Abstract
Background and Aims: Outbreaks of severe and chronic tick-borne diseases (TBDs) are on the rise. This is through the transmission of infectious disease agents to humans during tick feeding. The transmission rate and extent of microbial exchange, however, vary based on the tick microbiome composition. While select microbes are determined to be members of the normal tick microbiome and others are clearly recognized mammalian and/or avian pathogens, the status of many other members of the tick microbiota with respect to human and alternate host pathogenesis remains unclear. Moreover, the species-level 16S microbiome of prominent TBD vectors, including Ixodes pacificus, have not been extensively studied. To elucidate the I. pacificus microbiome composition, we performed a pan-domain species-specific characterization of the bacterial microbiome on adult I. pacificus ticks collected from two regional parks within Western California. Our methods provide for characterizing nuances within cohort microbiomes and their relationships to geo-locale of origin, surrounding fauna, and prevalences of known and suspected pathogens in relation to current TBD epidemiological zones.
Methods: Ninety-two adult I. pacificus bacterial microbiomes were characterized using a high-fidelity, pan-domain, species-specific, full-length 16S rRNA amplification method using circular consensus sequencing performed on the Pacific Biosciences Sequel platform. Data analyses were performed with the MCSMRT data analysis package and database.
Results: The species-specific I. pacificus microbiome composition illustrates a complex assortment of microflora, including over 900 eubacterial species with high taxonomic diversity, which was revealed to vary by sex and geo-locale, though the use of full-length 16S gene sequencing. The TBD-associated pathogens, such as Borrelia burgdorferi, Anaplasma phagocytophilum, and Rickettsia monacensis, were identified along with a host of bacteria previously unassociated with ticks.
Conclusion: Species-level taxonomic classification of the I. pacificus microbiome revealed that full-length bacterial 16S gene sequencing is required for the granularity to elucidate the microbial diversity within and among ticks based on geo-locale.
Keywords: 16S, microbiome, tick microbiome, coinfections, polymerase chain reaction, DNA sequencing, Ixodes pacificus
Introduction
The last half century has witnessed a rapid rise in the human acquisition of tick-borne diseases (TBDs) around the globe. This is exemplified by the prevalent bacterial TBD, Lyme borreliosis, within the United States (“Data and Surveillance | Lyme Disease | CDC” 2019). This TBD and others have become major public health concerns as they continue to spread beyond their traditional epidemiological zones (Sonenshine, 2018), in large part through the continual change in climate (Pfeiffer, 2018). The warming climate increases winter survival for ticks in their traditional habitats and also provides for their movement into regions that were previously too climatically harsh for their survival (Clover and Lane, 1995; Eisen et al., 2016; Hahn et al., 2016; Sonenshine, 2018; Gardner et al., 2020).
Ixodes pacificus, a hard ixodid tick found along the western coast of North America, undergoes several developmental life stages (egg, larvae, nymph, adult), which are regulated by blood meals (Padgett and Lane, 2001; Dingler et al., 2014; Eisen et al., 2016). Through these feedings, I. pacificus may acquire microbes, which are incorporated into a resident mutualistic and commensal microbial community. This microbiome assists ticks with key nutrient production, development, and general survival (Feldhaar, 2011; Nikoh et al., 2014; Hunter et al., 2015; Narasimhan and Fikrig, 2015; Bonnet et al., 2017; Kada et al., 2017; Kwan et al., 2017; Greay et al., 2018). As a host generalist, I. pacificus has been known to prey upon a broad range of hosts. This provides for greater opportunities to incorporate and transmit diverse microflora.
To better understand permanent and transient members of the I. pacificus microbiome, it is critical to fully characterize it. Previous studies have found that the I. pacificus microbiome is extremely diverse and a large portion is composed of endosymbionts, including Rickettsial species, which function as commensal, vertically transmitted organisms (Ahantarig et al., 2013; Eshoo et al., 2015; Kwan et al., 2017; Swei and Kwan, 2017). Other prevalent microbes were found to be primarily acquired through feeding on surrounding competent reservoir hosts such as birds, mice, squirrels, and deer (Lane et al., 1994; Brown and Lane, 1996; Castro and Wright, 2007; Salkeld et al., 2008; Ahantarig et al., 2013; Kwan et al., 2017). Bioinformatic removal of these prevalent microbes, primarily those in the Rickettsia genus, revealed a more diverse microbiome with decreased species evenness (Kwan et al., 2017). Additionally, the species richness of the I. pacificus microbiome was found to change considerably following blood feeding and life-stage progression (Swei and Kwan, 2017).
Microbiome studies on I. pacificus and other important tick vectors have demonstrated the prevalence of various TBD pathogens within the natural tick/host community (Henry, 2012; Eshoo et al., 2015; Narasimhan and Fikrig, 2015; Gall et al., 2016; Bonnet et al., 2017; Kwan et al., 2017; Swei and Kwan, 2017; Greay et al., 2018; Chicana et al., 2019; Thapa et al., 2019b). Within these studies, the exact bacterial species comprising the microbiome remains unknown. Limitations of short-read next-generation sequencing results in only partial 16S rRNA gene reads, which can only produce a genus-level resolution of the tick microbiome. The geographic spread of anthropophilic ticks, the potential transmission of TBDs, along with the high complexity of the tick microbiome, requires higher resolution analyses of the tick microbiome. In our research, we implemented our MCSMRT pipeline, which utilizes Pacific Biosciences (PacBio, Menlo Park, CA) long-read, circular consensus sequencing technology. This technology serves as a pan-domain molecular diagnostic assay that provides full-length bacterial 16S rRNA (FL16S) gene sequencing to attain species-specific resolution for any microbiome (Earl et al., 2018; Greathouse et al., 2018).
