ABSTRACT
Background
Physical activity may be a way to increase and maintain fat-free mass (FFM) in later life, similar to the prevention of fractures by increasing peak bone mass.
Objectives
A study is presented of the association between FFM and physical activity in relation to age.
Methods
In a cross-sectional study, FFM was analyzed in relation to physical activity in a large participant group as compiled in the International Atomic Energy Agency Doubly Labeled Water database. The database included 2000 participants, age 3–96 y, with measurements of total energy expenditure (TEE) and resting energy expenditure (REE) to allow calculation of physical activity level (PAL = TEE/REE), and calculation of FFM from isotope dilution.
Results
PAL was a main determinant of body composition at all ages. Models with age, fat mass (FM), and PAL explained 76% and 85% of the variation in FFM in females and males < 18 y old, and 32% and 47% of the variation in FFM in females and males ≥ 18 y old, respectively. In participants < 18 y old, mean FM-adjusted FFM was 1.7 kg (95% CI: 0.1, 3.2 kg) and 3.4 kg (95% CI: 1.0, 5.6 kg) higher in a very active participant with PAL = 2.0 than in a sedentary participant with PAL = 1.5, for females and males, respectively. At age 18 y, height and FM–adjusted FFM was 3.6 kg (95% CI: 2.8, 4.4 kg) and 4.4 kg (95% CI: 3.2, 5.7 kg) higher, and at age 80 y 0.7 kg (95% CI: −0.2, 1.7 kg) and 1.0 kg (95% CI: −0.1, 2.1 kg) higher, in a participant with PAL = 2.0 than in a participant with PAL = 1.5, for females and males, respectively.
Conclusions
If these associations are causal, they suggest physical activity is a major determinant of body composition as reflected in peak FFM, and that a physically active lifestyle can only partly protect against loss of FFM in aging adults.
Keywords: physical activity level, age, energy expenditure, body composition, doubly labeled water
See corresponding editorial on page 1579.
Introduction
Physical activity provides a variety of health benefits. Physically active individuals sleep better and function better (1). In addition, physical activity can be an effective lifestyle behavior to maximize fat-free mass (FFM), as a proxy for muscle mass, during growth. Youth physical activity is positively associated with bone mass accrual and bone structure (2, 3). Physical activity may be a way to increase and maintain FFM to prevent sarcopenia in later life, similar to the prevention of fractures by increasing peak bone mass (4–6).
Skeletal muscle accounts for about half of FFM. Muscle mass and bone mass are closely related throughout life, and FFM is the strongest determinant of whole-body bone mineral content. Modeling and remodeling processes that regulate bone strength potentially explain these relations, depending on the forces acting on the bones (7, 8). Physical activity positively affects FFM accretion from birth onwards (9). Physical activity during adolescence has been associated with greater FFM in both sexes (10). Habitual physical activity has been shown to have a significant independent effect on the growth of FFM during adolescence (11). These results support recommendations for sustained physical activity participation during the growing years (12).
FFM peaks in early adulthood (13). A cross-sectional analysis of a large multiethnic sample, ranging in age from 18 to 110 y, resulted in a quadratic model for FFM in relation to age with a peak FFM at similar ages for Caucasians, African Americans, Hispanics, and Asians. The estimated turning point, where growth ended and FFM started to decline, was in the mid-40s for females and mid-20s for males (13). Physical activity is likely to have a role in preventing FFM loss at later ages. A cross-sectional study showed that higher physical activity was associated with higher FFM in participants aged 60–64 y (14). A longitudinal study in participants aged 65–84 y showed that greater physical activity retained a greater FFM over 5 y of observation (15). On the other hand, a cross-sectional study in 529 participants aged 18–96 y suggested that greater physical activity was not associated with higher FFM (16). Two longitudinal studies, the first in 904 participants aged 67–84 y and the second in 302 participants aged 70–82 y, also showed that changes in FFM over 5 y were not associated with physical activity level (PAL), when controlled for potential confounding variables (17, 18). Thus, there is still controversy on the relation between physical activity and FFM at later ages.
Here, the focus is on physical activity and FFM accrual during early and later life. A cross-sectional analysis was performed in a large participant group, deriving physical activity from doubly labeled water–measured energy expenditure. Thus, physical activity was quantified with a criterion measure (19).
Methods
The analysis included daily total energy expenditure (TEE) measurements as compiled in the International Atomic Energy Agency Doubly Labeled Water database (established to pool doubly labeled water data across multiple studies), version 3.1.2 (20). All data were recalculated with the same standard methodology for human doubly labeled water studies as published recently (21). The analysis was restricted to TEE measures accompanied by measurements of resting energy expenditure (REE), to allow calculation of PAL (TEE/REE). REE was measured under postabsorptive, thermoneutral, and resting conditions with a ventilated hood, or during an early morning resting interval, directly after waking up and before having breakfast, in a respiration chamber.
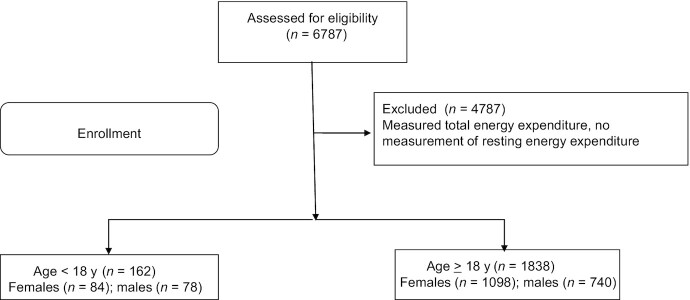
The database included 2000 participants (1182 females and 818 males) with measurements on TEE and REE to allow calculation of PAL (Figure 1). The age range of the participants was 3–96 y. The data analysis did not include participants with muscle wasting or participants with diseases affecting REE. All TEE measurements were performed under habitual daily life conditions, neutral energy balance, and before any study intervention. FFM was derived from total body water as measured with isotope dilution, a method directly derived from carcass analysis and thus 1 of the 2 single-indirect methods for body composition (22).
