Abstract
Background and Aims
There remains a historical misconception that inflammatory bowel disease [IBD] patients are underweight. However, recent data suggest rates of obesity in IBD parallel to those of the general population. The impact obesity has on the natural history of IBD is unclear. We aimed to determine obesity rates at the time of IBD diagnosis in a population-based cohort of ulcerative colitis [UC] patients.
Methods
Chart review was performed on patients diagnosed with UC over 1970–2010. Data were collected on demographics, body mass index [BMI], disease characteristics, IBD-specific hospitalisations, intestinal resection, and corticosteroid use. The proportion of patients who were obese at the time of their diagnosis was evaluated over time, and survival free of IBD-related complications was assessed using Kaplan‐Meier survival analysis.
Results
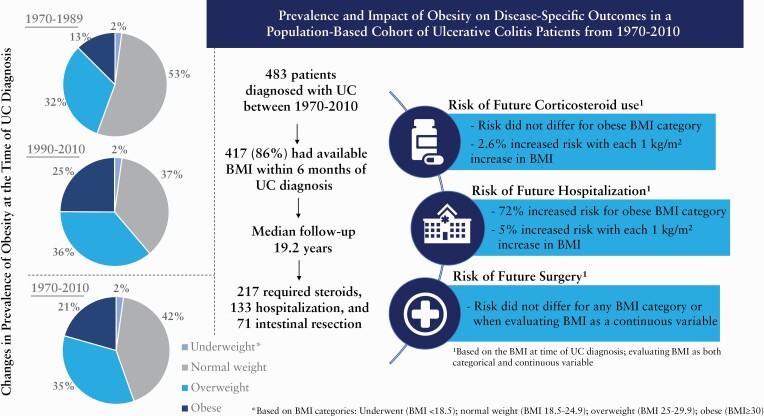
A total of 417 adults were diagnosed with UC over 1970–2010, 55.4% of whom were classified as either overweight [34.8%] or obese [20.6%]. The prevalence of obesity increased 2–3-fold over the 40-year study period. Obese patients had a 72% increased risk of hospitalisation (hazard ratio [HR],1.72; 95% confidence interval [CI], 1.10–2.71; p = 0.018) when compared with normal weight patients. Additionally, with each incremental increase in BMI by 1 kg/m2, the risk of hospitalisation increased by 5% [HR,1.05; 95% CI, 1.01–1.08; p = 0.008] and risk of corticosteroid use increased by 2.6% [HR,1.026; 95% CI, 1.00–1.05; p = 0.05].
Conclusions
The prevalence of obesity in the UC population is increasing and may have negative prognostic implications, specifically regarding risk of future hospitalisation and corticosteroid use. Additional prospective studies are necessary to more clearly define these associations.
Keywords: Ulcerative colitis[UC], inflammatory bowel disease [IBD], obesity
Graphical Abstract
1.Introduction
The prevalence of obesity within the general population has risen to unprecedented levels in recent decades, with estimates citing more than a third of the US population as obese.1,2 The inflammatory bowel disease [IBD] population has not been spared from this epidemic. In contrast to the conventional notion that many IBD patients are malnourished, several reports have documented a growing prevalence of obesity, with rates between 15% and 35%.3–10
Not only is obesity increasingly pervasive in this population, it has also become a putative risk factor for adverse outcomes in IBD, extrapolated from existing knowledge on the interaction between obesity and other immune-mediated conditions.11 Based on the theory that obesity itself is a pro-inflammatory state, it is conceivable that it may potentiate inflammation in IBD. Adipose tissue is not biologically inert, and instead is responsible for the production of a number of adipokines and pro-inflammatory cytokines fundamental to the inflammatory process of IBD, including tumour necrosis factor-alpha [TNF-α] and interleukin [IL]-6.12,13 Current data regarding the potential implications of obesity on IBD outcomes are sparse and largely inconclusive.3,4,7,8,14,15 Understanding specific risk factors that may portend a worse prognosis is essential, as it may allow for identification of high-risk individuals and prompt more aggressive medical therapy early on in their disease course—before the development of complications and further debility.