Materials and Methods
Tick collection
Ninety-two I. pacificus ticks were collected from low vegetation spots from two nearby locations characterized by differences in their fauna and overall ecology: the MacDonald Trail within Anthony Chabot Regional Park (37°48′04.7′′N 122°08′46.0′′W) and the Westridge Trail within Redwood Regional Park (37°47′47.4′′N 122°08′56.5′′W), in Oakland, California in the United States during the early spring of 2018 (Supplementary Table S1). Ticks were typed for species, sex, life stage, and feeding status (Table 1). After typing, all ticks were stored in 90% ethanol at −80°C before DNA extraction. The geo-locales, experimental setup, and process are illustrated in Figure 1.
Table 1.
Cohort Characteristics of Adult Ixodes pacificus Ticks Separated by Each Geo-Locale of Origin
| Location | Community characteristics |
|||
|---|---|---|---|---|
| Redwood regional | Redwood regional | Anthony chabot regional | Anthony chabot regional | |
| Sex | Male | Female | Male | Female |
| Life stage | Adult | Adult | Adult | Adult |
| Group size | 16 | 25 | 24 | 27 |
| Feeding status | Unfed | Unfed | Unfed | Unfed |
| Date collected | March 28, 2018 | March 28, 2018 | March 28, 2018 | March 28, 2018 |
FIG. 1.
An illustration of study design. (A) Adult Ixodes pacificus ticks were collected from two sites in Western California. A map of the geo-locales where ticks were collected was taken from Google Maps in Map view (scale bar = 200 ft). (B) The general process for FL16S on adult I. pacificus ticks is depicted. The illustration was created using Procreate 5.
DNA extraction
Individual whole, adult I. pacificus ticks were placed in a 2-mL bead beating tube (Matrix E) (MP Biomedicals LLC, Irvine, CA) and frozen using liquid nitrogen; homogenization was carried out using a SPEX 1600 MiniG (Fisher Scientific, Waltham, MA) for 10 min at 1500 Hz. DNAs from the homogenized ticks were extracted using the Qiagen™ DNeasy Blood & Tissue Kit according to the manufacturer's instructions (Qiagen, Hilden, DE). Extracted total metagenomic DNA was quantified using both a NanoDrop 2000 UV spectrophotometer and fluorometry using the AccuClear™ Ultra high-sensitivity dsDNA Quantification Kit as per the manufacturer's instructions (Biotium, Inc., Fremont, CA) and read on a BioTek® (FLx800) microplate fluorescence reader (BioTek, Winooski, VT).
Full-length 16S (FL16S) rDNA polymerase chain reactions
FL16S amplifications of the 16S rRNA gene (V1–V9 regions) were performed using total tick DNA extracts as template, the universal 16S forward primer F27 (5′-/5AmMC6/GCAGAACATGTAGCTGACTCAGGTCACGRAGAGTTTGATYMTGGCTCA/-3′), the universal 16S reverse primer R1492 (5′-/5AmMC6/TGGATCACTTGTGCAAGCATCACATCGTAGTACGGYTACCTTGTTACGACTT/-3′), and GoTaq Hot Start Master Mix (Promega, Madison, WI). All samples were amplified using the following thermocycler conditions: 95°C for 3 min followed by 35 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s followed by a final elongation step at 72°C for 5 min. The negative control for each FL16S amplification used molecular-grade water to replace the DNA specimen volume; all other reagents were the same as for the specimens. Polymerase chain reaction (PCR) amplifications were evaluated via electrophoresis through a 1% agarose gel. Specimens demonstrating amplicons of the appropriate size were then prepared for a secondary (nested) PCR using GoTaq Hot Start Master Mix (Promega) and Barcoded Universal F/R Primers from Plate-96v2 (PacBio). All samples were amplified a second time as follows: 95°C for 30 s followed by eight cycles of 95°C for 15 s, 64°C for 15 s, and 72°C for 2 min, and then a final elongation step at 72°C for 5 min. Barcoded samples were cleaned with the AxyPrep™ MagPCR (Corning Life Sciences, Pittson, PA) according to the manufacturer's protocol, and eluted in 40 μL sterile nuclease-free molecular-grade water. Cleaned PCR products were quantified by Qubit fluorometry using the AccuClear Ultra high-sensitivity dsDNA Quantification Kit. Amplicons with unique PacBio barcodes from individual ticks were normalized for pooling into multiplexed sets containing ∼24 samples per set.
PacBio circular consensus sequencing
Sequencing libraries were constructed using the PacBio SMRTbell™ Template Prep Kit v1.0-SPv3 on pooled FL16S amplicons, and sequencing was performed using the PacBio Sequel platform using the protocol “Procedure & Checklist—Amplicon template Preparation and Sequencing.” Movie times were 10 h for all SMRTcells. Sequencing performance and run statistics were collected using SMRT® link v9.0. Sequences were demultiplexed and converted to FastQ files using the pb_ccs and pb_ccs_demux protocols in the pbcromwell v1.0.4 software. Each read was labeled and used for subsequent filtering and clustering.
