ABSTRACT
Background
Angiotensin-converting enzyme 2 (ACE2) serves protective functions in metabolic, cardiovascular, renal, and pulmonary diseases and is linked to COVID-19 pathology. The correlates of temporal changes in soluble ACE2 (sACE2) remain understudied.
Objectives
We explored the associations of sACE2 with metabolic health and proteome dynamics during a weight loss diet intervention.
Methods
We analyzed 457 healthy individuals (mean ± SD age: 39.8 ± 6.6 y) with BMI 28–40 kg/m2 in the DIETFITS (Diet Intervention Examining the Factors Interacting with Treatment Success) study. Biochemical markers of metabolic health and 236 proteins were measured by Olink CVDII, CVDIII, and Inflammation I arrays at baseline and at 6 mo during the dietary intervention. We determined clinical and routine biochemical correlates of the diet-induced change in sACE2 (ΔsACE2) using stepwise linear regression. We combined feature selection models and multivariable-adjusted linear regression to identify protein dynamics associated with ΔsACE2.
Results
sACE2 decreased on average at 6 mo during the diet intervention. Stronger decline in sACE2 during the diet intervention was independently associated with female sex, lower HOMA-IR and LDL cholesterol at baseline, and a stronger decline in HOMA-IR, triglycerides, HDL cholesterol, and fat mass. Participants with decreasing HOMA-IR (OR: 1.97; 95% CI: 1.28, 3.03) and triglycerides (OR: 2.71; 95% CI: 1.72, 4.26) had significantly higher odds for a decrease in sACE2 during the diet intervention than those without (P ≤ 0.0073). Feature selection models linked ΔsACE2 to changes in α-1-microglobulin/bikunin precursor, E-selectin, hydroxyacid oxidase 1, kidney injury molecule 1, tyrosine-protein kinase Mer, placental growth factor, thrombomodulin, and TNF receptor superfamily member 10B. ΔsACE2 remained associated with these protein changes in multivariable-adjusted linear regression.
Conclusions
Decrease in sACE2 during a weight loss diet intervention was associated with improvements in metabolic health, fat mass, and markers of angiotensin peptide metabolism, hepatic and vascular injury, renal function, chronic inflammation, and oxidative stress. Our findings may improve the risk stratification, prevention, and management of cardiometabolic complications.
This trial was registered at clinicaltrials.gov as NCT01826591.
Keywords: angiotensin-converting enzyme 2, weight loss diet intervention, metabolic health, body composition, proteomics
Introduction
As part of the renin–angiotensin system (RAS), angiotensin-converting enzyme 2 (ACE2) is a key regulator of vascular tone and blood pressure (BP) and serves protective functions in metabolic, cardiovascular, renal, and pulmonary diseases (1, 2). Recently, research on the genetic, environmental, and metabolic determinants of ACE2 has increased exponentially, predominantly because of its strong link with COVID-19 pathology (3). Indeed, the SARS-CoV-2 virus binds to the ACE2 receptor for cell entry (4, 5). The measurement of ACE2 receptor tissue density is technically unfeasible on a large population level. Instead, sensitive assays now enable population-wide quantification of the soluble form of the catalytic ACE2 ectodomain [soluble ACE2 (sACE2)] in body fluids. sACE2 may be a relevant biomarker of COVID-19 susceptibility and prognosis (6).
Identifying factors modulating sACE2 may provide insights into susceptibility to COVID-19 infection. Previous observational studies have reported cross-sectional associations between higher sACE2 and clinical characteristics associated with higher likelihood of COVID-19 infection and worse prognosis of COVID-19-related complications (7–10), such as higher age, male sex, and metabolic disturbances including insulin resistance and hypertriglyceridemia (11–13). sACE2 is elevated in patients with cardiovascular (2, 14, 15) and metabolic disorders (16), most likely to counteract the adverse effects of increased local or systemic angiotensin II activity. Of note, higher plasma ACE2 was independently associated with increased cardiovascular mortality in a case–control analysis on 10,753 community-dwelling individuals (13).
Also, experimental studies have consistently linked ACE2 to the metabolic syndrome and associated aberrations in glucose and lipid metabolism (1, 17, 18). Indeed, animal studies suggest that RAS overactivation and improper compensation by ACE2 can contribute to organ-specific disorders associated with the metabolic syndrome, including pancreatic β-cell apoptosis, deficits in insulin secretion, and decreased insulin responsiveness in skeletal muscle and adipose tissue (1, 17, 18). In turn, metabolic disturbances can enhance the proinflammatory and profibrotic actions of angiotensin II, while hampering the compensatory actions of ACE2 (1).
However, the cross-sectional design of the aforementioned observational studies did not allow for inference of the causal relation between sACE2 and metabolic health profiles. Instead, interventional trials should evaluate the temporal and dynamic interplay between metabolic health and sACE2 in order to translate the experimental data on sACE2 dynamics to clinically relevant applications. However, to date, longitudinal evidence for linked dynamics in sACE2 concentrations and markers of metabolic health has been lacking. Moreover, no longitudinal study has yet explored the molecular associations underlying sACE2 fluctuations, whereas this may identify novel protein targets for better detection, prevention, and management of cardiovascular and COVID-19-related complications. Recent advancements in high-throughput proteomic profiling (19) and analytics capable of processing large protein networks (20, 21) have facilitated the search for pathologically relevant proteins, including the search for metabolic and inflammatory proteins associated with temporal fluctuations in sACE2.
Here, we hypothesized that perturbations in sACE2 are paralleled by alterations in distinct metabolic and proteomic health profiles. We investigated in generally healthy but overweight and obese individuals the association between sACE2, metabolic health, and body composition before, during, and after a weight loss diet intervention. In addition, we combined high-throughput proteomic profiling and multidimensional network analyses to identify metabolic, cardiovascular, and inflammatory protein dynamics associated with sACE2 changes during the dietary intervention.
Methods
Study design
The DIETFITS (Diet Intervention Examining the Factors Interacting with Treatment Success) study is a single-site, parallel-group weight loss diet trial that randomly assigned healthy adults to a healthy low-fat diet or a healthy low-carbohydrate diet (NCT01826591) (22, 23). The primary outcome of the DIETFITS study was 12-mo weight change (23). Men and premenopausal women aged 18–50 y with a BMI of 28–40 kg/m2 were recruited (22). The major criteria for exclusion were having uncontrolled hypertension or metabolic disease; diabetes; cancer; heart, renal, or liver disease; and being pregnant or lactating. Individuals were excluded if taking hypoglycemic, lipid-lowering, antihypertensive, psychiatric, or other medications known to affect body weight or energy expenditure. The Stanford University human subjects committee approved the study. All participants provided written informed consent.