FIGURE 1.
Participant flowchart.
Associations between physical activity and FFM can be confounded by fat mass (FM) because gains or losses in fat typically lead to respective gains or losses in FFM (23). Changes in body weight and body composition are primarily a function of energy balance. Consequently, changes in FM and FFM are not independent (24). Energy balance–related body mass changes are generally assumed to consist of 75% as FM and 25% as FFM, which is known as the “quarter FFM” rule (25). Refinements of the quarter FFM rule were developed for specific situations like diet-induced weight change in extremely lean participants or participants with obesity (26, 27). Whatever rule applies for the relation between energy balance–induced changes in FM and FFM, FM should be included as an independent variable in an analysis on physical activity and FFM.
Data analysis was performed separately for participants < 18 y old and for participants ≥ 18 y old. For participants < 18 y old, the relation between FFM and PAL was assessed in a multiple regression model accounting for FM and age. To allow body composition comparisons between participants ≥ 18 y old, FFM and FM were expressed as indexes, the fat-free mass index (FFMI) and fat mass index (FMI), respectively, where FFMI = FFM/height2 and FMI = FM/height2 (FFM and FM in kg and height in m). In this way we corrected for differences in height, in analogy with the BMI of Quetelet: BMI = FFMI + FMI (28). Unfortunately, the index fails to adjust for height differences in participants during growth (29). Thus, data analysis was performed separately for participants < 18 y old, using unadjusted FFM and FM as measures for body composition. Models were generated separately for females and males. In participants ≥ 18 y old, 4 models were applied in a top-down procedure, with FFMI as the outcome variable:
Model 1: age, FMI, PAL.
Model 2: age, FMI, PAL, age2.
Model 3: age, FMI, PAL, age2, age*PAL.
Model 4: age, FMI, PAL, age2, age*PAL, age2*PAL.
Because the linearity assumption for age was violated, a quadratic term (age2) needed to be included. The model explaining most variation in FFM from age-, FM-, and PAL-differences between participants was model 3. For females, model 3 was better than model 2, and model 4 was not better than model 3. For males, model 3 was as good as model 2, and model 4 was not better than model 3. Thus, model 3 was chosen for both sexes. Model 3 was checked for (multi)collinearity after centering for age, resulting in the same model fit and in condition indexes <30 (18.2 for females, 16.6 for males), indicating there was no collinearity problem.
Results
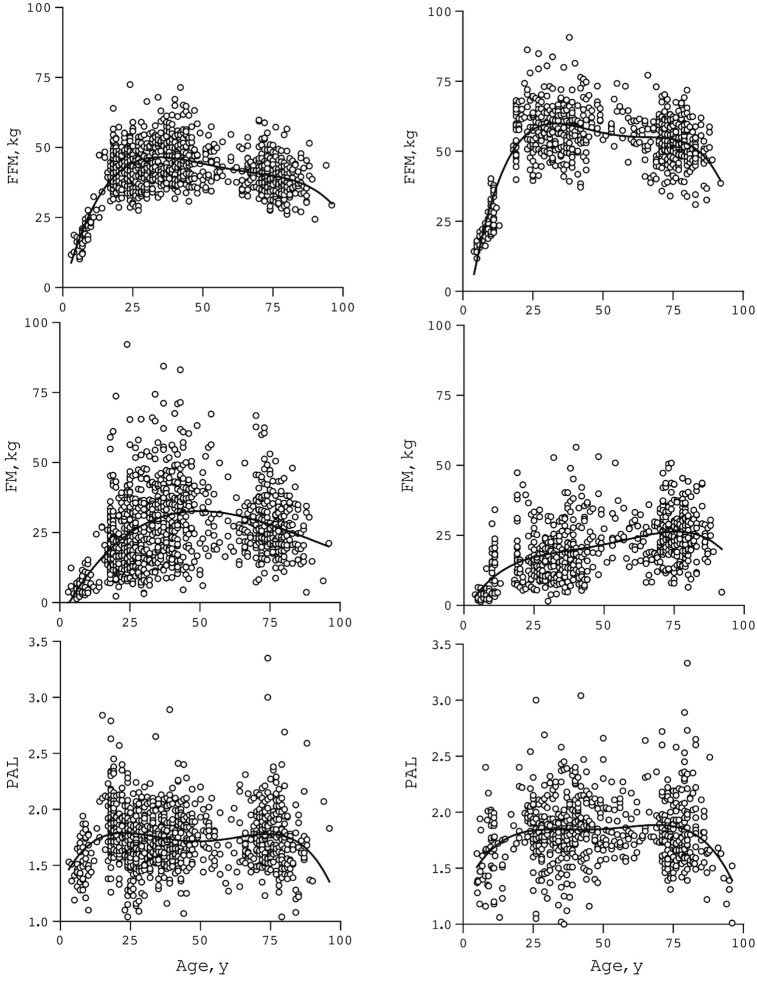
FFM was highest, around age 30 y, in females and males (Figure 2). The mean of peak FFM was 47 kg in females and 60 kg in males. Females showed a higher mean FM than males already at early ages. Mean PAL was similar in females and males at all ages (Table 1). The typical mean PAL value was ∼1.5 in the youngest (age < 10 y) and oldest (age > 80 y) participants (Figure 2). At adult age, from 18 to 80 y, PAL values generally ranged between a minimum of 1.1 and a maximum of 2.5 with a mean ± SD value of 1.71 ± 0.26 for females and 1.78 ± 0.30 for males.
