Abstract
Background: Significant gaps exist in access to evidence-based pediatric weight management interventions, especially for low-income families who are disproportionately affected by obesity. As a part of the Centers for Disease Control and Prevention's Childhood Obesity Research Demonstration project (CORD 3.0), the Missouri team (MO-CORD) aims to increase access to and dissemination of an efficacious pediatric obesity treatment, specifically family-based behavioral treatment (FBT), for low-income families.
Methods/Design: The implementation pilot study is a multisite matched-comparison group pilot of packaged FBT in pediatric clinics for low-income children with obesity, of ages 5 to 12 years old. The study is implemented in two Missouri pediatric primary care clinical sites, Freeman Health System Pediatric Clinics (rural Joplin) and Children's Mercy Hospital Pediatric Clinics (urban Kansas City). The design focuses on pragmatism through utilization of PRECIS (Pragmatic Explanatory Continuum Indicator Summary) domains, such as open eligibility criteria, limited follow-up intensity, reliance on medical records for creating a usual care comparison group data, and unobtrusive measurement of participant and provider adherence. The evaluation focuses on effectiveness as well as implementation outcomes and barriers to inform implementation scale up.
Conclusions: Findings from this study will advance both science and practice by providing novel and immediately useful information to families, health care providers, health care organizations, payers, and other state Medicaid plans by developing and optimizing evidence-based pediatric weight management treatment for implementation and dissemination in health systems to address health disparities among low-income populations most affected by overweight and obesity.
Keywords: childhood obesity, dissemination, family-based behavioral treatment, implementation, primary care
Introduction
Approximately 41.5% of youth in the United States meet criteria for overweight or obesity,1 and prevalence is higher among youth from low socioeconomic households and racial/ethnic minority youth.2,3 Although there are evidence-based treatments for childhood obesity delivered in primary care settings,4,5 low-income youth as well as racial/ethnic minority youth experience unique barriers to accessing quality care, including inadequate insurance coverage and high treatment costs.6–8 As such, scalable childhood obesity interventions are urgently needed to address disparities in obesity among at-risk youth.9
To address this gap, the Missouri Childhood Obesity Research Demonstration (MO-CORD) project aims to increase access to and dissemination of family-based behavioral obesity treatment (FBT) for low-income families. FBT is designed to help parents and children establish sustainable eating and physical activity changes across multiple socioenvironmental contexts (e.g., home, school, community, and work).10 FBT is consistent with the US Preventive Services Task Force (USPSTF) recommendation of ≥26 hours of individualized intensive intervention delivered for up to 12 months.11 Primary care offers an optimal setting for FBT delivery, as it capitalizes on the established relationship between primary care providers (PCPs) and families. Colocating interventionists within the primary care setting overcomes fragmentation of care and addresses provider time constraints and referral barriers. Furthermore, this delivery strategy dovetails with the obesity treatment benefit by the Missouri HealthNet Division of Missouri Medicaid published in March 2021 that covers intensive obesity behavioral counseling and medical nutrition therapy, thus setting the stage to scale FBT implementation across primary care.12
Missouri HealthNet Division's coverage changes will rectify lack of reimbursement as a major barrier to providing evidence-based treatment for obesity such as FBT for low-income families. However, for this change to achieve its desired effect, multiple barriers must be addressed. The delivery of FBT in primary care settings requires establishment of new roles to deliver the intervention. These interventionists then need to be trained, yet there is a lack of established and easily disseminated training and certification processes for FBT. PCPs also need training and guidance around diagnosis, referral, and coordination of care. In addition, there needs to be a better understanding of the contextual factors that promote uptake and sustainability of FBT in health care settings as well as the costs associated with implementation.
The MO-CORD project aims to address these barriers to care through three phases: (1) digitally package FBT behavioral health care interventionist training and intervention materials in a user-friendly scalable format with an emphasis on applying user-centered design methods; (2) conduct a multisite pilot implementation study in urban and rural pediatric primary care clinics involving establishment of new roles and training of interventionists and providers; and (3) develop a dissemination and sustainability plan for an optimized package incorporating lessons learned in the first two phases. This article describes the pilot implementation study protocol to accomplish the second aim mentioned.