We aimed to assess the prevalence of obesity within a population of newly diagnosed ulcerative colitis [UC] patients, as well as to explore the correlation that obesity may have with disease extent and future complications.
2.Methods
2.1. Study setting and participants
We identified and investigated a population-based cohort of Olmsted County, Minnesota, residents who received a diagnosis of UC between 1970 and 2010, using the Rochester Epidemiology Project [REP] based on previously defined criteria.16–18
The REP provides a unique medical records linkage system that allows for comprehensive review of medical information from health systems in Olmsted County Minnesota, which collectively see greater than 95% of county residents over any given 4-year period.19,20 This centralised system indexes diagnoses generated from outpatient episodes of care, emergency department visits, hospitalisations, nursing home visits, surgical and endoscopic procedures, autopsy examinations, and death certificates.
2.2. Data abstraction
With approval by the institutional review boards of Mayo Clinic and Olmsted Medical Center, medical records were reviewed for accuracy of diagnosis as well as obtainment of demographic data and recorded disease extent, IBD-related hospitalisations, intestinal resection, and corticosteroid use.
Surgical procedures included in the analysis are listed in Supplementary Table A, available as Supplementary data at ECCO-JCC online. Hospitalisations were recorded only when an inpatient encounter was associated with the patient’s IBD. Steroid use included both conventional corticosteroids and budesonide. The study cohort was followed through their medical records from the date of their UC diagnosis until date of death, last follow-up, or date of last medical record abstraction [November 16, 2017].
2.3. Weight characterisation: body mass index
Height and weight data were collected through chart review and body mass index [BMI] calculated within 6 months of the UC diagnosis, giving preference to data closest to the date of diagnosis. Weight status was classified based on World Health Organization [WHO]-defined BMI categories of underweight [BMI <18.5 kg/m2], normal weight [BMI 18.5–24.99 kg/m2], overweight [BMI 25–29.99 kg/m2], and obese [BMI ≥30 kg/m2]. Weight status was also reported as a numerical BMI value, referred to as a continuous variable within the remainder of this report.
2.4. Statistical analyses
Descriptive statistics are reported as number [percent] for discrete variables and as either mean (standard deviation [SD]) or median interquartile range as appropriate for continuous variables. Baseline patient demographics and disease characteristics were compared among the four categories of baseline BMI using a chi square test or analysis of variance [ANOVA] as appropriate. Changes in mean BMI at the time of UC diagnosis and over time were also reviewed within eight pre-defined time frames including 1970–1974, 1975–1979, 1980–1984, 1985–1989, 1990–1994, 1995–1999, 2000–2004, and 2005–2010.
The main outcomes of interest examined were first surgery, first IBD-related hospitalisation, and first corticosteroid use identified after 90 days from the initial UC diagnosis date. Any patients with any of these outcomes within the first 90 days were also considered to be at risk in the following 90-day period. Survival-free estimates were calculated using the Kaplan‐Meier survival method. Associations of BMI, both as a continuous measurement and as a four-level category, were examined as well as other baseline covariates of interest using Cox proportional hazards regression models. The alpha level was set at 0.05 for statistical significance.
3.Results
A total of 417 adult Olmsted County residents received a diagnosis of UC between 1970 and 2010 and had available BMI data within 6 months of their diagnosis. In a previous publication, we reported a total of 483 patients diagnosed with UC between 1970 and 2010.21 Thus, the 417 individuals represent 86% of the entire inception cohort. In that same paper, we reported that the overall incidence rate of UC in this time period was 10.7 cases per 100 000 person-years. The adjusted incidence rate was 9.2 per 100 000 in 1970–1979, and 12.2 per 100 000 in 2000–2010. Demographic information on the 417 included patients is outlined in Table 1. Median follow-up was 19.2 years.
Table 1.