FIGURE 2.
FFM, FM, and PAL, plotted as a function of age. Values for 2000 participants—1182 females (left) and 818 males (right)—with a 4th-order polynomial curve fit. FFM, fat-free mass; FM, fat mass; PAL, physical activity level.
TABLE 1.
Participant characteristics1
| Characteristics | Females | Males |
|---|---|---|
| <18 y old | ||
| n | 84 | 78 |
| Fat-free mass, kg | 26.7 ± 10.9 | 26.9 ± 10.2 |
| Fat mass, kg | 10.8 ± 10.4 | 9.2 ± 7.7 |
| PAL | 1.61 ± 0.30 | 1.62 ± 0.29 |
| ≥18 y old | ||
| n | 1098 | 740 |
| Fat-free mass index, kg/m2 | 16.2 ± 2.3 | 18.5 ± 2.2 |
| Fat mass index, kg/m2 | 10.3 ± 4.9 | 7.4 ± 3.6 |
| PAL | 1.71 ± 0.26 | 1.78 ± 0.30 |
Values are mean ± SD unless otherwise indicated. PAL, physical activity level.
In participants < 18 y old (n = 162), FFM was significantly higher in individuals with an older age, higher FM, and higher PAL (Table 2):
TABLE 2.
Sources of variation in FFM in participants <18 y old1
| Unstandardized coefficient (B) | 95% CI for B | P | |
|---|---|---|---|
| Females | |||
| (constant) | −1.53 | −6.21, 3.14 | 0.516 |
| Age | 1.90 | 1.63, 2.16 | <0.001 |
| FM | 0.21 | 0.10, 0.31 | <0.001 |
| PAL | 3.34 | 0.21, 6.47 | 0.037 |
| Males | |||
| (constant) | −7.42 | −14.45, −0.39 | 0.039 |
| Age | 2.00 | 1.57, 2.44 | <0.001 |
| FM | 0.39 | 0.22, 0.57 | <0.001 |
| PAL | 6.90 | 2.66, 11.15 | 0.002 |
Values are coefficients and P values from a multiple regression model of FFM (kg) as a function of age (y), FM (kg), and PAL, in females (n = 84, R2 = 0.85) and males (n = 78, R2 = 0.76). FFM, fat-free mass; FM, fat mass; PAL, physical activity level.
Females, FFM (kg) = −1.53 + 1.90 Age (y) + 0.21 FM (kg) + 3.34 PAL, R2 = 0.85;
Males, FFM (kg) = −7.42 + 2.00 Age (y) + 0.39 FM (kg) + 6.90 PAL, R2 = 0.76.
Thus, mean FM-adjusted FFM was 1.7 kg (95% CI: 0.1, 3.2 kg) and 3.4 kg (95% CI: 1.0, 5.6 kg) higher in a very active participant with PAL = 2.0 than in a sedentary participant with PAL = 1.5, for females and males, respectively.
In participants ≥ 18 y old (n = 1838), FFMI was significantly higher in participants with a higher FMI and PAL for both sexes (Table 3):
TABLE 3.
Sources of variation in FFMI in participants ≥ 18 y old1
| Unstandardized coefficient (B) | 95% CI for B | P | |
|---|---|---|---|
| Females | |||
| (constant) | 7.150 | 5.466, 8.838 | <0.001 |
| Age | 0.094 | 0.049, 0.140 | <0.001 |
| FMI | 0.312 | 0.290, 0.334 | <0.001 |
| PAL | 3.214 | 2.379, 4.050 | <0.001 |
| Age2 | −0.001 | −0.001, 0.000 | <0.001 |
| Age*PAL | −0.033 | −0.050, −0.017 | <0.001 |
| Males | |||
| (constant) | 9.084 | 6.674, 11.494 | <0.001 |
| Age | 0.141 | 0.080, 0.201 | <0.001 |
| FMI | 0.308 | 0.267, 0.349 | <0.001 |
| PAL | 3.557 | 2.442, 4.671 | <0.001 |
| Age2 | −0.001 | −0.001, −0.000 | <0.001 |
| Age*PAL | −0.036 | −0.056, −0.016 | <0.001 |
Values are coefficients and P values from a multiple regression model of FFMI (kg/m2) as a function of age (y), FMI (kg/m2), PAL, and interactions with age (y), in females (n = 1098, R2 = 0.47) and males (n = 740, R2 = 0.32). FFMI, fat-free mass index; FMI, fat mass index; PAL, physical activity level.
Females, FFMI (kg/m2) = 7.15 + 0.094 Age (y) − 0.001 Age2 (y) + 0.312 FMI (kg/m2) + 3.214 PAL − 0.033 Age*PAL (y), R2 = 0.47;
Males, FFMI (kg/m2) = 9.084 + 0.141 Age (y) − 0.001 Age2 (y) + 0.308 FMI (kg/m2) + 3.557 PAL − 0.036 Age*PAL (y), R2 = 0.32.