Method
Study Design
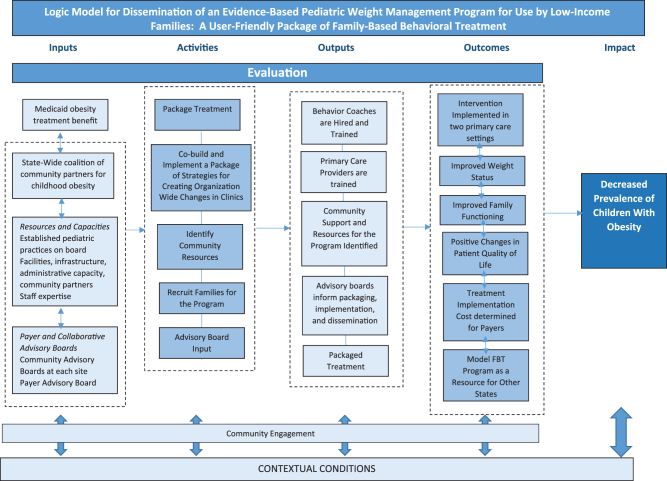
The implementation research study uses a nonrandomized multisite matched-comparison design to pilot a packaged FBT, delivery by clinic-based interventionists, in seven pediatric clinics that primarily serve low-income families. The target enrolment in the FBT arm is 208 children 5–12 years old with obesity. A matched-comparison group will leverage historical controls selected from electronic health records (EHRs) of similar proximal clinics. The design focuses on pragmatism through utilization of Pragmatic Explanatory Continuum Indicator Summary (PRECIS) domains, such as open eligibility criteria, limited follow-up intensity, reliance on medical records for creating a usual care comparison group data, and unobtrusive measurement of participant and provider adherence.13 The evaluation focuses on effectiveness as well as implementation outcomes and barriers to inform implementation scale up. The study logic model is shown in Figure 1. The Institutional Review Board approved the study (IRB ID #: 202103221-1070).
Figure 1.
Logic model for dissemination of an evidence-based pediatric weight management program for use by low-income families: a user-friendly package of family-based behavioral treatment.
Specific Aims
The aims of the pilot implementation study within the MO-CORD project are as follows.
Conduct a pilot study to test a scalable implementation model targeting increasing capacity of PCPs to refer children to evidence-based treatment, establishing behavioral interventionists in primary care settings to deliver treatment, and increasing referrals and reach through multisectoral stakeholder engagement. We will assess the impact of our pilot implementation through two subaims:
Sub-Aim a. Evaluate provider- and organizational-level reach, adoption, implementation, and maintenance, including implementation contextual factors that may impact these outcomes.
Sub-Aim b. Evaluate participant-level acceptability, engagement, and effectiveness.
Participants
Multisite sample
The study will be implemented in two Missouri pediatric health systems in rural Joplin and urban Kansas City. Three and four pediatric primary care clinics will participate in the study within the Joplin and Kansas City health systems, respectively. The sites were chosen, in part, because they have active practice-based research networks for study recruitment and implementation. Both health systems play a leading role in local healthy eating/active living coalitions through which the pilot can be supported and promoted. The study population will consist of low-income children with obesity of ages 5–12 years seen in an urban primary care clinic in Kansas City, MO (Children's Mercy Hospital's Pediatric Care Center—PCC) and rural primary care clinics (Freeman Health System) in Joplin, MO. Average characteristics of the two participating health systems over the past few years are presented in Table 1.
Table 1.
Characteristics of the Participating Health Systems
| Characteristic | Children's Mercy Hospital's Pediatric Care Center, Kansas City, MO | Freeman Health System, Joplin, MO |
|---|---|---|
| Physicians and nurse practitioners | 54 | 12 |
| Average patient visits per year | 52,000 | 4500 |
| Average eligible patients aged 5–12 years per year | 9900 | 5200 |
| Average percentage of patients 5–12 years with obesity | 23% (2300) | 19% (1000) |
| Average race/ethnicity of children with obesity | 10% White 36% Black/African American 45% Hispanic 9% Other race/ethnicity |
91% White 4% Black/African American 10% Hispanic 5% Other race/ethnicity |
| Average percentage of children with obesity on Medicaid | 82% | 37% |
Averages are of patients seen in outpatient settings (e.g., well child care visits) in the participating health systems from the past few years.
