Abstract
The nematode Caenorhabditis elegans (C. elegans) is a prevailing model which is commonly utilized in a variety of biomedical research arenas, including neuroscience. Due to its transparency and simplicity, it is becoming a choice model organism for conducting imaging and behavioral assessment crucial to understanding the intricacies of the nervous system. Here, the methods required for neuronal characterization using fluorescent proteins and behavioral tasks are described. These are simplified protocols using fluorescent microscopy and behavioral assays to examine neuronal connections and associated neurotransmitter systems involved in normal physiology and aberrant pathology of the nervous system. Our aim is to make available to readers some streamlined and replicable procedures using C. elegans models as well as highlighting some of the limitations.
Keywords: C. elegans, behavior, fluorescence microscopy, neurotransmitters, connectomes
1. Introduction: Strength of C. elegans in Neuronal System Evaluations
The Caenorhabditis elegans (C. elegans) model is a soil free-living nematode, whose advantages include small size (~1 mm in adulthood), simple anatomy, short life-cycle (~3 days at 20 °C), numerous progeny (300 per worm), simple maintenance, four larval stages (L1; L2; L3 and L4) and finally the adult. Under adverse conditions, the worm may assume a resistant larva stage, referred to as dauer. C. elegans compact and well-annotated genome presents considerable homology of 60 to 80% to mammalian genes [1]. The worm can be genetically modified to many target genes though established genetic methods such as mutagenesis and RNA interference (RNAi) knockdown, affording several options for the use of C. elegans for studies on gene-mediated processes [2]. In contrast to rodent models, C. elegans’s transparency allows for easy observations of intra-body structures and the use of labeled (e.g. fluorescence, etc.) biomolecules in vivo. Fluorescent proteins to tag specific proteins can elucidate the developmental process, neurotoxicity, fat storage, mitochondria integrity, and characterize protein expression [3–6]. Thus, the construction of transgenics expressing the green fluorescent protein (GFP) in specific neurons enables the evaluation of metal neurotoxicity in C. elegans with relative ease [6,7]. Laboratories interested in C. elegans research can quickly start the colony by ordering the strains (wild-type and transgenics) and Escherichia coli (E. coli) from Caenorhabditis Genetics Center (CGC). The wholly mapped C. elegans nervous system comprises 302 neurons in adult hermaphrodite worms and 383 in adult male worms resulting in approximately 8,000 synaptic connections. Most male-specific neurons are tail-associated and involved in mating behavior [8].
The above features provide quite a number of benefits to the continuous use of C. elegans biomedical for research. However, these benefits do not entirely eliminate its limitations as an ideal experimental model. First, its small body size and shortened length impedes proper biochemical investigations of oxidative markers and genetic products of nutrition. These have created a relatively limited usage of the nematode in research involving aging and some system-specific markers such as adaptive immunity and tissue-signaling source [9–11]. Furthermore, the simple anatomical plan and biology of C. elegans constrains its tissue organization into specialized organ-systems, thereby creating an evolutionary gap when compared to humans [12]. The C. elegans model further lacks the appropriate inflammatory cell types; microglial cell, that can equilibrate those expressed in humans during neurodegenerative changes [13,14]. Other difficulties encountered when adopting this model is in its use to imitate the underlying pathology in neurodegenerative disorders. This is especially due to mammalian cell interacting complexities which lead to a multifactorial disease nature in humans [15]. Despite the above limitations, the C. elegans model is proving vital to unravelling cellular and molecular mechanisms that underly normal and perturbed processes in the nervous system.
Since neurons are continuously activated by changes in ionic gradients leading to the transmission of information, any change in homeostatic physiological processes can impact their optimal function triggering neurodegeneration. The C. elegans genome encodes conserved neurotransmitter biosynthesis and releasing mechanisms and receptors to mammal neurotransmitters such as acetylcholine, glutamate, γ-aminobutyric acid (GABA), serotonin, and dopamine [16]. Glutamate is an excitatory neurotransmitter in vertebrates and invertebrates and its role and each specific neuron in the physiological orchestration of the worm are still being studied. In C. elegans, the vesicular glutamate transporter (VGluT / EAT-4) seems to be the only specific marker of glutamatergic neurons, since their synthesis does not occur only in these neurons and the glutaminases are distributed throughout the animal’s body. Hermaphrodite worms have 79 glutamatergic neurons and males approximately 98. Glutamatergic neurons which are distributed in the head, pharynx, ventral nerve cord and body and tail [17].
Acetylcholine (ACh) has been reported as the predominant neuromuscular transmitter in C. elegans. The unc-17 gene encodes the worm ACh vesicular transporter (VAChT) which is used to tag the GFP to cholinergic neuron assessment. Cholinergic neurons count 160 in hermaphrodites and 193 in males, and are thus located: head, ventral nerve cord and body, pharynx and tail [17,18].
The γ-Aminobutyric acid (GABA) is an amino acid neurotransmitter with both excitatory and inhibitory functions’ in C. elegans. The 26 GABA neurons comprised of 6 DD (motors), 13 VD (motors), 4 RME (head), RIS (interneuron), AVL, and DVB (enteric muscles). The serotoninergic system is not well established as to the number of neurons in this classification. Hermaphrodites have from 11 to 13 serotonergic neurons and males from 20 to 22 [19,20]. Dopaminergic neurons in adult worms are 8 in hermaphrodites (head: ADE (2), CEP (4); ventral body nerve: PDE (2)) and 16 in males (head: ADE (2), CEP (4); ventral nerve of the body: PDE (2), tail: R5A (2), R7A (2), R9A (2), SPSo (2)). Dopamine signaling has established roles in modulating locomotion behavior and learning 22 [19].
In this regard, the effects of metal exposures, including cadmium (Cd), manganese (Mn) [21], zinc (Zn) [22], mercury (Hg), and chromium (Cr) [23] on neurodegeneration has been evaluated in C. elegans. Among the parameters used to define a mature neuron are the position, physical connections (chemical and electrical synapses), electrophysiological properties, molecular means by which chemical signals are propagated and transmitted, and morphology.
Even in comparison to cell culture modeling, studies in C. elegans are of low cost and afford the advantage of using a complete organism. C. elegans is easy and inexpensive to maintain in laboratory conditions with a diet of E. coli OP50. The short, hermaphroditic life cycle (~3 days) and large number (300+) of offspring of C. elegans allows large-scale production of animals within a short period of time. Furthermore, rodent models require brain slices, paralyzed animals, high-technology equipment (e.g., Positron emission tomography (PET) scan), and complicated assays (e.g., electrophysiology) to evaluate neuronal dynamics in vivo [24,25]. Work with C. elegans has led to seminal discoveries in neuroscience, development, signal transduction, cell death, aging, and RNA interference, attracting increased attention including environmental toxicology. C. elegans is predictive of outcomes in higher eukaryotes both at the level of genetic and physiological similarity, as well as at the level of actual toxicity data. Many of the basic physiological processes in higher organisms (e.g. humans) are conserved in C. elegans and twelve out of seventeen known signal transduction pathways are conserved in C. elegans and human. Furthermore, the intensively studied genome, complete cell lineage map, knock-out mutant libraries, and established genetic methodologies including mutagenesis, transgenesis, and RNAi, provide a variety of options to manipulate and study C. elegans at the molecular level.
Here, we discuss the main neurotransmitter systems in C. elegans and how to perform characterizations of neuronal viability and activity. We also described behavioral assessment as measure of neuronal functions, and how neuronal connections and neurotransmitter systems can be associated behavioral outputs using the C. elegans model.
1.1-. Fluorescent Methods to Characterize the Neurons and Connectomes
1.1.1 –. Fluorescent proteins
In order to map the C. elegans nervous system, fluorescent methods are used, which are continually evolving and have been crucial for the development of research in this nematode. C. elegans adult hermaphrodite was the first multicellular organism used to express Green Fluorescent Protein (GFP) [3] so most of the reporter genes in use in C. elegans, including those available at the C. elegans Genetics Center (CGC), use some form of GFP. However, many fluorescent proteins have been developed and extensively modified in recent years [26]. There are currently several types of fluorescent proteins used in C. elegans, some of which are described in Table 1.
Table 1 -.
Fluorescent proteins used in C. elegans research
| Fluorescent proteins | Use | Reference |
|---|---|---|
| YFP, mNeonGreen, mYPet | Yellow fluorescence | [27,28] |
| CFP, mCerulean | Cian fluorescence | [27,29] |
| GFP, CrGFP, mNeonGreen, | Green fluorescence | [28,29,27] |
| BFP, mTagBFP | Blue fluorescence | [29] |
| RFP, KillerRed, mCherry, tdTomato, TagRFP-T, mRuby2, mKate2 | Red fluorescence | [28,27,30] |
The first step in C. elegans neurobiology study is to choose the right strain to evaluate neurons by observing whether fluorescent filters are available, and the target studied. It is essential to consider that the optical density quantified through the fluorescence can be influenced by the stability or degradation and synthesis of the fluorescent protein, which is sometimes not directly related to neuronal death. Transgenic strains for neuron-specific proteins tagged with fluorescent proteins are ideally suited for neurotoxic evaluations.