Demographics by BMI category
| Underweight | Normal Weight | Overweight | Obese | Total | |
|---|---|---|---|---|---|
| UC patients, n [%] | 9 [2.2%] | 177 [42.4%] | 145 [34.8%] | 86 [20.6%] | 417 [100%] |
| Age at Diagnosis | |||||
| Mean [SD] | 28.3 [9.3] | 36.6 [15.9] | 41.9 [16.2] | 46.9 [16.0] | 40.4 [16.4] |
| Sex, n [%] | |||||
| Female | 7 [77.8%] | 89 [50.3%] | 42 [29.0%] | 43 [50.0%] | 181 [43.4%] |
| Male | 2 [22.2%] | 88 [49.7%] | 103 [71.0%] | 43 [50.0%] | 236 [56.6%] |
| Race, n [%] | |||||
| White | 8 [88.9%] | 171 [96.6%] | 138 [95.2%] | 83 [96.5%] | 400 [95.9%] |
| Black | 0 [0.0%] | 1 [0.6%] | 4 [2.8%] | 2 [2.3%] | 7 [1.7%] |
| Asian | 0 [0.0%] | 3 [1.7%] | 1 [0.7%] | 0 [0.0%] | 4 [1.0%] |
| Unknown | 1 [11.1%] | 2 [1.1%] | 2 [1.4%] | 1 [1.2%] | 6 [1.4%] |
BMI categories: Underweight [BMI < 18.5]; normal weight [BMI 18.5–24.99]; overweight [BMI 25–29.99]; obese [BMI > 30]
3.1. Prevalence of obesity
Of 417 adults diagnosed with ulcerative colitis [UC] between 1970 and 2010, 231 [55.4%] were classified as either overweight [34.8%] or obese [20.6%] at the time of their diagnosis. Only nine [2.2%] patients were considered underweight.
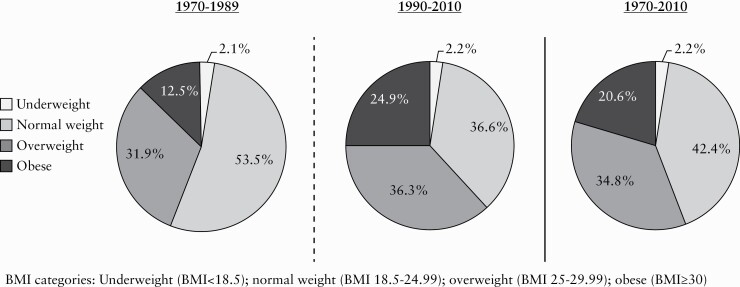
When BMI was evaluated over the eight pre-defined groups of patients based on year of UC diagnosis, there was a significant increase noted in mean BMI over time [p = 0.01] [Table 2]. Treating BMI as a categorical variable, the obesity rates increased 2–3-fold over the 40-year period [Figure 1]. The prevalence of obesity in patients diagnosed over 1970–1974 and 1975–1979 was 12.1% and 8.6%, respectively, with these numbers rising to 34.9% and 23.0% in the 2000–2004 and 2005–2010 cohorts, respectively. There was also a significant upward trajectory in mean BMI over time [p <0.001] [Table 2].
Table 2.
Trends in mean BMI at the time of UC diagnosis over the course of 40 years [1970–2010]
| Year of IBD Diagnosis | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1970–1974 | 1975–1979 | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2010 | ||
| Mean BMI* [SD] [n = 417] | 25.2 [3.7] | 23.8 [3.5] | 25.6 [5.0] | 25.6 [4.9] | 25.4 [4.3] | 26.7 [5.7] | 28.2 [5.5] | 27.0 [5.6] | <0.001 1 |
1ANOVA-F test
*BMI in kg/m2
Figure 1.
Changing prevalence of overweight and obesity at time of UC diagnosis from 1970–2010. BMI categories: Underweight [BMI < 18.5]; normal weight [BMI 18.5–24.99]; overweight [BMI 25–29.99]; obese [BMI≥30].