Filtering, clustering, and taxonomic classification of circular consensus sequence reads
Sequences were filtered based on their size, presence within sequencing negative controls, primer matching, expected error rates, and lack of human sequences, as described (Earl et al., 2018). Operational taxonomic units (OTUs) were classified using Utax with a curated FL16S rRNA database, MCSMRT, which provided species-level taxonomic classifications and confidence values at each taxonomic level (Earl et al., 2018).
Negative control handling
Following MCSMRT OTU clustering analysis, the 16S gene counts within the negative controls from FL16S sequencing were calculated and summed. The maximum read count of any OTU present in any negative control was removed from the count of all Ixodid tick samples, which also contained that specific OTU (or became zero, if this would have resulted in a negative count) Supplementary Figure S1.
OTU count preparation, clustering, and data visualization
The 16S gene counts from all I. pacificus ticks were converted into relative abundances for each tick (OTU count/total read count per sample). For further analysis only OTUs with at least 1% abundance in at least one sample were included. For figure generation this abundance cutoff was increased to 5% for clarity when required. Tick samples with fewer than 100 16S reads were removed. A pseudocount was added to approximate missing OTU counts and account for future log transformation (10–9abundance). Relative abundance values were log transformed. Euclidean distance matrices were calculated on both OTUs and samples. Hierarchical clustering was performed on both distance matrices using the complete linkage criteria (R v.3.6.3). All samples and controls, log-transformed abundance values, and hierarchical cluster dendrograms were visualized using pheatmap (v. 1.0.12) in R.
Population and diversity metrics
Alpha-level species diversity was examined by calculating Shannon's diversity index. To identify how different taxonomic levels affected the observed diversity of the samples, new tables were calculated, one each for species, genus, and family level. OTU counts were summed within samples if they belonged to the same named taxa (e.g., if multiple OTU were identified as Rickettsia monacensis, these would be consolidated to a single R. monacensis count for each sample at the species level, multiple OTU that belonged to the same genus Rickettsia would be summed to a single count of all Rickettsia at the genus level, etc.). Shannon diversity was calculated using the diversity function on the relative abundance values of these counts using the R package vegan v2.5–7. To interpret these index values, they were transformed to the “effective number” using the exponent function in R: specifically exp (diversity_index_value). The untransformed Shannon's diversity index is difficult to interpret and compare among samples. On the other hand, the “effective number” can be interpreted as “the number of equally abundant species dominating the community,” for example, an effective number of “5” would indicate there are approximately 5 equally abundant taxa dominating that sample. Beta diversity (comparison of diversity between samples) was examined with two different visualization techniques. A principal component analysis (PCA) and scree plot were calculated using the log-transformed abundance values as input (data as described previously), using the FactoMineR R package v2.4 function PCA. The other visualization we used was a nonmetric multidimensional scaling analysis (nmMDS) using the metaMDS function in the vegan R package. Additionally, the nmMDS stress plot was also plotted in R as aforementioned. Heatmaps were plotted using the pheatmap R package v1.0.12, otherwise data were plotted and visualized with the ggplot2 R package v3.3.4. To test for differences between geo-locale cohorts, a PERMANOVA test using the adonis function in the R package vegan was performed on the log-transformed relative abundance data (data as described previously).
Statistical analyses
The statistical difference between the geo-locale-defined tick cohorts was calculated using the Wilcoxon test in R (v 3.6.3) for a range of taxonomic levels (family, genus, and species). A PERMANOVA was also done for each taxonomic level evaluated (family, genus, species) in R (v 3.6.3).
Results
Pacificus collection and study design
I. pacificus ticks were collected from two separate sites within the two Oakland regional parks in Alameda County, CA (Fedorova et al., 2014; Margos et al., 2020).
Full-length 16S (FL16S) sequencing reveals a relatively large microbiome within adult I. pacificus ticks
FL16S sequencing and subsequent processing of the data through the MCSRMT pipeline was performed on two geo-diverse adult I. pacificus tick cohorts (Anthony Chabot Regional Park and Redwood Regional Park) from Western California. This analysis revealed a composite microbiome for the two I pacificus cohorts consisting of 965 unique OTU's. In both cohorts, a large variation in the overall abundance of identified OTUs was seen among the individual microbiomes (Fig. 2). As previously seen, the microbiomes were separated into two components (Kwan et al., 2017; Swei and Kwan, 2017; Chicana et al., 2019; Thapa et al., 2019b). The first component consisted of select, highly abundant microbes, and the second, yet numerically larger component on an OTU basis, contained a larger range of microbes with lower abundances.
FIG. 2.
Analysis of the composition of adult I. pacificus microbiome reveals extensive heterogeneity based on OTU composition and prevalence. Heatmap of bacterial species identified using the full-length 16S rRNA gene eubacterial pan-domain diagnostic utilizing single molecule error correcting circular consensus sequencing of I. pacificus ticks from Oakland, California. Y-axis: the list of the bacterial species identified in order of prevalence across all ticks. X-axis: the list of individual ticks. The key for the relative abundance parameters are in the upper right. The color bars above the heatmap signify the respective sex and geo-locale of origin for each tick used in this study. The left color bars signify the confidence in species and genus assignment for each OTU. Keys for these color bars are located on the upper right. OTU, operational taxonomic unit.