Recruitment and retention
Participant eligibility criteria include parent/caregiver 18 years of age or older and child of ages 5–12 years with BMI percentile ≥95th for age and gender, and enrolled in Missouri HealthNet Medicaid. Children will be recruited through referral by PCPs during any clinic visit (e.g., well or sick) at the pediatric clinics in both Kansas City and Joplin. Implementation mapping was used to inform the development of strategies and tools for supporting referrals, as described later. Additional recruitment methods will include letters and calls to eligible patients identified through EHR database-generated lists and study promotion information disseminated through community partners. Multiple retention strategies are employed throughout the study to maximize participant retention throughout their 12-month intervention including flexible session scheduling, frequent contact, identifying and problem-solving barriers to attendance, use of telemedicine/virtual modalities for group treatment sessions, and expectation setting from first contact.
Intervention: Family-Based Behavioral Treatment
An overview of the development and packaging of the FBT can be found elsewhere.14 The digital web-based FBT package includes content for training and certifying interventionists to deliver FBT, an interventionist portal to coordinate care with families and track progress, and a patient portal for children and caregivers to access digital resources and FBT materials to supplement their face-to-face group and individual sessions with interventionists. Over three decades of randomized-controlled trials have been used to develop, refine, and demonstrate the effectiveness of FBT, which provides a family-centered approach to help both caregivers and children build and establish lasting improvements in nutrition and physical activity and reductions in sedentary activity by applying self-regulatory skills, behavioral economics, and social learning theory principles to the practice of behaviors across multiple socioenvironmental contexts (e.g., home, school, community, and work).10,15–18 Licensed behavioral health care interventionists (e.g., social workers, professional counselors, registered dietitians, marriage/family therapists, and psychologists) serve as the FBT interventionists in this project. These interventionists are employed within each health system (1–2 per health system), their services are reimbursable by the Missouri Medicaid benefit, and can have other roles within the clinics (e.g., behavioral health specialist).
Formative Work to Inform Integration of the FBT into Each Health System
We used the iterative and participatory approach of implementation mapping19–22 to develop strategies and tools for supporting PCPs and other clinic staff to refer eligible patients to the program. This process is outlined in Table 2 and involved the creation of an implementation planning workgroup within each clinic and a series of meetings to discuss potential barriers to implementation and strategies for overcoming these barriers. The study team mapped the identified barriers to the constructs within the Consolidated Framework for Implementation Science (CFIR),23 each of which have been linked to promising implementation strategies in previous research.24,25 Although this mapping helped uncover broad implementation strategies to consider, the specifics of each strategy (e.g., how, who) were based on discussion among the implementation planning workgroups. The resulting strategies and tools for supporting referrals included referral training workshops and ongoing check-in meetings, weekly reviews of upcoming visits to identify and flag eligible patients, a notification to PCPs and clinic staff within each eligible patient's electronic previsit checklist, a single-page bulleted referral guide with talking points, an informational handout for eligible patients, an individualized follow-up call with a program recruiter, and periodic summaries of referral rates for PCPs and clinics.
Table 2.
Overview of Steps to Implementation Mapping Approach to Be Used in MO-CORD
| Establish an implementation planning workgroup within each clinic. |
| Conduct a needs assessment to identify clinic-level barriers and facilitators for embedding the implementation effort into the organization/system. |
| Identify the determinants (behavioral, environmental, and other social determinants of health) of the key barriers, based on CFIR (i.e., under which CFIR constructs do the barriers belong). |
| Identify potential implementation strategies for overcoming the selected barriers, using the list of strategies that have been mapped to CFIR |
| Operationalize each implementation strategy by adding specificity through workgroup discussion. |
| Create processes, documents, and other materials to guide implementation, based on the strategies identified. |
| Provide ongoing support to clinic implementation teams and adapt processes and materials as new lessons are learned. |
CFIR, Consolidated Framework for Implementation Science; MO-CORD, Missouri team-Childhood Obesity Research Demonstration.
Evaluation Data Collection and Measures
Evaluation frameworks and overview
Study measures are organized by the Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) framework,26 which emphasizes reach, effectiveness, adoption, implementation, and maintenance in scaling up interventions. The Sub-Aim a organizational level outcomes address the reach, adoption, implementation, and maintenance components of RE-AIM; Sub-Aim b participant-level outcomes address acceptability and effectiveness. The Sub-Aim a outcomes have often been conceptualized as implementation outcomes given that they are key indicators of implementation success and intermediate outcomes in relation to treatment effectiveness.27 MO-CORD will also use the CFIR28 to guide the qualitative assessment of implementation contextual factors to uncover potential barriers and facilitators to reach, adoption, implementation, and maintenance. Study measures are presented in Table 3. Participant demographics (e.g., gender, race/ethnicity) will also be assessed through questionnaire and obtained from EHRs from all pediatric clinic visits from the eligible clinics.
