ABSTRACT
Background
A growing body of literature suggests chronically higher bile acid (BA) concentrations may be associated with multiple health conditions. Diet may affect BA metabolism and signaling; however, evidence from human populations is lacking.
Objectives
We systematically investigated cross-sectional associations of a priori–selected dietary components (fiber, alcohol, coffee, fat) with circulating BA concentrations.
Methods
We used targeted, quantitative LC-MS/MS panels to measure 15 circulating BAs in a subset of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC; n = 2224) and Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO; n = 986) comprising Finnish male smokers and United States men and women, respectively. We used multivariable linear regression to estimate associations of each dietary component with log-transformed BAs; exponentiated coefficients estimate proportional differences. We included the median of the dietary component quartile in linear regression models to test for trend.
Results
In ATBC, fiber was inversely associated with multiple circulating BAs. The proportional difference was –10.09% (95% CI: −19.29 to 0.16; P-trend = 0.04) when comparing total BAs among those in the highest relative to the lowest fiber quartile. Alcohol, trans fat, and polyunsaturated fat were positively associated with BAs in ATBC. The proportional difference comparing total BAs among those in the highest relative to the lowest alcohol quartile was 8.76% (95% CI: –3.10 to 22.06; P-trend = 0.03). Coffee and monounsaturated fat were inversely associated with BAs. The proportional difference comparing total BAs among those in the highest relative to the lowest coffee quartile was –24.03% (95% CI: –31.57 to –15.66; P-trend < 0.0001). In PLCO, no dietary components were associated with BAs except fiber, which was inversely associated with tauroursodeoxycholic acid.
Conclusions
Alcohol, coffee, certain fat subtypes, and fiber were associated with circulating concentrations of multiple BAs among Finnish male smokers. Given the potential role of BAs in disease risk, further investigation of the effects of diet on BAs in humans is warranted.
Keywords: bile acids, food frequency questionnaire, diet history questionnaire, fiber, fat, coffee, alcohol
Introduction
Bile acids (BAs) are important cholesterol-derived signaling molecules that regulate a wide range of important physiological processes, including cholesterol, lipid, and glucose metabolisms (1, 2). Chronically higher levels of certain BAs may contribute to disease risks, including for colorectal and liver cancer (3–7) and for chronic liver disease (5). For example, several circulating conjugated primary and secondary BAs were strongly, positively associated with a higher colon cancer risk in a recent prospective study nested in the European Prospective Investigation into Cancer and Nutrition cohort (3). Furthermore, there is strong experimental evidence supporting secondary BAs, which are derived via the gut microbiota metabolism of primary BAs that escape enterohepatic circulation, as being strongly proinflammatory and procarcinogenic in the colon (7, 8).
A number of animal and human studies suggest that certain dietary constituents—particularly fiber, alcohol, coffee, and fat—may be associated with BA concentrations (2, 8–13). Dietary fiber was previously demonstrated to bind BAs and promote their excretion in stool (14, 15), whereas following consumption of fat, hepatocytes are stimulated to secrete BAs to regulate solubilization and absorption of lipids (2, 8, 9). Coffee was shown to suppress BA synthesis in animal studies through the action of its antioxidant constituents (10, 11), whereas alcohol was shown to stimulate BA synthesis via receptors such as cannabinoid receptor type 1 (Cb1r) and Crebh (12, 13). Collectively, these dietary components may represent important exposures that can be targeted for BA-mediated cancer and other chronic disease prevention. However, population-based studies investigating the associations of usual intakes of a priori–defined dietary components with circulating BAs are lacking.
To build upon the existing literature, we comprehensively investigated the association of a priori–selected dietary components with quantitative BA concentrations in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC).
Methods
Study population: ATBC
The ATBC, described previously (16), enrolled over 29,000 men aged 50–69 y who smoked ≥5 cigarettes/d in Finland from 1985 to 1988. The ATBC trial was registered at clinicaltrials.gov as NCT00342992. Written informed consent was obtained from all ATBC participants, and the study was approved by the Institutional Review Boards at both the National Cancer Institute and the Finnish National Public Health Institute.
The cross-sectional investigation in ATBC was conducted using pooled data from 4 nested case-control studies of liver cancer (247 cases/247 controls), biliary cancer (92 cases/92 controls), liver disease death (261 cases/261 controls), and colorectal cancer (CRC; 601 cases/601 controls). Controls were individually matched to cases based on the age at randomization (±5 y), baseline serum draw date (±30 d), and fasting status (<8 or ≥8 h fasting). From the pooled, nested case-control studies, we excluded those without valid completed FFQs (n = 161), those with estimated energy intakes greater than 2 IQRs outside the 25th and 75th percentiles of total energy intake (258.8 ≤ kcal/d ≤ 5027.2; n = 16), and those with missing BMI data (n = 1). The final sample size in ATBC was 2224 cases (Supplemental Figure 1).
At baseline, men self-reported general demographic characteristics, and their medical and smoking histories. The baseline diet was assessed in ATBC using a self-administered modified FFQ that assessed both the frequency of consumption over the previous year and the usual portion size of 276 food/beverage items (including beer, spirits, liquor, coffee, and mixed dishes), and included a picture booklet of 122 foods for portion size estimation (17). Estimated intakes of fat, fiber, and their subtypes were summed across food sources using the software and food composition database at the Finnish National Public Health Institute (18–20). In a reproducibility study among 121 men in ATBC with dietary measurements taken 3 mo apart, the intraclass correlation coefficients were 0.64, 0.73, and 0.88 for total fat, total fiber, and alcohol intakes, respectively. In a validity study among 168 men in ATBC, the Pearson correlations between total fat, total fiber, alcohol, and coffee intakes reported on the first administered dietary questionnaire and 24-d food recalls were 0.39, 0.72, 0.80, and 0.72, respectively (17).
Study population: PLCO
The PLCO is a multicenter, randomized screening trial of prostate, lung, colorectal, and ovarian cancers that was conducted among >155,000 US men and women aged 55–74 from 1993 to 2001 (21). Participants had no history of prostate, lung, colorectal, or ovarian cancer and were randomly assigned to a screening or control arm. The current study was conducted with data from the screening arm of the trial (n = 77,445). The PLCO was registered at clinicaltrials.gov as NCT00339495. The study was approved by the Institutional Review Boards at the National Cancer Institute and the 10 PLCO screening centers. All PLCO participants provided written informed consent.
The cross-sectional investigation in PLCO was conducted among participants selected for a nested case-control study of CRC (505 cases/505 controls). Controls were individually matched to cases based on the age at randomization (±5 y), sex, race, year of randomization, and season of blood draw. From the total nested case-control study, we excluded those without valid dietary questionnaires (DQx) with <8 questions missing, participants with estimated energy intakes in the highest or lowest 1% based on the sex-specific distribution (n = 15), and those with missing BMI data (n = 9). The final sample size in PLCO was 986 cases (Supplemental Figure 1).
At baseline, participants completed a risk-factor questionnaire assessing demographic characteristics, health-related behaviors, medical history, and other cancer risk factors. Participants in the screening intervention arm completed a 137-item FFQ, the PLCO DQx, assessing dietary intake over the past year (22–24). The DQx was similar in format to 2 FFQs that were previously validated (25, 26). A standard portion size and 10 possible frequency-of-consumption values were given, ranging from “never” to “2+ times/day,” with typical portion sizes assessed for 60 of the 137 items. The values of foods, beverages, and nutrients in grams were sex-specifically derived based on the participants’ responses and their reported serving sizes. Fat and fat subtype intakes were calculated by summing fats from food sources using the database from the USDA's 1994–1996 Continuing Survey of Food Intakes by Individuals (27). Fiber and fiber subtypes were calculated by summing fiber from food sources using the Nutrition Data System for Research (28).
