Morphologically and histologically, there are declines in lean skeletal muscle mass and accumulation of intramuscular fat44, reduced force-generating type 1 fibres, lower capillary to fibre ratio44, depressed high energy phosphate metabolism251, and reduced mitochondrial content252. A major determinant of exercise intolerance is insufficient oxygen extraction by the muscle45. Furthermore, while exercise training in HFrEF results in increased cardiac output reserve as well as improvement in skeletal muscle flow and oxygen extraction, in HFpEF the heart is not altered whereas skeletal muscle oxygen extraction is improved253. Thus, the benefits of exercise on raising peak oxygen consumption in HFpEF is largely peripheral at the level of skeletal muscle. Obesity also plays an important role in this pathophysiology, as intramuscular fat is pro-inflammatory and adversely impacts muscle metabolism impairing glucose utilization and thus muscle performance254. Percent fat mass is a strong correlate of exercise capacity and arterial–venous oxygen difference in HFpEF patients255.
In contrast to the heart, most data regarding skeletal muscle defects in HFpEF come from human studies, as animal models have not fully replicated the human pathophysiology. For example, while the ZSF-1 rat develops skeletal muscle with reduced fibre size, capillary density, and glycolytic metabolism, these are not reversed by endurance training256. The mouse l-NAME plus high-fat diet model also does not develop abnormal skeletal muscle perfusion or metabolism75. Skeletal muscle perfusion and metabolism have been little studied in hypertension–LVH models such as SSRs and DSSRs.
Pulmonary disease
Given the presence of elevated left ventricular diastolic pressures in HFpEF patients, it is not surprising that pulmonary disease including gas diffusion defects, vascular remodeling, and pulmonary hypertension (PH) are also common. Reduced alveolar-capillary membrane conductance and pulmonary capillary blood volume are associated with a 24% reduction in gas diffusion capacity at rest (versus controls), which rose to a 30% reduction with exercise contributing to exertional intolerance257. HFpEF patients with both PH and reduced diffusion capacity (<45% normal levels) have a markedly worse survival (36 vs 88% at 3 years)258. PH in HFpEF patients is primarily associated with post-capillary venous mechanisms, with only about 14% of patients present with both pre-capillary and post-capillary disease21. Autopsy studies report findings compatible similar to veno-occlusive disease259. Clinical studies have linked PH with reduced exercise capacity, in part due to right–left heart interdependence and adverse right ventricle–pulmonary artery interaction, as well as to worse disease progression20. Elevated right atrial and ventricular volumes and pressures increase pericardial pressure to reduce LV transmural pressure and thus filling volumes. The result is reduced stroke volume and thus cardiac output26. The right ventricle in HFpEF may be particularly sensitive to developing fibrosis with increased pulmonary vascular load244. There is far less research to date on the lung and PH in animal HFpEF models, though some have proposed mechanisms. For example, augmentation of AMPK activity by the prostacyclin analogue treprostinil or metformin improves pulmonary hypertension as well as metabolic status in the ZSF-1 rat260. Another intriguing mechanism relates to the expression of the NPRC receptor that is more increased in the right ventricle than the left ventricle or lung in a mouse model of HFpEF with obesity261. Whether this applies to humans remains unclear.
Kidney and liver
Fluid and electrolyte dys-homeostasis is a common feature in HFpEF and renal insufficiency is thought to play a major role262,263. Whether this represents a forme fruste of cardiorenal syndrome264 or a product of hypertension, diabetes, obesity, all common morbidities that adversely impact renal function265, depressed renal function is an important contributor to the syndrome. Renal insufficiency is often linked to elevated central venous pressures and thus right ventricular workload, contributing to right ventricular failure, a major predictor of adverse outcome in HFpEF243. One potential biological link between renal disease and HFpEF is presence of a variant allele in apolipoprotein 1 (APOL1) which is associated with an increased risk of chronic kidney disease primarily linked to hypertension266. The allele is particularly prevalent in African American women. In a study from the Women’s Health Initiative, postmenopausal women carrying the high-risk allele displayed a near 60% greater risk of hospitalization for HFpEF, though not of coronary artery disease, stroke or overall mortality267. With respect to therapy, there is ongoing interest in SGLT2 blockade, already shown to improve mortality in HFrEF patients by mechanisms that may involve the kidney. A trial of empagliflozin in HFpEF was recently completed, with results to be reported in the near future, although initial release from the sponsor suggests it did not achieve the primary outcome.
HFpEF therapeutics
The alarming state of affairs regarding HFpEF is that there exist so few therapies that impact its course and prognosis. Patients generally succumb to profound fluid volume overload that is difficult to ameliorate, dyspnoea, and severe exertional incapacity. Historically, treatment has focused on the heart and vessels, with neurohormonal blockade as has worked for HFrEF. However, with dozens of disappointing trials of RAAS blockers, this approach has been mostly abandoned. It remains possible that specific subgroups with constellations of clinical comorbidities likely coupled to molecular underlying mechanisms that are more responsive to these antagonists268, as was recently suggested in a spironolactone study95. As already mentioned, β-AR blockade is increasingly being abandoned given lack of evidence of efficacy, and selective sinus-node funny channel blockade may worsen symptoms.
A fair amount of effort has already focused on enhancing cGMP–PKG signalling, but translation in the clinic has been largely elusive to date. The multicentre RELAX trial of the PDE5 inhibitor sildenafil found no benefit on exercise performance (primary end point) nor any of a myriad of other parameters examined81. This may relate to lack of upregulation of PDE5 expression in HFpEF, and that cGMP primarily hydrolysed by PDE5 is coupled to nitric oxide stimulation155 which may be compromised in HFpEF29. Other approaches stimulated cGMP synthesis using inorganic nitrite269 or the sGC stimulator vericiguat270 and were neutral as well. A second sGC stimulator, paraliciguat, was also tested in a multicentre, placebo-controlled, phase II trial of 196 patients with HFpEF over a 12-week period. With the primary efficacy being exercise tolerance, there was no significant improvement, and the effort abandoned. Another approach is PDE9 inhibition, and trials were recently initiated in HFrEF patients. There are potential synergies with neprilysin inhibition, the latter enhancing NP-stimulated cGMP synthesis, while PDE9 inhibition would suppress hydrolysis of the synthesized cGMP. One caveat to this approach is that HFpEF patients often display low NP levels due in part to either to a lack of myocardial synthesis271 and/or to increased peripheral clearance by NPRC receptors in adipose tissue272.
Other recent trials tested the combination of sacubitril and valsartan (Entresto) in nearly 5,000 patients, and reported a 13% (P = 0.06) reduced rate ratio for combined HF hospitalization and cardiovascular death273. Greater effects were observed in those with lower EF, women, and those with recent decompensation requiring hospitalization273,274. The concept that this treatment may be efficacious in the appropriate subgroup is being further evaluated. Dopamine was tested for its potential role in enhancing glomerular filtration to improve diuresis in HFpEF patients hospitalized with fluid overload, but there was no benefit found275. Other recent studies include a test of an IL-1 blocker anakinra, inhaled β2-agonist albuterol, the mitochondrial fatty acid uptake inhibitor perhexiline, and SGLT2 inhibitor empagliflozin. Of these, only the albuterol data have reported, showing reduced pulmonary vascular resistance, increased compliance, and enhanced exercise reserve276. The EMPERIAL-Preserved study of empagliflozin in 315 HFpEF patients reported top-line results in late 2019, and was reportedly negative for the 6-min walking test primary end point. The larger EMPEROR-Preserved trial of nearly 6000 patients tests if this drug reduces the time to first composite endopint of cardiovascular death and/or heart failure hospitalization, and is expected to complete in late 2020. Other ongoing trials listed are testing the SGLT2 inhibitor dapagliflozin, inorganic nitrite, a monoamine oxidase inhibitor (AZD-4831), a xanthine oxidase inhibitor (allopurinol, Verinurad), and a cell-based therapy.
Studies involving inotropic modulation remain limited. One recent study tested the PDE3-inhibitor milrinone, a potent venous and arterial vasodilator that also increases heart rate and contractility. HFpEF patients receiving acute intravenous milrinone displayed lower central vascular pressures during exercise at greater cardiac output and heart rate 277. The investigators followed up with a new long-active form of milrinone tested in 23 patients, and while quality of life questionnaire results were improved, there was no significant change in 6-minute walk or other measures of cardiac function278. Another small trial (n=38) examined levosimendan, a PDE3-inhibitor but also a calcium sensitizer and peripheral vasodilator. Top line data reported it also lowered left sided filling pressures. The role of peripheral vascular versus cardiac impacts in both studies is uncertain. As previously discussed, the precise status of HFpEF contractility remains open to question, and likely varies among patient subgroups.