3.2. Disease extent based on BMI
When comparing UC disease extent among BMI categories, obese patients were more likely to have left-sided [E2] or extensive disease [E3] —at 38.4% and 38.4%, respectively—as opposed to isolated proctitis [23.3%] [Table 3]. Although 77.8% of patients in the underweight category also had extensive disease, only nine of 417 [2.2%] patients were considered underweight, making this a very small group. Overall, differences in disease location between BMI groups were not statistically significant [p = 0.06], nor were any differences noted when BMI was assessed as a continuous variable.
Table 3.
Effect of body mass index [BMI] on UC extent
| Body Mass Index [BMI] Category | Total | p 1 | ||||
|---|---|---|---|---|---|---|
| Underweight | Normal weight | Overweight | Obese | |||
| UC Patients | n = 9 | n = 177 | n = 145 | n = 86 | n = 417 | |
| Disease Extent | 0.06 | |||||
| E1 [proctitis] | 0 [0%] | 62 [35.0%] | 48 [33.1%] | 20 [23.3%] | 130 [31.2%] | |
| E2 [left-sided] | 2 [22.2%] | 47 [26.6%] | 41 [28.3%] | 33 [38.4%] | 123 [29.5%] | |
| E3 [extensive] | 7 [77.8%] | 68 [38.4%] | 56 [38.6%] | 33 [38.4%] | 164 [39.3%] |
1Chi-Square analysis; Bold indicates significant p-value [≤0.05]
Underweight [BMI < 18.5]; normal weight [BMI 18.5–24.99]; overweight [BMI 25–29.99]; obese [BMI≥30]
3.3. IBD-related complications
During a median follow-up of 19.2 years in 417 UC patients, 133 required hospitalisation, 71 underwent intestinal resection, and 217 required steroids. Risk of future IBD-related complications was assessed using BMI as both a continuous variable with the actual BMI value, as well as a categorical variable, based on the four WHO-defined BMI categories.
3.4. Corticosteroids
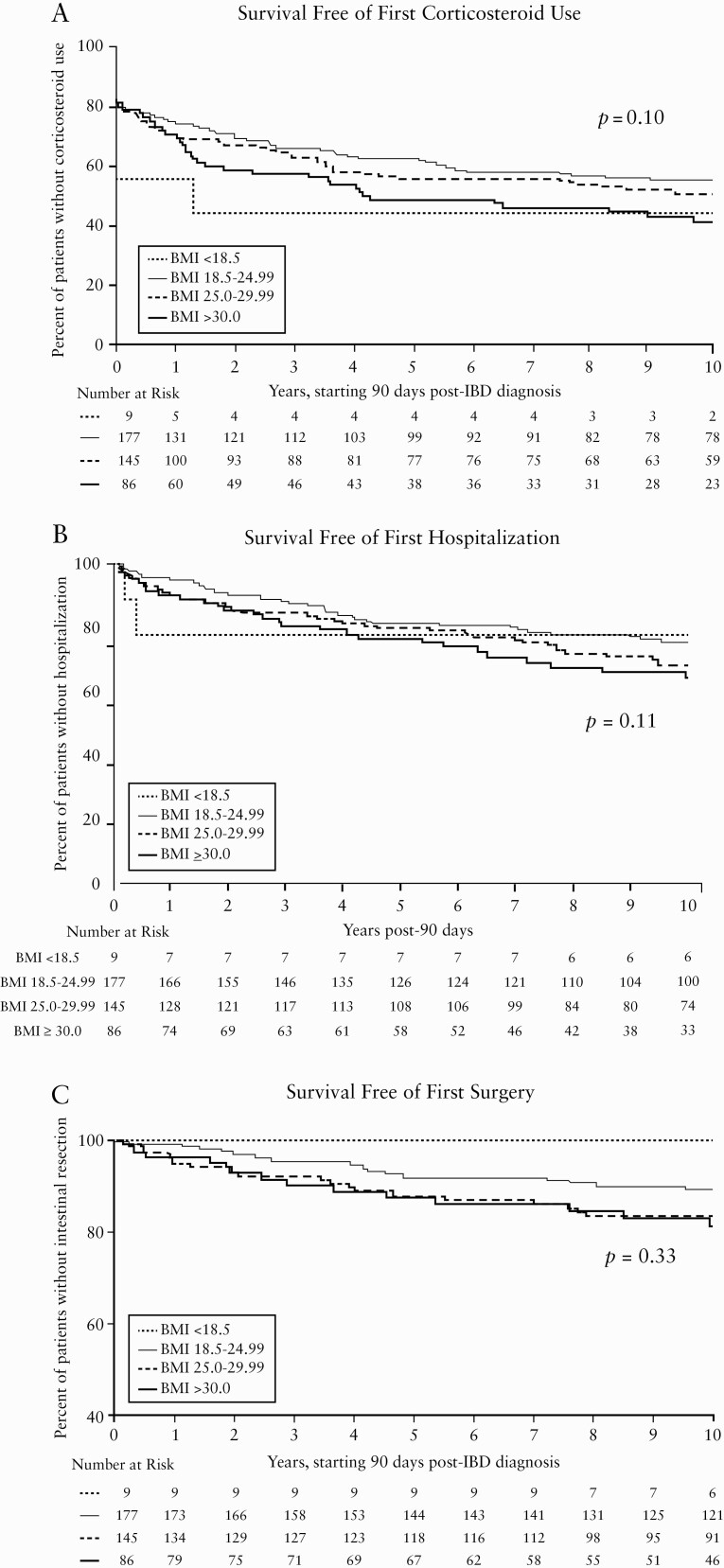