Table 3.
MO-CORD Measures Organized by the RE-AIM Framework
| Measure/construct | Level/unit of analysis | How used in analyses | Data source | Time point |
|---|---|---|---|---|
| Clinic-level measure (Sub-Aim a) | ||||
| 1 Reach | ||||
| 1.1 Percentage of eligible children who are (a) referred and (b) enrolled. | Clinics | Descriptive clinic-level outcome | Tracking records | End of study |
| 1.2 Representativeness of those (a) referred and (b) who complete the study to (c) those eligible and (d) the statewide population | Clinics, state | Descriptive clinic- and study-level outcome | Existing data—electronic health records and health surveillance systems | End of study |
| 2 Adoption | ||||
| 2.1 Percentage of providers within the clinic who (a) ever and (b) regularly refer eligible patients to the program. | Providers, clinics | Descriptive clinic-level outcome | Electronic health records | End of study |
| 2.2 Representativeness of participating clinics to all clinics in state | Clinics, state | Descriptive clinic-level outcome | Existing data—MO Primary Care Association | Baseline |
| 3 Implementation | ||||
| 3.1 Knowledge and weight bias | Interventionists | Descriptive | Surveys | Months 0, 3, 6, 12 |
| 3.2 Intervention fidelity and adaptations | Participants and interventionists | Participant-level predictor Clinic-level outcome |
Audio recording audits and interventionist checklists and interviews | Ongoing |
| 3.3 Costs (e.g., Implementation training and intervention delivery) | Clinics, interventionists, and participants | Descriptive, and clinic-level and participant-level outcome | Electronic health records; website metrics; surveys | Ongoing |
| 3.4 Implementation contextual factors | Clinics and health systems | Clinic-level predictor | Interventionist and medical director interviews | Postintervention |
| 4 Maintenance | ||||
| 4.1 Sustained delivery | Providers and clinics | Clinic-level outcome (also an implementation outcome) | Interventionist and medical director interviews | Postintervention |
| Participant-level measures (Sub-Aim b) | ||||
| 5 Acceptability | ||||
| 5.1 Acceptability | Participants (child and parent) | Patient-level outcome and predictor | Surveys | Postintervention |
| 5.1 Engagement | Participants (child and parent) | Patient-level outcome and predictor | Tracking records and surveys | Ongoing |
| 6 Effectiveness | ||||
| 6.1 Percent overweight | Participants (child and parent), Matched comparisons for children | Patient-level outcome | Stadiometer (height) and scale Matched comparisons will be from electronic health records |
Months 0, 3, 6, 12 |
| 6.2 Quality of life | Participants (child and parent) | Patient-level outcome | Surveys | Months 0, 3, 6, 12 |
RE-AIM, Reach, Effectiveness, Adoption, Implementation, Maintenance.
Reach and adoption (Sub-Aim a outcomes)
Calculating reach helps to describe who is willing to participate in the intervention and how similar or different (i.e., representative) participants are to those who are eligible but do not participate. A low reach value, based on the percentage of eligible children who are (a) referred and (b) enrolled, would indicate that greater efforts are needed to facilitate the identification and enrollment of participants. Indicators of representativeness can show whether specific population groups are being missed and how participation rates may translate to new geographic areas or settings with different populations. The MO-CORD team will calculate reach using data from the EHR of each participating health system, which involves tracking of patient eligibility, referrals, and enrollment. Since all seven sites are considered adopters, MO-CORD will calculate levels of adoption within each clinic, defined as the proportion of providers within the clinic who (a) ever and (b) regularly (e.g., ≥50% of the time) refer eligible patients to the program. The representativeness of the adopting clinics will be captured by comparing their clinic and patient characteristics with statewide data from health surveillance systems such as the MO Primary Care Association.29
Implementation (Sub-Aim a outcomes)
Interventionist knowledge and skills related to FBT delivery will be assessed using a 20-item questionnaire we developed for a previous study, before and after training of the interventionists. Fidelity to essential intervention components will be measured using regular supervision checklists completed by the study team and self-reported adherence completed by interventionists after each session. Adaptations to nonessential intervention components will be measured using postintervention interviews with interventionists.
Implementation costs (Sub-Aim a outcomes)
We will assess the costs to implement FBT inclusive of labor and nonlabor costs.30,31 We will use a societal perspective that includes providers, participants, and payers (insurers); each of those groups will also be reported separately.