At age 18 y, mean FMI-adjusted FFMI was 1.3 kg/m2 (95% CI: 1.0, 1.6 kg/m2) and 1.4 kg/m2 (95% CI: 1.0, 1.8 kg/m2) higher in a very active participant with PAL = 2.0 than in a sedentary participant with PAL = 1.5, for females and males, respectively. The differences in FFMI imply, for a typical female with height 1.65 m and male with height 1.75 m, a mean FM-adjusted FFM difference of 3.6 kg (95% CI: 2.8, 4.4 kg) and 4.4 kg (95% CI: 3.2, 5.7 kg), respectively. The positive association between FMI-adjusted FFMI and PAL was smaller the older the participant (Table 3). Thus, at age 80 y, the differences in FFM between a sedentary and very active female and male were 0.7 kg (95% CI: −0.2, 1.7 kg) and 1.0 kg (95% CI: −0.1, 2.1 kg), respectively.
Participants with a higher FM had a higher FFM. The mean of the coefficient was 0.21 and 0.39 kg FFM/kg FM, or 17% and 39% FFM/kg body mass, in females and males < 18 y old, respectively. At later ages (>18 y old), the mean of the coefficient was 0.312 and 0.308 kg FFM/kg FM in females and males, respectively, or 24% FFM/kg body mass.
Discussion
The data showed that physically active participants have higher FM-adjusted FFM already during growth under age 18 y. Thus, physical activity is a major determinant of body composition as reflected in FFM in this cross-sectional analysis. However, older age counteracted the positive association of physical activity with FFM. Peak FFM was observed around age 30 y, in females and in males (Figure 2).
Age of unadjusted peak FFM is clearly higher than age of peak bone mass, in females at 19–20 y and in males at 20–24 y, independently of race (5). The higher age for unadjusted peak FFM than for peak bone mass is probably explained by FM-associated FFM. FM was highest in females around age 50 y and in males around age 75 y (Figure 2). Thus, FM-associated FFM dominated the decrease in physical activity–associated FFM in participants with a higher FM.
A previous study found the age of unadjusted peak FFM to be in the mid-40s for females and mid-20s for males (13). In the current study, peak FFM was at ∼30 y old for both males and females, a difference possibly explained by differences in FM and thus in FM-associated FFM between the populations of study.
In adults, larger FFM in participants with a larger FM follows the quarter FFM rule (25). On average, 24% of the higher body mass was FFM. Factors confounding the quarter FFM rule, including an extreme imbalance between energy intake and energy expenditure and effects of differences in physical activity, were excluded in the model as presented. All participants were observed under neutral conditions of energy balance, and measured PAL was included in the model as an independent variable. Unfortunately, the quarter FFM rule still lacks a mechanistic explanation (27).
The controversy on FFM maintenance through physical activity at later age seems to be at least partly explained with inclusion of differences in FM between participants, in the model as presented. FFM adjusted for differences in FM was significantly higher in participants with a higher PAL, for females and males at younger age. The mean difference of 3.6 kg (95% CI: 2.8, 4.4 kg) and 4.4 kg (95% CI: 3.2, 5.7 kg) FFM at age 18 y and 0.7 kg (95% CI: −0.2, 1.7 kg) and 1.0 kg (95% CI: −0.1, 2.1) FFM at age 80 y, as calculated from the model presented, between a female and male with PAL = 1.5 and 2.0, respectively, is in line with an earlier cross-sectional analysis. Manini et al. (18) observed (mean ± SD) 2.0 ± 1.2 kg and 2.9 ± 1.3 kg greater FFM in older females and males, respectively, in participants in the first than in those in the third tertile of doubly labeled water–assessed activity energy expenditure. Differences in FM between participants in the first and third tertiles of activity energy expenditure were nonsignificant. However, despite a greater FFM in participants with a higher PAL, the age-related decline in FFM might not be prevented by a higher PAL.
In a 5-y follow-up of the participants 70–82 y old observed by Manini et al. (18), changes in physical activity did not affect the age-related change in body composition.
The average difference between peak FFM and FFM at age 80 y, an age interval where PAL remained the same, was −8 kg (Figure 2). The 8-kg difference between peak-FFM and FFM at age 80 y is similar to an earlier identical cross-sectional comparison resulting in −7.5 kg and −8.8 kg difference for females and males, respectively (30, 31). The mean difference in FM-adjusted FFM between a sedentary and a very active participant over the same age interval was between 3 and 4 kg. At older age, despite a greater routine physical activity, the inverse association of age*PAL counteracts the positive association of PAL with FFM.
Although aerobic exercise does not completely prevent the lower FFM in aging participants, resistance exercise may be more helpful (32). However, although resistance exercise elicited an ∼1-kg increase in FFM among older adults, this is modest compared with the differences with healthy young adults and with the 8-kg difference aforementioned (33). Exercise training in adults at older age has little or no effect on muscle mass but is important for physical fitness and performance (34). Physical activity and exercise training increase functional capacity, allowing individuals to maintain their independence with increasing age and participate in activities associated with daily living (35).
One major cause of muscle mass loss with aging appears to be the alteration in hormonal activity involved in muscle regeneration and protein synthesis (36). Hormone replacement therapy in women is shown to diminish age-associated muscle loss and to raise the synthesis rate in skeletal muscle after exercise training (37). Thus, age-associated hormonal activity is one explanation for the age-associated interaction between physical activity and FFM.
From a longitudinal point of view, physical activity during growth may provide lifelong benefits by reaching higher peak FFM, as shown for physical activity and lifelong bone health (38, 39). Development of FFM and bone mass may be coordinated (40). The growth phase is a window of opportunity for achieving higher peak FFM to maximize bone mass, through a physically active lifestyle (41). If longitudinally confirmed, early-life physical activity may contribute to prevention of disease in old age (42).