There was a somewhat higher risk of future corticosteroid use as BMI increased, in that with each 1-kg/m2 rise in BMI, the risk of requiring steroids increased by 2.6% [HR, 1.026; 95% CI, 1.00–1.05; p = 0.05][Table 4]. When BMI was assessed categorically, there was also a non-significant trend towards a higher risk of steroid use in the obese population when compared with their normal weight counterparts [HR, 1.37; 95% CI, 0.96–1.96; p = 0.08][Figure 2a and Table 4]. Although underweight patients made up a small percentage of the cohort, they did have a statistically significantly higher risk of steroid use as compared with normal weight patients [HR, 2.22; 95% CI, 1.03–4.81; p = 0.04][Table 4].
Table 4.
Risk of future UC-related complications based on BMI at diagnosis
| Categorical Variable | Continuous Variable | |||
|---|---|---|---|---|
| Hazard Ratio | p | Hazard Ratio | p | |
| Surgery | 0.33 [overall]1 | |||
| Underweight | 0.00 | 0.982 | 1.04 [0.99–1.08] | 0.09 |
| Overweight | 1.53 [0.90–2.62] | 0.12 | ||
| Obese | 1.67 [0.90–1.08] | 0.11 | ||
| Hospitalisation | 0.11 [overall]1 | |||
| Underweight | 1.42 [0.44–4.57] | 0.562 | 1.05 [1.01–1.08] | 0.008 |
| Overweight | 1.41 [0.95–2.10] | 0.09 | ||
| Obese | 1.72 [1.10–2.71] | 0.02 | ||
| Steroid use | 0.10 [overall]1 | |||
| Underweight | 2.22 [1.03–4.81] | 0.04 2 | 1.03 [1.00–1.05] | 0.05 |
| Overweight | 1.24 [0.91–1.69] | 0.17 | ||
| Obese | 1.37 [0.96–1.96] | 0.08 |
BMI categories: Underweight [BMI < 18.5]; overweight [BMI 25–29.99]; obese [BMI≥30]
Bold: Indicates significant p value [≤0.05]
aoverall p value reflects any differences between the three ‘abnormal’ BMI categories [underweight, overweight, obese]
bindividual p values evaluate differences between each specific BMI category and normal weight [BMI 18.5–24.99]
1univariate Cox-model.
Figure 2.
Kaplan Meier Curves for Survival Free of Corticosteroid Use, Hospitalization, and Intestinal Resection in UC Patients Based on BMI.