Constructions combining the GFP and the sodium-dependent DA transporter DAT-1 expressed in DAergic neurons in C. elegans represent the first choice to observe such neurons [31]. The strains BZ555 [egIs1 (Pdat-1::GFP)], BY200 [vtIs1 (Pdat-1::GFP) + pRF4 (rol-6(su1006))], and BY250 [vtIs7 (Pdat-1::GFP)] are available in Caenorhabditis Genetics Center (CGC) [32]. Another option is to use the OH7547 strain [otIs199 (cat-2::GFP + rgef-1(F25B3.3)::DsRed) + (rol-6(su1006)] which expresses GFP in DAergic neurons and DsRed pan-neuronally [31]. Intrinsic differences specific for each strain in the response to the exposures should be considered [32]. Transgenic constructions with the gene encoding the protein alpha-synuclein (α-Syn) are used for PD investigation in C. elegans. α-Syn is a key constituent of the Lewy bodies found in PD patients and a genetic risk factor in PD’s pathogenesis [33]. The strain UA44 [baIn11 (Pdat-1::α-syn + Pdat-1::GFP)] expresses both GFP and human α-Syn in the dopaminergic neurons [34].
Glutamatergic neurons are defined by examining the expression of a fosmid-based eat-4 reporter construct found in DA1240 [adIs1240 (eat-4::sGFP + lin-15(+)) X] and DA1243 [adIs1240 (eat-4::sGFP + lin-15(+) X)] strains [35].
The GABAergic neurons can be targeted with unc-47 [EG1285 strain, oxIs12 (Punc-47::GFP + lin-15(+))], serotoninergic with tph-1 [GR1366 strain, mgIs42 (tph-1::GFP + rol-6(su1006))], and cholinergic neurons with unc-17 [LX929, vsIs48 (unc-17::GFP)] [36]. Those are just a few examples amid many possible strains constructions already available that can be extensively crossed.
A relatively short incubation or treatment period is needed for examining the mortality or behavioral trials, which may range from a few minutes [37,38] to hours [39–41,7]. Even if the exposure was carried out in L1 larval stage, the neuronal development is only completed in L4 and that should be considered under the evaluations. After exposure to a compound or condition, the fluorescent worms can be transferred to the slides for microscopy observing.
1.2. Behavioral Assays as Indicators of Neuronal Damage
The shared homolog genome attribute of the C. elegans model with humans is indicative of close similarity in the cellular and molecular activities between humans and this nematode [42,43]. This knowledge has proved advantageous over the decades in studying the neuronal activity in various disease states [44]. Corresponding illustrations of the presence of neuronal damage can be monitored by the use of designated behavioral assays. These behavioral reactions are sometimes initiated by the interactive response following exposure of the nematode to environmental stimuli [45,46]. Some of these behavioral tools include: basal slowing response/ food sensing behavior, ethanol avoidance/preference, plasticity of chemotaxis, locomotor assay, shrinker and loopy foraging, area restricted searching (ARS) behavior, mechanosensory responses, habituation task/ tap withdrawal response, chemotaxis assay, fecundity, dauer-dependent behavior, swim to crawl transition and pharyngeal pumping and thrashing behaviors [47–51]. To avoid behavioral biases, certain factors have to be considered which are dependent on the assay to be carried out. These include: synchronized age, life cycle and sex of the nematode amongst other environmental factors [52]. Here we have described methods for the following behavioral assays.
1.2.1. Basal Slowing Response
This is a dopamine-mediated activity exhibited by C. elegans in the presence of food. It is adaptive conditioning that allows well-fed wild type (WT) nematodes to spend more time feeding. The dopaminergic neural circuits are triggered by a sensory response to the mechanical activity of bacteria (mechanosensation of DA circuits). On the other hand, the serotonergic circuits control another mechanism called “enhanced slowing response”. This heightened slow response involves the introduction of starved (wild-type; WT) worms to a food source, whereby the serotonergic circuits ignites an incessant need to remain within the region. In this assay, the rate of locomotion (number of bends) is the parameter to be quantified. This is a determinant for the functionality of dopaminergic neural circuits [53]. The bas-1, cat-2 and cat-4 mutant strains are defective in the production of these monoamines and can be used as positive controls during the assay [50].
1.2.2. Ethanol Avoidance/Ethanol Preference
This assay demonstrates a form of behavioral plasticity in the C. elegans model with emphasis on its tolerance level. Ethanol is a substance of addiction which under normal conditions is to a certain degree initially repulsive to the worms. Depending on the nature of the experiment conducted, C. elegans can either adapt/generate a preference to an ethanol contained environment (when pre-conditioned) or exhibit an avoidance behavior (when dopamine is depleted). Mutant worms like cat-2 and tph-1 that are defective in dopaminergic and serotonergic neurons respectively, can be used during the assay alongside control wildtype strains for comparison. However, in other mutant strains as ced-10, the presence of an alternative source may compensate for any dopaminergic loss present [54,55]. Ethanol pre-conditioned worms exhibit addictive behavior similar to alcohol intoxication/ addiction in humans. Under such environmental condition (also called conditioned place preference), C. elegans forms a good model of addiction [56]. This assay attests to the integrity of the above-mentioned neurotransmitters, although ethanol preference may not be entirely dependent on serotonin signalling. It could therefore be inferred that dopamine may be the primary neurotransmitter of concern while carrying out this assay. Both neurotransmitters are involved in the regulation of learning, depression, anxiety and addiction amongst other functions in humans. Whereas, in the nematode it controls locomotion, egg-laying, pharyngeal pumping and feeding [57,58].
1.2.3. Plasticity of Chemotaxis by NaCl
Plasticity is a crucial mechanism that promotes the formation of memories and ignites certain experience based behavioral responses from the perceived sensory neural transmission. Plasticity by NaCl is a conditioned associated behavior in which starved nematodes learn to withdraw from NaCl exposed regions after a prolonged time, also called gustatory plasticity. The starved worms under this condition learn to associate food deprivation with the presence of the salt [51]. Although naive worms are typically attracted to NaCl within a concentration of 0.1–200 Mm. However, they act aversively after an extended duration in a concentration of 100 Mm NaCl [59]. This behavior exhibited by C. elegans has been described as associative learning beyond adaptation, as it occurs in the absence of food source and presence of the salt to give a gradient corresponding response [60]. The following neurotransmitters dopamine, serotonin and glutamate play both active and passive roles in regulating gustatory plasticity [59,61]. To conduct this assay, four sets of plates are prepared to produce different pre-exposure environments for conditioning the worms. These plates include: conditioning plate, mock-conditioning plate, NaCl plug plate and assay plate [51]. Animals are either pre-exposed to the presence of NaCl or left naive by habituating them in a conditioned environment with or without a particular NaCl concentration. The parameter of focus in this assay is calculating the chemotaxis index of the worms.
1.2.4. Loopy Foraging and Shrinker Behaviors
C. elegans foraging ability involves a number of coordinated neuronal activities that enable the nematode search for available food source then feed sufficiently. The GABAergic system regulates the biomechanical coordination of the worm during foraging (head movement) and body wall movement [62]. This neuronal group forms the basis of examination in this behavioral phenotype. During foraging, the four RME GABAergic motor neurons found in the head control the slight arc-like movement of the nose from one side to the other. However, any defect in these motor neurons will result in a loopy (excess flexion) of the nose during foraging. Furthermore, the 19 D-type GABAergic motor neurons allow for a sinusoidal wave-like movement from head to tail when the nematode is gently touched or prodded. The sinusoidal bend is formed by contraction of muscles on one side of the body wall and relaxation by GABA on the other side [63]. These D-type neurons are found in the neuromuscular junction of the body wall. Lack of these neurons causes a shrinker or twitcher movement which is the pulling inward of the head of the worm, and shorten of body length (hyper contraction) when touched. Such behavioral alteration may negatively affect the survival instincts/defense mechanism of the worms.
1.2.5. Locomotor Assay
Locomotion is an essential part of existence and survival in all living organisms. C. elegans move in a sinusoidal (undulatory) motion in search of food and a conducive living environment. This movement is aided by the generation of dorsoventral body bends (controlled by the dorsal and ventral muscles) which spans along its entire body length against the direction of movement [64]. Several factors influence the pattern of motility including presence or absence of food and change in environmental/ external stimuli. Such alterations can be seen expressed in worms with a mutated dopaminergic system [47]. The locomotor assay is used to assess abnormal motion in C. elegans expressed as either irregular bends or thrashing motion, which depicts motility of nematode in liquid. Here, worms are transferred to unseeded NGM plates and total body bends (rate of locomotion) over a period of time is counted and scored.