BA assessment
BA concentrations in baseline serum samples, which were generally fasted in ATBC and nonfasted in PLCO, were analyzed by Metabolon using targeted assays for 15 BAs, including primary and secondary BAs and their respective glycine and taurine conjugates. In brief, serum samples were spiked with a solution of labeled internal standards for each of the BAs and were subjected to protein precipitation with acidified methanol. The samples were then centrifuged (2773 × g, 5 min, at room temperature), and a portion of the clear supernatant was evaporated to dryness in a gentle stream of nitrogen at 40°C. The dried extract was then reconstituted, and an aliquot was injected onto an Agilent 1290/Sciex QTrap 6500 LC-MS/MS system equipped with a C18 reverse-phase HPLC column with acquisition in negative ion mode. The peak area of each parent or product ion was measured against the peak area of the respective internal standard parent or product ion. Quantitation was then performed using a least squares regression analysis generated from fortified calibration standards prepared immediately prior to each run.
In both studies, serum samples from matched cases and controls were included adjacently in the same batch in random order. Interbatch CVs were estimated using replicate samples included with the ATBC and PLCO samples. For ATBC, 14 of the 15 BAs were quantifiable in 95% of quality controls (QCs). Interbatch CVs ranged from 3.4% for glycodeoxycholic acid to 15.4% for lithocholic acid (median = 4.4%). For PLCO, median CVs for the 10 of 15 BAs that were quantifiable in a sufficient number of QC samples ranged from 2.7% for chenodeoxycholic acid to 28.5% for glycochenodeoxycholic acid (median = 7.3%).
Values for BA concentrations below the limit of detection were replaced by the lowest detected value for that metabolite, divided by 2. We created summary BA scores for broader BA groups, including total, primary, secondary, glycine-conjugated, and taurine-conjugated groups. To do this, we summed the individual BA values and transformed the BA score by the natural logarithm.
Statistical analysis
We used the residual method to energy adjust nutrient intakes of fat, fiber, and their respective subtypes (29). Based on the distributions among controls, we calculated quartiles for alcohol and coffee intakes and energy-adjusted grams of fat, fiber, and fat and fiber subtypes. The quartiles were based on the study-specific distribution in ATBC and sex-specific distribution in PLCO.
The characteristics of the study populations were summarized and compared across study-specific quartiles of the total BA summary score, using chi-square tests for categorical variables, ANOVAs for normally distributed variables, or nonparametric Kruskal-Wallis tests for nonnormally distributed variables. We also estimated Pearson correlations between the natural logarithm–transformed individual BAs and plotted the correlations using the R package “corrplot” (30).
Next, we estimated the association of selected dietary components with log-transformed BA concentrations or scores. To do this, we fit this linear regression model:
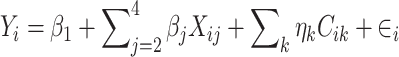 |
(1) |
Here, Yi is the log-transformed BA; Xi2, Xi3, and Xi4 are binary variables indicating the dietary quartile (e.g., Xi2 = 1 if the individual is in the second quartile); and Ci is a vector of covariates. Then, we approximated the proportional differences compared to the referent category as:
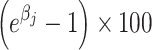 |
(2) |
That is, if 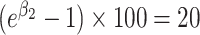
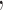 then individuals in the second quartile had approximately 20% higher BA concentrations. To formally test for a linear trend, we fit this linear regression model:
then individuals in the second quartile had approximately 20% higher BA concentrations. To formally test for a linear trend, we fit this linear regression model:
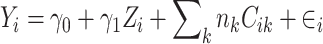 |
(3) |
Here, Zi is a continuous measure of the dietary component; we created a new variable (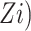 such that if an individual was in the kth gender/study-specific quartile for a dietary component, then their assigned value was the median of that quartile. We tested for a trend by considering the Wald-based P value (P-trend) for γ1
such that if an individual was in the kth gender/study-specific quartile for a dietary component, then their assigned value was the median of that quartile. We tested for a trend by considering the Wald-based P value (P-trend) for γ1 Combined and sex-stratified analyses were conducted with PLCO data.
Combined and sex-stratified analyses were conducted with PLCO data.
We selected covariates for the multivariable linear regression models based on the previous literature and biological plausibility. The covariates considered included future case status, age at baseline, sex (in PLCO only), total cigarettes smoked/d, total duration of smoking (y), physical activity level (none, moderate, or vigorous), BMI (kg/m2), level of education, and total energy intake (kcal/d). To assess the independent diet–BA associations, we also considered adjustment for coffee intake (servings/d), alcohol intake (drinks/d), total fat intake (energy residual-adjusted g/d), and total fiber intake (energy residual-adjusted g/d) in models where the dietary component covariate was not the primary exposure of interest in full or in part (e.g., we did not include total fat as a covariate in models for trans fat). We also conducted analyses stratified by future case status, age, and BMI category and, in PLCO, by sex and smoking status. We used the likelihood ratio test to calculate P values for interactions.
To test the sensitivity of our findings to various assumptions, we repeated the analyses 1) among controls only; 2) among daily drinkers of coffee and alcohol in PLCO, since intakes were much lower than in ATBC; 3) when excluding those who exited the cohort within 5, 10, and 15 y from baseline; and 4) when excluding those with <8 h of fasting in ATBC.
All analyses were conducted using R, version 3.6.1. All statistical tests were 2-sided, and all P values were corrected for multiple testing with false discovery rate (FDR) correction using the Benjamini-Hochberg method based on all possible tests in each study population. We considered FDR-corrected P values < 0.05 to be statistically significant.
Results
Characteristics of the participants in ATBC and PLCO are shown in Table 1. In ATBC, on average, those in the highest quartile of total BAs were older, had higher alcohol intake, and consumed less protein, carbohydrates, coffee, and fiber as compared to those in the lowest quartile. They were also more likely to have developed liver cancer or to have died from liver disease and were less likely to be a future CRC case. In PLCO, those in the highest quartile of total BAs trended toward higher BMIs. In both studies, on average, the summary scores for subtypes of BAs (primary, secondary, and glycine- and taurine-conjugated) were higher in the highest relative to lowest total BA quartile.
TABLE 1.