Training
We will use a microcosting approach,32 combining time spent on each component of FBT with wage/salary data including fringe benefits. We will assess FBT interventionists' time spent receiving FBT training using website usage metrics (e.g., logins and time spent using the website) and self-report survey of their time completing the web training and any additional training activities.
Delivery
During FBT delivery, we will collect EHR data on number of visits, who delivered the session, and when included in the note, the duration of the session. However, since additional time may be incurred for FBT that is not part of the record or bill, we will compare this with providers' self-reported time and adjust as necessary. As FBT will be reimbursable in Missouri, we will collect Medicaid claims data to account for provider and facility fees through the University of MO-Columbia Center for Health Policy, which contracts with MO Medicaid to provide this service and has agreed to assist with the project. We will continue to track time FBT providers spend using the treatment website for ongoing training and delivery support. Furthermore, we will assess the number of hours FBT providers, medical providers, and clinic administrators spend engaged in nonresearch implementation activities, such as participating in meetings to establish an implementation plan for the clinic and completing recertification, and any in-kind services that are provided to support implementation, derived through brief survey. Finally, participants will report through questionnaire any costs incurred as a result of treatment (e.g., buying a scale). Costs to maintain the FBT website will be collected from the vendor.
Implementation contextual factors (Sub-Aim a antecedents)
Implementation contextual factors will be assessed postintervention using qualitative interviews of interventionists and clinic-level medical directors/administrators based on the CFIR structured interview guide.28 These interviews will help uncover whether remaining implementation barriers exist and/or additional implementation strategies that may have been used within each clinic to support success around referrals and intervention delivery embedded within the existing health system.
Maintenance
The likelihood of sustained delivery of the intervention within each clinic will also be assessed during the postintervention qualitative interviews.
Acceptability (Sub-Aim b outcomes and antecedents)
Participant engagement and acceptability will be monitored by tracking session attendance and assessed using the Therapeutic Alliance Scale.33
Effectiveness (Sub-Aim b outcomes)
Children's and parents' height and weight (measured objectively) and quality of life will be assessed by FBT therapists at baseline, end of treatment, and 6 months follow-up. The primary effectiveness outcome will be change in child's percent overweight from baseline visit to post-treatment assessment, where child's percent overweight is calculated as
Median BMI for the child's age (in months) and gender is based on norms defined by Kuczmarski et al.34 and available from the Centers for Disease Control and Prevention. Change in parent/caregiver weight is also a primary outcome of the implementation study.
Secondary outcomes include participant quality of life assessed using the SF-12 and Sizing Them Up for adult and child quality of life, respectively.35,36 Adherence to diet, physical activity, and behavioral skills will additionally be assessed through caregiver report using the Family Nutrition and Physical Activity measure.37
Matched comparisons for evaluating changes in percent overweight
Each health system's EHR database will be used to retrospectively identify at least 1 comparison child for each study participant (total comparisons n = 208). The historical control match group data are drawn from the EHR at both external clinical sites from participants not eligible for the Medicaid benefit. Child-participant data are obtained from all pediatric clinic visits from the eligible clinics. Comparisons will be matched to the study participants based on age, gender, race/ethnicity, and percent overweight.
Statistical Analysis Plan
For Sub-Aim a, all data are at the clinic level (N = 7 clinics across the two sites). Each clinic's scores on reach, adoption, implementation cost, and maintenance, and each interventionists scores on implementation knowledge and fidelity, will be summarized descriptively. The implementation contextual factor (CFIR) interviews will be transcribed and coded using the established coding procedures developed by the CFIR team, which will involve creating memo summary for each CFIR construct and coding its presence ( = 1)/absence ( = 0) and valence rating (−2 to 2, strongly negative to strongly positive).28 Ratings will be compared across clinics and between clinics with higher vs. lower reach, adoption, implementation cost, and maintenance scores, and between interventionists with higher vs. lower implementation knowledge and fidelity scores (based on sample distributions). Narratives and quotations from the interview content will be used to provide more depth of information to guide future implementation efforts.