HFpEF device therapies are also being pursued, although few have trials completed and reported. One concept is to reduce sympathetic stimulation by either renal nerve denervation or splanchnic nerve resection, the latter potentially reducing venous return and thus central vascular congestion. Another is implantation of an interatrial shunt device that can reduce left atrial pressures at rest and during exercise by allowing blood to be transmitted to the right atrium279,280. This approach also improves pulmonary vascular function and RV load281 One study of 44 patients (REDUCE-LAP HF-1) reported a small (3-4 mmHg) but significant decline in PCWP that persisted during exercise282. The early reported data from non-randomized trials involved less than a dozen HFpEF patients but do suggest efficacy279. The CORolla TAA is a metal-wire structure with multiple springs that can be inserted into the LV chamber, compressed during systole, and then provides an elastic recoil to enhance cardiac filling. The primary target is diastolic dysfunction, and while this may find use in some forms of restrictive heart disease, it is unclear how this will help the phenotype in obese/diabetic multisystem HFpEF, where diastolic dysfunction is often mild. Physiological rate-responsive atrial pacing is being tested to determine if restoring some chronotropic response during exercise can improve symptoms. The role of pericardial constraint is being tested by performing pericardiectomy in HFpEF patients, the goal being to reduce interventricular crosstalk and enhance LV filling particular in patients with higher pulmonary pressures. One risk is converting a normal-volume ventricle into a larger one — essentially HFpEF into HFrEF, but that remains to be seen.
Lastly, there are therapies that do not target the heart at all. Exercise training is one of the very few interventions that has been successful, although the impact remains limited since such training in an individual with a BMI >35 is difficult to institute. Diet-induced weight loss has been tried, but again, morbid obesity is difficult to counteract with diet. Bariatric surgery is more impactful, and several limited trials have been performed examining the potential of this approach283,284. Table 3 lists current therapy trials that have not yet been published, including pharmaceuticals, and devices, though not life-style trials.
Table 3 ∣.
Ongoing clinical trials in HFpEF
| Status | Study Title | Interventions | Conditions |
|---|---|---|---|
| Pharmaceutical | |||
| Active | DETERMINE-preserved | Dapagliflozin | HFpEF |
| Active | Developing Oral LT3 Therapy for Heart Failure - HFpEF | liothyronine | Low Triiodothyronine Syndrome |
| Active | (KNO3CK OUT HFPEF) | Drug: Potassium Nitrate (KNO3), Drug: Potassium Chloride (KCl) | HFpEF |
| Active | (EMPEROR-Preserved) | Empagliflozin | HFpEF |
| Active | Oral Nitrite in Patients With Pulmonary Hypertension and Heart Failure With Preserved Ejection Fraction | Sodium Nitrite | HFpEF and secondary PH |
| Active | Metformin for Pulmonary Hypertension HFpEF | Metformin | HFpEF with Secondary PH |
| Active | AZD9977 and Spironolactone on Serum Potassium | AZD9977: Spironolactone | HFpEF |
| Active | Open-Label Rollover Study of Levosimendan in PH-HFpEF Patients | Levosimendan 2.5 mg/ml Injectable Solution | HFpEF with PH |
| Active | The Efficacy and Safety of Pirfenidone in HF and Preserved Left Ventricular Ejection Fraction | Pirfenidone | HFpEF |
| Recruiting | Safety and Tolerability of LCZ696 in Subjects Who Completed PARAGON-HF in Japan. | Drug: LCZ696 | HFpEF |
| Recruiting | Effect AZD4831 in Japanese and Chinese Healthy Volunteers | Drug: AZD4831 | HFpEF |
| Recruiting | Transthyretin Cardiac Amyloidosis in HFpEF | Drug: 99mTc-PYP | HFpEF |
| Recruiting | Circulating NEP and NEP Inhibition Study in Heart Failure With Preserved Ejection Fraction | Drug: Entresto™ 49Mg-51 mg tablet | HFpEF |
| Recruiting | Effect of IV Iron in Patients With Heart Failure With Preserved Ejection Fraction | Drug: Ferric Carboxymaltose 50Mg/Ml Inj 15Ml | Iron-deficiency, HFpEF |
| Recruiting | Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure. | Dapagliflozin | HFpEF |
| Recruiting | MPO Inhibitor A_Zeneca for HFpEF | Oral Myeloperoxidase Inhibitor | HFpEF |
| Recruiting | Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction | Spironolactone | HFpEF |
| Recruiting | LCZ696 in Advanced LV Hypertrophy and HFpEF | Drug: LCZ 696 Drug: Valsartan |
HFpEF with Essential Hypertension |
| Recruiting | A Study of Oral Nitrate in Adults With Pulmonary Hypertension With Heart Failure and Preserved Ejection Fraction | Drug: 15N Nitrate Drug: 14N Nitrate |
HFpEF, PH |
| Recruiting | A Trial to Study BAY1753011 in Patients With Congestive Heart Failure | Drug: BAY 1753011 Other: Placebo BAY 1753011 Drug: Furosemide Other: Placebo Furosemide |
HFrEF and HFpEF |
| Recruiting | Regression of Fibrosis & Reversal of Diastolic Dysfunction in HFPEF Patients Treated With Allogeneic CDCs | Biological: Allogeneic Derived Cells Biological: Placebo/Control Arm |
HFpEF with Diastolic Dysfunction |
| Recruiting | Dapagliflozin in PRESERVED Ejection Fraction Heart Failure | Drug: Dapagliflozin 10Mg Oral Tablet Drug: Dapagliflozin matching placebo |
HFpEF |
| Recruiting | β-blockers Withdrawal in Patients With HFpEF and Chronotropic Incompetence: Effect on Functional Capacity (Preserve-HR) | Drug: Controlled withdrawal of beta-blockers | HFpEF, Chronotropic Incompetence |
| Recruiting | Cell Therapy in HFpEF | Cell Therapy | HFpEF |
| Recruiting | INABLE-Training | Drug: Oral Sodium Nitrite Device: Accelerometer Other: Cardiac Exercise Training |
HFpEF |
| Recruiting | Changes in NT-proBNP and Outcomes, Safety, and Tolerability in HFpEF Patients With Acute Decompensated Heart Failure (ADHF) Who Have Been Stabilized During Hospitalization and Initiated In-hospital or Within 30 Days Post-discharge (PARAGLIDE-HF) Link |
Drug: sacubitril/valsartan Drug: valsartan |
HFpEF |
| Not yet recruiting | Effect of Dapagliflozin Plus Low Dose Pioglitazone on Hospitalization Rate in Patients With HF and HFpEF | Pioglitazone Plus dapaglifliozin | HFpEF and HFrEF |
| Enrolling by invitation | A Long Term Study to Find Out if Macitentan is an Effective and Safe Treatment for Patients With Heart Failure With Preserved Ejection Fraction and Pulmonary Vascular Disease (SERENADE OL) | Drug: macitentan 10 mg | HFpEF with Pulmonary Vascular Disease |
| Completed | A Randomized, Double-blind Controlled Study Comparing LCZ696 to Medical Therapy for Comorbidities in HFpEF Patients | Drug: sacubitril/valsartan Drug: Enalapril Drug: Valsartan |
HFpEF |
| Completed | The Efficacy and Safety of Pirfenidone in Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction | Drug: Pirfenidone Drug: Placebo |
HFpEF |
| Terminated | Oral Treprostinil in Subjects With Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction | Drug: Oral treprostinil Drug: Placebo |
HFpEF with PH |
| Not yet recruiting | Carvedilol SR Study for Biomarkers From Blood and Urine and Safety of in Patients With Heart Failure With Preserved Ejection Fraction | HFpEF | Carvedilol SR |
| Not yet recruiting | Study of Verinurad in Heart Failure With Preserved Ejection Fraction | HFpEF | Drug: Verinurad Drug: Allopurinol Drug: Placebo for verinurad Drug: Placebo for allopurinol |
| Devices | |||
| Recruiting | CORolla® TAA for Heart Failure With Preserved Ejection Fraction (HFpEF) and Diastolic Dysfunction (DD) | Diastolic Heart Failure, Diastolic Dysfunction | Device: CORolla™ TAA device |
| Recruiting | Endovascular GSN Ablation in Subjects With HFpEF | HFpEF | Device: Ablation |
| Recruiting | CCM in Heart Failure with Preserved Ejection Fraction | Heart Failure, Diastolic | Device: Optimizer SMART |
| Recruiting | Efficacy Study of Pacemakers to Treat Slow Heart Rate in Patients With Heart Failure | Heart Failure, Diastolic, Chronotropic Incompetence | Device: Rate adaptive atrial pacing using a dual-chamber pacemaker Device: Pacemaker system will be implanted but set to Pacing Off. |
| Active Not Recruiting | REDUCE LAP-HF RANDOMIZED TRIAL I | Device: Inter-Atrial Shunt Device Other: Intracardiac Echo |
Heart Failure |
| Not yet recruiting | 3-Month Home-based Training With Whole Body Vibration (WBV) Device in Patients With Heart Failure and Preserved Ejection Fraction (GALILEO-HFpEF-HOME) (GALILEOHOME) | HFpEF | Device: Group 1 GALILEO WBV Other: Group 2 Control |
| Recruiting | REDUCE LAP-HF TRIAL II | Device: VitalPatch Biosensor Device: DynaPort Move Monitor |
Heart Failure |
| Recruiting | Reducing Lung CongestIon Symptoms in Advanced Heart Failure | Device: V-Wave Interatrial Shunt Other: Control |
Heart Failure |
| Recruiting | Hemodynamic-GUIDEd Management of Heart Failure | Device: CardioMEMS™ HF System | Heart Failure Heart Failure, Systolic Heart Failure, Diastolic |
Perspectives and Future Directions
The expanding presence of HFpEF throughout the world and no truly impactful therapy options has intensified the focus on this syndrome like never before. The NIH recently presented a roadmap for research studies, identifying major gaps in knowledge and areas in sore need of new insight and advances4. One broad theme is that this syndrome is a systemic one, and we need to greatly broaden the focus of research and models to incorporate the complex interactions between multiple organ systems. While small rodents are undeniably the premier model system for unlocking new mechanisms and targets, few effective therapies in these models have yet been successfully translated to humans. The major push to human iPS-derived organoids has been one answer but we doubt this will be useful for HFpEF given the systems biology involved with complex inter-organ crosstalk. Perhaps this argues for much greater attention on human studies themselves, and at a mechanistic level. We need better-defined biological targets, and that is unlikely to come solely from biomarkers measured in the blood stream. This means more active pursuit of tissue procurement, as commonly done in oncology. HFpEF researchers need to consider doing the same, whether from the heart, skeletal muscle, fat, or other tissues. In the meantime, the resurgent interest in experimental models is moving in the right direction, with combination models that capture more of the multidimensionality that is HFpEF. There is much to uncover, and the need has never been more urgent.