1.2.6. Pharyngeal Pumping and Thrashing Behavior
Pharyngeal pumping in C. elegans is induced by extrinsic factors due to a lack of muscular pacemakers. These extrinsic factors are sometimes formed from the neuronal release from dopaminergic and cholinergic systems. The pharynx of this nematode regulates feeding via contraction (pumping) pattern of the neuromuscular organ in a rhythmic pattern [65]. There are three main parts of the pharynx which include: corpus, isthmus and terminal bulb. The isthmus connects the anteriorly positioned corpus to the posterior terminal bulb, which contains the grinder. Peristaltic movement within the terminal bulb is more visible than that of the corpus due to the crushing activity of the grinder. Hence, considering that contraction-relaxation cycles during feeding occurs through the entire length of the pharynx, the pumping rate can be counted from monitoring the movements of the grinder [66]. A maximum pumping rate is ~300 pumps per minute in two days old adult worms [50]. The thrashing assay is a basic motor performance test for C. elegans motility. A thrash is referred to as the movement of the head from the dorsal to the ventral side. It could also be referred to as a change in direction of the midbody as the worm struggles to get off a liquid medium. Adequate measurement of the number of directional changes is done accordingly. Normal worms can perform 90–100 thrashes in 30s in fluid medium [67,50].
1.3. Behavioral Assay Under Optogenetic Control for Assessing Connectomes
From a broader perspective, connectomes can be described as the science of mapping out the entirety of neural connections within an organism’s nervous system. There a number of techniques being used to detail both the structural and functional connectivity of the neural cells of the brain [68,69]. Understanding the organization of C. elegans connectomes has assisted in expanding knowledge on the molecular and structural basis of sex-based behavioral responses [70]. Earlier researches have made significant efforts to describe the interconnection between the neural circuitry (connectomes), muscular mechanics and behaviors.
This pioneered the use of advanced techniques called “optogenetics”, which is a combined use of light and genetically engineered cells to monitor neuronal activity. In optogenetics, genes are modified to produce specialized membrane-bound proteins called opsins (G-protein coupled receptor also referred to as actuators derived from microbial species), which are light sensitive. The most widely used opsin is the “Channelrhodopsin-2” (ChR-2); this is typically responsive to blue light, though there exists a rarely used subtype which is responsive to green light [71–74].
ChR-2 shares a similar excitation spectrum as Green Florescent Protein, it is used mostly during stimulation/ excitatory experiments. Some other opsins which express inhibitory properties include; NpHR/ Halorhodopsin, Archaerhodopsin-3 and Mac [72,75]. The optogenetics techniques aids in vivo regulation of neural activity through the use of those specific genetically encoded light-sensitive tools. For example, gated ion channels and calcium indicators are targeted to regulate neural cell excitation and activity respectively [76]. Therefore, the principal optogenetic tools consists of the actuators (opsins) and sensors. While the actuators manipulate neuronal activity, the sensors (calcium indicators: GECIs, vesicular release and pH sensors: synapto-pHluorins, voltage indicators: GEVIs), act as optical recorders to monitor the manipulated neural activity of actuators [77]. During retinoid metabolism in mammalian cells, exogenous vitamin A is used to produce “All-trans-Retinal”, an important cofactor for ChR-2 functions. Unfortunately, it is naturally absent in C. elegans, hence the need of an exogenous source. It is a photosensitizer that serves as a chromophore during polarization neuronal activity [78,79].
2. Materials
2.1. Preparing C. elegans for fluorescent Imaging
Worms are prepared on agarose pads, as they easily get dried if laying down directly on the slide). Furthermore, the pad of agarose is used to keep the same slide thickness, and to standardize analysis across treatment groups. Materials are outlined below
C. elegans strains
Slides
Coverslip
Plastic Pasteur pipettes
Agarose 2 % (some protocols have suggested 3 % or 4%) (http://wbg.wormbook.org/));
Drugs to immobilize the worms: 0.2 % tricaine/0.02 % tetramisole, 1 mM levamisole or 10 – 25 mM sodium Azide (NaN3)) depending on the behavior to be analyzed
Micropipette (2–20 μl)
Tips
Labeling tape
2.2. Behavioral assays
Behavioral assays described above require relatively simple tools to perform, and of great value in assessing neurotransmitter systems in C. elegans. Most behavioral assays have been performed manually under basic stereomicroscopes; however, the use of video recording system and tracking software is an added advantage that is encourage. Synchronous worm strain populations [51] are mostly used in behavioral assays. Several of the behavioral assays use similar materials that are outlined below.
Worm strains
Petri (culture) plates: 6 cm, 9 cm
Standard nematode growth media (NGM)
Genetically engineered E. coli bacteria strains: either OP50 or HB101
Reagents: Agar, CaCl2, NaCl, MgSO4, KH2PO4, Tween 20
Buffer solutions; either M9 buffer (KH2PO4, Na2HPO4, NaCl, dH2O, MgSO4) or S-basal buffer (NaCl, K2HPO4, KH2PO4, cholesterol, dH2O)
Glass culture tube
Capillary pipette and tip
Ethanol and flame source
Kimwipe
Pasteur Pipette
Kim wipe
2.3. Behavioral Assay Under Control of Optogenetics
Standard NGM
Worm Strains (transgenic strains expressing ChR2 (tph-1p::ChR2::GFP) available at Caenorhabditis Genetics Center)
Bacteria source; OP50 or HB101
100 mM all-trans-retinal (ATR) stock solution (diluted in ethanol and stored in a microcentrifuge tube for light-sensitive samples)
Petri plates; 6 cm
Blue light lens filter
Parafilm
Pasteur pipette
M9 buffer
DC power source
Multi-Worm Tracker
Custom-built LED light apparatus/ digital computerized LED lights (recommended for precision)
3. Methods
3.1. Fluorescence Imaging Methods for C. elegans
3.1.1. Mounting the worms (Fig. 1):
Fig. 1.

Procedure to mount C. elegans on slides.
Prepare the slide model with 2 two overlapping pieces of tape. These slides can be saved for the next experiments (1)
Heat the agarose in the microwave for 30 to 60 secs until start boiling (2). Take care of not heating for a long time because the agarose loses transparency and can implicate changes in the experiment
Place the slide model side by side and a new slide in the middle of a clean countertop near to the heated agarose. Use a plastic Pasteur pipet to place 4 to 5 drops of agarose in the middle of the slide (3)
Quickly covering puddle with another slide before agarose solidifies (4). Do not press, release the slide as quickly as possible to avoid the bubbles
Carefully discover the pad (1.2–1.6 cm across) which must be freshly prepared or discovered because it gets dry fast (5)
Cut the exceed pieces until it gets the coverslip shape (6)
The worms can be transferred by picking (hardest way) or by transfer from a resuspension in M9 buffer. Pipet about 10 μl of worms and 10 μl of the anesthetic (7)
Cover the pad with a coverslip (8)
Check the worms in the fluorescence or confocal microscope (9)
3.1.2. Fluorescence acquisition in C. elegans
The image acquisition can be acquired using confocal or fluorescence microscopy. As neurons are distributed in C. elegans cylindrically, Z-stacking should be considered for representative images. Since C. elegans body presents about 35–40 μm of diameter in L4 larval stage, the Z-stacking images should be taken at different focal distances, e.g., 2,5 μm, resulting in about 16 slices. Depending on the extent of photobleaching, 30–60 time points are collected. Concomitantly, the brightfield should be accessed considering the same set used to acquire the fluorescence. The image format is another crucial information and are generally exported and saved as 16-bit TIFF format. Numerous software can be used to process the images, the most used is ImageJ that is an platform for image processing designed for scientific multidimensional images and inspired by NIH Image. To acquire the whole body-length of C. elegans in adulthood (>1000 μm), it can be used the 2.5 × and 4 × objectives.
3.1.2.1. ImageJ download and setting:
Download ImageJ with Java on different operating systems using the link: https://imagej.nih.gov/ij/download.html
After installing, download the plugging “bioformats_package.jar”: https://downloads.openmicroscopy.org/bio-formats/5.0.3/
Copy the file and paste into the folder: imageJ > plugins > Jars
Open imageJ, click in plugins > install > bioformats_package.jar > save.
3.1.2.2. Z-stack image sequence processing in ImageJ (Fig. 2)
Fig. 2.

The step-by-step to process a z-stack image sequence in ImageJ.