Study protocol
Participants were recruited using media advertisements and email lists from previous recruitment for nutrition studies conducted by the Stanford Nutrition Studies Group. The screening, run-in, and randomization procedures as well as the dietary interventions have been described in detail previously (22). Briefly, 608 participants underwent a 1-mo run-in period during which they were instructed to maintain their habitual diet, physical activity level, and body weight. Random assignment to a healthy low-fat diet or a healthy low-carbohydrate diet was performed using an allocation sequence determined by random number generation (Blockrand in R version 3.4.0; R Foundation for Statistical Computing) by a statistician not involved in intervention delivery or data collection. The diet interventions consisted of intensive class-based education, a dietary strategy (a healthy low-fat diet or a healthy low-carbohydrate diet for 12 mo), promotion of physical activity, and behavioral modification (22). Participants underwent a clinical examination at baseline and at months 3, 6, and 12. All clinic visits took place at the Stanford Clinical and Translational Research Unit starting between 07:00 and 09:30 with the participants fasting for ≥10 h. Of note, there was no significant difference in weight loss between the healthy low-fat and healthy low-carbohydrate diets at the end of the 12-mo trial (23). For the current substudy on sACE2, we derived the following clinical measurements taken at baseline and at month 6: body height, body weight, and BP levels; routine biochemistry and high-throughput proteomics; and—in all but cohort 1 from the 5 study cohorts—body composition based on DXA. In total, 457 individuals with proteomic measurements available at baseline and at month 6 were included in our analysis (from hereon referred to as the full DIETFITS cohort), of whom 352 had available DXA measurements at baseline and month 6 (from hereon referred to as the DIETFITS subsample with available DXA) (Figure 1). The data set analyzed in this study will be made available upon reasonable request.
FIGURE 1.
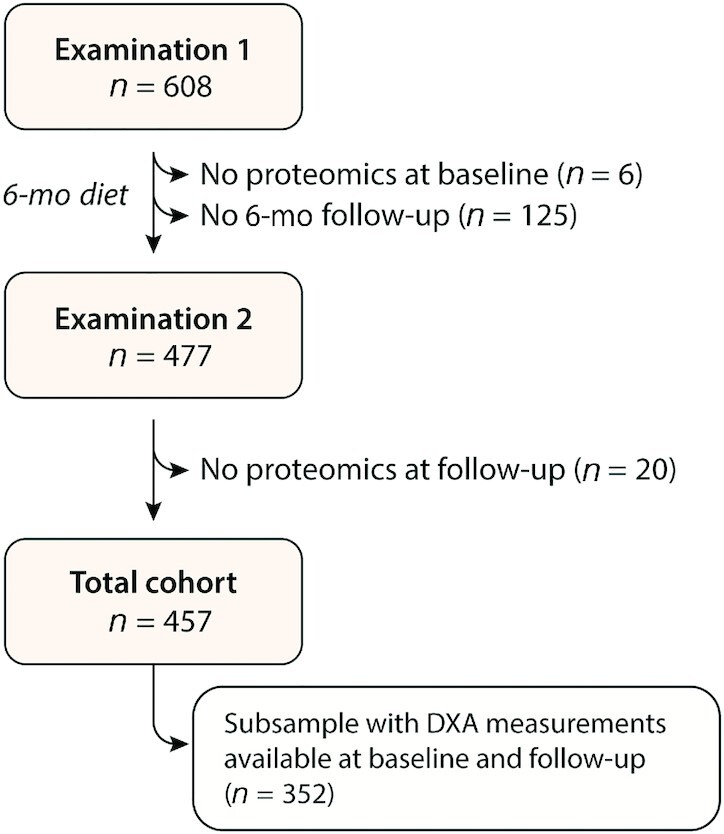
Flowchart of the DIETFITS (Diet Intervention Examining the Factors Interacting with Treatment Success) substudy.
Blood sampling and routine biochemistry
At all time points, fasting blood samples were taken via venipuncture, processed, and stored as plasma and serum samples in a −80°C freezer until biochemical analysis. Plasma triglycerides, total cholesterol, and HDL cholesterol were measured by enzymatic endpoint analysis on a clinical chemistry analyzer (Liasys 330) (22). LDL cholesterol was calculated using the Friedewald equation. Insulin concentrations were assessed by RIA by the Core Laboratory for Clinical Studies at Washington University School of Medicine, St. Louis, MO (22). Glucose concentrations were determined using a Beckman Glucose Analyzer II by electrochemical technique (22). We calculated the HOMA-IR as the product of fasting glucose (mg/dL) and serum insulin (μIU/L) divided by 405.
Proteomic measurements
At both time points, we performed high-throughput proteomic measurements on plasma samples using Proseek® multiplex platforms (Olink Proteomics). The proteomic profiling in DIETFITS has been described in detail before (24). To be specific, the arrays CVDII (which includes sACE2), CVDIII, and Inflammation I were used to determine 276 proteins (92/panel) related to immune regulation, metabolic pathways, and cardiovascular disease. The platforms apply proximity extension assay technology (19), where each protein gets linked to a unique pair of oligonucleotide-labeled antibodies. Next, hybridization, amplification, and quantification of the complementary oligonucleotide strands linked to the paired antibodies enabled protein quantification by real-time qPCR using a Fluidigm BioMark HD platform. Quantitation data were quality controlled with the help of internal and external controls. Data were normalized, providing Normalized Protein eXpression (NPX). NPX is an arbitrary unit on a log2 scale used to quantify relative changes in protein concentrations. Higher NPX corresponds to higher protein expression. The 3 panels used in this study have been thoroughly validated in terms of measurement range, specificity and precision, repeatability, reproducibility, and endogenous interference. Validation data documents and the list of biomarkers included in each panel are available at https://www.olink.com/resources-support/document-download-center/. In the DIETFITS study, the average intra- and interassay CVs were 8% and 15% (CVDII), 9% and 13% (CVDIII), and 6% and 13% (Inflammation I), respectively. For sACE2, the intra-assay CV was 9% and interassay CV was 17%. Of the 276 proteins, 40 were excluded owing to most measurements (>90%) being below the detection limit (n = 28), overlap between the panels (n = 11), or an irregular distribution (n = 1), leaving 236 proteins for analysis. Of the 215,704 individual proteomic data points, 64 missing protein concentrations (0.03%) were imputed as the cohort's mean NPX.
DXA
In a subset of 352 individuals, DXA scans were performed to assess bone mineral content, lean body mass, and whole-body adiposity at baseline and at 6 mo. A Hologic QDR-4500 W fan-beam scanner was used in compliance with the manufacturer's guidelines. Quality control procedures were carried out regularly and the scanner was calibrated weekly following the manufacturer's recommendations. To minimize observer variability, 1 technician completed all scans for all participants at both time points.
Other measurements
Body weight was recorded without shoes to the nearest 0.1 kg using a calibrated Scale-Tronix scale. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (Seca). After 5 min of rest, 3 BP readings were obtained on the right arm 1 min apart using a WelchAllyn, Spot Vital Signs LXi, with the participant in a seated position. The mean of the second and third BP measurements was used.
Statistical analysis
For database management and analysis, we used SAS version 9.4 and JMP Genomics 9.0 (SAS Institute). Means and proportions were compared by a large-sample z test and χ2 test, respectively. Significance was defined as P < 0.05 on a 2-sided test with 5% α error.