Key points.
While the historical focus of HFpEF pathophysiology has been on diastolic dysfunction, hypertrophy, and cardiac fibrosis, it actually engages many different components impacting systolic and diastolic heart function but also other organs and systems.
Preclinical studies, particularly those combining obesity/metabolic defects with hemodynamic/cardiac disease as exists in the majority of HFpEF patients, are beginning to reveal novel molecular mechanisms and therapeutic targets.
There is substantial overlap with proposed molecular/cellular abnormalities in HFpEF and that observed with diabetes and obesity, including metabolic defects in fuel utilization and efficiency, inflammatory responses, lipotoxicity, pathological growth, and loss of cytoprotection signaling.
The heart is but one feature of HFpEF, albeit the one for which the vast majority of basic biology focuses on, but the syndrome also engages lung, kidney, vascular, adipose, skeletal muscle, and other abnormalities.
In addition to exploring novel hemodynamic interventions with both drugs and devices, new therapies are targeting pleotropic signaling cascades to counter metabolic, inflammatory, and pathological-stress pathways.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Gladden JD, Chaanine AH & Redfield MM Heart Failure with Preserved Ejection Fraction. Annual review of medicine 69, 65–79, doi: 10.1146/annurev-med-041316-090654 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Lam CSP et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur Heart J 39, 1770–1780, doi: 10.1093/eurheartj/ehy005 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Zakeri R & Cowie MR Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart 104, 377–384, doi: 10.1136/heartjnl-2016-310790 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Shah SJ et al. Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 141, 1001–1026, doi: 10.1161/circulationaha.119.041886 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodek A, Kassebaum DG & Bristow JD Pulmonary edema in coronary-artery disease without cardiomegaly. Paradox of the stiff heart. N Engl J Med 286, 1347–1350, doi: 10.1056/nejm197206222862507 (1972). [DOI] [PubMed] [Google Scholar]
- 6.Dougherty AH, Naccarelli GV, Gray EL, Hicks CH & Goldstein RA Congestive heart failure with normal systolic function. Am.J.Cardiol 54, 778–782 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Soufer R, Wohlgelernter D, Vita NA & et al. Intact systolic left ventricular function in clinical congestive heart failure. Am.J.Cardiol 55, 1082–1086 (1985). [DOI] [PubMed] [Google Scholar]
- 8.Topol EJ, Traill TA & Fortuin NJ Hypertensive hypertrophic cardiomyopathy of the elderly. New England Journal of Medicine 312, 277–283 (1985). [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 33, 1948–1955, doi: 10.1016/s0735-1097(99)00118-7 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Owan TE et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N.Engl.J Med 355, 251–259 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Melenovsky V et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll.Cardiol 49, 198–207 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi M, Hay I, Fetics B & Kass DA Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107, 714–720 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ & Redfield MM Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 54, 410–418, doi: 10.1016/j.jacc.2009.05.013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borlaug BA et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114, 2138–2147 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Phan TT et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail 3, 29–34, doi: 10.1161/circheartfailure.109.877720 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Dominguez E et al. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail 5, 579–585, doi: 10.1002/ehf2.12281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam CS et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53, 1119–1126, doi: 10.1016/j.jacc.2008.11.051 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thenappan T, Prins KW, Cogswell R & Shah SJ Pulmonary hypertension secondary to heart failure with preserved ejection fraction. Can J Cardiol 31, 430–439, doi: 10.1016/j.cjca.2014.12.028 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA, Kane GC, Melenovsky V & Olson TP Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 37, 3293–3302, doi: 10.1093/eurheartj/ehw241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorter TM et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 20, 16–37, doi: 10.1002/ejhf.1029 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Levine AR, Simon MA & Gladwin MT Pulmonary vascular disease in the setting of heart failure with preserved ejection fraction. Trends Cardiovasc Med 29, 207–217, doi: 10.1016/j.tcm.2018.08.005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghio S et al. Pulmonary hypertension and right ventricular remodeling in HFpEF and HFrEF. Heart Fail Rev 25, 85–91, doi: 10.1007/s10741-019-09810-4 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Norman HS et al. Decreased cardiac functional reserve in heart failure with preserved systolic function. J Card Fail 17, 301–308, doi: 10.1016/j.cardfail.2010.11.004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56, 845–854, doi: 10.1016/j.jacc.2010.03.077 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlaug BA Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circulation journal : official journal of the Japanese Circulation Society 78, 20–32 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Gorter TM, Obokata M, Reddy YNV, Melenovsky V & Borlaug BA Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 39, 2825–2835, doi: 10.1093/eurheartj/ehy331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Buono MG et al. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol 73, 2209–2225, doi: 10.1016/j.jacc.2019.01.072 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Kitzman DW & Shah SJ The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol 68, 200–203, doi: 10.1016/j.jacc.2016.05.019 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Paulus WJ & Tschope C A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62, 263–271, doi: 10.1016/j.jacc.2013.02.092 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V & Borlaug BA Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 136, 6–19, doi: 10.1161/circulationaha.116.026807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman DE & Goodpaster BH Weighty Matters in HFpEF and Aging. JACC. Heart failure 6, 650–652, doi: 10.1016/j.jchf.2018.06.016 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Westermann D et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4, 44–52, doi: 10.1161/circheartfailure.109.931451 (2011). [DOI] [PubMed] [Google Scholar]
- 33.D'Elia E et al. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail 17, 1231–1239, doi: 10.1002/ejhf.430 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Abernethy A et al. Pro-Inflammatory Biomarkers in Stable Versus Acutely Decompensated Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc 7, doi: 10.1161/jaha.117.007385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirinos JA et al. Multiple Plasma Biomarkers for Risk Stratification in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 75, 1281–1295, doi: 10.1016/j.jacc.2019.12.069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DuBrock HM, AbouEzzeddine OF & Redfield MM High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS One 13, e0201836, doi: 10.1371/journal.pone.0201836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammed SF et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131, 550–559, doi: 10.1161/circulationaha.114.009625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JF et al. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart, doi: 10.1136/heartjnl-2015-308403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franssen C et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 4, 312–324, doi: 10.1016/j.jchf.2015.10.007 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Zeng H & Chen JX Microvascular Rarefaction and Heart Failure With Preserved Ejection Fraction. Frontiers in cardiovascular medicine 6, 15, doi: 10.3389/fcvm.2019.00015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Amario D et al. Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction. Frontiers in physiology 10, 1347, doi: 10.3389/fphys.2019.01347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haykowsky MJ et al. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci 68, 968–975, doi: 10.1093/gerona/glt011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzman DW et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 306, H1364–1370, doi: 10.1152/ajpheart.00004.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haykowsky MJ et al. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 113, 1211–1216, doi: 10.1016/j.amjcard.2013.12.031 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhakal BP et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 8, 286–294, doi: 10.1161/circheartfailure.114.001825 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirai DM, Musch TI & Poole DC Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309, H1419–1439, doi: 10.1152/ajpheart.00469.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tromp J et al. Heart failure with preserved ejection fraction in Asia. Eur J Heart Fail 21, 23–36, doi: 10.1002/ejhf.1227 (2019). [DOI] [PubMed] [Google Scholar]