Within this first component of the microbiome, we identified OTUs that were at a high abundance within a tick and found commonly across all cohort members. OTUs within this first component were known tick-associated and TBD microbes, including R. monacensis, Anaplasma phagocytophilum, and interestingly the emerging pathogen Stenotrophomonas maltophila (Denton and Kerr, 1998; Brooke, 2012; Wu et al., 2021). Additionally, identified tick- and/or insect-associated microbes, such as Diplorickettsia massiliensis and Variovorax ginsengisoli, were at a slightly lower abundance (Mediannikov et al., 2010; Narasimhan and Fikrig, 2015; Van Treuren et al., 2015; Bonnet et al., 2017; Petersen et al., 2019; Thapa et al., 2019b; Brinkerhoff et al., 2020). Other critical vertically transmitted endosymbionts, Spiroplasma ixodetis and Wolbachia spp., were also found at lower abundances (Tully et al., 1995; Qiu et al., 2014; Eshoo et al., 2015).
In the latter component of the microbiome, a wide array of low-abundance species was identified within all of the I. pacificus ticks. Interestingly, among the numerous detected microbes were the pathogens B. burgdorferi, Acinetobacter calcoaceticus, and Terrisporobacter mayombei (Denton and Kerr, 1998; Brooke, 2012; Cheng et al., 2016; Khaw et al., 2020; Wu et al., 2021). While there were a wide variety of identified OTUs present within both I. pacificus cohorts, there were notable differences between the composition of their respective composite microbiomes. These differences were centered on the microbial abundance distribution of a panel of microbes, including the aforementioned pathogens, R. monacensis, A. phagocytophilum, and S. maltophila. These pathogens and other detected microbes were not present in equal abundance across cohorts (Fig. 2).
Further investigation on pathogen distribution within both cohort microbiomes showed a great variance with respect to pathogen abundance (Fig. 3). The pathogens, R. monacensis, A. phagocytiphilum, and S. maltophilia, were found present in most I. pacificus microbiomes at high abundance. Additionally, the majority of the Anthony Chabot Regional ticks also had an additional group of pathogens found at high abundances. These microbes include the emerging pathogen Janthinobacterium lividum, the human pathogen Rhodococcus corynebacterioides, as well as the plant pathogens Xyophilus ampelinus and Curtobacterium flaccumfaciens (Grall and Manceau, 2003; Grall et al., 2005; Kitamura et al., 2012; Khalil et al., 2019; Oh et al., 2019). Interestingly, both J. lividum and R. corynebacterioides were found in select Redwood Regional ticks. Additionally, within the Redwood Regional cohort, one tick (t_217) did not have any relatively high abundance pathogens.
FIG. 3.
Known environmental and human pathogens were detected within adult I. pacificus but were not found ubiquitously between the collection sites. Heatmap of detected pathogens within adult I. pacificus microbiome. Y-axis: the list of the bacterial species identified in order of prevalence in all ticks. X-axis: the list of individual ticks. The key for relative abundance color heatmap is in upper right corner of the figure. The color bars above the heatmap signify the respective sex and geo-locale of origin for each tick used in this study. The left color bars signify the confidence in species and genus assignment to each OTU. Keys for all color bars are as noted on the upper right key.
To determine if the observed differences among tick samples clearly separated them by either geo-locale or sex, a PCA and an nmMDS analysis were performed (Fig. 4). The PCA showed a clear separation between geo-locale origin in the first dimension (x-axis, Dim.1), which explains the greatest degree of variance (15.3%) among the ticks (Fig. 4A). The second method of ordination, nmMDS, likewise showed clear separation between geo-locale cohorts, especially along the first dimension (x-axis MSD1) (Fig. 4B). Both figures were based on two dimensions for plotting, those dimensions in the PCA accounted for 22.5% of the variance (Fig. 4C), and stress for the nmMDS was ∼0.15 at two dimensions (Fig. 4D). This difference observed between geo-locale cohorts was statistically significant at the 0.05 level (PERMANOVA p = 0.001). Neither method showed a clear visual separation between sexes, and no statistically significant difference was observed (PERMANOVA p = 0.188). However, the members of each tick cohort clustered together with little overlap between the two geo-spatially-defined cohorts. (Supplementary Table S2). These microbes were highly enriched in their presence in the Anthony Chabot Regional cohort, as compared with Redwood Regional.
FIG. 4.
Comparison of I. pacificus log-transformed relative abundance OTU data using Euclidean distance among samples show significant separation (PERMANOVA test p-value = 0.001) between geo-locale-defined groups displayed with multidimensional scaling analyses. (A) First two components of PCA of all I. pacificus ticks in the study. (B) NMDS of all I. pacificus ticks using two dimensions. (C) Scree plot of PCA in (A) 22.5% of the variation is explained in the first two dimensions. (D) Stress plot as number of dimensions increased for NMDS, stress at two dimensions (plot B) ∼0.15. Geo-locale and sex are indicated on the upper right key. NMDS, non-metric multidimensional scaling; PCA, principal component analysis.