For economic evaluation, we will aggregate the labor and nonlabor cost data to measure the costs of implementation. These data will also be combined with effectiveness (below) to report costs per outcome, or average cost-effectiveness ratios. Labor costs from implementing and sustaining the intervention will be captured by deriving a per-hour salary rate including fringe benefits for each provider or organizational leader in the study. Total labor costs are the product of hourly rates plus fringe and the time spent engaged in each implementation activity. For costs measured over multiple years, an annual 3% discount rate will be applied. For FBT sessions in which the duration of the visit is unknown, we will impute the average session duration for that FBT provider or the clinic. Once total costs are calculated for the full sample of participants, we will calculate a per family cost of implementation and the cost-effectiveness per child, parent, and family to achieve changes in weight. We will assess the robustness of our results by conducting sensitivity analyses to evaluate the costs and cost-effectiveness if aspects of implementation were varied (e.g., level of training of the FBT provider).
For Sub-Aim b, our sample size of 208 children in the FBT arm with 208 (or more) in the historical matched-comparison cohort is sufficiently powered to test the hypothesis that FBT achieves a significant and clinically meaningful reduction in weight. Our estimated change in the FBT group begins with a review of seven studies.10 To ensure the study is well powered, we assume a 1% weight loss in the historical control matched group. An intraclass correlation was included in power calculations to account for the delivery of the FBT intervention in a group setting. A priori matching or stratification is not possible due to the pragmatic nature of this trial; however, the analyses take into account the intervention group as a random effect in the clustered design. This study is powered at 90% to detect a reduction in excess weight in children between 7% and 7.5% to make sure the study is well powered.
To evaluate effectiveness, we will use a mixed effect linear model to estimate the percent weight change in the children. These analysis groups will use the historical matched controls and be based on group level differences. Subanalysis will be done on the treatment group only since there are dyadic data between parent and child. Additional dyadic analyses will be conducted, on the treatment group only, to determine whether more information is obtained by modeling both responses.38 Analyses of the relationship between child and parent dyads will use linear mixed models with multiple levels. This analysis will primarily be within-subject comparisons and, in addition to percentage weight loss, includes quality of life. This study will also investigate possible differences in subgroups, such as gender and race, within the treatment group. Intent-to-treat analysis will be used so all participants will be used in the models. Sensitivity analysis will be conducted to determine how the missing data affect the results, using both completers and Markov Chain Monte Carlo algorithm methods.
Discussion
This project aims to increase accessibility and scale up of FBT, an evidence-based pediatric obesity treatment, among low-income families. The evaluation will provide critical insight into the challenges of implementing FBT within diverse community settings and approaches for overcoming these challenges. By testing this care delivery model in two distinct contexts—a rural and urban setting—this study will be able to advise future efforts to deliver care in diverse communities across the United States and reach more children in need.
The MO-CORD project is the first study of its kind to evaluate the implementation of a digitally packaged FBT delivered by interventionists embedded within a pediatric primary care setting. By capitalizing on the established relationship between providers and families, primary care offers an optimal setting for FBT delivery, reducing fragmented care that can occur through multiple providers and offices. This study will provide insight into the organizational, provider, and community connectedness factors underlying adoption of an evidence-based pediatric obesity treatment for low-income families and the relationship between patient and provider fidelity to the treatment protocol; other patient, provider, and organizational factors; and clinical outcomes. In addition, implementation in both a rural and an urban clinic will allow for evaluation across diverse settings and contribute to more rapid translation of FBT into primary care practices resulting in a more immediate public health impact than traditional effectiveness studies.39,40
The study is innovative in its key focus on sustainability and replicability/scale up through existing health care mechanisms designed to serve low-income families. Importantly, all children with obesity enrolled in Missouri Medicaid will be entitled to the timely benefit that covers behavioral obesity treatment. Insights from the implementation of the provider trainings can inform efforts to develop a workforce for delivery of this new benefit beyond the pilot sites. This study will also be the first to evaluate the costs associated with FBT implementation in pediatric clinics, thereby providing critical information for payers to improve their decisions regarding reimbursement for obesity treatment. The MO-CORD project will further equip other private and public payers across the United Sates with preliminary evidence on the extent to which the Medicaid reimbursement model is cost-effective and sustainable.
Conclusion
Data from this study will lead to the creation of a sustainability and dissemination plan. We will leverage established community and state advisory bodies who have prioritized childhood healthy weight to directly inform the scalability and sustainability of the packaged FBT. In this way, this project will serve as a model for other states to implement cost-effective evidence-based care for FBT with multisector supports that meet the needs of our evolving health care system.