- 48.McHugh K et al. Heart Failure With Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol 73, 602–611, doi: 10.1016/j.jacc.2018.11.033 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Shear FE Novel paradigms in the therapeutic management of heart failure with preserved ejection fraction: clinical perspectives. Am J Cardiovasc Dis 9, 91–108 (2019). [PMC free article] [PubMed] [Google Scholar]
- 50.Wintrich J et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin Res Cardiol, doi: 10.1007/s00392-020-01633-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulus WJ Unfolding Discoveries in Heart Failure. N Engl J Med 382, 679–682, doi: 10.1056/NEJMcibr1913825 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Okayama H et al. Alterations in expression of sarcoplasmic reticulum gene in Dahl rats during the transition from compensatory myocardial hypertrophy to heart failure. J Hypertens 15, 1767–1774, doi: 10.1097/00004872-199715120-00087 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Qu P et al. Time-course changes in left ventricular geometry and function during the development of hypertension in Dahl salt-sensitive rats. Hypertens Res 23, 613–623, doi: 10.1291/hypres.23.613 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Chen-Izu Y et al. Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am J Physiol Heart Circ Physiol 293, H3301–3310, doi: 10.1152/ajpheart.00259.2007 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Weber KT, Janicki JS, Pick R, Capasso J & Anversa P Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 65, 1G–7G (1990). [DOI] [PubMed] [Google Scholar]
- 56.Fillmore N et al. Uncoupling of glycolysis from glucose oxidation accompanies the development of heart failure with preserved ejection fraction. Mol Med 24, 3, doi: 10.1186/s10020-018-0005-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagata K et al. Early changes in excitation-contraction coupling: transition from compensated hypertrophy to failure in Dahl salt-sensitive rat myocytes. Cardiovasc Res 37, 467–477, doi: 10.1016/s0008-6363(97)00278-2 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Capasso JM, Palackal T, Olivetti G & Anversa P Left ventricular failure induced by long-term hypertension in rats. Circ Res 66, 1400–1412, doi: 10.1161/01.res.66.5.1400 (1990). [DOI] [PubMed] [Google Scholar]
- 59.Lupon J et al. Heart Failure With Preserved Ejection Fraction Infrequently Evolves Toward a Reduced Phenotype in Long-Term Survivors. Circ Heart Fail 12, e005652, doi: 10.1161/circheartfailure.118.005652 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Nishio M et al. Therapeutic effects of angiotensin II type 1 receptor blocker at an advanced stage of hypertensive diastolic heart failure. J Hypertens 25, 455–461, doi: 10.1097/HJH.0b013e328010d635 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto K et al. Roles of renin-angiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc Res 47, 274–283, doi: 10.1016/s0008-6363(00)00101-2 (2000). [DOI] [PubMed] [Google Scholar]
- 62.Wake R et al. Beneficial effect of candesartan on rat diastolic heart failure. J Pharmacol Sci 98, 372–379, doi: 10.1254/jphs.fp0050160 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Yoshida J et al. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension 43, 686–691, doi: 10.1161/01.HYP.0000118017.02160.fa (2004). [DOI] [PubMed] [Google Scholar]
- 64.Jeong MY et al. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med 10, doi: 10.1126/scitranslmed.aao0144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallner M et al. HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Science translational medicine 12, doi: 10.1126/scitranslmed.aay7205 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallet R et al. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci 1, 14–28, doi: 10.1016/j.jacbts.2016.01.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fein FS & Sonnenblick EH Diabetic cardiomyopathy. Prog Cardiovasc Dis 27, 255–270, doi: 10.1016/0033-0620(85)90009-x (1985). [DOI] [PubMed] [Google Scholar]
- 68.Jia G, Hill MA & Sowers JR Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res 122, 624–638, doi: 10.1161/circresaha.117.311586 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tofovic SP, Kusaka H, Kost CK Jr. & Bastacky S Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren Fail 22, 387–406, doi: 10.1081/jdi-100100882 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Leite S et al. Arterial Remodeling and Dysfunction in the ZSF1 Rat Model of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 12, e005596, doi: 10.1161/circheartfailure.118.005596 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Boustany-Kari CM et al. A Soluble Guanylate Cyclase Activator Inhibits the Progression of Diabetic Nephropathy in the ZSF1 Rat. J Pharmacol Exp Ther 356, 712–719, doi: 10.1124/jpet.115.230706 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Lai YC et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation 133, 717–731, doi: 10.1161/circulationaha.115.018935 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopf AE et al. Diabetes-Induced Cardiomyocyte Passive Stiffening Is Caused by Impaired Insulin-Dependent Titin Modification and Can Be Modulated by Neuregulin-1. Circ Res 123, 342–355, doi: 10.1161/circresaha.117.312166 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Salah EM, Bastacky SI, Jackson EK & Tofovic SP Captopril Attenuates Cardiovascular and Renal Disease in a Rat Model of Heart Failure With Preserved Ejection Fraction. J Cardiovasc Pharmacol 71, 205–214, doi: 10.1097/fjc.0000000000000561 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Schiattarella GG et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568, 351–356, doi: 10.1038/s41586-019-1100-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tong D et al. Female Sex Is Protective in a Preclinical Model of Heart Failure With Preserved Ejection Fraction. Circulation 140, 1769–1771, doi: 10.1161/circulationaha.119.042267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munagala VK, Hart CY, Burnett JC Jr., Meyer DM & Redfield MM Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation 111, 1128–1135, doi: 10.1161/01.Cir.0000157183.21404.63 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiter U et al. Early-stage heart failure with preserved ejection fraction in the pig: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 18, 63, doi: 10.1186/s12968-016-0283-9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorop O et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res 114, 954–964, doi: 10.1093/cvr/cvy038 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwarzl M et al. A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 309, H1407–1418, doi: 10.1152/ajpheart.00542.2015 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Redfield MM et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309, 1268–1277, doi: 10.1001/jama.2013.2024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy YNV, Carter RE, Obokata M, Redfield MM & Borlaug BA A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 138, 861–870, doi: 10.1161/circulationaha.118.034646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zile MR et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 124, 2491–2501, doi: 10.1161/circulationaha.110.011031 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Lindman BR et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol 64, 541–549, doi: 10.1016/j.jacc.2014.05.030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armstrong AC et al. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Echocardiography 31, 12–20, doi: 10.1111/echo.12303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura M & Sadoshima J Mechanisms of physiological and pathological cardiac hypertrophy. Nature reviews. Cardiology 15, 387–407, doi: 10.1038/s41569-018-0007-y (2018). [DOI] [PubMed] [Google Scholar]
- 87.Wettschureck N et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat.Med 7, 1236–1240 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Zhang W et al. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J Biol.Chem 281, 5811–5820 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Takimoto E et al. RGS2 mediates cardiac compensation to pressure-overload and antihypertrophic effects of PDE5 inhibition. J Clin Invest 119, 408–420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bishu K et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 164, 763–770 e763, doi: 10.1016/j.ahj.2012.08.014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tschope C, Van Linthout S & Kherad B Heart Failure with Preserved Ejection Fraction and Future Pharmacological Strategies: a Glance in the Crystal Ball. Curr Cardiol Rep 19, 70, doi: 10.1007/s11886-017-0874-6 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E & Zera T Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J Physiol Pharmacol 69, doi: 10.26402/jpp.2018.6.01 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Fraccarollo D et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 123, 400–408, doi: 10.1161/circulationaha.110.983023 (2011). [DOI] [PubMed] [Google Scholar]
