Abstract
The extracellular signals which regulate the myogenic program are transduced to the nucleus by mitogen-activated protein kinases (MAPKs). We have investigated the role of two MAPKs, p38 and extracellular signal-regulated kinase (ERK), whose activities undergo significant changes during muscle differentiation. p38 is rapidly activated in myocytes induced to differentiate. This activation differs from those triggered by stress and cytokines, because it is not linked to Jun–N-terminal kinase stimulation and is maintained during the whole process of myotube formation. Moreover, p38 activation is independent of a parallel promyogenic pathway stimulated by insulin-like growth factor 1. Inhibition of p38 prevents the differentiation program in myogenic cell lines and human primary myocytes. Conversely, deliberate activation of endogenous p38 stimulates muscle differentiation even in the presence of antimyogenic cues. Much evidence indicates that p38 is an activator of MyoD: (i) p38 kinase activity is required for the expression of MyoD-responsive genes, (ii) enforced induction of p38 stimulates the transcriptional activity of a Gal4-MyoD fusion protein and allows efficient activation of chromatin-integrated reporters by MyoD, and (iii) MyoD-dependent myogenic conversion is reduced in mouse embryonic fibroblasts derived from p38α−/− embryos. Activation of p38 also enhances the transcriptional activities of myocyte enhancer binding factor 2A (MEF2A) and MEF2C by direct phosphorylation. With MEF2C, selective phosphorylation of one residue (Thr293) is a tissue-specific activating signal in differentiating myocytes. Finally, ERK shows a biphasic activation profile, with peaks of activity in undifferentiated myoblasts and postmitotic myotubes. Importantly, activation of ERK is inhibitory toward myogenic transcription in myoblasts but contributes to the activation of myogenic transcription and regulates postmitotic responses (i.e., hypertrophic growth) in myotubes.
In the past decade, much has been learned about the molecular mechanisms that govern myogenesis owing mainly to the discovery of two groups of myogenic transcription factors (4, 45, 62). The first group includes the myogenic regulatory factors (MRFs), which belong to the basic helix-loop-helix (bHLH) protein family. This MRF group consists of four members: Myf5, MyoD, myogenin, and MRF4, all of which are specifically expressed in skeletal muscles. One of the unique features of these MRFs is that when they are ectopically expressed in fibroblasts or certain other nonmuscle cells, each has the ability to initiate the myogenic program and convert nonmuscle cells to myogenic derivatives (9, 59). Myogenic bHLH proteins heterodimerize with other ubiquitous bHLH proteins (like the E2A gene products, E12, and E47) to efficiently bind a consensus DNA site: CANNTG (also called the E box) (4, 33). The second group of transcription factors important in muscle differentiation consists of four different myocyte enhancer binding factor 2 (MEF2) proteins, which belong to the MADS box family (7). The MEF2 proteins (MEF2A, MEF2B, MEF2C, and MEF2D) form homo- or heterodimers which bind to a consensus AT-rich sequence (MEF2 site), found in the promoters of many muscle-specific genes. Myogenic bHLH and MEF2 cooperate to synergistically activate muscle-specific transcription through interactions mediated by the basic region and the MADS domain, respectively (44, 45).
The study of muscle differentiation has benefited from the availability of several myogenic cell lines which allow biochemical dissection of the myogenic pathway. These myogenic cell lines (e.g., mouse C2C12 and rat L6) can be induced to differentiate by withdrawal of mitogens, such as serum. Many negative regulators of myogenesis (e.g., Id, Twist, oncogenic Ras, and the viral proteins E1A and simian virus 40 T antigen) have been identified (1, 34). However, little is known about the intracellular components that positively regulate the activities of myogenic transcription factors, especially those that are involved in receiving and transducing extracellular cues. One extracellular signal that positively regulates myogenesis is insulin-like growth factor (IGF). IGF activates the phosphatidylinositol-3 kinase (PI3K) signaling pathway, which is required for myogenesis (11, 29, 30). However, how this signaling pathway influences myogenic transcription remains to be defined.
In eukaryotic cells, mitogen-activated protein kinases (MAPKs) are components of several important signaling pathways that relay extracellular cues to transcription factors in the nucleus (24, 25, 32, 41, 51). For mammals, three MAPK pathways, including the extracellular signal-regulated kinases (ERK1 and -2), the Jun–N-terminal kinases (JNK1, -2, and -3), and the p38 isoforms (α, β, γ, and δ) have been characterized (references 51 and 43 and references therein). In general, each group of MAPKs is activated by two homologous MAPK kinases (MKKs [also called MAPKKs]), including MEK1 and -2 for the ERKs, JNK kinase 1 and 2 (JNKK1 and -2) (or MKK4 and -7) for the JNKs, and MKK3 and -6 for the p38s (26, 51). Except for JNKK1, which can also activate p38 in vitro (14, 38), all other MKKs specifically activate their MAPK targets and have little activity on members of other groups. The ERK pathway has been implicated in the control of muscle differentiation, although its role remains controversial, with some reports suggesting a positive function (5, 11) and others suggesting a negative function (18). p38 has also been reported to activate certain MEF2 family members (21, 40, 48, 61, 67) and to stimulate muscle differentiation (12, 64). However, the upstream signals which regulate p38 activation at the onset of muscle differentiation and the mechanism(s) by which p38 activates myogenic transcription remain elusive.
Using a combination of different approaches, we found that the p38 kinase is rapidly activated in muscle cells induced to differentiate by serum withdrawal, through a pathway distinct from that activated in response to stress and cytokines. Specific inhibition of p38 prevents differentiation of both established muscle cell lines and human primary myoblasts, while deliberate p38 activation stimulates muscle-specific reporters, accelerates myotube formation, and induces the expression of myogenic markers despite the presence of serum, which otherwise inhibits muscle differentiation. p38 exerts its stimulatory effect on myogenesis by enhancing the transcriptional activities of both MyoD and MEF2A and -C through distinct mechanisms. While MEF2 proteins are activated by direct phosphorylation of residues located within the activation domain, p38-mediated activation of MyoD is likely to occur by an indirect mechanism. The p38 pathway is activated independently of the IGF-PI3K pathway, although the integrity of both these pathways is required to stimulate muscle differentiation. Conversely, the ERK pathway plays a dual role during myogenic differentiation, being inhibitory at early stages and stimulatory at late stages.
MATERIALS AND METHODS
Reagents.
Basic fibroblast growth factor (bFGF) was obtained from Sigma. The kinase inhibitors SB202190, SB203580, PD98059, LY294002, and rapamycin were purchased from Calbiochem.
Cell culture.
The myogenic cell line C2C12 derived from mouse muscle satellite cells and human primary myoblasts was cultured in growth medium (GM; Dulbecco modified Eagle medium [DMEM] with 20% fetal bovine serum [FBS]). Differentiation of these muscle cells was induced by replacing GM with differentiation medium (DM; DMEM with 2% horse serum) when cells were 90% confluent. 10T1/2 cells were maintained in DMEM supplemented with 10% FBS. Reporter cells (3T3 stable cell clones with an integrated MyoD-dependent reporter, 3T3 4RE-Luc) were generated by transfecting the 4RE-Luc template together with pcDNA3neo. G418-resistant colonies were grown and constituted a polyclonal population of reporter cells. Primary human myoblasts and p38−/− and wild-type mouse embryonic fibroblasts (mEFs) were isolated according to standard procedures.
Immunostaining.
Cells were washed twice with phosphate-buffered saline, fixed with 3.6% formaldehyde for 10 min, and permeabilized with 0.25% Triton X-100 for 10 min. After being blocked in 1% bovine serum albumin, the cells were incubated with diluted primary antibodies for 1 h. For immunohistochemical analysis, a Histomouse-SP kit (Zymed Laboratories Inc., South San Francisco, Calif.) was used according to the manufacturer's instructions. Indirect immunofluorescence was done as described previously (63) with a Nikon fluorescence microscope. Either fluorescein isothiocyanate- or rhodamine-conjugated secondary antibodies were used.
Transfection.
Transfections were carried out using Lipofectamine Plus as recommended by the manufacturer (Life Technologies, Inc.). When Gal4-Luc was used, the cells were first cultured in medium containing 10% FBS for 24 h immediately after transfection and then shifted to medium containing 0.1% FBS for another 24 h. Cells were then harvested to measure luciferase activity. Expression of Gal4 fusion proteins was quantitated by immunoblotting and phosphorimaging, and luciferase activity was normalized according to the immunoblot results. When transfected with muscle-specific reporters (4RE-Luc, MCK-Luc, or p21-Luc), cells were cultured in GM for 24 h after transfection and then shifted to DM for 36 to 48 h. When used, SB202190 was added to the medium immediately after transfection and the medium was changed every 12 to 24 h with fresh drug. The different reporters and activators were previously described (21, 53, 65).
Metabolic labeling and phosphopeptide mapping.
For transient transfections, the cells were labeled at 18 h posttransfection with 0.5 mCi of [32P]orthophosphate per ml for 5 h in the absence or presence of SB202190 as indicated in the figures. Alternatively, C2C12 cells transiently transfected with M2-tagged MEF2C were labeled 48 h after being cultured in DM for 36 h. The Gal4-MEF2C or M2-MEF2C proteins were immunoprecipitated with either anti-Gal4 (Santa Cruz) or M2 (Sigma) antibody, and after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to a polyvinylidene difluoride membrane for autoradiography and immunoblot analysis. Nontransfected C2C12 cells were kept in either GM or DM for 2 days and then labeled with 1 mCi of [32P]orthophosphate per ml for 5 h. Endogenous MEF2C was immunoprecipitated with anti-MEF antibody (kindly provided by J. Han). After being extensively washed in radioimmunoprecipitation assay buffer, the immune complexes were separated by SDS-PAGE and the MEF2C band was excised and analyzed by two-dimensional tryptic phosphopeptide mapping as described previously (21).
Immunoblot analysis.