The study has several strengths. It was conducted in a large participant group (i.e., 2000 participants) covering early to late life, obtaining physical activity from doubly labeled water–measured energy expenditure and FFM from total body water as measured with isotope dilution, both considered gold-standard methods. An obvious limitation is the observational design. In addition, the use of the 2-compartment model of body composition cannot discern the difference in changes of separate components of FFM, including muscle mass and FM-associated FFM.
In conclusion, physically active participants show higher FM-adjusted FFM, especially after growth at age 18 y. Thus, physical activity seems to be a major determinant of body composition as reflected in peak FFM. Older age counteracts the positive association of physical activity with FFM.
Acknowledgments
The doubly labeled water database, which can be found at https://doubly-labelled-water-database.iaea.org/home or https://www.dlwdatabase.org/, is generously supported by the International Atomic Energy Agency (IAEA), Taiyo Nippon Sanso, and SERCON. We are grateful to these companies for their support and especially to Takashi Oono for his tremendous efforts at fundraising on our behalf. The IAEA Doubly Labeled Water database group authorship contains the names of people whose data were contributed into the IAEA DLW database by the analysis laboratory but they later could not be traced, or they did not respond to emails to assent to inclusion among the authorship. The list also includes some researchers who did not assent to inclusion among the main authorship because they felt their contribution was not sufficient to merit authorship: Stefan Branth, University of Uppsala, Uppsala, Sweden; Lisa H Colbert, Kinesiology, University of Wisconsin, Madison, WI, USA; Niels C De Bruin, Erasmus University, Rotterdam, Netherlands; Alice E Dutman, TNO Quality of Life, Zeist, Netherlands; Sölve Elmståhl, Lund University, Lund, Sweden; Mikael Fogelholm, Department of Food and Nutrition, Helsinki, Finland; Tamara Harris, NIH, Bethesda, MD, USA; Rik Heijligenberg, Academic Medical Center of Amsterdam University, Amsterdam, Netherlands; Hans U Jorgensen, Bispebjerg Hospital, Copenhagen, Denmark; Christel L Larsson and Elisabet M Rothenberg, University of Gothenburg, Gothenburg, Sweden; Margaret McCloskey, Royal Belfast Hospital for Sick Children, Belfast, United Kingdom; Gerwin A Meijer, Daphne L Pannemans, Sabine Schulz, Rita Van den Berg-Emons, Wim G Van Gemert, Wilhelmine W Verboeket-van de Venne, and Jeanine A Verbunt, Maastricht University, Maastricht, Netherlands; Renaat M Philippaerts, Katholieke University Leuven, Leuven, Belgium; Amy Subar, Epidemiology and Genomics, Division of Cancer Control, NIH, Bethesda, MD, USA; Minna Tanskanen, University of Jyväskilä, Jyväskilä, Finland; Ricardo Uauy, Institute of Nutrition and Food Technology (INTA), University of Chile, Santiago, Chile; and Erica J Velthuis-te Wierik, TNO Nutrition and Food Research Institute, Zeist, Netherlands.
The authors’ responsibilities were as follows—KRW, YY, HS, AHL, HP, JR, DAS, WWW, and JRS: conceived the study; KRW: performed the data analysis and wrote the first draft; YY, HS, AHL, HP, JR, DAS, WWW, and JRS: commented on the manuscript; and all authors: contributed data to the study and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by US National Science Foundation grant BCS-1824466 (to HP). The funders played no role in the content of this article.
JP-R is deceased.
Abbreviations used: FFM, fat-free mass; FFMI, fat-free mass index; FM, fat mass; FMI, fat mass index; PAL, physical activity level; REE, resting energy expenditure; TEE, total energy expenditure.
Contributor Information
Klaas R Westerterp, School of Nutrition and Translational Research in Metabolism, University of Maastricht, Maastricht, The Netherlands.
Yosuke Yamada, National Institute of Health and Nutrition, National Institutes of Biomedical Innovation, Health and Nutrition, Tokyo, Japan; Institute for Active Health, Kyoto University of Advanced Science, Kyoto, Japan.
Hiroyuki Sagayama, Faculty of Health and Sport Sciences, University of Tsukuba, Tsukuba, Japan.
Philip N Ainslie, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Lene F Andersen, Department of Nutrition, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway.
Liam J Anderson, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom; Crewe Alexandra Football Club, Crewe, United Kingdom.
Lenore Arab, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Issaad Baddou, Unité Mixte de Recherche en Nutrition et Alimentation, CNESTEN–Université Ibn Tofail URAC39, Regional Designated Center of Nutrition Associated with African Regional Agreement for Research/International Atomic Energy Agency, Rabat, Morocco.
Kweku Bedu-Addo, Department of Physiology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Ellen E Blaak, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Stephane Blanc, Nutritional Sciences, University of Wisconsin, Madison, WI, USA; Institut Pluridisciplinaire Hubert Curien. CNRS Université de Strasbourg, UMR7178, Strasbourg, France.
Alberto G Bonomi, Phillips Research, Eindhoven, The Netherlands.
Carlijn V C Bouten, Department of Biomedical Engineering and Institute for Complex Molecular Systems, Eindhoven University of Technology, Eindhoven, The Netherlands.
Pascal Bovet, University Center for Primary Care and Public Health (Unisanté), Lausanne, Switzerland.