- 94.Rickard AJ et al. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 60, 1443–1450, doi: 10.1161/hypertensionaha.112.203158 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Cohen JB et al. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail 8, 172–184, doi: 10.1016/j.jchf.2019.09.009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pitt B et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370, 1383–1392, doi: 10.1056/NEJMoa1313731 (2014). [DOI] [PubMed] [Google Scholar]
- 97.de Denus S et al. Spironolactone Metabolites in TOPCAT - New Insights into Regional Variation. N Engl J Med 376, 1690–1692, doi: 10.1056/NEJMc1612601 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bristow MR et al. Detection and Management of Geographic Disparities in the TOPCAT Trial: Lessons Learned and Derivative Recommendations. JACC Basic Transl Sci 1, 180–189, doi: 10.1016/j.jacbts.2016.03.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ravassa S et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur J Heart Fail 20, 1290–1299, doi: 10.1002/ejhf.1194 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Maron MS et al. Effect of Spironolactone on Myocardial Fibrosis and Other Clinical Variables in Patients with Hypertrophic Cardiomyopathy. Am J Med 131, 837–841, doi: 10.1016/j.amjmed.2018.02.025 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Myhre PL et al. Mechanistic Effects of Spironolactone on Cardiovascular and Renal Biomarkers in Heart Failure With Preserved Ejection Fraction: A TOPCAT Biorepository Study. Circ Heart Fail 13, e006638, doi: 10.1161/circheartfailure.119.006638 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto K, Origasa H & Hori M Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 15, 110–118, doi: 10.1093/eurjhf/hfs141 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Pal N et al. Effect of Selective Heart Rate Slowing in Heart Failure With Preserved Ejection Fraction. Circulation 132, 1719–1725, doi: 10.1161/circulationaha.115.017119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komajda M et al. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur J Heart Fail 19, 1495–1503, doi: 10.1002/ejhf.876 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Mesubi OO & Anderson ME Atrial remodelling in atrial fibrillation: CaMKII as a nodal proarrhythmic signal. Cardiovasc Res 109, 542–557, doi: 10.1093/cvr/cvw002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anderson ME, Brown JH & Bers DM CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 51, 468–473, doi: 10.1016/j.yjmcc.2011.01.012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joiner ML et al. CaMKII determines mitochondrial stress responses in heart. Nature 491, 269–273, doi: 10.1038/nature11444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suetomi T, Miyamoto S & Brown JH Inflammation in nonischemic heart disease: initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am J Physiol Heart Circ Physiol 317, H877–h890, doi: 10.1152/ajpheart.00223.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rusciano MR et al. CaMKII Activity in the Inflammatory Response of Cardiac Diseases. Int J Mol Sci 20, doi: 10.3390/ijms20184374 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hegyi B, Bers DM & Bossuyt J CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J Mol Cell Cardiol 127, 246–259, doi: 10.1016/j.yjmcc.2019.01.001 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Shi J et al. Molecular determinants for cardiovascular TRPC6 channel regulation by Ca2+/calmodulin-dependent kinase II. J Physiol 591, 2851–2866, doi: 10.1113/jphysiol.2013.251249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuwahara K et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116, 3114–3126 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin BL et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc Natl Acad Sci U S A 116, 10156–10161, doi: 10.1073/pnas.1815354116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davis J, Burr AR, Davis GF, Birnbaumer L & Molkentin JD A TRPC6-Dependent Pathway for Myofibroblast Transdifferentiation and Wound Healing In Vivo. Developmental cell 23, 705–715, doi: 10.1016/j.devcel.2012.08.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang CL et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kong Y et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 113, 2579–2588 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ago T et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133, 978–993 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Doi R et al. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J.Hypertens 18, 111–120 (2000). [DOI] [PubMed] [Google Scholar]
- 119.Yamamoto K et al. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc.Res 55, 76–82 (2002). [DOI] [PubMed] [Google Scholar]
- 120.van Heerebeek L et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117, 43–51, doi: 10.1161/circulationaha.107.728550 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Fukui S et al. Diabetes mellitus accelerates left ventricular diastolic dysfunction through activation of the renin-angiotensin system in hypertensive rats. Hypertens Res 32, 472–480, doi: 10.1038/hr.2009.43 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Liu F et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor alpha. Circulation 131, 795–804, doi: 10.1161/circulationaha.114.012285 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Cavalera M, Wang J & Frangogiannis NG Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Translational research : the journal of laboratory and clinical medicine 164, 323–335, doi: 10.1016/j.trsl.2014.05.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alex L, Russo I, Holoborodko V & Frangogiannis NG Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 315, H934–h949, doi: 10.1152/ajpheart.00238.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Panchal SK et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 57, 611–624, doi: 10.1097/FJC.0b013e31821b1379 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Hahn VS et al. Endomyocardial Biopsy Characterization of Heart Failure with Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart Fail In Press (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fu X et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 128, 2127–2143, doi: 10.1172/jci98215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xiao Y et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev 33, 1491–1505, doi: 10.1101/gad.329763.119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DeLeon-Pennell KY, Meschiari CA, Jung M & Lindsey ML Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog Mol Biol Transl Sci 147, 75–100, doi: 10.1016/bs.pmbts.2017.02.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gonzalez A et al. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension 55, 1418–1424, doi: 10.1161/hypertensionaha.109.149112 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Kasner M et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol 57, 977–985, doi: 10.1016/j.jacc.2010.10.024 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Lopez B, Querejeta R, Gonzalez A, Larman M & Diez J Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension 60, 677–683, doi: 10.1161/hypertensionaha.112.196113 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Kanagala P et al. Relationship Between Focal and Diffuse Fibrosis Assessed by CMR and Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc Imaging 12, 2291–2301, doi: 10.1016/j.jcmg.2018.11.031 (2019). [DOI] [PubMed] [Google Scholar]
- 134.Zile MR et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131, 1247–1259, doi: 10.1161/CIRCULATIONAHA.114.013215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopez B et al. Is plasma cardiotrophin-1 a marker of hypertensive heart disease? J Hypertens. 23, 625–632 (2005). [DOI] [PubMed] [Google Scholar]
- 136.de Boer RA et al. Galectin-3 in heart failure with preserved ejection fraction. Eur J Heart Fail 15, 1095–1101, doi: 10.1093/eurjhf/hft077 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Polat V, Bozcali E, Uygun T, Opan S & Karakaya O Diagnostic significance of serum galectin-3 levels in heart failure with preserved ejection fraction. Acta Cardiol 71, 191–197, doi: 10.2143/ac.71.2.3141849 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Corden B, Adami E, Sweeney M, Schafer S & Cook SA IL-11 in cardiac and renal fibrosis: Late to the party but a central player. Br J Pharmacol 177, 1695–1708, doi: 10.1111/bph.15013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Du W et al. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics 8, 4155–4169, doi: 10.7150/thno.26055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luo M & Anderson ME Mechanisms of altered Ca(2)(+) handling in heart failure. Circ Res 113, 690–708, doi: 10.1161/circresaha.113.301651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nagayama T et al. Control of in vivo left ventricular contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation 116, 2399–2408 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Kapur S et al. Early development of intracellular calcium cycling defects in intact hearts of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 299, H1843–1853, doi: 10.1152/ajpheart.00623.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Primessnig U et al. Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. Eur J Heart Fail 18, 987–997, doi: 10.1002/ejhf.524 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Borbely A et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation 111, 774–781, doi: 10.1161/01.cir.0000155257.33485.6d (2005). [DOI] [PubMed] [Google Scholar]
