Abstract
Systemic candidiasis affects 1.6 to 4.5% of very low birth weight (≤1,500 g) infants. A specimen archive of Candida strains from intensive care nurseries was created; it currently houses 98 isolates from 17 institutions. Eight isolates (8.2%) were misidentified at the referring institution. The MICs of fluconazole for seven isolates (7.1%, all non-C. albicans species, one misidentified initially) were >8 μg/ml.
Very low birth weight (VLBW) (≤1,500 g) infants develop late-onset sepsis (variably defined as sepsis occurring >3, >4, or >7 days after birth) with high frequency. Two multicenter studies described late-onset sepsis in 16 and 25% of VLBW infants (6, 19). Nine percent of the bloodstream isolates in one study were fungi, predominantly Candida species (19). Systemic candidiasis in neonates is not limited to bloodstream infection: meningitis, urinary tract infection, and deep skin infection are other manifestations (4, 12, 17). The incidence of systemic candidiasis in this population is rising. In the 1980s, 1.6 to 4.5% of VLBW infants developed this infection (1, 2, 5, 22), whereas a recent study found that the incidence in one nursery had increased 11-fold between 1981 and 1995 (9).
Appropriate diagnosis and management of systemic candidiasis in neonates are controversial (16). The Neonatal Candidiasis Study Group (NCSG) is a consortium of pediatric infectious-disease specialists and neonatologists interested in exploring the epidemiology, treatment, and prophylaxis of this infection. The NCSG established a specimen archive to house Candida isolates involved in neonatal disease. Isolates were submitted to the archive before therapeutic or prophylactic trials were launched to provide a baseline of susceptibility patterns as a means to allow detection of shifts in these patterns following the introduction of new therapies. Evaluation of submitted isolates revealed that species identification was frequently incorrect and that resistance to fluconazole (MIC >8 μg/ml) was already present in nursery isolates.
Participants in the NCSG submitted at least five Candida isolates to the archive. Isolates were required to be from patients in the intensive care nursery. However, the isolates were not required to be from VLBW infants or from infants suffering from invasive disease. The specimens were streaked onto Sabouraud glucose agar slants and mailed to the Medical Mycology Research Center at the University of Texas Medical Branch in Galveston. Upon receipt, the isolates were checked for purity. Germ tube formation in plasma was assessed to identify Candida albicans. Non-C. albicans species were further identified by inoculation of either a Vitek yeast biochemical card or an API 20C strip and a Dalmau plate. Fluconazole MICs were determined by the M27 standard of the National Committee for Clinical Laboratory Standards (7).
A total of 98 isolates from 90 patients at 17 institutions were submitted. The median gestational age of the patients was 26 weeks (range, 23 to 41 weeks), the median birth weight was 820 g (range, 426 to 4,885 g), and the median age at the time of culture was 24 days (range, 1 to 198 days). Most of the isolates represented invasive disease. The source of the culture was blood in 56 samples, urine in 14, skin or soft tissue in 9, sputum or tracheal aspirates in 6, cerebrospinal fluid in 4, catheter tips in 3, and other sources in 6. Species included C. albicans (56%); C. parapsilosis (34%); C. tropicalis (5.3%); and C. guilliermondii, C. glabrata, and C. lusitaniae (collectively comprising the remaining 4.7%).
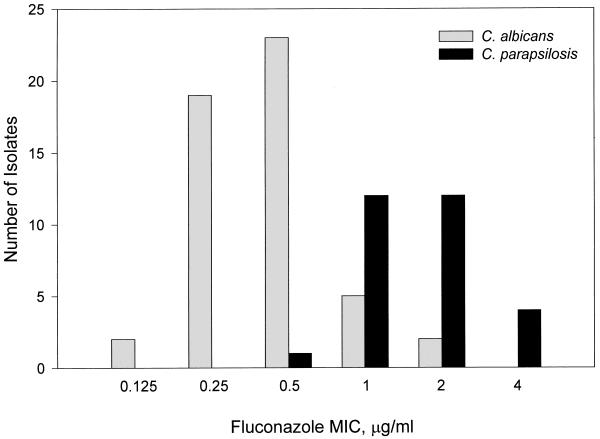
Seven institutions referred a total of eight isolates (8.2%) that were misidentified in their clinical laboratories (Table 1). Table 2 provides information about the seven isolates for which the fluconazole MICs were >8 μg/ml. Two of these isolates were from the blood and cerebrospinal fluid of the same patient. Two specimens with C. albicans contained two morphotypes, for which the fluconazole MICs were different (1 and 0.5 μg/ml and 0.25 and 2 μg/ml). The fluconazole MICs for the C. albicans and C. parapsilosis strains (the most common species isolated) are summarized in Fig. 1.