Characteristics of the 2224 participants in ATBC and 986 participants in PLCO
| Total BA score2 | |||||
|---|---|---|---|---|---|
| Study; characteristic1 | Quantile 1 | Quantile 2 | Quantile 3 | Quantile 4 | P 3 |
| ATBC | |||||
| n | 465 | 519 | 560 | 680 | |
| Total BA range, ng/mL | 52.05–378 | 277–640 | 447–1263 | 913–57,116 | |
| Future case type | — | — | — | — | 0.002 |
| Liver cancer, n (%) | 81 (17.4) | 91 (17.5) | 117 (20.9) | 172 (25.3) | |
| Biliary cancer, n (%) | 35 (7.5) | 41 (7.9) | 54 (9.6) | 46 (6.8) | |
| Liver disease death, n (%) | 90 (19.4) | 104 (20.0) | 116 (20.7) | 158 (23.2) | |
| Colorectal cancer, n (%) | 259 (55.7) | 283 (54.5) | 273 (48.8) | 304 (44.7) | |
| Demographics | |||||
| Age at baseline | 55.72 (4.43) | 56.34 (4.64) | 56.55 (4.87) | 57.01 (4.73) | <0.001 |
| More than some high school education, n (%) | 366 (78.7) | 405 (78.0) | 429 (76.6) | 504 (74.1) | 0.25 |
| Lifestyle characteristics | |||||
| Cigarettes/d, n (%) | 19.33 (7.82) | 19.45 (8.50) | 20.15 (8.94) | 20.55 (9.28) | 0.06 |
| Duration of smoking, y | 33.80 (8.25) | 34.74 (8.39) | 35.04 (8.40) | 35.58 (8.69) | 0.01 |
| Heavy leisure time exerciser, n (%) | 29 (6.2) | 40 (7.7) | 25 (4.5) | 32 (4.7) | 0.07 |
| BMI, kg/m2 | 26.18 (3.50) | 26.53 (3.62) | 26.65 (3.97) | 26.65 (3.89) | 0.15 |
| Dietary intake | |||||
| Energy intake, kcal/d | 2716 (689) | 2695 (716) | 2668 (667) | 2624 (731) | 0.13 |
| Cups coffee/d | 2.72 (1.46) | 2.54 (1.42) | 2.36 (1.36) | 2.11 (1.44) | <0.001 |
| Alcoholic drinks/d | 1.29 (1.40) | 1.38 (1.57) | 1.62 (1.71) | 1.92 (2.28) | <0.001 |
| % energy, fat | 40.42 (4.92) | 39.97 (5.41) | 40.19 (5.49) | 39.56 (6.30) | 0.06 |
| % energy, protein | 14.57 (1.89) | 14.53 (2.09) | 14.57 (1.99) | 14.10 (2.15) | <0.001 |
| % energy, carbohydrates | 40.82 (5.25) | 40.84 (5.70) | 39.79 (5.52) | 39.60 (5.90) | <0.001 |
| Saturated fat/1000 kcal/d | 19.21 (4.59) | 18.82 (4.93) | 18.66 (4.94) | 18.39 (5.00) | 0.04 |
| Trans fat/1000 kcal/d | 1.33 (0.69) | 1.32 (0.72) | 1.37 (0.74) | 1.39 (0.78) | 0.39 |
| Polyunsaturated fat/1000 kcal/d | 4.52 (2.20) | 4.55 (2.21) | 4.65 (2.33) | 4.72 (2.46) | 0.43 |
| Monounsaturated fat/1000 kcal/d | 12.21 (1.63) | 12.02 (1.74) | 12.11 (1.77) | 11.88 (1.98) | 0.01 |
| Fiber, g/1000 kcal/d | 7.19 (3.37) | 7.32 (3.45) | 6.97 (3.23) | 6.65 (3.47) | 0.004 |
| Insoluble fiber, g/1000 kcal/d | 4.30 (1.46) | 4.35 (1.48) | 4.20 (1.40) | 4.02 (1.52) | 0.001 |
| Soluble fiber, g/1000 kcal/d | 2.19 (0.60) | 2.20 (0.64) | 2.13 (0.58) | 2.05 (0.63) | <0.001 |
| BAs4 | |||||
| Primary BAs, ng/mL | 33.92 (30.14) | 75.53 (67.95) | 173 (149) | 681 (1044) | <0.001 |
| Secondary BAs, ng/mL | 54.36 (40.12) | 95.76 (61.91) | 135 (87.23) | 284 (276) | <0.001 |
| Glycine-conjugated BAs, ng/mL | 101 (46.89) | 208 (84.75) | 374 (171) | 1327 (2420) | <0.001 |
| Taurine-conjugated BAs, ng/mL | 20.33 (12.88) | 32.37 (23.96) | 52.58 (42.38) | 326.56 (1365) | <0.001 |
| PLCO | |||||
| n | 218 | 256 | 255 | 257 | |
| Total BA range, ng/mL | 13.19–369 | 318–747 | 615–1470 | 1250–14,151 | |
| Colorectal cancer case, n (%) | 94 (43.1) | 132 (51.6) | 133 (52.2) | 135 (52.5) | 0.14 |
| Demographics | |||||
| Age at baseline | 64.06 (5.12) | 63.85 (5.05) | 64.36 (5.18) | 64.18 (5.52) | 0.73 |
| Male, n (%) | 121 (55.5) | 147 (57.4) | 127 (49.8) | 126 (49.0) | 0.16 |
| College graduate or more, n (%) | 82 (37.6) | 88 (34.4) | 79 (31.0) | 105 (40.9) | 0.11 |
| Lifestyle characteristics | |||||
| Never smoker, n (%) | 111 (50.9) | 108 (42.2) | 121 (47.5) | 113 (44.0) | 0.08 |
| Vigorous activity >3 h/wk, n (%) | 97 (44.5) | 97 (37.9) | 91 (35.7) | 103 (40.1) | 0.25 |
| BMI, kg/m2 | 26.97 (4.60) | 27.44 (5.06) | 27.37 (4.58) | 28.12 (5.12) | 0.07 |
| Dietary intake | |||||
| Energy intake, kcal/d | 1948 (709) | 2049 (849) | 2067 (831) | 2043 (780) | 0.38 |
| Cups coffee/d | 1.76 (1.64) | 2.11 (1.93) | 1.90 (1.90) | 1.91 (1.91) | 0.23 |
| Alcoholic drinks/d | 0.95 (1.57) | 0.80 (1.55) | 0.87 (1.73) | 0.88 (1.66) | 0.79 |
| % energy, fat | 27.85 (5.77) | 29.66 (6.20) | 28.70 (6.06) | 28.83 (6.47) | 0.02 |
| % energy, protein | 15.43 (2.26) | 16.10 (2.62) | 15.46 (2.62) | 15.58 (2.51) | 0.01 |
| % energy, carbohydrates | 55.00 (8.21) | 53.26 (8.91) | 54.58 (8.86) | 54.34 (8.41) | 0.14 |
| Saturated fat/1000 kcal/d | 10.51 (2.78) | 11.20 (2.95) | 10.79 (2.93) | 10.93 (2.97) | 0.07 |
| Trans fat/1000 kcal/d | 2.66 (0.64) | 2.81 (0.67) | 2.80 (0.71) | 2.78 (0.73) | 0.07 |
| Monounsaturated fat/1000 kcal/d | 11.52 (2.63) | 12.38 (2.88) | 11.96 (2.73) | 11.98 (2.95) | 0.01 |
| Polyunsaturated fat/1000 kcal/d | 6.36 (1.42) | 6.67 (1.40) | 6.51 (1.51) | 6.47 (1.56) | 0.14 |
| Fiber, g/1000 kcal/d | 12.27 (3.63) | 11.49 (3.20) | 11.71 (3.59) | 11.65 (3.23) | 0.08 |
| Insoluble fiber, g/1000 kcal/d | 7.92 (2.63) | 7.33 (2.23) | 7.52 (2.53) | 7.49 (2.28) | 0.06 |
| Soluble fiber, g/1000 kcal/d | 4.25 (1.10) | 4.06 (1.06) | 4.09 (1.18) | 4.06 (1.05) | 0.18 |
| BAs4 | |||||
| Primary BAs, ng/mL | 17.54 (21.82) | 53.36 (69.25) | 112 (133) | 377 (824) | <0.001 |
| Secondary BAs, ng/mL | 47.59 (45.46) | 99.50 (75.33) | 161 (110) | 314 (328) | <0.001 |
| Glycine-conjugated BAs, ng/mL | 127 (64.72) | 304 (114) | 636 (229) | 1723 (1367) | <0.001 |
| Taurine-conjugated BAs, ng/mL | 15.94 (15.76) | 43.82 (32.80) | 89.16 (61.40) | 312 (406) | <0.001 |
Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
Presented as means (SDs) unless otherwise specified.
The total BA score is a sum of values for cholic acid, chenodeoxycholic acid, deoxycholic acid, lithocholic acid, ursodeoxycholic acid, glycocholic acid, glycochenodeoxycholic acid, glycodeoxycholic acid, glycolithocholic acid, glycoursodeoxycholic acid, taurocholic acid, taurochenodeoxycholic acid, taurodeoxycholic acid, taurolithocholic acid, and tauroursodeoxycholic acid.
The P values were calculated using a χ2 test for categorical variables, an ANOVA for normally distributed continuous variables, and a nonparametric Kruskal-Wallis test for continuous variables with nonnormal distributions.
The primary BA scores were a sum of values for cholic acid and chenodeoxycholic acid; the secondary BA scores were a sum of values for deoxycholic acid, lithocholic acid, and ursodeoxycholic acid; the glycine-conjugated BA scores were a sum of z scores for glycocholic acid, glycochenodeoxycholic acid, glycodeoxycholic acid, glycolithocholic acid, and glycoursodeoxycholic acid; and the taurine-conjugated BA scores were a sum of values for taurocholic acid, taurochenodeoxycholic acid, taurodeoxycholic acid, taurolithocholic acid, and tauroursodeoxycholic acid.
The medians and IQRs of dietary components and BA concentrations in ATBC and PLCO are shown in Table 2. There were some study population differences in the distributions of estimated daily coffee, alcohol, fat, and fiber intakes. For example, coffee and alcohol intakes were higher, on average, in ATBC than in PLCO. The distribution of BAs was similar between the populations, with the exception that concentrations of cholic acid, chenodeoxycholic, and ursodeoxycholic were higher and concentrations of glycocholic acid, glycodeoxycholic acid, and glycochenodeoxycholic acid were lower in ATBC.