First, we performed bidirectional stepwise regression to determine clinical correlates of baseline sACE2 and ΔsACE2. Baseline covariables considered were age, sex, race, body height and weight, systolic and diastolic BP, heart rate, regular alcohol consumption, and circulating markers of metabolic health (HOMA-IR, triglycerides, HDL cholesterol, and LDL cholesterol). For ΔsACE2, stepwise models also included 6-mo changes in these covariables as well as the diet intervention (low-carbohydrate compared with low-fat). In a subsample analysis, stepwise regression models in addition considered DXA measurements (bone mineral content, lean mass, and fat mass) at baseline and changes therein. We set the P values for variables to enter and stay in the stepwise regression models at 0.10 and 0.05, respectively. Next, we compared the mean ΔsACE2 per dichotomous change in metabolic health (i.e., ΔsACE2 by improvement or worsening in HOMA-IR, triglycerides, and HDL cholesterol), while accounting for the important correlates identified in stepwise regression previously. In addition, we assessed in multiple logistic regression the adjusted risk of experiencing an sACE2 decrease during the intervention as associated with a favorable change in metabolic health profile.
To explore sACE2 connectivity within the protein network, we constructed 2 weighted networks using NetworkX 2.5: one with all 236 protein concentrations at baseline, another with the 6-mo protein changes. The R package “Weighted Gene Co-Expression Network Analysis” or WGCNA 1.69 was used for the scale-free analysis (25). We constructed the protein network with edges weighted by Pearson correlation coefficients produced from the corr() method in pandas 1.1.4 (26). Edges with a Pearson correlation coefficient >0.4 were used for the network construction.
Next, we applied a 2-step approach to identify from the pool of 235 proteins those related to sACE2 (at baseline and follow-up) and ΔsACE2: 1) protein selection based on overlap between 2 different feature selection techniques capable of handling complex network data and 2) verification of selected proteins by traditional regression statistics. By considering the overlap between the feature selection techniques, we partially accounted for intermethod variability overall and incidental protein selection in specific. For feature selection, we used partial least squares (PLS) analysis (20) and eXtreme Gradient Boosting (XGBoost) (21), 2 dimension reduction techniques capable of handling large sets of interrelated biomarkers. PLS constructs linear combinations that maximize the covariance between the proteins and the outcome (here: sACE2). These latent factors then replaced the original features (proteins) in the sACE2/ΔsACE2 estimation. The number of latent factors was the number explaining a substantial proportion of the variation in features and outcome while not being significantly different from the model with the minimum predicted residual sum of squares. Per protein, we calculated the variable importance for projection (VIP) scores of Wold, reflecting the importance of each biomarker in the construction of the final PLS model. In XGBoost, the final model was an additive combination of a number of trees, with each subsequent tree trained on a negative gradient of a loss function (21). This approach decreased both variance and bias, and thus increased prediction performance. XGBoost was optimized with a Tree-structured Parzen Estimator Approach using hyperopt 0.2.5. Feature importance was assessed as a mean increase in accuracy resulting from the tree splits per given protein.
We assessed associations between ΔsACE2 and the protein changes considered influential in both PLS analysis (VIP >1.5) and XGBoost modeling (feature importance >0.010) for prediction of ΔsACE2, while accounting for age and important clinical correlates of ΔsACE2 identified in stepwise regression (i.e., baseline sACE2, sex, diet, HOMA-IR, LDL cholesterol, and longitudinal changes in HOMA-IR, triglycerides, and HDL cholesterol). P values were corrected for multiple testing using the Holm–Bonferroni method.
Results
Characteristics of the DIETFITS cohort
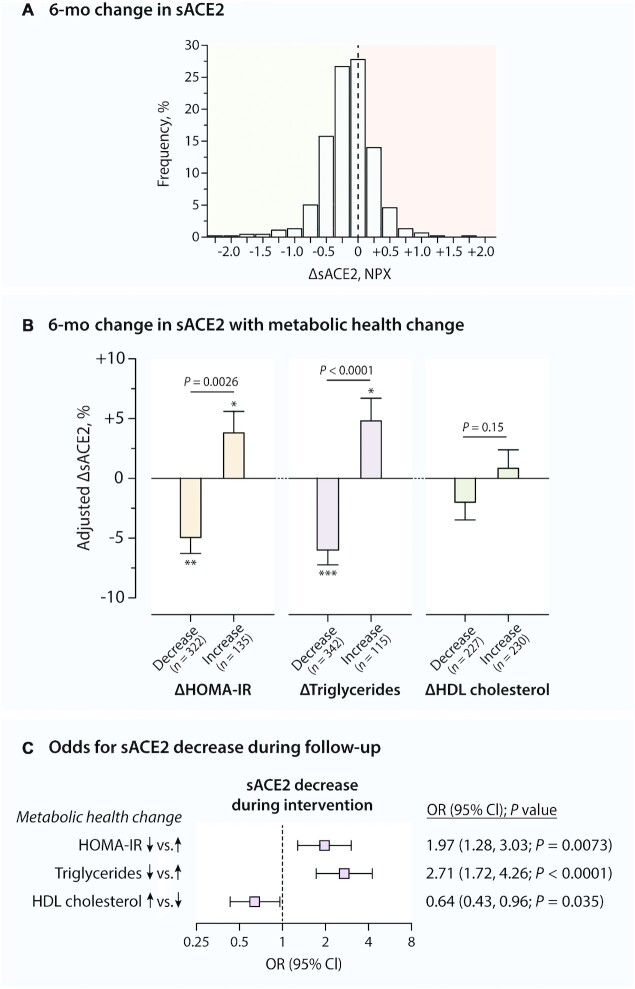
Clinical characteristics of the 457 participants in the DIETFITS trial (mean ± SD age: 39.8 ± 6.6 y; 59.1% women) are presented by examination phase in Table 1 and by sex in Supplemental Table 1. On average, BP levels, body composition, and biochemical markers of metabolic health improved, whereas sACE2 concentrations declined significantly at 6 mo in the diet intervention (P < 0.0001 for all) (Table 1, Figure 2A). sACE2 dropped significantly in both the low-fat and the low-carbohydrate diet intervention groups (P ≤ 0.0091 for both), but it declined stronger in the low-carbohydrate than in the low-fat diet group (P = 0.0004) (Supplemental Table 2).
TABLE 1.