FL16S sequencing shows that I. pacificus microbiome diversity varies by location
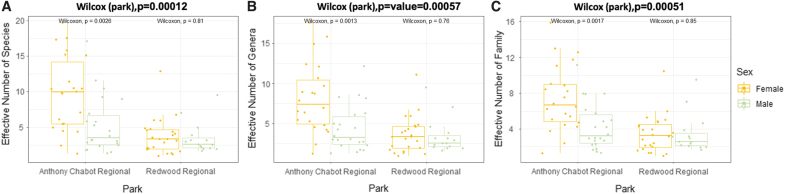
After differences among OTUs within these two cohorts were characterized, we examined differences in species richness within and between each cohort (Fig. 5). A boxplot of the effective number of species through Shannon's diversity of all samples at the species level showed a statistically significant difference between ticks by park (p = 0.00012 Wilcoxon), between sex stratified by park in Anthony Chabot (p = 0.0026 Wilcoxon), but not Redwood Regional (p = 0.81 Wilcoxon). The diversity difference between tick sexes (p = 0.056) without accounting for geo-locale was also not significant at the 0.05 confidence level (Fig. 5A). To examine if this difference continued to be observed at less-specific taxonomic levels, the effective diversity at the genus and family levels were also calculated, and the same statistical tests applied (Fig. 5B, C). Statistically significant (at 0.05 confidence level through Wilcoxon test) differences were likewise observed between Shannon's effective diversity numbers between samples grouped by park (genus-level p = 0.00057, family-level p-value = 0.00051) and between sexes within park [genus-level Anthony Chabot (p = 0.0013), Redwood Regional (p = 0.76) family-level Anthony Chabot (p = 0.0017), Redwood Regional (p = 0.85)]. Differences between the mean Shannon's effective diversity accounting only for sex was only barely significant at the genus level (p-value = 0.046) suggesting that the primary driving factor of difference in diversity among all of these samples was park location.
FIG. 5.
Bacterial diversity within adult I. pacificus ticks is impacted by geo-locale and sex. The effective number of species was calculated from the Shannon's diversity index and indicates the number of equally abundant species in a sample that would give the same Shannon's diversity index (the exponent of the index value). (A) Average bacterial diversity within the I. pacificus tick microbiomes at the species level varies by location. A box plot showing the differences in the expected diversity variation between each sex from both regional parks. Anthony Chabot Regional Park (blue) or Redwood Regional Park (orange) indicate the different regional parks collection points for ticks. (B) Average bacterial diversity within I. pacificus tick microbiomes at the genera level varies by location. (C) Average bacterial diversity within I. pacificus tick microbiome at the family level varies by location.
To visualize the beta diversity levels present among all tick samples, we created a heatmap of the Euclidean distance based on the log-transformed relative abundance OTU data (Fig. 6). After grouping the distance matrix using default parameter hierarchical clustering, tick samples again, were clearly separated by geo-locale. Likewise, we again observed a greater diversity within the Anthony Chabot Regional cohort in comparison to the Redwood Regional cohort, as we had seen in the PERMANOVA test (p = 0.001).
FIG. 6.
I. pacificus tick microbiome profiles vary based on geo-locale of origin. Beta diversity between I. pacificus ticks in Northern California. X- and Y-axis: the list of different adult I. pacificus ticks. Color scale: smaller distances are closer to blue, larger distances are closer to red. The origin and sex of each tick is indicated on the upper right key. A PERMANOVA test shows a significant difference between geo-locale groups (p-value = 0.001).
Discussion
PCR-based methods of detection have revolutionized microbial analyses beginning with simplex assays for single species detection (Kwok et al., 1987) and then progressing to the targeted analysis of multiple microflora beginning with panel-based multiplex tests covering a defined set of high-probability species (Ehrlich, 1996; Aul et al., 1998). Similarly, single nucleotide polymorphism-based PCR-based tests were developed to discriminate between and among very closely related microbial strains (Ehrlich et al., 1989; Shen et al., 2005; 2006a, b). Subsequently, nonbiased bacterial microbiome studies were developed using the ubiquitous rRNA 16S gene (Schloss and Handelsman, 2004). These initial microbiome assays provided vast amounts of information with regard to the approximate number of taxa present and their relative abundances, however, they lacked species specificity. Thus, what was needed was the development of a comprehensive, unbiased species-specific molecular diagnostic; this was accomplished by developing a long-read 16S microbiome assay that combined the breadth of traditional microbiome assays with the specificity of simplex/multiplex PCR-based assays (Earl et al., 2018; Greathouse et al., 2018). It was this latest development that provided the technology for the current study.
Herein, we conducted a FL16S microbiome analysis on the major human TBD vector I. pacificus from Western California and identified over 900 OTUs defined to the species level. Similar to previous publications, the I. pacificus microbiome was heavily dominated by select microbes (Gall et al., 2016; Chicana et al., 2019). Many of these microorganisms, particularly R. monacensis, A. phagocytophilum, S. ixodetis, and Wolbachia species, have been associated with I. pacificus and some are known to be vertically transmitted with each generation of Ixodid ticks (Hunter et al., 2015; Gall et al., 2016; Kwan et al., 2017; Chicana et al., 2019). Additionally, some of the detected microbes, including Anaplasma-like and Rickettsia-like species, were matched to their closest sequenced relative that is, A. phagocytophilum and R. monacensis. It is likely that this could have also occurred between the detected Wolbachia spp. and Rickettsia-like endosymbionts due to 16S sequence similarity as well as lack of full genome sequencing (Kent et al., 2011). Some of the abovementioned microbes, and many of low abundance species found within the I. pacificus microbiome could have been acquired through a variety of means.
Within our study, adult I. pacificus ticks were collected from previously surveyed regions on circulating TBD pathogens (Fedorova et al., 2014; Margos et al., 2020). There are some differences between the two collection site environments, which could also influence the I. pacificus microbial community (Supplementary Table S1). Both geo-locales reside along two popular hiking trails in the East Bay Regional Park District system within Alameda County. Although they are less than 1 mile apart, the distinct differences are in the elevation and sun exposure and corresponding differences among the alternate mammalian hosts. The differences in ecological surroundings in the collection sites could impact the acquisition of environmental microbes found within the tick microbiome. Furthermore, these geo-locales could provide more ideal shelters for different I. pacificus hosts.