Endogenous myogenin, myosin heavy chain (MHC), MyoD, p21, and actin in C2C12 cells were detected by immunoblotting using the monoclonal anti-myogenin (F5D), anti-MyoD (5.8), anti-MHC (MF20), anti-p21 (Ab-5; Oncogene), and anti-actin (Ab-1; Oncogene) antibodies. Antibodies against normal and phospho-p38, normal and phospho-ERK, and phospho-Akt were all from New England Biolabs. Transfected Gal-MyoD was detected by using anti-Gal4 antibody from Santa Cruz.
Kinase assays.
Fifty to 100 μg of cell extracts was incubated with antibodies against either p38α or JNK (recognizing all forms) in the presence of a 30-μl protein A-Sepharose bead suspension for 2 h at 4°C. The immune complexes were washed three times with lysis buffer (20 mM Tris [pH 7.6], 10% glycerol, 1% Triton X-100, 150 mM NaCl, 20 mM β-glycerolphosphate, 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail) and once with kinase buffer (20 mM HEPES [pH 7.6], 10 mM MgCl2, 20 mM β-glycerolphosphate, 20 μM ATP), with ATP omitted. Reactions were initiated by adding 1 μg of glutathione S-transferase (GST)–ATF2(1-92) (for p38) or GST–c-Jun(1-79) (for JNK) and 10 μCi of [γ-32P]ATP in 25 μl of kinase buffer. Reactions were carried out for 20 min at 30°C, and mixtures were analyzed by SDS-PAGE and autoradiography.
Generation of tetracycline-inducible stably transfected C2C12 cell lines.
cDNA fragments encoding either MKK6EE, JNKK2CA, or MEK1DD/ΔN4 were subcloned into pBPSTR1. A 1:1 ratio of the pBSPTR1-based expression vector and the pCLECO packaging vector (47) were cotransfected into 293T cells to generate retroviruses. Virus-containing supernatants were added to C2C12 cells for 6 h in the presence of 4 μg of Polybrene per ml. Cells were selected in a solution containing 1.5 μg of puromycin per ml and 2 μg of tetracycline per ml 24 h postinfection for 7 days. Mixtures of all puromycin-resistant clones were used for subsequent analysis.
RESULTS
Activation of p38 during muscle differentiation.
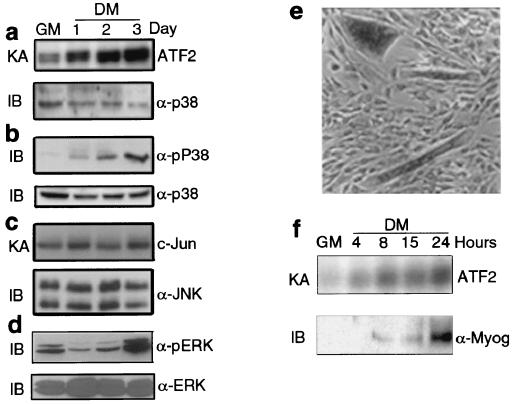
Given the importance of MAPKs in transmitting extracellular cues to the transcriptional machinery (25, 32), we examined the activities of the ERK, JNK, and p38 subgroups in C2C12 myoblasts undergoing differentiation. While serum removal, which induces C2C12 myoblasts to form multinucleated myotubes, does not alter JNK activity (Fig. 1c), it stimulates p38 kinase activity (Fig. 1a) and induces a biphasic change in ERK activity (Fig. 1d), which decreases at the onset of differentiation and increases at later stages, when myotubes are already formed. Activation of p38 is also detected by immunoblotting with antibodies specific for its activated form (Fig. 1b). p38 activity was rapidly elevated within 1 day of placing myoblasts in DM and continued to increase for at least 3 days (Fig. 1a and b), a point at which most myoblasts formed well-differentiated myotubes. Thus, sustained p38 activation, unlinked to JNK stimulation, is part of a differentiation-specific pathway which is distinct from that triggered by stress and cytokines. A rapid activation of p38 upon serum starvation is appreciable also in fibroblasts; however, unlike in muscle cells, this activity declines within the first 24 h (data not shown). Ectopic expression of MyoD is sufficient to sustain a persistent p38 activation in serum-starved fibroblasts (data not shown). This result suggests that the acquisition of the myogenic identity confers to the cells the ability to trigger a prolonged activation of p38 in response to serum withdrawal. Immunohistochemical analysis of a mixture of undifferentiated myoblasts and differentiated myotubes revealed high levels of active p38 only in myotubes (Fig. 1e). When examined in more detail, p38 activation was found to precede the accumulation of the myogenic transcription factor myogenin (Fig. 1f), an early differentiation marker in this cell system (3). Thus, p38 activation might be functionally linked to myogenic differentiation.
FIG. 1.
p38 activity increases upon muscle differentiation prior to induction of myogenin. (a) C2C12 myoblasts were cultured in GM until they were 90 to 95% confluent and then shifted to DM. Lysates were prepared from subconfluent cells in GM or cells kept for 1 to 3 days in DM as indicated. p38 was immunoprecipitated and its activity was measured by an immune complex kinase assay (KA) using AFT2 as a substrate. The amount of p38 was assessed by immunoblotting (IB). α-p38, anti-p38 antibody. (b) Equal amounts of whole-cell lysates were separated by SDS-PAGE in duplicate. Activation of p38 in C2C12 cell lysates was determined by immunoblotting with antibodies specific to phosphorylated and activated p38 (α-pP38) or total p38. (c) JNK activity and expression in C2C12 cell lysates were determined by an immune complex kinase assay using c-Jun as a substrate and by immunoblotting, respectively. α-JNK, anti-JNK antibody. (d) Samples (30 μg) of C2C12 extracts were analyzed by immunoblotting with anti-phospho-ERK antibody (α-pERK). The same membrane was stripped and reprobed with antibody against total ERK (α-ERK) to monitor the amounts of loaded proteins. (e) A coculture of undifferentiated C2C12 myoblasts and differentiated myotubes was prepared by mixing a population of myoblasts growing in GM and myoblasts previously cultured in DM for 24 h. This mixed population was cultured in GM for 1 day. Thereafter, cells were fixed and analyzed by immunohistochemistry with antibody specific to pP38. (f) Subconfluent C2C12 cells in GM were switched to DM and were collected at the indicated times (in hours). p38 activity was examined by an immune complex kinase assay, and expression of myogenin was determined by immunoblotting. α-Myog, anti-myogenin antibody.
Inhibition of p38 activity prevents myotube formation.
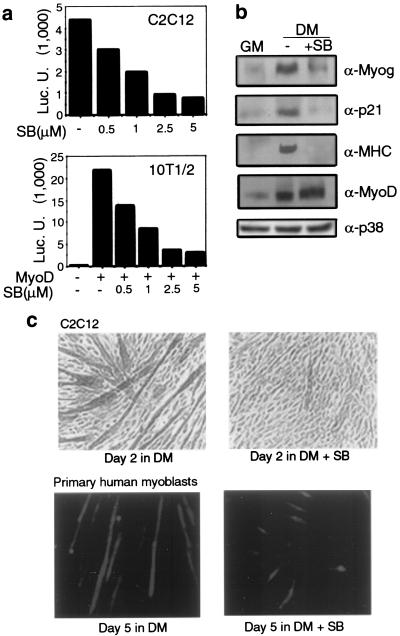
To establish a causal relationship between p38 and muscle differentiation, we employed the specific inhibitor SB202190, which fully inhibits p38 kinase activity at 5 to 10 μM without affecting JNK or ERK activities (reference 37 and our unpublished data). Instead, at concentrations higher than 20 μM, SB202190 may partially inhibit the activity of JNK and other protein kinases (8, 16, 27). We first examined the effect of this inhibition on expression of a luciferase reporter gene controlled by a synthetic promoter containing four E boxes (4RE-Luc). This reporter gene is activated only by myogenic bHLH proteins during muscle differentiation. The ability of SB202190 to inhibit p38 activity correlated with inhibition of 4RE-Luc activity in both C2C12 myoblasts and MyoD-expressing 10T1/2 fibroblasts (Fig. 2a). In both cases, reporter gene expression was induced by culturing cells in DM. Inhibition of p38 also prevented induction of both early (myogenin and p21) and late (MHC) myogenic markers but had no significant effect on MyoD or p38 expression (Fig. 2b). Most importantly, SB202190 at 5 μM blocked formation of MHC-positive myotubes by C2C12 myoblasts placed in DM (Fig. 2c, upper panels). To determine the physiological relevance of this effect, we examined the ability of SB202190 to inhibit myogenic differentiation of primary human myoblasts (satellite cells). A similar sensitivity to SB202190 was observed in these cells (Fig. 2c, lower panels) as well as in rat L6 myoblasts and MyoD-expressing 10T1/2 fibroblasts (data not shown). Collectively, these data indicate that p38 activity is necessary for activation of the myogenic differentiation program and place its site of action at a very early step in this process, preceding the induction of myogenin and the cell cycle inhibitor p21 (3).
FIG. 2.
p38 activity is required for myogenic differentiation. (a) The indicated cell lines were transfected with the myogenic reporter 4RE-Luc (together with MyoD in the case of 10T1/2 cells). The indicated concentrations of SB202190 (SB) were added immediately after transfection, and the cells were switched to DM for 36 h, after which they were collected to measure luciferase (Luc.) activity. (b) C2C12 cells in GM were switched to DM for 2 days with (5 μM) or without SB202190. Cell extracts were analyzed by immunoblotting for the presence of myogenic markers. α-Myog, α-p21, α-MHC, α-MyoD, and α-p38, anti-myogenin, anti-p21, anti-MHC, anti-MyoD, and anti-p38 antibodies, respectively. (c) Subconfluent C2C12 cells or primary human myoblasts were placed in DM with (5 μM) or without SB202190. At the indicated times, cells were fixed and immunostained with anti-MHC antibody to visualize differentiated cells. C2C12 cells were examined by immunohistochemistry and bright-field microscopy, and primary human myoblasts were examined by indirect immunofluorescence.