Clinical characteristics of the 457 DIETFITS (Diet Intervention Examining the Factors Interacting with Treatment Success) study participants by examination phase1
| Examination 1 (baseline) | Examination 2 (6-mo follow-up) | P value | |
|---|---|---|---|
| Anthropometrics | |||
| Age, y | 39.8 ± 6.6 | — | — |
| Body weight, kg | 95.7 ± 14.8 | 88.4 ± 14.3 | <0.0001 |
| Body height, cm | 169.3 ± 9.5 | — | — |
| BMI, kg/m2 | 33.3 ± 3.6 | 30.8 ± 3.7 | <0.0001 |
| Systolic BP, mm Hg | 123.8 ± 12.5 | 119.3 ± 11.5 | <0.0001 |
| Diastolic BP, mm Hg | 81.3 ± 7.6 | 78.3 ± 7.3 | <0.0001 |
| Heart rate, bpm | 70.4 ± 10.1 | 67.5 ± 10.1 | <0.0001 |
| Biochemical data | |||
| HOMA-IR | 3.26 (1.71–7.26) | 2.26 (1.43–6.39) | <0.0001 |
| Total cholesterol, mg/dL | 189.2 ± 33.9 | 183.6 ± 35.1 | <0.0001 |
| LDL cholesterol, mg/dL | 114.2 ± 28.9 | 113.2 ± 29.7 | 0.32 |
| HDL cholesterol, mg/dL | 48.9 ± 9.8 | 49.2 ± 9.6 | 0.31 |
| Triglycerides, mg/dL | 113.1 (64.9–223.0) | 92.5 (54.5–180.5) | <0.0001 |
| sACE2, NPX | 1.89 ± 0.63 | 1.74 ± 0.54 | <0.0001 |
| Race/ethnicity | |||
| White | 328 (71.8) | — | |
| Black/African American | 14 (3.1) | — | |
| Asian | 48 (10.5) | — | |
| Other | 67 (14.7) | — | |
| DXA scan2 | |||
| BMC mass, kg | 2.59 ± 0.44 | 2.59 ± 0.44 | 0.45 |
| Fat mass, kg | 33.4 ± 7.5 | 28.8 ± 8.0 | <0.0001 |
| Lean mass, kg | 56.0 ± 11.3 | 54.1 ± 10.8 | <0.0001 |
| Fat percentage, % | 36.5 ± 6.8 | 33.7 ± 7.6 | <0.0001 |
1Values are arithmetic mean ± SD, geometric mean (10th–90th percentile), or participant n (%). P values reflect the significance of 6-mo longitudinal changes according to a paired-sample t test. BMC, bone mineral content; BP, blood pressure; NPX, Normalized Protein eXpression; sACE2, soluble angiotensin-converting enzyme 2.
2Baseline and follow-up data for DXA scan measurements were available in 352 participants.
FIGURE 2.
Multivariable-adjusted association between 6-mo changes (Δ) in sACE2 and metabolic health during a diet intervention. (A) Histogram of ΔsACE2 in the entire cohort (n = 457). (B) Multivariable-adjusted change in sACE2 by change in metabolic health profile. Mean ± SE ΔsACE2 was adjusted for sACE2 at baseline, age, sex, and clinical correlates of ΔsACE2 (Table 2) in a linear mixed model and compared using an independent-samples t test. *,**,***Significant changes in sACE2 during follow-up according to paired-sample t test: *P < 0.05,**P < 0.001, ***P < 0.0001. (C) Multivariable-adjusted risk of decrease in sACE2 by change in metabolic health profile in logistic regression. NPX, normalized protein expression; sACE2, soluble angiotensin-converting enzyme 2.
sACE2 in relation to metabolic health and body composition
Clinical correlates of baseline sACE2
At baseline, higher sACE2 concentrations were associated independently with male sex and with higher systolic BP, HOMA-IR, triglycerides, and HDL cholesterol in stepwise regression (adjusted R2 = 30.9%; P ≤ 0.0056 for all) (Supplemental Table 2). In addition, no association was found between baseline sACE2 and total and LDL cholesterol (P > 0.091) (Supplemental Table 3). In subsample analysis considering DXA measurements, higher sACE2 concentrations were in addition correlated with lower fat mass at baseline (adjusted R2 = 31.6%) (Supplemental Table 2). The association between fat mass and sACE2 was inverse in both men and women (Psex interaction = 0.23), but only reached statistical significance in women (P < 0.0001 in women, P = 0.36 in men) (Supplemental Table 4).
Clinical correlates of longitudinal change in sACE2
Table 2 presents the correlates of the 6-mo change in sACE2 during the diet intervention. In stepwise regression, a stronger decline in sACE2 was associated with higher sACE2 at baseline, female sex, low-carbohydrate diet (as compared with low-fat), and lower HOMA-IR and LDL cholesterol at baseline as well as with a stronger decline in HOMA-IR, triglycerides, and HDL cholesterol during the diet intervention (Table 2). In subsample analysis including the DXA measurements, a larger drop in sACE2 was in addition correlated with a larger drop in fat mass (Table 2). Multivariable-adjusted analyses confirmed the association between ΔsACE2 and the 6-mo changes in HOMA-IR, triglycerides, HDL cholesterol, and fat mass (P ≤ 0.019 for all) (Supplemental Table 5). In support of this, after full adjustment, participants in whom HOMA-IR and triglycerides were decreased at month 6 of the diet intervention presented a significant drop in sACE2 (Figure 2B). In contrast, a significant rise in sACE2 was seen in those experiencing a 6-mo increase in HOMA-IR and triglycerides (Figure 2B). The multivariable-adjusted odds for experiencing a decrease in sACE2 during the diet intervention were significantly higher in participants with a 6-mo decrease in HOMA-IR (OR: 1.97; P = 0.0073) and triglycerides (OR: 2.71; P < 0.0001) than in those without (Figure 2C).
TABLE 2.
Clinical correlates of change in sACE2 during a diet intervention1
| ΔsACE2 | |||
|---|---|---|---|
| β ± SE | P value | Partial R2 | |
| Full DIETFITS cohort (n = 457) | |||
| Baseline correlates | |||
| sACE2 | −56.3 ± 4.2 | <0.0001 | 27.1% |
| Female sex | −29.3 ± 8.3 | 0.0005 | 2.2% |
| Low-carbohydrate diet (vs. low-fat) | −21.8 ± 7.8 | 0.0053 | 0.87% |
| HOMA-IR | 10.8 ± 4.6 | 0.018 | 0.67% |
| LDL cholesterol | 9.4 ± 3.7 | 0.011 | 0.74% |
| Change during intervention | |||
| ΔHOMA-IR | 19.1 ± 4.6 | <0.0001 | 1.7% |
| ΔTriglycerides | 17.7 ± 4.0 | <0.0001 | 5.9% |
| ΔHDL cholesterol | 14.0 ± 3.8 | 0.0003 | 1.4% |
| DIETFITS subsample with DXA measurements available (n = 352) | |||
| Baseline correlates | |||
| sACE2 | −31.1 ± 3.3 | <0.0001 | 33.4% |
| Female sex | −47.6 ± 9.2 | <0.0001 | 3.0% |
| LDL cholesterol | 10.3 ± 4.2 | 0.013 | 0.94% |
| Change during intervention | |||
| ΔHOMA-IR | 23.1 ± 6.6 | 0.0010 | 2.3% |
| ΔTriglycerides | 30.1 ± 9.0 | 0.0006 | 4.8% |
| ΔHDL cholesterol | 17.1 ± 4.1 | <0.0001 | 2.6% |
| ΔFat mass | 10.6 ± 4.5 | 0.019 | 1.2% |
1Values were standardized partial regression coefficients ± SEs standardized for the distribution of both the predictor and sACE2 retrieved from bidirectional stepwise regression. Covariables considered were age, sex, race, diet (low-carbohydrate compared with low-fat diet), body height and weight, systolic and diastolic blood pressure, heart rate, regular alcohol consumption, HOMA-IR, triglycerides, HDL and LDL cholesterol, and, if available, BMC mass, fat mass, and lean mass (as assessed by DXA) as well as longitudinal changes in these variables and sex interaction terms. Total adjusted R2 = 39.9% (full DIETFITS cohort) and 44.4% (DIETFITS subsample with DXA). None of the sex interaction terms were selected for the final stepwise regression models (P > 0.05 for all). DIETFITS, Diet Intervention Examining the Factors Interacting with Treatment Success; sACE2, soluble angiotensin-converting enzyme 2.