Acknowledgments
The authors thank the families, research staff, faculty, and institutions participating in the MO-CORD study. The following institutions and investigators constitute the MO-CORD Study Group: 3C Institute: Melissa DeRosier, PhD, Steve Grothmann, Sarah Winn; American Academy of Pediatrics, Institute for Child Weight and Wellness: Alison Baker, MS, Jeanne Lindros, MPH; Freeman Health System: Catherine Brown, Lisa Nelson, MA, Paul Petry, DO; Golisano Children's Hospital/University of Rochester Medical Center: Stephen Cook, MD; Children's Mercy Hospital: Sarah Hampl, MD, Meredith Dreyer Gillette, PhD, Jordan Carlson, PhD, Karen Stephens, MS, RD, Deborah Markenson, MA, Kelly Dunlap, RD; Northwestern University: Andrea Graham, PhD; Pennington Biomedical Research Center: Amanda Staiano, PhD, Chelsea Kracht, PhD, Robbie Beyl, PhD, William Johnson, PhD, Lindsay Hall, MA, Natalie Malek; University at Buffalo: Leonard Epstein, PhD; Washington University in St. Louis School of Medicine: Denise Wilfley, PhD, Lauren Fowler, PhD, Sherri Gabbert, PhD, Shaina Costello, MA, Angela Lima, MA, Fanice Thomas, PhD, Kelly Springstroh, MS, Aubrie Hampp, MSc, Robinson Welch, PhD; members of the Collaborative Advisory Board and Community Advisory Boards.
Contributor Information
The MO-CORD Study Group:
Melissa DeRosier, Steve Grothmann, Sarah Winn, Alison Baker, Jeanne Lindros, Catherine Brown, Lisa Nelson, Paul Petry, Stephen Cook, Sarah Hampl, Meredith Dreyer Gillette, Jordan Carlson, Karen Stephens, Deborah Markenson, Kelly Dunlap, Andrea Graham, Amanda Staiano, Chelsea Kracht, Robbie Beyl, William Johnson, Lindsay Hall, Natalie Malek, Leonard Epstein, Denise Wilfley, Lauren Fowler, Sherri Gabbert, Shaina Costello, Angela Lima, Fanice Thomas, Kelly Springstroh, Aubrie Hampp, and Robinson Welch
Collaborators: The MO-CORD Study Group
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding Information
This manuscript was supported by the Centers for Disease Control and Prevention of the US Department of Health and Human Services (HHS) (Award No.: U18DP006425). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the US Government. L.A.F., F.T., and A.C.G. are supported by grant T32 HL130357 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH). C.L.K. is supported by T32 DK064584 and A.K.G. is supported by K01 DK116925 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm
- 2. Weaver RG, Brazendale K, Hunt E, et al. Disparities in childhood overweight and obesity by income in the United States: An epidemiological examination using three nationally representative datasets. Int J Obes (Lond) 2019;43:1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogden CL, Fryar CD, Hales CM, et al. Differences in obesity prevalence by demographics and urbanization in US children and adolescents, 2013–2016. JAMA 2018;319:2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seburg EM, Olson-Bullis BA, Bredeson DM, et al. A review of primary care-based childhood obesity prevention and treatment interventions. Curr Obes Rep 2015;4:157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell TB, Amaro CM, Steele RG. Pediatric weight management interventions in primary care settings: A meta-analysis. Health Psychol 2016. [Epub ahead of print]; DOI: 10.1037/hea0000381. [DOI] [PubMed] [Google Scholar]
- 6. Brown L, Dolisca SB, Cheng JK. Barriers and facilitators of pediatric weight management among diverse families. Clin Pediatr (Phila) 2015;54:643–651. [DOI] [PubMed] [Google Scholar]
- 7. Johnson VR, Acholonu NO, Dolan AC, et al. Racial disparities in obesity treatment among children and adolescents. Curr Obes Rep 2021;10:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrd AS, Toth AT, Stanford FC. Racial disparities in obesity treatment. curr Obes Rep 2018;7:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dietz WH. We need a new approach to prevent obesity in low-income minority populations. Pediatrics 2019;143:e20190839. [DOI] [PubMed] [Google Scholar]
- 10. Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychol 2007;26:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017;317:2427–2444. [DOI] [PubMed] [Google Scholar]
- 12. Biopsychosocial Treatment of Obesity for Youth and Adults, 13 §208.201, 660.017, 208.152 (2020). https://casetext.com/regulation/missouri-administrative-code/title-13-department-of-social-services/division-70-mo-healthnet-division/chapter-25-physician-program/section-13-csr-70-25140-biopsychosocial-treatment-of-obesity-for-youth-and-adults