TABLE 2.
Medians and IQRs of dietary components and BAs among the 2224 participants in ATBC and 986 participants in PLCO
| Study | |||
|---|---|---|---|
| PLCO | |||
| ATBC | Men | Women | |
| Dietary components | |||
| Coffee, cups/d | 2.32 (1.39–3.16) | 1.00 (0.79–2.50) | 1.00 (0.43–2.50) |
| Alcoholic, drinks/d | 1.04 (0.33–2.13) | 0.40 (0.03–1.69) | 0.04 (0.00–0.43) |
| % kcal, fat | 40.09 (36.64–43.81) | 33.72 (28.91–37.91) | 30.10 (26.22–34.78) |
| Saturated fat/1000 kcal/d | 18.50 (15.02–22.27) | 11.26 (9.28–13.22) | 9.98 (8.44–12.09) |
| Trans fat/1000 kcal/d | 1.13 (0.88–1.58) | 2.81 (2.39–3.23) | 2.63 (2.22–3.10) |
| Monounsaturated fat/1000 kcal/d | 12.04 (10.89–13.20) | 12.75 (10.84–14.59) | 11.20 (9.57–12.83) |
| Polyunsaturated fat/1000 kcal/d | 0.80 (0.63–1.01) | 6.49 (5.54–7.61) | 6.26 (5.41–7.17) |
| Fiber/1000 kcal/d | 6.63 (4.67–8.92) | 10.58 (8.91–12.55) | 12.24 (10.24–14.62) |
| Insoluble fiber/1000 kcal/d | 4.02 (3.17–5.03) | 6.68 (5.53–8.16) | 7.82 (6.41–9.56) |
| Soluble fiber/1000 kcal/d | 2.07 (1.71–2.50) | 3.74 (3.24–4.38) | 4.35 (3.61–5.02) |
| BAs | |||
| Cholic acid, ng/mL | 28.40 (12.00–105) | 10.70 (4.12–37.05) | 8.33 (3.19–22.55) |
| Chenodeoxycholic acid, ng/mL | 56.70 (22.50–158) | 34.90 (10.04–94.25) | 21.50 (7.32–72.45) |
| Deoxycholic acid, ng/mL | 88.7 (45.1–160) | 88.65 (35.58–181) | 90.15 (41.27–171) |
| Lithocholic acid, ng/mL | 4.78 (3.51–7.16) | 3.54 (1.50–7.70) | 3.42 (1.54–6.85) |
| Ursodeoxycholic acid, ng/mL | 18.80 (9.81–40.80) | 8.10 (3.33–23.47) | 7.34 (3.04–21.20) |
| Glycocholic acid, ng/mL | 35.80 (18.00–78.35) | 66.80 (30.40–141) | 60.40 (24.80–148) |
| Glycochenodeoxycholic acid, ng/mL | 157 (79.47–327) | 234 (109–484) | 190 (90.80–401) |
| Glycodeoxycholic acid, ng/mL | 39.5 (18.7–83.55) | 83.55 (38.80–204.25) | 95.70 (38.60–218) |
| Glycolithocholic acid, ng/mL | 5.03 (3.40–9.22) | 4.48 (1.39–12.10) | 4.62 (1.55–11.30) |
| Glycoursodeoxycholic acid, ng/mL | 27.60 (13.30–57.60) | 22.25 (9.62–54.68) | 21.40 (8.20–53.60) |
| Taurocholic acid, ng/mL | 7.88 (4.39–16.80) | 6.36 (2.22–16.70) | 8.97 (3.21–25.50) |
| Taurochenodeoxycholic acid, ng/mL | 21.4 (11.7–45.05) | 25.70 (9.54–59.90) | 27.50 (12.20–74.90) |
| Taurodeoxycholic acid, ng/mL | 12.7 (7.77–23.90) | 10.70 (4.33–27.20) | 17.45 (5.67–46.53) |
| Taurolithocholic acid, ng/mL | 3.65 (2.95–5.64) | 1.47 (0.42–3.08) | 1.25 (0.46–2.93) |
| Tauroursodeoxycholic acid, ng/mL | 4.38 (3.17–7.05) | 1.20 (0.43–2.91) | 1.21 (0.39–4.24) |
Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
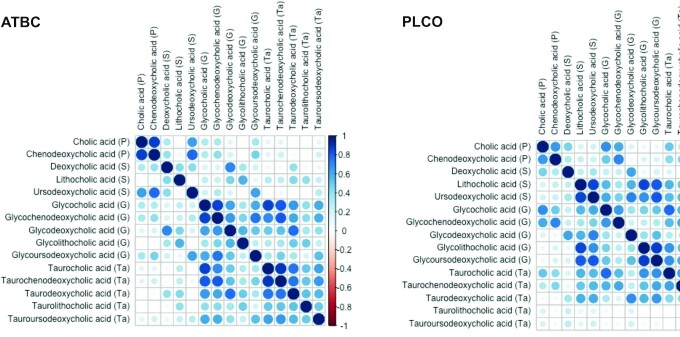
As shown in Figure 1, the BAs had similar patterns of correlations in ATBC and PLCO. The primary BAs, cholic and chenodeoxycholic acid, were strongly correlated with each other in both populations [R = 0.87 (P < 0.0001) in ATBC; R = 0.77 (P < 0.0001) in PLCO]. Most glycine-conjugated BAs were strongly correlated with their taurine-conjugated counterparts.
FIGURE 1.
Pearson correlations among BAs, measured among 2224 participants in ATBC and 986 participants in PLCO. The color intensity and sizes of the circles are proportional to the Pearson correlation coefficients (blank boxes represent non–statistically significant Pearson correlations). Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; G, glycine-conjugated bile acid; P, primary bile acid; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; S, secondary bile acid; T, tertiary bile acid; Ta, taurine-conjugated bile acid.
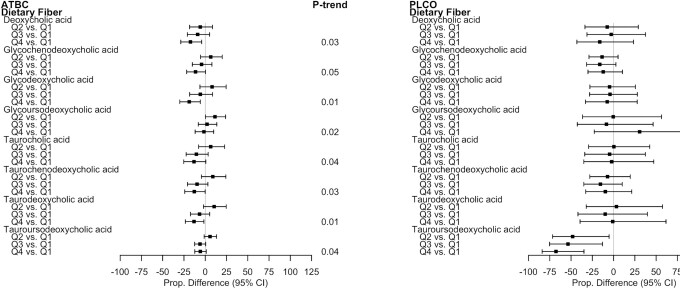
Fiber was inversely associated with concentrations of multiple secondary and glycine- and taurine-conjugated BAs in ATBC (Figure 2). For example, in ATBC, glycodeoxycholic acid and deoxycholic acid were 18.8% (95% CI: –29.91 to –5.82) and 17.4% (95% CI: –28.74 to –4.16) lower, respectively, among those in the highest relative to the lowest quartile of fiber (P-trends = 0.01 and 0.03, respectively; see Supplemental Tables 1and2 for ATBC adjusted mean estimates and all proportional differences for each diet component). In PLCO, there was a statistically significant, 67.7% lower mean of tauroursodeoxycholic acid for those in the highest relative to lowest quartile of fiber (95% CI: –84.01 to –34.80). The estimated fiber associations were similar among PLCO men and women. The directions and magnitudes of the associations for the summary BAs generally reflected those for the individual BAs in ATBC and in PLCO. For example, in ATBC, comparing those in the highest relative to the lowest quartile of fiber, there was a non–statistically significant proportional difference of –10.1% (95% CI: –19.29 to 0.16) in the total BA score (P-trend = 0.04) and there were statistically significant proportional differences of –14.4% (95% CI: –24.53 to –2.93) in the secondary BA score, –11.7% (95% CI: –21.48 to –0.73) in the glycine-conjugated BA score, and –12.0% (95% CI: –22.10 to –0.48) in the taurine-conjugated BA score (P-trends = 0.02, 0.03, and 0.02, respectively). The estimated associations for soluble and insoluble fibers with BAs were similar in both studies (Supplemental Tables 1 to 4).