Open ImageJ, click File > Import > image sequence (1)
Select folder containing images for treatment and set desired sequence options (2)
Z-stack images are collapsed into a maximum projection image: click image > stacks> Z-Project (3)
Select “start” slice and “stop” slice and the Projection type (e.g.: sum slice) (4)
For fluorescence quantification, click “polygon selections” and delimit the total area of the worm (5), then click analyze> measure (6, 7)
To save the image, click file >save as> TIFF> select the destination folder (8).
3.1.3. Neuronal viability analysis
The images processed can be analyzed in comparison to a control group. Some endpoints can be examined in vivo as indicatives of neurotoxicity including the neuronal development, punctum, neuronal absence or shrinkage, neuronal gaps, absence of cell bodies, and reduction in intensity of fluorescence [32,80,6,81]. Fig. 3, kindly provided by Tao Ke, showing neuronal changes at dopaminergic neurons induced by Methylmercury (5 μM MeHg) exposure.
Fig. 3.
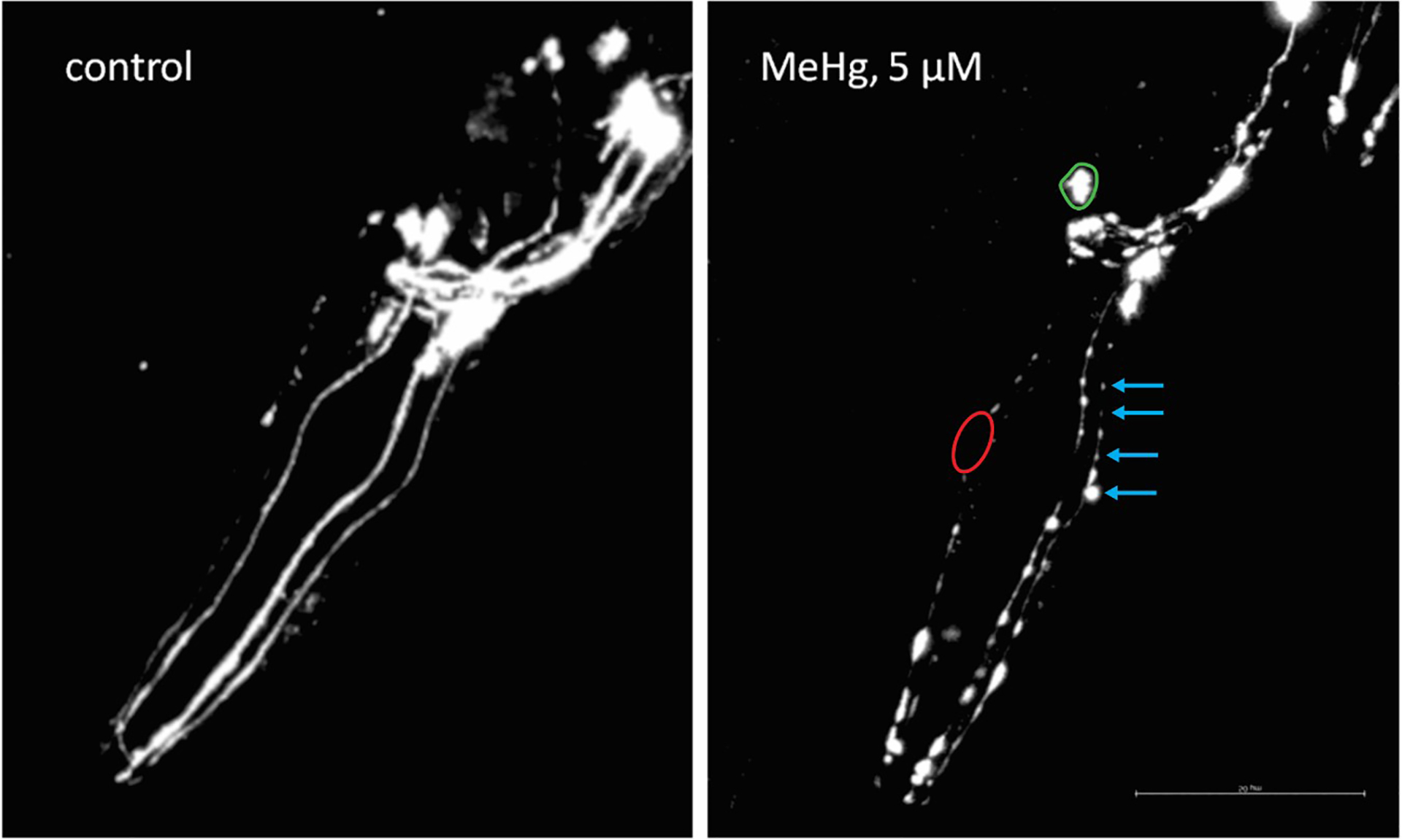
C. elegans dopaminergic neurodegeneration under Methylmercury (5 μM MeHg) exposure. Blue arrows indicate the punctum, red delimited area indicates neuronal absence, and green delimited area indicates the shrinking soma. BZ555 strain worms were focused on the head, L4 larval stage, 60 × objective. Kindly provided by Tao Ke.
Neurodegeneration can be also classified by range considering some of the endpoints discussed, as used by Schetinger and co-workers [82]. Cholinergic neurodegeneration was ranked from 0 to 3, where: 0 meant no degeneration;1 meant low degeneration; 2 means moderate degeneration, and 3 means high degeneration considering the head, body, and tail of C. elegans.
3.1.4. Neuronal activity imaging
Regarding neuronal activity, C. elegans is an ideal model mainly due to its already mapped nervous system for both hermaphrodites and males [83,84]. The development of imaging methods enables a fantastic expansion in C. elegans neuronal activity field. While in rodents expensive systems must be used to acquire neuronal function, the fluorescent GCaMP construct allows C. elegans in vivo four-dimensional imaging of neuronal calcium implicated in both behavior and neuronal activity patterns [ZM9078 strain; hpIs587 (Pflp-14::GCaMP6::wCherry + lin-15(+))] [85] and fluorescent voltage reporters can infer the postsynaptic responses [86]. For instance, GCaMP expression is limited to a set of neurons approaches, and the field of neuronal activity imaging is still limited by a lack of tools for robust assignment of all neurons at the same time.
3.2. Behavioral Assays
3.2.1. Basal Slowing Response
Procedure:
NGM plates can be prepared according to the protocol by [87]. These plates are stored at 20 °C 16–20 hrs prior to the assay
Two (2) sets of assay plates are prepared accordingly; bacteria spread (seeded) and un-spread (unseeded) plates.
- Assay plates to be seeded should be freshly spread with bacteria (OP50 or HB101) depending on the species used for cultivating the worms as follows [53,51]
- Drop approximately a drop of 2 μl bacteria within a circle (bacteria lawn: inner and outer rings of ~1 and 3.5 cm respectively) in the center of the NGM.
- Gently spread the bacteria within the boundary of the circle with the bottom of a glass culture tube (sterilize with 70% ethanol and flame)
- Place both sets of plates (spread and un-spread) in an incubator overnight at 37 °C
Take assay plates out of the incubator and allow to cool at room temperature before use, dry off lids with a Kimwipe.
- Already cultivated well-fed animals are washed in a buffer (~1.5 ml of M9 or S-basal) after being removed from culture plates before being transferred to assay plates. Washing, to remove bacteria from the worms, is done twice as follows:
- First, wash worms off plates using the buffer into Eppendorf tubes
- Spin in a centrifuge 30–60 secs at 1000 rpm or allow worms to settle to the bottom of the tube, then pull off buffer with pipette leaving 100–200 μl
- Transfer worms (5–10 worms) to the clear zone in the center of the assay plates as follows;
- In a drop of the buffer, use a capillary pipette or cut a 200 μl tip to transfer ~5 μl of worms. Ensure there is a minimum of 5 worms on the assay plate.
Gentle mop up the drop of the buffer used to transfer the worms from the assay plate with a Kimwipe
Allow assay plate sit undisturbed on a bench for about 5 mins of adaptation time
- Body movement count (movement rate) is counted thus;
- Count the number of bends per 20 secs for each worm i.e. change in body direction moving either forward or backward of the area behind the pharynx.
The analysis is calculated thus; Basal slowing response = [rate of movement in seeded plates – the rate of movement in unseeded plates] / [rate of movement in unseeded plates]
Notes -
Controls adapted for use during the assay should be well fed and similar in size relative to the thickness of the bacteria lawn in each plate.
Due caution should be taken purity (freshness) of reagents used as this can affect the assay
Transfer of worms to assay plates should be done one group at a time. This is to allow time to plate the worms and mop up the liquid (buffer) and efficient counting of bends.
To prevent worms from crawling off agar of unseeded assay plates, 100μl of 4 M fructose can be dropped on the edge of the plate. Tilt gently to spread the fructose all the way around the late. Allow drying for ~10mins before beginning the assay.
These experiments are done blindly and repeated at least 3 independent times.