Deliberate activation of p38 accelerates muscle differentiation.
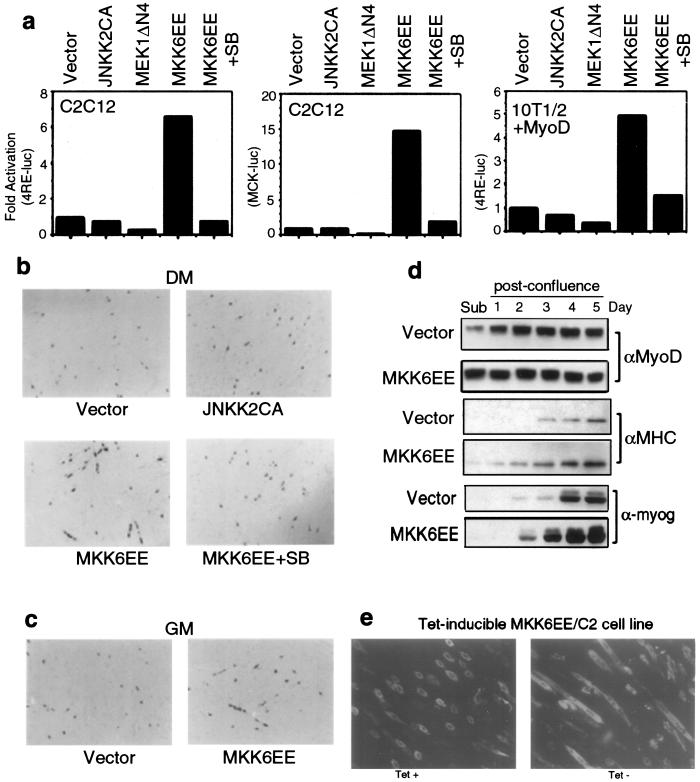
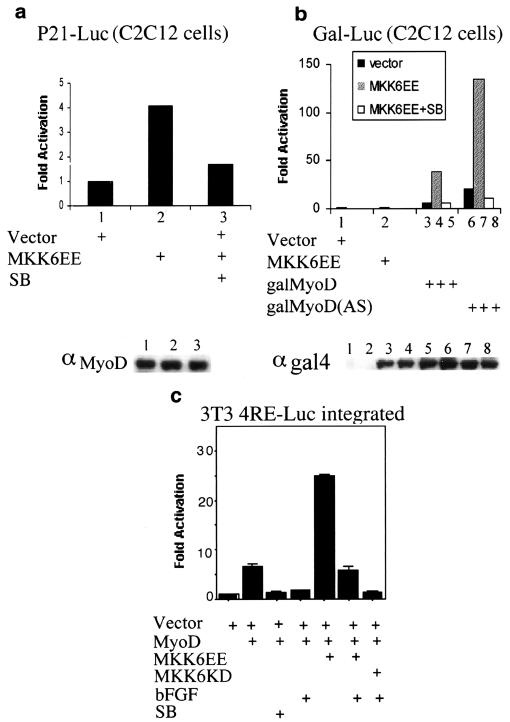
If p38 is causally involved in myogenic differentiation, then its deliberate activation should be sufficient for induction or at least acceleration of myoblast differentiation. Therefore, we examined the effect of a constitutively active mutant of MKK6 (MKK6EE), a p38-activating kinase (22, 50a ), on myogenic transcription. C2C12 or MyoD-expressing 10T1/2 cells were transfected with either 4RE-Luc or a luciferase gene under the control of the muscle creatine kinase (MCK) promoter (MCK-Luc) along with expression vectors encoding various constitutively active MKKs: MKK6EE (for p38), JNKK2CA (for JNK) (8), and MEK1DDΔN4 (for ERK) (39). The constitutive activities of these mutant MKKs were confirmed by both kinase and reporter gene assays using luciferase constructs driven by TRE- or SRE-containing promoters (data not shown). Among these vectors, only MKK6EE increased the activity of either the 4RE-Luc or the MCK-Luc reporter in C2C12 or 10T1/2 cells in a p38-dependent manner (Fig. 3a). The response of the 4RE-Luc reporter to MKK6EE in 10T1/2 fibroblasts required the expression of MyoD, demonstrating that activation of p38 enhances myogenic transcription by a mechanism dependent on the presence of myogenic activators.
FIG. 3.
Deliberate p38 activation induces myotube formation and expression of muscle differentiation markers. (a) Different muscle-specific reporters (4RE-Luc or MCK-Luc) were cotransfected with expression vectors encoding activated MKKs into either C2C12 or MyoD-expressing 10T1/2 cells. After growing in GM for 1 day, cells were shifted to DM for another 36 h and then harvested to measure luciferase activity. All determinations were done in duplicate, and the data shown are representative of three independent experiments. Fold activation is the ratio of luciferase activity in MKK-transfected cells to cells transfected with the empty vector. SB, SB202190. (b) C2C12 cells in GM were cotransfected with a construct encoding a β-galactosidase protein fused to a nuclear localization signal controlled by the MLC promoter. (see the text) and either an empty vector or a JNKK2CA or MKK6EE expression vector. After 1 day in DM without or with SB202190, the cells were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). In MKK6EE-transfected cells cultured without SB202190, a blue color appeared 12 h earlier than in the other samples. (c) Conditions were the same as those described above except that the cells were cultured in GM after transfection. (d) Subconfluent (sub) C2C12 clones stably transfected with either an empty vector or an MKK6EE expression vector were cultured in GM. The cells were lysed at the times indicated after reaching confluence and analyzed by immunoblotting for expression of MyoD, MHC, and myogenin. αMyoD, αMHC, and α-myog, anti-MyoD, anti-MHC, and anti-myogenin antibodies, respectively. (e) C2C12 cells stably expressing MKK6EE under the control of a tetracycline (Tet)-regulated promoter were cultured in GM until confluent. Tetracycline was either left in the culture medium or removed. After 48 h the cells were fixed, stained with anti-MHC antibody, and visualized by indirect immunofluorescence.
To couple activation of muscle transcription with induction of morphologic differentiation by deliberate p38 activation in myoblasts, C2C12 cells were transfected with a constitutively active MKK6EE or JNKK2CA vector together with a construct encoding a β-galactosidase protein fused to a nuclear localization signal controlled by the myosin light chain (MLC) promoter. Coexpression of MKK6EE, but not JNKK2CA, enhanced the expression of β-galactosidase in the nuclei of transfected cells and accelerated the formation of multinucleated myotubes upon induction of differentiation (DM) (Fig. 3b). Both the increases in reporter gene expression and myotube formation were prevented by SB202190. Strikingly, ectopic MKK6EE expression stimulated formation of β-galactosidase-positive myotubes even in cells cultured in 20% FBS (Fig. 3c), which normally inhibits in vitro myogenesis (10, 33). We further analyzed the effect of deliberate p38 activation on muscle differentiation by establishing stable C2C12 clones that constitutively express MKK6EE (MKK6EE/C2). These cells expressed higher basal levels of MyoD than those of vector-transfected cells during logarithmic growth (Fig. 3d) and exhibited faster and more pronounced induction of myogenin and MHC expression after reaching confluence (Fig. 3d). Formation of MHC-positive myotubes by postconfluent MKK6EE/C2 cells was inhibited by SB202190 (data not shown), supporting the notion that p38 activation accelerates myogenic differentiation. We also generated stably transfected C2C12 derivatives in which MKK6EE expression was controlled by a tetracycline-regulated system (47). Removal of the antibiotic resulted in MKK6EE induction and p38 activation (data not shown) and caused the appearance of multinucleated MHC-positive myotubes even in cells cultured in the presence of 20% FBS (Fig. 3e). These MKK6EE-induced C2C12 myotubes formed in the presence of serum were functionally indistinguishable from those formed by normal C2C12 in DM, as determined by the enzymatic activity of MCK (data not shown). Therefore, deliberate p38 activation is sufficient for triggering myogenic differentiation in serum-fed proliferating myoblasts.
Activation of MEF2A and MEF2C transcriptional activity by p38 through direct phosphorylation.