FIGURE 2.
Proportional differences in circulating BAs by quartiles of fiber intake in ATBC (n = 2224) and PLCO (n = 986). All statistically significant findings (FDR P value < 0.05) are presented with the corresponding BAs in the other study population to facilitate interpretation. ATBC study linear regression models were adjusted for case status (colorectal cancer, liver disease, biliary cancer, liver cancer, or control), age at randomization, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 times/wk, 1–2 times/wk, or <1 time/wk), BMI (kg/m2), level of education (greater than elementary to part of junior high school or less than junior high school), total energy intake (kcal/d), coffee intake (drinks/d), fat intake (energy residual-adjusted g/d), and alcohol intake (drinks/d). PLCO linear regression models were adjusted for colorectal cancer case status, age at diet questionnaire completion, sex, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 h/wk, 1–2 h/wk, or <1 h/wk), BMI (kg/m2), level of education (high school education or less, some college or postcollege training, college graduate or more), total energy intake (kcal/d), coffee intake (drinks/d), fat intake (energy residual-adjusted g/d), and alcohol intake (drinks/d). The P-trends were corrected using FDR correction with the Benjamini-Hochberg method; dietary component quartiles were based on energy-adjusted dietary estimates, using a residual method. Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; FDR, false discovery rate; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; Q, quartile.
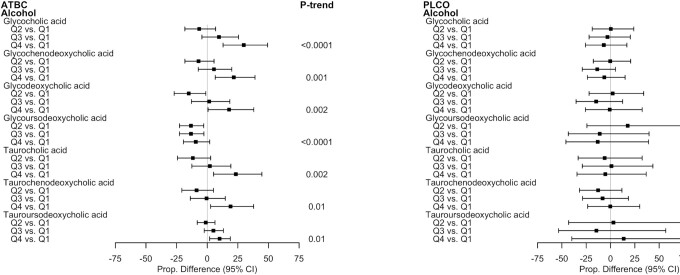
Consumption of alcoholic beverages was generally positively associated with concentrations of multiple glycine- and taurine-conjugated BAs in ATBC, but the associations were inconsistent and close to null in PLCO (Figure 3). Compared to those in the lowest quartile of alcohol intake in ATBC, the adjusted mean concentrations of all glycine- and taurine-conjugated primary BAs, glycodeoxycholic acid, and tauroursodeoxycholic acid were incrementally higher—and the concentrations of glycoursodeoxycholic acid were incrementally lower—with each higher quartile of alcohol intake (all P-trends ≤ 0.01). For example, men in the highest quartile of alcohol intake had statistically significant 29.8% (95% CI: 12.93–49.25), 21.7% (95% CI: 6.51–38.98), and 17.9% (95% CI: 0.65–37.99) higher glycocholic acid, glycochenodeoxycholic acid, and glycodeoxycholic acid concentrations, respectively, relative to those in the lowest quartile. Furthermore, alcoholic beverage consumption in ATBC was statistically significantly, positively associated with total BA, glycine-conjugated BA, and taurine-conjugated BA summary scores, reflective of the individual BA associations (Supplemental Tables 1 and 2). For example, the proportional difference comparing total BAs among those in the highest relative to lowest alcohol quartile was 8.76% (95% CI: –3.10 to 22.06; P-trend = 0.03).
FIGURE 3.
Proportional differences in circulating BAs by quartiles of alcohol intake in ATBC (n = 2224) and PLCO (n = 986). All statistically significant findings (FDR P value < 0.05) are presented with the corresponding BAs in the other study population to facilitate interpretation. ATBC study linear regression models were adjusted for case status (colorectal cancer, liver disease, biliary cancer, liver cancer, or control), age at randomization, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 times/wk, 1–2 times/wk, or <1 time/wk), BMI (kg/m2), level of education (greater than elementary to part of junior high school or less than junior high school), total energy intake (kcal/d), coffee intake (drinks/d), fat intake (energy residual-adjusted g/d), and fiber intake (energy residual-adjusted g/d). PLCO linear regression models were adjusted for colorectal cancer case status, age at diet questionnaire completion, sex, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 h/wk, 1–2 h/wk, or <1 h/wk), BMI (kg/m2), level of education (high school education or less, some college or postcollege training, college graduate or more), total energy intake (kcal/d), coffee intake (drinks/d), fat intake (energy residual-adjusted g/d), and fiber intake (energy residual-adjusted g/d). The P-trends were corrected using FDR correction with the Benjamini-Hochberg method. Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; FDR, false discovery rate; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; Q, quartile.
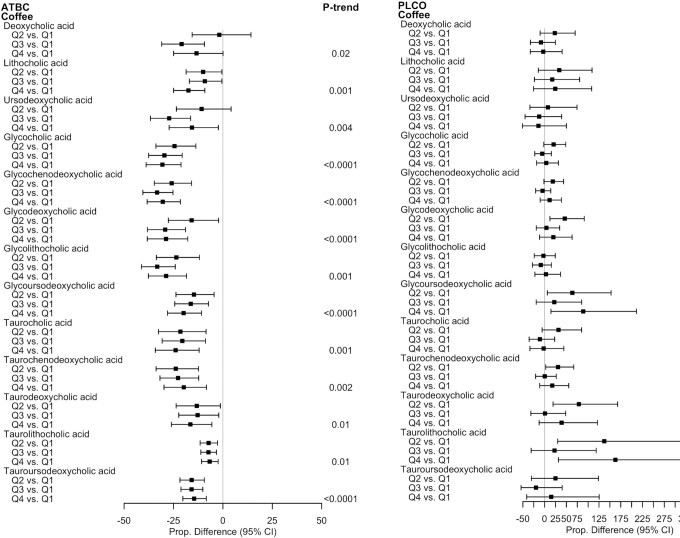
In ATBC, coffee consumption was strongly, inversely associated with concentrations of all secondary BAs and multiple glycine- and taurine-conjugated primary and secondary BAs; however, in PLCO, the associations were unstable and/or close to null (Figure 4). Coffee intake in ATBC was most strongly, inversely associated with glycine-conjugated BAs; relative to those in the lowest quartile, those in the highest quartile had statistically significant 30.6% (95% CI: –38.86 to –21.21) and 30.5% (95% CI: –38.34 to –21.54) lower mean concentrations of glycocholic and glycochenodeoxycholic acid, respectively (P-trends < 0.0001), and 28.8% (95% CI: –38.29 to –17.78) and 28.7% (95% CI: –37.72 to –18.43) lower means of the glycine-conjugated secondary BAs glycodeoxycholic and glycolithocholic acid, respectively (P-trend < 0.0001 and P-trend = 0.001, respectively). For total BAs, the proportional difference when comparing those in the highest relative to the lowest coffee quartile was –24.03% (95% CI: –31.57 to –15.66; P-trend < 0.0001).
FIGURE 4.
Proportional differences in circulating BAs by quartiles of coffee intake in ATBC (n = 2224) and PLCO (n = 986). All statistically significant findings (FDR P value < 0.05) are presented with the corresponding BAs in the other study population to facilitate interpretation. ATBC study linear regression models were adjusted for case status (colorectal cancer, liver disease, biliary cancer, liver cancer, or control), age at randomization, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 times/wk, 1–2 times/wk, or <1 time/wk), BMI (kg/m2), level of education (less than elementary to part of junior high school or greater than junior high school), total energy intake (kcal/d), fat intake (energy residual-adjusted g/d), alcohol intake (drinks/d), and fiber intake (energy residual-adjusted g/d). PLCO linear regression models were adjusted for colorectal cancer case status, age at diet questionnaire completion, sex, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 h/wk, 1–2 h/wk, or <1 h/wk), BMI (kg/m2), level of education (high school education or less, some college or postcollege training, college graduate or more), total energy intake (kcal/d), fat intake (energy residual-adjusted g/d), alcohol intake (drinks/d), and fiber intake (energy residual-adjusted g/d). The P-trends were corrected using FDR correction with the Benjamini-Hochberg method. Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; FDR, false discovery rate; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; Q, quartile.