- 145.van Heerebeek L et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113, 1966–1973 (2006). [DOI] [PubMed] [Google Scholar]
- 146.van Heerebeek L et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126, 830–839, doi: 10.1161/CIRCULATIONAHA.111.076075 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Kruger M et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ.Res 104, 87–94 (2009). [DOI] [PubMed] [Google Scholar]
- 148.Hamdani N et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail 6, 1239–1249, doi: 10.1161/circheartfailure.113.000539 (2013). [DOI] [PubMed] [Google Scholar]
- 149.Perreault CL, Bing OH, Brooks WW, Ransil BJ & Morgan JP Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ Res 67, 707–712, doi: 10.1161/01.res.67.3.707 (1990). [DOI] [PubMed] [Google Scholar]
- 150.Lin YH et al. Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J Mol Cell Cardiol 139, 135–147, doi: 10.1016/j.yjmcc.2020.01.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Foster DB et al. The cardiac acetyl-lysine proteome. PloS one 8, e67513, doi: 10.1371/journal.pone.0067513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Samant SA et al. HDAC3-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem 286, 5567–5577, doi: 10.1074/jbc.M110.163865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Holtwick R et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J.Clin.Invest 111, 1399–1407 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takimoto E et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat.Med 11, 214–222 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Lee DI et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 519, 472–476, doi: 10.1038/nature14332 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koitabashi N et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol 48, 713–724 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kinoshita H et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ.Res 106, 1849–1860 (2010). [DOI] [PubMed] [Google Scholar]
- 158.Takimoto E et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest 119, 408–420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tokudome T et al. Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation. 117, 2329–2339 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Ranek MJ et al. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 566, 264–269, doi: 10.1038/s41586-019-0895-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ranek MJ, Terpstra EJ, Li J, Kass DA & Wang X Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins. Circulation 128, 365–376, doi: 10.1161/CIRCULATIONAHA.113.001971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang WZ, Jones AW, Wang M, Durante W & Korthuis RJ Preconditioning with soluble guanylate cyclase activation prevents postischemic inflammation and reduces nitrate tolerance in heme oxygenase-1 knockout mice. Am J Physiol Heart Circ Physiol 305, H521–532, doi: 10.1152/ajpheart.00810.2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Inserte J & Garcia-Dorado D The cGMP/PKG pathway as a common mediator of cardioprotection: translatability and mechanism. British journal of pharmacology 172, 1996–2009, doi: 10.1111/bph.12959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kokkonen-Simon KM et al. Marked disparity of microRNA modulation by cGMP-selective PDE5 versus PDE9 inhibitors in heart disease. JCI insight 3, doi: 10.1172/jci.insight.121739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Collins S A heart-adipose tissue connection in the regulation of energy metabolism. Nature reviews. Endocrinology 10, 157–163, doi: 10.1038/nrendo.2013.234 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Mitschke MM et al. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J 27, 1621–1630, doi: 10.1096/fj.12-221580 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Layland J, Li JM & Shah AM Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J.Physiol 540, 457–467 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dunkerly-Eyring B & Kass DA Myocardial Phosphodiesterases and their Role in cGMP Regulation. J Cardiovasc Pharmacol, doi: 10.1097/FJC.0000000000000773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sasaki H et al. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest 124, 2464–2471, doi: 10.1172/jci70731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fukuma N et al. Estrogen Receptor-α Non-Nuclear Signaling Confers Cardioprotection and Is Essential to cGMP-PDE5 Inhibition Efficacy. JACC. Basic to translational science 5, 282–295, doi: 10.1016/j.jacbts.2019.12.009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Santos CX, Raza S & Shah AM Redox signaling in the cardiomyocyte: From physiology to failure. Int J Biochem Cell Biol 74, 145–151, doi: 10.1016/j.biocel.2016.03.002 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Faria A & Persaud SJ Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol Ther 172, 50–62, doi: 10.1016/j.pharmthera.2016.11.013 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Takimoto E & Kass DA Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Kaludercic N et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106, 193–202, doi: 10.1161/CIRCRESAHA.109.198366 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takimoto E et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J.Clin.Invest 115, 1221–1231 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Noordali H, Loudon BL, Frenneaux MP & Madhani M Cardiac metabolism - A promising therapeutic target for heart failure. Pharmacology & therapeutics 182, 95–114, doi: 10.1016/j.pharmthera.2017.08.001 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Karwi QG, Uddin GM, Ho KL & Lopaschuk GD Loss of Metabolic Flexibility in the Failing Heart. Front Cardiovasc Med 5, 68, doi: 10.3389/fcvm.2018.00068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS & Stanley WC Myocardial fatty acid metabolism in health and disease. Physiol Rev 90, 207–258, doi: 10.1152/physrev.00015.2009 (2010). [DOI] [PubMed] [Google Scholar]
- 179.Beer M et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol 40, 1267–1274, doi: 10.1016/s0735-1097(02)02160-5 (2002). [DOI] [PubMed] [Google Scholar]
- 180.Conway MA et al. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338, 973–976, doi: 10.1016/0140-6736(91)91838-l (1991). [DOI] [PubMed] [Google Scholar]
- 181.Kato T et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3, 420–430, doi: 10.1161/CIRCHEARTFAILURE.109.888479 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Nascimben L et al. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation 91, 1824–1833, doi: 10.1161/01.cir.91.6.1824 (1995). [DOI] [PubMed] [Google Scholar]
- 183.Neubauer S et al. Downregulation of the Na(+)-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation 100, 1847–1850, doi: 10.1161/01.cir.100.18.1847 (1999). [DOI] [PubMed] [Google Scholar]
- 184.Fillmore N & Lopaschuk GD Targeting mitochondrial oxidative metabolism as an approach to treat heart failure. Biochim Biophys Acta 1833, 857–865, doi: 10.1016/j.bbamcr.2012.08.014 (2013). [DOI] [PubMed] [Google Scholar]
- 185.Phan TT et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 54, 402–409, doi: 10.1016/j.jacc.2009.05.012 (2009). [DOI] [PubMed] [Google Scholar]
- 186.Neubauer S et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86, 1810–1818, doi: 10.1161/01.cir.86.6.1810 (1992). [DOI] [PubMed] [Google Scholar]
- 187.Arany Z et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1, 259–271, doi: 10.1016/j.cmet.2005.03.002 (2005). [DOI] [PubMed] [Google Scholar]
- 188.Berthiaume JM, Kurdys JG, Muntean DM & Rosca MG Mitochondrial NAD(+)/NADH Redox State and Diabetic Cardiomyopathy. Antioxid Redox Signal 30, 375–398, doi: 10.1089/ars.2017.7415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pillai VB et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 285, 3133–3144, doi: 10.1074/jbc.M109.077271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Horton JL et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2, doi: 10.1172/jci.insight.84897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee CF et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation 134, 883–894, doi: 10.1161/circulationaha.116.022495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Diguet N et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 137, 2256–2273, doi: 10.1161/circulationaha.116.026099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karamanlidis G et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18, 239–250, doi: 10.1016/j.cmet.2013.07.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Diakos NA et al. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl Sci 1, 432–444, doi: 10.1016/j.jacbts.2016.06.009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Doenst T, Nguyen TD & Abel ED Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113, 709–724, doi: 10.1161/CIRCRESAHA.113.300376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lei B et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36, 567–576, doi: 10.1016/j.yjmcc.2004.02.004 (2004). [DOI] [PubMed] [Google Scholar]
- 197.Tumova J, Andel M & Trnka J Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiological research / Academia Scientiarum Bohemoslovaca (2015). [DOI] [PubMed] [Google Scholar]