Assays can be recorded via digital camera and analyzed later.
3.2.2. Ethanol Avoidance/Ethanol Preference
Depending on the investigation being carried out, this behavioral procedure can either be tagged an ethanol avoidance or preference assay.
For the ethanol avoidance [90]
- Synchronized young adult worms (~63 hrs post-synchronization) to an assay plate which has been divided into four quadrants.
- Assay plate is prepared thus; Divide 10cm NGM petri dishes into four quadrants consisting of; A and B (ethanol quadrant), C and D (control quadrant), with each pair of quadrants opposite each other. Bore 9mm holes into quadrants A and B, in which~ 50μl of 100% ice-cold ethanol will be poured. Seal the plate with parafilm to allow ethanol equilibration.
- Transfer of worms is done by washing them off ethanol plate using S-basal (twice) then once in distilled water.
About 100–200 animals are transferred into the assay plate which contains a central marked spot (forms the origin). These worms are allowed to freely move for 30 mins, after which scoring is done. A video recording device of choice can be employed for use within this duration.
The preference index is calculated thus: [Number of worms in control quadrants - Number of worms in ethanol quadrants] / Total number of worms tested
For an ethanol preference; [58]
- Worms are pre-exposed to ethanol as follows; synchronized young adult worms (~63 hrs post-synchronization) are pretreated by incubating for about 4 hrs in a seeded NGM control or ethanol pre-exposure plate.
- Ethanol plate is prepared as follows; half of NGM plate is seeded with OP50 then allowed to dry for ~2 hrs. The other half is seeded with 300mM ice-cold ethanol. Plate is sealed with a parafilm so as to allow adequate diffusion of the ethanol within the agar for another 2 hrs.
Worms are transferred to assay plate as describe above, and procedure similarly performed as above.
The preference index is calculated thus: [Number of worms in ethanol quadrants - Number of worms in control quadrants] / Total number of worms tested
Notes:
Adequate care should be taken in quantifying the concentration of ethanol used during assay plate preparation.
Avoid overcrowding the assay plate in order to prevent clustering of worms.
Ensure the working bench is horizontal and avoid all external sources of disturbances such as; wind, centrifuge, and incubator, in order to eliminate vibrations
Do not count clumped worms left in the midline (center point) of the assay plate while calculating the chemotaxis index, as these consist of wash-injured animals.
3.2.3. Plasticity of Chemotaxis by NaCl
Procedure:
Prepare a culture media containing 14ml of NGM using a 6cm plate. This volume of NGM is the minimum requirement to get a sufficient spread of bacterial (over-night cultured E. coli) lawn. This should be incubated for a day at room temperature or overnight at 37°C, then stored at room temperature.
-
Prepare the four sets of plates as follows: [51,91,92]
- Conditioning Plate: This medium is made up of a combination of 25 g/L agar, 100 mM NaCl, 1 mM CaCl2, 1 mM MgSO4, 5 mM KH2PO4(pH 6.0). Autoclave, thereafter pour 8 ml into the 9 cm plate (or 9 ml into 10 cm plate).
- Mock-conditioning plate: This is prepared with similar constituents as the conditioning plate but with the exemption of NaCl
- NaCl plug plate: The plug medium consists of 17g/L agar, 100 mM NaCl, and 1 mM CaCl2, 1 mM MgSO4, 5 mM KH2PO4 (pH 6.0). Autoclave then pour 6 ml of the medium into a 6 cm plate
- Assay plate: This medium is prepared in a similar way as the plug plate but without NaCl. After autoclaving, pour ~3–3.5 ml of the medium into a 6 cm plate
Store all plates at 4°C and use within two-three weeks
- Create a NaCl concentration gradient on the assay plate. This can be done in two ways:
-
Use a wash buffer to collect well-fed animals from NGM. Wash buffer is made up of 1 mM CaCl2, 1 mM MgSO4, 5 mM KH2PO4 (pH 6.0), and 0.05% Tween 20].
NB-: Wash buffer with or without NaCl can be used for the pre-exposure conditions. Then animals are maintained at 20 °C for 15 mins in their respective pre-exposure wash solutions. Thereafter, wash once with buffer without NaCl for 60 secs at 450 × g for 60 secs; this is to remove the NaCl from the body of the worms [91,93]
Wash animals collected from NGM in a centrifuge three times using 1.5ml tubes for 20 secs at ~900 × g
Transfer washed animals to the conditioning or mock-conditioning plates respectively
Cover plates with lids then place in an incubator at 20 °C for 4 hrs
Use wash buffer to collect conditioned and mock-conditioned animals into 1.5 ml tubes
Gently place animals in the center of the assay plate (region having the established NaCl concentrated gradient), approximately 50–300 animals. Use a Kim wipe to mop off excess wash solution
Allow plate with lids to seat undisturbed on the working /experimental bench for 15 mins
Put several drops of chloroform on the lid to stop animal movement
Count the total number of animals on each fraction of the assay plate
- Calculation of chemotaxis index is done thus [51];
Where;- X= Number of animals on NaCl fraction of the assay plate
- Y= Number of animals on the other (control) fraction of the assay plate
Notes -
Ensure the working bench is horizontal and avoid all external sources of disturbances such as; wind, centrifuge, and incubator, in order to eliminate vibrations
Do not use old seeded nematode growth media (over 2 weeks)
Do not count clumped worms left in the midline (center point) of the assay plate while calculating the chemotaxis index, as these consist of wash-injured animals.
Presence of food during the assay may alter the results or create biases in the response of worms to the concentration gradient
Before commencement of the assay procedure, ensure animals are well fed and not from a starved agar chunk.
Ensure room temperature is kept at 25±1°C during the assay procedure.
The concentration of NaCl (between 50 mM-100 mM) used during the pre-exposure/ pre-conditioning stage, should be similar to that used for the assay [59,51]
3.2.4. Loopy Foraging and Shrinker Behaviors
Procedures are adapted from this previously described method [63,94].
Procedure for Loopy Foraging:
-
At young adult stage (~63 hrs post synchronization), pick 20 worms unto assay plate. Allow plate to seat on bench for 5 mins.
Assay Plate (prepared a day before assay):- Prepare thin seeded NGM plates by dropping ~30 μl OP50 bacterial into 60 mM plates, spread and allow to seat at room temperature for 24 hrs or overnight at 37°C.
Observe and count number of worms with “loopy” foraging per 20 worms
Procedure for Shrinker Behavior:
-
At young adult stage (~63 hrs post synchronization), pick 20 worms unto assay plate. Allow plate to seat on bench for 5 mins.
Assay Plate:- Prepare thin seeded NGM plates by dropping ~30μl OP50 bacterial into 60 mM plates, spread and allow to seat at room temperature for 24 hrs or overnight at 37 °C.
Gently prod or touch body of worm with a pick; and observe “shrinking” action (see fig below).
Count number of “shrinkers” per 20 worms
For examples of phenotypic pattern of loopy foraging and shrinker behaviors see Jorgenson 2005 [63].
Notes-
CB156 strain [unc-25(e156) III] worm strains can be used as appropriate controls. These worms which are unc-25 mutants show both loopy head and body shrinking behaviors.
Same worms can be used for both assays. First observe and count loopy head worms. Then prod worms for body shrinking.
3.2.5. Locomotion Assay
Procedure is modified [94] from the previously described method in [52].
Procedure:
Post synchronized young adult worms (~63 hrs) are collected by washing thrice in S-basal buffer.
Transfer ~7–10 worms with S-buffer into unseeded NGM assay plates using a Pasteur pipette. The use of unseeded plates is to eliminate the slowing of movements in bacteria lawn; particularly at the edge of the lawn, in order to assess the general locomotory rate.
Remove excess buffer with a Kimwipe and allow plate seat for 5 mins of acclimatization
- Count the number of body bends per 3 mins. The body bend count is denoted as each time the posterior bulb of the pharynx reaches a maximum turn in the opposing direction from the last count.
- The assay can be repeated several times and data collected over different experiments.
Data collected is expressed thus and the average calculated; Body bends/ 3mins
Notes-
Care should be taken not to mistake a worm body reversal in backward motion as a body bend. Such reversal occurs when during forward motion, the worm suddenly reverses thereby curving in the same direction it was previously bent/turned in the backward motion.
Prepare one assay session and finish count before preparing another.
Worms counted should be removed from the assay plate by gently picking up to prevent double counting.
If worms tend to crawl off Agar plate make fructose (4M) or glycerol (8M) rings around unseeded assay plates. Drop 60 – 100 μl of fructose or glycerol solution on edge of plate, tilt plate to coat the edge all the way around and allow to dry for about 10 mins.
These experiments are done blindly and repeated at least 3 independent times.
Assays can be recorded via digital camera and analyzed later.