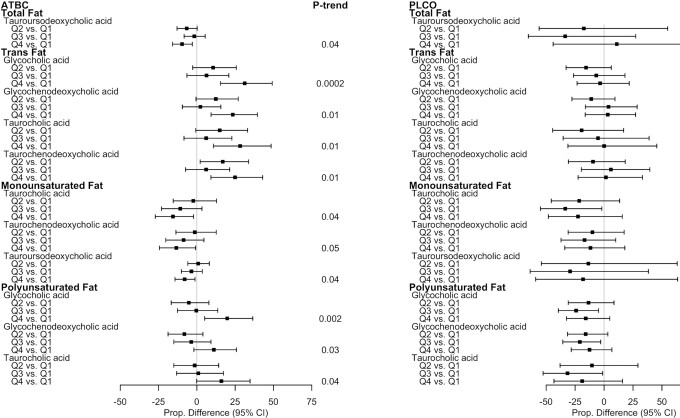
As shown in Figure 5, the findings for the associations of fat and fat subtypes with BAs were somewhat mixed in the ATBC study and were inconsistent and weak in the PLCO study. Trans fats were positively associated with glycine- and taurine-conjugated primary BAs in ATBC, but the corresponding associations in PLCO were close to null. Monounsaturated fats were weakly to moderately inversely associated with taurine-conjugated BAs in ATBC, and there were statistically significant 15.6% (95% CI: –27.17 to –2.18) and 8% (95% CI: –14.26 to –1.22) lower mean concentrations of taurocholic and tauroursodeoxycholic acids, respectively (both P-trends = 0.04) when comparing those in the highest relative to the lowest quartile. Polyunsaturated fats were moderately positively associated with glycine- and taurine-conjugated BAs in ATBC. For example, for those in the highest relative to the lowest quartile of polyunsaturated fats, mean glycocholic acid concentrations were statistically significantly 19.8% (95% CI: 5.13–36.50) higher (P-trend = 0.002). The saturated fat associations were inconsistent and weak in both studies.
FIGURE 5.
Proportional differences in circulating BAs by quartiles of fat and fat subtype intake in ATBC (n = 2224) and PLCO (n = 986). All statistically significant findings (FDR P value < 0.05) are presented with the corresponding BAs in the other study population to facilitate interpretation. ATBC study linear regression models were adjusted for case status (colorectal cancer, liver disease, biliary cancer, liver cancer, or control), age at randomization, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥3 times/wk, 1–2 times/wk, or <1 time/wk), BMI (kg/m2), level of education (less than elementary to part of junior high school or greater than junior high school), total energy intake (kcal/d), coffee intake (drinks/d), alcohol intake (drinks/d), and fiber intake (energy residual-adjusted g/d). PLCO linear regression models were adjusted for colorectal cancer case status, age at diet questionnaire completion, sex, total cigarettes smoked/d, total duration of smoking (y), physical activity level (≥ 3 h/wk, 1–2 h/wk, or <1 h/wk), BMI (kg/m2), level of education (high school education or less, some college or postcollege training, college graduate and more), total energy intake (kcal/d), coffee intake (drinks/d), alcohol intake (drinks/d), and fiber intake (energy residual-adjusted g/d). The P-trends were corrected using FDR correction with the Benjamini-Hochberg method. Dietary component quartiles were based on energy-adjusted dietary estimates, using a residual method. Abbreviations: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acid; FDR, false discovery rate; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; Q, quartile.
In our sensitivity analysis among daily alcohol drinkers in PLCO, alcohol consumption was statistically significantly, positively associated with glycoursodeoxycholic acid (proportional difference = 175.89%; 95% CI: 45.84–421.90; P-trend = 0.01), with the strongest associations among men, whereas the associations of coffee with BA concentrations among daily coffee drinkers were inconsistent and not statistically significant (Supplemental Table 5). There were no clear, consistent differences in the estimated associations of the dietary components with BAs by participant characteristics (all P-interactions > 0.05; Supplemental Table 6). In our sensitivity analyses among controls only in PLCO, we found that the associations were similar in inverse directions and magnitudes for fiber and tauroursodeoxycholic acid, and were similarly generally weak and/or close to null for all other dietary components (Supplemental Table 7). Among ATBC controls, the associations were in the same direction but were slightly weaker for alcohol, coffee, and monounsaturated fat, and were more similar for trans fat, polyunsaturated fat, and fiber. Finally, we found that excluding participants who were followed less than 5, 10, or 15 y or those with <8 h of fasting in ATBC minimally affected our findings (Supplemental Table 8).
Discussion
In a population of Finnish male smokers, we found that 1) alcohol, trans fat, and polyunsaturated fat intakes were positively associated with circulating BAs; and 2) coffee, monounsaturated fat, and fiber intakes were inversely associated with circulating BAs. The estimated associations were generally similar among highly correlated BAs, and were generally strongest for glycine- and taurine-conjugated BAs and some secondary BAs. Among a population of men and women in the United States, fiber was inversely associated with tauroursodeoxycholic acid, but no other ATBC findings were replicated. As discussed below, the study-specific differences in findings could be due, at least in part, to differences in fasting statuses, population demographics, geographic locations, and/or to the smaller sample size in PLCO. Nonetheless, notably, many of the diet-associated BAs in this study were previously associated with higher risks for colorectal neoplasms (3, 31, 32), liver disease mortality and cancer (5, 33, 34), and diabetes (35). These findings highlight that multiple dietary factors may influence circulating BA concentrations and, by extension, potential BA-mediated disease risks.
Accumulating evidence suggests that dietary fiber has multiple health benefits and is associated with lower risks for multiple chronic conditions and premature mortality (36). Accordingly, we found fiber was inversely associated with multiple conjugated and unconjugated BAs in ATBC and with tauroursodeoxycholic acid in PLCO. In in vitro studies, fiber was demonstrated to bind BAs, which in turn reduces BA reabsorption into the terminal ileum and promotes BA stool excretion (14, 15). Furthermore, diets high in fiber are associated with gut microbiota shifts away from a “bile-loving” composition (15, 37). In a clinical trial among 80 men and women randomized to a whole-grain or refined-grain diet, comprising 56 g or 25 g of fiber per day, respectively, the investigators found that the whole-grain diet increased plasma concentrations of taurocholic, glycocholic, and taurolithocholic acid (38), in contrast to our findings. The study differences may be due, at least in part, to an approximately 30-y difference in the average age between that study and ATBC/PLCO, a strict 12-h fasting status, and a more racially and ethnically diverse study population.
We found that alcohol was positively associated with concentrations of multiple glycine- and taurine-conjugated BAs in ATBC and in daily drinkers in PLCO. There is a strong biological plausibility that alcohol stimulates BA synthesis in humans, such as through activating hepatic Cb1r and Crebh (12) and interfering with gallbladder contraction, potentially leading to lower amounts of BAs being secreted into the duodenum (13). In a study of rats treated with ethanol, primary, secondary, and glycine-conjugated BAs were increased in the liver and gastrointestinal tract, but synthesis of taurine-conjugated BAs was suppressed. Of note, metabolisms of BAs among mice differ from those in humans, which may explain some of the conflicting findings (2, 39).
In ATBC, we found that coffee was strongly, inversely associated with multiple secondary and glycine- and taurine-conjugated BAs. Coffee contains cafestol, a compound found to decrease BA synthesis in rat hepatocytes (10) and in mice (11). In a cross-sectional investigation of the associations of untargeted metabolites with coffee consumption (n = 1664), coffee consumption was inversely associated with glycocholic acid, similar to our ATBC findings (40). Intriguingly, in an untargeted metabolomic platform investigation conducted among ATBC liver cancer and liver disease death cases and controls, overlapping with the data set discussed herein, coffee was similarly, inversely associated with glycocholic and glycochenodeoxycholic acids, which were in turn associated with higher risks for liver cancer and liver disease death (5). Continued investigation into the associations of coffee with BAs may lend novel insight into the mechanisms underlying the strong, inverse associations of coffee with disease (41–43).