Within Oakland California, several common I. pacificus hosts can be found, including Odocoileus hemionus columbianus (Colombian black-tailed deer), Neotoma fuscipes (Dusky-footed woodrats), Peromyscus maniculatus, and Sciurus griseus (Western gray squirrel). These fauna and others, however, are not found at equivalent abundances or rates within the collection sites used within this study. The most notable difference in fauna can be found throughout the Anthony Chabot Regional collection site, which had several Peromyscus species. The variety of fauna and differences in their availability could, in part, explain select differences in the composition of the I. pacificus microbiome, particularly in the case of TBD pathogens during feeding (Narasimhan and Fikrig, 2015; Van Treuren et al., 2015; Bonnet et al., 2017; Swei and Kwan, 2017; Zolnik et al., 2018; Thapa et al., 2019b; Brinkerhoff et al., 2020). Previous studies have found that through blood feeding and molting, a decrease in microbiome diversity can occur among adult ticks (Rynkiewicz et al., 2015; Zolnik et al., 2016, 2018; Kwan et al., 2017; Thapa et al., 2019a; Brinkerhoff et al., 2020). While our ticks were not documented to have fed immediately before FL16S sequencing, there remains a possibility that feeding on different hosts could create a difference. Additionally, feeding behavior differences between tick sexes could also promote microbial diversity. In our cohorts, we found an increase of diversity in females as supposed to males, contrasting previous reports on male microbiome (Van Treuren et al., 2015; Obregón et al., 2019; Thapa et al., 2019b; Brinkerhoff et al., 2020). This change was only partially diminished at higher taxonomic levels, suggesting the increased diversity was from having an increased number of species as opposed to genera (Fig. 5). Thus, it is likely that the difference between our diversity findings with regard to tick sex likely results from the improved resolution provided by FL16S sequencing. However, more work needs to be done to identify other direct contributors to microbiome diversity.
In this study, we solely focused on adult I. pacificus ticks from Alameda County, CA. These generalist feeders thrive on a wide array of vertebrate hosts and through changes within their surrounding local and global environments, have thrived beyond their “classical” endemic zone (Hahn et al., 2016; Gardner et al., 2020). This migration could alter normal I. pacificus processes and survival throughout their life cycle. Due to study limitations, we are unable to attain more ticks from different regions surrounding our study sites to fully appreciate microbiome fluctuation as impacted by geographic locale. Furthermore, we would need ticks that have recently fed to appreciate the nuance in microbiome diversity associated with feeding.
Future studies will include a wider range of geo-locales and the effect of blood feeding on the I. pacificus microbiome, as well as virulence investigations of prevalent species that are either presumed pathogens or closely related to known pathogens as what constitutes a pathogen is in part the environment, which the microbe finds itself (Ehrlich et al., 2008). In addition, we will be characterizing eukaryotic pathogens using a companion pan-domain 18S rDNA pipeline.
Conclusion
In this research, using our FL16S pipeline, we were able to characterize the microbiome of wild-caught I. pacificus ticks and highlight minute differences based on their sex and geo-locale of origin. Furthermore, we found that using taxonomic resolution has redefined the estimated diversity within I. pacificus ticks and between tick sexes.
Supplementary Material
Acknowledgments
This research could not have happened without the generous contributions of Timothy L. McGuire, MS; Joyce Kleinjan, MS; and Robert S. Lane, PhD.
Authors' Contributions
Designed experiments: K.M.S., J.E.K., J.P.E., J.C.M., A.B., and G.D.E.; Performed experiments: K.M.S., J.E.K., M.H.H., M.H., A.A., S.L., B.S., and M.P.; Analyzed the data: K.M.S., J.P.E., J.C.M., A.B., and G.D.E.; Contributed or created reagents/materials/analysis tools: J.E.K., A.B., J.P.E., J.C.M., K.V., S.P.L., M.H.H., M.H., and K.V.; Wrote the article: K.M.S., A.A., A.B., B.S., J.P.E., M.P., and G.D.E.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by Drexel University College of Medicine and the Institute of Molecular Medicine and Infectious Disease, The Bill and Marion Cook Foundation (GDE); and The Oskar Fisher Project—a gift or Dr. James Truchard (GDE).
