Rheumatology key message
Immunogenicity is particularly impaired after one, but not two doses of mRNA COVID-19 vaccine in patients with GCA.
Dear Editor, Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical to prevent coronavirus disease (COVID-19), especially in populations at higher risk of severe illness. Due to older age, frequent comorbidities and long-term immunosuppressive treatment, patients with giant cell arteritis (GCA) are considered particularly vulnerable in case of COVID-19; therefore, assessing vaccine immunogenicity in these patients becomes crucial.
Recent evidence provided reassuring seroconversion rates in rheumatic patients with heterogeneous diagnoses following mRNA vaccine [1], although concomitant immunosuppressants may potentially impair humoral response [2–4].
Here, we describe for the first time the immunogenicity of anti-SARS-CoV-2 mRNA vaccine in a large cohort of patients with treated GCA.
Patients with GCA followed at the Rheumatology Department of the University of Pavia, Italy, who received the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNtech) between 1 April 2021 and 30 April 2021 were recruited. First and second doses of vaccine were administered 3 weeks apart. A group of healthy controls was also included. Blood samples were obtained prior to vaccine administration, 3 weeks after the first dose and 3 weeks after the second dose. Serum samples were analysed using chemiluminescent immunoassay (LIAISON SARS-CoV-2 S1/S2 IgG; DiaSorin) for the quantification of SARS-CoV-2 anti-S1/anti-S2 IgG antibodies, with positive results identified by values >12 AU/ml. Patients with evidence of previous immunity due to COVID-19 at time of enrolment were excluded from the analysis. Ethical approval was obtained and patients provided informed consent.
Fifty-two patients with GCA and a group of 140 healthy controls completed the two-dose vaccination schedule. After the exclusion of 4 patients due to serological demonstration of previous COVID-19, 48 patients with GCA (female 72.9%, mean age 72.0±5.2 years) were enrolled. Mean disease duration at the time of vaccination was 51±39 months. Forty-four patients (91.7%) were treated with glucocorticoids (GC) (mean prednisone-equivalent dose 5.1±5.2 mg/day), with 11 patients (22.9%) receiving ≥7.5 mg/day. Seventeen (35.4%) and 5 patients (10.4%) were treated with methotrexate and subcutaneous tocilizumab, respectively (Supplementary Table S1, available at Rheumatology online). In order to minimise the risk of relapse in patients with GCA, immunosuppressive treatment was not withheld around vaccination.
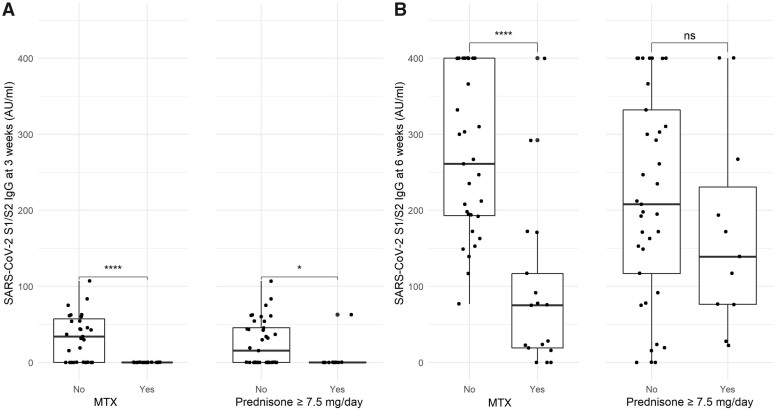
In our cohort, 20 (41.7%) patients developed positive S1/S2 IgG after the first dose, compared with 94.2% of healthy controls (P <0.0001). Compared to vaccine responders, patients without an adequate humoral response after the first dose were more likely on methotrexate (60.7% vs 0.0% P <0.0001) and/or on GC ≥ 7.5 mg/day (35.7% vs 5.0%, P = 0.01). Notably, no patients on methotrexate seroconverted after the first vaccine dose. After the first dose, patients receiving GC ≥7.5 mg/day developed significantly lower antibody titres compared with patients treated with GC <7.5 mg/day (median 0.0, IQR 0.0–0.0 AU/ml vs 15.4, IQR 0.0–45.5 AU/ml, P=0.03); likewise, lower antibody titres were observed in patients treated with methotrexate compared to patients not on methotrexate (median 0.0, IQR 0.0–0.0 AU/ml vs 33.9, IQR 0.0–57.3 AU/ml, P <0.0001; Fig. 1A).