TABLE 1.
Candida isolates misidentified by the referring institution
| Assigned species | Correct species identification | Source of isolate | Fluconazole MIC, μg/ml |
|---|---|---|---|
| C. albicans | C. parapsilosis | Eye | 2.0 |
| C. albicans | C. parapsilosis | Blood | 2.0 |
| C. albicans | C. parapsilosis | Blood | 1.0 |
| C. albicans | C. parapsilosis | Blood | 4.0 |
| C. parapsilosis | C. albicans | Right atrial mass | 0.5 |
| C. tropicalis | C. albicans | Groin | 0.5 |
| C. parapsilosis | C. tropicalis | Blood | 2.0 |
| C. albicans | C. glabrata | Blood | 16 |
TABLE 2.
Isolates for which the fluconazole MICs were >8 μg/ml
| Species | Source of isolate | MIC, μg/ml |
|---|---|---|
| C. glabrata | Blood | 16 |
| Tracheal aspirate | 16 | |
| Blood | 16 | |
| C. tropicalis | Blood | >64 |
| Urine | >64 | |
| C. guilliermondii | Blood | 32 |
| Cerebrospinal fluid | 16 |
FIG. 1.
Distribution of fluconazole MICs for the C. albicans and C. parapsilosis strains submitted to the archive.
The NCSG specimen archive contains a large number of invasive isolates from infants, with a wide geographic distribution (see footnote † to byline of this article). The National Epidemiology of Mycoses Survey, the only other multicenter study of Candida isolates from infants, included 147 isolates from intensive care nurseries, but this survey studied only 34 patients from six institutions (11). Much of the data from that study is presented in aggregate with isolate data from surgical intensive care units. Thus, the NCSG specimen archive is an excellent companion resource focusing on neonatal infection.
Although amphotericin B is considered the treatment of choice in neonatal systemic candidiasis, fluconazole is used by a substantial number of practitioners (16). Breakpoints for fluconazole susceptibility testing have been established through correlation with clinical outcomes (15). Isolates for which the MIC is ≤8 μg/ml are considered susceptible, whereas an MIC of ≥64 μg/ml indicates resistance. When the MIC is 16 or 32 μg/ml, the isolate has dose-dependent susceptibility. Data on fluconazole pharmacokinetics in VLBW infants is sparse, and no data exist concerning the safety of increasing the dose when the MIC is elevated (18, 20). In the specimens described in this report, elevated fluconazole MICs were found only for non-C. albicans species (Table 2). Although fluconazole resistance in C. albicans has been described, higher MICs are more common in non-C. albicans strains (10, 14). There is no published data correlating outcome to MIC in fluconazole-treated neonates; treatment failures, mostly due to C. albicans, have been described, but no MICs are included in the reports (3, 8, 21). The distribution of MICs shown in Fig. 1 is comparable to the distribution published in another large study of adult patients, suggesting that intensive care nursery isolates reflect those present in the community at large (15).
Several isolates in the archive were incorrectly identified at the referring institution's clinical laboratory (Table 1). In a recent survey of clinical laboratories in New York, blinded proficiency testing revealed misidentification of C. parapsilosis, C. tropicalis, and C. glabrata in 15% of specimens and C. albicans in 11% (13). Thus, the 8.2% error rate in this archive is not surprising. However, it does raise the specter of possible misidentification of isolates as C. albicans and subsequent complacency about the likelihood of resistance to azoles. Only 28% of clinicians obtain antifungal susceptibility testing data when treating neonates with invasive disease (16). We recommend that clinicians verify the competency of their clinical laboratories for species identification and for susceptibility testing if azole therapy is to be used. Additionally, whenever species identification is potentially critical (e.g., in treatment trials), we recommend that all isolates be submitted to a mycology specialty laboratory.
Acknowledgments
The NCSG specimen archive was established through a grant from the Pediatric Academic Societies Multicenter Clinical Trials Program.