sACE2 and circulating proteins related to inflammation and cardiovascular disease
Network of diet-induced protein changes
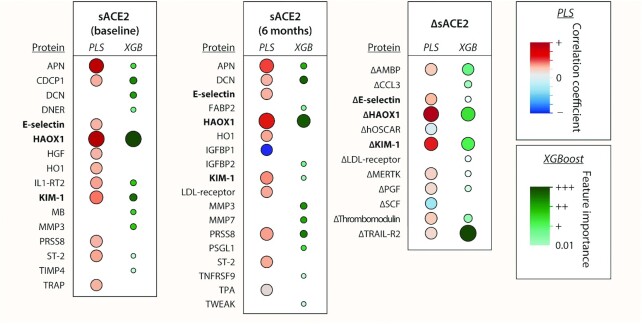
We constructed weighted networks of the 236 proteins at baseline (Supplemental Figure 1) and of their changes during the diet intervention (Supplemental Figure 2). Of note, ΔsACE2 was directly connected to the 6-mo change in α-1-microglobulin/bikunin precursor (AMBP), chemokine C-C motif ligand 3, E-selectin, hydroxyacid oxidase 1 (HAOX1), osteoclast-associated immunoglobulin-like receptor, IL-16 and IL-18, kidney injury molecule 1 (KIM-1), tyrosine-protein kinase Mer (MERTK), placental growth factor (PGF), prostasin (PRSS8), spondin-2, thrombospondin-2, thrombomodulin, tyrosine-protein kinase receptor (TIE2), and TNF receptor superfamily member 10B (TRAIL-R2) (Supplemental Figure 2).
Proteins linked to sACE2 and ΔsACE2
Figure 3 outlines the proteins identified by PLS and/or XGBoost as most likely to be associated with sACE2 and ΔsACE2. Of note, E-selectin, HAOX1, and KIM-1 consistently were selected as important proteins linked to baseline sACE2, follow-up sACE2, and ΔsACE2. The following 6-mo protein changes were related to ΔsACE2 in both PLS and XGBoost modeling: change in AMBP, E-selectin, HAOX-1, KIM-1, MERTK, PGF, thrombomodulin, and TRAIL-R2 (Figure 3). In support, ΔsACE2 remained independently associated with these protein changes in linear regression after adjustment for major clinical correlates identified previously (Supplemental Table 6). In addition, both baseline and follow-up sACE2 were found to be linked to aminopeptidase N (APN), decorin, heme oxygenase 1, matrix metalloproteinase 3, PRSS8, and soluble interleukin-1 receptor-like 1 (ST-2) (Figure 3). Summary data of the PLS models are presented in Supplemental Figure 3 (for ΔACE2) and Supplemental Table 7 (for sACE2 at baseline and follow-up).
FIGURE 3.
Proteins linked to sACE2 at baseline and follow-up and its changes at 6 mo in the diet intervention. The heat map presents the biomarkers that were important in PLS analysis (VIP >1.5) and/or XGBoost modeling (feature importance >0.010) for predicting the cross-sectional sACE2 and the 6-mo change (Δ) in sACE2. For PLS, red dots are positive and blue dots are negative correlation coefficients. Proteins selected for baseline sACE2, follow-up sACE2, and ΔsACE2 are in bold. Models for ΔsACE2 accounted for sACE2 at baseline. Larger dots reflect greater VIP score (for PLS) or greater feature importance (for XGBoost). AMBP, α-1-microglobulin/bikunin precursor; APN, aminopeptidase N; CCL3, C-C motif chemokine ligand 3; CDCP1, CUB domain containing protein 1; DCN, decorin; DNER, Δ and notch-like epidermal growth factor-related receptor; FABP2, fatty acid-binding protein 2; HAOX1, hydroxyacid oxidase 1; HGF, hepatocyte growth factor; hOSCAR, human osteoclast-associated immunoglobulin-like receptor; HO1, heme oxygenase 1; IGFBP, insulin-like growth factor-binding protein; IL1-RT2, interleukin 1 receptor type II; KIM-1, kidney injury molecule 1; MB, myoglobin; MERTK, tyrosine-protein kinase Mer; MMP, matrix metalloproteinase; PGF, placental growth factor; PLS, partial least squares; PRSS8, prostasin; PSGL1, P-selectin glycoprotein ligand-1; sACE2, soluble angiotensin-converting enzyme 2; SCF, stem cell factor; ST-2, soluble interleukin-1 receptor-like 1; TIMP4, tissue inhibitor of metalloproteinase 4; TNFRSF9, TNF receptor superfamily member 9; TPA, tissue-type plasminogen activator; TRAIL-R2, TNF receptor superfamily member 10B; TRAP, thrombospondin-related anonymous protein; TWEAK, TNF-related weak inducer of apoptosis; VIP, variable importance in projection; XGBoost, eXtreme Gradient Boosting.
Discussion
We uncovered metabolic and proteomic profiles associated with sACE2 changes. In this randomized weight loss diet intervention trial, a stronger reduction in sACE2 was associated with improvements in body composition and metabolic health. Moreover, sACE2 concentrations were associated with proteins related to angiotensin peptide metabolism (APN), vascular injury and inflammation (PGF, E-selectin, MERTK), blood coagulation (thrombomodulin), renal function (KIM-1), liver function and oxalate metabolism (HAOX1), and chronic inflammation and oxidative stress (AMBP, TRAIL-R2).
sACE2 and biological sex
Previous cross-sectional population studies have consistently reported higher sACE2 concentrations in men than in women (13). Even with lower sACE2 at baseline, women experienced a stronger reduction in sACE2 concentration than men during the weight loss diet intervention. Our findings suggest that sACE2 is less affected by dietary interventions and fat mass changes in men than in women. It remains to be studied to what extent sex differences in sACE2 dynamics may originate from sex differences in ACE2 gene regulation (a gene localized on the X chromosome). If true, such sex difference may encumber the benefits of a dietary intervention on cardiorenal health and COVID-19 susceptibility in men.
ACE2 as regulator of glucose and lipid metabolism
ACE2 catabolizes angiotensin II into angiotensin-(1–7) [Ang(1–7)], a peptide that exerts the protective actions of ACE2 on cardiorenal function and glucose and lipid metabolism. Of note, ACE2 may be upregulated in subjects with cardiometabolic risk factors to counteract the adverse effects of increased local or systemic angiotensin II activity. RAS and ACE2 have been linked experimentally and clinically to the metabolic syndrome and its associated aberrations in glucose and lipid metabolism (1, 17). As such, RAS overactivation and improper compensation by ACE2/Ang(1–7) could contribute to disorders associated with the metabolic syndrome, including pancreatic β-cell apoptosis, deficits in insulin secretion, and decreased insulin responsiveness in skeletal muscle and adipose tissue (1).