3.5. IBD-related hospitalisation
Compared with their normal weight counterparts, those classified as obese had a 72% increased risk [HR, 1.72; 95% CI, 1.10 – 2.71; p = 0.018] of requiring hospitalisation for their IBD [Figure 2b and Table 4]. Moreover, this trend remained when BMI was treated as a continuous variable, as with each 1-kg/m2 increase in BMI the risk of hospitalisation increased by 5% [HR, 1.05; 95% CI 1.01–1.08; p = 0.008] [Table 4].
3.6. Surgery
When comparing BMI as a categorical variable, there were no significant differences in the risk of future surgery between the three abnormal BMI groups [p = 0.33][Figure 2c and Table 4]. Although not significant, when BMI was treated as a continuous variable there was a trend toward elevated risk of surgery in those with higher BMIs, suggesting that with each 1-kg/m2 increase in BMI the risk of surgery rose by 3.7% [HR 1.037; 95% CI 0.99–1.08; p = 0.09][Table 4].
4.Discussion
£Within this population-based cohort of 417 patients diagnosed with UC between 1970 and 2010, the prevalence of obesity at the time of UC diagnosis increased significantly over time. While obesity was present in only 12.5% of UC patients in the first half of the study period [1970–1989], these numbers doubled for those diagnosed during the latter half [1990–2010], with 24.9% of UC patients classified as obese. When overweight patients were included in the more recent 1990–2010 cohort, a staggering 61.2% were considered to be above their ideal body weight [BMI≥25 kg/m2]. Our findings corroborate those from the other available data, which document obesity rates in IBD patients ranging from 15–35%, increasing further to 40–72% when overweight patients were included.3–5,7,8,10 To date, this is the only population-based study to explore the prevalence of obesity at the time of IBD diagnosis, as most others focus on patients with an established IBD diagnosis who are already undergoing therapy. Moreover, it is the first to review how the prevalence of overweight and obesity at the time of IBD diagnosis has changed over time.
Our data also represents one of only a few available studies reviewing whether BMI impacts UC disease location. While we demonstrated that numerically obese patients were more likely to have left-sided or pancolitis as opposed to isolated proctitis, this was not statistically significant, corroborating another available study suggesting no appreciable differences in disease location based on BMI.7
Whether the surge in obesity among IBD patients is simply a reflection of the trend seen within the general population, or if there is some underlying epidemiologic association is unclear. An environmentally-based hypothesis for the development of IBD is not a new concept, as multiple factors such as improved hygiene,22,23 increased antibiotic use,24,25 dietary changes,26,27 and alterations in the intestinal microbiome28–30 have previously been placed under scrutiny. As a result of the concurrent rise in IBD incidence alongside that of obesity,31 particularly in newly developed regions where IBD was previously uncommon,32–35 greater interest has also been paid to the possible pathogenic role that premorbid obesity may play in the development of IBD. The few studies that have explored this possibility have produced conflicting results.36–38
Even if no pathogenic role is ascertained, the impact obesity has on the natural history of IBD remains an important topic for investigative inquiry as this population is clearly suffering from higher rates of obesity. A limited number of studies have previously explored the clinical and prognostic implications of obesity in IBD with inconsistent results, the majority focusing on CD outcomes, and only a few including UC patients.3–5,7,8,14,15,39,40 Our data suggest that obesity in UC patients may portend a poorer prognosis. Specifically, we found that obese patients had a 72% higher risk of hospitalisation for their IBD compared with their normal-weight counterparts. Also demonstrated that with each increase in BMI by 1 kg/m2, the risk of hospitalisation increased by 4.5% and risk of steroid use increased by 2.6%. While sparse, the few additional studies that explore the effect of obesity on UC-related outcomes suggest that either the risk for complications is no different,8 less than,7,10 or greater than5,40 their normal weight counterparts. For instance, Steed et al previously demonstrated that overweight and obese UC patients have higher risk of surgery.5 Our findings, suggesting a higher risk of hospitalisation and corticosteroid use in obese UC patients, are consistent with another study which described a higher risk of hospitalisation, clinic visits and escalation of therapy in IBD patients with higher BMI – though note that CD patients were also included as part of this cohort.40 Therefore, our data provides much-needed insight into a currently understudied topic, within a population that is highly affected by obesity.