In alignment with multiple animal and human studies, we found that fat and some of its subtypes were associated with BA concentrations in ATBC. Dietary fat is mechanistically linked to the secretion and regulation of BAs and, following its consumption, hepatocytes are stimulated to secrete BAs into the duodenum to regulate solubilization and absorption of lipids (2, 8, 9). Consumption of animal-based, high-fat diets was previously associated with BA-resistant gut microbiota compositions in mice (44) and humans (37). For example, in a study of mice fed with milk-derived saturated fats, concentrations of taurine-conjugated BAs in gallbladder aspirates and abundances of “bile-loving” gut bacteria were increased (44).
Intriguingly, in ATBC, we found that trans and polyunsaturated fats were positively associated with BA concentrations, whereas monounsaturated fats were inversely associated with BA concentrations. The positive associations of trans fat with BAs were not surprising, given the strength of the evidence for positive associations of trans fat with LDL cholesterol (45) and inverse associations with HDL cholesterol (46, 47). In contrast, monounsaturated and polyunsaturated fats are generally thought to have beneficial, lipid-lowering properties (48–50). The positive polyunsaturated fat associations in ATBC may be explained in part by the predomination within polyunsaturated fat intakes of polyunsaturated omega-6 fatty acids, which increased generation of secondary BAs by activating bacterial 7-alpha-dehydroxylase in the colons of mice (51). Therefore, it is plausible that individual subtypes of fat may be differentially involved in BA synthesis and regulation; however, further investigation is needed.
Our study strengths include the quantitative, targeted BA platform, with high technical reproducibility and findings robust to multiple sensitivity analyses. Also, many previous studies only measured fecal BAs destined for excretion (37, 52–59). As compared with BAs measured in blood, BAs excreted in fecal samples may be prone to measurement issues, such as confounding by the fecal transit time and other standardization issues, and may have different associations with human health (4). Our study also had limitations. First, we estimated the diet-BA associations in a population enriched with future liver cancer/disease, biliary cancer, and colorectal cancer cases, and underlying disease could have affected our findings. However, all individuals were cancer-free at the time of BA measurement and dietary questionnaire completion, the associations were similar among controls only, and excluding those who were followed less than 5, 10, or 15 y after baseline did not meaningfully affect our findings. Inherent to studying dietary associations are known limitations of dietary questionnaires (e.g., respondent error); however, both questionnaires were validated and performed reasonably well for the dietary components assessed in this study (17, 25, 26). Finally, we investigated associations of a number of metabolites and, although we adjusted for multiple comparisons, there may still be concerns about chance findings.
Finally, we observed strong diet-BA associations in ATBC, with limited replication in PLCO. Each study had individual strengths and limitations, some of which may explain, at least in part, the differences in associations. PLCO only had nonfasting blood samples, which could have influenced estimated BA concentrations (60); in contrast, most ATBC participants (90.1%) fasted for ≥8 h, although excluding those fasting <8 h did not affect our findings. The 2 study populations were also different in their sample sizes, potentially affecting statistical power, and were different demographically: ATBC was conducted among Finnish male smokers and PLCO was conducted among men and women in the United States. Our differences in study findings could also be explained by differences in dietary measurements and reporting, differences in food availability, and geographical differences in gut microbiota compositions (and thus, in BA regulation) (1).
In conclusion, our findings, taken together with previous literature, suggest that alcohol, coffee, fat, and fiber consumption may be associated with circulating BA levels among Finnish male smokers. Given the potential importance of BA signaling in disease, future studies of diet and BAs are merited. Studies with longitudinal dietary data, serial collections of fasted blood, and stool samples to capture etiologic activities of both circulating and excreted BAs are needed to further understand the associations of habitual diet with systemic BAs.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DAB and EL: designed the research and wrote the paper; DAB: performed the statistical analysis; RS, SJW, DA, JS, and NDF: contributed to the data analysis plan and manuscript preparation; EL: provided study oversight and had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Supplemental Figure 1 and Supplemental Tables 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; BA, bile acids; Cb1r, cannabinoid receptor type 1; CRC, colorectal cancer; DQx, dietary questionnaire; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; QC, quality control.
Contributor Information
Doratha A Byrd, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Department of Cancer Epidemiology, Division of Population Science, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA.
Rashmi Sinha, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Stephanie J Weinstein, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Demetrius Albanes, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Neal D Freedman, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Joshua Sampson, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Erikka Loftfield, Division of Cancer Epidemiology & Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending proposal approval and completion of a Data Transfer Agreement (see https://atbcstudy.cancer.gov/ptsa/). For PLCO, approval should be obtained from: https://cdas.cancer.gov/learn/plco/instructions/?type=data.
References
- 1. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. [DOI] [PubMed] [Google Scholar]
- 2. De Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kühn T, Stepien M, López-Nogueroles M, Machado AD, Sookthai D, Johnson T, Roca M, Hüsing A, Maldonado SG, Cross AJet al. Pre-diagnostic plasma bile acid levels and colon cancer risk: a prospective study. J Natl Cancer Inst. 2019;112:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costarelli V. Bile acids as possible human carcinogens: new tricks from an old dog. Int J Food Sci Nutr. 2009;60(Suppl 6):116–25. [DOI] [PubMed] [Google Scholar]
- 5. Loftfield E, Rothwell JA, Sinha R, Keski-Rahkonen P, Robinot N, Albanes D, Weinstein SJ, Derkach A, Sampson J, Scalbert Aet al. Prospective investigation of serum metabolites, coffee drinking, liver cancer incidence, and liver disease mortality. J Natl Cancer Inst. 2020;112(3):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72. [DOI] [PubMed] [Google Scholar]
- 7. O'Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ocvirk S, O'Keefe SJ. Influence of bile acids on colorectal cancer risk: potential mechanisms mediated by diet-gut microbiota interactions. Curr Nutr Rep. 2017;6(4):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolt HM, Marchan R, Hengstler JG. Bile acids as colon carcinogens and coffee ingredients as antagonists. Arch Toxicol. 2011;85(8):859–60. [DOI] [PubMed] [Google Scholar]
- 10. Post SM, De Wit ECM, Princen HMG. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7α-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler Thromb Vasc Biol. 1997;17(11):3064–70. [DOI] [PubMed] [Google Scholar]
- 11. Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld G, Moen CJA, Müller M, Frants RR, Kasanmoentalib S, Post SM, Princen HMGet al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21(7):1603–16. [DOI] [PubMed] [Google Scholar]
- 12. Chanda D, Kim YH, Li T, Misra J, Kim DK, Kim JR, Kwon J, Jeong W Il, Ahn SH, Park TSet al. Hepatic cannabinoid receptor type 1 mediates alcohol-induced regulation of bile acid enzyme genes expression via CREBH. PLoS One. 2013;8(7):e68845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feng HY, Chen YC. Role of bile acids in carcinogenesis of pancreatic cancer: an old topic with new perspective. World J Gastroenterol. 2016;22(33):7463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunness P, Gidley MJ. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010;1(2):149–55. [DOI] [PubMed] [Google Scholar]
- 15. Singh J, Metrani R, Shivanagoudra SR, Jayaprakasha GK, Patil BS. Review on bile acids: effects of the gut microbiome, interactions with dietary fiber, and alterations in the bioaccessibility of bioactive compounds. J Agric Food Chem. 2019;67(33):9124–38. [DOI] [PubMed] [Google Scholar]
- 16. The ATBC Cancer Prevention Study Group. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 17. Pietinen P, Hartman AM, Haapa E, Råsånen L, Haapakoski J, Palmgran J, Albanes D, Virtamo J, Huttunen JK. Reproducibility and validity of dietary assessment instruments. Am J Epidemiol. 1988;128(3):655–66. [DOI] [PubMed] [Google Scholar]
- 18. Hyvönen L, Lampi A-M, Varo P, Koivistoinen P. Fatty acid analysis, TAG equivalents as net fat value, and nutritional attributes of commercial fats and oils. J Food Compos Anal. 1993;6(1):24–40. [Google Scholar]
- 19. Hyvönen L, Koivistoinen P. Fatty acid analysis, TAG equivalents as net fat value, and nutritional attributes of fish and fish products. J Food Compos Anal. 1994;7(1–2):44–58. [Google Scholar]
- 20. Englyst H. Determination of carbohydrate and its composition in plant materials. In: James WPT, Theander O, eds. The analysis of dietary fiber in food. New York (NY): Marcel Decker; 1981. p. 71–93. [Google Scholar]
- 21. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MAet al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6):273S–309S. [DOI] [PubMed] [Google Scholar]
- 22. Subar AF, Ziegler RG, Thompson FE, Johnson CC, Weissfeld JL, Reding D, Kavounis KH, Hayes RB. Is shorter always better? Relative importance of questionnaire length and cognitive ease on response rates and data quality for two dietary questionnaires. Am J Epidemiol. 2001;153(4):404–9. [DOI] [PubMed] [Google Scholar]
- 23. Thompson FE, Subar AF, Brown CC, Smith AF, Sharbaugh CO, Jobe JB, Mittl B, Gibson JT, Ziegler RG. Cognitive research enhances accuracy of food frequency. J Am Diet Assoc. 2002;102(2):212–25. [DOI] [PubMed] [Google Scholar]
- 24. Subar AF, Thompson FE, Smith AF, Jobe JB, Ziegler RG, Potischman N, Schatzkin A, Hartman A, Swanson C, Kruse Let al. Improving food frequency questionnaires. J Am Diet Assoc. 1995;95(7):781–8. [DOI] [PubMed] [Google Scholar]
- 25. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 26. Willett W. Reproducibility and validity of food-frequency questionnaires. In: Walter Willett, ed. Nutritional epidemiology. New York: Oxford University Press; 1998: p. 101–47. [Google Scholar]
- 27. Tippett KS, Cypel YS. Design and operation: the continuing survey of food intakes by individuals and the Diet and Health Knowledge Survey, 1994–96. U.S. Department of Agriculture Agricultural Research Service Nationwide Food Surveys Report; 1997. Washington (DC). [Google Scholar]
- 28. University of Minnesota. Nutrition Co-ordinating Center Nutrition Data System for Research descriptive overview. [Internet]. Version current 14 Jan 2021. Available from: http://www.ncc.umn.edu/.