Fig. 1.
SARS-COV-2 serological response after the first and second vaccine doses
(A) SARS-CoV-2 S1/S2 IgG antibody titre after the first vaccine dose, stratified according to the type of treatment (methotrexate and prednisone dose ≥7.5 mg/day); (B) SARS-CoV-2 S1/S2 IgG antibody titre after the second vaccine dose, stratified according to the type of treatment. ****P < 0.0001; *P < 0.01; ns: non-significant. Fourteen out of 17 patients treated with MTX received concomitant low-dose glucocorticoid treatment.
Positive humoral response rose up to 93.8% after the second dose, compared to 100% in healthy controls. All patients lacking serological response after two vaccine doses were on methotrexate. No significant difference in seroconversion rate was detected at lower GC doses or with tocilizumab. Notably, methotrexate, but not GC ≥ 7.5 mg/day, significantly affected antibody titres after the second dose (median 75.1, IQR 19.0–117.0 AU/ml vs 261, IQR 193.0–400.0 AU/ml, P <0.0001; Fig. 1B).
At multivariate analysis, methotrexate and GC ≥ 7.5 mg/day were independently associated with the inability to achieve immunogenicity after the first dose, while methotrexate was the only independent predictor of impaired response after the second dose (Supplementary Table S2, available at Rheumatology online).
This study provides, for the first time, real-world data on the immunogenicity of BNT162b2 COVID-19 vaccine in patients with GCA. Our results are of particular value, considering the highly vulnerable status of these elderly, immunocompromised patients during the pandemic. Reassuringly, almost all patients in our cohort achieved serological immunity after two vaccine doses, with seroconversion rates approaching those observed in healthy controls and in the BNT162b2 registration trial [5]. Conversely, immunogenicity after the first dose is poor and significantly hampered by GC and methotrexate. Therefore, particular caution is warranted until the completion of the full vaccine schedule. Moreover, the study provides evidence to discourage the practice of delaying the second vaccine dose in immunocompromised patients.
Further studies are needed to evaluate the immunogenic impact of different modalities of immunosuppressant withholding around vaccination, although the need to optimise immunogenicity should be weighed against the risk of disease relapse in severe systemic diseases, such as vasculitides. Continued monitoring of anti-SARS-CoV-2 antibodies in this category of highly vulnerable patients is warranted to assess the long-term duration of humoral response. Nevertheless, the degree of protection conferred by the achievement of an adequate humoral response, as well as the correlation between antibody levels and risk of infection in immunocompromised patients is still unclear. Finally, a more comprehensive assessment of T cell–mediated immunity, especially in those individuals with inadequate serological response, might contribute to the tailoring of vaccination strategies in rheumatic patients.
Funding: This work was supported by the Ministero della Salute, Ricerca Corrente (grant no. 80206) and Ricerca Finalizzata (grant BIAS no. 2020–12371760); by European Commission—Horizon 2020 (EU project 101003650—ATAC]; and by the Fondazione Cariplo (grantCoVIM, no. 2020–1374).
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data are available from the corresponding author on reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Furer V, Eviatar T, Zisman D et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 2. Haberman RH, Herati R, Simon D et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021; annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyarsky BJ, Ruddy JA, Connolly CM et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1098–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bugatti S, De Stefano L, Balduzzi S et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis 2021; annrheumdis-2021-220862. [DOI] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data are available from the corresponding author on reasonable request.