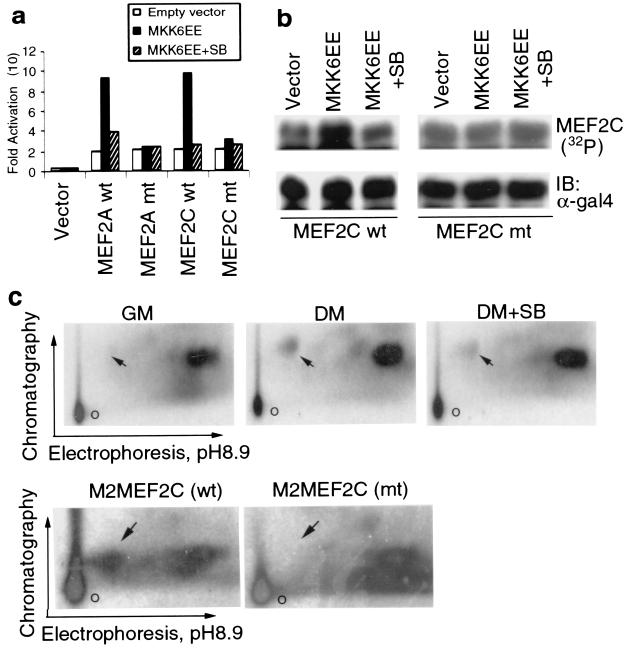
The results presented above suggest that p38 may directly or indirectly potentiate the activities of myogenic transcription factors. It has previously been shown that p38 phosphorylates MEF2C in lymphocytes, leading to its activation in response to endotoxin (21). Furthermore, p38 has been reported to phosphorylate other MEF2 family members (40, 61, 67). Despite their ubiquitous expression (except for MEF2C, which is expressed only in brain, skeletal muscle, and spleen) (42), MEF2 proteins play critical roles in myogenesis in both invertebrates and vertebrates (7). We examined the effect of p38 activation on the transcriptional activities of fusion proteins that contain the Gal4 DNA binding domain and MEF2-derived activation domains. MKK6EE enhanced the transcriptional activities of Gal4-MEF2A and Gal4-MEF2C, and that effect was blocked by SB202190 (Fig. 4a). Previous studies have identified threonines 312 and 319 in MEF2A and threonines 293, 300, and serine 387 in MEF2C as targets for p38-mediated phosphorylation in certain cellular systems (21, 48, 67). The replacement of threonines 312 and 319 with alanines in MEF2A abrogated the activation by MKK6EE. With MEF2C, we found that a single amino acid substitution (Thr293 to Ala) was sufficient to abolish the stimulatory effect of MKK6EE on Gal4-MEF2C (Fig. 4a). Other mutations of potential p38 phosphorylation sites that did not involve Thr293 failed to abrogate the positive effect of MKK6EE (data not shown). Metabolic labeling with 32P in C2C12 cells indicated that coexpression of MKK6EE stimulated the in vivo phosphorylation of wild-type Gal4-MEF2C and that this enhancement in phosphorylation was blocked by SB202190 (Fig. 4b). In contrast, MKK6EE had no effect on phosphorylation of a Gal4-MEF2C mutant (T293A) (Fig. 4b). These results suggest that the phosphorylation pattern of MEF2C by p38 during muscle differentiation differs from that found in other cellular systems (i.e., lymphoid cells) in which three residues are simultaneously phosphorylated (21). To further clarify the issue, we carried out a 32P metabolic labeling experiment with C2C12 cells and isolated endogenous MEF2C from either myoblasts or myotubes that were incubated with or without SB202190. When subjected to two-dimensional phosphopeptide mapping, only one tryptic phosphopeptide that is specific to myotubes and that contains Thr293 and -300 was detectable and its appearance was reduced by SB202190 (Fig. 4c, upper panels). In contrast, two p38-inducible tryptic phosphopeptides (one containing both Thr293 and Thr300 and the other containing Ser387) have been detected in lymphoid cells treated with lipopolysaccharide (21). To confirm that the inducible phosphorylation site on MEF2C during muscle differentiation is T293, Flag-tagged wild-type, and mutant MEF2C (T293A) were immunoprecipitated from 32P-labeled myotubes and subjected to tryptic phosphopeptide mapping. As shown in Fig. 4c (lower panels), the myotube-specific phosphopeptide seen in wild-type MEF2C was completely absent in MEF2C (T293A). It should be noted that in both the experiments with exogenous and endogenous MEF2C, other constitutive phosphopeptides detected by the mapping were not modified by changing the culture conditions, by SB treatment, or by threonine-to-alanine mutation. Collectively, these results support the concept that p38 kinase targets MEF2C at a unique site (Thr293), whose phosphorylation is required to stimulate MEF2-dependent transcription during muscle differentiation.
FIG. 4.
p38 phosphorylates MEF2C and increases its transcriptional activity during muscle differentiation. (a) 10T1/2 cells were cotransfected with a Gal4-Luc reporter and expression vectors for wild type (wt) and mutant (mt) Gal4-MEF2 fusion proteins [MEF2A(A312/A319) and MEF2C(A293)] along with either an empty vector or an MKK6EE expression vector. Fold activation is the ratio of the luciferase activity in cells transfected with the activator (Gal4-MEF2) to that of cells transfected with the reporter but no activators. The data are representative of three independent experiments. SB, SB202190. (b) C2C12 cells were transfected with either wild-type or mutant Gal4-MEF2C vectors along with either an empty vector or an MKK6EE expression vector. After in vivo labeling with 32P, Gal4-MEF2C was immunoprecipitated, separated by SDS-PAGE, and visualized by autoradiography. The level of Gal4-MEF2C expression was determined by immunoblotting (IB) with anti-Gal4 antibody (α-gal4). (c, upper panels) C2C12 cells kept in either GM or DM (2 days) were metabolically labeled with 32P in the absence or presence of SB202190 as indicated. Endogenous MEF2C was immunoprecipitated, the immune complexes were separated by SDS-PAGE, and the MEF2C band was excised and analyzed by two-dimensional tryptic phosphopeptide mapping. (lower panels), M2-tagged wild-type MEF2C or mutant MEF2C(A293) were transfected into C2C12 cells and were kept in DM for 2 days before being subjected to metabolic labeling as described for panel a. Tagged MEF2C proteins were immunoprecipitated and subjected to two-dimensional mapping. o, the origin of the chromatogram. The phosphopeptide containing T293 is indicated by an arrow.
p38 is an essential activator of MyoD-dependent gene transcription.
Since p38 activation can stimulate the activity of a promoter containing only E boxes (Fig. 2a and 3a) and lead to the induction of p21 (Fig. 2c), whose transcription is regulated by MyoD in muscle cells (19, 20, 49, 66), we conceived that myogenic bHLH factors may also be potential targets for p38. Consistent with this hypothesis, expression of MKK6EE enhanced MyoD-dependent transactivation of a luciferase reporter driven by either four E boxes (4RE-Luc) in 10T1/2 fibroblasts (data not shown) or a variant p21 promoter (p21-Luc) that lacks its p53 binding sites in C2C12 cells (Fig. 5a). Under these conditions, the p21 promoter is mostly responsive to MyoD (19, 20). The ectopic expression of MKK6EE did not change the protein levels of MyoD (Fig. 5a, bottom panel) and its effect on the activation of p21-Luc was inhibited by SB202190 (Fig. 5a). To more directly assess the effect of p38 activation on MyoD transcriptional activity, we examined its effect on Gal4-MyoD fusion proteins. MKK6EE enhanced the transcriptional activity of Gal4-MyoD in a p38-dependent manner (Fig. 5b), without changing the Gal4-MyoD levels (Fig. 5b, bottom panel). As a control, the low basal activity of a mutant containing only the bHLH region of MyoD was not stimulated by MKK6EE (data not shown). A MyoD mutant whose basic region was replaced by that of the Drosophila bHLH protein Achaete-scute (58) could still be stimulated by MKK6EE, an effect abrogated by SB202190 treatment (Fig. 5b). This finding ruled out the possibility that the activation of MyoD by MKK6EE was mediated through interactions with p38-phosphorylated MEF2 proteins, as it was demonstrated that MyoD without its own basic region does not associate with MEF2 proteins (6). Consistently, physical interactions between MyoD and MEF2C, as detected by a mammalian two-hybrid system, were not influenced by either MKK6EE cotransfection, SB202190 treatment, or mutation of threonine 293 in MEF2C (P. L. Puri and Z. Wu, unpublished results). These data also indicate that p38 regulates MyoD function through either the N-terminal or the C-terminal domain of MyoD, or both. These two domains have been previously shown to be capable of activating gene transcription in repressive chromatin by stimulating chromatin remodeling at binding sites in muscle gene enhancers (17a). This activity is blocked by antimyogenic factors, such as bFGF (17b, 23). To test whether p38 could stimulate MyoD-mediated chromatin remodeling, we carried out a set of experiments with 3T3 fibroblasts containing a chromosome-integrated MyoD-dependent reporter (4RE-Luc integrated). MyoD activates this integrated reporter (chromosomal template) with a lower efficiency than that of the same reporter transiently transfected (extrachromosomal template) (data not shown). Exposure to bFGF, which blocks MyoD-mediated chromatin remodeling (17a) abrogated the activation of the chromosomal template by MyoD (Fig. 5c). Inhibition of p38 by SB202190 resulted in a similar repression of this reporter (Fig. 5c). Importantly, activation of p38 by MKK6EE enhanced MyoD-dependent activation of chromosomal 4RE-Luc and partially counteracted the repressive effect exerted by bFGF on the same template (Fig. 5c). These results led to the speculations that p38 may stimulate myogenic transcription by enhancing the chromatin remodeling activity of MyoD and that it might bypass inhibitory signals (i.e., bFGF), which block this activity.
FIG. 5.
p38 activation enhances MyoD transcriptional activity. (a) A p21-Luc reporter lacking p53 binding sites was transfected into C2C12 cells along with either an empty vector or an MKK6EE expression vector in the presence or absence of SB202190 (SB) as indicated. After growing in GM for 1 day, cells were shifted to DM for another 36 h and then harvested to measure luciferase activity. Fold activation is the luciferase activity in MKK6EE-transfected cells relative to that in empty-vector-transfected cells. The levels of endogenous MyoD in each sample were monitored by Western blotting using the monoclonal MyoD antibody (αMyoD) 5.8 and are presented in the gel below the graph. (b) A Gal4-Luc reporter and various Gal4-MyoD activator constructs (galMyoD) (58) were cotransfected into C2C12 cells along with either an empty vector or an MKK6EE expression vector. After growing in GM for 1 day, cells were shifted to DM for another 36 h and then harvested to measure luciferase activity. galMyoD(AS), Gal4 DNA binding domain fused to a mutant MyoD whose basic region is replaced by that of the Drosophila bHLH protein Achaete-scute. The levels of wild-type galMyoD and galMyoD(AS) proteins were monitored by Western blotting using antibody against Gal4 and are presented in the gel below the graph. The sample order in the Western blot is the same as that in the bar graph. αgal4, anti-Gal4 antibody. (c) 3T3 clones with a chromosome-integrated MyoD-responsive reporter (3T3 4RE-Luc) were transfected with the indicated plasmids. After growing in GM for 1 day, cells were shifted to DM, in the presence or absence of bFGF (25 ng/ml) and SB202190, for an additional 36 h and then harvested to measure luciferase activity. All transfections and measurements were done in duplicate, and the results shown are representative of two independent experiments.