For instance, experimental studies demonstrated a protective role for ACE2 and Ang(1–7) in the regulation of pancreatic β-cell function and, in consequence, of insulin synthesis and secretion (18). Indeed, overexpression of ACE2 in the pancreas of db/db mice increased islet insulin content, reduced β-cell apoptosis, and improved glucose tolerance, whereas blocking the Ang(1–7) receptor Mas reversed these beneficial effects (27). Furthermore, ACE2 deficiency in vivo was associated with increased lipid accumulation in skeletal muscle, whereas ACE2 activation improved intramuscular fat regulation by improving endoplasmic reticulum and mitochondrial function (28). Also in murine muscle, ACE2 protected against high-calorie-induced insulin resistance by antagonizing angiotensin II–activated pathways (29, 30).
Conversely, disturbances in glucose and lipid metabolism may also affect RAS functioning. Indeed, hyperglycemia, hyperinsulinemia, dyslipidemia, and hypercholesterolemia were found to regulate RAS components required for the synthesis, degradation, secretion, and/or responsiveness of angiotensin peptides in various cell types, including pancreatic islet cells, adipocytes, and skeletal muscle cells (1). Such metabolic disturbances appear to enhance the proinflammatory and profibrotic actions of angiotensin II that are linked to the macrovascular complications of diabetes mellitus, while hampering the compensatory actions of ACE2 (1).
Clinical and epidemiological observations linking sACE2 to metabolic health
Clinical and epidemiological studies support the experimental evidence that links circulating ACE2 to metabolic health, albeit most of these studies were cross-sectional. For instance, plasma ACE2 was elevated in 171 individuals with metabolic syndrome as compared with 1880 healthy counterparts (12). Furthermore, sACE2 was associated with circulating markers of metabolic health, including glycated hemoglobin, HOMA-IR, triglycerides, and LDL cholesterol, in several large-scale population samples (12, 13). In support, urinary ACE2 concentrations were significantly higher in subjects with elevated fasting glucose, impaired glucose tolerance, and type 2 diabetes mellitus as compared with those with normal glucose tolerance (31). In a population study of 544 individuals, differences in insulin resistance as assessed by HOMA-IR explained most (17.4%) of the total variance in plasma ACE2 (11).
Adding to these cross-sectional population data, we provide the first longitudinal evidence of linked dynamics in sACE2 concentrations, fat mass, and markers of metabolic health, notably in relation to insulin resistance and lipid profile. During the weight loss diet intervention, participants with higher insulin resistance and LDL cholesterol at baseline experienced less reduction in sACE2, whereas a softer decline (or even an increase) in sACE2 was associated with less improvement (or even worsening) in insulin resistance, triglycerides, and fat mass. Overall, our longitudinal findings show the potency of a weight loss diet intervention to reduce sACE2 and present the tight interplay between metabolic health, body composition, and sACE2.
Of note, we observed a direct relation between sACE2 and HDL cholesterol. Intuitively, one would expect an inverse relation: higher/worse sACE2 related to lower/worse HDL. As in another community-based study (12), sACE2 was inversely correlated to HDL cholesterol in our unadjusted analyses, but the relation shifted to a direct one in the adjusted models. We cannot exclude the possibility of a hidden confounder that biased the sACE2–HDL relation. For instance, alcohol consumption is known to raise HDL cholesterol concentrations while inducing low-grade hepatic and pancreatic injury (32). Self-reported alcohol consumption did not alter the sACE2–HDL relation in our analyses, but the alcohol reporting may have been biased by cognitive and sociocultural factors (33). The question remains whether the ACE2–HDL association is confounded by hepatic and metabolic risk factors.
sACE2 and protein markers of vascular, renal, hepatic, and inflammatory health
In the second part of our analysis, we identified protein dynamics associated with the sACE2 changes. These proteins may highlight molecular mechanisms linked to the aforementioned ACE2 activities.
sACE2 concentrations and sACE2 changes during the diet intervention were most strongly associated with KIM-1 and HAOX1, which may reflect the link between elevated sACE2 activity and impaired kidney, liver, and pancreas function (34). KIM-1 is a biomarker of renal proximal tubular injury. In a rat model, increased KIM-1 protein expression was associated with the development of RAS-mediated renal damage (35). HAOX1 is a peroxisomal enzyme that converts glycolate into the oxalate precursor glyoxylate in the liver and pancreas (36, 37). HAOX1 could be released in the bloodstream upon hepatic and pancreatic injury, which may explain its strong connection with sACE2, a regulator of lipid and glucose metabolism in the liver. In support of this, a cross-sectional community study found strong correlations between sACE and other circulating markers of liver and pancreas damage such as γ-glutamyl transferase (12).
The strong relation between sACE2 and APN at baseline and follow-up is rather straightforward: the metalloproteinase APN catabolizes angiotensin III into angiotensin IV and is thus a regulator of angiotensin peptide metabolism like ACE2 (38). Interestingly, several alphacoronaviruses use APN as a cell-entry receptor (39), whereas human betacoronaviruses like SARS-CoV-2 rely on ACE2 for cell entry (4).
The associated dynamics in sACE2, PGF, MERTK, E-selectin, and thrombomodulin suggest a tight connection between RAS/ACE2 and pathways related to vascular remodeling, vascular inflammation, and blood coagulation. PGF is a member of the vascular endothelial growth factor family of angiogenic cytokines that is enhanced during vascular injury (40). Angiotensin II stimulates PGF production in human endothelial and vascular smooth muscle cells (41). Moreover, experimental data link RAS overactivation and consequent PGF overexpression to the pathogenesis of atherosclerosis. Indeed, PGF mediates aldosterone-induced vascular remodeling, leading to smooth muscle cell proliferation and vascular fibrosis and inflammation at sites of endothelial injury (42, 43). Similarly, both MERTK and E-selectin—a cell adhesion molecule expressed solely on activated endothelial cells—mediate the cascade of vascular inflammation leading to the formation of atherosclerotic lesions (44, 45). Thrombomodulin is an endothelial cell receptor with anticoagulatory, antifibrinolytic, and anti-inflammatory properties (46).
Lastly, the associations found between sACE2 dynamics and the biomarkers AMBP and TRAIL-R2 may reflect the counteracting role of ACE2 on systemic low-grade chronic inflammation and oxidative stress underlying cardiorenal and metabolic disorders. AMBP is a precursor of α1-microglobulin, a heme and radical scavenger with antioxidative and immunosuppressive properties, and was found to be involved in erythropoiesis and erythrocyte homeostasis (47). TRAIL-R2—also known as TNFRSF10B—is a member of the death receptor superfamily and a key modulator of apoptosis. High TRAIL-R2 was associated with incident cardiometabolic morbidity in a large cohort of 4742 community-dwelling individuals (48). Future studies should deeper investigate the connections between sACE2 and the highlighted set of proteins and explore whether the associated proteins represent clinically relevant targets for detection and management of cardiovascular, renal, and COVID-19-related diseases.