- 29. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(Suppl 4):120S–8S. [DOI] [PubMed] [Google Scholar]
- 30. Wei T, Simko V. R package “corrplot”: visualization of a correlation matrix (version 0.84). [Internet]. 2017. [Cited 2021 Jan 14]. Available from: https://github.com/taiyun/corrplot. [Google Scholar]
- 31. Bayerdörffer E, Mannes GA, Richter WO, Ochsenkühn T, Wiebecke B, Köpcke W, Paumgartner G. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology. 1993;104(1):145–51. [DOI] [PubMed] [Google Scholar]
- 32. Bayerdorffer E, Mannes G, Ochsenkuhn T, Dirschedl P, Wiebecke B, Paumgartner G. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut. 1995;36(2):268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo L, Aubrecht J, Li D, Warner RL, Johnson KJ, Kenny J, Colangelo JL. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS One. 2018;13(3):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25. [DOI] [PubMed] [Google Scholar]
- 35. Wewalka M, Patti ME, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99(4):1442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Partula V, Deschasaux M, Druesne-Pecollo N, Latino-Martel P, Desmetz E, Chazelas E, Kesse-Guyot E, Julia C, Fezeu LK, Galan Pet al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am J Clin Nutr. 2020;112(1):195–207. [DOI] [PubMed] [Google Scholar]
- 37. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MAet al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ginos BNR, Navarro SL, Schwarz Y, Gu H, Wang D, Randolph TW, Shojaie A, Hullar MAJ, Lampe PD, Kratz Met al. Circulating bile acids in healthy adults respond differently to a dietary pattern characterized by whole grains, legumes and fruits and vegetables compared to a diet high in refined grains and added sugars: a randomized, controlled, crossover feeding study. Metabolism. 2018;83:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Zet al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27(9):3583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papandreou C, Hernández-Alonso P, Bulló M, Ruiz-Canela M, Yu E, Guasch-Ferré M, Toledo E, Dennis C, Deik A, Clish Cet al. Plasma metabolites associated with coffee consumption: a metabolomic approach within the PREDIMED study. Nutrients. 2019;11(5):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inoue M, Tsugane S. Coffee drinking and reduced risk of liver cancer: update on epidemiological findings and potential mechanisms. Curr Nutr Rep. 2019;8(3):182–6. [DOI] [PubMed] [Google Scholar]
- 42. Wadhawan M, Anand AC. Coffee and liver disease. J Clin Exp Hepatol. 2016;6(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loftfield E, Freedman ND. Coffee and digestive cancers–what do we know, and where do we go?. Br J Cancer. 2020;122(9):1273–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487(7405):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–55. [DOI] [PubMed] [Google Scholar]
- 46. Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79(6):969–73. [DOI] [PubMed] [Google Scholar]
- 47. De Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001;21(7):1233–7. [DOI] [PubMed] [Google Scholar]
- 48. Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77(6):937–46. [DOI] [PubMed] [Google Scholar]
- 49. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KHO, AlAbdulghafoor FK, Summerbell CD, Worthington HVet al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7(7):CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91(3):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reddy BS, Simi B, Patel N, Aliaga C, Rao CV. Effect of amount and types of dietary fat on intestinal bacterial 7α-dehydroxylase and phosphatidylinositol-specific phospholipase C and colonic mucosal diacylglycerol kinase and PKC activities during different stages of colon tumor promotion. Cancer Res. 1996;56(10):2314–20. [PubMed] [Google Scholar]
- 52. Turjman N, Goodman GT, Jaeger B, Nair PP. Diet, nutrition intake, and metabolism in populations at high and low risk for colon cancer. Metabolism of bile acids. Am J Clin Nutr. 1984;40(4):937–41. [DOI] [PubMed] [Google Scholar]
- 53. Hylla S, Gostner A, Dusel G, Anger H, Bartram HP, Christl SU, Kasper H, Scheppach W. Effects of resistant starch on the colon in healthy volunteers: possible implications for cancer prevention. Am J Clin Nutr. 1998;67(1):136–42. [DOI] [PubMed] [Google Scholar]
- 54. Grubben M, Van Den Braak CCM, Essenberg M, Olthof M, Tangerman A, Katan MB, Nagengast FM. Effect of resistant starch on potential biomarkers for colonic cancer risk patients with colonic adenomas: a controlled trial. Dig Dis Sci. 2001;46(4):750–6. [DOI] [PubMed] [Google Scholar]
- 55. Heijnen MLA, Van Amelsvoort JMM, Deurenberg P, Beynen AC. Limited effect of consumption of uncooked (RS2) or retrograded (RS3) resistant starch on putative risk factors for colon cancer in healthy men. Am J Clin Nutr. 1998;67(2):322–31. [DOI] [PubMed] [Google Scholar]
- 56. Reddy BS. Diet and excretion of bile acids. Cancer Res. 1981;41(9 Pt 2):3766–8. [PubMed] [Google Scholar]
- 57. van Munster IP, Tangerman A, Nagengast FM. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis Sci. 1994;39(4):834–42. [DOI] [PubMed] [Google Scholar]
- 58. Trefflich I, Marschall HU, Di Giuseppe R, Ståhlman M, Michalsen A, Lampen A, Abraham K, Weikert C. Associations between dietary patterns and bile acids–results from a cross-sectional study in vegans and omnivores. Nutrients. 2020;12(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Faassen A, Bol J, Van Dokkum W, Pikaar NA, Ockhuizen T, Hermus RJJ. Bile acids, neutral steroids, and bacteria in feces as affected by a mixed, a lacto-ovovegetarian, and a vegan diet. Am J Clin Nutr. 1987;46(6):962–7. [DOI] [PubMed] [Google Scholar]
- 60. Fiamoncini J, Yiorkas AM, Gedrich K, Rundle M, Alsters SI, Roeselers G, Van Den Broek TJ, Clavel T, Lagkouvardos I, Wopereis Set al. Determinants of postprandial plasma bile acid kinetics in human volunteers. Am J Physiol Gastrointest Liver Physiol. 2017;313(4):G300–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending proposal approval and completion of a Data Transfer Agreement (see https://atbcstudy.cancer.gov/ptsa/). For PLCO, approval should be obtained from: https://cdas.cancer.gov/learn/plco/instructions/?type=data.