One notable limitation of this and other studies is the utilization of BMI as a measure of adiposity. It has been demonstrated that mesenteric adiposity, as opposed to subcutaneous fat, is likely the main driver of the CRP response and inflammatory cascade in CD patients.12 Studies assessing the utility of BMI as a surrogate measure for central adiposity in other disease processes have suggested a poor linear association, making its use as a corollary measurement unclear.41,42 It is possible that alternative methods of measuring adiposity, such as waist circumference, waist-to-hip ratio, skin-fold measurements, or radiographic modalities may have greater prognostic value. However, the retrospective nature of this study limits procurement of such measurements. Another potential limitation of this study relates to the idea that the available therapeutic options and thresholds for surgery within this population are likely heterogeneous given the lengthy time span that this cohort encompasses. Available data regarding the impact that obesity has on the efficacy of immunomodulator and biologic therapies are varied, and while this is an important question to answer, it is beyond the scope of this paper.
There are several strengths to report. Currently, this is the only population-based study to describe the prevalence of obesity within newly diagnosed UC patients. BMI measurements obtained at the time of IBD diagnosis reduce confounding by steroid use or smoking cessation, both of which are independently associated with disease activity and result in weight gain.43,44 The follow-up period for this population is significantly longer than what is available for most other studies, at nearly 20 years. It is also the first to review how the prevalence of overweight and obesity has changed over time. Finally, our report includes UC patients, a population for which there is extremely limited data.
In summary, the current study provides much-needed population-based data regarding the growing prevalence of obesity in patients with ulcerative colitis, adding to an expanding body of evidence that suggests this population is experiencing an obesity epidemic paralleling that of the general population. Our findings suggest that obesity may have negative prognostic implications for UC patients, specifically regarding IBD-related hospitalisation and potentially future corticosteroid use. Further prospective studies utilizing more accurate measurements of visceral adiposity may be better suited to more accurately capture the association between obesity and IBD-related outcomes. In the modern era of IBD care, gastroenterologists must remain cognizant of the potential new challenges that obesity generates in the management of our patients from both an IBD and a general health perspective.
Supplementary Material
Acknowledgments
Larry Timmons, Debra Jewell for data abstraction.
Funding
This work was supported in part by the Mayo Foundation for Medical Education and Research, the Rochester Epidemiology Project, as well as grants to include the following: [grants number R01 AG034676, AG052425] from the National Institute on Aging of the National Institutes of Health. The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Conflict of Interest
BKA—Consultant: Metamodix, BFKW, DyaMx, Boston Scientific, USGI medical; research support: Apollo Endosurgery, USGI, Spatz Medical, Boston Scientific, GI Dynamics, Cairn Diagnostics, Aspire Bariatrics, Medtronic; speaker: Johnson and Johnson, Endogastric solutions, Olympus. EVL—research support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene/Receptos, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Theravance, and Takeda; consulted for AbbVie, Allergan, Amgen, Arena, Boehringer Ingelheim, Bristol-Myers Squibb, Calibr, Celgene, Celltrion Healthcare, Eli Lilly, Genentech, Gilead, Iterative Scopes, Janssen, Ono Pharma, Pfizer, Sun Pharma, Takeda, and UCB.
Author Contributions
Study concept and design: AMJ, EVL; acquisition of data: AMJ, SA; statistical analysis: WSH; drafting of the manuscript: AMJ, EVL; critical revision of the manuscript: AMJ, WSH, SA, WJT, BKA, EVL.