Clinical implications
In particular, our findings may have implications on the risk stratification, prevention, and management of cardiometabolic and COVID-19-related disorders. For instance, improvements in insulin resistance and lipid profile may alleviate RAS contributions to vascular and metabolic pathologies. As such, weight loss diet interventions may be powerful prophylactic tools for both cardiovascular and COVID-19 risk reduction in the community. Improving body composition and metabolic health may particularly be an effective strategy for COVID-19 prophylaxis in people with poor cardiovascular disease risk profiles (7–10). In support, 33 patients with a prior history of metabolic surgery who tested positive for SARS-CoV-2 presented lower rates of hospital admission than SARS-CoV-2 positive patients with severe obesity (49). It remains to be studied whether an sACE2-reducing diet in individuals at high risk can lower SARS-CoV-2 incidence and disease severity. Future research should expand our analyses with genetic, environmental, and other metabolic factors to further unravel the mechanisms underlying sACE2 variability.
Study strengths and limitations
We acknowledge the study's strengths and limitations. First, a large number of proteins was measured in a large number of individuals. Technical variability may have influenced the proteomic measurements; however, the panels used in this study were validated extensively in terms of measurement ranges, assay specificity and precision, repeatability, reproducibility, and endogenous interference (https://www.olink.com/resources-support/document-download-center/). Second, although we determined a wide range of proteins linked to cardiovascular disease and inflammation, we may have missed hidden confounders or important proteins linked to sACE2. For instance, the protein panels did not include biochemical markers of overall liver and pancreas damage such as γ-glutamyl transferase, whereas such markers were found to correlate strongly with sACE2 in the community previously (12). Third, extrapolation of our findings may be limited by the conduct of the study in a geographic area with individuals who have attained relatively high education levels and high accessibility to high-quality food. However, the study was broadly advertised and successfully enrolled participants with relatively good ethnic and racial diversity and with a range of educational attainment.
Conclusions
A decrease in sACE2 at 6 mo in a weight loss diet intervention was associated with improvements in metabolic health, fat mass, and markers of angiotensin peptide metabolism, hepatic and vascular injury and inflammation, renal function, chronic inflammation, and oxidative stress. Our findings highlight the need for further studies on diet interventions for cardiorenal and COVID-19 disease prophylaxis in the community. Future research should investigate to what extent dietary sACE2 alterations can counteract the incidence and severity of cardiorenal and SARS-CoV-2-related diseases.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—NC, TK, and FH: performed the statistical analysis; NC, MP, and TK: drafted the manuscript; and all authors: contributed to the conception and design of the study, contributed to the data interpretation, revised the manuscript critically for important intellectual content, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the Sean N Parker Center for Allergy and Asthma Research (to KCN), Sunshine Foundation (to KCN), Crown Foundation (to KCN), National Institute of Environmental Health Sciences grant 1 R21 ES033049-01 (to KCN), Barakett Family (to KCN), National Institute of Diabetes and Digestive and Kidney Diseases grant NIH 1R01DK091831 (to CDG), Nutrition Science Initiative, NIH grant 1 K12 GM088033 (to CDG), and a Stanford Clinical and Translational Science Award (to CDG). TK, NC, and FS were supported by Research Foundation Flanders grants G.0880.13 (to TK), 1225021N (to NC), 1S07421N (to FS), and G0C5319N (to TK). None of the funders played a role in the design, implementation, analysis, and interpretation of the data.
Supplemental Tables 1–7 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ACE, angiotensin-converting enzyme; AMBP, α-1-microglobulin/bikunin precursor; Ang(1–7), angiotensin-(1–7); APN, aminopeptidase N; BP, blood pressure; DIETFITS, Diet Intervention Examining the Factors Interacting with Treatment Success; HAOX1, hydroxyacid oxidase 1; KIM-1, kidney injury molecule 1; MERTK, tyrosine-protein kinase Mer; NPX, Normalized Protein eXpression; PGF, placenta growth factor; PLS, partial least squares; PRSS8, prostasin; RAS, renin–angiotensin system; sACE2, soluble angiotensin-converting enzyme 2; TRAIL-R2, TNF receptor superfamily member 10B; VIP, variable importance for projection; XGBoost, eXtreme Gradient Boosting.
Contributor Information
Nicholas Cauwenberghs, Stanford Cardiovascular Institute, Department of Medicine, Stanford University, Stanford, CA, USA; Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Mary Prunicki, Sean N Parker Center for Allergy and Asthma Research, Stanford University, Stanford, CA, USA; Department of Medicine, Stanford University, Stanford, CA, USA.
František Sabovčik, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Dalia Perelman, Department of Genetics, Stanford University School of Medicine, Stanford, CA, USA.
Kévin Contrepois, Stanford Cardiovascular Institute, Department of Medicine, Stanford University, Stanford, CA, USA; Department of Genetics, Stanford University School of Medicine, Stanford, CA, USA.
Xiao Li, Department of Biochemistry, The Center for RNA Science and Therapeutics, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Department of Computer and Data Sciences, Case Western Reserve University, Cleveland, OH, USA.
Michael P Snyder, Stanford Cardiovascular Institute, Department of Medicine, Stanford University, Stanford, CA, USA; Department of Genetics, Stanford University School of Medicine, Stanford, CA, USA; Stanford Diabetes Research Center, Stanford University, Stanford, CA, USA.
Kari C Nadeau, Sean N Parker Center for Allergy and Asthma Research, Stanford University, Stanford, CA, USA; Department of Medicine, Stanford University, Stanford, CA, USA.
Tatiana Kuznetsova, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Francois Haddad, Stanford Cardiovascular Institute, Department of Medicine, Stanford University, Stanford, CA, USA.
Christopher D Gardner, Stanford Diabetes Research Center, Stanford University, Stanford, CA, USA; Stanford Prevention Research Center, Department of Medicine, Stanford University, Stanford, CA, USA.
Data availability
Data included in the article, code book, and analytic code will be made available upon reasonable request.