Maciej S Buchowski, Division of Gastroenterology, Hepatology and Nutrition, Department of Medicine, Vanderbilt University, Nashville, TN, USA.
Nancy F Butte, Department of Pediatrics, Baylor College of Medicine, USDA/Agricultural Research Service Children's Nutrition Research Center, Houston, TX, USA.
Stefan G J A Camps, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Graeme L Close, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Jamie A Cooper, Nutritional Sciences, University of Wisconsin, Madison, WI, USA.
Sai K Das, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Richard Cooper, Department of Public Health Sciences, Parkinson School of Health Sciences and Public Health, Loyola University, Maywood, IL, USA.
Lara R Dugas, Department of Public Health Sciences, Parkinson School of Health Sciences and Public Health, Loyola University, Maywood, IL, USA.
Ulf Ekelund, Department of Sport Medicine, Norwegian School of Sport Sciences, Oslo, Norway.
Sonja Entringer, Institute of Medical Psychology, Charité—Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), Berlin, Germany; Department of Pediatrics, University of California Irvine, Irvine, CA, USA.
Terrence Forrester, Solutions for Developing Countries, University of the West Indies, Mona, Kingston, Jamaica.
Barry W Fudge, Institute of Biomedical and Life Sciences, University of Glasgow, Glasgow, United Kingdom.
Annelies H Goris, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Michael Gurven, Department of Anthropology, University of California Santa Barbara, Santa Barbara, CA, USA.
Catherine Hambly, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen, United Kingdom.
Asmaa El Hamdouchi, Unité Mixte de Recherche en Nutrition et Alimentation, CNESTEN–Université Ibn Tofail URAC39, Regional Designated Center of Nutrition Associated with African Regional Agreement for Research/International Atomic Energy Agency, Rabat, Morocco.
Marije B Hoos, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Sumei Hu, State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
Noorjehan Joonas, Central Health Laboratory, Ministry of Health and Wellness, Port Louis, Mauritius.
Annemiek M Joosen, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Peter Katzmarzyk, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Kitty P Kempen, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Misaka Kimura, National Institute of Health and Nutrition, National Institutes of Biomedical Innovation, Health and Nutrition, Tokyo, Japan.
William E Kraus, Department of Medicine, Duke University, Durham, NC, USA.
Robert F Kushner, Department of Medicine, Northwestern University, Chicago, IL, USA.
Estelle V Lambert, Research Unit for Exercise Science and Sports Medicine, University of Cape Town, Cape Town, South Africa.
William R Leonard, Department of Anthropology, Northwestern University, Evanston, IL, USA.
Nader Lessan, Imperial College London Diabetes Centre, Imperial College London, London, United Kingdom.
Corby K Martin, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Anine C Medin, Department of Nutrition, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway; Department of Nutrition and Public Health, Faculty of Health and Sport Sciences, University of Agder, Kristiansand, Norway.
Erwin P Meijer, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
James C Morehen, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom; The FA Group, Burton-Upon-Trent, United Kingdom.
James P Morton, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Marian L Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center and School of Public Health, University of Washington, Seattle, WA, USA.
Theresa A Nicklas, Department of Pediatrics, Baylor College of Medicine, USDA/Agricultural Research Service Children's Nutrition Research Center, Houston, TX, USA.
Robert M Ojiambo, Department of Medical Physiology, Moi University, Eldoret, Kenya; Department of Biomedical Sciences, University of Global Health Equity, Butaro, Rwanda.
Kirsi H Pietiläinen, Helsinki University Central Hospital, Helsinki, Finland.
Yannis P Pitsiladis, Collaborating Centre of Sports Medicine, University of Brighton, Eastbourne, United Kingdom.
Jacob Plange-Rhule, Department of Physiology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Guy Plasqui, Department of Nutrition and Movement Sciences, Maastricht University, Maastricht, The Netherlands.
Ross L Prentice, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center and School of Public Health, University of Washington, Seattle, WA, USA.
Roberto A Rabinovich, Department of Respiratory Medicine, University of Edinburgh, Edinburgh, United Kingdom.
Susan B Racette, Program in Physical Therapy and Department of Medicine, Washington University School of Medicine, St Louis, MO, USA.
David A Raichlen, Biological Sciences and Anthropology, University of Southern California, Los Angeles, CA, USA.
Eric Ravussin, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Rebecca M Reynolds, Centre for Cardiovascular Sciences, Queen's Medical Research Institute, University of Edinburgh, Edinburgh, United Kingdom.
Susan B Roberts, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Albertine J Schuit, School of Social and Behavioural Sciences, University of Tilburg, Tilburg, The Netherlands.
Anders M Sjödin, Department of Nutrition, Exercise and Sports, Copenhagen University, Copenhagen, Denmark.
Eric Stice, Department of Psychiatry, Stanford University, Stanford, CA, USA.
Samuel S Urlacher, Department of Anthropology, Baylor University, Waco, TX, USA.
Giulio Valenti, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Ludo M Van Etten, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands.
Edgar A Van Mil, Faculty of Health, Medicine and Life Sciences, and Faculty of Science and Engineering, Maastricht University, Maastricht, The Netherlands.
Jonathan C K Wells, Population, Policy and Practice Research and Teaching Department, UCL Great Ormond Street Institute of Child Health, London, United Kingdom.
George Wilson, Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Brian M Wood, Department of Antropology, University of California Los Angeles, Los Angeles, CA, USA; Department of Human Behavior, Ecology, and Culture, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany.
Jack Yanovski, Section on Growth and Obesity, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Bethesda, MD, USA.