The molecular mechanism by which p38 stimulates MyoD transcriptional activity remains unclear. Although MyoD itself is a direct target for p38-mediated phosphorylation in vitro, mutation of a proline-directed serine (Ser5), which is in vivo phosphorylated in MKK6EE-induced myotubes, failed to abolish the stimulatory effect of MKK6EE on MyoD activity (P. J. Woodring, Z. Wu, and P. L. Puri, unpublished results). Thus, the regulatory function of this phosphorylation is not clear and p38 is rather likely to activate MyoD-dependent transcription by an indirect mechanism(s).
p38α and -β isoforms are the main activators of the myogenic program.
The repression of myogenic differentiation by SB202190, which selectively inhibits the p38α and -β kinases, suggests a specific role of these two isoforms in regulating myogenic differentiation. We have observed that transient overexpression of p38α and -β isoforms can increase the activation of the muscle-specific reporter gene by MyoD (data not shown). The availability of embryonic fibroblasts derived from p38α−/− embryos allowed us to test the importance of p38α in the activation of the myogenic program. We compared the extents of myogenic conversion by ectopic expression of MyoD in mEFs derived from p38α−/− and p38α wild-type embryos. In the absence of p38α, the efficiency of MyoD-dependent conversion was reduced by about 45%, as judged by the formation of MHC-positive myotubes (Table 1). Reintroduction of p38α by transient transfection completely restored the activation of MyoD-dependent transcription in p38α−/− mEFs (Table 1). These results demonstrate that p38α plays an important role in the activation of MyoD-dependent transcription. The presence of p38β might account for the residual activation of the myogenic program in the absence of p38α. Interestingly, we have observed that p38β can functionally replace p38α, since the overexpression of the p38β isoform, but not of the p38γ and -δ isoforms, could restore the MyoD-dependent myogenic conversion in p38α−/− mEFs (Z. Wu and P. L. Puri, unpublished observations). Although the specific role of p38α and -β remains to be defined, it is tempting for us to speculate that these two isoforms might exert a redundant function in activating the myogenic program.
TABLE 1.
Extents of ectopic expression of MyoD in mEFs derived from p38α−/− and wild-type p38α embryosa
| mEF type | Transfectant(s) | % MHC-positive cells in expt:
|
|
|---|---|---|---|
| 1 | 2 | ||
| wt + vector | GFP | <1 | <1 |
| wt | MyoD | 17 | 22 |
| −/− + vector | GFP | <1 | <1 |
| −/− | MyoD | 9 | 12 |
| −/− | MyoD + p38α | 15 | 23 |
mEFs were isolated from either p38 null (−/−) or wild-type (wt) embryos. Myogenic conversion was activated by transfection of MyoD with or without cotransfection of p38α kinase. After 3 days of culturing in (DMEM plus 2% horse serum), cells were fixed and the extent of myogenic conversion was evaluated by double staining for MyoD (using the monoclonal antibody 5.8) and a polyclonal anti-MHC antibody. Vector-transfected cells were visualized by transfection of the green fluorescent protein (GFP). The percentage of cells doubly positive for MHC and MyoD (or MHC and GFP) among 100 MyoD-positive cells was calculated.
p38 acts in parallel with the IGF-PI3K pathway.
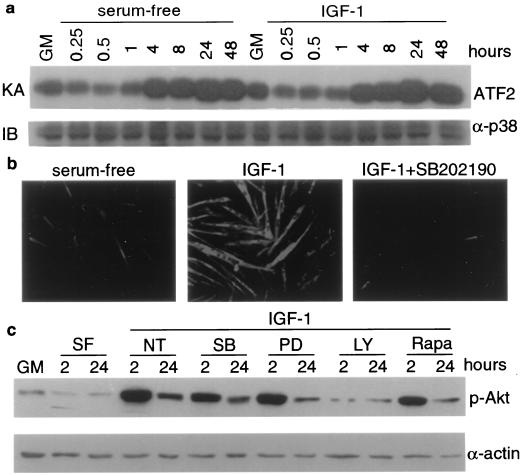
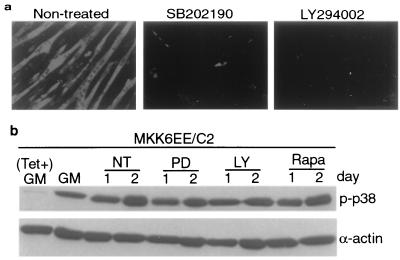
One exogenous factor that can stimulate muscle differentiation is IGF. The activities of IGF1 and IGF2 are transduced mostly through the PI3K-Akt (PKB) signaling pathway (11, 28, 29). We set out to investigate whether p38 MAPKs might function downstream of the IGF-PI3K-Akt pathway during muscle differentiation. This issue is pertinent because PI3K was shown to cause activation of the related JNK MAPK cascade in other cell systems (52) and can therefore also contribute to the activation of p38 MAPKs in muscle cells. As shown in Fig. 6a, the fold increases and the kinetics of p38 activation during muscle differentiation were very similar in the absence and presence of IGF1, although the extent of differentiation was dramatically increased in the presence of IGF1 (Fig. 6b). Furthermore, the addition of 10 μM SB202190 severely blocked both C2C12 and L6 (data not shown) differentiation induced by IGF, without affecting the integrity of the IGF-PI3K-Akt pathway as measured by Akt phosphorylation (Fig. 6b and c). As a control, the addition of 25 μM PD98059 or 10 ng of rapamycin per ml did not inhibit the stimulation of Akt phosphorylation by IGF1, while the addition of 25 μM LY294002, a specific inhibitor of PI3K (57), abolished IGF1-induced Akt phosphorylation (Fig. 6c). We also examined whether p38 activation has any effect on Akt phosphorylation using the MKK6EE/C2 stable cell line, and no measurable effect was detected (data not shown). Furthermore, the specific PI3K inhibitor LY294002 inhibited MKK6EE-induced differentiation without affecting p38 kinase activity in these cells (Fig. 7). As a control, PD98059 and rapamycin did not inhibit MKK6EE-mediated p38 activation (Fig. 7b). These results strongly suggest that the p38 MAPKs and the IGF-PI3K-Akt signaling pathways are independent but that they operate in parallel to stimulate myogenic differentiation. Inhibition of either pathway results in the abrogation of the myogenic program.
FIG. 6.
Inhibition of p38 blocks IGF-induced myogenic differentiation without affecting the IGF-PI3K-Akt pathway. Nearly confluent C2C12 cells were shifted to serum-free medium in either the absence or presence of IGF1 (50 μg/ml). (a) Cells were harvested at the indicated times, and 100-μg samples of cell lysates were used for a p38 immune complex kinase assay (KA) using AFT2 as a substrate. The level of p38 was determined by immunoblotting (IB) with anti-p38 antibody (α-p38). (b) C2C12 cells were cultured in serum-free medium without or with IGF1 (50 ng/ml). One of the cultures was also treated with SB202190, as indicated. Cells were fixed 24 h after treatment and subjected to immunofluorescence analysis with anti-MHC antibody. (c) Cells were either left untreated or pretreated with different kinase inhibitors for 30 min before IGF1 was added. Whole-cell extracts were analyzed by immunoblotting with antibodies against phospho-Akt (p-Akt) or α-actin. NT, nontreated; SF, serum free; SB, SB202190 (10 μM); PD, PD98059 (25 μM); LY, LY294002 (25 μM); Rapa, rapamycin (10 ng/ml).
FIG. 7.
Inhibition of PI3K blocks MKK6EE-induced myogenic differentiation without affecting p38 activity. Tetracycline (Tet)-inducible MKK6EE/C2 cells were cultured in the presence of tetracycline (2 μg/ml) until they were 70% confluent. Tetracycline was then removed to induce MKK6EE expression. Cells were cultured in tetracycline-free medium for 24 h and then shifted to DM. (a) Cells were treated with either the vehicle (dimethyl sulfoxide) or different kinase inhibitors for 36 h in DM. (b) Whole-cell extracts were analyzed by immunoblotting with antibodies against phospho-p38 (p-p38) and total p38. The final concentrations of inhibitors used were 10 μM for SB202190, 25 μM for PD98059 (PD), 25 μM for LY294002 (LY), and 10 ng/ml for rapamycin (Rapa). NT, nontreated.
Dual role of ERK activity during C2C12 myogenic differentiation.