3.2.6. Pharyngeal Pumping and Thrashing Behavior
Procedure:
Transfer at least 10 worms from synchronized population into freshly prepared NGM assay plate.
-
Count the number of times worms pump per minute (ppm) in two ways;
- Using a stereomicroscope, you may choose to manually count pumping rate, 10–20 secs per mins for 10 mins [66] OR
- If the pumping rate seems abnormal, one can decide to digitally count the pumping rate with a digital camera (at least 10 frames per secs). It is preferred to transfer a single worm into the NGM plate with a bacteria lawn of 5 mm
- Counts could also be done via another technique called the high power differential interference contrast [66].
Data is calculated thus; the rate of pharyngeal pumping = Total number of pumps / Total time
- For the thrashing assay, plates can be prepared in two ways:
- Put 10μl of dH2O in a shallow transparent Petri plate, then transfer worms with a Pasteur pipette into this assay plate [50] OR
- Place the worm in the middle of a droplet of the isotonic solution in a standard NGM. Counting is done as the worm tries to reach a solid medium [65]
- Thrashing assay is monitored via videotaping and assessed by use of any available recommended assay software [50].
Notes-
Make use of well-fed animals, as pharyngeal pumping is decreased in normal animals that are off food
Replay recorded videos of pumping assays at ½ to 1/3 of the original speed to aid accurate count
Make use of standard NGM that are well seeded with bacteria to avoid irregular increased motility of hungry worms. Such would make pump count difficult
L1 worms require a magnification of X100 for accurate viewing
These experiments are done blindly and repeated at least 3 independent times.
Assays can be recorded via digital camera and analyzed later.
3.3. Behavioral Assay Under Control of Optogenetics
For more detailed description see [79,76].
Procedure:
Prepare NGM using 12 ml of agar then store at room temperature for ≥48 hrs before use
Seed NGM with a 50 μl stock mixture of bacteria and ATR in a ratio 49.4 μl: 0.6 μl, onto the center of the NGM forming a square (4×4 cm area)
Store seeded plates in the dark (using a foil or kept in a drawer) or under red light to allow bacteria growth (~24 hrs)
- Age synchronize worms (use 3–4-day old young adult worms). This can be achieved by adapting the use of gravid strains that are allowed a 4hr egg-laying period within the plates. These are later removed to have 40–80 age synchronized worms.
- To achieve a strain of intense light response, worms expressing extrachromosomal array can be transferred into fresh ATR plates
Transfer worms into seeded plates by picking using a Pasteur pipette or pipette with M9 buffer
Seal worms with parafilm. Store in a controlled dark environment.
Attach the blue light filter over the lens of the Multi-Worm Tracker
The custom LED light (supplied by the DC power) should then be connected to the stimulus relay of the multi-worm tracker
Launch multi-worm tracker software, arrange the light stimulus parameter
Begin mounting of each plate on the tracker platform, focus the LED light accordingly at the center of the ring on the plates.
Analyze data using offline java-command-line Chorography software, use custom scripts to re-arrange output files as desired; investigating both neuronal activity and behavioral changes.
Notes-
Prepare extra seeded plates in case of contamination or uneven distribution of agar spread
Ensure to minimize the ATR exposure to light to avoid the photosensitizer losing its gating function on the opsins
The seeded plates should be used within 72hrs to avoid excess bacteria growth
Reset the worm tracker each time the plate is changed
4. Conclusions
The set of connections in neural systems called the connectome, form the communication in the brain. Detailed understanding of these connections, i.e., mapping high-resolution connectivity, is an essential first step in elucidating how the nervous system processes information and generates behavior [95]. C. elegans is an ideal model for studying neuronal activity due to its small, stereotyped yet relatively complex nervous system [84]. Furthermore, its optical transparency, homologues genomic constitution with humans and readily availability is an added advantage. Knowledge about nervous system connectivity is critical to understanding how its functions. Cook et al (2019) described in their studies the concepts of both adult sexual forms of the nematode C. elegans. Serial transmission electron microscopy and some previously related data were used to perform a reconstruction of circuits for the male head, including mainly the nerve ring and retro-vesicular globule [96]. The evaluation of whole-animal connectomes from sensory input to end-organ output across the worm showed a considerable number of connections’ differences between the male and hermaphrodites [96]. The connectivity profile can indicate how neurons work, whether by sensory perception or hormone secretion. A thorough understanding of the interconnections of various neurotransmitter systems of this nematode is imperative to understand certain behavioral outputs amongst species. Although varying protocols could be modified, the use of standard methods are essential to ensure reproducibility of results.
Acknowledgements
OMI acknowledges the 2019 Young IBRO Regions Connecting Awards. MA is supported by National Institute of Health (NIH), USA grants; NIEHS R01 10563, NIEHS R01 07331 and NIEHS R01 020852. We acknowledge Tao Ke, of the Albert Einstein College of Medicine for images of C. elegans dopaminergic neurodegeneration under MeHg exposure.
References:
- 1.Consortium CeS (1998) Genome sequence of the nematode C-elegans: A platform for investigating biology. Science 282 (5396):2012–2018. doi:DOI 10.1126/science.282.5396.2012 [DOI] [PubMed] [Google Scholar]
- 2.Antoshechkin I, Sternberg PW (2007) The versatile worm: genetic and genomic resources for Caenorhabditis elegans research. Nat Rev Genet 8 (7):518–532. doi: 10.1038/nrg2105 [DOI] [PubMed] [Google Scholar]
- 3.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green Fluorescent Protein as a Marker for Gene-Expression. Science 263 (5148):802–805. doi:DOI 10.1126/science.8303295 [DOI] [PubMed] [Google Scholar]
- 4.Boulin T, Pocock R, Hobert O (2006) A novel Eph receptor-interacting IgSF protein provides C. elegans motoneurons with midline guidepost function. Curr Biol 16 (19):1871–1883. doi: 10.1016/j.cub.2006.08.056 [DOI] [PubMed] [Google Scholar]
- 5.Feinberg EH, VanHoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmannl CI (2008) GFP reconstitution across synaptic partners (GRASP) defines cell contacts and Synapses in living nervous systems. Neuron 57 (3):353–363. doi: 10.1016/j.neuron.2007.11.030 [DOI] [PubMed] [Google Scholar]
- 6.Benedetto A, Au C, Avila DS, Milatovic D, Aschner M (2010) Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3-Dependent Manner in Caenorhabditis elegans. Plos Genet 6 (8). doi:ARTNe100108410.1371/journal.pgen.1001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubert P, Puntel B, Lehmen T, Fessel JP, Cheng P, Bornhorst J, Trindade LS, Avila DS, Aschner M, Soares FAA (2018) Metabolic effects of manganese in the nematode Caenorhabditis elegans through DAergic pathway and transcription factors activation. Neurotoxicology 67:65–72. doi: 10.1016/j.neuro.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 8.DiLoreto EM, Chute CD, Bryce S, Srinivasan J (2019) Novel Technological Advances in Functional Connectomics in C. elegans. J Dev Biol 7 (2). doi: 10.3390/jdb7020008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh EK, May RC (2012) Caenorhabditis elegans, a model organism for investigating immunity. Appl Environ Microbiol 78 (7):2075–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson TE (2003) Advantages and disadvantages of Caenorhabditis elegans for aging research. Experimental gerontology 38 (11–12):1329–1332 [DOI] [PubMed] [Google Scholar]
- 11.Gottschling D-C, Döring F (2019) Is C. elegans a suitable model for nutritional science? Genes & nutrition 14 (1):1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tissenbaum HA (2015) Using C. elegans for aging research. Invertebrate reproduction & development 59 (sup1):59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN (2008) Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicological sciences 106 (1):5–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Barclay JW, Burgoyne RD, Morgan A (2015) Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chemistry Central Journal 9 (1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander AG, Marfil V, Li C (2014) Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Frontiers in genetics 5:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobert O (2013) The neuronal genome of Caenorhabditis elegans. WormBook:1–106. doi: 10.1895/wormbook.1.161.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loer CMR, J.B. (2016) The evidence for classical neurotransmitters in C. elegans neurons (updated online review/database in WormAtlas 2.0; original in 2010). WormAtlas [Google Scholar]