Tsukasa Yoshida, Faculty of Health and Sport Sciences, University of Tsukuba, Tsukuba, Japan.
Xueying Zhang, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen, United Kingdom; State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China.
Alexia J Murphy-Alford, Nutritional and Health-Related Environmental Studies Section, Division of Human Health, International Atomic Energy Agency, Vienna, Austria.
Cornelia U Loechl, Nutritional and Health-Related Environmental Studies Section, Division of Human Health, International Atomic Energy Agency, Vienna, Austria.
Amy H Luke, Division of Epidemiology, Department of Public Health Sciences, Loyola University School of Medicine, Maywood, IL, USA.
Herman Pontzer, Evolutionary Anthropology, Duke University, Durham, NC, USA; Duke Global Health Institute, Duke University, Durham, NC, USA.
Jennifer Rood, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Dale A Schoeller, Biotech Center and Nutritional Sciences, University of Wisconsin, Madison, WI, USA.
William W Wong, Department of Pediatrics, Baylor College of Medicine, USDA/Agricultural Research Service Children's Nutrition Research Center, Houston, TX, USA.
John R Speakman, Institute of Biological and Environmental Sciences, University of Aberdeen, Aberdeen, United Kingdom; State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China; Center for Energy Metabolism and Reproduction, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China; CAS Center of Excellence in Animal Evolution and Genetics, Kunming, China.
International Atomic Energy Agency Doubly Labeled Water database group:
Stefan Branth, Lisa H Colbert, Niels C De Bruin, Alice E Dutman, Sölve Elmståhl, Mikael Fogelholm, Tamara Harris, Rik Heijligenberg, Hans U Jorgensen, Christel L Larsson, Elisabet M Rothenberg, Margaret McCloskey, Gerwin A Meijer, Daphne L Pannemans, Sabine Schulz, Rita Van den Berg-Emons, Wim G Van Gemert, W Wilhelmine, Venne Verboeket-van de, Jeanine A Verbunt, Renaat M Philippaerts, Amy Subar, Minna Tanskanen, Ricardo Uauy, and Erica J Velthuis-te Wierik
Data availability
Data All data used in these analyses are freely available via the International Atomic Energy Agency Doubly Labeled Water database (https://www.dlwdatabase.org/).
References
- 1. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington (DC): US Department of Health and Human Services; 2018. [Google Scholar]
- 2. Janz KF, Gilmore JM, Burns TL, Levy SM, Torner JC, Willing MC, Marshall TA. Physical activity augments bone mineral accrual in young children: the Iowa Bone Development study. J Pediatr. 2006;148(6):793–9. [DOI] [PubMed] [Google Scholar]
- 3. Janz KF, Letuchy EM, Eichenberger Gilmore JM, Burns TL, Tomer JC, Willing MC, Levy SM. Early physical activity provides sustained bone health benefits later in childhood. Med Sci Sports Exerc. 2010;42(6):1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Min S-K, Oh T, Kim SH, Cho J, Chung HY, Park D-H, Kim C-S. Position statement: exercise guidelines to increase peak bone mass in adolescents. J Bone Metab. 2019;26:225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xue S, Kemal O, Lu M, Lix LM, Leslie WD, Yang S. Age at attainment of peak bone mineral density and its associated factors: the National Health and Nutrition Examination Survey 2005–2014. Bone. 2020;131:1151–63. [DOI] [PubMed] [Google Scholar]
- 6. Westerterp KR. Changes in physical activity over the lifespan: impact on body composition and sarcopenic obesity. Obes Rev. 2018;19(Suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 7. Doyle F, Brown J, Lachance C. Relation between bone mass and muscle weight. Lancet. 1970;295:391–3. [DOI] [PubMed] [Google Scholar]
- 8. Vincente-Rodriguez G, Ara I, Perez-Gomez J, Dorado C, Calbet JAL. Muscular development and physical activity as major determinants of femoral bone mass acquisition during growth. Br J Sports Med. 2005;39:611–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGee M, Unger S, Hamilton J, Birken CS, Pausova Z, Vanderloo LM, Bando N, O'Connor DL. Lean mass accretion in children born very low birth weight is significantly associated with estimated changes from sedentary time to light physical activity. Pediatr Obes. 2020;15:e12610. [DOI] [PubMed] [Google Scholar]
- 10. Ramires VV, Dumith SC, Wehrmeister FC, Hallal PC, Menezes AMB, Gonçalves H. Physical activity throughout adolescence and body composition at 18 years: 1993 Pelotas (Brazil) birth cohort study. Int J Behav Nutr Phys Act. 2016;13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baxter-Jones AD, Eisenmann JC, Mirwald RL, Faulkner RA, Bailey DA. The influence of physical activity on lean mass accrual during adolescence: a longitudinal analysis. J Appl Physiol. 2008;105:734–41. [DOI] [PubMed] [Google Scholar]
- 12. Weeda J, Horan S, Beck B, Weeks BK. Lifetime physical activity, neuromuscular performance and body composition in healthy young men. Int J Sports Med. 2014;35:900–5. [DOI] [PubMed] [Google Scholar]