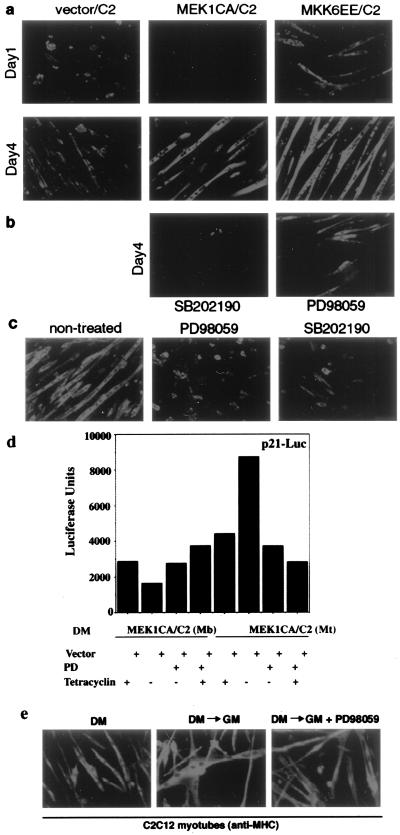
Several investigators have reported that the ERK group of MAPKs also plays a role in muscle differentiation. While some investigators indicated that ERK members inhibit differentiation (5, 11), one report indicated that ERKs are positive regulators of myogenesis (18). Our own observations of biphasic regulation of ERK activity during myogenic differentiation (Fig. 1d) suggest that the ERK pathway may play a dual role in this process. To examine this possibility, we generated an inducible C2 stable cell line overexpressing a constitutively active mutant of MEK1 (MEK1CA or MEK1DD/ΔN4) (39), a specific activator of the ERK1 and -2. As in the case of MKK6EE, we used the tetracycline system to achieve regulated MEK1CA expression and confirmed that its induction leads to specific activation of ERKs but not of p38 or JNK (data not shown). We observed that, when induced to differentiate by serum withdrawal, this stable cell line initially lagged behind vector-transfected cells in the formation of MHC-positive myotubes (Fig. 8a, day 1, middle panel). Consistently, forced activation of endogenous ERKs by transient expression of its activator, MEK1CA, reduced the activation of myogenic reporters in both C2C12 cells and 10T1/2 fibroblasts converted by MyoD (Fig. 3a). However, after a few days of lagging behind their wild-type counterparts in MHC expression, MEK1CA/C2 cells started to form multinucleated myotubes larger than those in vector-transfected cells (Fig. 8a, day 4, middle panel). In a distinct experiment, continuous treatment of either vector-transfected C2C12 cells or MEK1CA/C2 cells with PD98059, a specific inhibitor of MEK1 and -2 (15), initially (day 1 of growth in DM) increased the number of MHC-positive myocytes. However, continuous exposure to PD98059 for 3 days resulted in a reduced number of mature multinucleated myotubes (data not shown), in agreement with a previous report (5). Thus, ectopic activation of ERK, while initially interfering with terminal differentiation, eventually enhances the formation of multinucleated myotubes at late stages of differentiation. We also tested whether inhibition of one MAPK pathway could interfere with the differentiation program mediated by another MAPK. As shown in Fig. 8b, we found that treatment with SB202190 inhibited late myotube formation in MEK1CA/C2 cells. Treatment with PD98059 also reduced myotube formation in the C2 cell line expressing MKK6EE, albeit to a lesser extent (Fig. 8b). Furthermore, we tested the effect of either the p38 or ERK inhibitor in preformed multinucleated myotubes. Figure 8c shows that myotubes continuously exposed to either SB202190 or PD98059 displayed a reduced size, collapsed, and became round. These results collectively indicate that activation of both the p38 and ERK pathways is required for optimal differentiation and myotube survival. Also, these results suggest that the ERK pathway may play opposite roles in the regulation of myogenic transcription, depending on the particular stage of the myogenic program. We directly tested this hypothesis by investigating the effect of ERK activation on a myogenic reporter in undifferentiated versus differentiated muscle cells. Toward this aim, we used the MEK1CA/C2 cell line, in which the ERK pathway can be ectopically stimulated by inducing MEK1CA expression through tetracycline withdrawal. These cells were transfected with the p21 promoter lacking the p53 binding sites, which is regulated by MyoD in muscle cells and has been reported to be responsive to ERK in other cell types (4a, 38a). The expression of this reporter was reduced by MEK1CA activation in myoblasts induced to differentiate, and the effect was reversed by PD98059 treatment (Fig. 8d). In contrast, deliberate activation of ERK in formed myotubes, as achieved by tetracycline removal from the medium, increased the expression of the p21 promoter. Again, exposure to PD98059 abrogated this response (Fig. 8d). It is interesting that the activation of ERK is reminiscent of the effect of serum, which is inhibitory toward myogenic differentiation in myoblasts but which stimulates myogenic transcription in myotubes (P. L. Puri, unpublished results). This consideration prompted us to investigate whether the ERK pathway is also involved in growth factor-induced hypertrophy of terminally differentiated myotubes (50a). As already reported (52a), ERK activity can be stimulated about twofold by growth factors in myotubes contained in serum (data not shown). The stimulation of ERK activity was accompanied by an evident increase in the sizes of exposed myotubes (Fig. 8e, middle panel), which reflects the hypertrophic response of these cells. Inhibition of ERK by exposure to PD98059 completely abrogated serum-induced myotube hypertrophy (Fig. 8e, right panel), indicating an essential role of the ERK pathway in this process. Interestingly, treatment with SB202190 also inhibited both serum-dependent and MEK1-mediated hypertrophy of myotubes (P. L. Puri, unpublished results). These results implicate ERK and p38 kinases as components of a pathway which regulates postmitotic growth of terminally differentiated muscle cells.
FIG. 8.
Dual roles of ERK during myogenesis. (a) Nearly confluent C2C12 cells stably transfected with either an empty vector, an MEK1(CA) expression vector, or an MKK6EE expression vector were first induced by tetracycline removal from the culture medium for 1 day and then shifted to DM for various times as indicated. Cells were fixed and stained for the expression of MHC. (b) Conditions were the same as those described for panel a except that SB202190 (final concentration, 10 μM) was added to MEK1CA/C2 cells and PD98059 (final concentration, 25 μM) was added to MKK6EE/C2 cells at the time when cells were shifted to DM and inhibitors were kept for 4 days. Culture medium was replaced every 24 h with freshly added inhibitors. (c) C2C12 myotubes were exposed to either SB202190 (final concentration, 10 μM) or PD98059 (final concentration, 25 μM) for 36 h and then fixed and stained for the expression of MHC. (d) The MEK1CA/C2 cell line was transfected with the p53-site-deleted p21-Luc reporter. The ERK pathway was either stimulated by tetracycline removal or inhibited by PD98059 treatment of myoblasts (MEK1CA/C2 Mb) induced to differentiate by being cultured in DM and of myotubes (MEK1CA/C2 MT). Cells were harvested to measure luciferase activity. All determinations were done in duplicate, and the data shown are representative of three independent experiments. Fold activation is the ratio of luciferase activity in MKK-transfected cells to cells transfected with an empty vector. (e) C2C12 cells were allowed to differentiate into multinucleated myotubes by culturing them in DM for 3 days (left panel) and then restimulated by 20% FBS in the absence (middle image) or in the presence (right panel) of the ERK inhibitor PD98059. Cells were fixed at the indicated times and examined for MHC expression by indirect immunofluorescence. DM→GM, serum restimulation.
DISCUSSION
Selective activation of the p38 pathway during muscle differentiation.
The results described above illustrate a pathway by which extracellular factors can modulate myogenesis through activation of distinct MAPKs. p38 activation in muscle cells placed in DM displays features (i.e., persistent activation in the absence of parallel JNK stimulation) which distinguish this pathway from those activated in response to stress or cytokines. Since p38 is activated in muscle cells upon serum removal, it may be a part of a regulatory circuit by which serum growth factors silence the myogenic program in proliferating myoblasts. We have observed that serum starvation of confluent cells results in p38 activation also in 10T1/2 and NIH 3T3 fibroblasts (Z. Wu and P. L. Puri, unpublished data). However, only transient activation of p38 was observed in these cells in response to serum withdrawal. In contrast, fibroblasts stably expressing MyoD display an activation of p38 that persists along the whole process of myogenic conversion (Z. Wu and P. L. Puri, unpublished data), implicating a role for MyoD (or MyoD-dependent genes) in maintaining p38 activation in myogenic cells. This observation suggests the possible existence of a positive feedback between p38 and MyoD during the differentiation program.
At present we do not know the signal that triggers p38 activation in myogenic cells placed in DM. It is possible that p38 activity is mostly under a negative control factor in proliferating myogenic progenitors. Serum withdrawal and/or cell-to-cell contact may stimulate the p38 pathway by silencing the activity of a mitogen-dependent factor (e.g., a phosphatase). However, the findings that deliberate p38 activation overcomes the inhibitory effect of serum on muscle differentiation and stimulates myotube formation in the presence of mitogens also raise the possibility that the p38 pathway may be directly triggered by positive regulators of myogenesis that remain to be identified. In this regard, we have observed that IGF, which is known to stimulate muscle differentiation, does not activate the p38 cascade in muscle cells (Fig. 6a). Nevertheless, other upstream signaling molecules reported to stimulate myogenesis (e.g., the transmembrane protein CDO) (31a) may be implicated in the activation of the p38 pathway. With respect to this, it has been observed that the p38 pathway is more efficiently activated in confluent muscle cells placed in DM than in subconfluent myocytes cultured under the same conditions (P. L. Puri, unpublished results). This observation underscores the importance of cell density in the activation of p38 during muscle differentiation.
p38 MAPKs positively regulate myogenesis through MyoD and MEF2 proteins.
Once activated, p38 can phosphorylate and stimulate the activities of MEF2C and MEF2A (our data and references 21, 48, 61, and 67). Given the essential function of MEF2 proteins in muscle differentiation (7), the enhancement of their transcriptional activities by p38-mediated phosphorylation should result in activation of many myogenesis effector genes. While this work was in preparation, others reported that p38 MAPKs are required for myogenic differentiation of rat L8 and mouse C2C12 cells, respectively (12, 64). However, these conclusions rested mainly on the use of p38 inhibitors and do not provide insight into the mechanisms that underlie p38-dependent activation of the myogenic program. Although MEF2C and -A were shown to be p38 targets in nonmuscle cells as well as in muscle cells (21, 48, 61, 64), it was not demonstrated that endogenous MEF2C is actually phosphorylated by p38 in differentiating myoblasts. We therefore carried out a detailed mapping of the phosphorylation sites relevant to MEF2C activation during myogenesis. It has been previously shown that p38 can phosphorylate MEF2C on three residues (T293, T300, and S387) located in the activation domain (21, 61, 67). The simultaneous phosphorylation of these residues is important for MEF2C activation in nonmuscle cells (e.g., lymphoid cells) (21). The results presented in Fig. 4 indicate that the phosphorylation of threonine 293 is critical for the activation of MEF2C transcriptional function in muscle cells, suggesting that MEF2C regulation by p38 kinases occurs through selective phosphorylation at distinct residues in a tissue-restricted manner. MEF2 proteins affect muscle differentiation through synergistic interactions with myogenic bHLH factors (45). This raises the possibility that p38-mediated phosphorylation of MEF2 members may enhance the transcriptional synergy between MyoD and MEF2. Our data argue against this possibility, since we failed to detect any effect of p38 on MEF2C-MyoD interactions using a mammalian two-hybrid system (P. L. Puri and Z. Wu, unpublished data). In addition, we and others (47a) have observed that mutation of threonine 293 to alanine, which prevents in vivo p38-mediated phosphorylation and activation of MEF2C, does not affect MyoD-MEF2C functional synergism (P. L. Puri and Z. Wu, unpublished results). Finally, a Gal4-MyoD fusion protein without the basic domain and therefore impaired in associating with MEF2 proteins is still activated by MKK6EE. However, it is still formally possible that p38 stimulates bHLH-MEF2 functional synergism by an alternative mechanism, since a functional collaboration between MyoD and MEF2C has been reported also in the absence of their physical interaction (47a). In agreement with our results, Novitch et al. reported that the mutation of serine 387 to alanine does not affect the transcriptional activation of MEF2C by p38 kinase (47a). Moreover, they observed that this mutation abrogates the enhancement of MEF2C activity by MyoD (47a). This finding suggests that stimulation of the intrinsic transcriptional activity of MEF2C and induction of functional synergism between MEF2C and MyoD may be two separate processes controlled by distinct mechanisms. It is possible that, in muscle cells, p38-mediated phosphorylation of threonine 293 is important for induction of MEF2C intrinsic transcriptional activity but that phosphorylation of serine 387, which might not be mediated by p38, can be a signal required for functional synergism with myogenic bHLH factors.