- 18.Rand JB (2007) Acetylcholine. WormBook:1–21. doi: 10.1895/wormbook.1.131.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chase DL, Koelle MR (2007) Biogenic amine neurotransmitters in C. elegans. WormBook:1–15. doi: 10.1895/wormbook.1.132.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal-Gadea AG, Davis S, Becker L, Pierce-Shimomura JT (2012) Coordination of behavioral hierarchies during environmental transitions in Caenorhabditis elegans. Worm 1 (1):5–11. doi: 10.4161/worm.19148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang B, Tong P, Xue KS, Williams PL, Wang JS, Tang L (2019) High-throughput assessment of toxic effects of metal mixtures of cadmium(Cd), lead(Pb), and manganese(Mn) in nematode Caenorhabditis elegans. Chemosphere 234:232–241. doi: 10.1016/j.chemosphere.2019.05.271 [DOI] [PubMed] [Google Scholar]
- 22.Baesler J, Kopp JF, Pohl G, Aschner M, Haase H, Schwerdtle T, Bornhorst J (2019) Zn homeostasis in genetic models of Parkinson’s disease in Caenorhabditis elegans. J Trace Elem Med Biol 55:44–49. doi: 10.1016/j.jtemb.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing X, Guo Y, Wang D (2009) Using the larvae nematode Caenorhabditis elegans to evaluate neurobehavioral toxicity to metallic salts. Ecotoxicol Environ Saf 72 (7):1819–1823. doi: 10.1016/j.ecoenv.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Melo-Thomas L, Engelhardt KA, Thomas U, Hoehl D, Thomas S, Wohr M, Werner B, Bremmer F, Schwarting RKW (2017) A Wireless, Bidirectional Interface for In Vivo Recording and Stimulation of Neural Activity in Freely Behaving Rats. J Vis Exp (129). doi: 10.3791/56299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann T, Kurth J, Hawlitschka A, Stenzel J, Lindner T, Polei S, Hohn A, Krause BJ, Wree A (2018) [(18)F]fallypride-PET/CT Analysis of the Dopamine D(2)/D(3) Receptor in the Hemiparkinsonian Rat Brain Following Intrastriatal Botulinum Neurotoxin A Injection. Molecules 23 (3). doi: 10.3390/molecules23030587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sands B, Burnaevskiy N, Yun SR, Crane MM, Kaeberlein M, Mendenhall A (2018) A toolkit for DNA assembly, genome engineering and multicolor imaging for C. elegans. Transl Med Aging 2: 1–10. doi: 10.1016/j.tma.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green RA, Audhya A, Pozniakovsky A, Dammermann A, Pemble H, Monen J, Portier N, Hyman A, Desai A, Oegema K (2008) Expression and imaging of fluorescent proteins in the C-elegans gonad and early embryo. Method Cell Biol 85:179-+. doi: 10.1016/S0091-679x(08)85009-1 [DOI] [PubMed] [Google Scholar]
- 28.Heppert JK, Dickinson D, Pani AM, Higgins CD, Goldstein B (2016) Comparative assessment of fluorescent proteins for in vivo imaging in an animal model system. Molecular Biology of the Cell 27. doi:<Go to ISI>://WOS:000396046900067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molino JVD, de Carvalho JCM, Mayfield S (2018) Evaluation of secretion reporters to microalgae biotechnology: Blue to red fluorescent proteins. Algal Res 31:252–261. doi: 10.1016/j.algal.2018.02.018 [DOI] [Google Scholar]
- 30.Kobayashi J, Shidara H, Morisawa Y, Kawakami M, Tanahashi Y, Hotta K, Oka K (2013) A method for selective ablation of neurons in C. elegans using the phototoxic fluorescent protein, KillerRed. Neurosci Lett 548:261–264. doi: 10.1016/j.neulet.2013.05.053 [DOI] [PubMed] [Google Scholar]
- 31.Flames N, Hobert O (2009) Gene regulatory logic of dopamine neuron differentiation. Nature 458 (7240):885–889. doi: 10.1038/nature07929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nass R, Hall DH, Miller DM 3rd, Blakely RD (2002) Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99 (5):3264–3269. doi: 10.1073/pnas.042497999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39 (6):889–909 [DOI] [PubMed] [Google Scholar]
- 34.Harrington AJ, Yacoubian TA, Slone SR, Caldwell KA, Caldwell GA (2012) Functional analysis of VPS41-mediated neuroprotection in Caenorhabditis elegans and mammalian models of Parkinson’s disease. Journal of Neuroscience 32 (6):2142–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano-Saiz E, Poole RJ, Felton T, Zhang F, De La Cruz ED, Hobert O (2013) Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell 155 (3):659–673. doi: 10.1016/j.cell.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieke T, Steinberg CE, Ju J, Saul N (2015) Natural Marine and Synthetic Xenobiotics Get on Nematode’s Nerves: Neuro-Stimulating and Neurotoxic Findings in Caenorhabditis elegans. Mar Drugs 13 (5):2785–2812. doi: 10.3390/md13052785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares FA, Fagundez DA, Avila DS (2017) Neurodegeneration Induced by Metals in Caenorhabditis elegans. Adv Neurobiol 18:355–383. doi: 10.1007/978-3-319-60189-2_18 [DOI] [PubMed] [Google Scholar]
- 38.Jacques MT, Bornhorst J, Soares MV, Schwerdtle T, Garcia S, Avila DS (2019) Reprotoxicity of glyphosate-based formulation in Caenorhabditis elegans is not due to the active ingredient only. Environ Pollut 252 (Pt B):1854–1862. doi: 10.1016/j.envpol.2019.06.099 [DOI] [PubMed] [Google Scholar]
- 39.Zhang RQ, Hong Y, and Xiao JS (2013) Separation and determination of pyrrolidinium ionic liquid cations by ion chromatography with direct conductivity detection. Chinese Chemical Letters 24 (6):503–505 [Google Scholar]
- 40.Rohn I, Raschke S, Aschner M, Tuck S, Kuehnelt D, Kipp A, Schwerdtle T, Bornhorst J (2019) Treatment of Caenorhabditis elegans with Small Selenium Species Enhances Antioxidant Defense Systems. Mol Nutr Food Res 63 (9). doi: 10.1002/mnfr.201801304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gubert P, Puntel B, Lehmen T, Bornhorst J, Avila DS, Aschner M, Soares FAA (2016) Reversible reprotoxic effects of manganese through DAF-16 transcription factor activation and vitellogenin downregulation in Caenorhabditis elegans. Life Sci 151:218–223. doi: 10.1016/j.lfs.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 42.Kim W, Underwood RS, Greenwald I, Shaye DD (2018) OrthoList 2: A New Comparative Genomic Analysis of Human and Caenorhabditis elegans Genes. Genetics 210 (2):445–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corsi AK, Wightman B, Chalfie M (2015) A transparent window into biology: a primer on Caenorhabditis elegans. Genetics 200 (2):387–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman GA, Luke CJ, Bhatia SR, Long OS, Vetica AC, Perlmutter DH, Pak SC (2009) Modeling molecular and cellular aspects of human disease using the nematode Caenorhabditis elegans. Pediatric research 65 (1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metaxakis A, Petratou D, Tavernarakis N (2018) Multimodal sensory processing in Caenorhabditis elegans. Open biology 8 (6):180049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mujika A, Leškovský P, Álvarez R, Otaduy MA, Epelde G (2017) Modeling behavioral experiment interaction and environmental stimuli for a synthetic C. elegans. Frontiers in neuroinformatics 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maulik M, Mitra S, Bult-Ito A, Taylor BE, Vayndorf EM (2017) Behavioral Phenotyping and Pathological Indicators of Parkinson’s Disease in C. elegans Models. Frontiers in genetics 8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotroff DM, Joubert BR, Marvel SW, Håberg SE, Wu MC, Nilsen RM, Ueland PM, Nystad W, London SJ, Motsinger-Reif A (2016) Maternal smoking impacts key biological pathways in newborns through epigenetic modification in Utero. BMC genomics 17 (1):976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raley-Susman KM, Chou E, Lemoine H (2018) Use of the Model Organism Caenorhabditis elegans to Elucidate Neurotoxic and Behavioral Effects of Commercial Fungicides. Neurotoxins:37 [Google Scholar]
- 50.Chen P, Martinez-Finley EJ, Bornhorst J, Chakraborty S, Aschner M (2013) Metal-induced neurodegeneration in C. elegans. Frontiers in aging neuroscience 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ijomone OM, Miah MR, Peres TV, Nwoha PU, Aschner M (2016) Null allele mutants of trt-1, the catalytic subunit of telomerase in Caenorhabditis elegans, are less sensitive to Mn-induced toxicity and DAergic degeneration. Neurotoxicology 57:54–60. doi: 10.1016/j.neuro.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 52.Hart AC (2006) Behavior (July 3, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook. 1.87. 1. [Google Scholar]
- 53.Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26 (3):619–631 [DOI] [PubMed] [Google Scholar]