References
- 1. Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 2014;5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hooper NM, Lambert DW, Turner AJ. Discovery and characterization of ACE2 – a 20-year journey of surprises from vasopeptidase to COVID-19. Clin Sci. 2020;134:2489–501. [DOI] [PubMed] [Google Scholar]
- 4. Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–92.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong PJ, Look DC, Tan P, Shi L, Hickey M, Gakhar L, Chappell MC, Wohlford-Lenane C, McCray PB Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng H, Wang Y, Wang G-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SLet al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du Ket al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, Yu X, Zhang S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. 2020;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 11. Kuznetsova T, Cauwenberghs N. Determinants of circulating angiotensin-converting enzyme 2 protein levels in the general population. Eur J Intern Med. 2020;84:104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kornilov SA, Lucas I, Jade K, Dai CL, Lovejoy JC, Magis AT. Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit Care. 2020;24:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Narula S, Yusuf S, Chong M, Ramasundarahettige C, Rangarajan S, Bangdiwala SI, van Eikels M, Leineweber K, Wu A, Pigeyre Met al. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396:968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Úri K, Fagyas M, Siket IM, Kertész A, Csanádi Z, Sándorfi G, Clemens M, Fedor R, Papp Z, Édes Iet al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS One. 2014;9:e87845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shao Z, Schuster A, Borowski AG, Thakur A, Li L, Wilson Tang WH. Soluble angiotensin converting enzyme 2 levels in chronic heart failure is associated with decreased exercise capacity and increased oxidative stress-mediated endothelial dysfunction. Transl Res. 2019;212:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop P-H. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens. 2012;30:375–83. [DOI] [PubMed] [Google Scholar]
- 17. Chhabra KH, Chodavarapu H, Lazartigues E. Angiotensin converting enzyme 2: a new important player in the regulation of glycemia. IUBMB Life. 2013;65:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernardi S, Tikellis C, Candido R, Tsorotes D, Pickering RJ, Bossi F, Carretta R, Fabris B, Cooper ME, Thomas MC. ACE2 deficiency shifts energy metabolism towards glucose utilization. Metabolism. 2015;64:406–15. [DOI] [PubMed] [Google Scholar]
- 19. Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 2011;39:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee LC, Liong C-Y, Jemain AA. Partial least squares-discriminant analysis (PLS-DA) for classification of high-dimensional (HD) data: a review of contemporary practice strategies and knowledge gaps. Analyst. 2018;143:3526–39. [DOI] [PubMed] [Google Scholar]
- 21. Chen T, Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, August 13–17. New York (NY): Association for Computing Machinery; 2016. p. 785–94. [Google Scholar]
- 22. Stanton M, Robinson J, Kirkpatrick S, Farzinkhou S, Avery E, Rigdon J, Offringa L, Trepanowski J, Hauser M, Hartle Jet al. DIETFITS study (diet intervention examining the factors interacting with treatment success) – study design and methods. Contemp Clin Trials. 2017;53:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gardner CD, Trepanowski JF, Gobbo LCD, Hauser ME, Rigdon J, Ioannidis JPA, Desai M, King AC. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319:667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figarska SM, Rigdon J, Ganna A, Elmståhl S, Lind L, Gardner CD, Ingelsson E. Proteomic profiles before and during weight loss: results from randomized trial of dietary intervention. Sci Rep. 2020;10:7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKinney W. Data structures for statistical computing in Python. [Internet]. In: van der Walt S, Millman J, editors. Proceedings of the 9th Python in Science Conference. Austin (TX), June 28–30. 2010. p. 56–61.. [Accessed 2021 Jul 27]. Available from: https://conference.scipy.org/proceedings/scipy2010/mckinney.html. [Google Scholar]
- 27. Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I–converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao X, Lu X-M, Tuo X, Liu J-Y, Zhang Y-C, Song L-N, Cheng Z-Q, Yang J-K, Xin Z. Angiotensin-converting enzyme 2 regulates endoplasmic reticulum stress and mitochondrial function to preserve skeletal muscle lipid metabolism. Lipids Health Dis. 2019;18:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeda M, Yamamoto K, Takemura Y, Takeshita H, Hongyo K, Kawai T, Hanasaki-Yamamoto H, Oguro R, Takami Y, Tatara Yet al. Loss of ACE2 exaggerates high-calorie diet–induced insulin resistance by reduction of GLUT4 in mice. Diabetes. 2013;62:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamamoto K, Takeshita H, Rakugi H. ACE2, angiotensin 1-7 and skeletal muscle: review in the era of COVID-19. Clin Sci. 2020;134:3047–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park SE, Kim WJ, Park SW, Park JW, Lee N, Park C-Y, Youn B-S. High urinary ACE2 concentrations are associated with severity of glucose intolerance and microalbuminuria. Eur J Endocrinol. 2013;168:203–10. [DOI] [PubMed] [Google Scholar]
- 32. De Oliveira e Silva ER, Foster D, Harper MM, Seidman CE, Smith JD, Breslow JL, Brinton EA. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–52. [DOI] [PubMed] [Google Scholar]
- 33. Northcote J, Livingston M. Accuracy of self-reported drinking: observational verification of “last occasion” drink estimates of young adults. Alcohol Alcohol. 2011;46:709–13. [DOI] [PubMed] [Google Scholar]
- 34. Wolke C, Teumer A, Endlich K, Endlich N, Rettig R, Stracke S, Fiene B, Aymanns S, Felix SB, Hannemann Aet al. Serum protease activity in chronic kidney disease patients: the GANI_MED renal cohort. Exp Biol Med. 2017;242:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Borst MH, van Timmeren MM, Vaidya VS, de Boer RA, van Dalen MBA, Kramer AB, Schuurs TA, Bonventre JV, Navis G, van Goor H. Induction of kidney injury molecule-1 in homozygous Ren2 rats is attenuated by blockade of the renin-angiotensin system or p38 MAP kinase. Am J Physiol Renal Physiol. 2007;292:F313–20. [DOI] [PubMed] [Google Scholar]
- 36. Recalcati S, Tacchini L, Alberghini A, Conte D, Cairo G. Oxidative stress-mediated down-regulation of rat hydroxyacid oxidase 1, a liver-specific peroxisomal enzyme. Hepatology. 2003;38:1159–66. [DOI] [PubMed] [Google Scholar]
- 37. Murray MS, Holmes RP, Lowther WT. Active site and loop 4 movements within human glycolate oxidase: implications for substrate specificity and drug design. Biochemistry. 2008;47:2439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wevers BA, Hoek L. Renin–angiotensin system in human coronavirus pathogenesis. Future Virol. 2010;5:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reguera J, Santiago C, Mudgal G, Ordoño D, Enjuanes L, Casasnovas JM. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8:e1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koenig JB, Jaffe IZ. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep. 2014;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan P, Fu H, Zhang L, Huang H, Luo F, Wu W, Guo Y, Liu X. Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol. 2010;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Carmeliet P, Ehsan A, Mendelsohn ME. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest. 2010;120:3891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McShane L, Tabas I, Lemke G, Kurowska-Stolarska M, Maffia P. TAM receptors in cardiovascular disease. Cardiovasc Res. 2019;115:1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roldán V, Marín F, Lip GYH, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007–20. [DOI] [PubMed] [Google Scholar]
- 46. Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304:H1585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kristiansson A, Gram M, Flygare J, Hansson SR, Åkerström B, Storry JR. The role of α1-microglobulin (A1M) in erythropoiesis and erythrocyte homeostasis—therapeutic opportunities in hemolytic conditions. Int J Mol Sci. 2020;21:7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mattisson IY, Björkbacka H, Wigren M, Edsfeldt A, Melander O, Fredrikson GN, Bengtsson E, Gonçalves I, Orho-Melander M, Engström Get al. Elevated markers of death receptor-activated apoptosis are associated with increased risk for development of diabetes and cardiovascular disease. EBioMedicine. 2017;26:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aminian A, Fathalizadeh A, Tu C, Butsch WS, Pantalone KM, Griebeler ML, Kashyap SR, Rosenthal RJ, Burguera B, Nissen SE. Association of prior metabolic and bariatric surgery with severity of coronavirus disease 2019 (COVID-19) in patients with obesity. Surg Obes Relat Dis. 2021;17:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in the article, code book, and analytic code will be made available upon reasonable request.