The observation that the p38 pathway is essential for activation of both the MyoD-dependent promoter and the MyoD-mediated conversion of fibroblasts into myogenic cells suggests the possibility that MyoD might also be a target of p38. Due to the use of a weak p38 activator (the wild-type MKK6 construct), previous works failed to detect a p38-mediated enhancement of MyoD transcriptional activity (64). By using the constitutive activated p38 activator MKK6EE, we could consistently detect a positive effect of p38 on several MyoD-responsive reporters (Fig. 3a and 5). As discussed above, it is unlikely that the stimulatory effect of MKK6EE on MyoD is mediated through physical interactions with MEF2 proteins. Experiments carried out with reporter cell lines and chromatin-integrated MyoD-responsive templates suggest that p38 might stimulate MyoD-dependent chromatin remodeling. Although we have observed efficient phosphorylation of MyoD by p38 in vitro and we have detected a p38-dependent phosphorylation of serine 5 at the N terminus of MyoD during myotube formation (P. J. Woodring, Z. Wu, and P. L. Puri, unpublished results), the replacement of this serine with an alanine did not significantly alter MyoD transcriptional activity. Thus, it is likely that p38 stimulates MyoD-dependent transcription by an indirect mechanism. Consistent with this, it has been observed that the activities of other myogenic bHLH proteins, which do not share in vitro putative p38 phosphorylation sites (like serine 5 in MyoD), can also be stimulated by MKK6EE (P. L. Puri, unpublished results). This may also explain the functional relevance of the p38 pathway in muscle cell lines deficient in MyoD expression, like rat L6 myoblasts (64). One possibility is that p38 indirectly stimulates myogenic transcription by targeting bHLH transcriptional coactivators, such as p300 and PCAF (50, 53).
As there are four p38 isoforms encoded by separate genes, another relevant question is which of the four isozymes is involved in myogenic activation. Since SB202190 inhibits only p38α and p38β (37), it is likely that one or both of these isozymes may be involved in myogenic differentiation. Congruently, transient overexpression of either isozyme leads to activation of muscle-specific reporter genes (Z. Wu and P. L. Puri, unpublished data). Although the p38γ isozyme was reported to be expressed exclusively in skeletal muscle (36), it is insensitive to SB202190 and therefore is an unlikely major mediator of the stimulatory effect of MKK6 in muscle cells. Similar arguments can be made against p38δ. In accordance with that, overexpression of either the p38γ or p38δ isoform did not enhance myogenic transcription (Z. Wu and P. L. Puri, unpublished data). Genetic evidence provided by experiments performed with p38α−/− mEFs demonstrates that in the absence of p38α the ability of MyoD to activate the myogenic program in cultured nonmuscle cells is reduced and that reexpression of p38α may restore this activity. While these results indicate an essential role for p38α in promoting MyoD-dependent myogenic conversion of fibroblasts, the presence of p38β might account for the residual activation of the myogenic program in the absence of p38α and can explain the lack of an apparent muscle phenotype in p38 knockout mice (K. Tamura and M. Karin, unpublished data). In agreement with this, it was recently shown that both p38α and p38β phosphorylate MEF2C and MEF2A and enhance their transcriptional activities. In contrast, p38γ only weakly phosphorylates MEF2A and MEF2C in vitro and barely stimulates their transcriptional activities in vivo while p38δ does not phosphorylate MEF2A or MEF2C at all (61, 65). These results suggest that the different p38 isoforms may have their own preferred substrates, as previously reported for different isoforms of JNK (31).
The MKK6-p38 pathway and IGF-regulated signaling are two parallel cascades involved in myogenesis.
IGFs are the only peptide hormones known to induce myogenic differentiation in mammalian systems (11). We investigated whether p38 could be an effector of IGF's myogenic activity. Such a relationship seemed reasonable, because PI3K, whose activity is strongly stimulated by IGF (60), was previously shown to lead to activation of JNK (52), whose activity is usually regulated similarly to that of p38 (43). Much to our surprise, activation of p38 during muscle differentiation was independent of IGF action (Fig. 6a). Furthermore, p38 activation had no effect on Akt activity, an important component of the IGF-PI3K signaling pathway. Nevertheless, specific inhibition of p38 with SB202190 blocked the myogenic effect of IGF1 in both mouse C2C12 cells and rat L6 cells, without interfering with activation of Akt. Recently, ectopic expression of Akt was found to stimulate myogenic differentiation (17, 28). Accordingly, a constitutively activated form of PI3K enhances both MyoD- and MEF2-dependent transcription (Z. Wu, unpublished results). Specific inhibition of PI3K by LY294002 abrogated myogenic differentiation not only in response to IGF1 but also in response to MKK6EE, without severely affecting p38 activation (Fig. 7). Taken together, these results indicate that these two signaling pathways must act in parallel and that both are absolutely required for myogenic differentiation, at least in cultured cells.
Another target for IGF-PI3K signaling is the p70 S6 kinase (p70S6K) (10, 46a, 56). A specific inhibitor of IGF- and PI3K-mediated p70S6K activation is rapamycin (13). As rapamycin was found to inhibit myogenic differentiation, p70S6K is another protein kinase implicated in the control of myogenesis (11, 12). The relationship between Akt and p70S6K in PI3K signaling is still not clear. Our results suggest that p70S6K acts either downstream of Akt or in a parallel branch downstream of PI3K, because IGF induced Akt phosphorylation was not inhibited by rapamycin (Fig. 6c). Interestingly, rapamycin also blocked MKK6EE-mediated myogenic differentiation (unpublished data) without affecting p38 activity. Thus, IGF may stimulate myogenesis via either one linear PI3K-to-Akt-to-p70S6K pathway or via a branched pathway in which Akt and p70S6K both serve essential functions downstream of PI3K. Although these pathways have no effect on p38 activity, they are also required for induction of differentiation in response to MKK6EE expression. One interesting question that remains to be answered is whether the signals from the two pathways converge on the same or different targets.
Dual role of ERK during the myogenic program.
The ERK pathway has also been implicated in the control of myogenesis, but its functions seem to be controversial, as some reports have proposed an inhibitory role at the beginning of the myogenic program (5, 11) while another report proposed a positive regulatory function (18). We were able to unify these seemingly conflicting results by demonstrating that ERK activity is subject to biphasic regulation and has a dual function during myogenesis. The concomitant decline in ERK activity and stimulation of p38 kinase during the early phase of terminal differentiation may facilitate a reduction in cyclin D1 levels (5, 35), thereby leading to activation of MyoD (54, 65), followed by the induction of myogenic markers, including p21. The combined effects of cyclin D1 downregulation and p21 upregulation contribute to the permanent withdrawal of myoblasts from the cell cycle. Forced ERK activation at this stage, as achieved by using a constitutively active MEK1 mutant, interferes with initiation of the differentiation program. During later stages of myogenic differentiation, ERK activity increases and seems to cooperate with p38 in promoting differentiation and/or myotube fusion. This explains why prolonged inactivation of ERK either by PD98059 (data not shown and reference 18) or by overexpression of the dual-specificity phosphatase MKP-1 (5) prevents formation of mature myotubes. Furthermore, results presented in Fig. 8 implicate ERK and p38 in the process of myotube survival and in the hypertrophic growth of myotubes following growth factor stimulation. These results illustrate a multistep regulation of the myogenic program by p38 and ERK, with p38 being an essential activator of myogenic transcription and ERK exerting two opposite functions depending on the stage of the differentiation program. In proliferating myoblasts, the ERK pathway represses myogenic transcription and contributes to the maintenance of the undifferentiated phenotype. The reduction of ERK activity upon serum removal can therefore relieve that repression and allow p38-mediated muscle-specific transcription. Once the activation of the myogenic program is initiated, ERK activation is no longer repressive and cooperates with p38 in promoting postmitotic responses in differentiated myotubes. It is therefore likely that the antimyogenic function of ERK depends on its mitogenic potential in undifferentiated myoblasts. After the acquisition of their postmitotic state, myotubes become refractory to the mitogenic activity of ERK and the ERK pathway is converted into a promyogenic one. At the stage of multinucleated myotubes, ERK activation by serum contributes to p21 activation (Fig. 8d), and this can be important in rendering myotubes refractory to mitogenic stimuli. Remarkably, a dual function has also been described for IGF1 (15a). Sarbassov et al. have reported the ability of IGFs to activate ERKs in muscle cells (52a). Since IGFs are secreted by muscle cells in culture, the ERK pathway must be an important mediator of such an autocrine loop, which regulates myogenic differentiation and myocyte hypertrophy (46).