- 13. Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): A tool to help trial designers. J Clin Epidemiol 2009;62:464–475. [DOI] [PubMed] [Google Scholar]
- 14. Fowler LA, Hampl SE, Dreyer Gillette ML, et al. Translating family-based behavioral treatment for childhood obesity into a user-friendly digital package for delivery to low-income families through primary care partnerships: The MO-CORD Study. Child Obes. 2021;17S1:S-30–S-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA 2007;298:1661–1673. [DOI] [PubMed] [Google Scholar]
- 16. Wilfley DE, Saelens BE, Stein RI, et al. Dose, content, and mediators of family-based treatment for childhood obesity: A multisite randomized clinical trial. JAMA Pediatr 2017;171:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Epstein LH, Wing RR, Koeske R, et al. Child and parent weight loss in family-based behavior modification programs. J Consult Clin Psychol 1981;49:674–685. [DOI] [PubMed] [Google Scholar]
- 18. Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol 1994;13:373–383. [DOI] [PubMed] [Google Scholar]
- 19. Bartholomew Eldrigde LK, Markham CM, Ruiter RAC, et al. Planning Health Promotion Programs: An Intervention Mapping Approach, 4th ed. Jossey-Bass: San Francisco, CA, 2016. [Google Scholar]
- 20. Bartholomew LK, Markham C, Mullen P, Fernandez ME. Planning models for theory-based health promotion interventions. In: Glantz K, Rimer BK, Viswanath K (eds). Health Behavior: Theory, Research, and Practice. John Wiley & Sons: San Francisco, CA, 2015, p. 359. [Google Scholar]
- 21. Intervention Mapping: Welcome. Available at https://interventionmapping.com Last accessed January 15, 2021.
- 22. Powell BJ, Beidas RS, Lewis CC, et al. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res 2017;44:177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waltz TJ, Powell BJ, Fernández ME, et al. Choosing implementation strategies to address contextual barriers: Diversity in recommendations and future directions. Implement Sci 2019;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health 1999;89:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Consolidated Framework for Implementation Research. 2014. http://cfirguide.org Last accessed August 30, 2018.
- 29. Missouri Primary Care Association. Who We Are. Available at https://www.mo-pca.org/about. Last accessed January 30, 2021.
- 30. Ritzwoller DP, Sukhanova A, Gaglio B, Glasgow RE. Costing behavioral interventions: A practical guide to enhance translation. Ann Behav Med 2009;37:218–227. [DOI] [PubMed] [Google Scholar]
- 31. Raghavan R. The role of economic evaluation in dissemination and implementation research. In: Brownson RC, Colditz GA, Proctor EK (eds). Dissemination and Implementation Research in Health: Translating Science to Practice, 2nd ed. Oxford University Press: New York, NY, 2018, pp. 89–106. [Google Scholar]
- 32. Muennig P, Bounthavong M. Cost-Effectiveness Analysis in Health: A Practical Approach, 3rd ed. Wiley: San Francisco, CA, 2016. [Google Scholar]
- 33. Accurso EC, Hawley KM, Garland AF. Psychometric properties of the Therapeutic Alliance Scale for Caregivers and Parents. Psychol Assess 2013;25:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000:1–27. [PubMed] [Google Scholar]
- 35. Modi AC, Zeller MH. Validation of a parent-proxy, obesity-specific quality-of-life measure: Sizing them up. Obesity (Silver Spring) 2008;16:2624–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ware J Jr., Kosinski M, Keller SD.. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 37. Ihmels MA, Welk GJ, Eisenmann JC, Nusser SM. Development and preliminary validation of a Family Nutrition and Physical Activity (FNPA) screening tool. Int J Behav Nutr Phys Act 2009;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kenny D, Kashy D, Cook W. Dyadic Data Analysis. Guilford Press: New York, NY, 2006. [Google Scholar]
- 39. Dietz WH, Baur LA, Hall K, et al. Management of obesity: Improvement of health-care training and systems for prevention and care. Lancet 2015;385:2521–2533. [DOI] [PubMed] [Google Scholar]
- 40. Glasgow RE. RE-AIMing research for application: Ways to improve evidence for family medicine. J Am Board Fam Med 2006;19:11–19. [DOI] [PubMed] [Google Scholar]