- 13. Hull HR, Thornton J, Wang J, Pierson RN, Kaleem Z, Pi-Sunyer X, Heymsfield S, Albu J, Fernandez JR, VanItallie TBet al. Fat-free mass index: changes and race/ethnic differences in adulthood. Int J Obes. 2011;35:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bann D, Kuh D, Wills AK, Adams J, Brage S, Cooper R. Physical activity across adulthood in relation to fat and lean body mass in early old age: findings from the Medical Research Council National Survey of Health and Development, 1946–2010. Am J Epidemiol. 2014;179:1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shephard RJ, Park H, Park S, Aoyagi Y. Objectively measured physical activity and progressive loss of lean tissue in older Japanese adults: longitudinal data from the Nakanojo study. J Am Geriatr Soc. 2013;61:1887–93. [DOI] [PubMed] [Google Scholar]
- 16. Speakman JR, Westerterp KR. Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am J Clin Nutr. 2010;92(4):826–34. [DOI] [PubMed] [Google Scholar]
- 17. Bouchard DR, Beliaeff S, DionneI J, Brochu M. Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: nutrition as a determinant of successful aging (NuAge)—the Quebec Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2007;62(12):1382–8. [DOI] [PubMed] [Google Scholar]
- 18. Manini TM, Everhart JE, Anton SD, Schoeller DA, Cummings SR, Mackey DC, Delmonico MJ, Bauer DC, Simonsick EM, Colbert LHet al. Activity energy expenditure and change in body composition in late life. Am J Clin Nutr. 2009;90(5):1336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melanson EL, Freedson PS. Physical activity assessment: a review of methods. Crit Rev Food Sci Nutr. 1996;36:385–96. [DOI] [PubMed] [Google Scholar]
- 20. Speakman JR, Pontzer G, Rood J, Sagayama H, Schoeller DA, Westerterp KR, Wong WW, Yamada Y, Loechl C, Murphy-Alford AJ. The International Atomic Energy Agency international doubly labeled water database: aims, scope and procedures. Ann Nutr Metab. 2019;75(2):114–18. [DOI] [PubMed] [Google Scholar]
- 21. Speakman JR, Yamada Y, Sagayama H, Berman ES, Ainslie PN, Andersen LF, Anderson LJ, Arab L, Baddou I, Bedu-Addo Ket al. A standard calculation methodology for human doubly labeled water studies. Cell Rep Med. 2021;2:100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schoeller DA. Hydrometry. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human body composition. Champaign, IL: Human Kinetics; 1996. pp 25–43. [Google Scholar]
- 23. Forbes GB. Longitudinal changes in adult fat-free mass: influence of body weight. Am J Clin Nutr. 1999;70(6):1025–31. [DOI] [PubMed] [Google Scholar]
- 24. Forbes GB. The companionship of lean and fat. In: Ellis KJ, Eastman JD, editors. Human body composition. New York: Plenum Press; 1993. p. 3–143. [Google Scholar]
- 25. Webster JD, Hesp R, Garrow JS. The composition of excess weight in obese women estimated by body density, total body water and total body potassium. Hum Nutr Clin Nutr. 1984;38:299–306. [PubMed] [Google Scholar]
- 26. Thomas D, Das SK, Levine JA, Martin CK, Mayer L, McDougall A, Strauss BJ, Heymsfield SB. New fat-free mass - fat mass model for use in physiological energy balance equations. Nutr Metab (Lond). 2010;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15:310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quetelet LA. Antropométrie ou mesure des différentes faculties de l'homme. Brussels, Belgium: Muquardt; 1871. [Google Scholar]
- 29. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohn SH, Vartsky D, Yasumura S, Sawitsky A, Zanzi I, Vaswani A, Ellis KJ. Compartmental body composition based on total-body nitrogen, potassium, and calcium. Am J Physiol. 1980;239:E524–30. [DOI] [PubMed] [Google Scholar]
- 31. Cohn SH, Vaswant A, Yasumura S, Yuen K, Ellis KJ. Assessment of cellular mass and lean body mass by non-invasive nuclear techniques. J Lab Clin Med. 1985;105:305–11. [PubMed] [Google Scholar]
- 32. Jackson AS, Janssen I, Sui X, Church TS, Blair SN. Longitudinal changes in body composition associated with healthy ageing; men, aged 20–96 years. Br J Nutr. 2012;107:1085–91. [DOI] [PubMed] [Google Scholar]
- 33. Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westerterp KR, Meijer EP. Physical activity and parameters of aging: a physiological perspective. J Geront A Biol Sci Med Sci. 2001;56:7–12. [DOI] [PubMed] [Google Scholar]
- 35. Mazzeo RS, Tanaka H. Exercise prescription for the elderly. Sports Med. 2001;31:809–18. [DOI] [PubMed] [Google Scholar]
- 36. Vitale G, Cesari M, Mari D. Aging of the endocrine system and its potential impact on sarcopenia. Eur J Int Med. 2016;35:10–15. [DOI] [PubMed] [Google Scholar]
- 37. Sipilä S, Narici M, Kjaer M, Pöllänen A, Atkinson RA, Hansen M, Kovanen V. Sex hormones and skeletal muscle weakness. Biogerontology. 2013;14:231–45. [DOI] [PubMed] [Google Scholar]
- 38. Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, Fuchs RK. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111:5337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Warden SJ, Mantila Roosa SM. Physical activity completed when young has residual bone benefits at 94 years of age: a within-subject controlled case study. J Musculoskelet Neuronal Interact. 2014;14:239–43. [PMC free article] [PubMed] [Google Scholar]
- 40. Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls, a seven-year longitudinal study. J Bone Miner Res. 2009;24:1693–8. [DOI] [PubMed] [Google Scholar]
- 41. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later?. Trends Ecol Evol. 2001;16:254–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data All data used in these analyses are freely available via the International Atomic Energy Agency Doubly Labeled Water database (https://www.dlwdatabase.org/).