- 198.Lauzier B et al. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol 55, 92–100, doi: 10.1016/j.yjmcc.2012.11.008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sowton AP, Griffin JL & Murray AJ Metabolic Profiling of the Diabetic Heart: Toward a Richer Picture. Front Physiol 10, 639, doi: 10.3389/fphys.2019.00639 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davila-Roman VG et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40, 271–277, doi: 10.1016/s0735-1097(02)01967-8 (2002). [DOI] [PubMed] [Google Scholar]
- 201.Mahmod M et al. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 20, 88, doi: 10.1186/s12968-018-0511-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wei J et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol 310, H14–19, doi: 10.1152/ajpheart.00612.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Djousse L et al. Plasma free fatty acids and risk of heart failure: the Cardiovascular Health Study. Circ Heart Fail 6, 964–969, doi: 10.1161/CIRCHEARTFAILURE.113.000521 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hunter WG et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J Am Heart Assoc 5, doi: 10.1161/jaha.115.003190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hage C et al. Inflammatory Biomarkers Predict Heart Failure Severity and Prognosis in Patients With Heart Failure With Preserved Ejection Fraction: A Holistic Proteomic Approach. Circ Cardiovasc Genet 10, doi: 10.1161/CIRCGENETICS.116.001633 (2017). [DOI] [PubMed] [Google Scholar]
- 206.Zordoky BN et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One 10, e0124844, doi: 10.1371/journal.pone.0124844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Aubert G et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 133, 698–705, doi: 10.1161/circulationaha.115.017355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mizuno Y et al. The diabetic heart utilizes ketone bodies as an energy source. Metabolism 77, 65–72, doi: 10.1016/j.metabol.2017.08.005 (2017). [DOI] [PubMed] [Google Scholar]
- 209.Ho KL et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 115, 1606–1616, doi: 10.1093/cvr/cvz045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zordoky BN et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One 10, e0124844, doi: 10.1371/journal.pone.0124844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Newman JC & Verdin E Ketone bodies as signaling metabolites. Trends Endocrinol Metab 25, 42–52, doi: 10.1016/j.tem.2013.09.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ferrannini E, Mark M & Mayoux E CV Protection in the EMPA-REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes Care 39, 1108–1114, doi: 10.2337/dc16-0330 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Xia Y et al. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131, 471–481, doi: 10.1007/s00418-008-0541-5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Suetomi T et al. Inflammation and NLRP3 Inflammasome Activation Initiated in Response to Pressure Overload by Ca(2+)/Calmodulin-Dependent Protein Kinase II delta Signaling in Cardiomyocytes Are Essential for Adverse Cardiac Remodeling. Circulation 138, 2530–2544, doi: 10.1161/circulationaha.118.034621 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Patel B et al. CCR2(+) Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC Basic Transl Sci 3, 230–244, doi: 10.1016/j.jacbts.2017.12.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Laroumanie F et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129, 2111–2124, doi: 10.1161/circulationaha.113.007101 (2014). [DOI] [PubMed] [Google Scholar]
- 217.Kallikourdis M et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun 8, 14680, doi: 10.1038/ncomms14680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Borlaug BA et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll.Cardiol 50, 1570–1577 (2007). [DOI] [PubMed] [Google Scholar]
- 219.Mohammed SF et al. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail 7, 580–589, doi: 10.1161/circheartfailure.114.001192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kass DA & Kelly RP Ventriculo-arterial coupling: Concepts, assumptions, and applications. Annals of Biomedical Engineering 20, 41–62 (1992). [DOI] [PubMed] [Google Scholar]
- 221.Kelly RP, Tunin R & Kass DA Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 71, 490–502 (1992). [DOI] [PubMed] [Google Scholar]
- 222.Chirinos JA et al. Impact of Diabetes Mellitus on Ventricular Structure, Arterial Stiffness, and Pulsatile Hemodynamics in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc 8, e011457, doi: 10.1161/jaha.118.011457 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bache RJ, Arentzen CE, Simon AB & Vrobel TR Abnormalities in myocardial perfusion during tachycardia in dogs with left ventricular hypertrophy: metabolic evidence for myocardial ischemia. Circulation 69, 409–417 (1984). [DOI] [PubMed] [Google Scholar]
- 224.Bache RJ, Dai XZ, Alyono D, Vrobel TR & Homans DC Myocardial blood flow during exercise in dogs with left ventricular hypertrophy produced by aortic banding and perinephritic hypertension. Circulation 76, 835–842 (1987). [DOI] [PubMed] [Google Scholar]
- 225.Toyota E et al. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am J Physiol Heart Circ Physiol 288, H1598–1603, doi: 10.1152/ajpheart.01103.2003 (2005). [DOI] [PubMed] [Google Scholar]
- 226.Wei T et al. Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis. J Am Heart Assoc 6, doi: 10.1161/jaha.117.006114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sundaresan NR et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119, 2758–2771, doi: 10.1172/jci39162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.He X et al. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J Mol Cell Cardiol 112, 104–113, doi: 10.1016/j.yjmcc.2017.09.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee JF et al. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart 102, 278–284, doi: 10.1136/heartjnl-2015-308403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Marechaux S et al. Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. J Card Fail 22, 3–11, doi: 10.1016/j.cardfail.2015.09.003 (2016). [DOI] [PubMed] [Google Scholar]
- 231.Yang JH et al. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 22, 432–441, doi: 10.1002/ejhf.1671 (2020). [DOI] [PubMed] [Google Scholar]
- 232.Taqueti VR et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 39, 840–849, doi: 10.1093/eurheartj/ehx721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.AbouEzzeddine OF et al. Myocardial Energetics in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 12, e006240, doi: 10.1161/circheartfailure.119.006240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI & Kitzman DW Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 119, 739–744, doi: 10.1152/japplphysiol.00049.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wong LL et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 17, 393–404, doi: 10.1002/ejhf.223 (2015). [DOI] [PubMed] [Google Scholar]
- 236.Yan H et al. miRNAs as biomarkers for diagnosis of heart failure: A systematic review and meta-analysis. Medicine (Baltimore) 96, e6825, doi: 10.1097/md.0000000000006825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wong LL et al. Combining Circulating MicroRNA and NT-proBNP to Detect and Categorize Heart Failure Subtypes. J Am Coll Cardiol 73, 1300–1313, doi: 10.1016/j.jacc.2018.11.060 (2019). [DOI] [PubMed] [Google Scholar]
- 238.Chen YT, Wong LL, Liew OW & Richards AM Heart Failure with Reduced Ejection Fraction (HFrEF) and Preserved Ejection Fraction (HFpEF): The Diagnostic Value of Circulating MicroRNAs. Cells 8, doi: 10.3390/cells8121651 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Obokata M, Reddy YNV, Melenovsky V, Pislaru S & Borlaug BA Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 40, 689–697, doi: 10.1093/eurheartj/ehy809 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mohammed SF et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 130, 2310–2320, doi: 10.1161/circulationaha.113.008461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Melenovsky V, Hwang SJ, Lin G, Redfield MM & Borlaug BA Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 35, 3452–3462, doi: 10.1093/eurheartj/ehu193 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zakeri R & Mohammed SF Epidemiology of Right Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction. Current heart failure reports, doi: 10.1007/s11897-015-0267-3 (2015). [DOI] [PubMed] [Google Scholar]
- 243.Kanjanahattakij N et al. High Right Ventricular Stroke Work Index Is Associated with Worse Kidney Function in Patients with Heart Failure with Preserved Ejection Fraction. Cardiorenal Med 8, 123–129, doi: 10.1159/000486629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Patel RB et al. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail 7, 253–263, doi: 10.1002/ehf2.12565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Williams JL et al. Defining the molecular signatures of human right heart failure. Life Sci 196, 118–126, doi: 10.1016/j.lfs.2018.01.021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mancini DM, Davis L, Wexler JP, Chadwick B & LeJemtel TH Dependence of enhanced maximal exercise performance on increased peak skeletal muscle perfusion during long-term captopril therapy in heart failure. J Am Coll Cardiol 10, 845–850, doi: 10.1016/s0735-1097(87)80279-6 (1987). [DOI] [PubMed] [Google Scholar]
- 247.Sullivan MJ, Knight JD, Higginbotham MB & Cobb FR Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation 80, 769–781, doi: 10.1161/01.cir.80.4.769 (1989). [DOI] [PubMed] [Google Scholar]
- 248.LeJemtel TH, Maskin CS, Lucido D & Chadwick BJ Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation 74, 245–251, doi: 10.1161/01.cir.74.2.245 (1986). [DOI] [PubMed] [Google Scholar]