The results presented here may have biological implications in therapeutic strategies aimed at stimulating the differentiation of satellite cells after muscle injury (2) or in muscle degenerative diseases. Recruitment of satellite cells followed by fusion into multinucleated myofibers is regulated by paracrine growth factors, whose effects are transduced by MAPK pathways. Therefore, proper modulation of these pathways can be used to stimulate muscle regeneration. Similarly, modulation of the activities of MAPKs can be useful in improving the efficiency of muscle-mediated gene therapy. For instance, it may be possible to stimulate proliferation of transplanted muscle satellite cells by first increasing ERK activity in order to expand the pool of myoblasts further available for p38-induced differentiation. Moreover, the described role of the ERK pathway in mediating the hypertrophic response of postmitotic myocytes might also have implications in designing strategies aimed at enhancing both the size and activity of muscle fibers in patients with myopathies. Finally, the ability of p38 to activate MyoD can be used to stimulate the differentiation of muscle-derived tumors (rhabdomyosarcomas) in which MyoD is functionally latent (55) and the p38 pathway is not activated (50b).
ACKNOWLEDGMENTS
Z.W. and P.L.P. contributed equally to this work.
We thank J. Han, V. Sartorelli, L. Kedes, F. Tatò, W. Wright, E. Bengal, J. Houghton, and R. Henry for reagents and B. Thompson for manuscript preparation.
This work was supported by grants from the National Institutes of Health (M.K.) and NCI (J.Y.J.W.) and by grants from the California Department of Health Services Cancer Research Program (M.K. and J.R.F.) and Telethon (P.L.P.). M.K. is an American Cancer Society research professor. P.L.P. was partially supported by a postdoctoral fellowship from the Human Frontier Science Program. Z.W. was supported by postdoctoral fellowships from the Medical Research Council of Canada and the Human Frontier Science Program, and P.J.W. was supported by a postdoctoral fellowship from the National Institutes of Health.
REFERENCES
- 1.Alemà S, Tatò F. Oncogenes and muscle differentiation: multiple mechanism of interference. Semin Cancer Biol. 1994;5:147–156. [PubMed] [Google Scholar]
- 2.Anderson J E. Murray L. Barr Award lecture. Studies of the dynamics of skeletal muscle regeneration: the mouse came back! Biochem Cell Biol. 1998;76:13–26. [PubMed] [Google Scholar]
- 3.Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold H H, Winter B. Muscle differentiation: more complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8:539–544. doi: 10.1016/s0959-437x(98)80008-7. [DOI] [PubMed] [Google Scholar]
- 4a.Beier F, Taylor A C, La Valle P. The Raf-1/MEK/ERK pathway regulates the expression of the p21 cip1/waf1 gene in chondrocytes. J Biol Chem. 1999;274:30273–30279. doi: 10.1074/jbc.274.42.30273. [DOI] [PubMed] [Google Scholar]
- 5.Bennett A M, Tonks N K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 6.Black B L, Molkentin J D, Olson E N. Multiple roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with MEF2. Mol Cell Biol. 1998;18:69–77. doi: 10.1128/mcb.18.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black B L, Olson E N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Chen C Y, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Costa M L, Mermelstein C S, Chagas C, Holtzer S, Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci USA. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 11.Coolican S A, Samuel D S, Ewton D Z, McWade F J, Florini J R. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 12.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 13.Dennis P B, Fumagalli S, Thomas G. Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev. 1999;9:49–54. doi: 10.1016/s0959-437x(99)80007-0. [DOI] [PubMed] [Google Scholar]
- 14.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 15.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Engert J C, Berglund E B, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyers P A, Craxton M, Morrice N, Cohen P, Goedert M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem Biol. 1998;5:321–328. doi: 10.1016/s1074-5521(98)90170-3. [DOI] [PubMed] [Google Scholar]
- 17.Fujio Y, Guo K, Mano T, Mitsuuchi Y, Testa J R, Walsh K. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol. 1999;19:5073–5083. doi: 10.1128/mcb.19.7.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Gerber A N, Klesert T R, Bergstrom D A, Tapscott S J. Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 1997;11:436–450. doi: 10.1101/gad.11.4.436. [DOI] [PubMed] [Google Scholar]
- 18.Gredinger E, Gerber A N, Tamir Y, Tapscott S J, Bengal E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J Biol Chem. 1998;273:10436–10444. doi: 10.1074/jbc.273.17.10436. [DOI] [PubMed] [Google Scholar]
- 19.Guo K, Wang J, Andres V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Jiang Y, Li Z, Kravchenko V V, Ulevitch R J. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 23.Hannon K A, Kudla J, McAvoy M J, Clase K L, Olwin B. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J Cell Biol. 1996;132:1151–1159. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 25.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 26.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 27.Jacinto E, Werlen G, Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/s1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
- 28.Jiang B H, Aoki M, Zheng J Z, Li J, Vogt P K. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaliman P, Canicio J, Shepherd P R, Beeton C A, Testar X, Palacin M, Zorzano A. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol Endocrinol. 1998;12:66–77. doi: 10.1210/mend.12.1.0047. [DOI] [PubMed] [Google Scholar]
- 30.Kaliman P, Vinals F, Testar X, Palacin M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- 31.Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–939. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- 31a.Kang J-S, Mulieri P J, Miller C, Sassoon D A, Krause R. CDO, a Robo-related surface protein that mediates myogenic differentiation. J Cell Biol. 1998;143:403–413. doi: 10.1083/jcb.143.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos Trans R Soc Lond Ser. 1996;351:127–134. doi: 10.1098/rstb.1996.0008. [DOI] [PubMed] [Google Scholar]
- 33.Lassar A, Munsterberg A. Wiring diagrams: regulatory circuits and the control of skeletal myogenesis. Curr Opin Cell Biol. 1994;6:432–442. doi: 10.1016/0955-0674(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 34.Lassar A B, Skapek S X, Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 35.Lavoie J N, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 36.Lechner C, Zahalka M A, Giot J F, Moller N P, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 38.Lin A, Minden A, Martinetto H, Claret F X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 38a.Liu Y, Martindale J L, Gorospe M, Holbrook N J. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signalling pathway. Cancer Res. 1996;56:31–35. [PubMed] [Google Scholar]
- 39.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 40.Marinissen M J, Chiariello M, Pallante M, Gutkind J S. A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol Cell Biol. 1999;19:4289–4301. doi: 10.1128/mcb.19.6.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 42.McDermott J C, Cardoso M C, Yu Y, Andres V, Leifer D, Krainc D, Lipton S A, Nadal-Ginard B. hMEF2C gene encodes skeletal muscle- and brain-specific transcription factors. Mol Cell Biol. 1993;13:2564–2577. doi: 10.1128/mcb.13.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 44.Molkentin J D, Black B L, Martin J F, Olson E N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 45.Molkentin J D, Olson E N. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc Natl Acad Sci USA. 1996;93:9366–9373. doi: 10.1073/pnas.93.18.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musarò A, McCullagh K J A, Naya F, Olson E N, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 46a.Myers M G, Jr, Grammer T C, Wang L M, Sun X J, Pierce J H, Blenis J, White M F. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J Biol Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- 47.Naviaux R K, Costanzi E, Haas M, Verma I M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Novitch B G, Spicer D B, Kim P S, Cheung W L, Lassar A B. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr Biol. 1999;9:449–459. doi: 10.1016/s0960-9822(99)80210-3. [DOI] [PubMed] [Google Scholar]
- 48.Ornatasky O I, Cox D M, Tangirala P, Andreucci J J, Quinn Z A, Wrana J L, Prywes R, Yu Y-T, McDermott J. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 1999;21:2646–2654. doi: 10.1093/nar/27.13.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledge S J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 50.Puri P L, Sartorelli V, Yang X J, Hamamori Y, Ogrizko V V, Howard B H, Kedes L, Wang J Y, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 50a.Puri P L, Avantaggiati M L, Burgio V L, Chirillo P, Natoli G, Balsano C, Levrero M. Reactive oxygen intermediates (ROIs) mediate angiotensin II-induced AP1-DNA binding increase and proliferative/hypertrophic response in myogenic C2C12 cells. J Biol Chem. 1995;270:22129–22134. doi: 10.1074/jbc.270.38.22129. [DOI] [PubMed] [Google Scholar]
- 50b.Puri P L, Wu Z, Zhang P, Wood L D, Bhakta K S, Han J, Feramisco J R, Karin M, Wang J Y J. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–587. [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 52.Rodrigues G A, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Sarbassov D D, Jones L G, Peterson C. Extracellular signal-regulated kinase-1 and 2 respond differently to mitogenic and differentiative signalling pathways in myoblasts. Mol Endocrinol. 1997;11:2038–2047. doi: 10.1210/mend.11.13.0036. [DOI] [PubMed] [Google Scholar]
- 53.Sartorelli V, Huang J, Hamamori Y, Kedes L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol Cell Biol. 1997;17:1010–1026. doi: 10.1128/mcb.17.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skapek S X, Rhee J, Spicer D B, Lassar A B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 55.Tapscott S J, Thayer M J, Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–1453. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- 56.Thomas G, Hall M N. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 57.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 58.Weintraub H, Dwarki V J, Verma I, Davis R, Hollenberg S, Snider L, Lassar A, Tapscott S J. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991;5:1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- 59.Weintraub H, Tapscott S J, Davis R L, Thayer M J, Adam M A, Lassar A B, Miller A D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto K, Lapetina E G, Moxham C P. Insulin like growth factor-I induces limited association of phosphatidylinositol 3-kinase to its receptor. Endocrinology. 1992;130:1490–1498. doi: 10.1210/endo.130.3.1311242. [DOI] [PubMed] [Google Scholar]
- 61.Yang S H, Galanis A, Sharrocks A D. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19:4028–4038. doi: 10.1128/mcb.19.6.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun K, Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr Opin Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- 63.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 64.Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J-M, Wei Q, Zhao X, Paterson B M. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 1999;18:926–933. doi: 10.1093/emboj/18.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P, Wong C, Liu D, Finegold M, Harper J W, Elledge S J. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao M, new L, Kravchenko V V, Kato Y, Gram H, di Padova F, Olson E N, Ulevitch R J, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]