References
- 1. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief, no 288. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 2. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–50. [DOI] [PubMed] [Google Scholar]
- 3. Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O’Morain C, O’Sullivan M. High prevalence of overweight and obesity in adults with Crohn’s disease: associations with disease and lifestyle factors. J Crohns Colitis 2013;7:e241–8. [DOI] [PubMed] [Google Scholar]
- 4. Moran GW, Dubeau MF, Kaplan GG, Panaccione R, Ghosh S. The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis 2013;19:2949–56. [DOI] [PubMed] [Google Scholar]
- 5. Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts 2009;2:370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long MD, Crandall WV, Leibowitz IH, et al. ; ImproveCareNow Collaborative for Pediatric IBD. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci 2015;60:2436–45. [DOI] [PubMed] [Google Scholar]
- 8. Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis 2015;21:2857–63. [DOI] [PubMed] [Google Scholar]
- 9. Sousa Guerreiro C, Cravo M, Costa AR, et al. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: a case-control study. Am J Gastroenterol 2007;102:2551–6. [DOI] [PubMed] [Google Scholar]
- 10. Stabroth-Akil D, Leifeld L, Pfützer R, Morgenstern J, Kruis W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis 2015;30:237–42. [DOI] [PubMed] [Google Scholar]
- 11. Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13:981–1000. [DOI] [PubMed] [Google Scholar]
- 12. Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 2012;61:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 2009;54:1847–56. [DOI] [PubMed] [Google Scholar]
- 14. Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn’s disease clinical course and severity in obese patients. Clin Nutr 2002;21:51–7. [DOI] [PubMed] [Google Scholar]
- 15. Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:482–8. [DOI] [PubMed] [Google Scholar]
- 16. Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology 1998;114:1161–8. [DOI] [PubMed] [Google Scholar]
- 17. Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut 2000;46:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loftus CG, Loftus EV Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis 2007;13:254–61. [DOI] [PubMed] [Google Scholar]
- 19. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol 2017;15:857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dupas JL, Debeugny S, Salomez JL, et al. Environmental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut 2005;54:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cholapranee A, Ananthakrishnan AN. Environmental hygiene and risk of inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis 2016;22:2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am J Gastroenterol 2011;106:2133–42. [DOI] [PubMed] [Google Scholar]
- 25. Aniwan S, Tremaine WJ, Raffals LE, Kane SV, Loftus EV Jr. Antibiotic use and new-onset inflammatory bowel disease in Olmsted County, Minnesota: a population-based case-control study. J Crohns Colitis 2018;12:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tjonneland A, Overvad K, Berglund G, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut 2009;58:1606–11. [DOI] [PubMed] [Google Scholar]
- 27. Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013;145:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 2017;152:327–39.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011;60:631–7. [DOI] [PubMed] [Google Scholar]
- 31. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 32. Benchimol EI, Mack DR, Guttmann A, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol 2015;110:1–11. [DOI] [PubMed] [Google Scholar]
- 33. Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol 2013;5:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ng SC, Tang W, Ching JY, et al. ; Asia–Pacific Crohn’s and Colitis Epidemiologic Study [ACCESS] Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013;145:158–65.e2. [DOI] [PubMed] [Google Scholar]
- 35. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 36. Chan SS, Luben R, Olsen A, et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: data from a European Prospective Cohort Study [The IBD in EPIC Study]. Am J Gastroenterol 2013;108:575–82. [DOI] [PubMed] [Google Scholar]
- 37. Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2015;21:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harpsøe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014;43:843–55. [DOI] [PubMed] [Google Scholar]
- 39. Pringle PL, Stewart KO, Peloquin JM, et al. Body mass index, genetic susceptibility, and risk of complications among individuals with Crohn’s disease. Inflamm Bowel Dis 2015;21:2304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pavelock N, Masood U, Minchenberg S, Heisig D. Effects of obesity on the course of inflammatory bowel disease. Proc [Bayl Univ Med Cent] 2019;32:14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dimala CA, Ngu RC, Kadia BM, Tianyi FL, Choukem SP. Markers of adiposity in HIV/AIDS patients: agreement between waist circumference, waist-to-hip ratio, waist-to-height ratio and body mass index. PLoS One 2018;13:e0194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens 2013;7:85–94. [DOI] [PubMed] [Google Scholar]
- 43. Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systematic review and meta-analysis of prospective cohort studies. Obes Rev 2015;16:883–901. [DOI] [PubMed] [Google Scholar]
- 44. Berthon BS, MacDonald-Wicks LK, Wood LG. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr Res 2014;34:179–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.