- 249.Adams V, Linke A & Winzer E Skeletal muscle alterations in HFrEF vs. HFpEF. Curr Heart Fail Rep 14, 489–497, doi: 10.1007/s11897-017-0361-9 (2017). [DOI] [PubMed] [Google Scholar]
- 250.Tucker WJ, Haykowsky MJ, Seo Y, Stehling E & Forman DE Impaired Exercise Tolerance in Heart Failure: Role of Skeletal Muscle Morphology and Function. Curr Heart Fail Rep 15, 323–331, doi: 10.1007/s11897-018-0408-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Weiss K et al. Fatigability, Exercise Intolerance, and Abnormal Skeletal Muscle Energetics in Heart Failure. Circ Heart Fail 10, doi: 10.1161/circheartfailure.117.004129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Molina AJ et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail 4, 636–645, doi: 10.1016/j.jchf.2016.03.011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tucker WJ et al. Impact of Exercise Training on Peak Oxygen Uptake and its Determinants in Heart Failure with Preserved Ejection Fraction. Card Fail Rev 2, 95–101, doi: 10.15420/cfr.2016:16:2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wu H & Ballantyne CM Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest 127, 43–54, doi: 10.1172/jci88880 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zamani P et al. Peripheral Determinants of Oxygen Utilization in Heart Failure With Preserved Ejection Fraction: Central Role of Adiposity. JACC Basic Transl Sci 5, 211–225, doi: 10.1016/j.jacbts.2020.01.003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bowen TS et al. Effects of Endurance Training on Detrimental Structural, Cellular, and Functional Alterations in Skeletal Muscles of Heart Failure With Preserved Ejection Fraction. J Card Fail 24, 603–613, doi: 10.1016/j.cardfail.2018.08.009 (2018). [DOI] [PubMed] [Google Scholar]
- 257.Olson TP, Johnson BD & Borlaug BA Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 4, 490–498, doi: 10.1016/j.jchf.2016.03.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hoeper MM et al. Diffusion Capacity and Mortality in Patients With Pulmonary Hypertension Due to Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 4, 441–449, doi: 10.1016/j.jchf.2015.12.016 (2016). [DOI] [PubMed] [Google Scholar]
- 259.Fayyaz AU et al. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction. Circulation 137, 1796–1810, doi: 10.1161/circulationaha.117.031608 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang L et al. Treatment With Treprostinil and Metformin Normalizes Hyperglycemia and Improves Cardiac Function in Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Arterioscler Thromb Vasc Biol, Atvbaha119313883, doi: 10.1161/atvbaha.119.313883 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Agrawal V et al. Natriuretic peptide receptor C contributes to disproportionate right ventricular hypertrophy in a rodent model of obesity-induced heart failure with preserved ejection fraction with pulmonary hypertension. Pulm Circ 9, 2045894019878599, doi: 10.1177/2045894019895452 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Shah KS & Fang JC Is Heart Failure with Preserved Ejection Fraction a Kidney Disorder? Curr Hypertens Rep 21, 86, doi: 10.1007/s11906-019-0993-0 (2019). [DOI] [PubMed] [Google Scholar]
- 263.van de Wouw J et al. Chronic Kidney Disease as a Risk Factor for Heart Failure With Preserved Ejection Fraction: A Focus on Microcirculatory Factors and Therapeutic Targets. Front Physiol 10, 1108, doi: 10.3389/fphys.2019.01108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Agrawal A, Naranjo M, Kanjanahattakij N, Rangaswami J & Gupta S Cardiorenal syndrome in heart failure with preserved ejection fraction-an under-recognized clinical entity. Heart Fail Rev 24, 421–437, doi: 10.1007/s10741-018-09768-9 (2019). [DOI] [PubMed] [Google Scholar]
- 265.Upadhya B, Amjad A & Stacey RB Optimizing The Management of Obese HFpEF Phenotype: Can We Mind Both The Heart and The Kidney? J Card Fail 26, 108–111, doi: 10.1016/j.cardfail.2019.11.018 (2020). [DOI] [PubMed] [Google Scholar]
- 266.Robinson TW & Freedman BI The Impact of APOL1 on Chronic Kidney Disease and Hypertension. Adv Chronic Kidney Dis 26, 131–136, doi: 10.1053/j.ackd.2019.01.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Franceschini N et al. Association of APOL1 With Heart Failure With Preserved Ejection Fraction in Postmenopausal African American Women. JAMA Cardiol 3, 712–720, doi: 10.1001/jamacardio.2018.1827 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ferreira JP et al. Covariate adjusted reanalysis of the I-Preserve trial. Clin Res Cardiol, doi: 10.1007/s00392-020-01632-x (2020). [DOI] [PubMed] [Google Scholar]
- 269.Borlaug BA et al. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. Jama 320, 1764–1773, doi: 10.1001/jama.2018.14852 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pieske B et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 38, 1119–1127, doi: 10.1093/eurheartj/ehw593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hahn VS et al. Abstract 16227: Myocardial Transcriptomics Reveal Distinct Gene Expression in Human Heart Failure With Preserved Ejection Fraction. Circulation 140, A16227–A16227, doi:doi: 10.1161/circ.140.suppl_1.16227 (2019). [DOI] [Google Scholar]
- 272.Khush KK et al. Obese patients have lower B-type and atrial natriuretic peptide levels compared with nonobese. Congestive heart failure (Greenwich, Conn.) 12, 85–90 (2006). [DOI] [PubMed] [Google Scholar]
- 273.McMurray JJ et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371, 993–1004, doi: 10.1056/NEJMoa1409077 (2014). [DOI] [PubMed] [Google Scholar]
- 274.Vaduganathan M et al. Prior Heart Failure Hospitalization, Clinical Outcomes, and Response to Sacubitril/Valsartan Compared with Valsartan in HFpEF. J Am Coll Cardiol, doi: 10.1016/j.jacc.2019.11.003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sharma K et al. Randomized Evaluation of Heart Failure With Preserved Ejection Fraction Patients With Acute Heart Failure and Dopamine: The ROPA-DOP Trial. JACC Heart Fail 6, 859–870, doi: 10.1016/j.jchf.2018.04.008 (2018). [DOI] [PubMed] [Google Scholar]
- 276.Reddy YNV et al. The beta-Adrenergic Agonist Albuterol Improves Pulmonary Vascular Reserve in Heart Failure With Preserved Ejection Fraction. Circ Res 124, 306–314, doi: 10.1161/circresaha.118.313832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kaye DM, Nanayakkara S, Vizi D, Byrne M & Mariani JA Effects of Milrinone on Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 67, 2554–2556, doi: 10.1016/j.jacc.2016.03.539 (2016). [DOI] [PubMed] [Google Scholar]
- 278.Nanayakkara S et al. Extended-Release Oral Milrinone for the Treatment of Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc 9, e015026, doi: 10.1161/jaha.119.015026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rodes-Cabau J et al. Interatrial Shunting for Heart Failure: Early and Late Results From the First-in-Human Experience With the V-Wave System. JACC Cardiovasc Interv 11, 2300–2310, doi: 10.1016/j.jcin.2018.07.001 (2018). [DOI] [PubMed] [Google Scholar]
- 280.Kaye DM & Nanayakkara S Interatrial Shunt Device for Heart Failure With Preserved Ejection Fraction. Front Cardiovasc Med 6, 143, doi: 10.3389/fcvm.2019.00143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Obokata M et al. Effects of Interatrial Shunt on Pulmonary Vascular Function in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 74, 2539–2550, doi: 10.1016/j.jacc.2019.08.1062 (2019). [DOI] [PubMed] [Google Scholar]
- 282.Feldman T et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation 137, 364–375, doi: 10.1161/circulationaha.117.032094 (2018). [DOI] [PubMed] [Google Scholar]
- 283.Rodriguez Flores M, Aguilar Salinas C, Piche ME, Auclair A & Poirier P Effect of bariatric surgery on heart failure. Expert review of cardiovascular therapy 15, 567–579, doi: 10.1080/14779072.2017.1352471 (2017). [DOI] [PubMed] [Google Scholar]
- 284.Mikhalkova D et al. Bariatric Surgery-Induced Cardiac and Lipidomic Changes in Obesity-Related Heart Failure with Preserved Ejection Fraction. Obesity (Silver Spring, Md.) 26, 284–290, doi: 10.1002/oby.22038 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.de Boer RA et al. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol 3, 215–224, doi: 10.1001/jamacardio.2017.4987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hedman AK et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart 106, 342–349, doi: 10.1136/heartjnl-2019-315481 (2020). [DOI] [PubMed] [Google Scholar]
- 287.Chan MM et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 18, 81–88, doi: 10.1002/ejhf.431 (2016). [DOI] [PubMed] [Google Scholar]
- 288.Putko BN et al. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS One 9, e99495, doi: 10.1371/journal.pone.0099495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Gohar A et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur J Heart Fail 19, 1638–1647, doi: 10.1002/ejhf.911 (2017). [DOI] [PubMed] [Google Scholar]
- 290.Sanders-van Wijk S et al. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 17, 1006–1014, doi: 10.1002/ejhf.414 (2015). [DOI] [PubMed] [Google Scholar]
- 291.Salah K et al. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 105, 1182–1189, doi: 10.1136/heartjnl-2018-314173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pandey A et al. Factors Associated With and Prognostic Implications of Cardiac Troponin Elevation in Decompensated Heart Failure With Preserved Ejection Fraction: Findings From the American Heart Association Get With The Guidelines-Heart Failure Program. JAMA Cardiol 2, 136–145, doi: 10.1001/jamacardio.2016.4726 (2017). [DOI] [PubMed] [Google Scholar]