REFERENCES
- 1.Baley J E, Kleigman R M, Fanaroff A A. Disseminated fungal infections in very low-birth-weight infants: clinical manifestations and epidemiology. Pediatrics. 1984;73:144–152. [PubMed] [Google Scholar]
- 2.Butler K M, Baker C J. Candida: an increasingly important pathogen in the nursery. Pediatr Clin N Am. 1988;35:543–563. doi: 10.1016/s0031-3955(16)36471-9. [DOI] [PubMed] [Google Scholar]
- 3.Driessen M, Ellis J B, Cooper P A, Wainer S, Muwazi F, Hahn D, Gous H, De Villiers F P R. Fluconazole vs. amphotericin B for the treatment of neonatal fungal septicemia: a prospective randomized trial. Pediatr Infect Dis J. 1996;15:1107–1112. doi: 10.1097/00006454-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Faix R G. Systemic Candida infections in infants in intensive care nurseries: high incidence of central nervous system involvement. J Pediatr. 1984;105:616–622. doi: 10.1016/s0022-3476(84)80433-3. [DOI] [PubMed] [Google Scholar]
- 5.Faix R G, Kovarik S M, Shaw T R, Johnson R V. Mucocutaneous and invasive candidiasis among very low birth weight (<1,500 grams) infants in intensive care nurseries: a prospective study. Pediatrics. 1989;83:101–107. [PubMed] [Google Scholar]
- 6.Fanaroff A A, Korones S B, Wright L L, Verter J, Poland R L, Bauer C R, Tyson J E, Philips III J B, Edwards W, Lucey J F, Catz C S, Shankaran S, Oh W for the National Institute of Child Health and Human Development Neonatal Research Network. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. Pediatr Infect Dis J. 1998;17:593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Galgiani J N, Bartlett M S, Ghannoum M A, Espinel-Ingroff A, Lancaster M V, Odds F C, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Reference method for broth dilution antifungal susceptibility testing of yeasts. Vol. 17 1997. , no. 9, p. 1–32. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 8.Huttova M, Hartmanova I, Kralinsky K, Filka J, Uher J, Kurak J, Krizan S, Krcmery V., Jr Candida fungemia in neonates treated with fluconazole: report of forty cases, including eight with meningitis. Pediatr Infect Dis J. 1998;17:1012–1015. doi: 10.1097/00006454-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kossoff E H, Buescher E S, Karlowicz M G. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J. 1998;17:504–508. doi: 10.1097/00006454-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen M H, Peacock J E, Jr, Morris A J, Tanner D C, Nguyen M L, Snydman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller M A, Messer S A, Houston A, Rangel-Frausto M S, Wilbin T, Blumberg H M, Edwards J E, Jarvis W, Martin M A, Neu H C, Saiman L, Patterson J E, Dibb J C, Roldan C M, Rinaldi M G, Wenzel R P. National epidemiology of mycoses survey: a multicenter study of strain variation and antifungal susceptibility among isolates of Candida species. Diagn Microbiol Infect Dis. 1998;31:289–296. doi: 10.1016/s0732-8893(97)00245-9. [DOI] [PubMed] [Google Scholar]
- 12.Phillips J R, Karlowicz M G. Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190–194. doi: 10.1097/00006454-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Reilly A A, Salkin I F, McGinnis M R, Gromadzki S, Pasarell L, Kemna M, Higgins N, Salfinger M. Evaluation of mycology laboratory proficiency testing. J Clin Microbiol. 1999;37:2297–2305. doi: 10.1128/jcm.37.7.2297-2305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C D for the NIAID Mycoses Study Group and the Candidemia Study Group. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, intraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 16.Rowen J L, Tate J M for the Neonatal Candidiasis Study Group. Management of neonatal candidiasis. Pediatr Infect Dis J. 1998;17:1007–1011. doi: 10.1097/00006454-199811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowen J L, Atkins J T, Levy M L, Baer S C, Baker C J. Invasive fungal dermatitis in the ≤1000-gram neonate. Pediatrics. 1995;95:682–687. [PubMed] [Google Scholar]
- 18.Saxén H, Hoppu K, Pohjavuori M. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther. 1993;54:269–277. doi: 10.1038/clpt.1993.147. [DOI] [PubMed] [Google Scholar]
- 19.Stoll B J, Gordon T, Korones S B, Shankaran S, Tyson J E, Bauer C R, Fanaroff A A, Lemons J A, Donavan E F, Oh W, Stevenson D K, Ehrenkranz R A, Papile L, Verter J, Wright L L. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:63–71. doi: 10.1016/s0022-3476(96)70191-9. [DOI] [PubMed] [Google Scholar]
- 20.van den Anker J N, van Popele N M L, Sauer P J J. Antifungal agents in neonatal systemic candidiasis. Antimicrob Agents Chemother. 1995;39:1391–1397. doi: 10.1128/aac.39.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wainer S, Cooper P A, Gouws H, Akierman A. Prospective study of fluconazole therapy in systemic neonatal fungal infection. Pediatr Infect Dis J. 1997;16:763–767. doi: 10.1097/00006454-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Weese-Mayer D E, Fondriest D W, Brouillette R T, Shulman S T. Risk factors associated with candidemia in the neonatal intensive care unit: a case-control study. Pediatr Infect Dis J. 1987;6:190–196. doi: 10.1097/00006454-198702000-00009. [DOI] [PubMed] [Google Scholar]