Supplementary Material
References
- Ahantarig A, Trinachartvanit W, Baimai V, Grubhoffer L (2013) Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol 58:419–428. [DOI] [PubMed] [Google Scholar]
- Aul JJ, Anderson KW, Wadowsky R, et al. (1998) Comparative evaluation of culture and PCR for the detection and determination of persistence of bacterial strains and DNAs in the Chinchilla laniger model of otitis media. Ann Otol Rhinol Laryngol 107:508–513. [DOI] [PubMed] [Google Scholar]
- Bonnet SI, Binetruy F, Hernández-Jarguín AM, Duron O (2017) The tick microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front Cell Infect Microbiol 7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Clark C, Ocasio K, et al. (2020) Factors affecting the microbiome of ixodes scapularis and Amblyomma Americanum. PLoS One 15:e0232398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke JS (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RN, Lane RS (1996) Reservoir competence of four Chaparral-Dwelling rodents for Borrelia Burgdorferi in California. Am J Trop Med Hygiene 54:84–91. [DOI] [PubMed] [Google Scholar]
- Castro MB, Wright SA (2007) Vertebrate hosts of Ixodes pacificus (Acari: Ixodidae) in California. J Vector Ecol 32:140–149. [DOI] [PubMed] [Google Scholar]
- Cheng MP, Domingo M-C, Lévesque S, Yansouni CP (2016) A case report of a deep surgical site infection with Terrisporobacter glycolicus/T. Mayombei and review of the literature. BMC Infect Dis 16:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicana B, Couper LI, Kwan JY, et al. (2019) Comparative microbiome profiles of sympatric tick species from the Far-Western United States. Insects 10:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clover JR, Lane RS (1995) Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of lyme disease in California. Am J Trop Med Hygiene 53:237–240. [DOI] [PubMed] [Google Scholar]
- Data and Surveillance | Lyme Disease | CDC (2019) February 5, 2019. https://www.cdc.gov/lyme/datasurveillance/index.html, accessed July 28, 2021.
- Denton M, Kerr KG (1998) Microbiological and clinical aspects of infection associated with stenotrophomonas maltophilia. Clin Microbiol Rev 11:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingler RJ, Wright SA, Donohue AM, et al. (2014) Surveillance for Ixodes pacificus and the tick-borne pathogens anaplasma phagocytophilum and borrelia burgdorferi in birds from California's inner coast range. Ticks Tick Borne Dis 5:436–445. [DOI] [PubMed] [Google Scholar]
- Earl JP, Adappa ND, Krol J, et al. (2018) Species-level bacterial community profiling of the healthy sinonasal microbiome using pacific biosciences sequencing of full-length 16S rRNA genes. Microbiome 6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich GD (1996) Syndromic illnesses demand multiplex PCR-based assays. Mol Diagn 1:83–87. [DOI] [PubMed] [Google Scholar]
- Ehrlich GD, Glaser J, Lavigne K, et al. (1989) Prevalence of human T-cell leukemia/lymphoma virus type II infection among high risk individuals: type-specific identification of HTLVs by polymerase chain reaction. Blood 74:1658–1664. [PubMed] [Google Scholar]
- Ehrlich GD, Hiller NL, Hu FZ (2008) What makes pathogens pathogenic. Genome Biol 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB (2016) County-scale distribution of ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol 53:349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshoo MW, Carolan HE, Massire C, et al. (2015) Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread anaplasmataceae species. PLoS One 10:e0135828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova N, Kleinjan JE, James D, et al. (2014) Remarkable diversity of tick or mammalian-associated borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick Borne Dis 5:951–961. [DOI] [PubMed] [Google Scholar]
- Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543. [Google Scholar]
- Gall CA, Reif KE, Scoles GA, et al. (2016) The bacterial microbiome of dermacentor andersoni ticks influences pathogen susceptibility. ISME J 10:1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AM, Pawlikowski NC, Hamer SA, et al. (2020) Landscape features predict the current and forecast the future geographic spread of lyme disease. Proc Biol Sci Royal Soc 287:20202278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall S, Manceau C (2003) Colonization of vitis vinifera by a green fluorescence protein-labeled, Gfp-marked strain of xylophilus ampelinus, the causal agent of bacterial necrosis of grapevine. Appl Environ Microbiol 69:1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall S, Roulland C, Guillaumès J, Manceau C (2005) Bleeding sap and old wood are the two main sources of contamination of merging organs of vine plants by xylophilus ampelinus, the causal agent of bacterial necrosis. Appl Environ Microbiol 71:8292–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greathouse KL, White JR, Vargas AJ, et al. (2018) Microbiome-TP53 Gene interaction in human lung cancer. Genome Biol 19:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greay TL, Gofton AW, Paparini A, et al. (2018) Recent insights into the tick microbiome gained through next-generation sequencing. Parasites Vectors 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, Eisen RJ (2016) Modeling the geographic distribution of ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J Med Entomol 53:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L (2012) Host Meal Affects the Tick Microbiome. Bard College. https://digitalcommons.bard.edu/senproj_s2012/46/, accessed July 28, 2021.