- 54.Aschner M, Chen P, Martinez-Finley EJ, Bornhorst J, Chakraborty S (2013) Metal-induced neurodegeneration in C. elegans. Frontiers in aging neuroscience 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H, Calatayud C, Guha S, Fernández-Carasa I, Berkowitz L, Carballo-Carbajal I, Ezquerra M, Fernández-Santiago R, Kapahi P, Raya Á (2018) The Small GTPase RAC1/CED-10 Is Essential in Maintaining Dopaminergic Neuron Function and Survival Against α-Synuclein-Induced Toxicity. Molecular neurobiology 55 (9):7533–7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engleman EA, Katner SN, Neal-Beliveau BS (2016) Caenorhabditis elegans as a model to study the molecular and genetic mechanisms of drug addiction. In: Progress in molecular biology and translational science, vol 137. Elsevier, pp 229–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Lu H, Bargmann CI (2005) Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438 (7065):179–184 [DOI] [PubMed] [Google Scholar]
- 58.Lee J, Jee C, McIntire SL (2009) Ethanol preference in C. elegans. Genes, Brain and Behavior 8 (6):578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hukema RK, Rademakers S, Jansen G (2008) Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learning & Memory 15 (11):829–836 [DOI] [PubMed] [Google Scholar]
- 60.Saeki S, Yamamoto M, Iino Y (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. Journal of Experimental Biology 204 (10):1757–1764 [DOI] [PubMed] [Google Scholar]
- 61.Oda S, Tomioka M, Iino Y (2011) Neuronal plasticity regulated by the insulin-like signaling pathway underlies salt chemotaxis learning in Caenorhabditis elegans. Journal of neurophysiology 106 (1):301–308 [DOI] [PubMed] [Google Scholar]
- 62.Zhou X, Bessereau J-L (2019) Molecular architecture of genetically-tractable GABA synapses in C. elegans. Frontiers in Molecular Neuroscience 12:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jorgensen EM (2005) GABA (August 31, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook. 1.14. 1. [Google Scholar]
- 64.Gjorgjieva J, Biron D, Haspel G (2014) Neurobiology of Caenorhabditis elegans locomotion: where do we stand? Bioscience 64 (6):476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trojanowski NF, Raizen DM, Fang-Yen C (2016) Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Scientific reports 6:22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raizen D, Song B-m, Trojanowski N, You Y-J (2018) Methods for measuring pharyngeal behaviors. In: WormBook: The Online Review of C. elegans Biology [Internet]. WormBook, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kompoliti K, Verhagen L (2010) Encyclopedia of movement disorders, vol 1. Academic Press, [Google Scholar]
- 68.Shi Y, Toga AW (2017) Connectome imaging for mapping human brain pathways. Molecular psychiatry 22 (9):1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azulay A, Itskovits E, Zaslaver A (2016) The C. elegans connectome consists of homogenous circuits with defined functional roles. PLoS computational biology 12 (9):e1005021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, Nguyen KC, Tang LT-H, Bayer EA, Duerr JS (2019) Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571 (7763):63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim DH, LeDue J (2017) What is Optogenetics And How Can We Use It To Discover More About The Brain? Frontiers Young Minds 5 [Google Scholar]
- 72.Fang-Yen C, Alkema MJ, Samuel AD (2015) Illuminating neural circuits and behaviour in Caenorhabditis elegans with optogenetics. Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1677):20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guru A, Post RJ, Ho Y-Y, Warden MR (2015) Making sense of optogenetics. International Journal of Neuropsychopharmacology 18 (11):pyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schild LC, Glauser DA (2015) Dual color neural activation and behavior control with chrimson and CoChR in Caenorhabditis elegans. Genetics 200 (4):1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Husson SJ, Gottschalk A, Leifer AM (2013) Optogenetic manipulation of neural activity in C. elegans: from synapse to circuits and behaviour. Biology of the Cell 105 (6):235–250 [DOI] [PubMed] [Google Scholar]
- 76.Yu AJ, McDiarmid TA, Ardiel EL, Rankin CH (2019) High‐Throughput Analysis of Behavior Under the Control of Optogenetics in Caenorhabditis elegans. Current protocols in neuroscience 86 (1):e57. [DOI] [PubMed] [Google Scholar]
- 77.Rost BR, Schneider-Warme F, Schmitz D, Hegemann P (2017) Optogenetic tools for subcellular applications in neuroscience. Neuron 96 (3):572–603 [DOI] [PubMed] [Google Scholar]
- 78.Yu J, Chen K, Lucero RV, Ambrosi CM, Entcheva E (2015) Cardiac optogenetics: enhancement by all-trans-retinal. Scientific reports 5:16542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pokala N, Glater EE (2018) Using optogenetics to understand neuronal mechanisms underlying behavior in C. elegans. Journal of Undergraduate Neuroscience Education 16 (2):A152. [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Finley EJ, Avila DS, Chakraborty S, Aschner M (2011) Insights from Caenorhabditis elegans on the role of metals in neurodegenerative diseases. Metallomics 3 (3):271–279. doi: 10.1039/c0mt00064g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qu M, Kong Y, Yuana Y, Wang D (2019,) Neuronal damage induced by nanopolystyrene particles in nematode Caenorhabditis elegans. Environ Sci: Nano 6:2591–2601. doi: 10.1039/C9EN00473D [DOI] [Google Scholar]
- 82.Schetinger MRC, Peres TV, Arantes LP, Carvalho F, Dressler V, Heidrich G, Bowman AB, Aschner M (2019) Combined exposure to methylmercury and manganese during L1 larval stage causes motor dysfunction, cholinergic and monoaminergic up-regulation and oxidative stress in L4 Caenorhabditis elegans. Toxicology 411:154–162. doi: 10.1016/j.tox.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314 (1165):1–340. doi: 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- 84.Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, Hall DH, Emmons SW (2012) The connectome of a decision-making neural network. Science 337 (6093):437–444. doi: 10.1126/science.1221762 [DOI] [PubMed] [Google Scholar]
- 85.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499 (7458):295–300. doi: 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piatkevich KD, Jung EE, Straub C, Linghu C, Park D, Suk HJ, Hochbaum DR, Goodwin D, Pnevmatikakis E, Pak N, Kawashima T, Yang CT, Rhoades JL, Shemesh O, Asano S, Yoon YG, Freifeld L, Saulnier JL, Riegler C, Engert F, Hughes T, Drobizhev M, Szabo B, Ahrens MB, Flavell SW, Sabatini BL, Boyden ES (2018) A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat Chem Biol 14 (4):352–360. doi: 10.1038/s41589-018-0004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaudhuri J, Parihar M, Pires-daSilva A (2011) An introduction to worm lab: from culturing worms to mutagenesis. JoVE (Journal of Visualized Experiments) (47):e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rivard L, Srinivasan J, Stone A, Ochoa S, Sternberg PW, Loer CM (2010) A comparison of experience-dependent locomotory behaviors and biogenic amine neurons in nematode relatives of Caenorhabditis elegans. BMC neuroscience 11 (1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swierczek NA, Giles AC, Rankin CH, Kerr RA (2011) High-throughput behavioral analysis in C. elegans. Nature methods 8 (7):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cooper JF, Dues DJ, Spielbauer KK, Machiela E, Senchuk MM, Van Raamsdonk JM (2015) Delaying aging is neuroprotective in Parkinson’s disease: a genetic analysis in C. elegans models. NPJ Parkinson’s disease 1 (1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsuura T, Urushihata T (2015) Chronic nicotine exposure augments gustatory plasticity in Caenorhabditis elegans: involvement of dopamine signaling. Bioscience, biotechnology, and biochemistry 79 (3):462–469 [DOI] [PubMed] [Google Scholar]
- 92.Urushihata T, Wakabayashi T, Osato S, Yamashita T, Matsuura T (2016) Short-term nicotine exposure induces long-lasting modulation of gustatory plasticity in Caenorhabditis elegans. Biochemistry and biophysics reports 8:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Urushihata T, Takuwa H, Higuchi Y, Sakata K, Wakabayashi T, Nishino A, Matsuura T (2016) Inhibitory effects of caffeine on gustatory plasticity in the nematode Caenorhabditis elegans. Bioscience, biotechnology, and biochemistry 80 (10):1990–1994 [DOI] [PubMed] [Google Scholar]
- 94.Ijomone OM, Miah MR, Akingbade GT, Bucinca H, Aschner M (2020) Nickel-Induced Developmental Neurotoxicity in C. elegans Includes Cholinergic, Dopaminergic and GABAergic Degeneration, Altered Behaviour, and Increased SKN-1 Activity. Neurotoxicity Research 37:1010–1028. doi: 10.1007/s12640-020-00175-3 [DOI] [PubMed] [Google Scholar]
- 95.Bhattacharya A, Aghayeva U, Berghoff EG, Hobert O (2019) Plasticity of the Electrical Connectome of C. elegans. Cell 176 (5):1174–1189 e1116. doi: 10.1016/j.cell.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, Nguyen KCQ, Tang LT, Bayer EA, Duerr JS, Bulow HE, Hobert O, Hall DH, Emmons SW (2019) Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571 (7763):63–71. doi: 10.1038/s41586-019-1352-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