- Hunter DJ, Torkelson J, Bodnar J, et al. (2015) The rickettsia endosymbiont of Ixodes pacificus contains all the genes of de novo folate biosynthesis. PLoS One 10:e0144552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kada S, McCoy KD, Boulinier T (2017) Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents. Parasites Vectors 10:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BN, Funkhouser LJ, Setia S, Bordenstein SR (2011) Evolutionary genomics of a temperate bacteriophage in an obligate intracellular bacteria (Wolbachia). PLoS One 6:e24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Corker L, Powell EA, Mortensen JE (2019) Neonatal bacteremia and oligoarthritis caused by Rhodococcus corynebacterioides/Rhodococcus Kroppenstedtii. Diagn Microbiol Infect Dis 94:395–397. [DOI] [PubMed] [Google Scholar]
- Khaw TH, Wong SNM, Herle G, et al. (2020) Identification of bithionol, dichlorophen, and miconazole as antibacterial agents against acinetobacter calcoaceticus. ACS Omega 5:23951–23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Sawabe E, Ohkusu K, et al. (2012) First report of sepsis caused by rhodococcus corynebacterioides in a patient with myelodysplastic syndrome. J Clin Microbiol 50:1089–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan JY, Griggs R, Chicana B, et al. (2017) Vertical vs. horizontal transmission of the microbiome in a key disease vector, Ixodes pacificus. Mol Ecol 26:6578–6589. [DOI] [PubMed] [Google Scholar]
- Kwok S, Mack DH, Mullis KB, et al. (1987) Identification of human immunodefiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol 61:1690–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Brown RN, Piesman J, Peavey CA (1994) Vector competence of Ixodes pacificus and dermacentor occidentalis (Acari: Ixodidae) for various isolates of lyme disease spirochetes. J Med Entomol 31:417–424. [DOI] [PubMed] [Google Scholar]
- Margos G, Fedorova N, Becker NS, et al. (2020) Borrelia Maritima Sp. Nov., a novel species of the borrelia burgdorferi sensu lato complex, occupying a basal position to North American species. Int J Syst Evol Microbiol 70:849–856. [DOI] [PubMed] [Google Scholar]
- Mediannikov O, Sekeyová Z, Birg M-L, Raoult D (2010) A novel obligate intracellular gamma-proteobacterium associated with ixodid ticks, diplorickettsia massiliensis. PLoS One 5:e11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Fikrig E (2015) Tick microbiome: the force within. Trends Parasitol 31:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh N, Hosokawa T, Moriyama M, et al. (2014) Evolutionary origin of insect-Wolbachia Nutritional Mutualism. Proc Natl Acad Sci U S A 111:10257–10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregón D, Bard E, Abrial D, et al. (2019) Sex-specific linkages between taxonomic and functional profiles of tick gut microbiomes. Front Cell Infect Microbiol 9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WT, Giri SS, Yun S, et al. (2019) Janthinobacterium lividum as an emerging pathogenic bacterium affecting rainbow trout (oncorhynchus mykiss) fisheries in Korea. Pathogens 8:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett KA, Lane RS (2001) Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J Med Entomol 38:684–693. [DOI] [PubMed] [Google Scholar]
- Petersen A, Rosenstierne MW, Rasmussen M, et al. (2019) Field samplings of ixodes ricinus ticks from a tick-borne encephalitis virus micro-focus in Northern Zealand, Denmark. Ticks Tick Borne Dis 10:1028–1032. [DOI] [PubMed] [Google Scholar]
- Pfeiffer MB (2018) Lyme: the first epidemic of climate change. Island Press.
- Qiu Y, Nakao R, Ohnuma A, et al. (2014) Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS One 9:e103961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz EC, Hemmerich C, Rusch DB, et al. (2015) Concordance of bacterial communities of two tick species and blood of their shared rodent host. Mol Ecol 24:2566–2579. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Leonhard S, Girard YA, et al. (2008) Identifying the reservoir hosts of the lyme disease spirochete borrelia burgdorferi in California: the role of the western gray squirrel (Sciurus Griseus). Am J Trop Med Hygiene 79:535–540. [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Handelsman J (2004) Status of the microbial census. Microbiol Mol Biol Rev 68:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Antalis P, Gladitz J, et al. (2005) Identification, distribution, and expression of novel (nonRd) genes in ten clinical isolates of nontypeable haemophilus influenzae. Infect Immun 73:3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Antalis P, Gladitz J, et al. (2006a) Characterization, distribution and expression of novel genes among eight clinical isolates of Streptococcus pneumoniae. Infect Immun 74:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Sayeed S, Antalis P, et al. (2006b) Extensive genomic plasticity in Pseudomonas aeruginosa revealed by identification and distribution studies of novel (nonPAO1) genes among clinical isolates. Infect Immun 74:5272–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE (2018) Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int J Environ Res Public Health 15:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A, Kwan JY (2017) Tick microbiome and pathogen acquisition altered by host blood meal. ISME J 11:813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa S, Zhang Y, Allen MS (2019a) Effects of temperature on bacterial microbiome composition in ixodes scapularis ticks. Microbiol Open 8:e00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa S, Zhang Y, Allen MS (2019b) Bacterial microbiomes of ixodes scapularis ticks collected from Massachusetts and Texas, USA. BMC Microbiol 19:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully JG, Rose DL, Yunker CE, et al. (1995) Spiroplasma ixodetis Sp. Nov., a new species from Ixodes pacificus ticks collected in Oregon. Int J Syst Bacteriol 45:23–28. [DOI] [PubMed] [Google Scholar]
- Van Treuren W, Ponnusamy L, Brinkerhoff RJ, et al. (2015) Variation in the microbiota of ixodes ticks with regard to geography, species, and sex. Appl Environ Microbiol 81:6200–6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R-X, Yu C-M, Hsu S-T, Wang CH (2021) Emergence of concurrent levofloxacin- and Trimethoprim/sulfamethoxazole-resistant stenotrophomonas maltophilia: risk factors and antimicrobial sensitivity pattern analysis from a single medical center in Taiwan. J Microbiol Immunol Infect [Epub ahead of print]; DOI: 10.1016/j.jmii.2020.12.012. [DOI] [PubMed] [Google Scholar]
- Zolnik CP, Falco RC, Daniels TJ, Kolokotronis S-O (2018) Transient influence of blood meal and natural environment on blacklegged tick bacterial communities. Ticks Tick Borne Dis 9:563–572. [DOI] [PubMed] [Google Scholar]
- Zolnik CP, Prill RJ, Falco RC, et al. (2016) Microbiome changes through ontogeny of a tick pathogen vector. Mol Ecol 25:4963–4977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.